Introduction
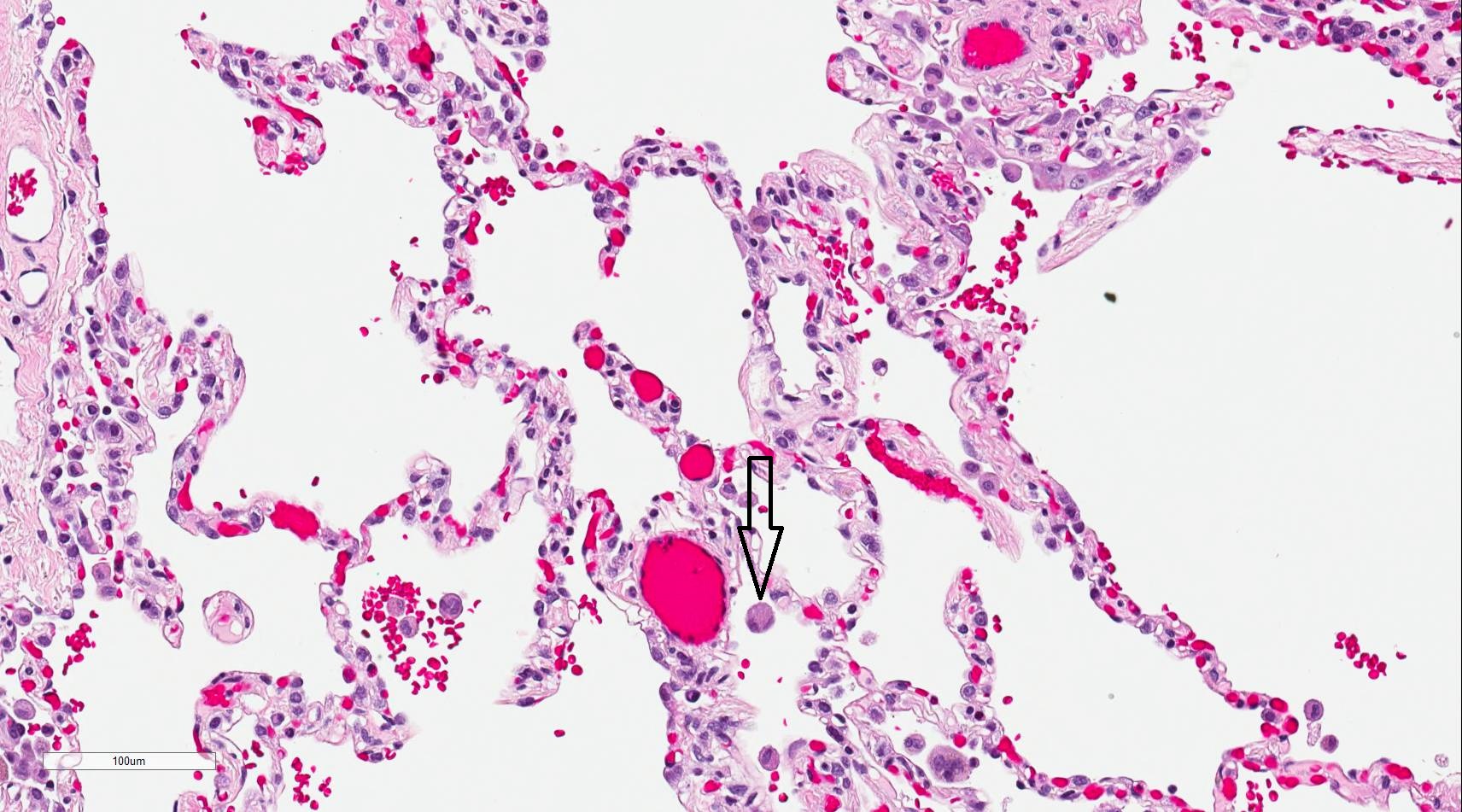
Alveolar macrophages, also known as dust cells, are phagocytic cells that play a crucial role in the immune defense of the respiratory system (see Image. Alveolar Macrophage). As part of the innate immune system, alveolar macrophages serve as the first line of defense against inhaled pathogens and particulate matter in the pulmonary alveoli and interalveolar septae near pneumocytes.
The alveoli, the terminal units of the respiratory system, are responsible for gas exchange between the lungs and the bloodstream. The alveolar structure consists of 3 primary cell types.
Type I pneumocytes form the alveolar wall and are essential for gas exchange, covering approximately 95% of the alveolar surface. Type I pneumocytes cannot replicate.[1]
Type II pneumocytes secrete pulmonary surfactant, a lipoprotein that lowers surface tension, preventing alveolar collapse during exhalation. Additionally, type II pneumocytes function as progenitor cells, capable of differentiating into either type I or new type II pneumocytes in response to injury, which is crucial for maintaining the structural integrity and function of the alveoli.[2][3]
Alveolar macrophages function as vital immune cells, clearing the alveoli of debris and pathogens through phagocytosis while producing cytokines and chemokines to recruit and activate other immune cells.[4] These roles make them pivotal in maintaining both immune defense and tissue homeostasis.[5] Alveolar macrophages are also essential for tissue remodeling and repair.[6]
All cells from the mononuclear phagocyte system originate from hematopoietic stem cells in the bone marrow, which give rise to both myeloid and lymphoid progenitors.[7] Common myeloid progenitors differentiate into various myeloid cells, including granulocyte-monocyte progenitors, which further develop into monocytes.[8]
Monocytes circulate in the bloodstream and subsequently migrate into tissues, differentiating into macrophages.[9] However, specific tissue-resident macrophages, including alveolar macrophages in the lungs, may develop on-site during embryonic stages and maintain their population independently of circulating monocytes through local proliferation.[10][11] Monocytes have a short lifespan in the blood, typically around 1 to 3 days. In contrast, tissue macrophages, such as alveolar macrophages, can persist for months to years under steady-state conditions.[12][13]
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
Alveolar macrophages' unique morphology and molecular characteristics allow them to interact effectively with inhaled particles and the local lung environment. The morphology of alveolar macrophages, including their size and shape, may vary due to phagocytic activity differences and influences from the lung microenvironment.[14]
Cell Shape and Size
Alveolar macrophages are relatively large cells, typically ranging between 15 μm and 21 μm in diameter, which allows them to engulf larger pathogens, debris, and particulate matter. These cells exhibit a round or oval shape when in resting states, but their shape can become more irregular with extended pseudopods when actively phagocytosing foreign particles. This dynamic ability to change shape is essential for their migratory and phagocytic activities.
Cytoplasmic Features
The cytoplasm of alveolar macrophages is abundant and contains numerous lysosomes, which are crucial for degrading engulfed material. The presence of phagosomes and secondary lysosomes indicates active phagocytosis and degradation of pathogens or inhaled particles. Electron microscopy reveals the dense cytoplasmic granularity in these cells, indicative of engulfed particulates like dust, bacteria, or carbon particles, hence the name "dust cells."[15] Alveolar macrophages also contain various vacuoles and lipid droplets, reflecting their role in metabolizing lipids, especially in conditions where lipid accumulation, such as pulmonary alveolar proteinosis (PAP) or chronic exposure to lipid-rich air pollutants, occurs.[16]
Plasma Membrane and Surface Receptors
The surfaces of alveolar macrophages house various receptors essential for recognizing pathogens, apoptotic cells, and signaling molecules. These receptors include pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), as well as scavenger receptors like CD204 and CD206. These molecules enable the recognition and binding of foreign particles and pathogens, facilitating phagocytosis and activation of the immune response.[17]
Fc receptors for immunoglobulins, as well as complement and cytokine receptors, are also abundant in this region, allowing the macrophages to respond to opsonized pathogens and inflammatory signals effectively. The CD11c receptor is highly expressed in alveolar macrophages. This receptor distinguishes alveolar macrophages from other macrophage populations and plays a crucial role in their antigen-presenting function and adhesion to the alveolar epithelium.[18]
Cytoskeletal Structure
The cytoskeleton of alveolar macrophages comprises actin filaments and microtubules, which provide structural integrity and facilitate their mobility within the alveolar spaces. The actin cytoskeleton undergoes rapid reorganization during phagocytosis, allowing macrophages to extend pseudopods and engulf particles. These cytoskeletal components are also essential for chemotaxis, the directed migration of alveolar macrophages toward signals of tissue injury or infection.[19][20][21][22]
Nucleus
The nucleus of an alveolar macrophage is typically oval-shaped and centrally located. This organelle contains euchromatin and heterochromatin, indicating a balance between active transcription—for responding to environmental stimuli—and resting states. The nuclear structure enables the macrophage to respond to external signals rapidly, produce cytokines, and initiate transcriptional changes necessary for inflammation and immune regulation.[23]
Interaction with the Alveolar Environment
Alveolar macrophages reside in the alveolar lumen, interacting closely with the alveolar epithelium, particularly type I and type II pneumocytes. Alveolar macrophages maintain an anti-inflammatory environment under normal conditions but can activate immune responses during pathogen invasion. The structure of these cells allows for constant surveillance and rapid response to any breach in the epithelial barrier.[24]
Function
Alveolar macrophages are crucial components of the immune system, residing within the alveolar spaces of the lungs. These cells are pivotal in maintaining lung homeostasis and protecting the respiratory system from inhaled pathogens, particulate matter, and environmental toxins. The functions of alveolar macrophages can be broadly classified into immune defense, tissue remodeling, and homeostasis.
Phagocytosis and Immune Defense
The primary function of alveolar macrophages is phagocytosis, which involves engulfing and digesting pathogens, apoptotic cells, and particulate matter entering the lungs through inhalation. Alveolar macrophages act as the first line of defense in the innate immune system by detecting and neutralizing foreign materials, including bacteria, viruses, and pollutants.
Pattern recognition receptors, including toll-like and scavenger receptors, recognize foreign invaders, prompting alveolar macrophages to initiate phagocytosis. During this process, the alveolar macrophages secrete reactive oxygen species and nitric oxide, which help neutralize pathogens. Additionally, alveolar macrophages release cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), interleukin-6, and interleukin-1β, to recruit and activate other immune cells, including neutrophils, monocytes, and lymphocytes, to the site of infection or injury. This immune communication is critical for coordinating the body's defense against pathogens and initiating inflammation to contain infections.
Clearing Cellular Debris and Surfactant Turnover
Besides pathogen clearance, alveolar macrophages are responsible for removing apoptotic cells, dead epithelial cells, and cellular debris from the alveolar spaces, preventing inflammation and preserving lung function. These roles are essential for maintaining a healthy alveolar environment, particularly in conditions of ongoing cellular turnover. Alveolar macrophages also participate in pulmonary surfactant clearance. Type II pneumocytes produce surfactant and help reduce surface tension in the alveoli, preventing alveolar collapse during exhalation. Alveolar macrophages phagocytose excess surfactant to maintain optimal surfactant levels and ensure proper gas exchange.[25] This surfactant clearance is crucial for preventing diseases such as PAP, characterized by impaired macrophage function leading to surfactant accumulation.
Anti-Inflammatory and Pro-Resolution Functions
While alveolar macrophages are essential in mounting an immune response, they also play a critical role in resolving inflammation. In times when infection is absent or inflammation is resolving, alveolar macrophages exhibit anti-inflammatory properties to prevent excessive immune reactions that could damage lung tissue. Alveolar macrophages produce anti-inflammatory cytokines, such as interleukin-10 and transforming growth factor-beta (TGF-β), which help downregulate inflammatory processes and promote tissue repair.[26] Alveolar macrophages of the M2 phenotype are primarily engaged in tissue repair and regeneration. Releasing growth factors and anti-inflammatory signals promote fibroblast activation and extracellular matrix deposition, aiding tissue remodeling after injury or infection.
Tissue Homeostasis and Immune Tolerance
Beyond immunity activation, alveolar macrophages are essential for maintaining tissue homeostasis and immune tolerance in the lungs. These cells continuously monitor the alveolar environment to ensure minor irritants or inhaled particles do not provoke unnecessary immune responses. Under normal conditions, alveolar macrophages actively suppress immune activation through various mechanisms, including the secretion of prostaglandins and TGF-β, which prevent immune cell infiltration and the release of pro-inflammatory cytokines.[27] This regulatory function is essential in the lungs, which are constantly exposed to external elements through breathing. By promoting immune tolerance to harmless particles and preventing overactive immune responses, alveolar macrophages help maintain lung integrity and function over time.[28]
Tissue Preparation
Alveolar macrophages are essential immune cells that reside in the alveolar spaces of the lungs, where they clear inhaled pathogens, debris, and particulate matter. Preparing tissue samples for studying alveolar macrophages requires careful techniques to preserve their structure, function, and immunological markers. Tissue preparation for alveolar macrophages involves several critical steps, including sample collection, fixation, staining, and, in some cases, advanced techniques like immunohistochemistry and electron microscopy.
Sample Collection
The first step in preparing tissue for alveolar macrophage analysis involves obtaining bronchoalveolar lavage (BAL) fluid or lung tissue. An overview of these procedures is provided below.
- Bronchoalveolar lavage: This minimally invasive technique involves injecting sterile saline into the airways and retrieving the fluid containing alveolar macrophages. BAL is widely used for isolating live alveolar macrophages for functional assays, flow cytometry, or in vitro studies. The cells may be immediately processed or cultured for further analysis.
- Lung tissue sectioning: Lung tissue may be obtained from experimental animals or clinical biopsies for studies requiring a more detailed anatomical context. Lung tissue is collected and prepared for histological examination after euthanasia or surgery. Lung tissue sectioning allows for studying alveolar macrophages within their natural tissue environment.
Fixation
Fixation is crucial for preserving cellular morphology and preventing autolysis or degradation. This step also immobilizes proteins and nucleic acids essential for subsequent staining or antibody labeling. The fixation methods employed when studying alveolar macrophages include the following:
- Formalin fixation with paraffin embedding: Lung tissue is often fixed in 10% neutral-buffered formalin to preserve alveolar macrophage architecture. The tissue is dehydrated and embedded in paraffin for sectioning, which is suitable for long-term preservation and immunohistochemical analysis.[29]
- Cryopreservation: In cases where preserving specific protein markers is necessary for studies like flow cytometry or immunofluorescence, tissues may be flash-frozen in optimal cutting temperature compound without formalin fixation. This step allows for immediate sectioning and analysis while maintaining functional proteins and cell surface markers.
- Glutaraldehyde fixation: Glutaraldehyde is a suitable fixative for high-resolution ultrastructural studies using electron microscopy. Glutaraldehyde preserves intracellular organelles, such as phagosomes and lysosomes, which is crucial for studying the phagocytic activity of alveolar macrophages.[30]
Staining Techniques
Various staining methods are employed to visualize alveolar macrophages in lung tissues after fixation and sectioning. The choice of staining depends on the type of analysis required (see section on Microscopy, Light).
Histochemistry and Cytochemistry
Immunohistochemistry and immunofluorescence are powerful techniques for identifying alveolar macrophages, both targeting cell surface or intracellular markers using antibodies. CD68 and CD11b are commonly used markers for macrophages, including alveolar macrophages. These glycoproteins are expressed in macrophage lysosomes and are reliably used to locate and quantify macrophages in lung sections.[31][32][33] Mouse F4/80 and human CD163, as well as CD204 or CD206, are additional markers for identifying alveolar macrophages. These molecules are widely used to study macrophages in both healthy and diseased lung tissue.[34]
Immunofluorescence allows for the simultaneous detection of multiple markers in alveolar macrophages, providing insights into their interactions with other lung immune cells or structural components. Confocal microscopy combined with immunofluorescence offers high-resolution, 3-dimensional images, which can be particularly useful for studying the distribution and behavior of alveolar macrophages in vivo.[35][36]
Microscopy, Light
Stains enhance the visibility of macrophages under light microscopy. Staining techniques essential for light microscopic visualization of the macrophages are discussed below.
Hematoxylin and Eosin Staining
Hematoxylin and eosin staining is the most commonly used staining technique for general histological examination. Hematoxylin stains the nuclei blue, while eosin stains the cytoplasm pink. Alveolar macrophages appear granular due to their phagocytic function, although they may be challenging to distinguish from other cells in resting states without specific markers.[37]
Periodic Acid-Schiff Staining
Periodic acid-Schiff staining highlights polysaccharides and glycoproteins in alveolar macrophages, enhancing these cells' visibility in lung tissue sections. This technique is especially useful when macrophages contain phagocytosed material, such as pathogens and environmental particles.[38]
Oil Red O Staining
Studies investigating lipid accumulation in alveolar macrophages, particularly in relation to conditions like lipid pneumonia and exposure to lipophilic substances, employ oil red O staining to identify lipid-laden macrophages.[39]
Microscopy, Electron
Transmission electron microscopy (TEM) or scanning electron microscopy (SEM) may be used for detailed ultrastructural analysis. These techniques provide highly detailed images of the internal structures of alveolar macrophages, such as phagosomes and lysosomes. Electron microscopy is particularly useful for studying macrophage-pathogen interactions or the ingestion of inhaled particulate matter.
Pathophysiology
Alveolar macrophages are central to maintaining lung homeostasis and immune defense, but alterations in their function can lead to various pathophysiological conditions. Alveolar macrophage dysregulation can contribute to both acute and chronic lung diseases through inappropriate immune activation, tissue damage, or impaired clearance of pathogens and debris.
Immune Response Dysregulation
In conditions such as chronic obstructive pulmonary disease (COPD) and asthma, alveolar macrophages can become excessively activated, producing large amounts of pro-inflammatory cytokines like TNF-α and interleukin-1β. This excessive immune response leads to chronic inflammation, airway remodeling, and tissue destruction. In COPD, alveolar macrophages also exhibit impaired phagocytic function, reducing their ability to clear pathogens and exacerbating infection risks.
Surfactant Accumulation and Alveolar Proteinosis
Alveolar macrophages are crucial in clearing excess pulmonary surfactant. In conditions like PAP, alveolar macrophage dysfunction leads to the accumulation of surfactant, impairing gas exchange and causing respiratory distress. Granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling, which is essential for the surfactant clearance function of alveolar macrophages, is defective.
Fibrotic Responses and Lung Fibrosis
Alveolar macrophages contribute to the development of pulmonary fibrosis by promoting excessive tissue remodeling and extracellular matrix deposition. In diseases like idiopathic pulmonary fibrosis, alveolar macrophages shift toward a profibrotic phenotype, secreting growth factors such as transforming growth factor-β and platelet-derived growth factor, which drive fibroblast activation and collagen deposition, ultimately leading to lung tissue scarring and loss of function.
Impaired Phagocytosis in Acute Lung Injury
Alveolar macrophages can become overwhelmed in acute conditions, such as acute respiratory distress syndrome (ARDS), leading to impaired clearance of pathogens and apoptotic cells. This dysfunction contributes to the persistence of inflammation and the development of diffuse alveolar damage, which characterizes acute respiratory distress syndrome. In such scenarios, macrophages may produce high levels of inflammatory cytokines and oxidative stress mediators, exacerbating lung injury.
Walling Off of Bacteria
Alveolar macrophages play an essential role in tuberculosis infections. Mycobacterium tuberculosis has developed mechanisms that resist macrophage phagocytosis. Alveolar macrophages gather around M tuberculosis and form a multinucleated giant cell, the Langerhans giant cell, surrounded by T-cells. TNF-α and interferon-γ are essential in forming granuloma.[40][41]
Tuberculosis is one of the leading causes of mortality and morbidity in patients with HIV, especially in underdeveloped countries where highly active antiretroviral therapy is not widely available. Additionally, the hallmark finding of noncaseating granulomas in certain systemic diseases, such as sarcoidosis, results from alveolar macrophages aggregating to isolate the infectious process. Alveolar macrophages also secrete vitamin D, which can lead to hypercalcemia in sarcoidosis—a clinical criterion that aids in diagnosing the condition.[42][43]
Alveolar macrophages also engulf environmental particles that harm them in the process. Carbon accumulation leading to a condition known as pneumoconiosis is often reported among coal mine workers. Crystalline silica particles can impair the immunologic response of alveolar macrophages, mimicking tuberculosis. For this reason, patients with a history of silica exposure should undergo periodic tuberculosis testing.
Clinical Significance
Alveolar macrophages are clinically significant due to their involvement in various lung diseases and serve as potential therapeutic targets for treatment. Some notable examples are explained below.
Biomarkers for Lung Disease
Alveolar macrophages can aid in diagnosing and monitoring lung diseases. In COPD and asthma, elevated levels of pro-inflammatory cytokines and impaired phagocytic function of alveolar macrophages can be detected in BAL fluid (BALF), helping to gauge disease severity and progression. Alveolar macrophages are also found in higher numbers in the BALF collected from the lungs of patients who smoke and have COPD. The macrophage count obtained by BAL is 4 to 6 times greater in smokers than in nonsmokers. Further, alveolar macrophages in smokers are morphologically different and contain more harmful pigment and free radicals than in nonsmokers.[44].
Emphysema is a chronic lung disease caused by the destruction of terminal airways due to elastases secreted by neutrophils. Interestingly, alveolar macrophages also secrete elastase, leading to elevated levels of elastases in the BALF of smokers.[45][46] Studies on the role of alveolar macrophages in emphysema provide valuable insight into the disease process and present new avenues for research. In pulmonary fibrosis, the presence of M2 macrophages in lung biopsies or BALF may indicate disease progression, as these macrophages are associated with tissue remodeling and fibrosis.
Role in Infection Control and Immune Modulation
Alveolar macrophages are critical in controlling respiratory infections such as tuberculosis, pneumonia, and viral infections like influenza and SARS-CoV-2 (COVID-19). In tuberculosis, alveolar macrophages are the primary cells infected by M tuberculosis, and their ability to contain the infection is central to disease progression or control. Targeting alveolar macrophages for therapeutic modulation may enhance the immune system’s ability to clear infections more effectively.
Therapeutic Targets in Lung Disease
Alveolar macrophages represent potential therapeutic targets in various lung diseases. In PAP, therapies aimed at restoring GM-CSF signaling in alveolar macrophages, such as recombinant GM-CSF administration, have been effective in reducing surfactant buildup and improving lung function. Similarly, in pulmonary fibrosis, therapies that modulate the fibrotic activity of M2 macrophages, such as TGF-β inhibitors, are being explored to slow disease progression and reduce scarring.[47]
Role in Cancer Immunotherapy
In lung cancer, alveolar macrophages can adopt a tumor-promoting phenotype and become "tumor-associated macrophages" (TAMs), which facilitate tumor growth by suppressing antitumor immune responses. Modulating the activity of these macrophages to restore their antitumor function is a promising area in cancer immunotherapy. Several strategies, including immune checkpoint inhibitors and macrophage-targeted therapies, are currently under investigation to harness the power of alveolar macrophages in combating lung cancer.
Media
(Click Image to Enlarge)
References
Schiller HB, Montoro DT, Simon LM, Rawlins EL, Meyer KB, Strunz M, Vieira Braga FA, Timens W, Koppelman GH, Budinger GRS, Burgess JK, Waghray A, van den Berge M, Theis FJ, Regev A, Kaminski N, Rajagopal J, Teichmann SA, Misharin AV, Nawijn MC. The Human Lung Cell Atlas: A High-Resolution Reference Map of the Human Lung in Health and Disease. American journal of respiratory cell and molecular biology. 2019 Jul:61(1):31-41. doi: 10.1165/rcmb.2018-0416TR. Epub [PubMed PMID: 30995076]
Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. American journal of physiology. Cell physiology. 2014 Jun 1:306(11):C987-96. doi: 10.1152/ajpcell.00321.2013. Epub 2014 Apr 16 [PubMed PMID: 24740535]
Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, Cui Z, Herriges MJ, Morley MP, Zhou S, Lu MM, Morrisey EE. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell reports. 2016 Nov 22:17(9):2312-2325. doi: 10.1016/j.celrep.2016.11.001. Epub [PubMed PMID: 27880906]
Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015 Dec:70(12):1189-96. doi: 10.1136/thoraxjnl-2015-207020. Epub 2015 Aug 18 [PubMed PMID: 26286722]
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014:6():13. doi: 10.12703/P6-13. Epub 2014 Mar 3 [PubMed PMID: 24669294]
Joshi N, Watanabe S, Verma R, Jablonski RP, Chen CI, Cheresh P, Markov NS, Reyfman PA, McQuattie-Pimentel AC, Sichizya L, Lu Z, Piseaux-Aillon R, Kirchenbuechler D, Flozak AS, Gottardi CJ, Cuda CM, Perlman H, Jain M, Kamp DW, Budinger GRS, Misharin AV. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. The European respiratory journal. 2020 Jan:55(1):. doi: 10.1183/13993003.00646-2019. Epub 2020 Jan 16 [PubMed PMID: 31601718]
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews. Immunology. 2005 Dec:5(12):953-64 [PubMed PMID: 16322748]
Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016 Mar 15:44(3):439-449. doi: 10.1016/j.immuni.2016.02.024. Epub [PubMed PMID: 26982352]
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013 Apr 25:496(7446):445-55. doi: 10.1038/nature12034. Epub [PubMed PMID: 23619691]
Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013 Apr 18:38(4):792-804. doi: 10.1016/j.immuni.2013.04.004. Epub [PubMed PMID: 23601688]
Guilliams M, Scott CL. Does niche competition determine the origin of tissue-resident macrophages? Nature reviews. Immunology. 2017 Jul:17(7):451-460. doi: 10.1038/nri.2017.42. Epub 2017 May 2 [PubMed PMID: 28461703]
Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews. Immunology. 2011 Oct 10:11(11):762-74. doi: 10.1038/nri3070. Epub 2011 Oct 10 [PubMed PMID: 21984070]
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nature immunology. 2013 Oct:14(10):986-95. doi: 10.1038/ni.2705. Epub 2013 Sep 18 [PubMed PMID: 24048120]
Malainou C, Abdin SM, Lachmann N, Matt U, Herold S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting. The Journal of clinical investigation. 2023 Oct 2:133(19):. doi: 10.1172/JCI170501. Epub 2023 Oct 2 [PubMed PMID: 37781922]
Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity. 2022 Sep 13:55(9):1564-1580. doi: 10.1016/j.immuni.2022.08.010. Epub [PubMed PMID: 36103853]
Bush A, Pabary R. Pulmonary alveolarproteinosis in children. Breathe (Sheffield, England). 2020 Jun:16(2):200001. doi: 10.1183/20734735.0001-2020. Epub [PubMed PMID: 32684993]
Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nature immunology. 2015 Jan:16(1):36-44. doi: 10.1038/ni.3052. Epub [PubMed PMID: 25521683]
Zeng D, Hu Z, Yi Y, Valeria B, Shan G, Chen Z, Zhan C, Lin M, Lin Z, Wang Q. Differences in genetics and microenvironment of lung adenocarcinoma patients with or without TP53 mutation. BMC pulmonary medicine. 2021 Oct 11:21(1):316. doi: 10.1186/s12890-021-01671-8. Epub 2021 Oct 11 [PubMed PMID: 34635074]
Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annual review of pathology. 2012:7():61-98. doi: 10.1146/annurev-pathol-011811-132445. Epub 2011 Sep 9 [PubMed PMID: 21910624]
Tong CS, Su M, Sun H, Chua XL, Xiong D, Guo S, Raj R, Ong NWP, Lee AG, Miao Y, Wu M. Collective dynamics of actin and microtubule and its crosstalk mediated by FHDC1. Frontiers in cell and developmental biology. 2023:11():1261117. doi: 10.3389/fcell.2023.1261117. Epub 2024 Mar 19 [PubMed PMID: 38567385]
May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. Journal of cell science. 2001 Mar:114(Pt 6):1061-77 [PubMed PMID: 11228151]
Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, Sharma L, Yildirim AÖ, Dela Cruz CS, Hogaboam CM. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. Journal of innate immunity. 2018:10(5-6):487-501. doi: 10.1159/000487057. Epub 2018 Feb 13 [PubMed PMID: 29439264]
Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia H, Yacoub TJ, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. The Journal of experimental medicine. 2017 Aug 7:214(8):2387-2404. doi: 10.1084/jem.20162152. Epub 2017 Jul 10 [PubMed PMID: 28694385]
Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nature reviews. Immunology. 2014 Feb:14(2):81-93. doi: 10.1038/nri3600. Epub 2014 Jan 21 [PubMed PMID: 24445666]
Morales-Nebreda L, Misharin AV, Perlman H, Budinger GR. The heterogeneity of lung macrophages in the susceptibility to disease. European respiratory review : an official journal of the European Respiratory Society. 2015 Sep:24(137):505-9. doi: 10.1183/16000617.0031-2015. Epub [PubMed PMID: 26324812]
Wynn TA,Vannella KM, Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016 Mar 15; [PubMed PMID: 26982353]
Gopalakrishnan A, Joseph J, Shirey KA, Keegan AD, Boukhvalova MS, Vogel SN, Blanco JCG. Protection against influenza-induced Acute Lung Injury (ALI) by enhanced induction of M2a macrophages: possible role of PPARγ/RXR ligands in IL-4-induced M2a macrophage differentiation. Frontiers in immunology. 2022:13():968336. doi: 10.3389/fimmu.2022.968336. Epub 2022 Aug 16 [PubMed PMID: 36052067]
Bhattacharya J, Westphalen K. Macrophage-epithelial interactions in pulmonary alveoli. Seminars in immunopathology. 2016 Jul:38(4):461-9. doi: 10.1007/s00281-016-0569-x. Epub 2016 May 12 [PubMed PMID: 27170185]
Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of experimental medicine. 2013 Sep 23:210(10):1977-92. doi: 10.1084/jem.20131199. Epub 2013 Sep 16 [PubMed PMID: 24043763]
Bosmann M, Ward PA. The inflammatory response in sepsis. Trends in immunology. 2013 Mar:34(3):129-36. doi: 10.1016/j.it.2012.09.004. Epub 2012 Oct 2 [PubMed PMID: 23036432]
Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. Journal of immunology (Baltimore, Md. : 1950). 2007 Feb 15:178(4):2000-7 [PubMed PMID: 17277103]
Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney international. 2005 Jun:67(6):2488-93 [PubMed PMID: 15882296]
Level 3 (low-level) evidenceLe Hir M, Kaissling B. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney international. 2005 Nov:68(5):2400; author reply 2400-1 [PubMed PMID: 16221249]
Level 3 (low-level) evidenceGordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010 May 28:32(5):593-604. doi: 10.1016/j.immuni.2010.05.007. Epub [PubMed PMID: 20510870]
Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014 Feb 27:506(7489):503-6. doi: 10.1038/nature12902. Epub 2014 Jan 19 [PubMed PMID: 24463523]
Kunz LI, Lapperre TS, Snoeck-Stroband JB, Budulac SE, Timens W, van Wijngaarden S, Schrumpf JA, Rabe KF, Postma DS, Sterk PJ, Hiemstra PS, Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respiratory research. 2011 Mar 22:12(1):34. doi: 10.1186/1465-9921-12-34. Epub 2011 Mar 22 [PubMed PMID: 21426578]
Level 2 (mid-level) evidenceSoroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. The Journal of experimental medicine. 2013 Apr 8:210(4):775-88. doi: 10.1084/jem.20121849. Epub 2013 Apr 1 [PubMed PMID: 23547101]
Ogawa T, Shichino S, Ueha S, Matsushima K. Macrophages in lung fibrosis. International immunology. 2021 Nov 25:33(12):665-671. doi: 10.1093/intimm/dxab040. Epub [PubMed PMID: 34270737]
Zhu Y, Choi D, Somanath PR, Zhang D. Lipid-Laden Macrophages in Pulmonary Diseases. Cells. 2024 May 22:13(11):. doi: 10.3390/cells13110889. Epub 2024 May 22 [PubMed PMID: 38891022]
Ufimtseva E, Eremeeva N, Bayborodin S, Umpeleva T, Vakhrusheva D, Skornyakov S. Mycobacterium tuberculosis with different virulence reside within intact phagosomes and inhibit phagolysosomal biogenesis in alveolar macrophages of patients with pulmonary tuberculosis. Tuberculosis (Edinburgh, Scotland). 2019 Jan:114():77-90. doi: 10.1016/j.tube.2018.12.002. Epub 2018 Dec 6 [PubMed PMID: 30711161]
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004 Dec 17:119(6):753-66 [PubMed PMID: 15607973]
Level 3 (low-level) evidenceMohan A, Malur A, McPeek M, Barna BP, Schnapp LM, Thomassen MJ, Gharib SA. Transcriptional survey of alveolar macrophages in a murine model of chronic granulomatous inflammation reveals common themes with human sarcoidosis. American journal of physiology. Lung cellular and molecular physiology. 2018 Apr 1:314(4):L617-L625. doi: 10.1152/ajplung.00289.2017. Epub 2017 Dec 6 [PubMed PMID: 29212802]
Level 3 (low-level) evidenceMortaz E, Masjedi MR, Abedini A, Matroodi S, Kiani A, Soroush D, Adcock IM. Common features of tuberculosis and sarcoidosis. International journal of mycobacteriology. 2016 Dec:5 Suppl 1():S240-S241. doi: 10.1016/j.ijmyco.2016.09.031. Epub 2016 Nov 9 [PubMed PMID: 28043581]
Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. American journal of respiratory and critical care medicine. 2002 Aug 1:166(3):382-5 [PubMed PMID: 12153975]
Lee KH, Jeong J, Koo YJ, Jang AH, Lee CH, Yoo CG. Exogenous neutrophil elastase enters bronchial epithelial cells and suppresses cigarette smoke extract-induced heme oxygenase-1 by cleaving sirtuin 1. The Journal of biological chemistry. 2017 Jul 14:292(28):11970-11979. doi: 10.1074/jbc.M116.771089. Epub 2017 Jun 6 [PubMed PMID: 28588027]
Lee KH, Lee J, Jeong J, Woo J, Lee CH, Yoo CG. Cigarette smoke extract enhances neutrophil elastase-induced IL-8 production via proteinase-activated receptor-2 upregulation in human bronchial epithelial cells. Experimental & molecular medicine. 2018 Jul 6:50(7):1-9. doi: 10.1038/s12276-018-0114-1. Epub 2018 Jul 6 [PubMed PMID: 29980681]
Huang S, Goplen NP, Zhu B, Cheon IS, Son Y, Wang Z, Li C, Dai Q, Jiang L, Xiang M, Carmona EM, Vassallo R, Limper AH, Sun J. Macrophage PPAR-γ suppresses long-term lung fibrotic sequelae following acute influenza infection. PloS one. 2019:14(10):e0223430. doi: 10.1371/journal.pone.0223430. Epub 2019 Oct 4 [PubMed PMID: 31584978]