Introduction
Acral lentiginous melanoma (ALM), also sometimes referred to as, simply, acral melanoma, is melanoma occurring on the hands and feet (palms, soles, fingers, toes, and nail units). The word acral derives from the Greek word referring to the highest or topmost portion of the limbs (extremities). The same stem word is used for the city of Akron (in Summit County, Ohio, the highest elevation on the Ohio and Erie Canal), and the acropolis (the rocky outcrop in Greece on which the Parthenon sits). Lentiginous refers to the initial origin of these tumors as a macular (flat) brown spot, resembling a benign lentigo.
The term melanoma refers to a malignancy of melanocytes, the pigment-producing cells in the epidermis. Acral melanoma was first separated as a distinct subtype of cutaneous malignant melanoma (CMM) by Arrington et al. in 1977.[1] It is the least common subtype of melanoma, comprising only 2 to 3% of total melanoma diagnoses. Unlike other CMM, UV-radiation is not thought to play a significant part in the development of ALM. It is most commonly on the lower extremities and is often advanced at the time of presentation leading to a high level of morbidity and mortality.[2][3][4] It has a higher proportional incidence in non-white populations compared to other subtypes of melanoma.[2] In this article, we will discuss the unique aspects of this disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Multiple studies proposed stress, or shearing force as a mechanism for the induction of ALMs, as the incidence is higher in the weight-bearing areas of the foot, such as the heel, forefoot, and lateral side of the foot.[5][6][7] Melanoma in the arch is rarer, however, was found to occur more frequently in obese patients.[8] However, most recently, Ghanavatian et al. found that once corrected for surface area, ALM was inversely proportional to atypical acral nevi and benign acral nevi in weight-bearing areas of the foot.[9] ALM may have an increased incidence on plantar surfaces compared to the palm, due to 50% more melanocytic density on the sole.[10] There has been some correlation in the literature with penetrative injuries increasing the likelihood of ALM compared to control patients without penetrative injury.[11][12] Typical mutations identified in other types of CMM, which are UV-induced, are not identified in ALM. The pathogenesis of ALM remains unclear, but genetic factors have been found to contribute.
Epidemiology
ALM accounts for 2 to 3% of all new melanomas. The average age of diagnosis for ALM is 62.8 years. The incidence of ALM in all populations increases with age, and a sharp increase is seen in incidence per person year after the age of 80. Similar numbers of males and females are affected; however, females are more commonly diagnosed at an earlier stage than males. ALM has a disproportionately higher incidence in non-white patients compared to other melanoma subtypes.[3] It does not represent the most common type of melanoma in any racial group in United States-based studies.[4]
Although studies from Mexico, Taiwan, and China have found it to be the most common subtype in their populations.[13][14][15] In two United States population-based registry studies, Bradford et al. and Huang et al. found that ALM accounted for 33-36% of CMM in Blacks. ALM represented 18-23.1% of total CMM in Asian/Pacific Islanders. ALM represented 9% of CMM diagnosed in Hispanic Whites and only 1% in non-Hispanic Whites.[3] Incidence rates of ALM were highest in Hispanic Whites at 2.5 per 1,000,000 person-years and significantly lower in Blacks, Non-Hispanic Whites, and Asian/Pacific Islanders at 1.8, 1.8, and 1.1 per 1,000,000 person-years, respectively.[2] Melanoma specific survival (MSS) was lower in Black and Hispanic White populations at 5 and 10 years than whites; however, when controlled for Breslow depth, no difference was seen. This is thought to be attributable to the advanced stage at presentation in these minority groups.[3]
Pathophysiology
ALM, like mucosal melanomas, is a low mutational burden sub-type of melanoma and does not carry UV-radiation induced mutations that are commonly seen in other CMMs.[16][17][18] Common gene mutations in ALM include KIT, BRAF, NRAS, and NF1.[16][19] Specifically, KIT copy number gains are seen in up to 36% of acral melanomas, which is thought to be the most common mutation in ALM, which is much higher than other CMM. In one study, the most commonly identified molecular aberration was chromosomal instability in the Cyclin D1 gene and was seen in 45% of ALM. Cytologically typical melanocytes which surround the atypical lesion often harbor KIT copy number gains and Cyclin D1 mutations, which is a concept known as 'field effect' and may explain local recurrences after excision of the clinical lesion.
In a paper on FISH analysis of ALM, CCND1 gains, MYC gains, and CDKN2A loss were common alterations seen in their cohort. TERT promoter mutations, commonly found in UV-radiation induced melanomas, are not prevalent in ALM; however, other mutations involving the TERT gene including copy gains, translocations, missense, and promoter mutations were found in up to 41% of cases.[16] It has been proposed by Su, J. et al. that the carcinogenesis of ALM could be ethnicity dependent, which might help explain the large variety of genetic aberrations seen in the literature.[20][21]
Histopathology
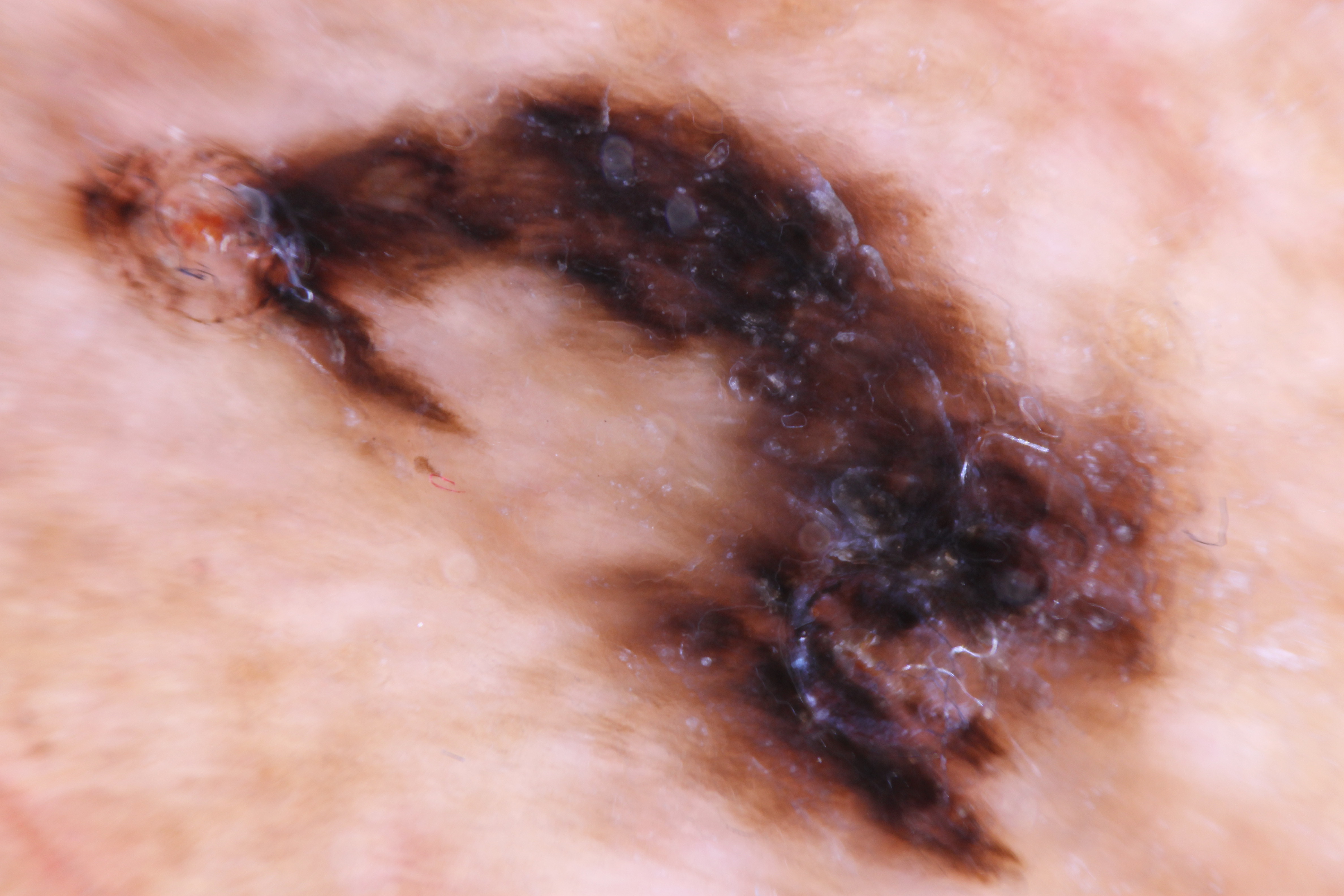
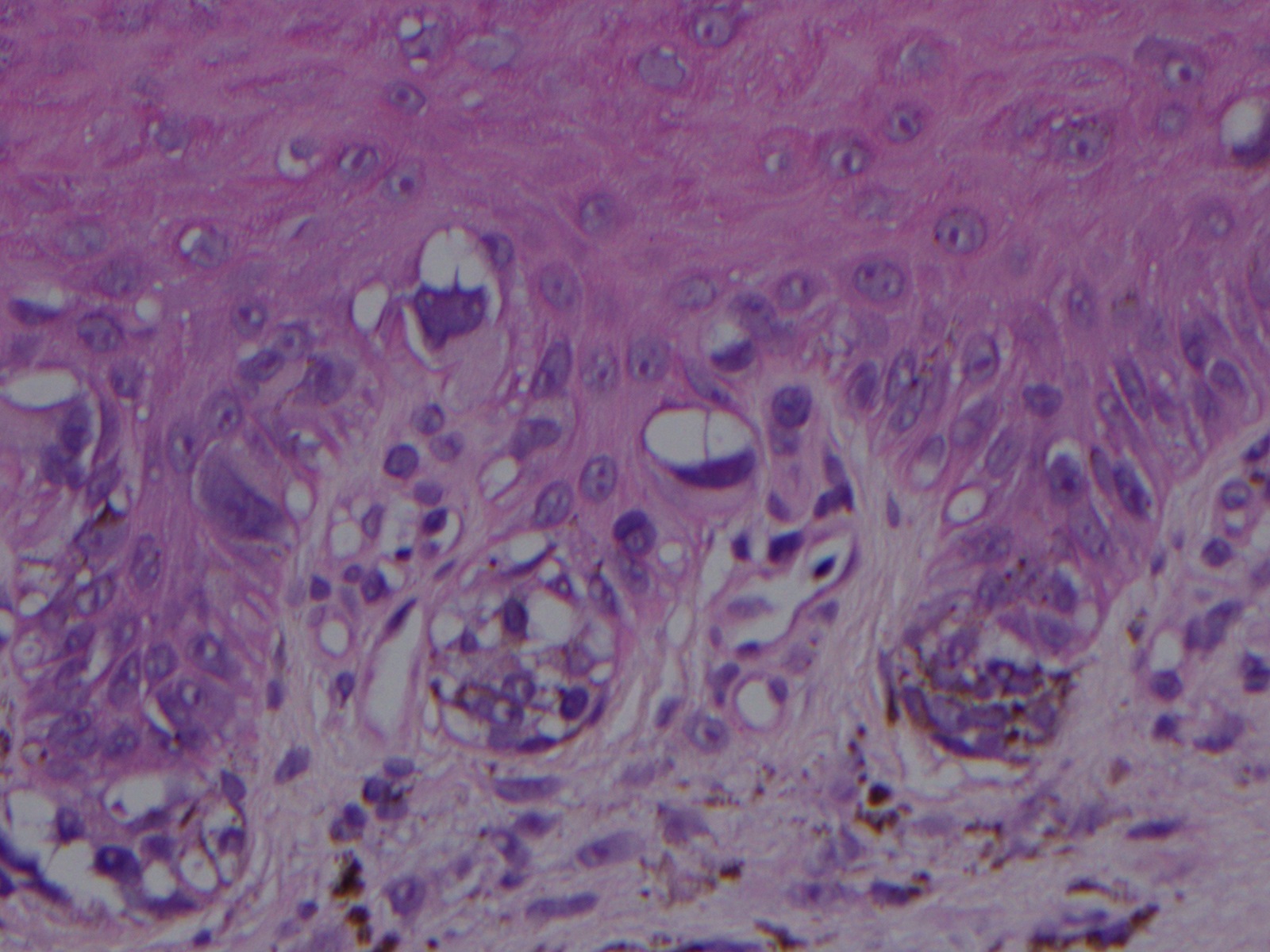
ALM is characterized by a broad radial growth phase in which the melanocytic neoplasm remains in-situ. In this phase, single atypical melanocytes are increased predominately at the dermo-epidermal junction in a lentiginous pattern with a propensity to involve the adnexal structures.[22] In an advanced in-situ lesion, rare nests can be seen, and they are typically larger, more discohesive, and variable in size and shape compared to their benign counterparts.[4] Pagetoid spread, a common feature of both benign and malignant acral melanocytic lesions can be prominent at the peripheral edges and within the ridges, which impart the characteristic dermoscopic findings of “parallel ridges” that is associated with acral melanocytic malignancy.[4][23] Cytologic atypia of melanocytes can be prominent in ALM and manifests as nuclear hyperchromasia, increased size, and variable shape. Invasion occurs typically many years after the lesion begins and consists of nested or single melanocytes invading the dermis. The invasive portion of ALM lesions typically resembles superficial spreading melanoma but may progress to a more nodular morphology with time.
Histopathologic evaluation of acral melanocytic lesions is difficult as the lesions may be subtle and heterogenous microscopically.[24] This is further complicated by the partial sampling of lesions due to the large lesion size at presentation.[25] Additionally, because of the unique diagnostic features of acral melanocytic lesions, special attention must be paid when gross dissection is performed to ensure sectioning perpendicular to the dermatoglyphics and is best accomplished by experienced laboratory personnel.
History and Physical
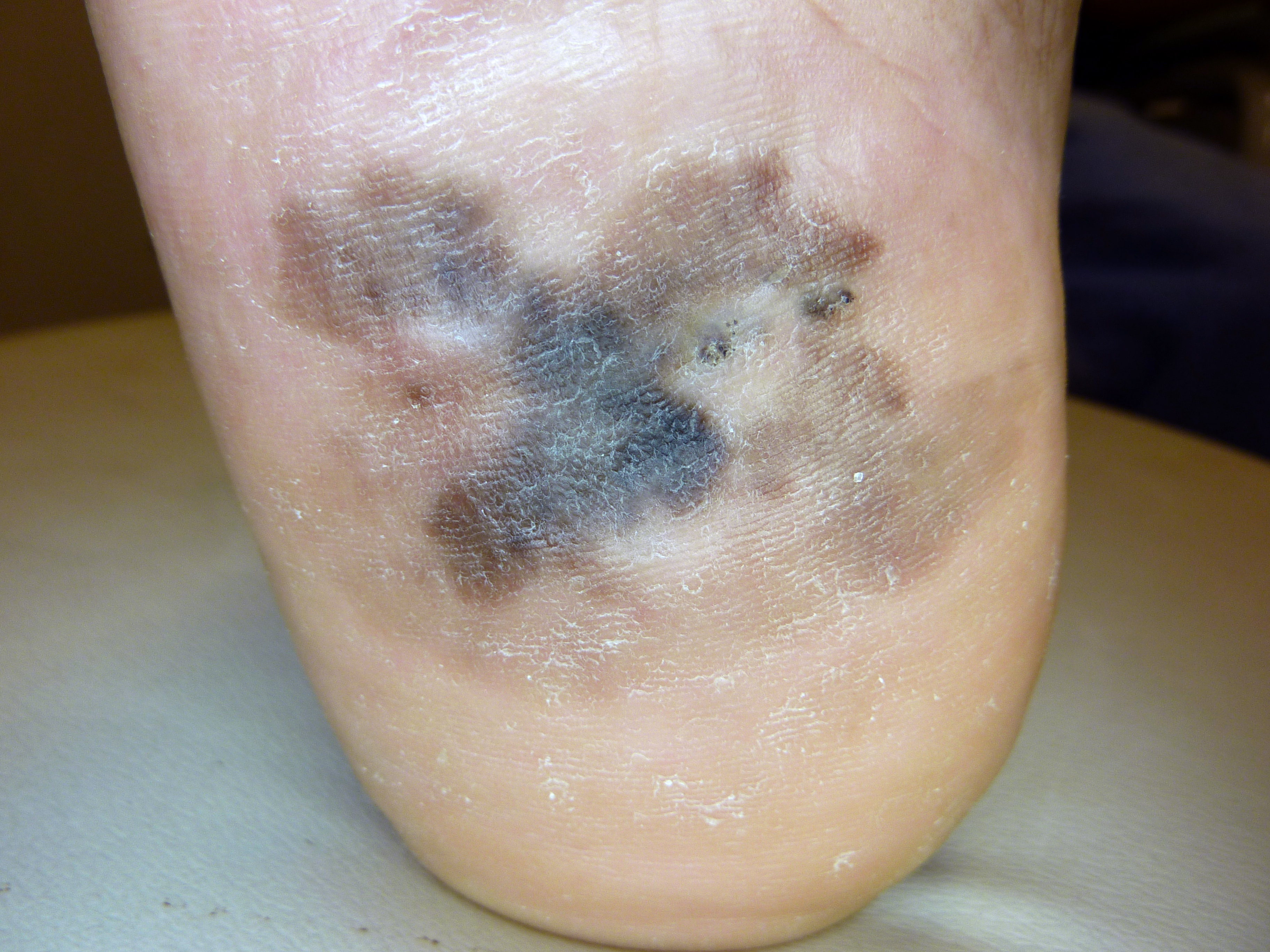
ALM is most commonly an acquired lesion and is associated with a previously existing nevus in less than 11% of cases.[26] It typically begins as a macule progressing to a patch with variable light to dark brown pigment. The edges are typically angular, often following the ridges of the dermatoglyphics. As the lesion progresses and invades, it may become nodular and darkly pigmented, appearing blue to black. Ulceration may occur as the lesion becomes more nodular.
It has been proposed the that classical “ABCDE” approach for melanoma be replaced on acral surfaces with the “CUBED” acronym which stands for colored lesions where any part is a different color, uncertain diagnosis or a lesion without a clear clinical diagnosis, bleeding lesions, enlargement of a lesion, and a delay in healing beyond two months.[27]
The dermoscopic examination is a useful adjunct for pigmented acral lesions to help identify worrisome pigment patterns.[28] Two systems to assess pigmented acral lesions on dermoscopy have been proposed. The first by Koga and Saida is a 3-step algorithm. Concerning features of an acral lesion on dermoscopy, include a parallel ridge pattern and/or lack of typical features of an acral nevus (i.e., fibrillar, lattice-like, and parallel furrow patterns) and a size greater than 7 mm.[29] The second, by Lallas et al., uses six dermatoscopic variables to assess the likelihood of ALM under the acronym BRAFF.[30] Four positive variables include blotches, ridge pattern, asymmetry of structures, and colors, and two negative variables include furrow pattern and fibrillar pattern. Despite the dermoscopic and clinical appearance, lesions greater than 7 mm in diameter should be biopsied, and all lesions greater than 9 mm are likely to be melanoma.[28][31]
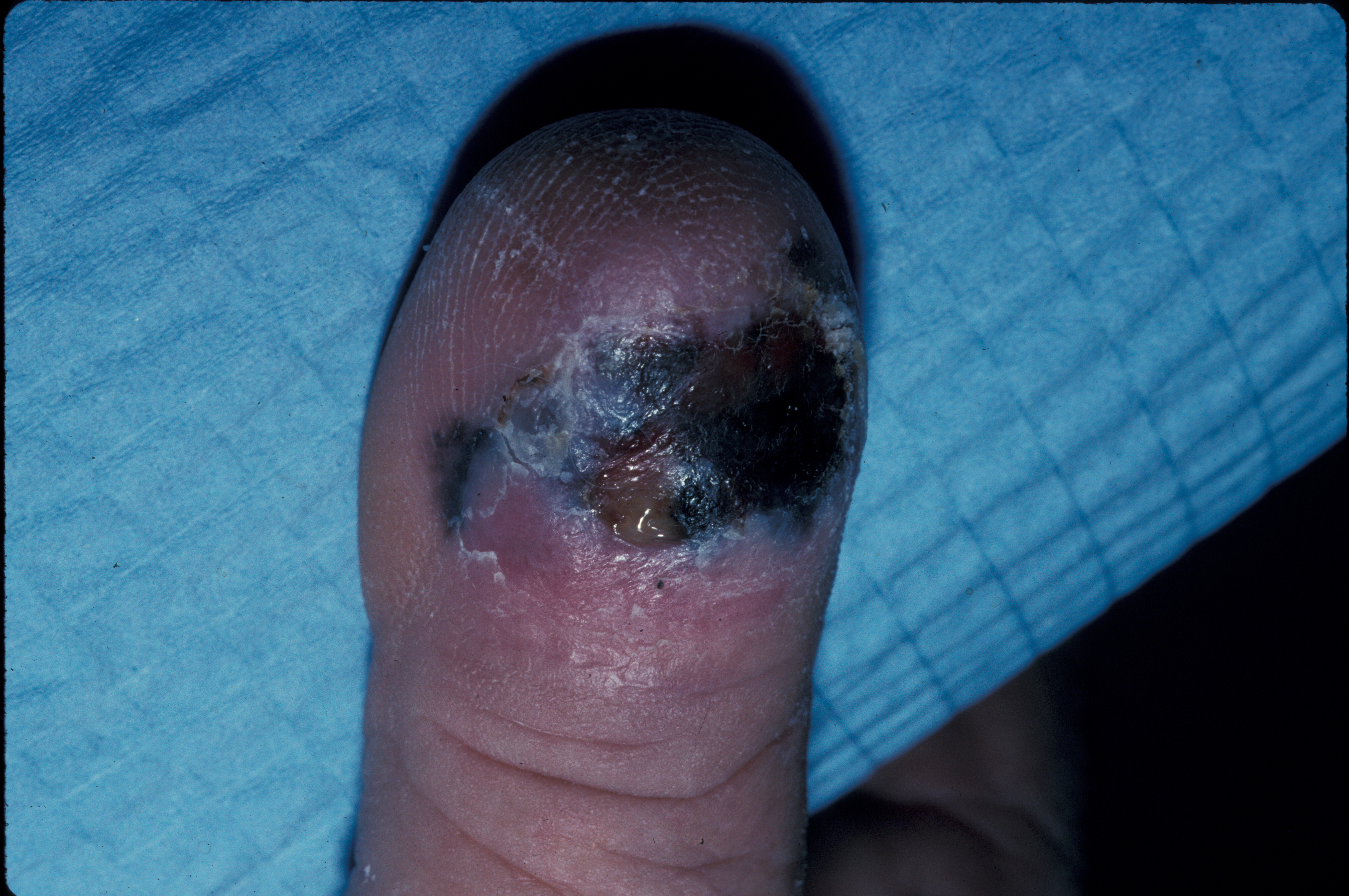
ALM of the nail unit will often present as diffuse pigmentation of the nail, pigmented striping of the nail, or other non-pigmented changes, such as ulceration. The thumb and great toe are the site of subungual ALM in 92% of cases.[10] When pigment from a subungual ALM extends into the nail fold, it is known as Hutchinson sign and is a worrisome clinical feature. The lesion may begin as a subungual ALM with pigment isolated to the nail fold; however, extension into the adjacent glabrous skin is common with advanced lesions.
Evaluation
For clinically suspected melanomas, narrow margin excisional biopsy is recommended; however, it is acknowledged in the recommendations that this may be impractical on an acral site.[32] A partial sampling of the lesion is then considered an acceptable alternative, although the main risk with partial sampling is that it may result in an inaccurate histologic diagnosis. The most nodular portion of the lesion should be sampled so that an accurate Breslow depth can be determined. With suspicious nail lesions, nail matrix sampling is necessary. The surgical techniques in nail sampling are complicated and should be referred to a provider skilled in these techniques to ensure diagnostic accuracy.[32] Subsequently, biopsy evaluation by a dermatopathologist is often required as the findings in ALM may be subtle and require a high level of clinicopathologic correlation.
If the patient is shown to have the local cutaneous disease and is otherwise asymptomatic, imaging studies and other laboratory tests such as blood work are not recommended.[32] In later stage disease, lactate dehydrogenase levels, while not usually the sole indicator of metastatic disease, are a good predictor of survival in patients with stage IV disease.[33]
Radiologic assessment to detect occult nodal disease or distant metastases has a high rate of false positives. However, if imaging is obtained, a chest x-ray should be considered as a cost-effective option. Gratuitous imaging may result in unnecessary invasive procedures. The American Academy of Dermatology suggests yearly skin exam and regional lymph node palpation as sufficient to assess for recurrence.[32]
Sentinel lymph node biopsies have high prognostic value in intermediate and thick melanomas. In patients with intermediate thickness melanomas and sentinel lymph node metastases, the melanoma-specific survival (MSS) was 62.1% when sentinel lymph node biopsy and completion lymphadenectomy were performed, but only 41.5% in the observation group who had a completion lymphadenectomy after clinical nodal metastases were identified.[34] The MSS was not affected significantly in patients with thick melanomas who underwent sentinel lymph node biopsy. Despite the previously mentioned study, these findings do remain controversial, with many authors questioning the survival benefit of sentinel lymph node biopsies.[32]
Treatment / Management
Wide local excisions are the mainstay for ALM. Definitive surgical margin recommendations are based on tumor thickness at the time of biopsy, which is further discussed in the surgical oncology section. Foregoing surgical excision of in-situ lesions when surgery is not practical can be considered.
Off label use of topical imiquimod has been studied; however, the persistence of in-situ lesions, failure to detect invasive lesions due to lack of excision, the progression of lesions, and the high cost of treatment are all potential pitfalls of this treatment. If metastases are detected clinically, by palpation of local lymph node beds, or by sentinel lymph node biopsy, systemic treatment with chemotherapeutic agents, targeted mutational therapy, and immune checkpoint inhibitors may be indicated which is further discussed below in the oncology section. Patients who have asymptomatic high stage disease do not show a survival benefit from treatment before symptoms arise.[32]
Differential Diagnosis
The differential diagnosis for ALM is relatively broad. The differential includes other melanocytic neoplasms such as lentigo, congenital acral nevi, and acquired acral nevi. The differential for subungual ALM includes ethnic pigmentation, lentigo, and nevi. Other non-melanocytic lesions that may mimic ALM on the glabrous skin and subungual nail include fungal and bacterial infections, trauma-related hemorrhage (talon noir), terra firma-forme dermatosis, chronic wounds, verrucae, and other skin cancers which may be secondarily pigmented such as squamous cell carcinoma or porocarcinoma.[35][36]
Surgical Oncology
Wide local excision is the primary treatment for ALM.
The suggested excision depth is to the fascia or deep adipose, depending on the location. The AAD and AJCC guidelines suggest variable clinical, surgical margins depending on Breslow depth[32]:
- In-situ lesions: 0.5 to 1 cm clinical margins
- Breslow depth less than 1 mm: 1 cm clinical margin
- Breslow depth between 1.01 to 2 mm: 1 to 2 cm clinical margin
- Breslow depth greater than 2 mm: 2 cm clinical margin
Large defects on acral surfaces are hard to close primarily and may require skin grafting or flap reconstruction. Primary closure has better functional results, heals faster, leads to fewer patient complications, and has a lower cost.[37] Healing by secondary intention is an acceptable alternative. With tissue defects larger than 1.5 cm, an alternative form of closure will help prevent painful scars and contractures.[38] For ALM located on the digits, wide local excisions have been shown to have a similar prognosis compared to amputations.[39]
Subungual melanomas are difficult to resect due to the proximity to the phalanx. The previous gold standard of treatment involved digit amputation.[40] More recently, a review of 67 cases of conservative surgical excision of subungual melanoma determined wide local excision without removal of bone, followed by full-thickness skin graft had better functional outcomes for the patient with good cosmesis and achieved the same disease-free survival interval.[41] A prospective multi-institutional trial is currently underway with the goal to confirm that conservative non-amputation treatment of subungual melanoma is a safe and efficacious treatment.[40]
Radiation Oncology
Radiation therapy is not commonly viewed as a primary approach to treating these lesions since melanocytes are not highly radiosensitive. However, this treatment has been used for adjuvant treatment, palliation, and for patients with recurrent disease.[42]
Medical Oncology
Dacarbazine had been the mainstay of metastatic melanoma chemotherapy, until the recent advent of immune checkpoint inhibitors and mutation-driven therapy; however, it never showed a clear survival benefit.[43][44]
Metastatic ALM has been shown to be less susceptible to immune checkpoint inhibitors owing to a poor immune response to the tumor.[43] PDL1 was found to be expressed in 31% of ALMS with a lower number of tumor-infiltrating lymphocytes (TILs) than other types of melanoma.[45] Immune stimulators such as imiquimod, dacarbazine, and interferon may help increase TILs within ALM and thus increase the ability of the immune checkpoint inhibitors to function.[43] Imiquimod on its own has been shown to prevent progression and even regression of ALM.[46][47] However, utility in acral skin with thick stratum corneum may be limited.[43]
While other types of CMM harbor frequent BRAF mutations, ALM rarely has these mutations and is thus less susceptible to the commonly used BRAF/MEK inhibitors. NRAS mutations may cause the resultant tumor to be susceptible to BRAF and MEK inhibitors due to the location of the NRAS upstream of BRAF.[43] Increased KIT mutations seen in ALM can be targeted with KIT inhibitors. However, studies have shown imatinib, KIT inhibitor, to be more successful in treating CMM with KIT mutations than with KIT copy number gains, as seen in ALM.[48]
Staging
Staging for all subtypes of melanoma is the same and based on the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th Edition.[49] There are four stages, 1 to 4, which are based on various parameters of the TNM pathologic grading system. Below is a brief summary.
- Stage 1: T1a, T1b, T2a primary lesions with no nodal, regional, or distant metastases
- Stage 2: T2b, T3a, T3b, T4a, T4b primary lesions with no nodal, regional, or distant metastases
- Stage 3: Any primary lesion with regional nodal or in-transit metastases
- Stage 4: Any primary lesion with distant metastases.
Prognosis
Prognosis in ALM depends on the evaluation of multiple clinicopathologic factors. Disease-specific survival is affected by gender, race, age, Breslow thickness, ulceration, pathologic stage, and sentinel lymph node positivity.[3] In two studies, through multivariant analysis, positive sentinel lymph nodes were the strongest predictor of disease recurrence and death from melanoma.[34][15] Compared to CMM at the same stage and depth, ALM has lower respective survival rates [3][50][51][50][52] with few studies finding that survival rates are similar.[53]. ALMs often present at a more advanced stage, which is thought to be multifactorial in cause. Contributing to this is difficulty in observation of the soles of the feet by the elderly, lack of knowledge in minority populations about their risk for melanoma, decreased access to care, and other cultural factors.
Complications
Large excisions of ALM, particularly on the hands, can lead to contractures and painful scarring, which can lead to significant morbidity.[38] If digit or limb amputation is required for treatment, patients can experience significant interference with activities of daily living, loss of function of the affected limb, phantom pain, and poor cosmetic outcomes.[40]
Deterrence and Patient Education
Because the causation of ALM cannot be attributed to any specific exposure, avoidance is difficult. Patient education and early detection make the most difference in disease-specific survival. Increased education is necessary for populations who have a low incidence of sun-induced melanoma as they have decreased MSS compared to non-Hispanic White populations and may be less aware of the warning signs of melanoma.[53]
Pearls and Other Issues
1. Benign acral nevi are more likely to have pigment found in the furrows of the dermatoglyphic lines, as opposed to acral melanomas, which are more likely to have pigment accentuated in the elevated ridges. There is an adage saying "furrows are fine, ridges are risky," referring to the pigment accentuation viewed with magnification or dermoscopy.
2. Dirt on the soles (terra firma-forme dermatosis) can resemble acral nevi, but usually will rub off with an alcohol prep.
3. Tinea nigra, a pigmented fungus found in warm climates, has been mistaken for melanoma, and unnecessary surgeries have been performed on these lesions. A simple KOH-preparation will reveal the hyphae.
4. Talon noir (French for black heel) can resemble acral melanoma. This is simply blood in the stratum corneum from trauma, common from running or other activity. Examination with dermoscopy can often eliminate the need for biopsy.
5. Small incomplete biopsies can be misinterpreted as benign by the pathologist. It is best to sample the entire lesion, when possible. If the lesion is large, then alert the pathologist of the total lesion size on the requisition, to increase suspicion.
6. Shave biopsies are difficult to perform on thick acral skin and often result in samples that are too superficial. Usually, a punch biopsy or excisional biopsy is better.
7. Benign acral nevi can have pagetoid melanocytes (cells that ascend upward through the epidermis as do adenocarcinoma cells in Paget's disease) in one-third of cases, causing a misdiagnosis of acral melanoma. In benign acral nevi, the pagetoid cells are more likely to be limited to the central portion of the lesion, whereas in melanomas, the pagetoid cells are more likely to extend into the margins. Acral nevi have been called MANIAC nevi (melanocytic acral nevus with the intraepidermal ascent of melanocytes).
Enhancing Healthcare Team Outcomes
An interprofessional approach is paramount in providing care for patients with ALM. Primary care providers play an important role in patient education and the detection of early changing skin lesions. Once detected, dermatologists are often consulted to confirm the suspicious nature of the lesion and perform the initial biopsies. Evaluation of the lesional biopsy by a trained dermatopathologist is also necessary as the diagnosis is difficult and requires significant clinicopathologic correlation. Treatment may involve multiple specialties include Mohs surgeons, general surgeons, or orthopedic surgeons for definitive excision of the lesion. If metastases are expected, surgical oncology and hematology/oncology should be consulted to facilitate sentinel lymph node biopsy and consider systemic therapies as warranted.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Arrington JH 3rd, Reed RJ, Ichinose H, Krementz ET. Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma. The American journal of surgical pathology. 1977 Jun:1(2):131-43 [PubMed PMID: 602975]
Level 2 (mid-level) evidenceBradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Archives of dermatology. 2009 Apr:145(4):427-34. doi: 10.1001/archdermatol.2008.609. Epub [PubMed PMID: 19380664]
Huang K, Fan J, Misra S. Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006-2015, an Analysis of the SEER Registry. The Journal of surgical research. 2020 Jul:251():329-339. doi: 10.1016/j.jss.2020.02.010. Epub 2020 Mar 21 [PubMed PMID: 32208196]
Fernandez-Flores A, Cassarino DS. Histopathological diagnosis of acral lentiginous melanoma in early stages. Annals of diagnostic pathology. 2017 Feb:26():64-69. doi: 10.1016/j.anndiagpath.2016.08.005. Epub 2016 Aug 20 [PubMed PMID: 27601330]
Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA dermatology. 2013 Nov:149(11):1281-8. doi: 10.1001/jamadermatol.2013.5853. Epub [PubMed PMID: 24067997]
Level 2 (mid-level) evidenceMinagawa A, Omodaka T, Okuyama R. Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. The New England journal of medicine. 2016 Jun 16:374(24):2404-6. doi: 10.1056/NEJMc1512354. Epub [PubMed PMID: 27305207]
Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Tseng YJ, Lee CH, Chang YL, Chu CY. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Scientific reports. 2017 Jul 17:7(1):5564. doi: 10.1038/s41598-017-05809-9. Epub 2017 Jul 17 [PubMed PMID: 28717212]
Costello CM, Pittelkow MR, Mangold AR. Acral Melanoma and Mechanical Stress on the Plantar Surface of the Foot. The New England journal of medicine. 2017 Jul 27:377(4):395-396. doi: 10.1056/NEJMc1706162. Epub [PubMed PMID: 28745985]
Ghanavatian S, Costello CM, Buras MR, Cumsky HJL, Pittelkow MR, Swanson DL, Mangold AR. Density and distribution of acral melanocytic nevi and acral melanomas on the plantar surface of the foot. Journal of the American Academy of Dermatology. 2019 Mar:80(3):790-792.e2. doi: 10.1016/j.jaad.2018.07.019. Epub 2018 Jul 25 [PubMed PMID: 30055202]
Feibleman CE, Stoll H, Maize JC. Melanomas of the palm, sole, and nailbed: a clinicopathologic study. Cancer. 1980 Dec 1:46(11):2492-504 [PubMed PMID: 7438021]
Level 2 (mid-level) evidenceGreen A, McCredie M, MacKie R, Giles G, Young P, Morton C, Jackman L, Thursfield V. A case-control study of melanomas of the soles and palms (Australia and Scotland). Cancer causes & control : CCC. 1999 Feb:10(1):21-5 [PubMed PMID: 10334638]
Level 2 (mid-level) evidenceLundberg R, Brytting M, Dahlgren L, Kanter-Lewensohn L, Schloss L, Dalianis T, Ragnarsson-Olding B. Human herpes virus DNA is rarely detected in non-UV light-associated primary malignant melanomas of mucous membranes. Anticancer research. 2006 Sep-Oct:26(5B):3627-31 [PubMed PMID: 17094377]
Lino-Silva LS, Domínguez-Rodríguez JA, Aguilar-Romero JM, Martínez-Said H, Salcedo-Hernández RA, García-Pérez L, Herrera-Gómez Á, Cuellar-Hubbe M. Melanoma in Mexico: Clinicopathologic Features in a Population with Predominance of Acral Lentiginous Subtype. Annals of surgical oncology. 2016 Dec:23(13):4189-4194 [PubMed PMID: 27401447]
Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC, Kuo TT, Yang CH. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma research. 2004 Dec:14(6):537-41 [PubMed PMID: 15577327]
Level 3 (low-level) evidenceWei X, Wu D, Li H, Zhang R, Chen Y, Yao H, Chi Z, Sheng X, Cui C, Bai X, Qi Z, Li K, Lan S, Chen L, Guo R, Yao X, Mao L, Lian B, Kong Y, Dai J, Tang B, Yan X, Wang X, Li S, Zhou L, Balch CM, Si L, Guo J. The Clinicopathological and Survival Profiles Comparison Across Primary Sites in Acral Melanoma. Annals of surgical oncology. 2020 Sep:27(9):3478-3485. doi: 10.1245/s10434-020-08418-5. Epub 2020 Apr 6 [PubMed PMID: 32253677]
Level 2 (mid-level) evidenceLiang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, Craig DW, Adkins J, Cuyugan L, Manojlovic Z, Halperin RF, Helland A, Nasser S, Legendre C, Hurley LH, Sivaprakasam K, Johnson DB, Crandall H, Busam KJ, Zismann V, Deluca V, Lee J, Sekulic A, Ariyan CE, Sosman J, Trent J. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome research. 2017 Apr:27(4):524-532. doi: 10.1101/gr.213348.116. Epub [PubMed PMID: 28373299]
Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. Journal of cutaneous pathology. 2014 Jun:41(6):504-8. doi: 10.1111/cup.12323. Epub 2014 Apr 2 [PubMed PMID: 24588324]
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, Jakrot V, Kazakoff S, Holmes O, Leonard C, Sabarinathan R, Mularoni L, Wood S, Xu Q, Waddell N, Tembe V, Pupo GM, De Paoli-Iseppi R, Vilain RE, Shang P, Lau LMS, Dagg RA, Schramm SJ, Pritchard A, Dutton-Regester K, Newell F, Fitzgerald A, Shang CA, Grimmond SM, Pickett HA, Yang JY, Stretch JR, Behren A, Kefford RF, Hersey P, Long GV, Cebon J, Shackleton M, Spillane AJ, Saw RPM, López-Bigas N, Pearson JV, Thompson JF, Scolyer RA, Mann GJ. Whole-genome landscapes of major melanoma subtypes. Nature. 2017 May 11:545(7653):175-180. doi: 10.1038/nature22071. Epub 2017 May 3 [PubMed PMID: 28467829]
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. The New England journal of medicine. 2005 Nov 17:353(20):2135-47 [PubMed PMID: 16291983]
Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Laboratory investigation; a journal of technical methods and pathology. 2017 Jun:97(6):630-635. doi: 10.1038/labinvest.2016.147. Epub 2017 Jan 16 [PubMed PMID: 28092366]
Su J, Yu W, Liu J, Zheng J, Huang S, Wang Y, Qi S, Ma X, Chen J, Zhang Y. Fluorescence in situ hybridisation as an ancillary tool in the diagnosis of acral melanoma: a review of 44 cases. Pathology. 2017 Dec:49(7):740-749. doi: 10.1016/j.pathol.2017.08.006. Epub 2017 Oct 14 [PubMed PMID: 29037804]
Level 2 (mid-level) evidenceKuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. The British journal of dermatology. 2000 Aug:143(2):275-80 [PubMed PMID: 10951133]
Boyd AS, Rapini RP. Acral melanocytic neoplasms: a histologic analysis of 158 lesions. Journal of the American Academy of Dermatology. 1994 Nov:31(5 Pt 1):740-5 [PubMed PMID: 7929919]
Level 2 (mid-level) evidenceScolyer RA, Thompson JF, McCarthy SW, Strutton GM, Elder DE. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. The Australasian journal of dermatology. 2006 Feb:47(1):71-3; author reply 74-5 [PubMed PMID: 16405491]
Level 3 (low-level) evidenceDarmawan CC, Jo G, Montenegro SE, Kwak Y, Cheol L, Cho KH, Mun JH. Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. Journal of the American Academy of Dermatology. 2019 Sep:81(3):805-812. doi: 10.1016/j.jaad.2019.01.081. Epub 2019 Feb 5 [PubMed PMID: 30731177]
Pampena R, Kyrgidis A, Lallas A, Moscarella E, Argenziano G, Longo C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. Journal of the American Academy of Dermatology. 2017 Nov:77(5):938-945.e4. doi: 10.1016/j.jaad.2017.06.149. Epub 2017 Aug 29 [PubMed PMID: 28864306]
Level 1 (high-level) evidenceBristow IR, de Berker DA, Acland KM, Turner RJ, Bowling J. Clinical guidelines for the recognition of melanoma of the foot and nail unit. Journal of foot and ankle research. 2010 Nov 1:3():25. doi: 10.1186/1757-1146-3-25. Epub 2010 Nov 1 [PubMed PMID: 21040565]
Tan A, Stein JA. Dermoscopic patterns of acral melanocytic lesions in skin of color. Cutis. 2019 May:103(5):274-276 [PubMed PMID: 31233579]
Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Archives of dermatology. 2011 Jun:147(6):741-3. doi: 10.1001/archdermatol.2011.136. Epub [PubMed PMID: 21690544]
Level 3 (low-level) evidenceLallas A, Kyrgidis A, Koga H, Moscarella E, Tschandl P, Apalla Z, Di Stefani A, Ioannides D, Kittler H, Kobayashi K, Lazaridou E, Longo C, Phan A, Saida T, Tanaka M, Thomas L, Zalaudek I, Argenziano G. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. The British journal of dermatology. 2015 Oct:173(4):1041-9. doi: 10.1111/bjd.14045. Epub 2015 Oct 1 [PubMed PMID: 26211689]
Saida T, Yoshida N, Ikegawa S, Ishihara K, Nakajima T. Clinical guidelines for the early detection of plantar malignant melanoma. Journal of the American Academy of Dermatology. 1990 Jul:23(1):37-40 [PubMed PMID: 2365875]
Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, Sober AJ, Thompson JA, Wisco OJ, Wyatt S, Hu S, Lamina T. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology. 2019 Jan:80(1):208-250. doi: 10.1016/j.jaad.2018.08.055. Epub 2018 Nov 1 [PubMed PMID: 30392755]
Neuman HB, Patel A, Ishill N, Hanlon C, Brady MS, Halpern AC, Houghton A, Coit DG. A single-institution validation of the AJCC staging system for stage IV melanoma. Annals of surgical oncology. 2008 Jul:15(7):2034-41. doi: 10.1245/s10434-008-9915-0. Epub 2008 May 9 [PubMed PMID: 18465172]
Level 1 (high-level) evidenceMorton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Puleo CA, Coventry BJ, Kashani-Sabet M, Smithers BM, Paul E, Kraybill WG, McKinnon JG, Wang HJ, Elashoff R, Faries MB, MSLT Group. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. The New England journal of medicine. 2014 Feb 13:370(7):599-609. doi: 10.1056/NEJMoa1310460. Epub [PubMed PMID: 24521106]
Level 1 (high-level) evidenceHaneke E, Baran R. Longitudinal melanonychia. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Jun:27(6):580-4 [PubMed PMID: 11442597]
Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. Journal of the American Academy of Dermatology. 2003 Feb:48(2):183-8 [PubMed PMID: 12582386]
Level 2 (mid-level) evidenceTseng JF, Tanabe KK, Gadd MA, Cosimi AB, Malt RA, Haluska FG, Mihm MC Jr, Sober AJ, Souba WW. Surgical management of primary cutaneous melanomas of the hands and feet. Annals of surgery. 1997 May:225(5):544-50; discussion 550-3 [PubMed PMID: 9193182]
Level 2 (mid-level) evidenceGoydos JS, Shoen SL. Acral Lentiginous Melanoma. Cancer treatment and research. 2016:167():321-9. doi: 10.1007/978-3-319-22539-5_14. Epub [PubMed PMID: 26601870]
Roh MR, Kim J, Chung KY. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei medical journal. 2010 Jul:51(4):562-8. doi: 10.3349/ymj.2010.51.4.562. Epub [PubMed PMID: 20499423]
Level 2 (mid-level) evidenceTanaka K, Nakamura Y, Mizutani T, Shibata T, Tsutsumida A, Fukuda H, Matsushita S, Aoki M, Namikawa K, Ohe S, Fukushima S, Yamazaki N, Dermatologic Oncology Group of the Japan Clinical Oncology Group. Confirmatory trial of non-amputative digit preservation surgery for subungual melanoma: Japan Clinical Oncology Group study (JCOG1602, J-NAIL study protocol). BMC cancer. 2019 Oct 25:19(1):1002. doi: 10.1186/s12885-019-6248-2. Epub 2019 Oct 25 [PubMed PMID: 31653251]
Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. The British journal of dermatology. 2011 Oct:165(4):852-8. doi: 10.1111/j.1365-2133.2011.10477.x. Epub 2011 Aug 4 [PubMed PMID: 21812768]
Level 3 (low-level) evidenceSeegenschmiedt MH, Keilholz L, Altendorf-Hofmann A, Urban A, Schell H, Hohenberger W, Sauer R. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: a 20-year experience. International journal of radiation oncology, biology, physics. 1999 Jun 1:44(3):607-18 [PubMed PMID: 10348291]
Level 2 (mid-level) evidenceNakamura Y, Fujisawa Y. Diagnosis and Management of Acral Lentiginous Melanoma. Current treatment options in oncology. 2018 Jun 27:19(8):42. doi: 10.1007/s11864-018-0560-y. Epub 2018 Jun 27 [PubMed PMID: 29951919]
Hadash-Bengad R, Hajaj E, Klein S, Merims S, Frank S, Eisenberg G, Yakobson A, Orevi M, Caplan N, Peretz T, Lotem M, Cohen JE. Immunotherapy Potentiates the Effect of Chemotherapy in Metastatic Melanoma-A Retrospective Study. Frontiers in oncology. 2020:10():70. doi: 10.3389/fonc.2020.00070. Epub 2020 Feb 14 [PubMed PMID: 32117727]
Level 2 (mid-level) evidenceKaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH, Kraft S, Gerstenblith MR, Thompson CL, Honda K, Cuda JD, Eberhart CG, Handa JT, Lipson EJ, Taube JM. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Laboratory investigation; a journal of technical methods and pathology. 2017 Sep:97(9):1063-1071. doi: 10.1038/labinvest.2017.64. Epub 2017 Jul 24 [PubMed PMID: 28737763]
Savarese I, Papi F, D'Errico A, Gori A, Grazzini M, Vannucchi M, Massi D, De Giorgi V. Acral lentiginous melanoma treated with topical imiquimod cream: possible cooperation between drug and tumour cells. Clinical and experimental dermatology. 2015 Jan:40(1):27-30. doi: 10.1111/ced.12469. Epub 2014 Sep 23 [PubMed PMID: 25252087]
Level 3 (low-level) evidenceOcampo-Garza J, Gioia Di Chiacchio N, Haneke E, le Voci F, Paschoal FM. Subungual Melanoma In Situ Treated With Imiquimod 5% Cream After Conservative Surgery Recurrence. Journal of drugs in dermatology : JDD. 2017 Mar 1:16(3):268-270 [PubMed PMID: 28301623]
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O'Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Sep 10:31(26):3182-90. doi: 10.1200/JCO.2012.47.7836. Epub 2013 Jun 17 [PubMed PMID: 23775962]
Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Annals of surgical oncology. 2018 Aug:25(8):2105-2110. doi: 10.1245/s10434-018-6513-7. Epub 2018 May 30 [PubMed PMID: 29850954]
Duarte CA, Flórez JP, López HG, Meneses MX, de Vries E. Survival of acral lentiginous melanoma in the National Cancer Institute of Colombia. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 Mar:31(3):438-442. doi: 10.1111/jdv.13913. Epub 2016 Dec 4 [PubMed PMID: 27518480]
Lv J, Dai B, Kong Y, Shen X, Kong J. Acral Melanoma in Chinese: A Clinicopathological and Prognostic Study of 142 cases. Scientific reports. 2016 Aug 22:6():31432. doi: 10.1038/srep31432. Epub 2016 Aug 22 [PubMed PMID: 27545198]
Level 3 (low-level) evidenceBello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, Ariyan CE. Prognosis of acral melanoma: a series of 281 patients. Annals of surgical oncology. 2013 Oct:20(11):3618-25. doi: 10.1245/s10434-013-3089-0. Epub 2013 Jul 10 [PubMed PMID: 23838913]
Lino-Silva LS, Zepeda-Najar C, Salcedo-Hernández RA, Martínez-Said H. Acral Lentiginous Melanoma: Survival Analysis of 715 Cases. Journal of cutaneous medicine and surgery. 2019 Jan/Feb:23(1):38-43. doi: 10.1177/1203475418800943. Epub 2018 Sep 15 [PubMed PMID: 30221995]
Level 3 (low-level) evidence