Introduction
Abdominoperineal resection (APR) is a surgical procedure that involves the removal of the rectum and anus, resulting in the creation of a permanent end colostomy. This procedure is typically carried out for low rectal cancers where it is not possible to spare the sphincters during curative resection, as well as for anal cancers that do not respond to chemoradiation or recur. In some cases, benign conditions such as perianal Crohn disease, complex anorectal fistulae, and severe trauma may also necessitate an APR.[1]
During an APR, the rectum, surrounding mesorectum, anal sphincter complex, and anus are removed, and the perineal opening is closed (see Image. Abdominoperineal Resection Specimen). The distal colon is then brought out as a permanent end colostomy. The extent of the operation and resection of surrounding structures depends on the specific pathology, patient factors, and the stage of the disease. Due to advancements in diagnostic techniques, radiation, and chemotherapeutic improvements, APRs are becoming less common in favor of more sphincter-sparing approaches. While this procedure was traditionally performed with a laparotomy and a separate perineal incision, it is now commonly carried out using laparoscopy or robotic surgery.[1] APR is associated with significant morbidity. In addition, patients undergoing an APR have a permanent colostomy and may experience a considerable rate of genitourinary and sexual dysfunction that can affect their quality of life. Therefore, appropriate counseling, psychosocial support, good surgical technique, and perioperative care are essential for positive patient outcomes.[2]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
APR is a surgical procedure often performed for rectal cancers located in the distal third of the rectum. The procedure removes several key anatomical structures within the pelvis and lower gastrointestinal tract. Understanding the anatomy of the left colon, sigmoid colon, rectum, pelvic floor, and anus, along with the anal sphincter complex, is essential for appreciating the procedure’s complexity and its impact on patient outcomes.[3]
Left Colon and Sigmoid Colon
The left colon, which begins at the splenic flexure, where the transverse colon transitions into the descending colon, includes the distal portion of the descending colon, extending to the sigmoid colon. The descending colon travels along the left side of the abdomen and ends at the sigmoid colon, a segment that is characteristically S-shaped and suspended by a mesocolon. The sigmoid colon is typically located within the pelvis and transitions into the rectum at the level of the third sacral vertebra (S3). The blood supply of the left colon and sigmoid colon is primarily derived from the left colic artery and the sigmoid arteries, which are branches of the inferior mesenteric artery (IMA). Venous drainage parallels the arterial supply and empties into the inferior mesenteric vein. Lymphatic drainage follows a similar path along the vessels, ultimately draining into the IMA lymph node chain. During an APR, the left and sigmoid colon are mobilized, and the blood supply is divided at the appropriate level to ensure adequate perfusion and prevent ischemia in the remaining bowel.
Rectum
The rectum continues the sigmoid colon, measures approximately 12 cm to 15 cm long, and is situated within the pelvic cavity, lying anterior to the sacrum and coccyx. The rectum is divided into 3 distinct parts: the upper third, middle third, and lower third. The upper third is covered anteriorly and laterally by the peritoneum, the middle third is only covered anteriorly, and the lower third is entirely extraperitoneal. The rectum terminates at the anorectal junction, typically defined as the point where the puborectalis muscle creates an angle known as the anorectal angle. In men, the rectum is situated posteriorly to the seminal vesicles, prostate, and bladder, while in women it lies posterior to the vagina.[4] The blood supply to the rectum is mainly derived from the superior rectal artery (a continuation of the IMA), the middle rectal artery (a branch of the internal iliac artery), and the inferior rectal artery (a branch of the internal pudendal artery). The venous drainage includes the superior rectal vein (into the portal system) and the middle and inferior rectal veins (into the systemic circulation). Lymphatic drainage of the rectum is complex and varies depending on the segment involved, with the upper rectum draining into the pararectal and inferior mesenteric lymph nodes and the lower rectum draining into the internal iliac and presacral lymph nodes.
Anal Canal, Anus, and Anal Sphincter Complex
The anal canal, measuring approximately 4 cm in length, is the terminal segment of the gastrointestinal tract, extending from the rectum to the anus. The canal is enveloped by the internal anal sphincter in its upper two-thirds and by the external anal sphincter in its lower third. A key anatomical landmark for surgeons is the anorectal ring, which marks the transition between the rectum and the anal canal. This ring is formed by the confluence of the puborectalis muscle, the deep external sphincter, the conjoined longitudinal muscle, and the upper portion of the internal anal sphincter. This area can be palpated during a digital rectal examination to evaluate for low-lying rectal lesions.
The pectinate (dentate) line, an irregular ring formed by the anal valves, further divides the anal canal based on its embryological origin. This line separates the canal into upper and lower sections, each with distinct structural and neurovascular characteristics. The upper section, derived from the hindgut, receives blood from the superior rectal artery, a branch of the IMA. In contrast, the lower section, which originates from the ectoderm, is supplied by the inferior rectal artery, a branch of the pudendal artery.[5] Lymphatic drainage of the anal canal follows distinct pathways: above the pectinate line, it drains into the internal iliac lymph nodes, while below the pectinate line, it drains into the inguinal lymph nodes.
The autonomic nervous system innervates the anal canal above the pectinate line through the inferior hypogastric plexus. Parasympathetic fibers from the S2-S4 nerve roots reduce the tone of the internal anal sphincter, facilitating defecation. Conversely, sympathetic fibers from the L1-L2 nerve roots increase the tone of the internal anal sphincter, contributing to continence. The pudendal nerve provides somatic innervation below the pectinate line. The inferior anal nerve, a branch of the pudendal nerve, innervates the external anal sphincter and supplies sensory input to the area below the pectinate line, making it sensitive to pain, temperature, and touch. The anus is the terminal portion of the anal canal, is approximately 2 to 4 cm long, and is surrounded by the anal sphincter complex, which is responsible for maintaining continence. The anal sphincter complex comprises 2 main components, the internal and external anal sphincters.
The internal anal sphincter, a continuation of the inner circular muscle layer of the rectum, is composed of smooth muscle under involuntary control. This sphincter provides continuous basal tone, contributing to approximately 70% of resting anal pressure, and its function is crucial for maintaining continence at rest and responds to the rectoanal inhibitory reflex during defecation, where it relaxes in response to rectal distension. The external anal sphincter, composed of striated muscle under voluntary control, is subdivided into 3 parts: deep, superficial, and subcutaneous. This structure is crucial in maintaining continence, especially during increased intraabdominal pressure or voluntary contraction. The ability to contract it consciously allows for additional closure of the anal canal, providing an essential mechanism for preventing fecal incontinence when the internal anal sphincter relaxes or when there is a sudden increase in abdominal pressure, such as during coughing or lifting.
Pelvic Floor
The pelvic floor forms a muscular base that supports the pelvic organs, including the rectum and anal canal. The pelvic floor consists of several muscles, primarily the levator ani muscle group (pubococcygeus, puborectalis, and iliococcygeus muscles) and the coccygeus muscle.[6] These muscles create a funnel-shaped structure that helps maintain continence and support intraabdominal contents. During an APR, the pelvic floor is approached from the perineal side and divided to allow removal of the distal rectum and anal canal. This requires careful dissection to preserve the surrounding nerves and ensure complete resection of the cancerous tissue while minimizing postoperative complications such as pelvic floor dysfunction.
Pelvic Innervation
The pelvis is innervated by an extensive network of sympathetic and parasympathetic nerves that are intricately related to the rectum and anal canal. Sympathetic innervation originates from the sympathetic trunk and the inferior mesenteric, superior, and hypogastric plexuses. These nerves traverse behind the IMA, traveling within the presacral fascia to supply the pelvic viscera. Parasympathetic fibers, derived from the S2-S4 nerve roots, either directly innervate the target organs or reach them via the hypogastric nerves. These autonomic nerves are at risk of injury during surgical procedures, potentially leading to complications such as impaired bowel and bladder function or sexual dysfunction. The sacral plexus and the pudendal nerve provide somatic innervation in the pelvis. The pudendal nerve, arising from S2-S4, plays a critical role in the sensory and motor innervation of the perineum and external anal sphincter.[7] This complex neural supply is essential for coordinated motor control and sensation, and its preservation during surgery is crucial to maintaining continence and pelvic organ function.
Surgical Considerations in APR
During an APR, the surgical dissection includes the complete removal of the rectum and anus, along with a portion of the pelvic floor, necessitating a permanent colostomy. The procedure involves meticulous dissection to ensure clear margins, particularly around the distal rectum and the sphincter complex. Careful preservation of the autonomic nerves within the pelvis is necessary to reduce the risk of complications such as urinary dysfunction or sexual dysfunction. Understanding the vascular and lymphatic anatomy is crucial for achieving an oncologically sound resection and minimizing intraoperative blood loss and postoperative complications. The complexity of APR necessitates a comprehensive understanding of pelvic and anorectal anatomy, and mastery of these anatomical structures is essential for optimizing patient outcomes and reducing complications.
Indications
APR is indicated in the following conditions:
- Anal cancer
- Patients with recurrent anal cancer or those who do not respond to chemoradiation
- Rectal cancer
- Patients with low rectal cancer abutting or involving the anal sphincter complex or levator muscles
- Patients who require an ultralow resection but have weak sphincter tone
- Rectal adenocarcinoma located within 4 cm to 6 cm of the anal verge where:
- Sphincter preservation would result in inadequate oncological margins
- Sphincter preservation would cause impaired postoperative function
- Nonmalignant conditions
- Recurrent anorectal fistulae with extensive tissue destruction that are not responsive to conservative management or less extensive surgeries
- Inflammatory bowel disease
- Ulcerative colitis and Crohn disease with significant anal or rectal disease who are not candidates for ileal pouch reconstruction or who have poor functional results post-reconstruction
- Fecal incontinence not amenable to any less invasive treatment
- Severe anal trauma with destruction of the sphincter complex [8][9]
Contraindications
APR is contraindicated in patients who are not considered fit for general anesthesia. Relative contraindications include those with an increased risk of poor postoperative outcomes such as poorly controlled diabetes, morbid obesity, frailty, and immunosuppression.
Equipment
The equipment needed for an APR depends on whether the procedure is done through open surgery or a minimally invasive approach. Regardless of the approach, an operating table that can adjust for lithotomy and prone jack-knife positions is essential. Open, laparoscopic, and robotic procedures require the standard equipment used for major abdominal cases. Additional instruments that should be available for an APR include lighted pelvic retractors (eg, St Mark retractors), perineal retractors (eg, Lone Star retractors), and energy devices.
Personnel
The essential operating team for an APR consists of the surgeon, surgical assistant, anesthesiologist, and surgical technician or operating room nurse. Urologic or gynecologic assistance may be required in patients requiring extended resection and for placement of preoperative ureteral stents. Therefore, patient education and counseling regarding the resulting stoma are equally crucial. The stoma site should be carefully marked to ensure it is in the most usable and convenient location for the patient. Preoperative counseling should address the realities of managing a stoma, including the psychological effects and practical considerations. An enterostomal nurse therapist is indispensable in this process, helping patients understand what to expect and providing guidance on stoma management to enhance patients' quality of life post-surgery.
Preparation
Perioperative Preparation
Patients should receive a preoperative evaluation similar to that of any major abdominal operation but tailored to the individual. A thorough review of preoperative imaging, especially rectal magnetic resonance imaging (MRI) in cancer cases, is critical. As previously stated, an enterostomal nurse therapist should meet with the patient to mark the stoma site and counsel them on what to expect with a stoma, answering all questions and addressing any concerns. Most patients undergoing colorectal surgery are part of an enhanced recovery after surgery (ERAS) pathway that streamlines perioperative care.[10][11] ERAS protocols typically include the following preoperative components:
- Mechanical and antibiotic bowel preparation [12]
- High carbohydrate clear fluids until 2 hours before surgery (preoperative carbohydrate loading) [13]
- Multimodal opioid-sparing analgesia
- Maintaining pre- and intraoperative normothermia [14]
- Fluid restriction to target euvolemia
- Using opioid receptor blockers such as alvimopan [15]
Preoperative Care
The following steps are taken just before beginning the procedure:
- Patients are placed in a lithotomy position with well-padded stirrups. The perineum should be easily accessible for the perineal portion of the operation. Some surgeons prefer a prone jack-knife position for the perineal portion of the operation, in which case the patient is repositioned after the abdominal portion.[15]
- Broad-spectrum antibiotics are administered within 30 minutes of the incision.
- In cases where there is significant concern for ureteral injury, preoperative ureteral stent placement may be considered. While these stents do not reduce the risk of ureteral injury, they allow for earlier injury identification and repair.[16]
- A Foley catheter is placed in all cases.
- A digital rectal exam is performed to identify the target lesion and the operative landmarks. In women, the vagina is included in the operative field in case uterine manipulation or palpation of the posterior vaginal wall is necessary.
Technique or Treatment
The principles of an APR described below apply to all techniques regardless of whether the procedure is performed via an open, laparoscopic, or robotic approach.[17] The procedure can be divided into 2 phases, which can be carried out synchronously or sequentially.
Abdominal Operation
Access to the abdomen is achieved via a lower midline incision or through the use of up to 6 laparoscopic ports for laparoscopic or robotic techniques. Proper use of lighted retractors is essential for optimal exposure in open surgery. At the same time, in minimally invasive procedures, a steep Trendelenburg position combined with firm retraction on the rectum is crucial for adequate visualization. An initial survey of the abdomen is conducted to detect any signs of metastasis. The small bowel is retracted into the upper quadrants, and any redundant sigmoid colon is reduced from the pelvis. Dissection begins by entering the plane between the mesocolon and retroperitoneum to mobilize the sigmoid colon. This can be approached medially by elevating the vascular pedicle (superior rectal artery) and incising the peritoneum behind it. The mesocolon is separated from the retroperitoneum from the lateral side while ensuring the left ureter and gonadal vessels are identified and preserved. The superior rectal or IMA is ligated. Some surgeons advocate routine high ligation of the inferior mesenteric vein, identified by retracting the transverse mesocolon and duodenojejunal flexure toward the midline. The inferior mesenteric vein can reliably be found lateral to the ligament of Treitz and, in most cases, can be ligated near the IMA rather than divided at its origin.
For the lateral-to-medial approach, the peritoneum is incised along the line of Toldt, and lateral attachments are divided. The same avascular plane is entered, and the dissection proceeds medially, ligating the vascular pedicle as the final step. Minimally invasive surgeries more often employ the medial-to-lateral approach. Mobilization of the splenic flexure is usually unnecessary, as there is no anastomosis in an APR, unlike in a low anterior resection. Total mesorectal excision begins by entering the avascular plane between the presacral and mesorectal fascia at the level of the sacral promontory.[18] Care must be taken to avoid entering the presacral plane at this stage, as doing so risks significant bleeding from the sacral plexus and damage to autonomic nerves. The ureters are identified and protected. In female patients, the uterus may need to be retracted anteriorly, which can be achieved by suturing or suspending it from the anterior abdominal wall.
Once the posterior dissection is complete, lateral dissection begins. The parasympathetic nerves, located along the pelvic sidewall near the lateral stalks containing the middle rectal arteries, should be preserved. The final step involves the anterior dissection. The anterior peritoneum is opened, and careful attention is paid to separate the bladder, cervix, posterior vaginal wall (in women), or seminal vesicles (in men) from the dissection plane. Care is taken to ensure that the mesorectum maintains a cylindrical shape, avoiding coning at the level of the levator muscles. Mobilization of the rectum is completed anteriorly just below the level of the seminal vesicles in men or the cervix in women and posteriorly at the upper border of the coccyx. The mesorectum is not separated from the levator muscles at this point. The colon is transected with a linear cutting stapler, at least 5 cm proximal to the tumor, ensuring enough mobility to prevent tension at the stoma site. This transection usually occurs in the descending or sigmoid colon.
An omental flap is mobilized if feasible. The greater omentum is dissected from the transverse colon, and the greater curvature of the stomach is preserved while the gastroepiploic vessels are preserved. Care must be taken to avoid devascularizing the omentum. Typically, the omental flap is pedicled on the left gastroepiploic artery and is brought down along the lateral abdominal wall into the pelvis. A well-vascularized omental flap may help improve perineal wound healing and adhesions of the small bowel deep in the pelvis.[19][20] The abdominal wall at the designated stoma marking creates an opening sufficient to accommodate the bowel without excessive laxity or narrowing. Following this, the transected colon is brought out through the stoma site. If the procedure is planned in a prone jackknife position, the abdomen is typically closed, and the stoma is matured at this stage. In contrast, if the patient remains in the lithotomy position, the stoma can be created after completing the primary surgical procedure.
Perineal Operation
The eralevator abdominoperineal excision can be performed with the patient in either the prone jackknife or lithotomy position.[21][22] The prone position offers superior exposure and visualization, especially for anterior tumors.[23][24] The perineal area is exposed by taping the buttocks apart, and it is essential to ensure excellent lighting and a reliable retractor system. A purse-string suture is placed around the anus to prevent fecal leakage. A skin incision is made around the anus, typically just outside the anal verge. In cases of bulky anal tumors, a wider incision may be necessary. The incision is deepened until the ischiorectal fossa is entered bilaterally, with dissection proceeding outside the external sphincter muscles. Dissection typically begins posteriorly, where the anatomical landmarks are more distinct, and the risk of damaging surrounding structures is minimized. The anococcygeal raphe is carefully divided while ensuring the incision remains anterior to the coccyx. This technique facilitates the identification of the levator muscles, which are critical for subsequent steps in the procedure.
Lateral dissection follows, with care taken to either ligate or seal the vascular pedicles (eg, inferior rectal and pudendal arteries) using energy devices. The right and left levator muscles are then divided near their insertion on the pelvic sidewall, connecting the lateral dissection with the posterior plane. Anterior dissection is performed last and requires great caution to avoid injury to the urethra in men or the posterior vaginal wall in women. Once the rectum and disconnected sigmoid colon are delivered through the perineal incision, the wound is irrigated, and hemostasis is ensured. If an omental flap is created, it is brought down and sutured into the perineal wound. The pelvic floor is then closed in layers over a surgical drain. In patients with large perineal wounds or significant risk factors for wound dehiscence—such as neoadjuvant radiation therapy, malnutrition, chronic steroid use, obesity, or diabetes—tissue coverage with flap closure may be required. Myocutaneous flaps, such as the gracilis, rectus abdominis, or pedicled gluteus maximus flaps, are frequently employed in these cases.[25]
APR Variations
These include:
- Extrasphincteric resection
- This is the traditional approach to APR. The abdominal portion of the operation is the same. During the perineal dissection, the plane of dissection is immediately external to the sphincter muscles through the ischiorectal fat. The levator muscle is not divided close to its attachment to the lateral pelvic walls, resulting in a more funnel-shaped specimen. This approach can increase the risk of positive margins when performed for cancer.[1]
- Intersphincteric resection
- In this approach, perineal dissection is conducted between the internal and external sphincters, preserving the external sphincter. This method is useful for benign disease, as it spares more muscle and allows for better perineal closure.
Complications
Complications associated with APR are similar to those seen in other major abdominal and perineal surgeries. They include:
- Wound infections
- Intraabdominal abscesses
- Postoperative ileus or small bowel obstruction
- Ureteral injury
- Sexual or urinary dysfunction
- Stoma-related complications
Unlike low anterior resection and proctectomy, which restore intestinal continuity through an anastomosis, APR does not involve an anastomosis, thus eliminating the risk of anastomotic complications. However, practitioners should be aware of several specific complications related to APR:
- Perineal wound dehiscence
- The removal of the anal sphincter complex creates a wide perineal defect, which can be a site of weakness, especially in patients with risk factors for poor wound healing. These risk factors include smoking, uncontrolled diabetes, obesity, prior radiation therapy, and malnutrition. Wound complications are also more common in patients undergoing surgery for anal cancer or inflammatory bowel disease compared to rectal cancer. Dehiscence may present with gaping wound edges and serous or salmon-colored discharge. A careful physical examination is necessary to assess the integrity of deeper tissue layers, as dehiscence is often associated with infection. Superficial dehiscence can usually be managed with close monitoring, local wound care, and possibly negative pressure dressings. More severe cases, particularly those involving deeper tissues, often require surgical intervention, such as secondary closure with tissue transfer. The most severe form of dehiscence involves herniation of abdominal contents through the perineum, which is a surgical emergency.[26][27]
- Perineal wound infections
- These are relatively common after APR, particularly in patients with underlying inflammatory bowel disease or irradiated tissues. Superficial infections can be managed with antibiotics targeting common skin and soft tissue organisms. However, deeper infections, especially those associated with abscesses or subcutaneous collections, require more aggressive treatment. Wound drainage or debridement, combined with antibiotics, is typically needed. Abscesses larger than 5 cm generally do not respond well to percutaneous drainage alone and often require surgical intervention in the operating room.[28][29]
- Urethral injury
- The urethra is anatomically close to the anterior anal canal, particularly in men, making it vulnerable during APR. Careful dissection and frequent palpation of the Foley catheter are essential to avoid urethral injury. Intraoperative awareness of the urethra’s location is crucial to prevent this complication.[30][31]
- Nerve injuries
- Autonomic nerve injuries can occur at different points of the operation. They most commonly occur during high inferior mesenteric artery ligation, retrorectal dissection during total mesorectal excision, division of the lateral stalks (containing the nervi erigentes), and anterior dissection, especially at the seminal vesicles in men. Injuries often affect sympathetic and parasympathetic nerves together and present with sexual and bladder dysfunction, including retrograde ejaculation, erectile dysfunction, vaginal dryness, and dyspareunia.[32][33]
Clinical Significance
APR is a major surgical procedure for cancers of the lower rectum and anus and occasionally for benign diseases in this region. The anus, anal canal with the surrounding sphincter muscles, and rectum are removed, with the creation of a permanent end colostomy. With improvements in medical and radiation therapies, APR is less commonly performed. However, it remains an essential tool in the surgeon's armamentarium in treating anal and rectal pathology. Careful patient selection is critical, with sphincter-sparing operations preferred whenever feasible. For patients requiring APR, thorough counseling and psychosocial support are essential to help them adjust to living with a permanent colostomy.
Enhancing Healthcare Team Outcomes
Effective management of patients undergoing APR necessitates a collaborative approach emphasizing interprofessional communication, care coordination, and shared decision-making among various healthcare providers, including advanced clinicians, nurses, pharmacists, and allied health professionals. Clinicians are crucial in developing comprehensive treatment plans, guiding the surgical procedure, and overseeing postoperative care. They must communicate the surgical indications, risks, and benefits to ensure patients and their families understand the procedure. Nurses are instrumental in preoperative education, addressing patient concerns, and providing emotional support throughout the surgical journey. Their ability to relay critical information about patient status and potential complications to the surgical team is essential for timely interventions and enhanced patient safety.
Pharmacists contribute significantly to the perioperative management of patients by optimizing medication regimens, ensuring proper pain management, and preventing drug interactions. Their expertise in pharmacotherapy is vital in caring for patients with comorbidities and minimizing adverse drug events. The healthcare team must engage in regular interdisciplinary meetings to discuss patient progress, address challenges, and adjust care plans as needed. Such collaborative strategies enhance patient-centered care and improve outcomes, safety, and overall team performance by fostering a culture of mutual respect, continuous learning, and shared accountability. By working cohesively, the healthcare team can ensure that patients undergoing APR receive holistic care that addresses their physical, emotional, and psychosocial needs, ultimately leading to better recovery experiences and long-term health outcomes.
Media
(Click Image to Enlarge)
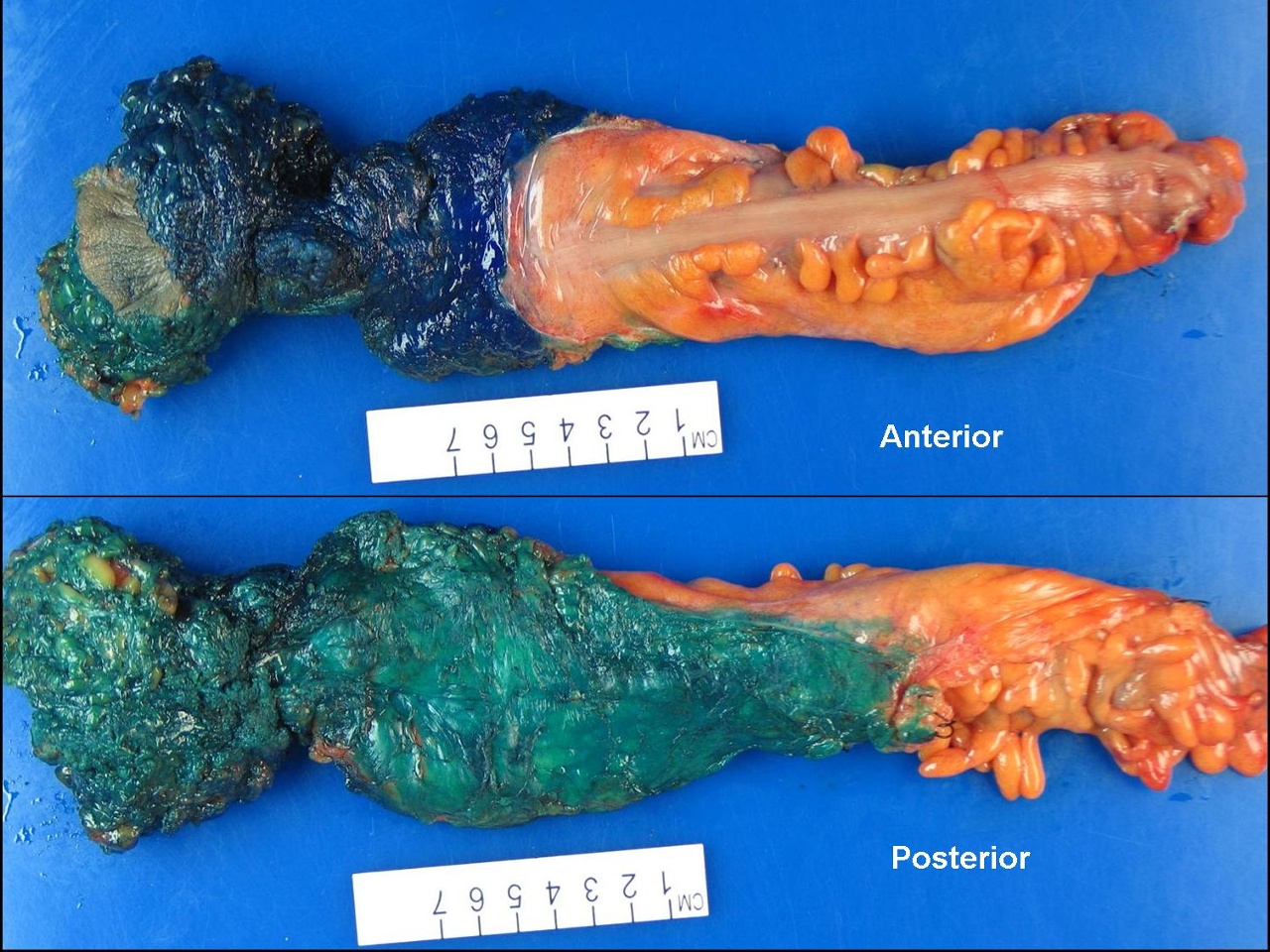
Abdominoperineal Resection Specimen. This image shows a dissected section of the bowel after the abdominoperineal procedure, which involves the complete removal of the anal canal, rectum, and a portion of the sigmoid colon.
Hagemani, Public Domain, via Wikimedia Commons
References
Hawkins AT, Albutt K, Wise PE, Alavi K, Sudan R, Kaiser AM, Bordeianou L, Continuing Education Committee of the SSAT. Abdominoperineal Resection for Rectal Cancer in the Twenty-First Century: Indications, Techniques, and Outcomes. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2018 Aug:22(8):1477-1487. doi: 10.1007/s11605-018-3750-9. Epub 2018 Apr 16 [PubMed PMID: 29663303]
Tooley JE, Sceats LA, Bohl DD, Read B, Kin C. Frequency and timing of short-term complications following abdominoperineal resection. The Journal of surgical research. 2018 Nov:231():69-76. doi: 10.1016/j.jss.2018.05.009. Epub 2018 Jun 9 [PubMed PMID: 30278971]
Mike M, Kano N. Reappraisal of the vascular anatomy of the colon and consequences for the definition of surgical resection. Digestive surgery. 2013:30(4-6):383-92. doi: 10.1159/000343156. Epub 2013 Oct 10 [PubMed PMID: 24135859]
Wang YHW, Wiseman J. Anatomy, Abdomen and Pelvis, Rectum. StatPearls. 2024 Jan:(): [PubMed PMID: 30725930]
Ahmed A, Arbor TC, Qureshi WA. Anatomy, Abdomen and Pelvis: Anal Canal. StatPearls. 2024 Jan:(): [PubMed PMID: 32119418]
Bordoni B, Sugumar K, Leslie SW. Anatomy, Abdomen and Pelvis, Pelvic Floor. StatPearls. 2025 Jan:(): [PubMed PMID: 29489277]
Park SB, Garg R, Singh P. Anatomy, Abdomen and Pelvis, Nerves. StatPearls. 2024 Jan:(): [PubMed PMID: 31194402]
Holden J, Nayak JG, Botkin C, Helewa RM. Abdominoperineal Resection with Absorbable Mesh Repair of Perineal Defect for Fournier's Gangrene: A Case Report. International medical case reports journal. 2021:14():133-138. doi: 10.2147/IMCRJ.S295099. Epub 2021 Feb 26 [PubMed PMID: 33664599]
Level 3 (low-level) evidenceMeima-van Praag EM, Buskens CJ, Hompes R, Bemelman WA. Surgical management of Crohn's disease: a state of the art review. International journal of colorectal disease. 2021 Jun:36(6):1133-1145. doi: 10.1007/s00384-021-03857-2. Epub 2021 Feb 2 [PubMed PMID: 33528750]
Tan JKH, Ang JJ, Chan DKH. Enhanced recovery program versus conventional care after colorectal surgery in the geriatric population: a systematic review and meta-analysis. Surgical endoscopy. 2021 Jun:35(6):3166-3174. doi: 10.1007/s00464-020-07673-7. Epub 2020 May 28 [PubMed PMID: 32468264]
Level 1 (high-level) evidenceFavuzza J, Madiedo AM, Schultz K, Rasic G, Phatak UR, Hall J. Colorectal ERAS: Years Later. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2022 Jul:26(7):1506-1508. doi: 10.1007/s11605-022-05242-y. Epub 2022 Jan 19 [PubMed PMID: 35048259]
Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for Antibiotic Administration for Bowel Preparation Among Patients Undergoing Elective Colorectal Surgery: A Network Meta-analysis. JAMA surgery. 2022 Jan 1:157(1):34-41. doi: 10.1001/jamasurg.2021.5251. Epub [PubMed PMID: 34668964]
Level 1 (high-level) evidenceLu J, Khamar J, McKechnie T, Lee Y, Amin N, Hong D, Eskicioglu C. Preoperative carbohydrate loading before colorectal surgery: a systematic review and meta-analysis of randomized controlled trials. International journal of colorectal disease. 2022 Dec:37(12):2431-2450. doi: 10.1007/s00384-022-04288-3. Epub 2022 Dec 6 [PubMed PMID: 36472671]
Level 1 (high-level) evidenceSchwenk W. Optimized perioperative management (fast-track, ERAS) to enhance postoperative recovery in elective colorectal surgery. GMS hygiene and infection control. 2022:17():Doc10. doi: 10.3205/dgkh000413. Epub 2022 Jun 23 [PubMed PMID: 35909653]
Murtaza R, Clarke O, Sivakanthan T, Al-Sarireh H, Al-Sarireh A, Raza MM, Navid AZ, Ali B, Hajibandeh S, Hajibandeh S. Effect of Alvimopan on Postoperative Ileus and Length of Hospital Stay in Patients Undergoing Bowel Resection: A Systematic Review and Meta-Analysis. The American surgeon. 2024 Dec:90(12):3272-3283. doi: 10.1177/00031348241265149. Epub 2024 Jul 20 [PubMed PMID: 39031053]
Level 1 (high-level) evidenceHeimberger M, Stocchi L, Brennan E, Spaulding A, DeLeon M, Merchea A, Dozois E, Colibaseanu D. Can preoperative ureteral stent placement help in the intraoperative identification of iatrogenic ureteral injury? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2024 Jun:28(6):903-909. doi: 10.1016/j.gassur.2024.03.028. Epub 2024 Mar 28 [PubMed PMID: 38555016]
Holm T. Abdominoperineal Excision: Technical Challenges in Optimal Surgical and Oncological Outcomes after Abdominoperineal Excision for Rectal Cancer. Clinics in colon and rectal surgery. 2017 Nov:30(5):357-367. doi: 10.1055/s-0037-1606113. Epub 2017 Nov 27 [PubMed PMID: 29184471]
Knol J, Keller DS. Total Mesorectal Excision Technique-Past, Present, and Future. Clinics in colon and rectal surgery. 2020 May:33(3):134-143. doi: 10.1055/s-0039-3402776. Epub 2020 Apr 28 [PubMed PMID: 32351336]
Killeen S, Devaney A, Mannion M, Martin ST, Winter DC. Omental pedicle flaps following proctectomy: a systematic review. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2013 Nov:15(11):e634-45. doi: 10.1111/codi.12394. Epub [PubMed PMID: 24034172]
Level 1 (high-level) evidenceDe Broux E, Parc Y, Rondelli F, Dehni N, Tiret E, Parc R. Sutured perineal omentoplasty after abdominoperineal resection for adenocarcinoma of the lower rectum. Diseases of the colon and rectum. 2005 Mar:48(3):476-81; discussion 481-2 [PubMed PMID: 15714245]
Garcia-Henriquez N, Galante DJ, Monson JRT. Selection and Outcomes in Abdominoperineal Resection. Frontiers in oncology. 2020:10():1339. doi: 10.3389/fonc.2020.01339. Epub 2020 Aug 18 [PubMed PMID: 33014775]
Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, Dixon MF, Mapstone NP, Sebag-Montefiore D, Scott N, Johnston D, Sagar P, Finan P, Quirke P. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Annals of surgery. 2005 Jul:242(1):74-82 [PubMed PMID: 15973104]
Kumar P, Mishra TS, Sarthak S, Sasmal PK. Lithotomy versus prone position for perianal surgery: a randomized controlled trial. Annals of coloproctology. 2022 Apr:38(2):117-123. doi: 10.3393/ac.2020.12.16. Epub 2021 Jun 7 [PubMed PMID: 34098632]
Level 1 (high-level) evidenceLiu P, Bao H, Zhang X, Zhang J, Ma L, Wang Y, Li C, Wang Z, Gong P. Better operative outcomes achieved with the prone jackknife vs. lithotomy position during abdominoperineal resection in patients with low rectal cancer. World journal of surgical oncology. 2015 Feb 12:13():39. doi: 10.1186/s12957-015-0453-5. Epub 2015 Feb 12 [PubMed PMID: 25889121]
Copeland-Halperin LR, Stewart T, Chen Y, Funderburk CD, Freed GL. Perineal reconstruction following abdominoperineal resection: Comprehensive review of the literature. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2020 Nov:73(11):1924-1932. doi: 10.1016/j.bjps.2020.08.090. Epub 2020 Sep 10 [PubMed PMID: 32958425]
Morita M, Fujimoto N, Sekido Y, Ogino T, Hata T, Takahashi H, Miyoshi N, Uemura M, Doki Y, Eguchi H. [Perineal Wound Complications after Abdominoperineal Resection for Anorectal Lesions]. Gan to kagaku ryoho. Cancer & chemotherapy. 2022 Dec:49(13):1926-1928 [PubMed PMID: 36733046]
Buscail E, Canivet C, Shourick J, Chantalat E, Carrere N, Duffas JP, Philis A, Berard E, Buscail L, Ghouti L, Chaput B. Perineal Wound Closure Following Abdominoperineal Resection and Pelvic Exenteration for Cancer: A Systematic Review and Meta-Analysis. Cancers. 2021 Feb 10:13(4):. doi: 10.3390/cancers13040721. Epub 2021 Feb 10 [PubMed PMID: 33578769]
Level 1 (high-level) evidenceHákonarson A, Algethami N, Lydrup ML, Buchwald P. Perineal wound healing after abdominoperineal resection for rectal cancer: a retrospective cohort study. International journal of colorectal disease. 2022 May:37(5):1029-1034. doi: 10.1007/s00384-022-04141-7. Epub 2022 Apr 8 [PubMed PMID: 35396618]
Level 2 (mid-level) evidenceKhattak MA, Khan AN, Jafferi S, Iqbal Y, Abdulrasheed H, McArthur D. Perineal Wound Healing Following Abdominoperineal Resection of the Rectum. Cureus. 2024 Aug:16(8):e66318. doi: 10.7759/cureus.66318. Epub 2024 Aug 6 [PubMed PMID: 39238678]
Hasegawa S, Kajitani R, Munechika T, Matsumoto Y, Nagano H, Taketomi H, Komono A, Aisu N, Yoshimatsu G, Morimoto M, Yoshida Y. Avoiding urethral and rectal injury during transperineal abdominoperineal resection in male patients with anorectal cancer. Surgical endoscopy. 2020 Oct:34(10):4679-4682. doi: 10.1007/s00464-020-07655-9. Epub 2020 May 19 [PubMed PMID: 32430530]
Sylla P, Knol JJ, D'Andrea AP, Perez RO, Atallah SB, Penna M, Hompes R, Wolthuis A, Rouanet P, Fingerhut A, International taTME Urethral Injury Collaborative. Urethral Injury and Other Urologic Injuries During Transanal Total Mesorectal Excision: An International Collaborative Study. Annals of surgery. 2021 Aug 1:274(2):e115-e125. doi: 10.1097/SLA.0000000000003597. Epub [PubMed PMID: 31567502]
Perry WRG, Abd El Aziz MA, Duchalais E, Grass F, Behm KT, Mathis KL, Kelley SR. Sexual dysfunction following surgery for rectal cancer: a single-institution experience. Updates in surgery. 2021 Dec:73(6):2155-2159. doi: 10.1007/s13304-021-01124-1. Epub 2021 Jul 8 [PubMed PMID: 34236596]
Li K, He X, Tong S, Zheng Y. Risk factors for sexual dysfunction after rectal cancer surgery in 948 consecutive patients: A prospective cohort study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2021 Aug:47(8):2087-2092. doi: 10.1016/j.ejso.2021.03.251. Epub 2021 Mar 29 [PubMed PMID: 33832775]