Introduction
Hemangioblastomas are rare, benign, highly vascularized tumors classified as WHO grade 1, primarily affecting the central nervous system. They most commonly occur in the cerebellum, followed by the spinal cord and brainstem. These tumors can be sporadic or associated with von Hippel-Lindau (VHL) disease, an autosomal dominant genetic disorder that predisposes individuals to various tumors.[1][2] Approximately 45% of people with VHL disease develop CNS hemangioblastomas, and an estimated 20% of those with CNS hemangioblastomas have concurrent VHL disease.[3][4] Hemangioblastomas are uncommon, representing 1% to 2.5% of all brain tumors and 2% to 10% of spinal cord tumors, typically manifesting between the ages of 30 and 60, with a slight male predominance.[5][6]
The clinical presentation of hemangioblastomas varies depending on their location and can significantly impact the quality of life. Cerebellar tumors often cause headaches, nausea, vomiting, and signs of increased intracranial pressure, while brainstem tumors can lead to motor and sensory deficits, ataxia, and potentially fatal hemorrhages. Spinal hemangioblastomas may result in localized pain, motor weakness, sensory disturbances, and bowel or bladder dysfunction.[6]
Diagnosis is primarily made through magnetic resonance imaging, which typically reveals a cystic mass with an enhancing mural nodule. The primary treatment is surgical resection, with preoperative embolization sometimes necessary due to the tumor's vascularity. Stereotactic radiosurgery and novel agents targeting vascular endothelial growth factor pathways are emerging treatments for multiple or surgically inaccessible tumors.[7][8] The prognosis after surgery is generally favorable, with low recurrence rates of less than 25%, although regular follow-up is crucial, especially in patients with VHL disease.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Hemangioblastoma can occur sporadically (75%) or in association with VHL disease. VHL is caused by a germline mutation of the VHL gene inherited in an autosomal dominant fashion. However, somatic mutations of VHL, amongst other genomic aberrations, have been identified in sporadic cases.[10] VHL is a tumor-suppressor gene located on chromosome 3p25-26 that encodes VHL protein (pVHL) of 213 amino acids. Identified VHL gene alterations include missense, splice-site, and frameshift (deletions > insertions) mutations, as well as epigenetic suppression of VHL through promoter methylation.[10][11][12] VHL mutation or deletion is more common in VHL-associated hemangioblastomas, whereas promoter methylation has only been detected in sporadic cases. In addition, loss of heterozygosity in chromosomes 6 and 10, even in the absence of VHL mutation, has been identified in sporadic cases, suggesting alternative pathways for sporadic disease development.[10]
Epidemiology
Hemangioblastomas contribute to approximately 1% to 2.5% of all intracranial tumors and about 2% to 10% of primary spinal cord tumors. The overall incidence rate of CNS hemangioblastomas is approximately 0.141 per 100,000 person-years. These tumors are rare, with only 1 person out of a million estimated to develop hemangioblastomas each year. They are more commonly diagnosed in adults between the ages of 30 and 65, with a slight predominance in males. The male-to-female ratio ranges from 1.3:1 to 2.6:1.[9][13]
Anatomic Distribution
Although they can occur anywhere in the central nervous system, most hemangioblastomas arise in the posterior fossa of the brain, with the cerebellum being the most common site. Approximately 45% to 50% of these tumors are found in the cerebellum, 30% to 40% in the spinal cord, and 5% to 10% in the brainstem.[14] Sporadic hemangioblastomas are typically solitary; multiple tumors within the neuraxis should raise suspicion for VHL disease. In patients with sporadic hemangioblastomas, isolated cerebellar lesions are more common. However, individuals with VHL may have multiple lesions in the spinal cord, cerebellum, and brainstem.[9]
Pathophysiology
The pVHL plays an essential role in cell cycle regulation and is involved in the degradation of hypoxia-inducible factors. Amongst several downstream targets, hypoxia-inducible factors are known to activate the transcription of erythropoietin, vascular endothelial growth factor, and platelet-derived growth factor in hypoxic conditions. Loss of pVHL function mimics hypoxia, and consequently, cells are unable to degrade hypoxia-inducible factors despite the presence of oxygen.[15] This results in the upregulation of downstream targets, contributing to the characteristic hypervascularity and erythropoietin overproduction of hemangioblastoma tumors. The 2-hit theory of biallelic inactivation of the VHL gene is thought to initiate tumorigenesis.
In VHL syndrome, affected organs reveal microscopic developmental changes and the formation of preneoplastic lesions. Precursor cells are developmentally arrested in hemangioblasts that have biallelic VHL inactivation. However, these cells begin to cause reactive angiogenesis through unknown triggering mechanisms, and a subset may grow faster, progressing to hemangioblastoma. During growth, hemangioblastoma cells imitate the morphology of embryonic hemangioblasts, the precursors of hematopoietic and endothelial cells.[16]
Histopathology
The hallmark histological feature of hemangioblastomas is their biphasic tissue composition comprising stromal cells and capillary networks. Stromal cells, which are large and polygonal, compose the neoplastic element, driving tumor growth and neovascularization. They have clear cytoplasm full of lipid vacuoles, giving them a foamy look with round or oval nuclei and prominent nucleolus.[13] The vascular component of hemangioblastomas has dense capillary networks and sinusoids. These blood vessels have a single layer of endothelial cells that are often dilated, contributing to the tumor's highly vascular nature (see Image. Hemangioblastoma). The vascular structure helps the tumor grow by supplying nutrients and oxygen. Additionally, these capillaries have pericytes and smooth muscle cells which provide structural support.[13]
Immunohistochemical staining is crucial in helping to differentiate hemangioblastomas from other central nervous system (CNS) tumors. Stromal cells in hemangioblastomas show several markers, including alpha-inhibin, S100 protein, and vimentin. Alpha-inhibin helps distinguish hemangioblastomas from metastatic clear cell renal cell carcinoma, which can have a similar microscopic appearance. In addition, stromal cells are negative for epithelial markers like cytokeratin, typically noted in clear cell renal cell carcinoma. Endothelial markers (eg, CD31 and CD34) highlight the tumor's vascular network.[17]
History and Physical
Clinical History
Hemangioblastoma evaluation begins with a comprehensive history and thorough physical examination, which guides the physician to an intracranial pathology. In patients with hemangioblastoma, clinical presentation depends upon the location of the tumor and compressed areas. Clinical symptoms result once these slow-growing tumors have exerted enough of a significant mass effect to disrupt the function of the surrounding area. The most common location for these tumors is the posterior fossa, which causes an obstructive effect on the fourth ventricle or the cerebral aqueduct and increases intracranial pressure, which presents with symptoms such as headache and vomiting. Additionally, patients may report dizziness, ataxia, and gait disturbances due to compromised balance and coordination functions.[1][2] Brainstem hemangioblastomas present with motor and sensory deficits, nausea, vomiting, ataxia, and sometimes fatal hemorrhage. Spinal hemangioblastomas, on the other hand, can cause localized pain, motor weakness, sensory disturbances, and bowel or bladder dysfunction.
A thorough family history is advised to identify incidents that may indicate a genetic linkage. Personal or family history may reveal VHL-related tumors, including retinal angiomas, also called benign vascular hamartomas, clear cell renal cell carcinomas, pheochromocytomas, and pancreatic neuroendocrine neoplasms. Additionally, a history of VHL-related tumor surgical resection in the patient or a family member is relevant.[3][4]
Physical Examination
In patients with hemangioblastomas, typical findings include signs of increased intracranial pressure and cerebral mass effect. Additional examination findings vary depending on the tumor location within the CNS.[4] Neurological examination is essential for assessing the impact of the tumor on various CNS functions. The following findings are associated with hemangioblastomas located in these CNS areas:
- Ataxia: Difficulty with coordination and balance that leads to gait disturbances and difficulty performing delicate motor tasks.
- Dysmetria: Dysmetria is tested using finger-to-nose or heel-to-shin tests and is primarily present with tumors located in the cerebellum.
- Nystagmus: Cerebellar or brainstem involvement may indicated by nystagmus.[4]
- Back Pain: Localized or radicular back pain may radiate along the distribution of the affected nerve roots.
- Motor weakness and myelopathy: Weakness commonly arises in the muscles innervated by the affected spinal level.
- Sensory Disturbances: Loss of sensation or paresthesias in the dermatomes typically corresponds to the involved spinal levels.[7]
Additionally, a systemic examination is warranted in patients with VHL disease to identify other syndrome manifestations, including:
Evaluation
Diagnostic Imaging Studies
Clinical findings can guide clinicians in determining the optimal imaging study for the diagnostic evaluation of a suspected tumor.
Magnetic resonance imaging
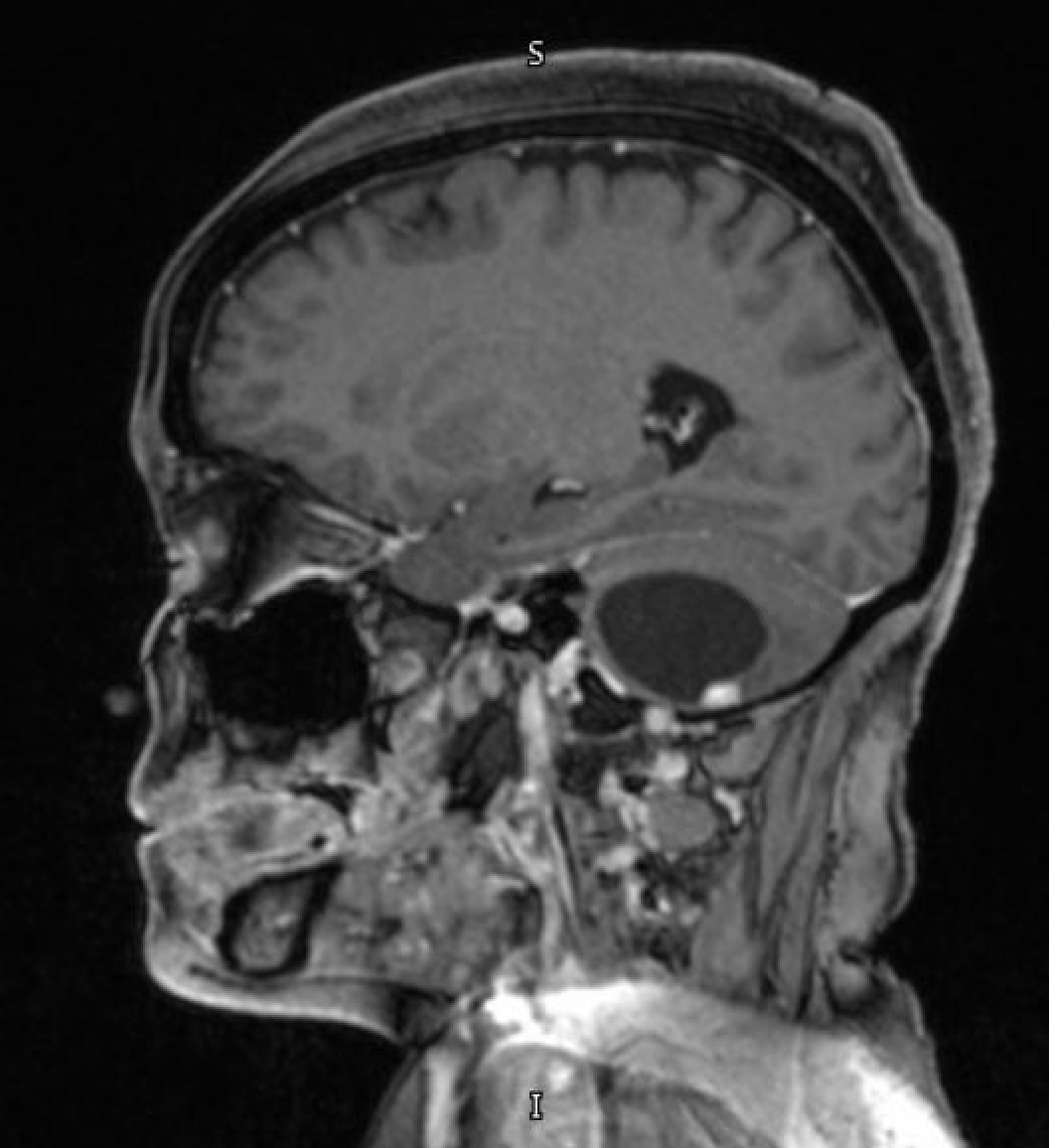
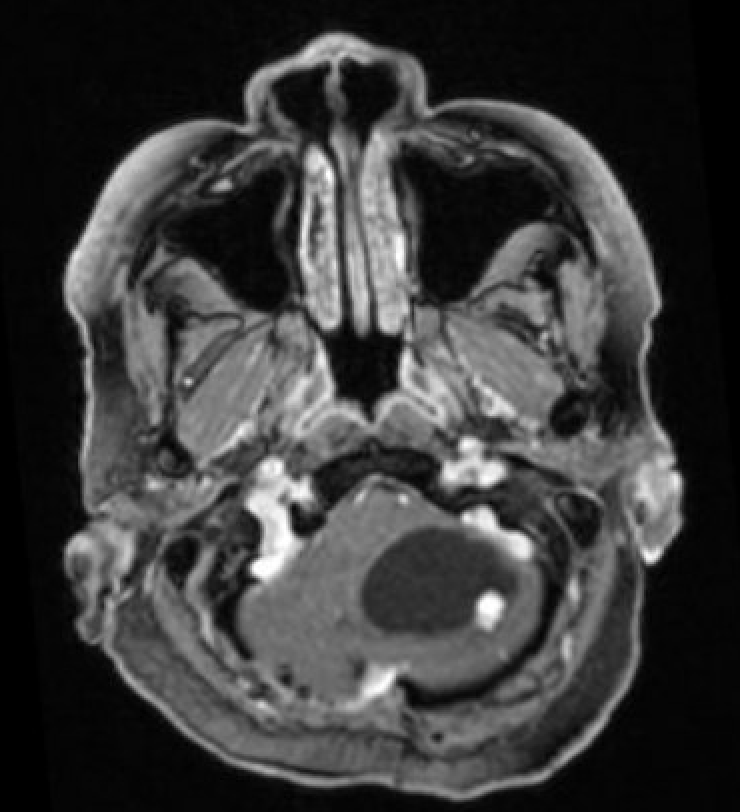
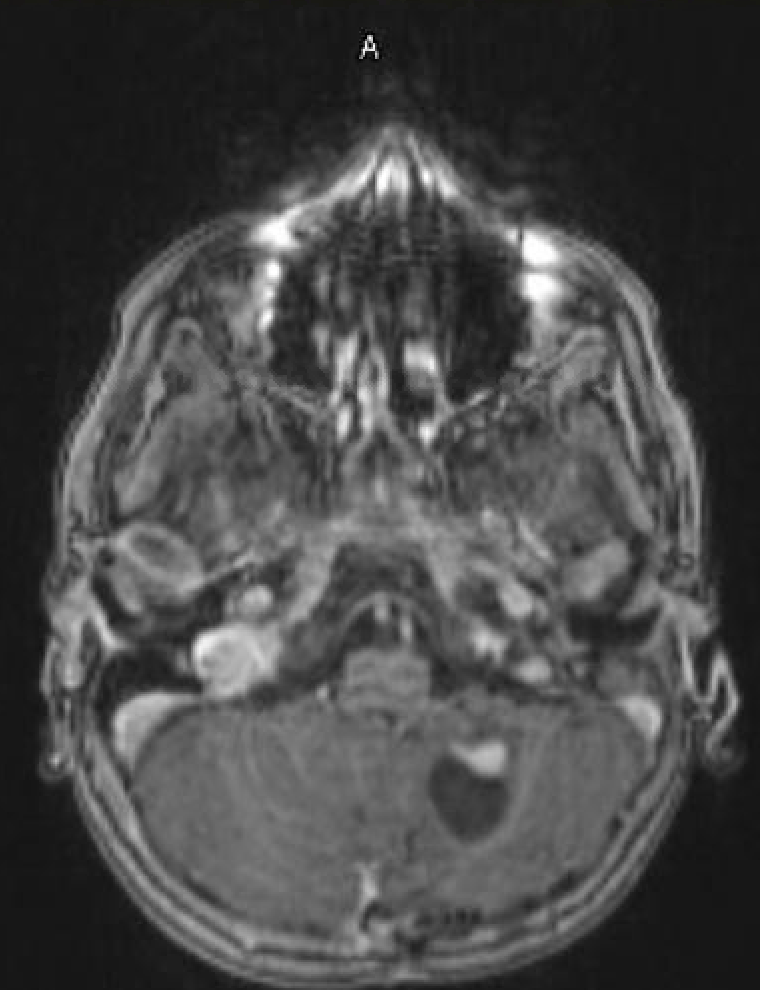
Magnetic resonance imaging (MRI) is the preferred modality for diagnosing CNS hemangioblastomas due to its detailed visualization of CNS structures. A characteristic MRI finding of hemangioblastomas is a mural nodule within a cyst. This feature typically appears as a well-circumscribed lesion enhanced by contrast with an associated cystic component.[6][18] The following findings are frequently observed in hemangioblastoma MRIs:
- T1: The mural nodule appears hypointense to isointense. The cyst content shows a signal similar to cerebrospinal fluid.
- T1 with contrast: The mural nodule shows vivid enhancement with gadolinium contrast; however, the cyst wall does not enhance (see Images. Axial MRI Head With Contrast and Sagittal MRI Head With Contrast).
- T2: The mural nodule appears hyperintense. Flow voids, seen in 60% to 70% of cases, occur due to enlarged vessels, especially at the periphery of the cyst. The cyst is fluid-filled and has a signal similar to cerebrospinal fluid.
- MR perfusion: High relative cerebral blood volume ratios are better observed with this study.
Computed tomography imaging
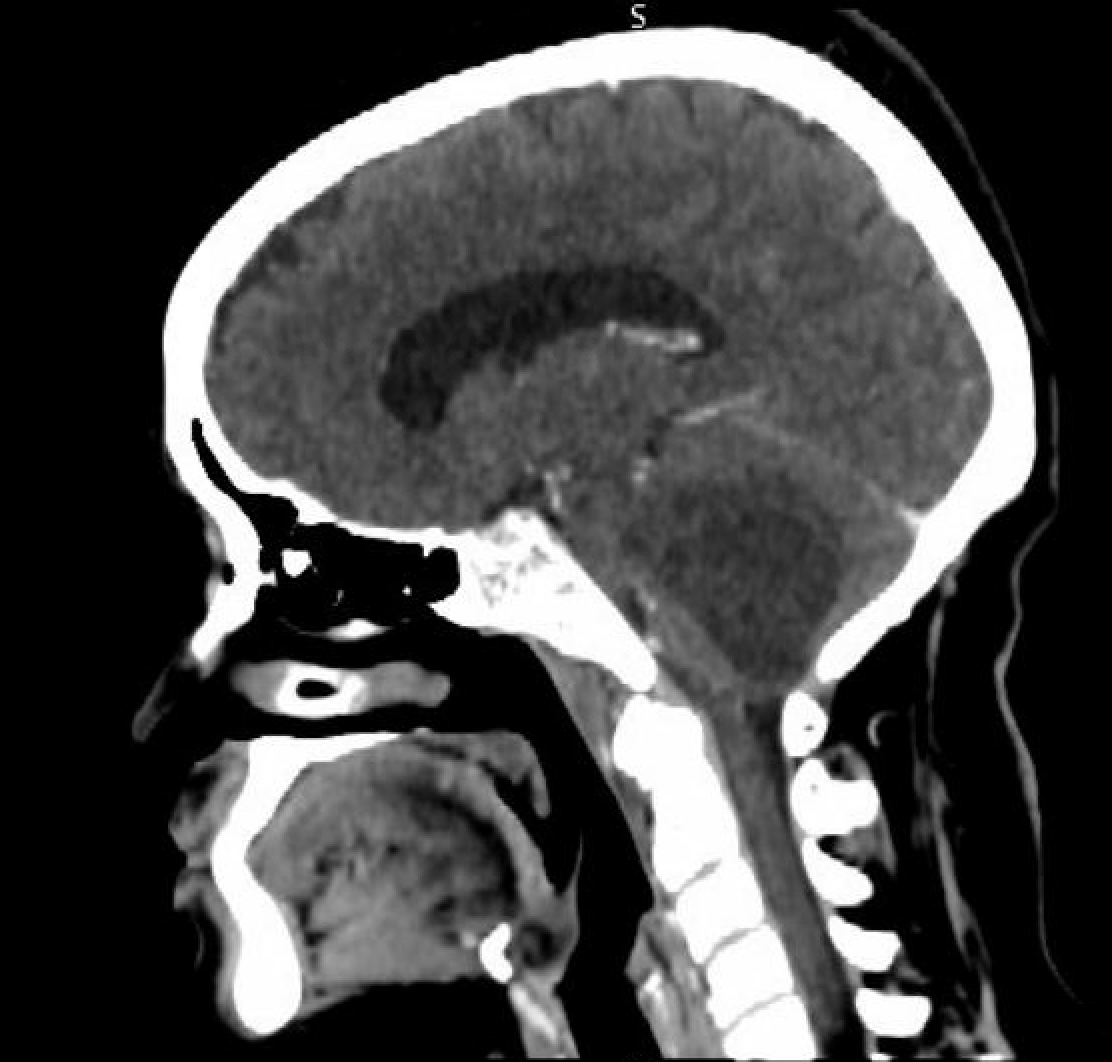
In computed tomography (CT) studies, the mural nodule in hemangioblastomas appears isodense to the brain on noncontrast images surrounded by cystic fluid. The mural nodule also demonstrates intense homogeneous enhancement (see Image. Computed tomography With Contrast). The cyst walls usually do not enhance. Calcification is not present in these tumors.[6]
Angiography
Studies using angiography facilitate visualization of enlarged feeding arteries and dilated draining veins often seen in hemangioblastomas. A dense tumor blush is commonly observed centrally, demonstrating the tumor's rich blood supply.[6]
Laboratory Studies
Laboratory studies are primarily performed for preoperative evaluation to assess the patient's overall health and readiness for surgery, including complete blood count, liver function tests, renal function tests, and coagulation profiles.[4] In cases where hemangioblastoma is associated with Von Hippel Lindau disease, genetic testing for VHL gene mutation is recommended.[4]
Treatment / Management
Surgical Treatment
Surgical resection is the treatment of choice for CNS hemangioblastoma, especially for symptomatic tumors secondary to mass effect. The goal of the surgery is complete tumor removal while minimizing damage to surrounding neural structures. Due to the high vascularity of such tumors, preoperative planning is essential to minimize intraoperative bleeding. Preoperative embolization can be considered to reduce the tumor's blood supply and facilitate safer resection. As most tumors arise in the posterior fossa, the usual surgical approach is a suboccipital craniotomy, which provides an excellent corridor to resect this lesion.[2] Microsurgical techniques are used to resect the tumor with the help of a microscope. Intraoperative neurophysiological monitoring can help preserve neurological function during spinal and cranial surgery. Image-guided navigation systems may also be used to improve the accuracy of tumor localization and resection.[2]
Medical Management
Medical management of CNS hemangioblastoma is usually supportive because these tumors are benign lesions that do not invade the surrounding structures or metastasize. Hence, chemotherapy has a limited role. Novel therapeutic agents, including antiangiogenic factors, have demonstrated promising results in managing these tumors, especially in patients with VHL gene mutations.[4] Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor and has been investigated for its ability to inhibit tumor growth by reducing angiogenesis. Other tyrosine kinase inhibitors (eg, sunitinib and sorafenib) have also been explored for their antiangiogenic properties.[4][8](B3)
Radiation Therapy
Stereotactic radiosurgery (SRS) is a noninvasive treatment option that delivers high doses of radiation precisely to the tumor while sparing surrounding healthy tissue. SRS is beneficial for small, inaccessible hemangioblastomas or residual tumors following surgery. Gamma knife and CyberKnife are common modalities used for SRS.[19](B2)
Tumor Surveillance
Regular follow-up with MRI is essential for detecting tumor recurrence or progression. The follow-up imaging duration varies based on the initial tumor characteristics, including the extent of resection achieved and the presence of VHL mutation. MRI scans are usually done every 6 to 12 months postoperatively for the first few years and then yearly if no recurrence is detected.[9]
Similar surveillance is recommended for VHL disease. A follow-up assessment is essential due to the risk of tumor recurrence or the development of new lesions. Follow-up usually includes regular imaging as well as ophthalmologic examination for retinal hemangioblastomas. Genetic counseling and testing are recommended for patients and their families to identify at-risk individuals and implement early surveillance strategies.[4][12]
Differential Diagnosis
Metastatic Carcinoma
Metastatic brain tumors from various primaries, especially renal cell carcinoma, can easily mimic hemangioblastomas in the posterior fossa of the brain. Metastatic lesions are the most common lesions in the posterior fossa of the brain. Renal cell carcinoma closely resembles hemangioblastomas, both radiologically and histologically. Renal cell carcinoma metastasis can present as a well-circumscribed hypervascular lesion with similar clear cell morphology. Immunohistochemical staining is needed to differentiate between both pathologies. Hemangioblastomas typically express alpha-inhibin and S100 protein, while RCC metastases express epithelial markers such as cytokeratin and epithelial membrane antigen.[20][21]
Pilocytic Astrocytoma
Pilocytic astrocytoma is a common benign tumor in children and young adults. Radiographically, it can present with cystic and solid components similar to hemangioblastomas. Both lesions are well-circumscribed and can be enhanced with contrast. On histopathological examination, pilocytic astrocytomas show Rosenthal fibers and eosinophilic granular bodies, which are absent in hemangioblastomas. Immunohistochemical staining can further help differentiate hemangioblastomas from pilocytic astrocytomas, which usually express GFAP.[22] (see Image. Pilocytic Astrocytoma).
Ependymoma
Ependymomas arise from the fourth ventricle lining. Due to the similar location and sometimes cystic appearance, ependymomas can be confused with hemangioblastomas. Ependymomas are differentiated from hemangioblastomas by the characteristic perivascular pseudorosettes and ependymal rosettes. In addition, ependymomas express GFAP and EMA, which are absent in hemangioblastomas.[22][23]
Vascular Malformations
Vascular lesions such as arteriovenous malformations and cavernous malformations can mimic these lesions on imagining due to their high vascular nature. Arteriovenous malformations on imaging have a nidus with direct arteriovenous shunting, while cavernous malformations consist of dilated and thin-walled capillaries without intervening brain tissue. These features distinguish vascular lesions from the organized capillary network and stromal cells of hemangioblastomas and, in most cases, the cystic lesion. Imaging modalities (eg, MRI and angiography) can aid in identifying these vascular malformations.[24]
Other Cystic Lesions
Other CNS cystic lesions, such as arachnoid cysts and neuroenteric cysts, should be considered as differential diagnoses when present in the cerebellum. Arachnoid cysts are benign with fluid-filled sacs that arise within the arachnoid membrane and are generally asymptomatic unless they are large in size and cause symptoms related to mass effect. Unlike hemangioblastomas, they do not enhance with contrast on MRI. Neuroenteric cysts are rare congenital lesions that can also appear cystic but have distinct histological features, including a lining of gastrointestinal or respiratory epithelium.[25]
Treatment Planning
Radiation Therapy Planning
Treatment planning is a necessary process wherein a patient's clinical presentation and images must be studied for the best outcomes. Therapy planning considers several factors, including tumor size, location, vascularity, and the patient's overall health. Because these tumors are highly vascular, the treatment plans must be precise and individualized to optimize outcomes and minimize complications.
Simulation imaging
Simulation imaging is a process that uses imaging to plan for radiation by defining the treatment area and preparing the radiation delivery. A CT scan is performed with the patient in the treatment position, thus providing detailed anatomical information and helping to outline the tumor and surrounding structures. MRI is often combined with CT images to enhance soft tissue contrast and further improve the accuracy of tumor delineation. This combination is especially important for CNS tumors where precise targeting is critical to avoid damaging healthy brain tissue.[26]
After the simulation is complete and the tumor margins are perfectly delineated, treating clinicians assess the target volumes for the radiation therapy. The gross tumor volume includes the visible tumor in imaging studies. The clinical target volume encompasses the gross tumor and any potential microscopic disease spread. The planning target volume adds margin to the clinical target volume to compensate for patient movement or other variations and uncertainties in radiation delivery. This ensures that the prescribed dose covers the target area despite these variables.[26]
Radiation therapy for hemangioblastomas primarily involves SRS. Other common methods include Gamma knife, CyberKnife, and Linear Accelerator with Gamma knife being the most published method for treating hemangioblastoma. Different authors have mentioned different target volumes for radiation therapy that change from center to center. Median marginal doses for SRS range from 12 to 29.9 Gy, with a maximum dose of 13.3 to 40 Gy. Median isodose lines vary between 50% and 90%. Follow-up periods range from 12 to 102 months. This shows satisfactory tumor control rates with pooled 5-year progression-free survival of around 88.4%.[27][28][29][30]
SRS is recommended for incompletely resected and recurrent tumors and is also suitable for patients with unfavorable anatomy for surgical resection or medical comorbidities, which makes surgery risky. Patients with VHL disease who have had multiple prior surgical resections are usually good candidates for SRS. Common adverse effects include hydrocephalus, radiation necrosis, peritumoral edema, headache, nausea, and vomiting. Tumor control rates are 92% at 3 years, 89% at 5 years, and 79% at 10 years. Overall survival rates are 94% at 3 years, 90% at 5 years, and 74% at 10 years.[29][30][31]
Various studies report a range of radiation doses for hemangioblastoma treatment via SRS. The dose falls between 14 and 35 Gy, with a median dose around 18 to 21 Gy. Dose selection will depend on factors like tumor size and location and patient factors such as age and overall health. Smaller tumors receive higher doses per fraction. Doses near critical structures like the brainstem or optic nerves need careful planning to minimize side effects. SRS is a single-fraction treatment where the entire radiation dose is delivered in a single session. The treatment takes only a few minutes, but the planning and preparation process can take several hours. The long-term effects of SRS are still under investigation. These patients need Regular follow-up MRIs to monitor for tumor control and potential side effects of the radiation therapy.[32][33]
Prognosis
The prognosis of patients with hemangioblastoma depends on numerous factors, including the location of the tumor, its size at the time of presentation, and whether it is sporadic or associated with VHL disease. Postoperatively, the success of the surgery and the extent of resection will help determine the patient's prognosis. In sporadic hemangioblastomas that are not linked to any genetic syndrome, the prognosis is usually excellent after complete surgical resection. These tumors grow slowly; complete resection often leads to long-term disease-free survival. Recurrence rates are low if the tumor is completely removed. Studies show that patients with sporadic hemangioblastomas have a high long-term survival rate, with a 5-year survival rate exceeding 90%.[1][4][9] The prognosis of patients with VHL is more varied and complex as patients can have other tumors like renal cell carcinoma, pheochromocytoma, and pancreatic tumors. When such patients develop CNS involvement, their outcome tends to be worse.[3][4]
Complications
Hemangioblastomas in the cerebellum or brainstem can obstruct the cerebrospinal fluid pathways, which can cause hydrocephalus, necessitating further surgical interventions such as having a ventriculoperitoneal shunt or third ventriculostomy. Tumors in distinct locations lead to specific neurological deficits: cerebellar tumors cause cerebellar signs, spinal cord tumors result in motor weakness and sensory disturbances, and brainstem tumors affect cranial nerves, causing symptoms like facial weakness, dysphagia, and diplopia.[1][2][4][14]
Surgical resection of hemangioblastomas carries a considerable risk of significant intraoperative bleeding due to their rich vascular supply. Preoperative embolization can reduce but not eliminate this risk. Complete resection is challenging, especially in critical areas like the brainstem and spinal cord, and can result in postoperative complications such as new or worsened neurological deficits. Surgery in the posterior fossa also carries the risk of cerebrospinal fluid leak, which can lead to meningitis if not managed promptly. Adequate closure techniques and vigilant postoperative monitoring are essential.[2][18]
Patients with VHL disease are at high risk for multiple lesions and tumor recurrence, requiring regular MRI surveillance. Recurrence or new tumors might necessitate repeated surgeries, increasing the cumulative risk of complications. This genetic disease is also linked to other neoplasms like pheochromocytomas, renal cell carcinoma, and pancreatic neuroendocrine tumors, leading to complications such as hypertension and renal dysfunction. A multidisciplinary approach is essential for managing these issues. The chronic nature of the disease and ongoing surveillance impact patients' quality of life, thus making psychological support and rehabilitation services crucial.[5][4][16]
Deterrence and Patient Education
Like any other disease, patient education is crucial, especially in patients with von Hippel Landau disease. These patients must understand that they have a critical condition with some genetic predisposition that makes them vulnerable to other tumors in other parts of the body. A regularly scheduled MRI and imaging studies are essential to monitor tumors. Early detection and treatment are essential, and therefore, the patient should have adequate information about their disease and the genetic knowledge of their condition to help them recognize their symptoms early and plan their future accordingly, such as having genetic counseling before starting a family.
Enhancing Healthcare Team Outcomes
Effective management of hemangioblastoma necessitates a cohesive interprofessional team involving physicians, advanced practitioners, nurses, pharmacists, and other health professionals. In collaboration with other specialists, neurosurgeons should regularly meet to develop and refine personalized treatment and follow-up surveillance plans, ensuring accurate and early diagnosis. Each patient’s unique medical history, including family history, must be thoroughly evaluated. Interprofessional communication is critical in educating patients and their families about the disease and treatment options, empowering them to participate in care decisions, and improving outcomes. Physicians and surgeons must stay current with the latest research and guidelines, providing evidence-based care. Continuous quality improvement processes, alongside ongoing education and training for all healthcare professionals, are essential for enhancing patient-centered care, ensuring safety, and optimizing team performance.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
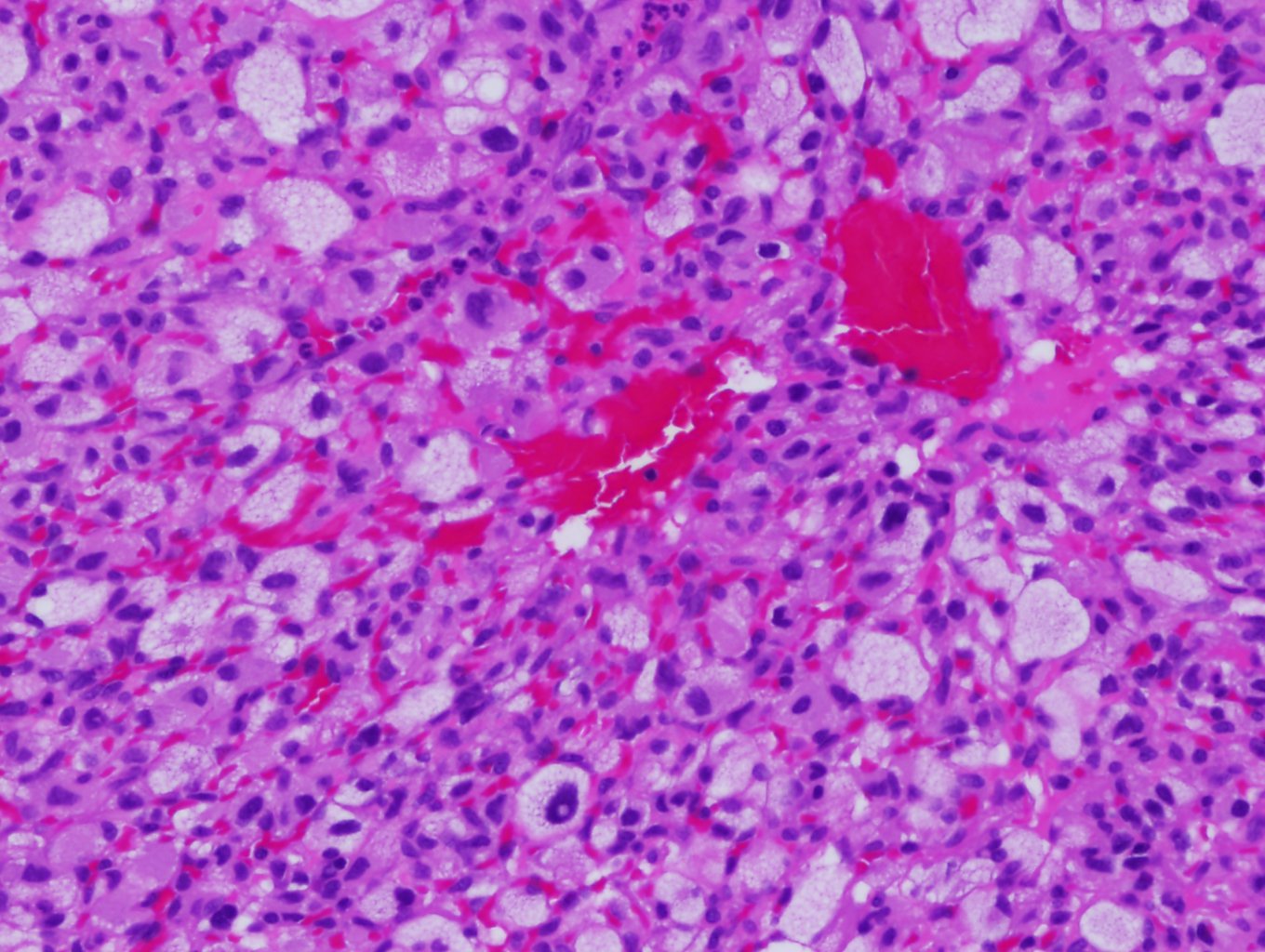
Hemangioblastoma. Hemangioblastomas have a hallmark histological feature: their biphasic tissue composition comprising stromal cells and capillary networks. The vascular component of hemangioblastomas has dense capillary networks and sinusoids. These blood vessels have a single layer of endothelial cells that are often dilated, contributing to the tumor's highly vascular nature.
Contributed by D Neelon, MD
References
Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001 Jan:48(1):55-62; discussion 62-3 [PubMed PMID: 11152361]
Bründl E, Schödel P, Ullrich OW, Brawanski A, Schebesch KM. Surgical resection of sporadic and hereditary hemangioblastoma: Our 10-year experience and a literature review. Surgical neurology international. 2014:5():138. doi: 10.4103/2152-7806.141469. Epub 2014 Sep 22 [PubMed PMID: 25317353]
Friedrich CA. Von Hippel-Lindau syndrome. A pleomorphic condition. Cancer. 1999 Dec 1:86(11 Suppl):2478-82 [PubMed PMID: 10630173]
Ganeshan D, Menias CO, Pickhardt PJ, Sandrasegaran K, Lubner MG, Ramalingam P, Bhalla S. Tumors in von Hippel-Lindau Syndrome: From Head to Toe-Comprehensive State-of-the-Art Review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 May-Jun:38(3):849-866. doi: 10.1148/rg.2018170156. Epub 2018 Mar 30 [PubMed PMID: 29601266]
Farrukh HM. Cerebellar hemangioblastoma presenting as secondary erythrocytosis and aspiration pneumonia. The Western journal of medicine. 1996 Feb:164(2):169-71 [PubMed PMID: 8775737]
Ho VB, Smirniotopoulos JG, Murphy FM, Rushing EJ. Radiologic-pathologic correlation: hemangioblastoma. AJNR. American journal of neuroradiology. 1992 Sep-Oct:13(5):1343-52 [PubMed PMID: 1414827]
Cvek J, Knybel L, Reguli S, Lipina R, Hanzlikova P, Šilhán P, Resova K, Blazek T, Palicka M, Feltl D. Stereotactic radiotherapy for spinal hemangioblastoma - disease control and volume analysis in long-term follow up. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2022:27(1):134-141. doi: 10.5603/RPOR.a2022.0003. Epub 2022 Mar 22 [PubMed PMID: 35402025]
Mak G, Algird A, Greenspoon J, Provias J, Hirte H. Cervicomedullary hemangioblastoma treated with bevacizumab. Neuro-oncology advances. 2020 Jan-Dec:2(1):vdaa076. doi: 10.1093/noajnl/vdaa076. Epub 2020 Sep 3 [PubMed PMID: 32908970]
Level 3 (low-level) evidenceYin X, Duan H, Yi Z, Li C, Lu R, Li L. Incidence, Prognostic Factors and Survival for Hemangioblastoma of the Central Nervous System: Analysis Based on the Surveillance, Epidemiology, and End Results Database. Frontiers in oncology. 2020:10():570103. doi: 10.3389/fonc.2020.570103. Epub 2020 Sep 9 [PubMed PMID: 33014882]
Takayanagi S, Mukasa A, Tanaka S, Nomura M, Omata M, Yanagisawa S, Yamamoto S, Ichimura K, Nakatomi H, Ueki K, Aburatani H, Saito N. Differences in genetic and epigenetic alterations between von Hippel-Lindau disease-related and sporadic hemangioblastomas of the central nervous system. Neuro-oncology. 2017 Sep 1:19(9):1228-1236. doi: 10.1093/neuonc/nox034. Epub [PubMed PMID: 28379443]
Takami H, Graffeo CS, Perry A, Brown DA, Meyer FB, Burns TC, Parney IF. Presentation, imaging, patterns of care, growth, and outcome in sporadic and von Hippel-Lindau-associated central nervous system hemangioblastomas. Journal of neuro-oncology. 2022 Sep:159(2):221-231. doi: 10.1007/s11060-022-04021-8. Epub 2022 Jul 28 [PubMed PMID: 35902552]
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. European journal of human genetics : EJHG. 2011 Jun:19(6):617-23. doi: 10.1038/ejhg.2010.175. Epub 2011 Mar 9 [PubMed PMID: 21386872]
Kim H, Park IS, Jo KW. Meningeal supratentorial hemangioblastoma in a patient with von hippel-lindau disease mimicking angioblastic menigioma. Journal of Korean Neurosurgical Society. 2013 Nov:54(5):415-9. doi: 10.3340/jkns.2013.54.5.415. Epub 2013 Nov 30 [PubMed PMID: 24379949]
Vetrano IG, Gioppo A, Faragò G, Pinzi V, Pollo B, Broggi M, Schiariti M, Ferroli P, Acerbi F. Hemangioblastomas and Other Vascular Origating Tumors of Brain or Spinal Cord. Advances in experimental medicine and biology. 2023:1405():377-403. doi: 10.1007/978-3-031-23705-8_14. Epub [PubMed PMID: 37452946]
Level 3 (low-level) evidenceWiesener MS, Eckardt KU. Erythropoietin, tumours and the von Hippel-Lindau gene: towards identification of mechanisms and dysfunction of oxygen sensing. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2002 Mar:17(3):356-9 [PubMed PMID: 11865075]
Klingler JH, Gläsker S, Bausch B, Urbach H, Krauss T, Jilg CA, Steiert C, Puzik A, Neumann-Haefelin E, Kotsis F, Agostini H, Neumann HPH, Beck J. Hemangioblastoma and von Hippel-Lindau disease: genetic background, spectrum of disease, and neurosurgical treatment. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020 Oct:36(10):2537-2552. doi: 10.1007/s00381-020-04712-5. Epub 2020 Jun 7 [PubMed PMID: 32507909]
Yoda RA, Cimino PJ. Neuropathologic features of central nervous system hemangioblastoma. Journal of pathology and translational medicine. 2022 May:56(3):115-125. doi: 10.4132/jptm.2022.04.13. Epub 2022 May 3 [PubMed PMID: 35501672]
Bonneville F, Sarrazin JL, Marsot-Dupuch K, Iffenecker C, Cordoliani YS, Doyon D, Bonneville JF. Unusual lesions of the cerebellopontine angle: a segmental approach. Radiographics : a review publication of the Radiological Society of North America, Inc. 2001 Mar-Apr:21(2):419-38 [PubMed PMID: 11259705]
Kano H, Shuto T, Iwai Y, Sheehan J, Yamamoto M, McBride HL, Sato M, Serizawa T, Yomo S, Moriki A, Kohda Y, Young B, Suzuki S, Kenai H, Duma C, Kikuchi Y, Mathieu D, Akabane A, Nagano O, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. Journal of neurosurgery. 2015 Jun:122(6):1469-78. doi: 10.3171/2014.10.JNS131602. Epub 2015 Mar 27 [PubMed PMID: 25816088]
Level 2 (mid-level) evidenceLee C, Park JW, Suh JH, Nam KH, Moon KC. Histologic variations and immunohistochemical features of metastatic clear cell renal cell carcinoma. Korean journal of pathology. 2013 Oct:47(5):426-32. doi: 10.4132/KoreanJPathol.2013.47.5.426. Epub 2013 Oct 25 [PubMed PMID: 24255630]
Kojima F, Musangile FY, Matsuzaki I, Yorita K, Kuroda N, Nagashima Y, Murata SI. Current Knowledge and Prospects for Renal Hemangioblastoma and Renal Cell Carcinoma with Hemangioblastoma-like Features. Biomedicines. 2023 May 17:11(5):. doi: 10.3390/biomedicines11051467. Epub 2023 May 17 [PubMed PMID: 37239138]
Suri VS, Tatke M, Singh D, Sharma A. Histological spectrum of ependymomas and correlation of p53 and Ki-67 expression with ependymoma grade and subtype. Indian journal of cancer. 2004 Apr-Jun:41(2):66-71 [PubMed PMID: 15318011]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun:131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9 [PubMed PMID: 27157931]
Mulligan PR, Prajapati HJ, Martin LG, Patel TH. Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. The British journal of radiology. 2014 Mar:87(1035):20130392. doi: 10.1259/bjr.20130392. Epub [PubMed PMID: 24588666]
Epelman M, Daneman A, Blaser SI, Ortiz-Neira C, Konen O, Jarrín J, Navarro OM. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006 Jan-Feb:26(1):173-96 [PubMed PMID: 16418251]
Pereira GC, Traughber M, Muzic RF Jr. The role of imaging in radiation therapy planning: past, present, and future. BioMed research international. 2014:2014():231090. doi: 10.1155/2014/231090. Epub 2014 Apr 10 [PubMed PMID: 24812609]
Hanakita S, Koga T, Shin M, Takayanagi S, Mukasa A, Tago M, Igaki H, Saito N. The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro-oncology. 2014 Mar:16(3):429-33. doi: 10.1093/neuonc/not201. Epub 2013 Dec 12 [PubMed PMID: 24335701]
Johnson S, Niranjan A, Kano H, Lunsford LD. Leksell Radiosurgery for the 3 H Tumors: Hemangiomas, Hemangioblastomas, and Hemangiopericytomas. Progress in neurological surgery. 2019:34():223-231. doi: 10.1159/000493068. Epub 2019 May 16 [PubMed PMID: 31096251]
Yoo KH, Park DJ, Marianayagam NJ, Gu X, Pollom EL, Soltys SG, Chang SD, Meola A. Stereotactic Radiosurgery for Cranial and Spinal Hemangioblastomas: A Single-Institution Retrospective Series. Neurosurgery. 2024 Mar 1:94(3):630-642. doi: 10.1227/neu.0000000000002728. Epub 2023 Oct 17 [PubMed PMID: 37967154]
Level 2 (mid-level) evidencePan J, Ho AL, D'Astous M, Sussman ES, Thompson PA, Tayag AT, Pangilinan L, Soltys SG, Gibbs IC, Chang SD. Image-guided stereotactic radiosurgery for treatment of spinal hemangioblastoma. Neurosurgical focus. 2017 Jan:42(1):E12. doi: 10.3171/2016.10.FOCUS16361. Epub [PubMed PMID: 28041328]
Kano H, Niranjan A, Mongia S, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008 Sep:63(3):443-50; discussion 450-1. doi: 10.1227/01.NEU.0000313120.81565.D7. Epub [PubMed PMID: 18812955]
Pan J, Jabarkheel R, Huang Y, Ho A, Chang SD. Stereotactic radiosurgery for central nervous system hemangioblastoma: systematic review and meta-analysis. Journal of neuro-oncology. 2018 Mar:137(1):11-22. doi: 10.1007/s11060-017-2697-0. Epub 2017 Dec 4 [PubMed PMID: 29204841]
Level 1 (high-level) evidenceAsthagiri AR, Mehta GU, Zach L, Li X, Butman JA, Camphausen KA, Lonser RR. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel-Lindau disease. Neuro-oncology. 2010 Jan:12(1):80-6. doi: 10.1093/neuonc/nop018. Epub 2009 Dec 23 [PubMed PMID: 20150370]