Introduction
Tuberculosis (TB) is an ancient infectious disease with many varied presentations. Although prevention and treatment are available, between 8 and 10 million people still develop TB, and 2 to 3 million people die from it globally, according to the 2022 estimates from the World Health Organization (WHO).[WHO 2023 Global TB Report] For years, children have been thought to contribute little to the global epidemic as they are rarely infectious, and they often clear the infection without treatment. However, without testing and treatment for those children with exposure, a potential reservoir is created from which new future cases will develop, and global efforts at control and eradication will fail.[1] Significant gaps are present between the recognition of children at risk for acquiring TB and their subsequent diagnosis and management. Children, especially those younger than 5, develop TB more readily and in more severe forms than older children and adults. According to the most recent WHO data for children younger than 15, 1.3 million cases of TB were diagnosed, and nearly a quarter million TB-related deaths were reported.[WHO 2023 Global TB Report]
The developed world has seen some improvement in diagnosing and preventing TB cases, but no evidence of a decline in high-burden nations has been seen.[WHO 2023 Global TB Report] In fact, high-burden countries have seen significant increases in not only infection and disease but also major increases in multidrug-resistant TB.[1] Poverty, lack of access to adequate medical care, malnutrition, and concurrent infections such as human immunodeficiency virus continue to fuel the infection rates in high-burden countries and pockets throughout the developed world. Pediatric TB case rates are likely underreported in many high-burden countries, and study results have suggested that children may represent as many as 50% of TB cases worldwide.[WHO 2023 Global TB Report] Much work is needed in the diagnosis and management of pediatric TB, as well as addressing the underlying social causes that create the perfect environment for the transmission of TB and the comorbidities that facilitate worse outcomes following infection.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Mycobacterium tuberculosis is an aerobic, slow-growing, nonmotile, acid-fast bacillus. Unlike other aerobes, M tuberculosis has a cell wall containing an elevated amount of high molecular weight lipids.[2] M tuberculosis is part of a group of closely related organisms (including Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium canetti) associated with human disease.[2] M tuberculosis is primarily found in the United States, while M bovis, M africanum, and M canetti are rare in the United States and are not routinely identified by clinical laboratories. If necessary, reference laboratories can distinguish M bovis from M tuberculosis. This differentiation may be relevant for a given case presentation since the epidemiology, treatment, and prevention are different, even though the clinical presentation is similar to M tuberculosis. Other mycobacteria in the M tuberculosis complex primarily cause animal disease.
Epidemiology
Most M tuberculosis infections and disease in children are found in areas of the world where adults have the greatest incidence of TB infection. The highest M tuberculosis disease burden is borne in countries in sub-Saharan Africa and Asia, particularly India and the Western Pacific. In the United States, which is a low-incidence country, children who develop TB are often either born outside the United States or have been exposed to close contact who are from outside the United States, particularly from a high-burden country. Another small percentage of cases result from travel to high-burden areas.[WHO 2023 Global TB Report][3] Drug resistance to first- and even second-line medications is an emerging public health problem globally. While data collection is variable, children with multidrug-resistant TB in some areas of the world reach as high as 13%.[WHO 2023 Global TB Report][4][5]
The 2023 WHO Global TB report indicated that 1.3 million children younger than 14 contracted TB in 2022, about 12% of the global total TB disease burden, while a quarter million have succumbed to TB-related disease.[WHO 2023 Global TB Report] Most likely, this is an underrepresentation of the actual number of pediatric cases and deaths, as childhood TB is often misdiagnosed or miscategorized.[1] Deaths from TB in many high-burden regions are frequently ascribed to pneumonia or meningitis without a specific diagnosis.[1]
The human immunodeficiency virus (HIV) epidemic has also affected the epidemiology of M tuberculosis, as coinfection with HIV is not an uncommon occurrence.[6] Although many children in the developing world have access to antiretroviral therapy (ART), children with HIV have a higher risk for M tuberculosis-associated disease even with a normal cluster of differentiation 4 lymphocyte counts and strong viral suppression compared with their non-HIV-infected cohort.[7] Several studies conducted in African nations documented that even though ART reduces TB risk and improves outcomes among children with HIV in sub-Saharan Africa, the risk of TB remains highly elevated compared to children without HIV in the same settings.[7] Children younger than 14 represent the lowest percentage of HIV-infected persons on ART (57% vs 77% for those older than 14).[7] This discrepancy also occurs at a time when both international and domestic funding for HIV initiatives has declined significantly [Global HIV and AIDS Statistics] and will impact not only HIV cases but also coinfections, including M tuberculosis that can accompany severe HIV disease.[8][9][10]
Pathophysiology
The pathophysiology of TB is well-documented. Please see StatPearls' companion resource, "Tuberculosis," for an overview of TB. However, clinicians should note that differences exist between children and adults with TB in terms of host control of the disease. Healthy adults with TB infection have only a 5% to 10% risk of developing TB disease during their lifetime and tend to develop their disease in the first 1 to 2 years after infection is established. However, infants and toddler-age children have a much higher risk of disease progression after exposure than school-age children if left untreated (40% to 50%, 25%, and 10% to 15%, respectively).[11]
Infants and young children have different immunologic responses to TB exposure than adults.[12] An infant's immune system has altered inflammatory responses to accommodate the transition to an extrauterine environment with inherent contact with beneficial and virulent pathogens.[12][13] They typically have lower numbers of macrophages, neutrophils, and tissue dendritic cells, and the function of these cells is different compared to that of adult cells. Production of proinflammatory cytokines, such as tumor necrosis factor and interleukins 1 and 12, is reduced, while anti-inflammatory cytokines are increased, including interleukin 10.[14] Infant T cells are less able to differentiate into interferon gamma-producing T cells, a key factor in M tuberculosis control.[14]
Therefore, infants are 5 to 10 times more likely to develop active TB than adults, and they have significantly higher rates of severe disseminated disease, including miliary TB and meningitis.[15] Lack of T-cell responses with interferon-gamma release also interferes with tuberculin diagnostic testing for interferon-gamma release assay and tuberculin skin tests, even in the face of overwhelming disease. Children with immune deficiencies, particularly HIV, are at even greater risk for TB progression and severe disease.[7][Global HIV and AIDS Statistics]
Histopathology
Children tend to have paucibacillary disease yet a very brisk immunologic response to M tuberculosis.[10] The hallmark of M tuberculosis infection in tissue specimens is a caseating granuloma; children readily produce granulomas in M tuberculosis tissue disease. This granuloma comprises epithelioid histiocytes surrounding a central necrotic area and is accompanied by multinucleated giant cells and lymphocytes.[16] The function of these cells is to contain the mycobacterial infection and prevent its spread to surrounding healthy tissues and other organs.[16]
Ziehl–Neelsen staining for acid-fast organisms can be applied to tissue samples for children, but their disease's paucibacillary nature makes visual diagnosis difficult. Fluorescent techniques utilizing auramine-rhodamine staining are often used for the ease of visualization in specimens with low numbers of mycobacteria. In situ, techniques using ribonucleic acid (RNA)scope adapted for M tuberculosis to identify intact and disintegrated mRNA in necrotic and nonnecrotic granulomata may also be useful as an adjunct in Ziehl–Neelsen-negative or fluorescence–negative tissue samples from children with suspected TB disease.[17]
History and Physical
TB has a wide variety of presentations in children and can produce disease in nearly all organ systems. Most TB disease in children takes a pulmonary form, with only approximately 20% manifesting as extrathoracic disease. An overlap is often seen between pulmonary disease and extrapulmonary manifestations, especially in children younger than 2, primarily including the following areas:
- Intrathoracic
- Pulmonary
- Pleural
- Cardiac (1% to 4%)
- Extrathoracic
- Disseminated (lymphohematogenous)
- Lymphatic
- Central nervous system
- Osteoarticular
- Abdominal and gastrointestinal
- Genitourinary
- Cutaneous
- Congenital
- Other
Intrathoracic Manifestations
Pulmonary disease
The pulmonary system is the most affected in children with TB. After exposure, silent pulmonary infection without any obvious signs, symptoms, or imaging abnormalities ensues for many children. Some may have a prodrome consisting of several days of low-grade fever and cough resembling a viral illness, but these symptoms will resolve over several weeks. Ninety percent of newly infected older children will have an asymptomatic infection, whereas up to 50% of infected infants develop respiratory or constitutional symptoms with radiographic abnormalities during primary infection.[18] Clinicians must be aware of the age discrepancy in developing progressive severe disease.
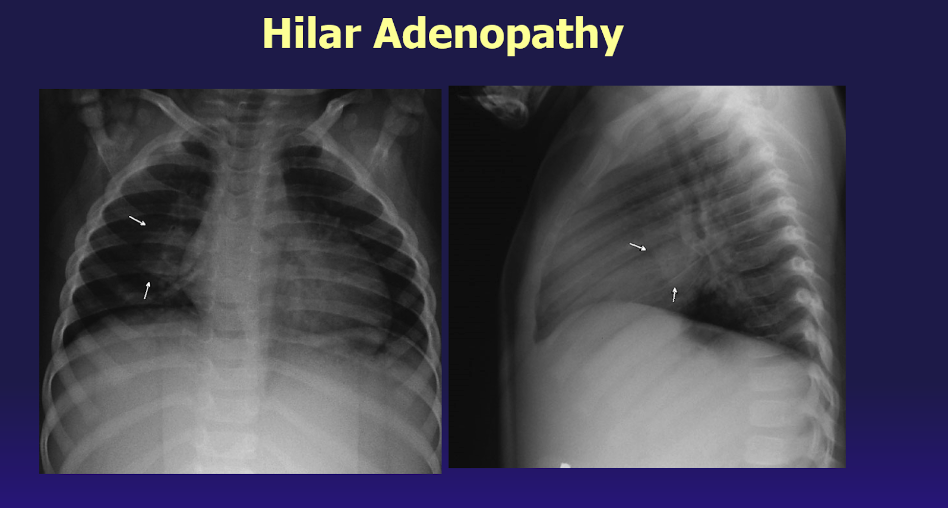
Primary pulmonary infection in children includes infection of the lung tissue coupled with hyperplasia of regional lymph nodes, called the primary pulmonary complex. Unlike adults, all lung lobes are equally affected in children, and a quarter of the cases can have multiple parenchymal foci. Radiographic findings may note disproportionately enlarged regional lymph nodes compared with little to no parenchymal focus (see Image. Hilar Adenopathy).[19][20]
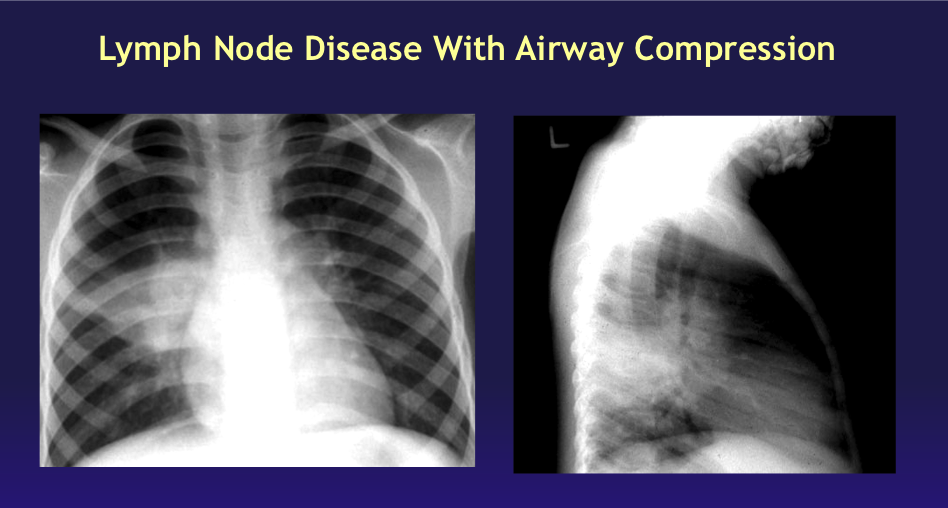
Radiographic changes often resolve quickly, but in some children, particularly those younger than 2, lymph nodes will continue to enlarge, resulting in partial or complete bronchial obstruction from external compression. Such children may present with wheezing and shortness of breath due to the small caliber of their airways, similar to a foreign body aspiration or other obstructive disorders. Chest radiographs may demonstrate localized hyperinflation with atelectasis of contiguous parenchyma areas known as segmental lesions (see Image. Lymph Node Disease With Airway Compression).[19] Clinicians and radiologists must know that this finding is not typical of other types of bacterial pneumonia but can be M tuberculosis-related.
Adolescents are frequently more symptomatic from pulmonary TB disease than school-aged children, especially when the disease is acquired near puberty. Adolescents may also present with reactivation disease like that seen in adults, with a classical picture consisting of progressive fatigue, loss of appetite, night sweats, weight loss, and fevers.[21] Chest pain and productive or nonproductive cough may occasionally be accompanied by hemoptysis. Physical findings may be absent, but chest radiographs demonstrate extensive upper lobe infiltrates and possibly even cavities (see Image. Adult-Type Cavitary Tuberculosis Disease).[18] Fortunately, M tuberculosis is localized well by the immune system if the teen is not immunocompromised, and anti-tuberculous treatment clears the signs and symptoms of infection rapidly if diagnosed and treated promptly.[22]
Pleural disease
Occasionally, pleural disease is seen in pediatric TB and may happen without segmental or miliary lesions in the lung parenchyma. This occurs when bacilli form a subpleural focus and erode into the pleural space. A hypersensitivity response ensues, stimulating the formation of a pleural effusion. Due to the brisk immune response, these collections may become large enough to be associated with abrupt onset of fever, chest pain, and shortness of breath with rapid respiratory compromise depending on the child's size and relative pleural effusion size. The physical examination will reveal dullness to percussion and diminished breath sounds on the affected side. Despite appropriate treatment, fevers are often very high and may persist for several weeks.[23][24] Microbiologic confirmation is often difficult due to low numbers of bacteria.[25] Pleural biopsy may demonstrate granuloma formation but requires surgical intervention or a fine needle biopsy. Newer techniques, such as polymerase chain reaction, may prove useful in such situations, but TB detection rates still fall below 50%.[26] Because true empyema rarely forms, it must be considered in the differential diagnosis of any culture-negative pleural specimen, especially in high-burden countries.[26]
Cardiac disease
Infection involving the cardiac system is unusual, occurring in less than 4% of all cases of pediatric TB.[27] The most common site of infection is pericardial tissue, which results in pericarditis and pericardial effusion. The presentation of pericardial involvement is similar to other forms of pericarditis with symptoms of fever, malaise, and weight loss; children are less likely to complain of chest pain than adults with TB pericarditis.[27] Generally, other organ systems are involved, and these features may overshadow the pericardial disease.[27] A physical examination may demonstrate a pericardial friction rub, distant heart sounds, and pulsus paradoxus, especially if the disease has progressed to the fibrinous stage. Chest radiography reveals a large globular heart, and ultrasonography demonstrates fluid collection in the pericardial space. When aspirated, pericardial fluid is serofibrinous or mildly hemorrhagic but rarely is acid-fast bacillus smear positive. Nonetheless, cultures are positive in 30% to 70% of cases, and a biopsy of the pericardium reveals caseating granulomas in 50% to 75% of the cases. Without early recognition and treatment of this condition, constrictive pericarditis from fibrosis over months to years will develop. In contrast to the outcomes of TB pericardial disease in adults, results from a recent study in South Africa found that the mortality was low and the residual morbidity among children in their cohort primarily resulted more from the concurrent disease at the time of presentation, particularly that of TB meningitis, than the pericardial involvement.[20]
Extrathoracic Manifestations
Disseminated disease
TB can disseminate to distant anatomic sites following the establishment of primary infection in young children or during reactivation disease in older children and adolescents, often triggered by the erosion of a previously healed primary pulmonary lesion. Depending upon the host's age and immune function, infection from this seeding to distant sites may become readily apparent or quiescent for years. Miliary TB is a highly lethal form of disseminated TB that results from a massive lymphohematogenous dissemination of M tuberculosis from an infected focus. The presentation varies depending on the organs involved and may resemble other common childhood diseases.[28] Fortunately, pediatric miliary TB is rare, comprising less than 1% of all pediatric TB cases, but this may be underestimated due to lack of recognition. A recent case report from Tunisia highlighted the complexities of miliary disease masquerading as multisystem inflammatory disease secondary to COVID-19 yet found to be caused by M tuberculosis.[29]
During early hematogenous dissemination of M tuberculosis, there may be an acute onset of high-spiking fevers, but a prolonged and intermittent fever is also possible. Classic features of TB infection may develop, including chronic weight loss, hepatosplenomegaly, and superficial or deep node lymphadenitis. Other unusual features, such as crops of skin lesions called papulonecrotic tuberculids and choroid tubercles seen on ophthalmological evaluation, are possible. Later in the clinical course, meningitis may also develop. Pulmonary disease, which may have been mild initially, can become more severe over time (see Image. Chest Radiograph and Computed Tomography, Tuberculosis). Due to the atypical multiple-organ involvement, a high index of suspicion is required for early recognition, followed by comprehensive investigation and prompt initiation of appropriate treatment for disseminated TB to mitigate the potential for severe complications and to reduce morbidity and mortality associated with this complex disease.[28][30]
Lymphatic disease
Tuberculous lymphadenitis (previously known as scrofula) is the most common form of extrapulmonary TB disease in children and constitutes nearly 60% of extrapulmonary cases. In the past, tuberculous lymphadenitis was often caused by drinking unpasteurized cow's milk contaminated by M bovis; pasteurization of milk and milk products has nearly eliminated M bovis as a cause of adenitis in low-burden countries. However, in high-burden regions of the world, particularly in pastoral areas, M bovis and other members of the M tuberculosis complex still warrant consideration.[31][32] Now, most cases of lymphadenitis are caused by M tuberculosis primary pulmonary infection acquired by aerosol or droplet contact.
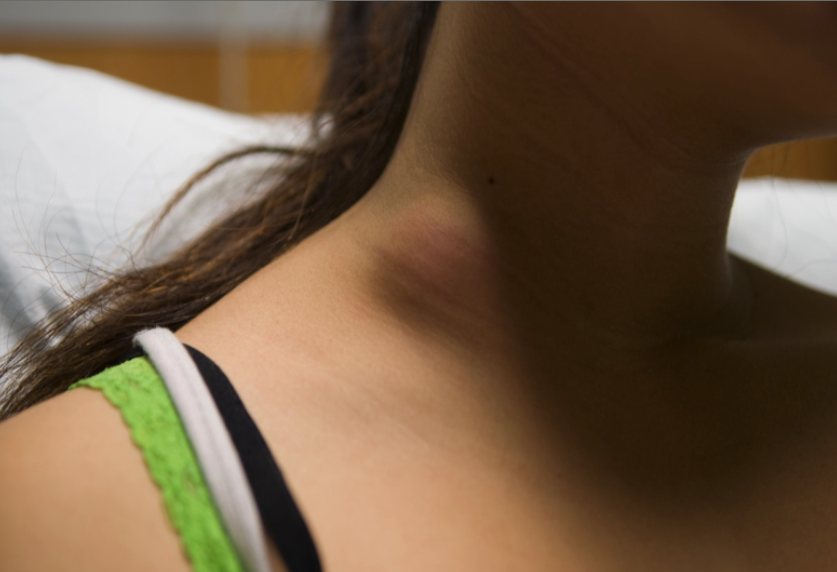
Lymph nodes of the anterior cervical, supraclavicular, tonsillar, and submandibular areas are commonly involved due to extension from the primary lesion in the upper lung fields or the abdomen (see Image. Tuberculosis Disease, Cervical Lymph Node). Other areas of lymph node involvement include the inguinal, epitrochlear, and axillary chains. These generally are associated with skin lesions secondary to the M tuberculosis complex. Involved nodes often enlarge slowly but can become massive in size. They are discrete and firm but not hard or tender to touch. However, these affected nodes can feel fixed to adjacent tissue, suggesting a malignancy. Even though a primary pulmonary focus is invariably present, it is often asymptomatic and may be radiographically inapparent in as many as 30% of cases. Untreated lymphadenitis may resolve spontaneously or progress with fluctuance and an overlying purplish red hue indistinguishable from nontuberculous mycobacterial infections in children (see Image. Lymph Node Disease). A chest radiograph may be negative in tuberculous and nontuberculous lymphadenitis, but an interferon-gamma release assay will be unequivocally positive.[33] If untreated, the node may rupture and create a draining sinus tract that will require surgical resection. Clinicians need to maintain a high index of suspicion in low-burden countries to arrive at the proper diagnosis and treatment, especially for children of parents from high-burden nations.[34]
Central nervous system disease
The most deadly and debilitating form of TB is meningitis, which disproportionately affects young children. Without antibiotic therapy, corticosteroids, and critical care management of fluids, electrolytes, and elevated intracranial pressure, tuberculous meningitis is rapidly fatal within several weeks.[35] Despite advances in the care of children with tubercular meningitis, mortality is still high; more than 50% of survivors have neurodevelopmental disabilities.[35][36][37][38] Areas of the world with a high burden of TB may be more likely to recognize the disease than others but lack some tools for fully successful diagnosis and treatment. In contrast, high-resource areas lack knowledge and experience with the disease to recognize signs and symptoms early on.[38]
The clinical onset of tuberculous meningitis can be acute or gradual in the early stages. Nonspecific symptoms, such as flu-like illness, low-grade fever, malaise, and pulmonary symptoms, may overshadow central nervous system findings. Many children are initially seen with pneumonia that will not resolve, poor appetite, and vomiting before more serious signs of central nervous system disease become manifest. Advanced disease may demonstrate signs of meningeal irritation (eg, elevated intracranial pressure with a bulging fontanelle, sunsetting sign, and papilledema), cranial nerve palsies, neurologic deficits and loss of milestones, altered sensorium, and seizures.[39][40][41] The Glasgow Coma Scale can help assess the child's consciousness level and aid in determining the ultimate prognosis.[35]
Urgent evaluation of the central nervous system with head imaging is crucial, followed by lumbar puncture for cultures and fluid analysis.[42] Brain imaging commonly shows basilar meningeal enhancement, hydrocephalus, enhancement of basal ganglion, infarcts, or a combination of these.[43][44] Magnetic resonance imaging (MRI) is preferred over computed tomography for its improved ability to identify early infarcts, basal ganglia enhancement, and exudates in the basal cisterns.[45][46][47] However, MRI is not widely available in low-resource countries (see Image. Tuberculosis Meningitis).
The treatment of tubercular meningitis requires a regimen of prolonged antituberculosis therapy, initially with 4 drugs. (Please refer to the Treatment section for more information on antituberculosis regimens). Management of increased intracranial pressure may require additional medications, including steroids, mannitol, and acetazolamide. The placement of a temporary or permanent ventriculoperitoneal shunt may be needed.[42][41] Close outpatient follow-up is required to manage adherence and potential adverse events from non TB medications, particularly electrolyte disturbances with the use of acetazolamide.
Skeletal disease
Osteoarticular TB also behaves differently in pediatrics than in adults. The primary focus of infection in children is generally the pulmonary system with dissemination to the osteoarticular system, but extension from either a caseous regional lymph node or an adjacent bone is also possible. Metaphyseal involvement develops initially, followed by necrosis from the pressure of granulation tissue and caseation. Extension into the joint space and adjacent soft tissues can complicate established bone infection, but the swollen joint makes the disease clinically apparent.[48]
Skeletal TB mainly involves weight-bearing bones and joints; the knee, hip, spine, elbow, and ankle are the most affected. The spectrum of presentation ranges from mild joint effusion without any bony destruction to significant destruction of bone and restriction of joint mobility caused by chronic synovial fibrosis. A single joint or many may be involved.[48][49][50][51] This slow process may evolve over months to years, causing mild pain, stiffness, limping, and restricted movement. A tuberculin skin test or interferon-gamma release assay is often reactive, and joint fluid culture or bone biopsy will usually be positive for M tuberculosis.
Dactylitis is more common in young infants and toddlers but is occasionally seen in older children and adults.[48][50] Distal endarteritis from M tuberculosis dissemination through the bloodstream leads to painless swelling of the hands or feet. In the case of digital involvement, a typical lesion involves the proximal phalanx of the index and middle fingers. Fusiform swelling of the digits may resemble juvenile idiopathic arthritis of the hands or feet but is painless. Radiographs showing lytic lesions in the involved digits, called spina ventosa, are typical for M tuberculosis dactylitis.[52]
The vertebral bodies are a particular target of TB (Pott disease), and multiple vertebral bodies may become involved.[53] Infection in the lower thoracic or the upper lumbar vertebrae can lead to bone necrosis, with wedging and collapse; this can lead to gibbous or kyphotic spinal deformity (see Image. Vertebral Osteomyelitis Due to Tuberculosis). Rupture of the infection into adjacent soft tissue may lead to local abscess formation in the psoas muscle, paraspinal muscles, or retropharyngeal space. Paraspinal muscle spasms in children can be associated with low-grade fever, irritability, back pain, abnormal positioning of the back, refusal to bear weight, and movement difficulty. If significant bone destruction and collapse ensues, neurological complications are highly likely to occur, and neurologic impairment may be permanent.[54]
Radiographs of the involved bones generally reveal a lytic lesion, but an MRI should be performed to obtain further information.[48] Several characteristic MRI findings are often present, including synovial thickening, fluid collections, and bony erosions. T1 images show a low signal on the thickened synovium, and T2 images show a hypointense synovium. A confirmatory biopsy should be performed by either needle or open methods, but often, the bony cavity requires debridement of the necrotic bone and caseous material.
Cultures are frequently negative, as in other pediatric TB infections elsewhere in the body. However, biopsy material is critical to the diagnosis since caseating giant cell granulomas seen on histopathology are highly specific for TB and should be treated as such.[48] A history of TB contact is critical when evaluating a child with skeletal disease from a high-burden country or a child with HIV or other immunocompromising conditions who may have had an infectious contact. History is critical in younger age groups presenting with dactylitis.[48]
Abdominal and gastrointestinal disease
Abdominal TB in children may be more common than previously appreciated since it has nonspecific abdominal signs and symptoms, and the lack of available diagnostic tools makes the diagnosis difficult.[55][56] Abdominal TB tends to occur in slightly older children, usually older than 5 years. Abdominal TB can involve the mesenteric and intra-abdominal lymph nodes, the peritoneum, the intestines, and the intra-abdominal and pelvic organs. Lymphatic or hematogenous seeding, spread from adjacent lymph nodes, and the introduction of mycobacteria orally through M tuberculosis-contaminated animal milk, breast milk, or swallowed pulmonary secretions are the most common acquisition routes. The presentation may resemble other abdominal diseases such as appendicitis, intestinal perforation, and inflammatory bowel disease.[57]
TB enteritis generally results from hematogenous spread to the gut or from swallowing mycobacteria in contaminated milk or respiratory secretions. The jejunum and the ileum are the most common sites involved and may present with shallow ulcers that cause localized pain, diarrhea or constipation, and weight loss. A diagnosis of Crohn disease may be entertained before TB testing is completed as the signs and symptoms are similar. Mesenteric lymphadenitis is often present but generally not palpable on abdominal exam. Enlarged nodes can obstruct the intestines or erode through the omentum, resulting in generalized peritonitis. Tuberculous peritonitis can also result from TB of the genitourinary tract with extension, especially from infection in the fallopian tubes.[58] Intestinal obstruction is a common complication of intestinal TB resulting from mural thickening, ileal stricture, or adhesions.
Abdominal ultrasound may aid in identifying enlarged lymph nodes, visceral disease with abscess formation, and the presence or absence of ascites.[56] Peritoneal fluid may demonstrate neutrophil or lymphocyte predominance. In a South African series, aspirated peritoneal fluid with an elevated adenosine deaminase level was present in three-quarters of the children with TB peritonitis; the children in this study were significantly more ill.[56]
Genitourinary disease
The genitourinary tract can be seeded by M tuberculosis during direct hematogenous or lymphatic spread from primary M tuberculosis lesions or reactivation disease elsewhere in the body.[59] M tuberculosis organisms can lodge in any portion of the genitourinary tract in both sexes. The disease progression is slow and insidious and is often missed clinically until the disease has caused significant organ damage.
Renal TB is the most common clinical presentation of urogenital TB. In the past, renal TB was believed to spare young children as the progression of the disease was slow. However, currently, several case series underscore the fact that renal TB may not be clinically obvious, though slow destruction of the organ is ongoing, even in infants and younger children.[60][61][62]
The intense vascularity of the kidney makes it an easy target for hematogenous spread, and M tuberculosis establishes foci with a granulomatous response. Small caseous tubercles develop and shed M tuberculosis into the tubules and, hence, into the urine. Large caseous masses can also develop and erode into the renal pelvis. Infection can readily spread to the ureters, prostate, epididymis, and adjacent organs like the uterus and fallopian tubes. Untreated, renal TB can lead to the destruction of the renal parenchyma, obstructive uropathy, and end-stage renal failure.[59][63]
The genital tract can be infected with M tuberculosis in both sexes. In young women of reproductive age (15 and older), infertility is the most common presentation of TB disease involving the genital tract in high-burden areas of the world.[59] In vitro fertilization for a woman with unrecognized genital tract TB can have severe consequences for the outcome of the procedure.[64] Adolescent females may complain of lower abdominal pain and experience dysmenorrhea or amenorrhea.[58] Adolescent males may present with unilateral, nodular, and painless scrotal swelling due to epididymitis or orchitis.[65] All parts of the male genital tract, including the prostate, seminal vesicles, bladder, urethra, and the penis, can be infected with M tuberculosis.[59] Fortunately, this is a rare situation for children.
Congenital and perinatal disease
Congenital TB is rare, but clinicians must always maintain a high index of suspicion when faced with a patient diagnosed with active TB during pregnancy. In-utero infections can occur following maternal bacteremia and have also been reported following in vitro fertilization of women from high-burden countries who likely had unrecognized or subclinical genital TB infection without treatment.[66] Perinatal TB carries up to a 50% mortality since many of the typical features, such as fever, respiratory distress, pulmonary infiltration, lethargy, enlarged liver and spleen, and hematologic abnormalities, cannot distinguish TB from other types of serious systemic infections in a newborn.[67][68] Further complicating the problem is that tuberculin skin test results are usually negative in infants with congenital or perinatally acquired infection, and the sensitivity of interferon-gamma release assay testing is unknown but also likely to be low due to the infant's immune response to M tuberculosis. If M tuberculosis is suspected, regardless of the tuberculin skin test or interferon-gamma release assay results, therapy for the infant should be initiated immediately with a 4-drug regimen. The placenta should be evaluated for granulomas and acid-fast bacilli and cultured for M tuberculosis. The parent should also have testing for tuberculosis as well as HIV since the risk of coinfection with HIV and TB in high-burden countries is significant.[67][68]
For patients with known or suspected TB, the following management of the parent-infant dyad is recommended depending on whether the parent has latent or active TB:
- The maternal parent has been diagnosed with TB infection in pregnancy (positive tuberculin skin test or interferon-gamma release assay with negative chest radiograph):
- This scenario suggests that the pregnant individual has TB infection (formerly latent TB) as opposed to TB disease. The pregnant patient does not have active TB and is noninfectious. Generally, the pregnant patient will be treated after the postpartum period for latent TB.
- In this scenario, the infant needs no special testing and need not be separated from the maternal parent, who may breastfeed if she desires. However, the entire household and other potential contacts of the infant should be questioned and tested, especially if the index case for the maternal parent's positive TB test is not known since it will place the infant at risk from exposure.[69]
- The maternal parent has been diagnosed or treated for active TB disease during pregnancy (positive TST or IGRA with an abnormal chest radiograph consistent with tuberculosis):
- This is a high-risk situation for the infant to acquire TB disease through respiratory droplets or airborne transmission. Unfortunately, the infant and maternal parent need to be separated until both have been evaluated thoroughly for TB disease and placed on therapy.
- The postpartum patient should begin antituberculosis therapy targeting her specific disease location. She needs to wear a mask and adhere to infection control measures. She may not breastfeed, but the infant could be fed expressed milk if the mother has no evidence of TB mastitis. Once the mother has been on therapy for 2 or more weeks AND she is no longer considered contagious, ie, has smear-negative sputum, she may breastfeed.
- The infant requires evaluation for congenital tuberculosis, and if excluded, the infant should be started on isoniazid (INH) unless the M tuberculosis isolate is known or suspected to be INH resistant until the infant is 3 or 4 months of age when the TST should be performed. If this 3- to 4-month TST is negative AND the child has not developed any signs or symptoms of TB disease, INH can be discontinued as long as the postpartum parent has been adherent to medication and remains noninfectious. However, if the infant's 3-month TST is positive, they should be reassessed for M tuberculosis disease. Even if TB disease is excluded, the infant still requires further treatment for the positive TST. A full 9 months of INH, or 4 months of rifampin, is warranted.
- Consultation with a specialist in pediatric infectious disease or a clinician with the local TB branch of the health department is recommended to ensure proper care.[69]
Other disease manifestations
TB can also present with rare and varied features that are often part of disseminated disease but may occasionally be seen in isolation. Otitis media and mastoiditis [70][71], cutaneous lesions called papulonecrotic tuberculids [72], and ophthalmologic findings of vitritis, keratitis, choroid tuberculids are the result of dissemination of M tuberculosis to distant sites.[73][74]
Evaluation
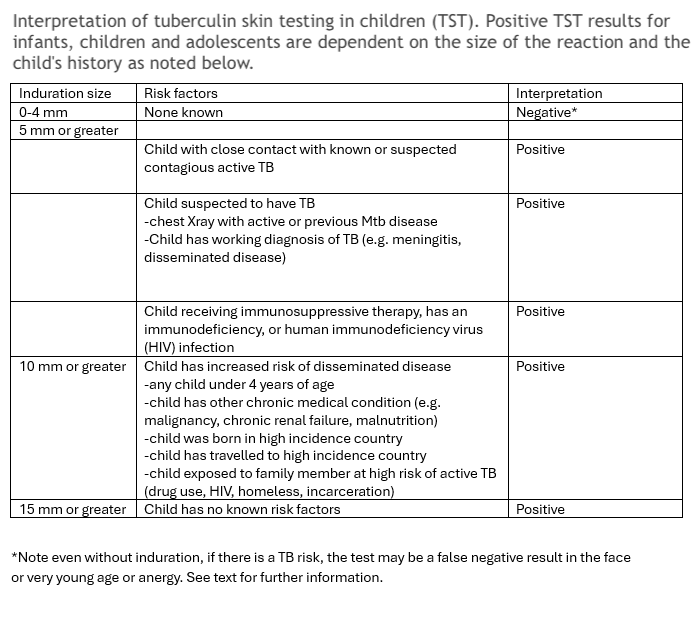
The initial step for TB evaluation should involve a TST or IGRA test. Proper placement, measurement, and interpretation are very important for accurate results (see Images. Mantoux Tuberculin Skin Test, Accurate Measurement). The TST should be read in the context of the child's history and potential exposure to active TB (see Table. Interpretation of Tuberculin Skin Testing in Children). Positive reactions should generate a chest radiograph.
Depending on the site of suspected M tuberculosis infection and the urgency of the evaluation, these tests may also be performed in conjunction with specimens from the most affected organ systems. For pulmonary TB, sputum collection is always of primary importance for a child who can expectorate. Children older than 10 years are generally able to provide an adequate amount of sputum, but children between 5 and 10 years of age may require assistance with induced sputum. For children younger than 5 years, specimens are generally collected by gastric aspiration or lavage of first morning specimens since young children usually swallow their respiratory secretions.
Specimens should always be sent for culture, drug susceptibilities, and for more rapid testing techniques where available. Culture of clinical specimens may take 3 to 8 weeks for visual growth to appear on solid media. Recent methods utilizing liquid media, such as the mycobacteria growth indicator tube (MGIT) system, have facilitated more rapid organism identification.[6][3] PCR technology remains a more useful and rapid modality but may not be widely available, especially in high-burden countries. Other types of detection methods are evolving, such as CRISPR detection of M tuberculosis in blood samples, that may prove to be of great benefit for children as they have paucibacillary disease, similar to that of reactivation disease for some adults.[75]
Another collection technique involves using a "string test," a highly absorbent nylon yarn packaged inside a gelatin capsule that is swallowed.[76] Feces may also contain swallowed M tuberculosis, and a nucleic acid amplification test (NAAT), eg, GeneXpert® MTB/RIF (Cepheid, United States of America), can confirm approximately 45% of clinically diagnosed cases of pulmonary TB with less trauma for the child.[77][78] Bronchoalveolar lavage fluid (BAL) may also provide useful specimens for analysis in children suspected of having pulmonary TB, but less invasive methods should always be attempted first.[79] The utility of NAAT BAL has been demonstrated in adults with paucibacillary recurrent TB and may apply to the analysis of pediatric specimens.[80]
Similar techniques of in situ NAAT testing with the expert MTB/RIF assay can be performed on extrapulmonary specimens, fine needle aspirate or excisional biopsy specimens for lymph nodes, and in formalin-fixed, paraffin-embedded tissues for other types of extrapulmonary TB disease.[81][82] WHO recommends the use of GeneXpert® testing to diagnose extrapulmonary TB, including TB meningitis of cerebral spinal fluid specimens for both adults and children.[83]
Treatment / Management
Treatment decisions regarding TB management are made based on a detailed medical history and a comprehensive physical examination. After careful consideration for TB in the differential diagnosis of a presenting child, one of the first tests that should be performed is a tuberculin test, either a purified protein derivative (PPD)/TST or a NAAT (eg, IGRA). (Please refer to the Evaluation section for more information.) Information from this test will guide subsequent testing.
A chest x-ray is warranted for evaluation following a positive PPD/TST or NAAT test to determine if pulmonary involvement is present; in cases of high suspicion for M tuberculosis in the absence of a positive screening test, imaging may be performed to assess for silent intraparenchymal disease or adenopathy. Other studies depend upon the location of potential infectious foci and can include ultrasonography of enlarged lymph nodes, imaging of the central nervous system followed by lumbar puncture, skeletal x-rays, imaging of the gastrointestinal or genitourinary tract, or a combination of these tests. Unfortunately, the lack of a positive reaction to a tuberculin test does not always exclude TB infection or TB disease, nor does a positive reaction distinguish between latent TB and actual disease. Up to 40% of immunocompetent children with culture-positive TB disease did not initially react to the tuberculin test. Host factors, particularly young age (younger than 2 years), poor nutrition, immunosuppression, and concurrent viral infections, can adversely affect a positive result. Recent TB infection and overwhelming disease (eg, those seen in disseminated disease) may also result in a negative reaction. Immunosuppression, particularly in children with advanced HIV disease, can also lead to a false negative PPD/TST result.
Treatment protocols for children parallel those of adults, except weight-based dosing is employed for all the needed medications. If directly observed therapy (DOT) is available or is required, as is the case for some regimens, it can be a helpful adjunct to adherence when children transition from the intensive phase with 3 or 4 drugs to the continuation phase with 2 drugs that can be administrated several times per week rather than daily (see Tables. Recommendations for Regimens to Treat Latent TB Infection, Recommended Drug Regimens and Therapy Length for Drug-Susceptible Tuberculosis Treatment, and Pediatric Drugs and Dosing for Drug Susceptible Tuberculosis Infection and Disease).[84][85][[86]
Table. Recommended Drug Regimens and Therapy Length for Drug-Susceptible Tuberculosis Treatment
|
Disease Category |
Subgroups |
Drug Regimen |
Comments |
|
Pulmonary and Extrapulmonary Tuberculosis Disease (excluding meningitis and osteoarticular disease) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Isolated Lymphadenitis |
|
|
|
|
Osteoarticular Tuberculosis |
|
|
|
|
Meningitis |
|
|
|
|
|
|
|
|
*Some experts recommend the use of a 3-drug regimen without ethambutol if the risk of drug-resistant Mtb (DR-Mtb) is low
¶ substitution of ethionamide or levofloxacin is more commonly used in practice, although WHO recommends the use of ethambutol
Φ Dose of Rifampin for meningitis is 30 mg/kg/d up to a max of 600 mg/d [84][85][[86]
Table. Recommended Pediatric Drugs and Dosing for Drug-Susceptible M tuberculosis Infection and Disease
|
Drug |
Form |
Daily Dosage (mg/kg) |
Weekly Dosing Regimen |
Maximum Daily Dosage |
Most Common Adverse Effects |
|
Ethambutol |
|
|
|
|
|
|
Isoniazid |
|
|
|
|
|
|
Pyrazinamide |
|
|
|
|
|
|
Rifampin |
|
|
|
|
|
†Dosing is based on lean body weight; doses for obese patients are not well-documented
Treatment of DR-Mtb in children is more difficult as some of the medications are not well-studied in children. Children are more likely to experience possible DR-Mtb if they or a close contact fit the following criteria:
- Reside in or emigrate from a country with high rates of resistance (eg, Russia and former Soviet bloc countries, Asia, Africa, and Latin America)
- Personal or contact history of prior incomplete drug treatment
- Personal or contact history of treatment for DR-Mtb
- Personal or contact history of persistently positive smear 2 months into therapy
The attached references can guide the treatment of DR-Mtb therapy, but consultation with an expert experienced in the treatment of DR-Mtb is highly recommended to prevent inadequate or partial treatment.[87][84] (A1)
Adherence can be significantly aided by home health nursing assistance where available. A care team consisting of the prescribing physicians, social workers, mental health clinicians, and local health department personnel is often needed to aid the family in completing successful treatment for a young child.[88] Home visits for DOT and intermittent visits to the office for the child and family to assess adverse effects, adherence, potential therapeutic barriers, and response to therapy may be needed for some types of TB treatment protocols. Unlike adults, children do not necessarily require monitoring of laboratory values, such as liver enzymes, unless they initially had abnormal testing or underlying conditions that may make tolerance of treatment regimens difficult without adverse effects.
Differential Diagnosis
Unfortunately, the differential diagnosis of TB is highly dependent upon the location of the disease. As noted in the clinical description of TB, many more common childhood diseases may be mistakenly diagnosed in a child with TB. Other viral, bacterial, and fungal types of pneumonia can present similarly, and M tuberculosis should always be considered in these cases. Cervical lymphadenitis can be viral, especially if multiple nodes are involved. Bacterial pathogens, eg, Bartonella and atypical mycobacteria, can also cause cervical, axillary, and inguinal lymphadenitis; atypical mycobacteria, like M tuberculosis, may produce purplish-red skin discoloration during the progression of the infection. M tuberculosis meningitis is often missed until late as it can mimic other more common childhood viral and bacterial infections that affect the central nervous system.
Furthermore, in high-burden countries, numerous vector-borne diseases (eg, malaria and encephalitis viruses) are present, in addition to parasites that infect the central nervous system. Disseminated TB, abdominal TB disease, and genitourinary tract TB disease can mimic inflammatory bowel disease, severe systemic viral, bacterial, and fungal infections, including multisystem inflammatory disease secondary to COVID-19, and even malignancy. Additionally, HIV should always be tested for in a patient diagnosed with M tuberculosis, and all persons with HIV should have yearly testing for M tuberculosis.
Prognosis
Tuberculosis is both curable and preventable. The overall prognosis for TB should be excellent for a healthy child with an early diagnosis and adequate treatment. Sadly, that is not often the case; morbidity and mortality from tuberculosis take their toll. Many complications, noted below, of late diagnosis or inadequate treatment occur worldwide, but especially in high-burden countries. This is especially true for children with disseminated disease with or without meningitis and for tuberculosis when coinfecting a child with impaired immunity, particularly HIV.[89] Overall mortality in children with HIV as a subset of high-risk children who are coinfected with TB have a disproportionate share of the overall TB mortality, estimated to be as high as 17%.[90]
For children lacking access to early diagnosis and treatment, mortality can reach 21%, rivaling the M tuberculosis pretreatment era before 1946.[89] Mortality in tuberculous meningitis, even with treatment, can reach as high as 19%, and neurodisabilities are seen in >50% of survivors.[36] Survivors may suffer from significant complications related to their disease, the stage at which they started treatment, and any underlying conditions that compromise their immune system. Delays in diagnosis of other organ-specific involvement, eg, renal disease, can progress slowly with significant damage once the disease manifests.[91]
Complications
Most children with proper diagnosis and treatment will experience no complications related to their disease. However, children with immunodeficiencies, particularly HIV, children whose diagnosis was delayed, or children with poor adherence to therapy may lead to the following complications:
- Pulmonary
- Extensive lung damage with an increased risk of subsequent wheezing
- Reduced lung function
- Cavitary disease
- Lymphadenitis
- Development of chronic fistula
- Central nervous system:
- Hydrocephalus
- Cerebrovascular diseases such as stroke and vasculitis
- Tuberculoma
- Coma
- Skeletal
- Pott disease
- Paraplegia related to spinal disease
- Epiphysial invasion, deformity, and bone shortening
- Deformities of the affected limb
- Spinal deformities
- Abdominal
- Generalized peritonitis
- Intestinal obstruction (from enlarged nodes and erosions)
- Genitourinary
- Infertility in females
- Hydronephrosis
- Ureteral strictures
- Miliary spread (development of disseminated disease)
Consultations
Since TB is relatively rare in the United States, many clinicians do not have sufficient experience or feel comfortable with many aspects of TB diagnosis and treatment. Clinicians should always have a low threshold for consulting a pediatric TB expert, either a pediatric infectious disease specialist or a physician working with the TB branch of the local county or state health department. Therefore, questions regarding interpretations of chest radiographs, TB testing in immunocompromised children, and more complex treatment regimens for those with high levels of drug resistance or compromised immune systems should be directed to these experts.
Deterrence and Patient Education
The prevention of TB disease has been available for 100 years using the Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine. This vaccine remains the first and only licensed vaccine against tuberculosis and is utilized in over 180 countries worldwide. The BCG vaccine is administered in infancy and can provide newborns with nearly 90% protection against severe disseminated tuberculosis disease in the first year of life. Although neonates generate a poor interferon-gamma response to M tuberculosis in vivo, BCG vaccination activates CD4 T-cell responses to produce TH1 cytokines, including interferon-gamma, which is lacking in a newborn’s responses to natural tuberculosis infection. BCG provides excellent protection against TB meningitis and miliary TB but cannot prevent pulmonary disease.[100] However, the BCG vaccine is also a live attenuated vaccine and, therefore, is contraindicated in immunocompromised individuals, especially those with untreated HIV.
Over a dozen new candidate vaccines are currently in clinical trials. A complete discussion is beyond the scope of this course; the new candidate vaccine platforms include:
- Recombinant fusion proteins delivered with an adjuvant
- Viral vectors that express M tuberculosis antigens
- A new live BCG vaccine replacement
Strategies for infants have included providing an initial BCG vaccination as a priming dose with a subunit vaccine boost or the reverse strategy with the subunit vaccine first followed by BCG. Unfortunately, this model has only produced modest immunogenicity in infants compared with parallel trials in adults.[101][102] Some were concerned that the coadministration of the vaccine with the routine infant vaccination series might blunt responses to the M tuberculosis vaccine. However, while lower immunogenicity to the M tuberculosis vaccine was seen in vaccinated infants, no effect on the responses to routine infant vaccines was noted.[103]
Another target group for vaccination is the adolescent and young adult population since this age group (15 to 25 years) has had a sharp increase in the incidence of tuberculosis disease worldwide. Many of these individuals present with cavitary disease and are highly infectious. Therefore, mathematical modeling suggests that the prevention of this condition potentially has a significant impact on the TB epidemic.[104] In adults, an adenovirus vector vaccine produced excellent responses and is felt to be sufficient for protective immunity.[102] Safety, efficacy, and immunogenicity trials are ongoing in infants, adolescents, and adults focused on ending the TB pandemic with new preventative strategies.
Pearls and Other Issues
Key facts to keep in mind for TB in children:
- Children with TB infection are a small but often overlooked population and represent a repository for future cases if not treated to prevent reactivation disease.
- Any child younger than 5 years with a contact who has active disease requires a TB evaluation and should be placed on preventive medication pending a reevaluation in several months. This is especially true for an infant born to a mother with active M tuberculosis disease who has either been newly diagnosed or is not yet on therapy.
- The presentation of TB in children is varied and can involve all organ systems. In low-burden countries, a high index of suspicion is required to reach the proper diagnosis, as M tuberculosis disease can masquerade as many common childhood illnesses. A child with TB always represents a secondary case; the index case adult must be located and treated.
- TB treatment regimens are designed for adults, and while they are highly effective for treatment, they are difficult to administer in children and require close follow-up to ensure a good outcome.
Enhancing Healthcare Team Outcomes
In treating pediatric tuberculosis, a collaborative, interprofessional team is essential to deliver effective, patient-centered care that maximizes patient safety and improves outcomes. Physicians, advanced practitioners, nurses, pharmacists, clinical microbiologists, and public health experts work together to coordinate care, ensuring adherence to complex TB regimens and educating families on the risks of incomplete treatment to prevent the development of resistance and the possibility of worsening disease is critical.
Additionally, directly observed therapy (DOT), facilitated by local health departments, can support adherence, especially with twice-weekly regimens. Nurses and public health professionals play a crucial role in monitoring adverse effects and tracking weight changes, as these can impact dosing requirements. Pharmacists and pharmacologists ensure proper dosing and often prepare custom formulations, particularly in cases where pediatric doses or suspensions are unavailable. Interprofessional teams can address adherence challenges and adjust therapies as needed through coordinated communication and education, enhancing patient safety and promoting effective treatment outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
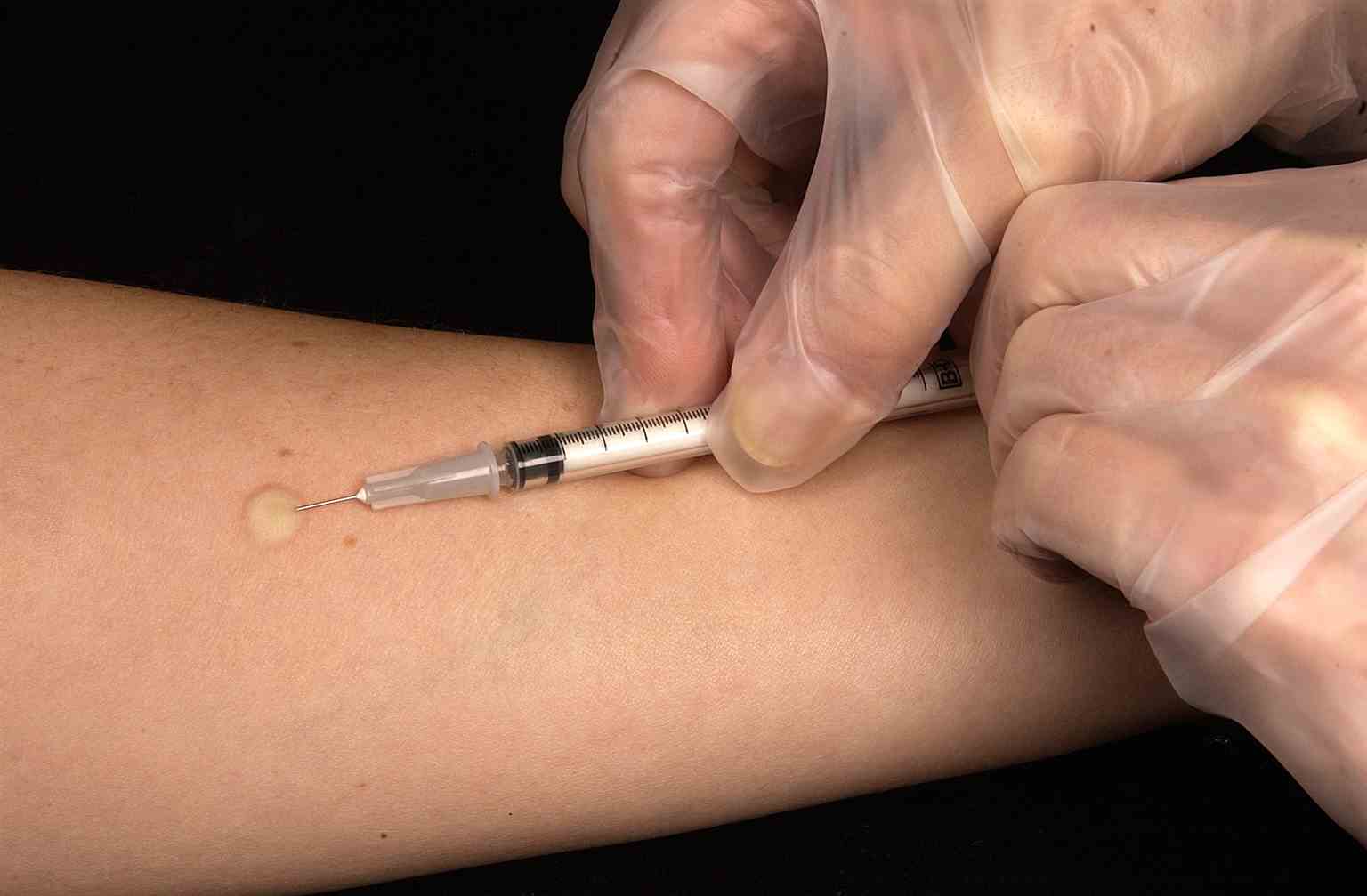
Mantoux Tuberculin Skin Test. This technician is in the process of correctly placing a Mantoux tuberculin skin test in this recipient’s forearm, which will cause a 6 mm to 10 mm wheal, or a raised area of skin surface, to form at the injection site. The Mantoux tuberculin skin test evaluates people for latent tuberculosis infection. In the United States, this skin test consists of an intradermal injection of exactly one-tenth of a milliliter of tuberculin containing 5 tuberculin units. Correct placement of this intradermal injection involves inserting the needle bevel slowly at a 5° to 15° angle. The needle bevel is advanced through the epidermis, the superficial layer of skin, approximately 3 mm so that the entire bevel is covered and lies just under the skin surface. A tense, pale wheal that is 6 mm to 10 mm in diameter appears over the needle bevel.
Greg Knobloch, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
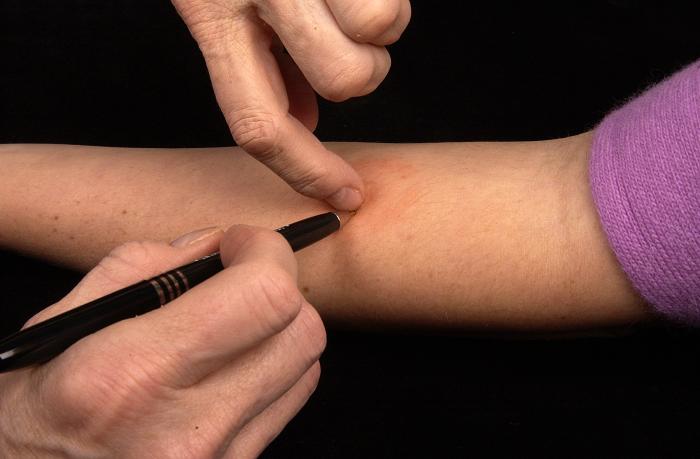
Mantoux Tuberculin Skin Test, Accurate Measurement. The image demonstrates a technician marking the widest edges of the patient's induration, a hard, dense, or raised formation, for an accurate measurement of Mantoux tuberculin skin test reaction.
Contributed by Gabrielle Benenson, Centers for Disease Control and Prevention
(Click Image to Enlarge)
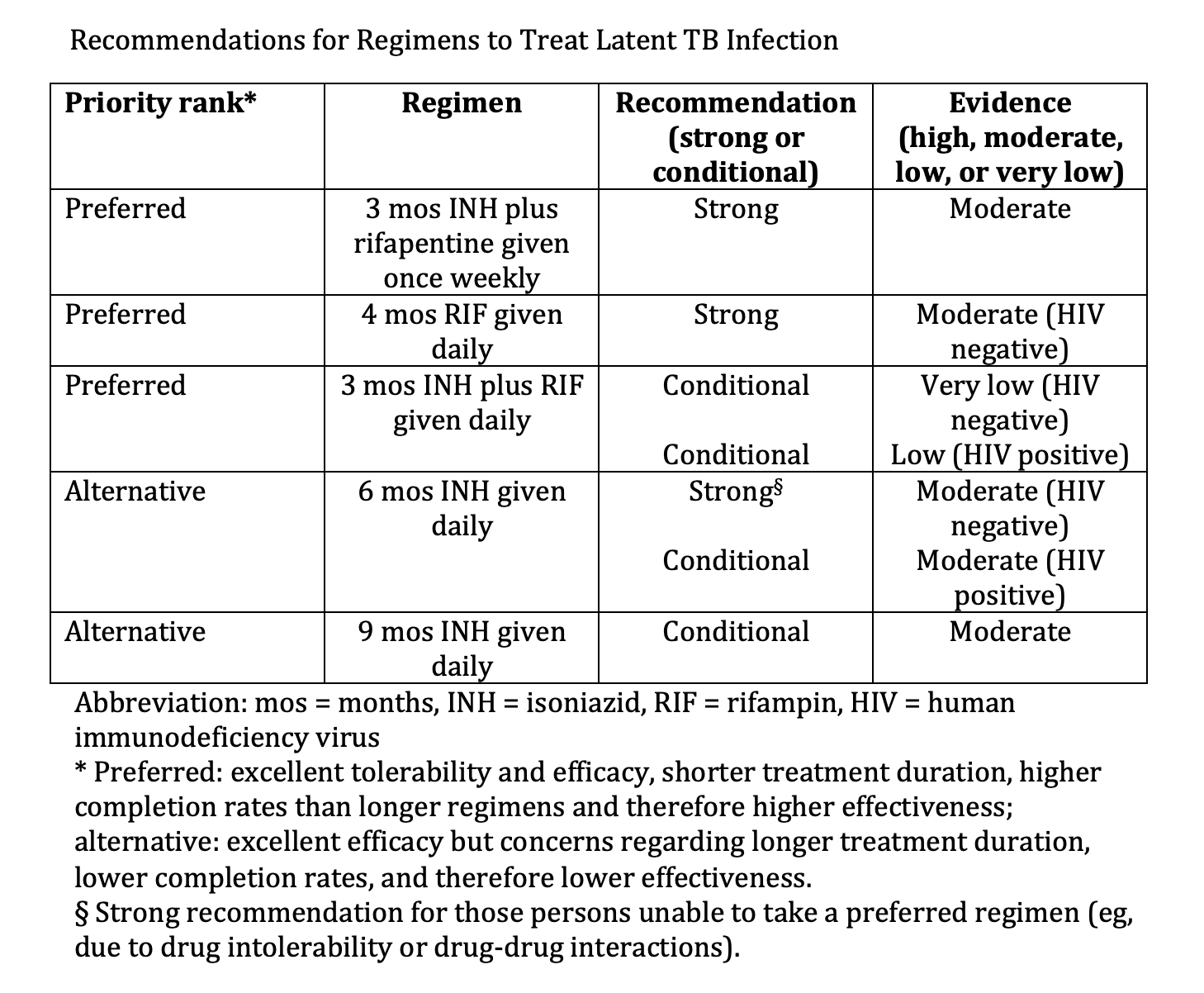
Recommendations for Regimens to Treat Latent TB Infection. Various regimens comprise treatment options for 3 to 9 months.
Adapted from: Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1-11. doi: 10.15585/mmwr.rr6901a1.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
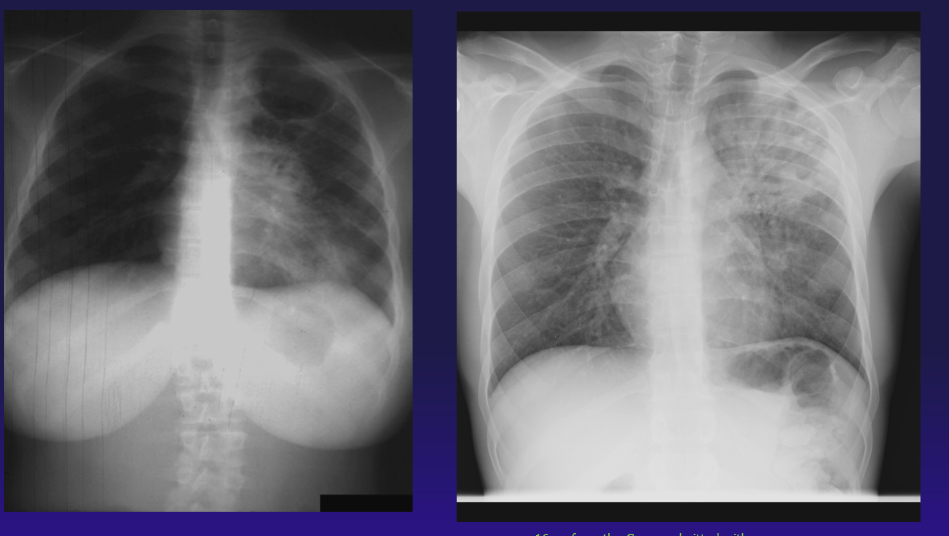
Adult-Type Cavitary Tuberculosis Disease. The chest radiograph on the left demonstrates adult-type cavitary M tuberculosis disease in a 16-year-old pregnant female from Liberia; the radiograph on the right is from a 16-year-old from the Congo admitted with hemoptysis and fever.
Contributed by A Ahmed, MD
(Click Image to Enlarge)
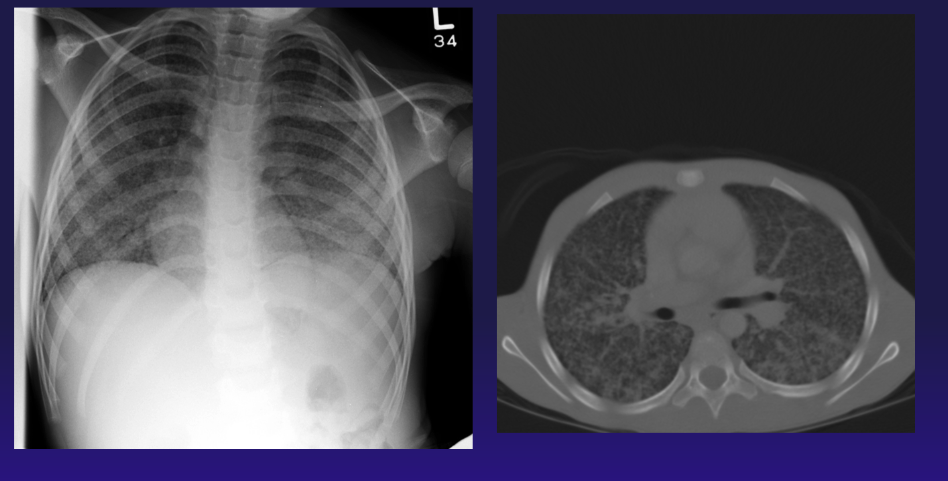
Chest Radiograph and Computed Tomography, Tuberculosis. The image is a chest radiograph and computed tomography scan of a 13-year-old adolescent who was diagnosed with juvenile idiopathic arthritis on methotrexate therapy. He developed respiratory distress, and sputum samples revealed MTB. His arthritis was later found to be caused by MTB.
Contributed by A Ahmed, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
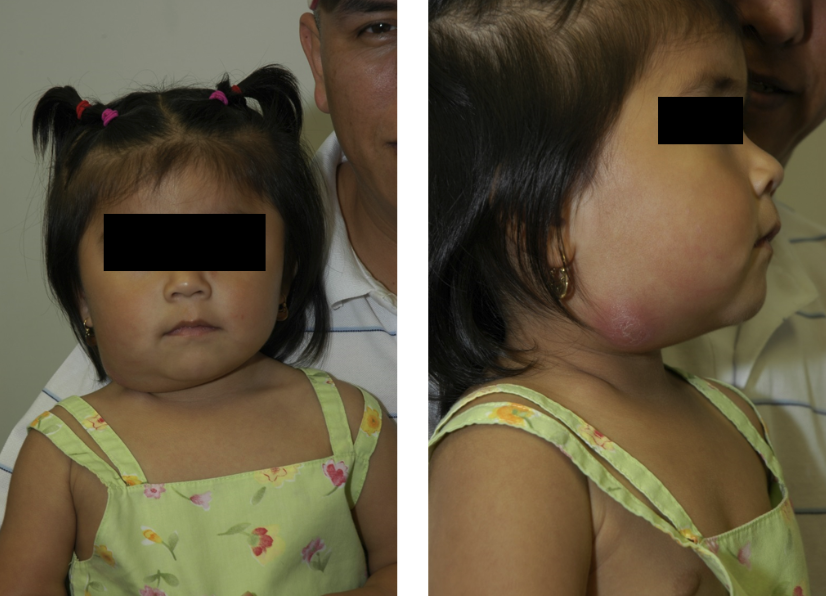
Lymph Node Disease. This is an image of a 4-year-old Hispanic child with persistent right neck adenitis who was unresponsive to an Amoxicillin course. Progression of the lymph node disease presenting with a reddish-purple hue is also seen in the atypical mycobacterial disease. Interferon-gamma release assay testing for tuberculosis was positive.
Contributed by A Ahmed, MD
(Click Image to Enlarge)
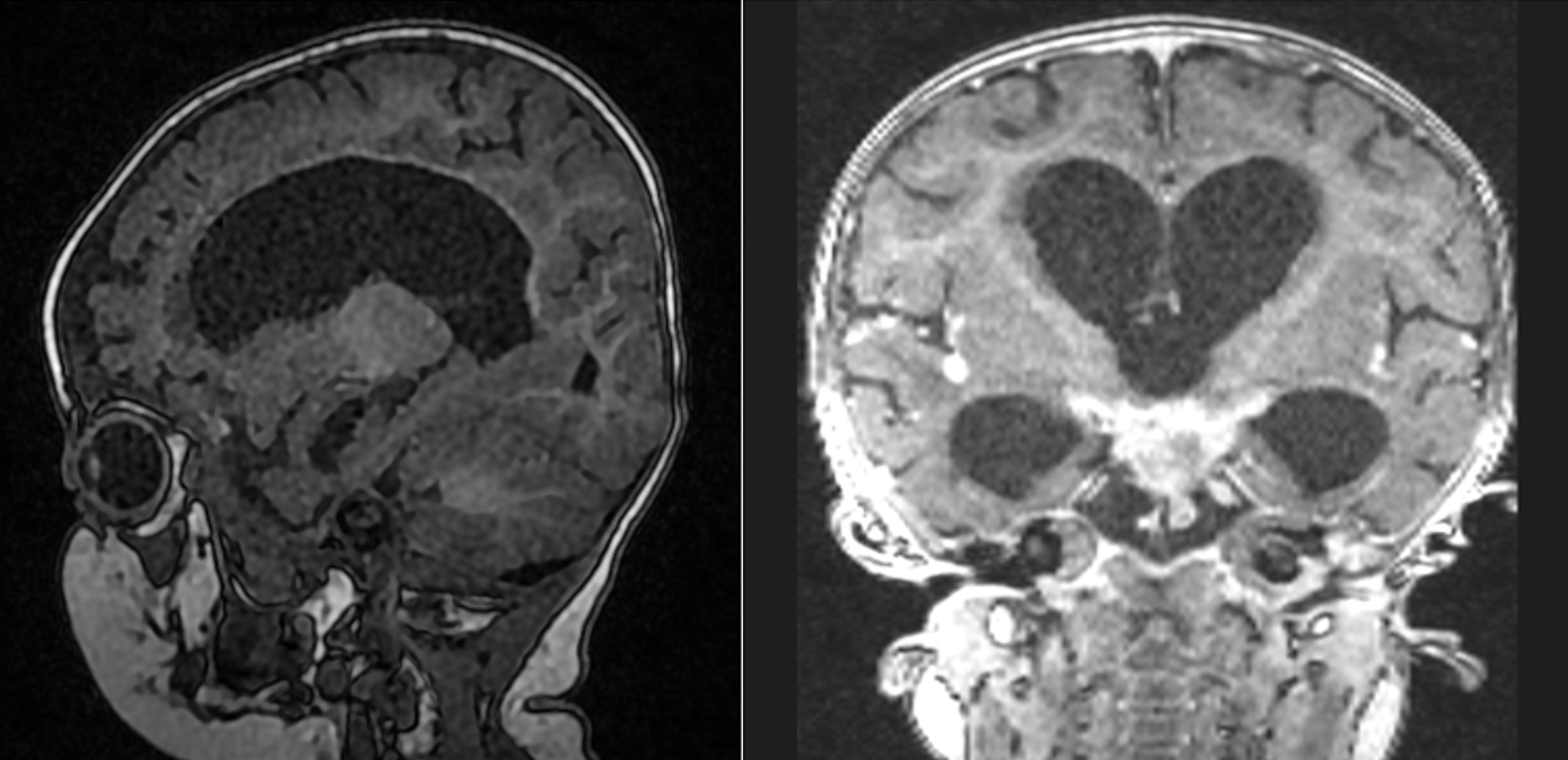
Tuberculosis Meningitis. Magnetic resonance imaging of an 8-month-old infant with recent travel to South Africa, presenting with unremitting fever, pneumonia, and progressive lethargy. MRI demonstrating hydrocephalus and basilar enhancement characteristic of M tuberculosis meningitis.
Contributed by A Ahmed, MD
(Click Image to Enlarge)
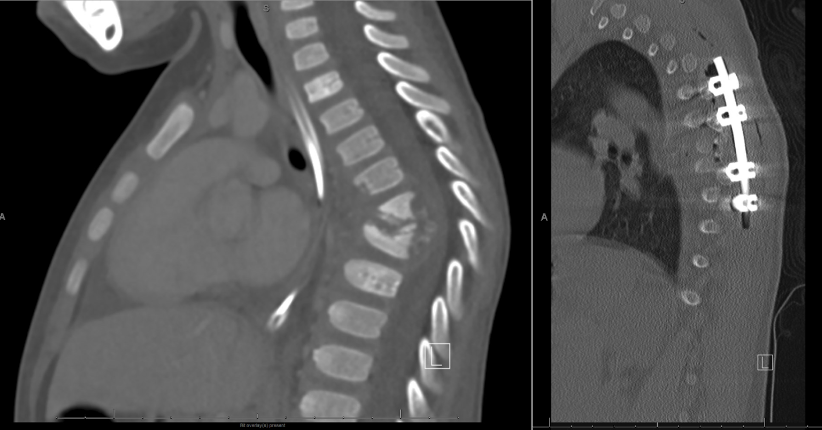
Vertebral Osteomyelitis Due to Tuberculosis. A radiograph of a 3-year-old child presenting with long-standing back pain and limping. The radiograph demonstrates progressive degeneration of the thoracic vertebra with wedging and collapse on the left frame. The right frame is a postoperative spinal fusion that was completed after the successful eradication of his M tuberculosis disease.
Contributed by A Ahmed, MD
References
Mane SS, Shrotriya P. Current Epidemiology of Pediatric Tuberculosis. Indian journal of pediatrics. 2024 Jul:91(7):711-716. doi: 10.1007/s12098-023-04910-4. Epub 2023 Nov 3 [PubMed PMID: 37919487]
Bespiatykh D, Bespyatykh J, Mokrousov I, Shitikov E. A Comprehensive Map of Mycobacterium tuberculosis Complex Regions of Difference. mSphere. 2021 Aug 25:6(4):e0053521. doi: 10.1128/mSphere.00535-21. Epub 2021 Jul 21 [PubMed PMID: 34287002]
Halse TA, Edwards J, Cunningham PL, Wolfgang WJ, Dumas NB, Escuyer VE, Musser KA. Combined real-time PCR and rpoB gene pyrosequencing for rapid identification of Mycobacterium tuberculosis and determination of rifampin resistance directly in clinical specimens. Journal of clinical microbiology. 2010 Apr:48(4):1182-8. doi: 10.1128/JCM.02149-09. Epub 2010 Jan 27 [PubMed PMID: 20107097]
Harichander S, Wiafe E, Mensah KB, Bangalee V, Oosthuizen F. The incidence of TB and MDR-TB in pediatrics and therapeutic options: a systematic review. Systematic reviews. 2022 Aug 4:11(1):157. doi: 10.1186/s13643-022-02023-1. Epub 2022 Aug 4 [PubMed PMID: 35927752]
Level 1 (high-level) evidenceHarichander S, Wiafe E, Mensah KB, Bangalee V, Oosthuizen F. Correction: The incidence of TB and MDR-TB in pediatrics and therapeutic options: a systematic review. Systematic reviews. 2022 Oct 22:11(1):228. doi: 10.1186/s13643-022-02101-4. Epub 2022 Oct 22 [PubMed PMID: 36273176]
Level 1 (high-level) evidenceDunn JJ, Starke JR, Revell PA. Laboratory Diagnosis of Mycobacterium tuberculosis Infection and Disease in Children. Journal of clinical microbiology. 2016 Jun:54(6):1434-1441. doi: 10.1128/JCM.03043-15. Epub 2016 Mar 16 [PubMed PMID: 26984977]
Kay AW, Rabie H, Maleche-Obimbo E, Sekadde MP, Cotton MF, Mandalakas AM. HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening, Prevention and Management Strategies. Pathogens (Basel, Switzerland). 2021 Dec 29:11(1):. doi: 10.3390/pathogens11010033. Epub 2021 Dec 29 [PubMed PMID: 35055981]
Enane LA, Christenson JC. Global emerging resistance in pediatric infections with TB, HIV, and gram-negative pathogens. Paediatrics and international child health. 2021 Feb:41(1):65-75. doi: 10.1080/20469047.2020.1853350. Epub 2020 Dec 11 [PubMed PMID: 33305992]
Albert H, Manabe Y, Lukyamuzi G, Ademun P, Mukkada S, Nyesiga B, Joloba M, Paramasivan CN, Perkins MD. Performance of three LED-based fluorescence microscopy systems for detection of tuberculosis in Uganda. PloS one. 2010 Dec 28:5(12):e15206. doi: 10.1371/journal.pone.0015206. Epub 2010 Dec 28 [PubMed PMID: 21203398]
Thomas TA. Tuberculosis in Children. Pediatric clinics of North America. 2017 Aug:64(4):893-909. doi: 10.1016/j.pcl.2017.03.010. Epub [PubMed PMID: 28734517]
Nolt D, Starke JR. Tuberculosis Infection in Children and Adolescents: Testing and Treatment. Pediatrics. 2021 Dec 1:148(6):. pii: e2021054663. doi: 10.1542/peds.2021-054663. Epub [PubMed PMID: 34851422]
Vanden Driessche K, Persson A, Marais BJ, Fink PJ, Urdahl KB. Immune vulnerability of infants to tuberculosis. Clinical & developmental immunology. 2013:2013():781320. doi: 10.1155/2013/781320. Epub 2013 May 13 [PubMed PMID: 23762096]
Semmes EC, Chen JL, Goswami R, Burt TD, Permar SR, Fouda GG. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Frontiers in immunology. 2020:11():595297. doi: 10.3389/fimmu.2020.595297. Epub 2021 Jan 21 [PubMed PMID: 33552052]
Level 3 (low-level) evidenceBerns SA, Isakova JA, Pekhtereva PI. Therapeutic potential of interferon-gamma in tuberculosis. ADMET & DMPK. 2022:10(1):63-73. doi: 10.5599/admet.1078. Epub 2022 Feb 14 [PubMed PMID: 35360672]
Stewart P, Patel S, Comer A, Muneer S, Nawaz U, Quann V, Bansal M, Venketaraman V. Role of B Cells in Mycobacterium Tuberculosis Infection. Vaccines. 2023 May 6:11(5):. doi: 10.3390/vaccines11050955. Epub 2023 May 6 [PubMed PMID: 37243059]
Saunders BM, Cooper AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunology and cell biology. 2000 Aug:78(4):334-41 [PubMed PMID: 10947857]
Nargan K, Naidoo T, Msimang M, Nadeem S, Wells G, Hunter RL, Hutton A, Lumamba K, Glasgow JN, Benson PV, Steyn AJ. Detection of Mycobacterium tuberculosis in human tissue via RNA in situ hybridization. bioRxiv : the preprint server for biology. 2023 Oct 5:():. pii: 2023.10.04.560963. doi: 10.1101/2023.10.04.560963. Epub 2023 Oct 5 [PubMed PMID: 37873458]
Del Castillo-Barrientos H, Centeno-Luque G, Untiveros-Tello A, Simms B, Lecca L, Nelson AK, Lastimoso C, Shin S. Clinical presentation of children with pulmonary tuberculosis: 25 years of experience in Lima, Peru. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014 Sep:18(9):1066-73. doi: 10.5588/ijtld.13.0458. Epub [PubMed PMID: 25189554]
Concepcion NDP, Laya BF, Andronikou S, Daltro PAN, Sanchez MO, Uy JAU, Lim TRU. Standardized radiographic interpretation of thoracic tuberculosis in children. Pediatric radiology. 2017 Sep:47(10):1237-1248. doi: 10.1007/s00247-017-3868-z. Epub 2017 Aug 29 [PubMed PMID: 29052771]
Obihara NJ, Walters E, Lawrenson J, Garcia-Prats AJ, Hesseling AC, Schaaf HS. Tuberculous Pericardial Effusions in Children. Journal of the Pediatric Infectious Diseases Society. 2018 Dec 3:7(4):346-349. doi: 10.1093/jpids/pix087. Epub [PubMed PMID: 29096017]
Lotfian F, Bolursaz MR, Khalilzadeh S, Baghaie N, Hassanzad M, Velayati A. Features of Adolescents Tuberculosis at a Referral TB's Hospital in Tehran, Iran. Mediterranean journal of hematology and infectious diseases. 2016:8(1):e2016005. doi: 10.4084/MJHID.2016.005. Epub 2016 Jan 1 [PubMed PMID: 26740866]
Snow KJ, Cruz AT, Seddon JA, Ferrand RA, Chiang SS, Hughes JA, Kampmann B, Graham SM, Dodd PJ, Houben RM, Denholm JT, Sawyer SM, Kranzer K. Adolescent tuberculosis. The Lancet. Child & adolescent health. 2020 Jan:4(1):68-79. doi: 10.1016/S2352-4642(19)30337-2. Epub 2019 Nov 18 [PubMed PMID: 31753806]
Fischer GB, Andrade CF, Lima JB. Pleural tuberculosis in children. Paediatric respiratory reviews. 2011 Mar:12(1):27-30. doi: 10.1016/j.prrv.2010.11.001. Epub 2010 Dec 8 [PubMed PMID: 21172672]
Gong HZ, Han C, Yang FL, Wang CF, Wang JL, Wang MS. Treatment delay in childhood pleural tuberculosis and associated factors. BMC infectious diseases. 2020 Oct 27:20(1):793. doi: 10.1186/s12879-020-05496-4. Epub 2020 Oct 27 [PubMed PMID: 33109109]
Bayhan GI, Sayir F, Tanir G, Tuncer O. Pediatric pleural tuberculosis. International journal of mycobacteriology. 2018 Jul-Sep:7(3):261-264. doi: 10.4103/ijmy.ijmy_91_18. Epub [PubMed PMID: 30198507]
Rosso F, Michelon CT, Sperhacke RD, Verza M, Olival L, Conde MB, Guerra RL, Zaha A, Rossetti ML. Evaluation of real-time PCR of patient pleural effusion for diagnosis of tuberculosis. BMC research notes. 2011 Aug 5:4():279. doi: 10.1186/1756-0500-4-279. Epub 2011 Aug 5 [PubMed PMID: 21819571]
Imazio M, Gaita F, LeWinter M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA. 2015 Oct 13:314(14):1498-506. doi: 10.1001/jama.2015.12763. Epub [PubMed PMID: 26461998]
Level 1 (high-level) evidenceSharma SK, Mohan A. Miliary Tuberculosis. Microbiology spectrum. 2017 Mar:5(2):. doi: 10.1128/microbiolspec.TNMI7-0013-2016. Epub [PubMed PMID: 28281441]
Kolsi R, Ammar M, Sfaihi L, Charfi F, Maaloul I, Kammoun T. Miliary tuberculosis mimicking COVID-19 multisystemic inflammatory syndrome in children. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 2023 Oct:30(7):521-523. doi: 10.1016/j.arcped.2023.08.003. Epub 2023 Sep 11 [PubMed PMID: 37704522]
Bonnet M, Nordholm AC, Ssekyanzi B, Byamukama O, Orikiriza P, Tusabe T, Nyehangane D, Taremwa IM, Turyashemererwa E, Wobudeya E, Mwanga-Amumpaire J, Marais BJ, Nampijja D. Mortality and Cause of Death in Children With Presumptive Disseminated Tuberculosis. Pediatrics. 2023 Apr 1:151(4):. pii: e2022057912. doi: 10.1542/peds.2022-057912. Epub [PubMed PMID: 36950924]
Oloya J, Opuda-Asibo J, Kazwala R, Demelash AB, Skjerve E, Lund A, Johansen TB, Djonne B. Mycobacteria causing human cervical lymphadenitis in pastoral communities in the Karamoja region of Uganda. Epidemiology and infection. 2008 May:136(5):636-43 [PubMed PMID: 17599779]
Borham M, Oreiby A, El-Gedawy A, Hegazy Y, Khalifa HO, Al-Gaabary M, Matsumoto T. Review on Bovine Tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species. Pathogens (Basel, Switzerland). 2022 Jun 21:11(7):. doi: 10.3390/pathogens11070715. Epub 2022 Jun 21 [PubMed PMID: 35889961]
Martínez-Planas A, Baquero-Artigao F, Santiago B, Fortuny C, Méndez-Echevarría A, Del Rosal T, Bustillo-Alonso M, Gale I, Guerrero C, Blázquez-Gamero D, Canet A, Lillo M, Calavia O, Núñez Cuadros E, Falcón-Neyra L, Soriano-Arandes A, Van Ingen J, Tebruegge M, Noguera-Julian A, Spanish Pediatric TB Research Network (pTBred) and the European NontuberculouS MycoBacterial Lymphadenitis in childrEn (ENSeMBLE) Study. Interferon-Gamma Release Assays Differentiate between Mycobacterium avium Complex and Tuberculous Lymphadenitis in Children. The Journal of pediatrics. 2021 Sep:236():211-218.e2. doi: 10.1016/j.jpeds.2021.05.008. Epub 2021 May 10 [PubMed PMID: 33984332]
Xu JJ, Peer S, Papsin BC, Kitai I, Propst EJ. Tuberculous lymphadenitis of the head and neck in Canadian children: Experience from a low-burden region. International journal of pediatric otorhinolaryngology. 2016 Dec:91():11-14. doi: 10.1016/j.ijporl.2016.09.035. Epub 2016 Sep 28 [PubMed PMID: 27863623]
Schaaf HS, Seddon JA. Management of tuberculous meningitis in children. Paediatrics and international child health. 2021 Nov:41(4):231-236. doi: 10.1080/20469047.2021.1952818. Epub 2021 Nov 16 [PubMed PMID: 34783305]
Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, Becerra MC. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2014 Oct:14(10):947-57. doi: 10.1016/S1473-3099(14)70852-7. Epub 2014 Aug 6 [PubMed PMID: 25108337]
Level 1 (high-level) evidenceKhambati N, Hou M, Kelly D, Song R. Fatal tuberculous meningitis in an infant presenting with seizures in the UK. BMJ case reports. 2021 Aug 19:14(8):. doi: 10.1136/bcr-2021-243573. Epub 2021 Aug 19 [PubMed PMID: 34413037]
Level 3 (low-level) evidenceGuillem L, Espinosa J, Laporte-Amargos J, Sánchez A, Grijota MD, Santin M. Mortality and sequelae of tuberculous meningitis in a high-resource setting: A cohort study, 1990-2017. Enfermedades infecciosas y microbiologia clinica (English ed.). 2024 Mar:42(3):124-129. doi: 10.1016/j.eimce.2023.01.005. Epub 2023 Feb 1 [PubMed PMID: 36737367]
Daniel BD, Grace GA, Natrajan M. Tuberculous meningitis in children: Clinical management & outcome. The Indian journal of medical research. 2019 Aug:150(2):117-130. doi: 10.4103/ijmr.IJMR_786_17. Epub [PubMed PMID: 31670267]
Kasinathan A, Serane VK, Palanisamy S. Tuberculous meningitis manifesting with neuroregression in a eleven month child. The Indian journal of tuberculosis. 2020 Jan:67(1):136-138. doi: 10.1016/j.ijtb.2020.01.001. Epub 2020 Jan 8 [PubMed PMID: 32192608]
Hill J, Marais B. Improved treatment for children with tuberculous meningitis: acting on what we know. Archives of disease in childhood. 2022 Jan:107(1):68-69. doi: 10.1136/archdischild-2021-322660. Epub [PubMed PMID: 34911685]
Kumar K, Mathew JL. World Health Organization Guideline on the Management of Tuberculosis in Children: Critical Appraisal, Concerns, and Caution. Indian journal of pediatrics. 2023 Aug:90(8):811-816. doi: 10.1007/s12098-023-04584-y. Epub 2023 May 17 [PubMed PMID: 37193925]
Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE, Tuberculous Meningitis International Research Consortium. Tuberculous meningitis. Nature reviews. Neurology. 2017 Oct:13(10):581-598. doi: 10.1038/nrneurol.2017.120. Epub 2017 Sep 8 [PubMed PMID: 28884751]
Méchaï F, Bouchaud O. Tuberculous meningitis: Challenges in diagnosis and management. Revue neurologique. 2019 Sep-Oct:175(7-8):451-457. doi: 10.1016/j.neurol.2019.07.007. Epub 2019 Aug 2 [PubMed PMID: 31383464]
Mezochow A, Thakur K, Vinnard C. Tuberculous Meningitis in Children and Adults: New Insights for an Ancient Foe. Current neurology and neuroscience reports. 2017 Sep 20:17(11):85. doi: 10.1007/s11910-017-0796-0. Epub 2017 Sep 20 [PubMed PMID: 28932979]
Paliwal VK, Garg RK. Hydrocephalus in Tuberculous Meningitis - Pearls and Nuances. Neurology India. 2021 Nov-Dec:69(Supplement):S330-S335. doi: 10.4103/0028-3886.332275. Epub [PubMed PMID: 35102984]
García-Grimshaw M, Gutiérrez-Manjarrez FA, Navarro-Álvarez S, González-Duarte A. Clinical, Imaging, and Laboratory Characteristics of Adult Mexican Patients with Tuberculous Meningitis: A Retrospective Cohort Study. Journal of epidemiology and global health. 2020 Mar:10(1):59-64. doi: 10.2991/jegh.k.191023.001. Epub [PubMed PMID: 32175711]
Level 2 (mid-level) evidenceAgarwal A. Paediatric osteoarticular tuberculosis: A review. Journal of clinical orthopaedics and trauma. 2020 Mar-Apr:11(2):202-207. doi: 10.1016/j.jcot.2020.01.005. Epub 2020 Jan 22 [PubMed PMID: 32099280]
Verma H, Rajvanshi N, Rathaur VK, Pathania M, Bhat NK. Poncet's Disease: A Case Report. Journal of tropical pediatrics. 2021 Jan 29:67(1):. pii: fmaa116. doi: 10.1093/tropej/fmaa116. Epub [PubMed PMID: 33306806]
Level 3 (low-level) evidenceRao GN, Gali JH, Rao SN. Tuberculous Dactylitis: An Uncommon Presentation of a Common Infection. Case reports in pediatrics. 2016:2016():4013471. doi: 10.1155/2016/4013471. Epub 2016 Jan 17 [PubMed PMID: 26885427]
Level 3 (low-level) evidenceYadav S. Tuberculosis of the Elbow Joint in an Indian Boy: A Rare Entity With a Diagnostic Challenge. Cureus. 2024 Apr:16(4):e58184. doi: 10.7759/cureus.58184. Epub 2024 Apr 13 [PubMed PMID: 38741885]
Agarwal A, Agarwal S, Singh S, Nandwani S. Spina Ventosa: An often Missed Diagnosis. Journal of global infectious diseases. 2021 Jan-Mar:13(1):36-37. doi: 10.4103/jgid.jgid_198_20. Epub 2021 Jan 29 [PubMed PMID: 33911451]
Benzagmout M, Boujraf S, Chakour K, Chaoui Mel F. Pott's disease in children. Surgical neurology international. 2011 Jan 11:2():1. doi: 10.4103/2152-7806.75459. Epub 2011 Jan 11 [PubMed PMID: 21297923]
Nussbaum ES, Rockswold GL, Bergman TA, Erickson DL, Seljeskog EL. Spinal tuberculosis: a diagnostic and management challenge. Journal of neurosurgery. 1995 Aug:83(2):243-7 [PubMed PMID: 7616269]
Sartoris G, Seddon JA, Rabie H, Nel ED, Schaaf HS. Abdominal Tuberculosis in Children: Challenges, Uncertainty, and Confusion. Journal of the Pediatric Infectious Diseases Society. 2020 Apr 30:9(2):218-227. doi: 10.1093/jpids/piz093. Epub [PubMed PMID: 31909804]
Sartoris G, Seddon JA, Rabie H, Nel ED, Losurdo G, Schaaf HS. Abdominal Involvement in Children With Bacteriologically Confirmed Tuberculosis: A Five-year Experience From Cape Town, South Africa. The Pediatric infectious disease journal. 2020 Oct:39(10):914-919. doi: 10.1097/INF.0000000000002749. Epub [PubMed PMID: 32496408]
Lancella L, Abate L, Cursi L, Chiopris G, Nicoletti L, Principi N, Villani A, Esposito S. Abdominal Tuberculosis in Children: A Case Series of Five Patients. Microorganisms. 2023 Mar 12:11(3):. doi: 10.3390/microorganisms11030730. Epub 2023 Mar 12 [PubMed PMID: 36985303]
Level 2 (mid-level) evidenceKesharwani H, Mohammad S, Pathak P. Tuberculosis in the Female Genital Tract. Cureus. 2022 Sep:14(9):e28708. doi: 10.7759/cureus.28708. Epub 2022 Sep 2 [PubMed PMID: 36204039]
Muneer A, Macrae B, Krishnamoorthy S, Zumla A. Urogenital tuberculosis - epidemiology, pathogenesis and clinical features. Nature reviews. Urology. 2019 Oct:16(10):573-598. doi: 10.1038/s41585-019-0228-9. Epub 2019 Sep 23 [PubMed PMID: 31548730]
Dhua AK, Borkar N, Ghosh V, Aggarwal SK. Renal tuberculosis in infancy. Journal of Indian Association of Pediatric Surgeons. 2011 Apr:16(2):69-71. doi: 10.4103/0971-9261.78136. Epub [PubMed PMID: 21731237]
Zhang X, Xie Y, Huang G, Fu H. Analysis of 17 children with renal abscess. International journal of clinical and experimental pathology. 2019:12(9):3179-3184 [PubMed PMID: 31934162]
Santra A, Mandi F, Bandyopadhyay A. Renal Tuberculosis Presenting as a Mass Lesion in a Two-year-old Girl: Report of a rare case. Sultan Qaboos University medical journal. 2016 Feb:16(1):e105-8. doi: 10.18295/squmj.2016.16.01.020. Epub 2016 Feb 2 [PubMed PMID: 26909199]
Level 3 (low-level) evidenceChitnis AS, Schecter GF, Cilnis M, Robsky K, Flood JM, Barry PM. Epidemiology of tuberculosis cases with end-stage renal disease, California, 2010. American journal of nephrology. 2014:39(4):314-21. doi: 10.1159/000360183. Epub 2014 Apr 15 [PubMed PMID: 24751696]
Level 3 (low-level) evidenceTjahyadi D, Tjandraprawira KD. Poor in vitro fertilisation outcomes in genital tuberculosis - Case report. Annals of medicine and surgery (2012). 2022 Sep:81():104438. doi: 10.1016/j.amsu.2022.104438. Epub 2022 Aug 19 [PubMed PMID: 36147182]
Level 3 (low-level) evidenceCesilia C, Nugraha HG, Siregar S, Nataprawira HM. The challenges in diagnosing isolated epididymal tuberculosis (TB) in an adolescent male: a case report. BMC urology. 2024 Mar 19:24(1):61. doi: 10.1186/s12894-024-01442-7. Epub 2024 Mar 19 [PubMed PMID: 38504239]
Level 3 (low-level) evidence. Notes from the Field: Undiagnosed Tuberculosis During Pregnancy Resulting in a Neonatal Death-United States, 2021. The Pediatric infectious disease journal. 2024 Mar 13:():. doi: 10.1097/INF.0000000000004324. Epub 2024 Mar 13 [PubMed PMID: 38502883]
Hageman J, Shulman S, Schreiber M, Luck S, Yogev R. Congenital tuberculosis: critical reappraisal of clinical findings and diagnostic procedures. Pediatrics. 1980 Dec:66(6):980-4 [PubMed PMID: 7454491]
Sobhy S, Babiker Z, Zamora J, Khan KS, Kunst H. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology. 2017 Apr:124(5):727-733. doi: 10.1111/1471-0528.14408. Epub 2016 Nov 11 [PubMed PMID: 27862893]
Level 1 (high-level) evidenceShao Y, Hageman JR, Shulman ST. Congenital and Perinatal Tuberculosis. NeoReviews. 2021 Sep:22(9):e600-e605. doi: 10.1542/neo.22-9-e600. Epub [PubMed PMID: 34470761]
Sebastian SK, Vijayan V, Kumar VB, Garg P. Tuberculous otitis media in children: Series of 4 cases. International journal of pediatric otorhinolaryngology. 2020 Aug:135():110118. doi: 10.1016/j.ijporl.2020.110118. Epub 2020 May 13 [PubMed PMID: 32446040]
Level 3 (low-level) evidenceMcMaster D, Din WB, Ramalingaiah B, Agrawal S. Tuberculous mastoiditis in a 2-month-old infant presenting with facial nerve palsy. BMJ case reports. 2020 Dec 9:13(12):. doi: 10.1136/bcr-2020-237606. Epub 2020 Dec 9 [PubMed PMID: 33298491]
Level 3 (low-level) evidenceSantos JB, Figueiredo AR, Ferraz CE, Oliveira MH, Silva PG, Medeiros VL. Cutaneous tuberculosis: epidemiologic, etiopathogenic and clinical aspects - part I. Anais brasileiros de dermatologia. 2014 Mar-Apr:89(2):219-28 [PubMed PMID: 24770496]
Vieira PM, Zilhão C, Miranda V. Ocular Tuberculosis in Pediatrics: A Case Report. Acta medica portuguesa. 2023 Oct 2:36(10):683-686. doi: 10.20344/amp.19245. Epub 2023 Apr 19 [PubMed PMID: 37080196]
Level 3 (low-level) evidenceAlam S, Thallam MV. Primary eyelid tuberculosis mimicking as chalazion in an immunocompetent child. Orbit (Amsterdam, Netherlands). 2020 Dec:39(6):454. doi: 10.1080/01676830.2019.1704034. Epub 2019 Dec 17 [PubMed PMID: 31847663]
Huang Z, LaCourse SM, Kay AW, Stern J, Escudero JN, Youngquist BM, Zheng W, Vambe D, Dlamini M, Mtetwa G, Cranmer LM, Njuguna I, Wamalwa DC, Maleche-Obimbo E, Catanzaro DG, Lyon CJ, John-Stewart G, DiNardo A, Mandalakas AM, Ning B, Hu TY. CRISPR detection of circulating cell-free Mycobacterium tuberculosis DNA in adults and children, including children with HIV: a molecular diagnostics study. The Lancet. Microbe. 2022 Jul:3(7):e482-e492. doi: 10.1016/S2666-5247(22)00087-8. Epub 2022 May 31 [PubMed PMID: 35659882]
Imperiale BR, Nieves C, Mancino B, Sanjurjo M, Tártara S, Di Giulio ÁB, Palomino JC, Morcillo NS, Martin A. String test: A new tool for tuberculosis diagnosis and drug-resistance detection in children. International journal of mycobacteriology. 2018 Apr-Jun:7(2):162-166. doi: 10.4103/ijmy.ijmy_54_18. Epub [PubMed PMID: 29900894]
Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF Assay: a pilot study. The Pediatric infectious disease journal. 2012 Dec:31(12):1316. doi: 10.1097/INF.0b013e318266c21c. Epub [PubMed PMID: 23188101]
Level 3 (low-level) evidenceNicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, Zemanay W, Zar HJ. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Aug:57(3):e18-21. doi: 10.1093/cid/cit230. Epub 2013 Apr 11 [PubMed PMID: 23580738]
Goussard P, Eber E, Venkatakrishna S, Frigati L, Greybe L, Janson J, Schubert P, Andronikou S. Interventional bronchoscopy in pediatric pulmonary tuberculosis. Expert review of respiratory medicine. 2023 Dec:17(12):1159-1175. doi: 10.1080/17476348.2023.2299336. Epub 2023 Dec 28 [PubMed PMID: 38140708]
Jafari C, Olaru ID, Daduna F, Lange C, Kalsdorf B. Rapid Diagnosis of Recurrent Paucibacillary Tuberculosis. Pathogens & immunity. 2022:7(2):189-202. doi: 10.20411/pai.v7i2.565. Epub 2023 Apr 19 [PubMed PMID: 37207169]
Bholla M, Kapalata N, Masika E, Chande H, Jugheli L, Sasamalo M, Glass TR, Beck HP, Reither K. Evaluation of Xpert® MTB/RIF and Ustar EasyNAT™ TB IAD for diagnosis of tuberculous lymphadenitis of children in Tanzania: a prospective descriptive study. BMC infectious diseases. 2016 Jun 6:16():246. doi: 10.1186/s12879-016-1578-z. Epub 2016 Jun 6 [PubMed PMID: 27268404]
Polepole P, Kabwe M, Kasonde M, Tembo J, Shibemba A, O'Grady J, Kapata N, Zumla A, Bates M. Performance of the Xpert MTB/RIF assay in the diagnosis of tuberculosis in formalin-fixed, paraffin-embedded tissues. International journal of mycobacteriology. 2017 Jan-Mar:6(1):87-93. doi: 10.4103/2212-5531.201892. Epub [PubMed PMID: 28317811]
Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, Alland D, Meya DB, Rhein J, Boulware DR. Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015 Oct:19(10):1209-15. doi: 10.5588/ijtld.15.0253. Epub [PubMed PMID: 26459535]
. WHO consolidated guidelines on tuberculosis: Module 5: Management of tuberculosis in children and adolescents. 2022:(): [PubMed PMID: 35404556]
Carr W, Kurbatova E, Starks A, Goswami N, Allen L, Winston C. Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the Treatment of Drug-Susceptible Pulmonary Tuberculosis - United States, 2022. MMWR. Morbidity and mortality weekly report. 2022 Feb 25:71(8):285-289. doi: 10.15585/mmwr.mm7108a1. Epub 2022 Feb 25 [PubMed PMID: 35202353]
Turkova A, Wills GH, Wobudeya E, Chabala C, Palmer M, Kinikar A, Hissar S, Choo L, Musoke P, Mulenga V, Mave V, Joseph B, LeBeau K, Thomason MJ, Mboizi RB, Kapasa M, van der Zalm MM, Raichur P, Bhavani PK, McIlleron H, Demers AM, Aarnoutse R, Love-Koh J, Seddon JA, Welch SB, Graham SM, Hesseling AC, Gibb DM, Crook AM, SHINE Trial Team. Shorter Treatment for Nonsevere Tuberculosis in African and Indian Children. The New England journal of medicine. 2022 Mar 10:386(10):911-922. doi: 10.1056/NEJMoa2104535. Epub [PubMed PMID: 35263517]
Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, Cattamanchi A, Cegielski JP, Chen L, Daley CL, Dalton TL, Duarte R, Fregonese F, Horsburgh CR Jr, Ahmad Khan F, Kheir F, Lan Z, Lardizabal A, Lauzardo M, Mangan JM, Marks SM, McKenna L, Menzies D, Mitnick CD, Nilsen DM, Parvez F, Peloquin CA, Raftery A, Schaaf HS, Shah NS, Starke JR, Wilson JW, Wortham JM, Chorba T, Seaworth B. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2019 Nov 15:200(10):e93-e142. doi: 10.1164/rccm.201909-1874ST. Epub [PubMed PMID: 31729908]
Level 1 (high-level) evidenceRamos JP, Vieira M, Pimentel C, Argel M, Barbosa P, Duarte R. Building bridges: multidisciplinary teams in tuberculosis prevention and care. Breathe (Sheffield, England). 2023 Sep:19(3):230092. doi: 10.1183/20734735.0092-2023. Epub 2023 Sep 12 [PubMed PMID: 37719241]
Jenkins HE, Yuen CM, Rodriguez CA, Nathavitharana RR, McLaughlin MM, Donald P, Marais BJ, Becerra MC. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2017 Mar:17(3):285-295. doi: 10.1016/S1473-3099(16)30474-1. Epub 2016 Dec 8 [PubMed PMID: 27964822]
Level 1 (high-level) evidenceChiang SS, Jenkins HE. Shedding Light on Tuberculosis Deaths in Children and Adolescents. Pediatrics. 2021 Apr:147(4):. doi: 10.1542/peds.2020-039404. Epub 2021 Mar 10 [PubMed PMID: 33692160]
Nataprawira HM, Pratama AA, Widiasta A, Sibarani J, Hilmanto D, Sekarwana N, Rachmadi D. Complicated Urinary Tract Tuberculosis in a 13-Year-Old Adolescent with Chronic Kidney Disease and Antituberculous Drug-Induced Hepatotoxicity. Case reports in infectious diseases. 2019:2019():7370150. doi: 10.1155/2019/7370150. Epub 2019 Oct 23 [PubMed PMID: 31781434]
Level 3 (low-level) evidenceVonasek BJ, Rabie H, Hesseling AC, Garcia-Prats AJ. Tuberculosis in Children Living With HIV: Ongoing Progress and Challenges. Journal of the Pediatric Infectious Diseases Society. 2022 Oct 31:11(Supplement_3):S72-S78. doi: 10.1093/jpids/piac060. Epub [PubMed PMID: 36314545]
Githinji L, Zar HJ. Respiratory Complications in Children and Adolescents with Human Immunodeficiency Virus. Pediatric clinics of North America. 2021 Feb:68(1):131-145. doi: 10.1016/j.pcl.2020.09.016. Epub [PubMed PMID: 33228928]
Muzumdar D, Vedantam R, Chandrashekhar D. Tuberculosis of the central nervous system in children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2018 Oct:34(10):1925-1935. doi: 10.1007/s00381-018-3884-9. Epub 2018 Jul 5 [PubMed PMID: 29978252]
Murthy PR, A K AK, N N, K V VG. Adolescent tuberculosis in the ICU. The Indian journal of tuberculosis. 2023:70 Suppl 1():S24-S28. doi: 10.1016/j.ijtb.2023.06.020. Epub 2023 Jun 28 [PubMed PMID: 38110256]
Duvenhage J, Draper HR, Garcia-Prats AJ, Winckler J, Hesseling AC, Schaaf HS. Hepatocellular Injury in Children Treated for Rifampicin-resistant Tuberculosis: Incidence, Etiology and Outcome. The Pediatric infectious disease journal. 2022 Dec 1:41(12):953-958. doi: 10.1097/INF.0000000000003690. Epub 2022 Sep 6 [PubMed PMID: 36102699]
Eisen S, Honywood L, Shingadia D, Novelli V. Spinal tuberculosis in children. Archives of disease in childhood. 2012 Aug:97(8):724-9. doi: 10.1136/archdischild-2011-301571. Epub 2012 Jun 25 [PubMed PMID: 22734017]
Soni N, Kumar S, Shimle A, Ora M, Bathla G, Mishra P. Cerebrovascular complications in tuberculous meningitis-A magnetic resonance imaging study in 90 patients from a tertiary care hospital. The neuroradiology journal. 2020 Feb:33(1):3-16. doi: 10.1177/1971400919881188. Epub 2019 Oct 7 [PubMed PMID: 31589101]
Prakash J, Mehtani A. Hand and wrist tuberculosis in paediatric patients - our experience in 44 patients. Journal of pediatric orthopedics. Part B. 2017 May:26(3):250-260. doi: 10.1097/BPB.0000000000000325. Epub [PubMed PMID: 27111553]
Cranmer LM, Cotton MF, Day CL, Nemes E. What's Old and New in Tuberculosis Vaccines for Children. Journal of the Pediatric Infectious Diseases Society. 2022 Oct 31:11(Supplement_3):S110-S116. doi: 10.1093/jpids/piac078. Epub [PubMed PMID: 36314550]
Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet (London, England). 2013 Mar 23:381(9871):1021-8 [PubMed PMID: 23391465]
Level 1 (high-level) evidenceAbel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. American journal of respiratory and critical care medicine. 2010 Jun 15:181(12):1407-17. doi: 10.1164/rccm.200910-1484OC. Epub 2010 Feb 18 [PubMed PMID: 20167847]
Ota MO, Odutola AA, Owiafe PK, Donkor S, Owolabi OA, Brittain NJ, Williams N, Rowland-Jones S, Hill AV, Adegbola RA, McShane H. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Science translational medicine. 2011 Jun 22:3(88):88ra56. doi: 10.1126/scitranslmed.3002461. Epub [PubMed PMID: 21697532]
Level 1 (high-level) evidenceHarris RC, Sumner T, Knight GM, White RG. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Human vaccines & immunotherapeutics. 2016 Nov:12(11):2813-2832 [PubMed PMID: 27448625]
Level 1 (high-level) evidence