 Mohs Micrographic Surgery Management of Melanoma and Melanoma In Situ
Mohs Micrographic Surgery Management of Melanoma and Melanoma In Situ
Introduction
Mohs micrographic surgery (MMS) is a precise, tissue-sparing procedure used to remove skin malignancies such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).[1] Although MMS is predominantly used for BCC and SCC, it has shown promise as a treatment for melanoma in situ (MIS) and invasive melanoma, with the potential for superior outcomes in disease management and aesthetic preservation.[1][2]
This surgical method, named after its pioneer, Dr Frederic Mohs, enables precise microscopic control of the entire tumor edge while maximizing the preservation of normal tissue, which makes it especially useful for skin malignancies with a high risk of incomplete excision, recurrence, or risk of significant local tissue loss.[1][2] Initially dubbed "chemosurgery," the treatment originally required the application of a chemical fixative (eg, zinc chloride) to the in vivo tumor, which was subsequently removed and examined microscopically.[1] The technique has evolved to involve processing fresh tissue, then freezing and sectioning it in a cryostat microtome, permitting faster processing, reduced patient discomfort, and increased tissue conservation.[1]
MMS enables microscopic control of the deep and peripheral tumor margins while conserving normal tissue, which makes it particularly useful for skin cancers with a high risk of incomplete excision or recurrence and when tissue conservation is crucial.[2] MMS has shown promise in the treatment of melanoma, as indicated by numerous studies.[3] The complete microscopic margin evaluation associated with MMS makes it an appropriate option for managing MIS and invasive melanoma, given their propensity for subclinical spread; MMS is also linked with superior outcomes in tumor extirpation and cosmesis.[1][2]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The tissue-sparing capabilities of MMS make this technique especially useful in functionally and aesthetically sensitive areas such as the head and neck, hands, feet, genitalia, and pretibial leg.[1] Melanomas in these anatomic sites are also prone to subclinical extension, which can be detected with MMS. In addition, free margins, such as the lip and eyelid, are functionally important and facially defining characteristics, which, when altered, lead to significant disfigurement.[4] When tissue redundancy is limited, repairing a Mohs defect becomes more challenging due to the possibility of substantial distortion of surrounding anatomical structures.[4][5]
Indications
MMS is warranted for skin malignancies with a high risk of recurrence, incomplete excision, or when tissue preservation is critical. The Mohs appropriate use criteria guidelines serve as a tool for dermatologists to use in determining the appropriateness of MMS for a specific tumor, accounting for patient characteristics, anatomic location, and tumor features.[6]
MMS is effective for MIS or invasive melanoma, particularly when combined with immunostains such as melanoma antigen recognized by T cells 1 (MART-1) (see Image. Melanama-Associated Antigen Recognized by T-Cells-1 [MART-1] Staining).[7][8] MMS allows for a thorough histopathologic evaluation of tumor margins prior to reconstruction, thus reducing recurrence rates.[6] MMS is a viable therapy option for both MIS and invasive melanoma, with most evidence describing its use for MIS.[2][9] MMS is particularly effective for treating melanomas in specialty sites such as the head, neck, hands, feet, genitalia, and pretibial leg due to its complete margin assessment, tissue conservation, and low recurrence rates.[2]
MMS fills a gap in the National Comprehensive Cancer Network (NCCN) guidelines for melanoma excision, based on the results of 6 randomized prospective trials in which 99.1% were on the trunk and proximal extremities. The “rule of 10s” and “rule of 2s” are vital concepts that outline complication rates based on anatomic site after melanoma excision. Wide local excision (WLE) effectively treats melanomas on the trunk and proximal extremities, with rates of positive margins and local recurrence of only 2% (rule of 2s).[5] However, melanoma margin trials included only 37 melanomas (<1%) on specialty sites. The paucity of data on specialty-site melanomas in current guidelines is problematic because the anatomical location is a crucial predictor of complications and complexity of WLE. For specialty-site melanomas, the rates of positive margins and local recurrence after WLE are approximately 10% (rule of 10s).[5] The 10% risk of incomplete excision after WLE complicates the timing of reconstruction because confirmation of tumor removal is important before complex reconstruction, and flaps and grafts are 10 times more likely to be necessary on specialty sites versus the trunk and proximal extremities.[5]
MMS can be utilized using WLE margins recommended by NCCN guidelines. For example, a 0.5-mm margin is advised for MIS, and a 1.0-mm margin is recommended for T1a/b melanomas.[10] However, a smaller initial margin during MMS can be used if the melanoma is in a critical anatomic location or near a free margin.[9] Based on a prospective study of 1,120 patients with melanoma in situ who underwent MMS, 9-mm margins were superior to 6-mm margins since the former resulted in 99% removal of melanomas; in contrast, the latter resulted in 86% removal.[11][12] MMS can also be performed on invasive melanoma with a Breslow depth greater than 0.8 mm. However, in this scenario, a sentinel lymph node biopsy (SLNB) may be indicated before MMS, thus necessitating 2 separate operative sessions.[13][14] Additionally, given the potential for adnexal involvement, the depth of the MMS excision should extend at least to the superficial fascia, as recommended for WLE per NCCN guidelines.[14][15] While further retrospective studies are needed for margins and depth, 5-mm margins at a minimum are often needed for complete histologic clearance, especially for the lentigo maligna subtype.[16][17][18][19][20]
MART-1, SRY (sex-determining region Y)-box 10 (SOX-10), and microphthalmia-associated transcription factor (MITF) are 3 commonly utilized immunohistochemical stains in MMS for melanoma. MMS with MART-1, a sensitive and specific cytoplasmic melanocyte stain, is effective for both MIS and invasive melanoma, with low local recurrence rates and equivalent or greater survival rates than WLE.[7][21] SOX-10, a nuclear transcription factor, exhibits high sensitivity in various subtypes of melanoma, including desmoplastic melanoma, and can also be used in MMS.[13] Both SOX-10 and another nuclear melanocyte immunostain, MITF, offer a distinct outline of melanocyte nuclei and represent alternatives to MART-1 during MMS.[2]
When performing MMS for melanoma, the debulked material may be viewed on frozen sections but must be sent for traditional breadloafing pathology and permanent sectioning per NCCN guidelines.[14][15] Permanent sections for excised melanoma is considered the gold standard for appropriate histologic assessment. This is important because frozen section analysis of the debulking material may be less precise in assessing Breslow depth or finding lymphovascular invasion or desmoplastic melanoma. This rare but aggressive melanoma can be difficult to diagnose and treat.[14][15][22] Debulking for permanent sections is thus a key step in establishing appropriate tumor staging, which is used to decide whether adjuvant treatment or diagnostic/staging techniques, such as sentinel lymph node biopsy, are required.[22][23]
Despite many successful studies showing the value of MMS for melanoma and melanoma in situ, significant controversy exists on its use. Based on the NCCN guidelines, MMS is not recommended for invasive cutaneous melanoma where standard clinical margins are obtainable and should be considered for minimally invasive (eg, T1a) melanomas in anatomically complex areas (eg, ears, hands). When large or poorly defined T1a melanoma undergoes MMS, larger surgical margins may be needed with peripheral and deep margin assessment. Frozen immunohistochemical stains may aid in preventing progression or local recurrence by providing an accurate interpretation of margins.
Contraindications
In patients deemed fit for surgery, there are no absolute contraindications to MMS for melanoma.[1] Patients on chronic anticoagulation can typically continue their medication during the perioperative period.[24]
Equipment
MMS requires specialized equipment in the procedure room and the laboratory, where tissue is excised, processed, and inspected microscopically. In the procedure room, proper lighting and adjustable seating in the operating area aid in visualization and access to the tumor. During the procedure, local anesthesia with a buffer of 0.5% lidocaine and 1:200,000 epinephrine is often used before surgery. A scalpel with a #15 blade for incisions, gauze for absorbing blood and other fluids, and an electrosurgical device for hemostasis are often used.[1][25]
The excised tissue is processed and inspected microscopically in the Mohs histology laboratory. This laboratory requires a cryotome or cryostat, devices in which tissue can be frozen and then cut into very thin slices to be placed on glass slides. Once the tissue has been mounted on slides, it is either placed in an automatic stainer or stained by hand with hematoxylin and eosin (H&E) and immunostains, if applicable. A ventilation hood may reduce exposure to the chemicals used in the staining process. After slide preparation, they are viewed under light microscopy by the Mohs surgeon to identify if residual cancer is visible. Some Mohs laboratories also feature dedicated stainers to facilitate immunohistochemistry (IHC).[1] For wound closure, sutures of various sizes measured in United States Pharmacopeia sizes are routinely used in repairing the MMS defect. A surgical repair tray with needle drivers for suturing, scissors for cutting sutures, fine forceps for handling delicate tissues, skin hooks for retracting skin, and a scalpel for making incisions are used during surgical closure.[1]
Personnel
The Mohs surgeon serves as both the surgeon and pathologist, excising the tumor, evaluating the histopathologic margins, and performing the reconstruction.[1] Additionally, a surgical assistant and a histotechnician are needed in the surgical suite and for tissue processing, respectively.[1] A pathologist may also be involved in permanent section evaluation.
Preparation
Preparation for MMS for melanoma involves the administration of local anesthesia to ensure patient comfort throughout the treatment.[1][25] A typical anesthetic is buffered 1% lidocaine with 1:100,000 epinephrine.[25]
Technique or Treatment
MMS for melanoma requires a multidisciplinary team-based approach to optimize outcomes. Before surgery, the Mohs surgeon may consult with other surgical subspecialties, such as otolaryngology or surgical oncology, to arrange for preoperative SLNB for invasive melanomas meeting the criteria for SLNB.[14] Discussion with the dermatopathology team preoperatively and intraoperatively can be vital in visualizing the features of melanoma from the initial biopsy and when findings are equivocal on frozen sections during MMS. Collaboration with plastic surgery is also warranted in cases such as acral lentiginous melanoma, nail unit melanoma, or desmoplastic melanoma requiring removal of the periosteum for complete extirpation of the tumor, amputation, or reconstruction.[26][27] Close collaboration with these subspecialties is essential for preoperative planning, tumor staging, interpretation of Mohs layers, and reconstruction during MMS for complex melanoma cases.
The following steps outline the MMS procedure for melanoma:
- Outlining the tumor: The surgeon first outlines the tumor, the applicable margin, and corresponding hashes on the patient with a skin marker for mapping purposes. This step is performed before the injection of a local anesthetic.[1] A Mohs map depicting the outline performed on the patient is drawn on paper.
- Debulking: After the area has been disinfected and anesthetized, any visible tumor is removed or "debulked" using a scalpel, curette, or flexible blade.[1] This technique not only aids in tumor removal but also relaxes the tissue, aiding in subsequent tissue processing.[28]
- Orientation of tissue layer: Before removal, the orientation of the tissue is marked by placing small superficial hash marks with a scalpel around the tissue layer, often at 12 o'clock, 3 o'clock, 6 o'clock, and 9 o'clock positions.[1]
- Removal of tissue layer: A thin margin of tissue is then removed circumferentially around and deep into the debulked tumor defect. This "layer" of tissue is traditionally removed with a beveled angle of approximately 45 degrees, which facilitates tissue processing, but can be removed without an angle when a debulk is performed since the sharp debulking relaxes the tissue.[1]
- Marking and flattening of tissue: Once removed, the tissue layer is often divided into halves or quadrants and then inked with colored dyes to facilitate precise mapping of the tumor. The tissue is pressed flat, so the epidermal edge occupies the same tissue plane as the deep margin.[1]
- Tissue processing: The tissue is then frozen, cut, and processed into horizontal en-face sections, enabling nearly 100% of the peripheral and deep margins to be examined on the same tissue section under the microscope.[1][29] This sectioning method contrasts the traditional vertical (ie, breadloafed) tissue processing performed with WLE, which examines only 0.5% of the tumor margin.[29][30] Vertical sections may be performed on the debulking specimen to visualize the tumor and evaluate it for upstaging. For example, this may alter management and necessitate an SLNB if the invasion is detected on an MIS. According to NCCN guidelines, the debulking specimen should be sent for permanent vertical sections to evaluate for features such as Breslow depth, lymphovascular invasion, or desmoplastic melanoma that can be more difficult to assess on frozen sections.[10] While the Breslow depth is the most important predictor, ulceration and high mitotic index is also important to prognosticate melanoma-specific survival.
- Microscopic examination: When treating melanoma, the debulking specimen and the tissue layer are examined under a microscope using H&E staining and applicable immunostains such as MART-1, SOX-10, or MITF. If any residual tumor is identified, then this area with the residual tumor is marked on the Mohs map and removed from the patient's skin.[1][31]
- Repeat removal of tissue layer: This process is repeated until the tumor margins are clear, ensuring complete tumor removal (see Image. Cleared Mohs Micrographic Surgical Margin).[1][32][33]
- Closure of defect: Once the tumor has been removed, various reconstructive techniques can close the defect, such as primary closure, flaps, grafts, or second intention healing.[1] The Mohs surgeon or another surgical subspecialist, such as a plastic surgeon or otolaryngologist, may perform the reconstruction.
- Postoperative evaluation: After the surgery, the patient is monitored for potential complications such as hematoma, infection, or dehiscence.[34]
Slow MMS, or formalin-fixed tissue MMS, is a modified procedure in which excised tissue is processed into formalin-fixed, paraffin-embedded sections rather than frozen sections.[35] This approach can be used when rapid frozen section analysis is unavailable or IHC quality or interpretation is inadequate on frozen sections.[35][36] The excised tissue is still examined with horizontal en-face sections, with both the deep and the peripheral margins in the same plane; however, the slide preparation can take several days since frozen sections are not used.[35]
Staged excision is a similar technique that also involves excising the tumor and processing the tissue with permanent sections; however, the tissue is "breadloafed" rather than processed as horizontal sections and is typically examined by a dermatopathologist.[37] In slow MMS and staged excision, the wound is left open while the tissue is processed and the slides are prepared.[37] If the margins remain positive, the patient returns for additional removal and subsequent delayed margin evaluation, and the wound is not closed until clear margins are confirmed.[37] These methods differ from standard MMS, in which the tissue is frozen and analyzed immediately, and WLE, in which the tumor is excised and immediate repair is performed before a dermatopathologist's permanent section analysis of "breadloafed" tissue.[35][37]
Based on a Delphi consensus of Mohs surgeons, the following recommendations were made for performing MMS for MIS:[38]
- A Wood lamp should not be used to identify surgical margins.
- Dermatoscopes may be used to identify the surgical margin, though this is left to each surgeon's discretion.
- A negative tissue control from a site with equivalent sunlight exposure of more than 10 cm from the MMS site should be used to decrease false-positive rates.[23][39]
- Vertical section use is left to the discretion of each surgeon.
- A starting margin of at least 5 mm should be used.
- Resection to subcutaneous fat (deep to follicular bulbs) but not to fascia with en-face deep margin assessment should be performed.
- Frozen sections are preferred to permanent sections for final margin confirmation.
- At least 1 immunohistochemical stain should be used to assess each specimen.
Complications
Several complications may arise during the MMS procedure. These include challenges with frozen section quality, interpretation of these sections, issues with fragmented tissue margins, or problems with tissue orientation.[40] Furthermore, histologically differentiating between melanocyte hyperplasia in chronically sun-damaged skin versus melanoma can be difficult, especially with acral lentiginous melanoma.[41]
Wound necrosis (1.9%) and dehiscence (1%) are postoperative complications associated with larger defects and complex repairs.[42] Infections, delayed wound healing, and bleeding are also common complications.[43] Since they lie in the subcutaneous fat, sensory nerves are often disrupted during MMS, leading to transient or possibly permanent anesthesia at the operative site.[2] Disruption of motor nerves from resection of deeply invasive tumors is less common but may result in temporary or permanent muscle weakness.[2]
MMS is considered a generally safe procedure given the low incidence and complication severity.[44] According to a multicenter prospective cohort analysis of 20,821 cases at 23 locations, MMS is associated with an adverse event rate of 0.72% and an undetectable death rate.[43] Most complications can be addressed in the Mohs clinic.[44]
Recurrence of the tumor or incomplete resection remains an unlikely but possible complication of MMS for melanoma and melanoma in situ.[45][46] Further resection should be considered in these cases. In patient who are unable to undergo further resection, topical imiquimod (for MIS or lentigo maligna melanoma) or radiation therapy may be considered.[47][48]
Clinical Significance
MMS has emerged as an effective option in the treatment of melanoma.[2] During the MMS procedure, immunohistochemical stains, such as MART-1, have enabled Mohs surgeons to assess melanoma margin status on frozen section specimens.[7][14][49] This advancement addresses the challenge of identifying features of melanoma, such as pagetoid spread, confluence, nesting, and melanocyte atypia, on frozen sections with H&E staining alone.[49]
The value of MMS for melanoma extends beyond providing a potentially tissue-sparing approach in patients with lesions in cosmetically sensitive locations.[2] Given its complete circumferential and deep margin assessment, MMS for melanoma is associated with low recurrence rates. A retrospective cohort analysis of 123 melanomas excised with MMS with MART-1 immunostains noted local recurrence in only 1.63% of cases, with overall survival of 95.12% and disease-specific survival of 100%.[40] Importantly, MMS may offer lower recurrence rates for early-stage melanomas of the head and neck compared to conventional WLE and staged excision.[13] According to 1 meta-analysis, treatment with MMS conferred a pooled recurrence risk of 0.8%, compared with 2.5% for staged excision and 8.7% for WLE.[13] Another systematic review and meta-analysis found that local recurrence of melanoma was lower after MMS (1%) than after staged excision (3%) or WLE (7%).[50]
Despite its utility, MMS for melanoma is associated with several challenges. MMS for melanoma is a relatively new approach for which further evidence is needed, especially prospective data evaluating whether MMS improves survival compared with WLE or staged excision. In addition, many Mohs surgeons have not received formal training in MMS for melanoma and thus may not be proficient in the procedure and interpreting slides with IHC. Furthermore, MMS for melanoma requires a well-equipped Mohs laboratory and personnel capable of performing IHC.[51] Even with skilled personnel, the lengthy staining process and the propensity for subclinical spread with MIS and invasive melanoma may result in MMS cases that can last several hours. Despite these obstacles, MMS for melanoma permits same-day reconstruction and may improve patient outcomes by minimizing incomplete excision and recurrence rates.[51][52]
Enhancing Healthcare Team Outcomes
Effective management of melanoma and melanoma in situ using Mohs micrographic surgery (MMS) requires a coordinated, interprofessional approach to ensure optimal patient-centered care, outcomes, and safety. Physicians, including dermatologists, surgical oncologists, and plastic surgeons, must possess advanced skills in diagnosing and treating melanoma, with a focus on precise surgical techniques to achieve clear margins while preserving healthy tissue. Advanced practitioners, such as nurse practitioners and physician assistants, play a critical role in preoperative assessments, patient education, and postoperative care. Nurses ensure comprehensive patient support, managing wound care and monitoring for complications, while pharmacists assist in managing medications, including pain management and antibiotics.
Interprofessional communication and care coordination are vital to the success of MMS for melanoma. Physicians, advanced practitioners, and nurses must collaborate closely, sharing patient information and surgical plans to ensure seamless care transitions. Regular multidisciplinary team meetings, including pathologists for immediate histopathologic evaluation and radiologists for imaging needs, are essential for developing comprehensive treatment plans. This collaborative approach, combined with clear communication and documentation, enhances patient safety by minimizing errors and ensuring all team members know the patient's status and treatment progress. Ultimately, this strategy improves clinical outcomes and enhances the patient experience by providing coordinated, compassionate care.
Media
(Click Image to Enlarge)
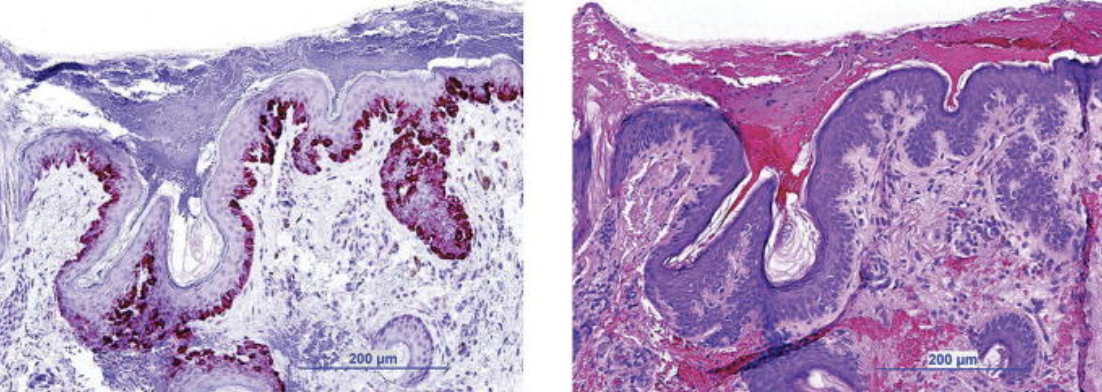
Melanama-Associated Antigen Recognized by T-Cells-1 (MART-1) Staining. Lentigo maligna histology with MART-1 staining (left) or hematoxylin and eosin staining (right) during Mohs micrographic surgery.
Kasprzak JM, Xu YG. Diagnosis and management of lentigo maligna: a review. Drugs Context. 2015;4:212281.
(Click Image to Enlarge)
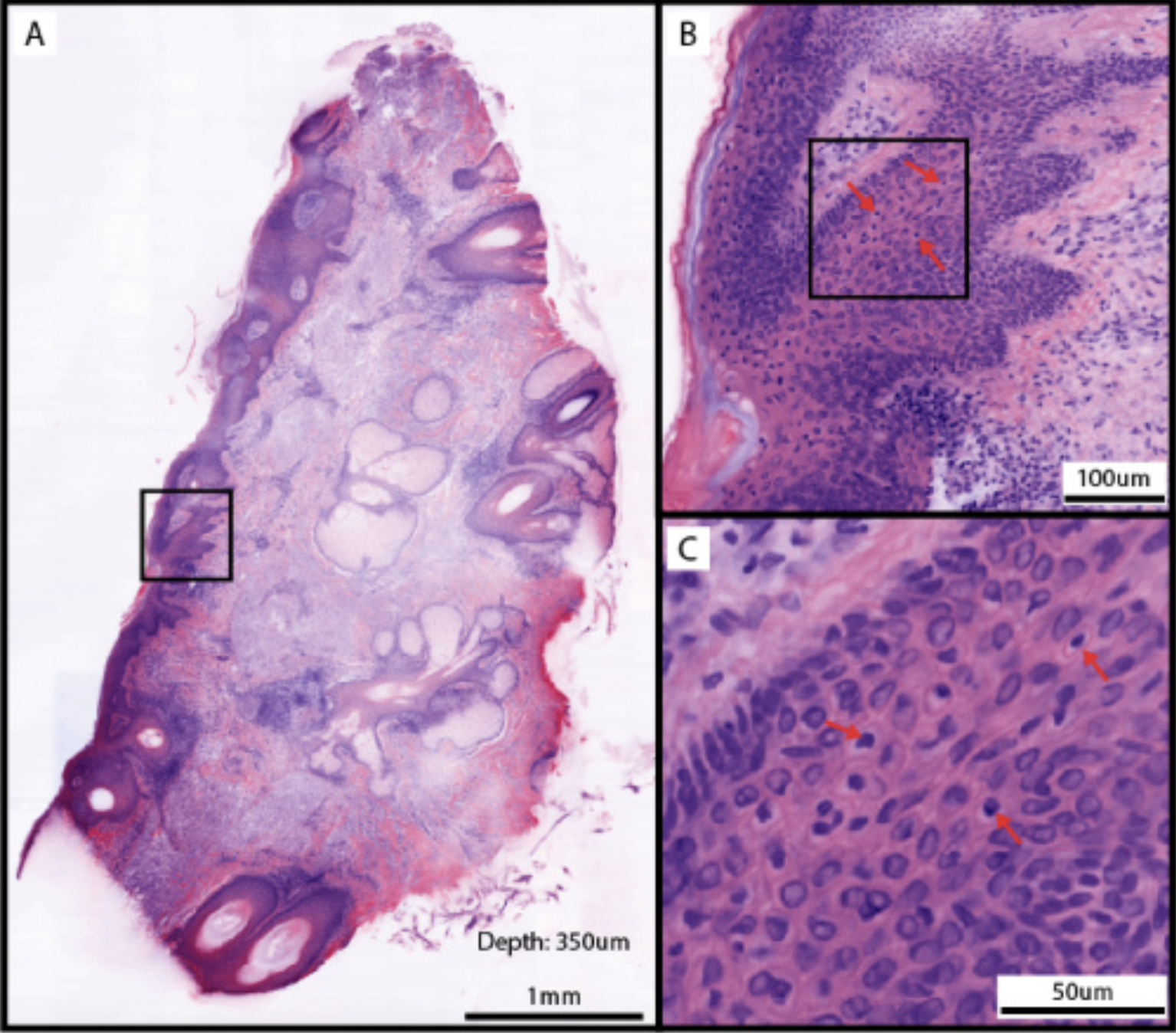
Cleared Mohs Micrographic Surgical Margin. This is a clear surgical margin at 350 μm depth from the tumor margin in a Mohs micrographic surgery (MMS). A: Full layer mosaic of MMS surgical margin. B: Magnified epidermis region boxed in A, with potential melanocytes indicated by arrows. C: The boxed region in B, with arrows indicating the same potential melanocytes in B.
Huang CZ, Montague JE, Ching-Roa VD, Drage MG, Ibrahim SF, Giacomelli MG. Rapid clearing and imaging of Mohs and melanoma surgical margins using a low-cost tissue processor. Biomed Opt Express. 2024;15(2):700-714. doi: 10.1364/BOE.510132.
PMID: 38404330; PMCID: PMC10890881.
References
Prickett KA, Ramsey ML. Mohs Micrographic Surgery. StatPearls. 2024 Jan:(): [PubMed PMID: 28722863]
Clements S, Khachemoune A. Mohs Micrographic Surgery for Melanoma: A Convincing Treatment Modality. Indian journal of dermatology. 2022 Jul-Aug:67(4):479. doi: 10.4103/ijd.ijd_1074_20. Epub [PubMed PMID: 36578768]
Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: A prospective multicenter study. Journal of the American Academy of Dermatology. 2019 Sep:81(3):767-774. doi: 10.1016/j.jaad.2019.05.057. Epub 2019 May 28 [PubMed PMID: 31150700]
Level 2 (mid-level) evidenceDewey J, Bermudez R. Mohs Micrographic Surgery Design and Execution of Rotation Flaps. StatPearls. 2024 Jan:(): [PubMed PMID: 37983335]
Rzepecki AK, Hwang CD, Etzkorn JR, Shin TM, Sobanko JF, Howe NM, Miller CJ. The rule of 10s versus the rule of 2s: High complication rates after conventional excision with postoperative margin assessment of specialty site versus trunk and proximal extremity melanomas. Journal of the American Academy of Dermatology. 2021 Aug:85(2):442-452. doi: 10.1016/j.jaad.2018.11.008. Epub 2018 Nov 14 [PubMed PMID: 30447316]
Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Smith Begolka W, Ratings Panel, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, McBurney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct:67(4):531-50. doi: 10.1016/j.jaad.2012.06.009. Epub 2012 Sep 5 [PubMed PMID: 22959232]
Valentín-Nogueras SM, Brodland DG, Zitelli JA, González-Sepúlveda L, Nazario CM. Mohs Micrographic Surgery Using MART-1 Immunostain in the Treatment of Invasive Melanoma and Melanoma In Situ. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2016 Jun:42(6):733-44. doi: 10.1097/DSS.0000000000000725. Epub [PubMed PMID: 27158886]
Kasprzak JM, Xu YG. Diagnosis and management of lentigo maligna: a review. Drugs in context. 2015:4():212281. doi: 10.7573/dic.212281. Epub 2015 May 29 [PubMed PMID: 26082796]
Brodland DG. Mohs Micrographic Surgery for Melanoma: Evidence, Controversy, and a Critical Review of Excisional Margin Guidelines. Dermatologic clinics. 2023 Jan:41(1):79-88. doi: 10.1016/j.det.2022.07.008. Epub 2022 Oct 28 [PubMed PMID: 36410985]
Level 3 (low-level) evidencePathak S, Zito PM. Clinical Guidelines for the Staging, Diagnosis, and Management of Cutaneous Malignant Melanoma. StatPearls. 2024 Jan:(): [PubMed PMID: 34283515]
Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ: when 5-mm margins are really 9 mm. Journal of the American Academy of Dermatology. 2015 Apr:72(4):745. doi: 10.1016/j.jaad.2014.12.016. Epub [PubMed PMID: 25773425]
Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. Journal of the American Academy of Dermatology. 2019 Jul:81(1):204-212. doi: 10.1016/j.jaad.2019.01.051. Epub 2019 Apr 20 [PubMed PMID: 31014825]
Wright FC, Souter LH, Kellett S, Easson A, Murray C, Toye J, McCready D, Nessim C, Ghazarian D, Hong NJL, Johnson S, Goldstein DP, Petrella T, Melanoma Disease Site Group. Primary excision margins, sentinel lymph node biopsy, and completion lymph node dissection in cutaneous melanoma: a clinical practice guideline. Current oncology (Toronto, Ont.). 2019 Aug:26(4):e541-e550. doi: 10.3747/co.26.4885. Epub 2019 Aug 1 [PubMed PMID: 31548823]
Level 1 (high-level) evidenceMacArthur KM, Baumann BC, Sobanko JF, Etzkorn JR, Shin TM, Higgins HW 2nd, Giordano CN, McMurray SL, Krausz A, Newman JG, Rajasekaran K, Cannady SB, Brody RM, Karakousis GC, Miura JT, Cohen JV, Amaravadi RK, Mitchell TC, Schuchter LM, Miller CJ. Compliance with sentinel lymph node biopsy guidelines for invasive melanomas treated with Mohs micrographic surgery. Cancer. 2021 Oct 1:127(19):3591-3598. doi: 10.1002/cncr.33651. Epub 2021 Jul 22 [PubMed PMID: 34292585]
Nahhas AF, Scarbrough CA, Trotter S. A Review of the Global Guidelines on Surgical Margins for Nonmelanoma Skin Cancers. The Journal of clinical and aesthetic dermatology. 2017 Apr:10(4):37-46 [PubMed PMID: 28458773]
Felton S, Taylor RS, Srivastava D. Excision Margins for Melanoma In Situ on the Head and Neck. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2016 Mar:42(3):327-34. doi: 10.1097/DSS.0000000000000648. Epub [PubMed PMID: 26866286]
Hilari H, Llorca D, Traves V, Villanueva A, Serra-Guillén C, Requena C, Llombart B, Sanmartín O, Guillén C, Nagore E. Conventional surgery compared with slow Mohs micrographic surgery in the treatment of lentigo maligna: a retrospective study of 62 cases. Actas dermo-sifiliograficas. 2012 Sep:103(7):614-23 [PubMed PMID: 22572575]
Level 2 (mid-level) evidenceDuffy KL, Truong A, Bowen GM, Andtbacka RH, Hyngstrom J, Bowles T, Grossmann K, Khong H, Hyde M, Florell SR, Bowen AR, Wada D, Grossman D. Adequacy of 5-mm surgical excision margins for non-lentiginous melanoma in situ. Journal of the American Academy of Dermatology. 2014 Oct:71(4):835-8. doi: 10.1016/j.jaad.2014.06.044. Epub [PubMed PMID: 25219711]
Akhtar S, Bhat W, Magdum A, Stanley PR. Surgical excision margins for melanoma in situ. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2014 Mar:67(3):320-3. doi: 10.1016/j.bjps.2013.11.014. Epub 2013 Dec 12 [PubMed PMID: 24444795]
Walling HW, Scupham RK, Bean AK, Ceilley RI. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 2007 Oct:57(4):659-64 [PubMed PMID: 17870430]
Etzkorn JR, Sobanko JF, Elenitsas R, Newman JG, Goldbach H, Shin TM, Miller CJ. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. Journal of the American Academy of Dermatology. 2015 May:72(5):840-50. doi: 10.1016/j.jaad.2015.01.007. Epub 2015 Mar 13 [PubMed PMID: 25774012]
Finley EM. The principles of mohs micrographic surgery for cutaneous neoplasia. Ochsner journal. 2003 Spring:5(2):22-33 [PubMed PMID: 22826680]
Beaulieu D, Fathi R, Srivastava D, Nijhawan RI. Current perspectives on Mohs micrographic surgery for melanoma. Clinical, cosmetic and investigational dermatology. 2018:11():309-320. doi: 10.2147/CCID.S137513. Epub 2018 Jun 20 [PubMed PMID: 29950878]
Level 3 (low-level) evidenceAlcalay J, Alkalay R. Controversies in perioperative management of blood thinners in dermatologic surgery: continue or discontinue? Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Aug:30(8):1091-4; discussion 1094 [PubMed PMID: 15274698]
Firoz B, Davis N, Goldberg LH. Local anesthesia using buffered 0.5% lidocaine with 1:200,000 epinephrine for tumors of the digits treated with Mohs micrographic surgery. Journal of the American Academy of Dermatology. 2009 Oct:61(4):639-43. doi: 10.1016/j.jaad.2009.07.005. Epub [PubMed PMID: 19751881]
Zhang J, Yun SJ, McMurray SL, Miller CJ. Management of Nail Unit Melanoma. Dermatologic clinics. 2021 Apr:39(2):269-280. doi: 10.1016/j.det.2020.12.006. Epub 2021 Feb 11 [PubMed PMID: 33745639]
Ran NA, Nugent ST, Veerabagu SA, Chu EY, Modi MB, Sobanko JF, Etzkorn JR, Shin TM, Higgins HW 2nd, Giordano CN, McMurray SL, Walker JL, Stull CM, Miller CJ. Desmoplastic melanoma treated with wide local excision or Mohs micrographic surgery: Rates of positive margins, local recurrence, and repeat surgeries. Journal of the American Academy of Dermatology. 2022 Dec:87(6):1376-1378. doi: 10.1016/j.jaad.2022.07.058. Epub 2022 Aug 10 [PubMed PMID: 35963290]
Brady M, Ramsey M. Preparation of the Mohs Debulk Specimen for More Efficient, Accurate Laboratory Processing. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2020 Sep:46(9):1227-1228. doi: 10.1097/DSS.0000000000002113. Epub [PubMed PMID: 31567584]
Rapini RP. Comparison of methods for checking surgical margins. Journal of the American Academy of Dermatology. 1990 Aug:23(2 Pt 1):288-94 [PubMed PMID: 2212126]
van Delft LCJ, Nelemans PJ, van Loo E, Abdul Hamid M, Kelleners-Smeets NWJ. The illusion of conventional histological resection margin control. The British journal of dermatology. 2019 May:180(5):1240-1241. doi: 10.1111/bjd.17510. Epub 2019 Jan 18 [PubMed PMID: 30515776]
Humphreys TR, Nemeth A, McCrevey S, Baer SC, Goldberg LH. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1996 Aug:22(8):693-7 [PubMed PMID: 8780761]
Level 3 (low-level) evidenceKrishnan A, Xu T, Hutfless S, Park A, Stasko T, Vidimos AT, Leshin B, Coldiron BM, Bennett RG, Marks VJ, Brandt R, Makary MA, Albertini JG, the American College of Mohs Surgery Improving Wisely Study Group. Outlier Practice Patterns in Mohs Micrographic Surgery: Defining the Problem and a Proposed Solution. JAMA dermatology. 2017 Jun 1:153(6):565-570. doi: 10.1001/jamadermatol.2017.1450. Epub [PubMed PMID: 28453605]
Huang CZ, Montague JE, Ching-Roa VD, Drage MG, Ibrahim SF, Giacomelli MG. Rapid clearing and imaging of Mohs and melanoma surgical margins using a low-cost tissue processor. Biomedical optics express. 2024 Feb 1:15(2):700-714. doi: 10.1364/BOE.510132. Epub 2024 Jan 8 [PubMed PMID: 38404330]
Cottrell J, Raggio BS. Facial Reconstruction for Mohs Defect Repairs. StatPearls. 2024 Jan:(): [PubMed PMID: 31971739]
Lawrence CM, Haniffa M, Dahl MG. Formalin-fixed tissue Mohs surgery (slow Mohs) for basal cell carcinoma: 5-year follow-up data. The British journal of dermatology. 2009 Mar:160(3):573-80. doi: 10.1111/j.1365-2133.2008.09021.x. Epub 2009 Jan 28 [PubMed PMID: 19210500]
Asilian A, Honarjou N, Faghihi G, Saber M, Mozafarpoor S, Hafezi H. An experience of slow-Mohs micrographic surgery for the treatment of Dermatofibrosarcoma protuberans: A long-term cohort study. Journal of cosmetic dermatology. 2020 Oct:19(10):2701-2705. doi: 10.1111/jocd.13319. Epub 2020 Feb 10 [PubMed PMID: 32039548]
Liu A, Botkin A, Murray C, Solish N, Kitchen J, Chan AW. Outcomes of Staged Excision With Circumferential en Face Margin Control for Lentigo Maligna of the Head and Neck. Journal of cutaneous medicine and surgery. 2021 Jan-Feb:25(1):18-24. doi: 10.1177/1203475420952425. Epub 2020 Sep 10 [PubMed PMID: 32911979]
Curtis KK, Fakult NJ, Strunck JL, Aasi SZ, Ahn CS, Alam M, Bar AA, Behshad R, Bichakjian CK, Bolotin D, Boone SL, Bordeaux JS, Brewer JD, Carr DR, Carucci JA, Castillo JR, Christensen SR, Clark MA, Collins LK, Demer AM, Eisen DB, Feng H, Firoz BF, Grekin RC, Hirshburg JM, Holmes TE, Huang CC, Jennings TA, Jiang SIB, Konda S, Leitenberger JJ, Lewin JM, Maher IA, Ng E, Orengo IF, Samie FH, Saylor DK, Sharon VR, Soleymani T, Swetter SM, Tate JA, Van Beek MJ, Vidal NY, Vij A, Wysong A, Xu YG, Carroll BT, Yu WY. Establishing Consensus for Mohs Micrographic Surgical Techniques in the Treatment of Melanoma in Situ for Future Clinical Trials: A Modified Delphi Study. Journal of the National Comprehensive Cancer Network : JNCCN. 2024 Jul 30:():1-6. doi: 10.6004/jnccn.2024.7036. Epub 2024 Jul 30 [PubMed PMID: 39079545]
Level 3 (low-level) evidenceChang KH, Finn DT, Lee D, Bhawan J, Dallal GE, Rogers GS. Novel 16-minute technique for evaluating melanoma resection margins during Mohs surgery. Journal of the American Academy of Dermatology. 2011 Jan:64(1):107-12. doi: 10.1016/j.jaad.2010.02.055. Epub [PubMed PMID: 21167405]
Rapini RP. Pitfalls of Mohs micrographic surgery. Journal of the American Academy of Dermatology. 1990 Apr:22(4):681-6 [PubMed PMID: 2180998]
Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Feb:19 Suppl 2():S34-40 [PubMed PMID: 16446714]
Ruiz-Salas V, Sanmartin-Jiménez O, Garcés JR, Vilarrasa E, Miñano-Medrano R, Escutia-Muñoz B, Flórez-Menéndez Á, Artola-Igarza JL, Alfaro-Rubio A, Redondo P, Delgado-Jiménez Y, Sánchez-Schmidt J, Allende-Markixana I, García Bracamonte B, de la Cueva-Dobao P, Ciudad C, Carnero-González L, Vázquez-Veiga H, Sánchez-Sambucety P, Estebaranz JL, Botella-Estrada R, González-Sixto B, Martorell A, Morales-Gordillo V, Toll-Abelló A, Mayor-Arenal M, Suárez-Fernández R, Sainz-Gaspar L, Descalzo MA, Garcia-Doval I, REGESMOHS (Registro Español de Cirugía de Mohs). Complications Associated with Mohs Micrographic Surgery: Data from the Nationwide Prospective Cohort REGESMOHS. Dermatology (Basel, Switzerland). 2022:238(2):320-328. doi: 10.1159/000517010. Epub 2021 Aug 11 [PubMed PMID: 34380138]
Alam M, Ibrahim O, Nodzenski M, Strasswimmer JM, Jiang SI, Cohen JL, Albano BJ, Batra P, Behshad R, Benedetto AV, Chan CS, Chilukuri S, Crocker C, Crystal HW, Dhir A, Faulconer VA, Goldberg LH, Goodman C, Greenbaum SS, Hale EK, Hanke CW, Hruza GJ, Jacobson L, Jones J, Kimyai-Asadi A, Kouba D, Lahti J, Macias K, Miller SJ, Monk E, Nguyen TH, Oganesyan G, Pennie M, Pontius K, Posten W, Reichel JL, Rohrer TE, Rooney JA, Tran HT, Poon E, Bolotin D, Dubina M, Pace N, Kim N, Disphanurat W, Kathawalla U, Kakar R, West DP, Veledar E, Yoo S. Adverse events associated with mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA dermatology. 2013 Dec:149(12):1378-85. doi: 10.1001/jamadermatol.2013.6255. Epub [PubMed PMID: 24080866]
Level 3 (low-level) evidenceBittner GC, Cerci FB, Kubo EM, Tolkachjov SN. Mohs micrographic surgery: a review of indications, technique, outcomes, and considerations. Anais brasileiros de dermatologia. 2021 May-Jun:96(3):263-277. doi: 10.1016/j.abd.2020.10.004. Epub 2021 Mar 24 [PubMed PMID: 33849752]
de Vries K, Greveling K, Prens LM, Munte K, Koljenović S, van Doorn MB, Prens EP. Recurrence rate of lentigo maligna after micrographically controlled staged surgical excision. The British journal of dermatology. 2016 Mar:174(3):588-93. doi: 10.1111/bjd.14325. Epub 2016 Jan 27 [PubMed PMID: 26616840]
Hou JL, Reed KB, Knudson RM, Mirzoyev SA, Lohse CM, Frohm ML, Brewer JD, Otley CC, Roenigk RK. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2015 Feb:41(2):211-8. doi: 10.1097/DSS.0000000000000248. Epub [PubMed PMID: 25590473]
Naylor MF, Crowson N, Kuwahara R, Teague K, Garcia C, Mackinnis C, Haque R, Odom C, Jankey C, Cornelison RL. Treatment of lentigo maligna with topical imiquimod. The British journal of dermatology. 2003 Nov:149 Suppl 66():66-70 [PubMed PMID: 14616356]
Powell AM, Russell-Jones R, Barlow RJ. Topical imiquimod immunotherapy in the management of lentigo maligna. Clinical and experimental dermatology. 2004 Jan:29(1):15-21 [PubMed PMID: 14723712]
Theunissen CCW, Lee MH, Murad FG, Waldman AH. Systematic Review of the Role of Mohs Micrographic Surgery in the Management of Early-Stage Melanoma of the Head and Neck. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2021 Sep 1:47(9):1185-1189. doi: 10.1097/DSS.0000000000003126. Epub [PubMed PMID: 34148999]
Level 1 (high-level) evidencePride RLD, Miller CJ, Murad MH, Erwin PJ, Brewer JD. Local Recurrence of Melanoma Is Higher After Wide Local Excision Versus Mohs Micrographic Surgery or Staged Excision: A Systematic Review and Meta-analysis. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2022 Feb 1:48(2):164-170. doi: 10.1097/DSS.0000000000003309. Epub [PubMed PMID: 34889212]
Level 1 (high-level) evidenceSiscos SM, Neill BC, Seger EW, Hocker TLH. Factors Deterring the Utilization of Mohs Surgery for Treatment of Melanoma: A Nationwide Cross-Sectional Survey of Mohs Surgeons. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2021 May 1:47(5):709-710. doi: 10.1097/DSS.0000000000002711. Epub [PubMed PMID: 33905398]
Level 2 (mid-level) evidenceWang W, Lee J, Qin S, Brown MD, Doerr T. Indications and Outcomes of Mohs Micrographic Surgery Using a Multidisciplinary Approach: A Decade of Experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2021 Jan 1:47(1):10-15. doi: 10.1097/DSS.0000000000002467. Epub [PubMed PMID: 32541342]
Level 2 (mid-level) evidence