Introduction
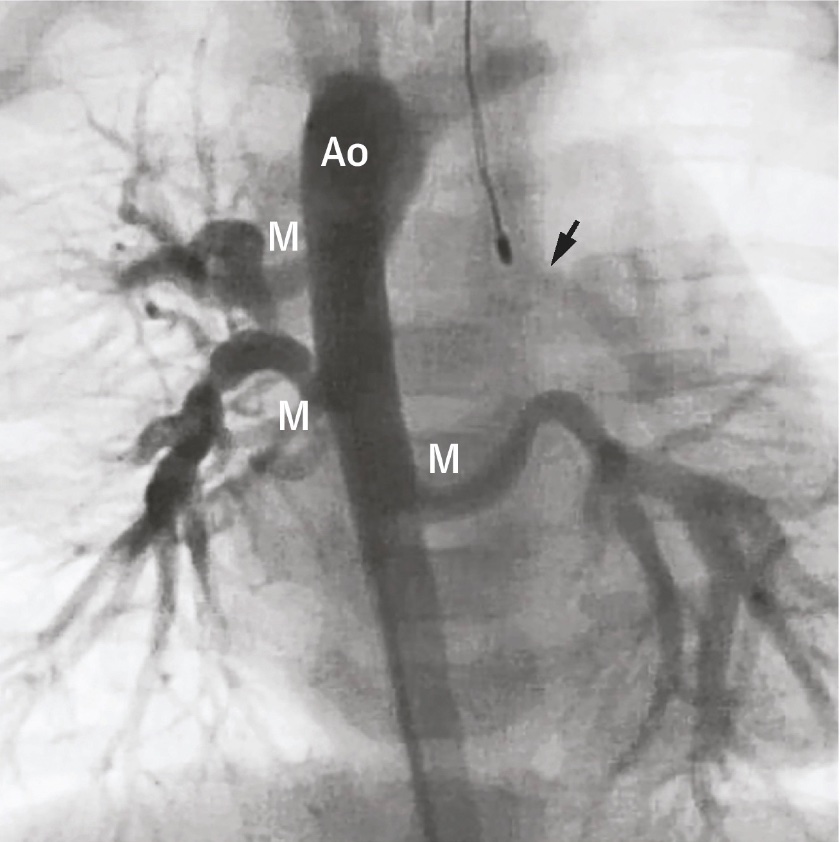
Major aortopulmonary collateral arteries (MAPCAs) are congenital cardiac lesions that result from persistent embryonic connections between the aorta or its branches and the pulmonary vascular system (see Image. Major Aortopulmonary Collateral Arteries Angiogram).[1] MAPCAs are usually associated with other congenital heart defects with complete or partial pulmonary blood flow occlusion. These abnormal systemic-to-pulmonary arterial connections provide the pulmonary perfusion necessary to sustain life in such conditions. MAPCAs are extremely rare. Some studies estimate that pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (PA/VSD/MAPCAs) occurs in approximately 10 out of 100,000 live births.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
MAPCAs develop early in embryonic life yet regress as soon as normal pulmonary circulation develops. The pulmonary arteries fail to develop properly in some congenital heart conditions. Consequently, the collaterals continue to grow, with some potentially becoming the main blood supply to the lungs. This condition often accompanies congenital heart diseases that reduce pulmonary blood flow, like tetralogy of Fallot with pulmonary atresia (pulmonary atresia with VSD), though it can also occur by itself on rare occasions. Such an anomaly is known as an isolated aortopulmonary collateral artery and is a potential cause of congestive heart failure in neonates.[3][4][5] Different genetic syndromes are incriminated in the development of MAPCAs. DiGeorge syndrome is historically most notoriously associated with MAPCAs, followed by VATER (vertebrae, anus, trachea, esophagus, and renal) syndrome and Alagille syndromes. Children with MAPCAs due to Alagille syndrome have a poor prognosis, mainly due to multiorgan system involvement.[6][7][8]
Epidemiology
PA/VSD/MAPCAs is a complex congenital cardiac anomaly that manifests in approximately 10 out of 100,000 live births. The condition affects the sexes equally, but data is insufficient to demonstrate ethnic preponderance. Nevertheless, at least 40% of patients with 22q11 deletion or DiGeorge syndrome have MAPCAs.[9]
Pathophysiology
MAPCAs are often present in various CHDs that compromise pulmonary circulation, either due to severe pulmonary valvular or arterial stenosis or complete pulmonary atresia. Several pathophysiological mechanisms have been proposed, such as monosomy 22q11, hypoxemia, and abnormal pulmonary blood pressure. During fetal development, the well-oxygenated blood initially flows into the lungs, leading to pulmonary vasodilation and reducing blood flow across the ductus arteriosus and foramen ovale. Consequently, the pulmonary circulation and left heart become isolated from the rest of the circulation, resulting in hypoxemia. This low oxygen level drives the nonregression of aortopulmonary collateral vessels.[9][10][11]
Lesions frequently showing MAPCAs in their pathology are called "pulmonary atresia with ventricular septal defect." However, it is more descriptive to consider them as tetralogy of Fallot (TOF) with pulmonary atresia (TOF/PA). Patients with varying phenotypic arrangements can also be correctly described as having pulmonary atresia with ventricular septal defect (PA/VSD). For example, patients with transposition of the great arteries, congenitally corrected or otherwise, with isomeric atrial appendages, double-inlet ventricle, or atrioventricular valvar atresia, have pulmonary atresia in the presence of deficient ventricular septation. A patent ductus arteriosus (PDA) supplies the pulmonary arteries within the heart's pericardial sac in these cases. However, the subgroup with a TOF and a stenosed or blocked subpulmonary outflow tract demonstrate pulmonary arteries only supplied by true congenital systemic-to-pulmonary collateral arteries. Most of these aortopulmonary collaterals originate from the subclavian and internal mammary arteries. Nevertheless, this condition is relatively rare.
TOF/PA is a congenital heart defect that can also be referred to as "pseudo-truncus arteriosus." This condition prevents blood from flowing directly from the right ventricle to the pulmonary artery. Thus, blood from both ventricles goes into the aorta. The pulmonary circulation is supplied through multiple MAPCAs or a PDA. Neonates can develop hypoxic symptoms if the PDA closes or if the MAPCAs are narrow or stenosed in severe cases.
History and Physical
The clinical presentation of patients with duct-dependent pulmonary circulation and confluent pulmonary arteries is highly variable. Patients may develop cyanosis as the ductus closes, requiring prostaglandin resuscitation if not diagnosed prenatally. Those with pulmonary circulation dependent on MAPCAs and an atretic ductus arteriosus may be asymptomatic at birth due to collateral blood flow meeting normal metabolic demand. However, cyanosis can develop later as growth and activity outpace aortopulmonary circulation. Some infants and neonates may exhibit pulmonary overcirculation and congestive heart failure. Notably, MAPCAs rarely occur as an isolated lesion and are usually associated with various congenital cardiac and noncardiac anomalies. Thus, patients with MAPCAs can present with a wide range of pulmonary blood flow issues, from severely inadequate to excessively high.
Patients with duct-dependent pulmonary circulation and confluent pulmonary arteries often experience cyanotic spells due to reduced systemic vascular resistance or increased pulmonary blood flow. Undiagnosed patients may present later with polycythemia and clubbing or, in severe cases, recurrent pulmonary or cerebral infections, abscesses, or stroke. Tachypnea, tachycardia, irritability, excessive crying, and intercostal and subcostal retractions are typical early symptoms and signs. Precordial auscultation may yield a loud P2 sound or harsh continuous murmur, which may vary depending on the accompanying congenital anomaly (eg, VSD or PDA).
Evaluation
MAPCAs are classified as type I, II, or III based on radiological findings regarding the vessel of origin:
- Type I: Indirect MAPCAs from major aorta branches (eg, subclavian and celiac)
- Type II: Indirect MAPCAs from the bronchial artery
- Type III: Direct MAPCAs from the aorta
MAPCAs can also arise from the coronary arteries.[12][13][14] The tests routinely employed in diagnosing MAPCAs are discussed below. These modalities enable clinicians to assess pulmonary blood flow, detect associated anomalies, plan surgical interventions, and monitor postoperative outcomes, ultimately enhancing patient care and treatment efficacy.
Cardiac Catheterization
Cardiac catheterization continues to be the benchmark method for identifying collateral vessel structure, branching patterns, lung areas, pulmonary artery connections, and the presence of narrowing. This modality also enables examining duct-dependent blood flow in the lungs and strategizing for endovascular embolization procedures. The structure of all aortopulmonary collateral vessels must be delineated during the initial cardiac catheterization. Identifying the anatomy of the true pulmonary arteries can also be challenging, except in cases where they are duct-dependent and connected or have a proximal association with a collateral vessel. Retrograde venous wedge angiography is often required, which may only provide limited visibility of the small, central true pulmonary arteries.
The angiographer must meticulously inspect for the distinctive “seagull” morphology of the true pulmonary arteries in either a lateral or left anterior oblique view. In this analogy, the right and left pulmonary arteries correspond to the seagull’s wings, while the diminutive main pulmonary artery resembles the bird’s body and head. With each cardiac contraction, the main pulmonary artery moves inferiorly, simulating the flapping of wings. The angiographer’s task, in this case, is to identify a branch with a diameter of merely 1 to 2 mm, often comparable to the catheter’s diameter.
Detailed intracardiac anatomy during initial catheterization is generally unnecessary unless the initial echocardiogram indicates multiple VSDs or other rare anomalies. After the surgical establishment of continuity between the right ventricle and pulmonary arteries in the rehabilitative phase, subsequent catheterizations can navigate from the right ventricle into these pulmonary arteries. Determining the blood supply to each of the 18 to 20 bronchopulmonary segments is crucial, identifying whether true pulmonary arteries, collateral vessels, or both supply each segment. Identifying any connections between true pulmonary arteries and collateral vessels is also essential.
The surgeon needs to comprehend the collateral vessels' spatial relationships with other mediastinal structures, notably the trachea and the esophagus.[15] Collateral vessels may run posterior to the esophagus, between the trachea and esophagus, or anterior to the trachea and bronchi. A single vessel can bifurcate, with one branch between the trachea and esophagus and the other either behind the esophagus or in front of the trachea. This complex anatomy requires careful analysis using lateral or anterior oblique views, as relying on a single projection could mislead the surgeon about the feasibility of a straightforward unifocalization procedure.
Cardiac catheterization also provides crucial anatomical insights and vital hemodynamic data. Distal pressure in MAPCAs should be evaluated. Pulmonary vascular disease may be present or likely to develop if the mean pressure exceeds 20 to 25 mm Hg. Cardiac catheterization helps determine the timing for VSD closure. A left-to-right shunt observed after dilating peripheral pulmonary artery stenoses and successful unifocalization indicates the pulmonary artery tree can handle moderate cardiac output, allowing VSD closure without causing the right ventricle to exceed systemic pressures. Postoperatively, the pressure within the right ventricle is a critical measure when evaluating the efficacy of the overall treatment approach for individuals with TOF/PA and MAPCAs. Ideally, right ventricular pressure should be below 50% of systemic pressure or, at the very least, under 75%. However, attaining this objective can be exceedingly challenging.
Echocardiography
Diagnosing MAPCAs should not rely solely on echocardiography. Other methods, like cardiac catheterization, are more reliable and necessary, eg, when examining the descending aorta for aortopulmonary collaterals. While echocardiography is sensitive in detecting these collaterals, it cannot adequately delineate their anatomy or identify peripheral pulmonary artery stenoses, often seen with TOF/PA. Despite these limitations, echocardiography is valuable for detailing intracardiac anatomy, especially in identifying multiple VSDs. High blood flow velocity in the pulmonary veins on echocardiography may indicate MAPCAs, warranting further investigation. Echocardiography also helps identify atypical coronary anatomies or a secundum ASD instead of a patent foramen ovale.[16]
Computed Tomography
Computed tomography (CT), taken in high resolution and with the aid of 3-dimensional reconstruction and printing, is very useful in visualizing the complex pulmonary arterial map before surgical intervention. Nevertheless, CT falls short of cardiac catheterization due to its inability to measure intravascular and cardiac chamber pressures.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is increasingly valuable for diagnosing complex MAPCAs, as angiography alone often fails to capture the intricate relationships between collateral vessels and pulmonary arteries. MRI's 3-dimensional reconstruction capability is particularly useful for planning unifocalization procedures in young infants after initial surgery. Additionally, some research groups have found magnetic resonance angiography helpful in planning neonatal procedures.[17]
Chest Radiographs
Radiographic lung imaging typically shows congestive and plethoric appearances with excessive pulmonary blood flow, and heart enlargement is often seen with congestive heart failure. Conversely, insufficient pulmonary blood flow results in darker, oligemic lung fields (with reduced blood flow), with the heart size normal or smaller than usual. Some lung segments may receive excessive blood flow while others have inadequate supply, causing regional perfusion variations that may be discernible on chest x-rays. Despite these variations, overall oxygen saturation in the child generally remains between 75% and 85%.
Electrocardiography
In MAPCAs, electrocardiography usually appears normal at birth but exhibits signs of right ventricular hypertrophy over time. This hypertrophy is secondary to systemic pressure within the right ventricle, stemming from the unrestricted anterior malalignment VSD.
Photoplethysmography
Pulse oximetry is useful for assessing the balance between pulmonary and systemic blood flow. In children with excessive collateral vessel formation, potentially causing congestive heart failure, resting oxygen saturation typically exceeds 85% to 90%. Conversely, insufficient pulmonary blood flow—due to ductal closure, collateral vessel stenosis, true pulmonary artery stenosis, or pulmonary vascular disease—results in oxygen saturation readings below 75% to 80%.
Treatment / Management
The optimal surgical management for patients hinges on several significant factors, including the size and distribution of the intrapericardial and intrapulmonary arteries and their blood supply from a PDA or MAPCAs. Other critical considerations include the patient's clinical condition, age, weight, and prior surgical history. A thorough and individualized evaluation of each patient's anatomy, physiology, and overall health is essential to ensure the best possible outcome. This approach allows for a tailored surgical plan that accounts for the complex interplay between the various factors in managing CHDs with MAPCAs. Ultimately, this approach ensures patients receive the most effective and appropriate surgical intervention to optimize their long-term postoperative outcome and quality of life.
Results from a study showed neonatal age and low body weight had adverse effects, while simultaneous or staged ventricular septal defect closure positively affected outcomes. Additionally, survival was independently affected by chromosome 22q11 deletion.[18]
MAPCAs can be categorized into 3 main groups:
- Normal arterial distribution to both lungs with normal-sized intrapericardial confluent pulmonary arteries
- Normal arterial distribution to both lungs with moderate hypoplasia of intrapericardial pulmonary arteries
- Marked arterial abnormalities in lung parenchyma, pulmonary atresia, a VSD, and underdeveloped or absent intrapericardial pulmonary arteries
The first 2 groups comprise 75% of cases and depend on a PDA that supplies the aortopulmonary vessels. The 3rd group, the rarest at 25%, depends on MAPCAs for lung blood supply and presents a management challenge.[19] Another essential feature of MAPCAs is their communication with the native pulmonary arteries. MAPCAs may or may not be necessary to supply blood to the lungs. Nonessential MAPCAs terminate by forming an anastomosis with the distal pulmonary arteries, resulting in a dual blood supply to the corresponding lung segments. Conversely, essential MAPCAs supply blood to the relevant lung segment without a native pulmonary artery blood supply; essential MAPCAs may affect the surgical plan.[19]
Surgical Management
The McGoon ratio or the Nakata index estimates the degree of confluence in MAPCAs with data obtained from cardiac catheterization or imaging. The Nakata index is the cross-sectional area of both pulmonary arteries divided by body surface area (BSA). The measurements for the pulmonary branches are taken at the hilum in the craniocaudal axis before the origin of the first ramus. The equation used is:
π x 2/PA diameter/BSA
Standard measurements are around 330 mm/m2, with 30 mm/m2 as the standard deviation. Generally, patients with an index greater than or equal to 150 mm/m2 undergo complete correction (unifocalization) with good postoperative outcomes.
Pulmonary artery rehabilitation
In patients with MAPCAs and diminished pulmonary blood flow or low Nakata scoring, hypoplastic, confluent central pulmonary arteries can be enlarged by establishing right-ventricle-to-pulmonary-artery continuity using a valveless conduit or patching the main pulmonary artery and infundibulum. The ventricular septal defect is left open, resulting in wide pulse pressure due to the absence of a valve in the conduit. Results from a retrospective study of over 1000 patients between 1964 and 2001 found that valveless conduits are more durable than porcine-valved Dacron conduits. Proponents of pulmonary artery rehabilitation prefer surgical intervention within the first few weeks of life.[20][21](B2)
Unifocalization
Unifocalization techniques aim to create a single, unified source of blood flow to the lungs. The unifocalization process is thought to result in all lung areas receiving adequate blood flow for efficient oxygen exchange, normalizing the pressures and flow of blood in the heart and lungs.
Interventional Catheterization
Collateral vessels that provide duplicate blood supply to bronchopulmonary segments must occasionally be occluded. The procedure usually requires steel, platinum, or titanium coils with thrombogenic fabric tufts. Additionally, balloon dilation catheters dilate multiple peripheral stenoses commonly present in the true pulmonary arteries. Balloon dilation is also necessary for anastomotic stenoses that occur in collateral vessels after unifocalization has been performed surgically.
Medical Management
In today's era of advanced surgical techniques, medical therapy plays a limited role in managing congenital cardiac lesions with MAPCAs, often serving as palliative treatment. An exception is ductus-dependent MAPCAs, where continuous prostaglandin E1 infusion may be necessary to maintain ductal patency, potentially saving lives. When managing severe cyanosis, long-term prophylactic β-blocker use is not recommended. Phenylephrine can temporarily manage severe cyanosis in the intensive care setting. However, caution is needed to avoid vasoconstriction immediately before cardiopulmonary bypass, especially in TOF/PA cases.
Differential Diagnosis
MAPCAs are complex vascular structures that can present with congenital heart defects, particularly those involving reduced pulmonary blood flow. The differential diagnosis for MAPCAs is broad, encompassing several other congenital and acquired cardiovascular conditions that might mimic or complicate the presentation of MAPCAs. Clinicians must consider these differential diagnoses when evaluating patients suspected of having MAPCAs to ensure accurate diagnosis and optimal management.
Pulmonary Atresia with Ventricular Septal Defect
Pulmonary atresia with VSD is frequently associated with MAPCAs, as these collateral arteries often develop as a compensatory mechanism to provide blood flow to the lungs when the pulmonary valve is atretic. However, a true pulmonary trunk and confluent pulmonary arteries distinguish typical pulmonary atresia from cases predominantly characterized by MAPCAs.
Tetralogy of Fallot with Absent Pulmonary Valve
This TOF variant features the absence or severe underdevelopment of the pulmonary valve, leading to pulmonary regurgitation and aneurysmal dilation of the pulmonary arteries. Although MAPCAs are not a primary feature, the disruption of normal pulmonary arterial anatomy can initially suggest a similar clinical and radiographic appearance.
Truncus Arteriosus
Truncus arteriosus involves a single arterial trunk that arises from the heart, supplying the systemic, pulmonary, and coronary circulations. The differentiation from MAPCAs lies in identifying a common arterial trunk in the truncus arteriosus, as opposed to multiple separate collateral arteries arising from the aorta or its major branches.
Complex Single Ventricle Defects
Patients with single ventricle physiology, such as hypoplastic left heart syndrome or tricuspid atresia, may have various shunts or conduits placed that can be confused with MAPCAs on imaging studies. Comprehensive imaging and historical data are critical for differentiating these surgically modified circulations from naturally occurring collateral networks.
Congenital Pulmonary Artery Stenosis
Isolated or multiple congenital stenoses of the pulmonary arteries can lead to the development of collateral circulation similar to MAPCAs. The key diagnostic feature distinguishing true pulmonary artery stenosis from MAPCAs is the primary involvement of the main pulmonary arteries and branches rather than abnormal systemic-to-pulmonary connections.
Acquired Pulmonary Artery Stenosis
Acquired stenoses resulting from chronic inflammation, surgical interventions, or external compression, eg, by tumors or enlarged lymph nodes, may also lead to compensatory collateral artery formation. A detailed patient history and sequential imaging are necessary to distinguish these acquired conditions from congenital MAPCAs.
Williams Syndrome
Williams syndrome involves elastin gene deletions leading to cardiovascular abnormalities, including supravalvular aortic stenosis and peripheral pulmonary stenosis. The condition can occasionally mimic the hemodynamic effects seen in MAPCAs due to the development of collateral networks. In clinical practice, evaluating a patient with suspected MAPCAs should include a thorough diagnostic workup involving echocardiography, cardiac MRI, and, often, cardiac catheterization. These studies help map the extent of the collateral network, assess the functionality of the pulmonary circulation, and distinguish MAPCAs from other differential diagnoses. Accurate identification of the underlying condition is crucial for planning appropriate surgical or interventional treatments, including unifocalization of collaterals, repair of associated defects, or, in some cases, transplantation.
Pertinent Studies and Ongoing Trials
The anatomy of MAPCAs exhibits significant variability in their number, anatomical origin, path, and relationship with the native pulmonary arteries. Certain MAPCAs traverse behind the esophagus (retroesophageal) and bronchus before reaching the lung tissue. A recent study examined the physiological and surgical characteristics of retroesophageal MAPCAs in comparison to those located anteriorly. The results reveal distinct anatomical and physiological differences between gastroesophageal and anterior MAPCAs. Consequently, the results indicate that nearly all gastroesophageal MAPCAs should be unifocalized to ensure the incorporation of their supply lung segments.
Treatment Planning
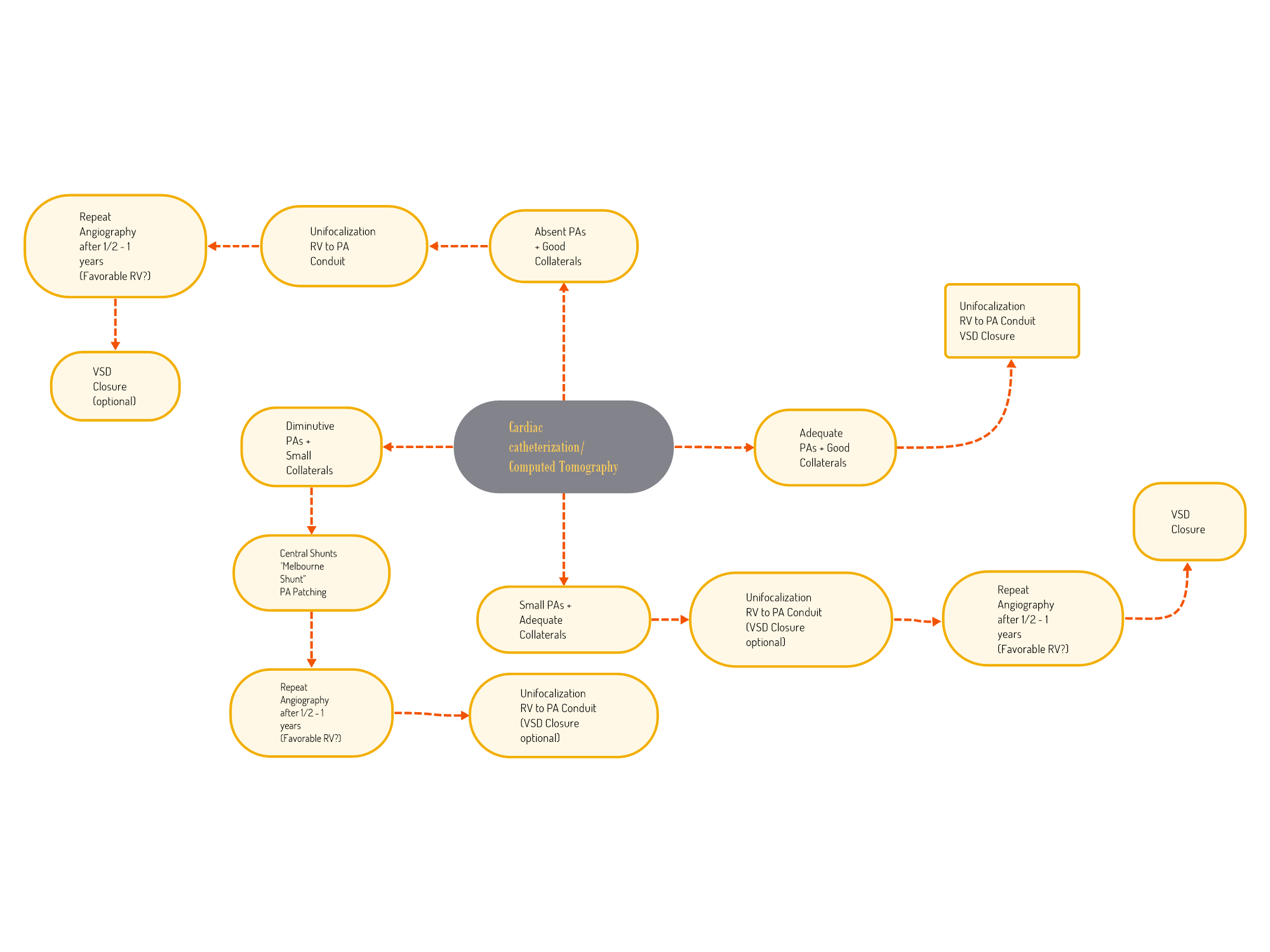
The surgical treatment planning of MAPCAs is guided by multiple factors, such as the quality of native pulmonary arteries versus collaterals, cardiac chamber pressures, and MAPCA confluences. Generally, single-stage repair is preserved for patients with an almost normal pulmonary arterial tree and good collaterals. On the other end of the spectrum, patients with absent native pulmonary arteries and diminutive collaterals are not good candidates for surgery (see Image. Surgical Algorithm and Treatment Planning for Major Aortopulmonary Collateral Arteries).
Prognosis
A recent study at Lucille Packard Children's Hospital (LPCH), Stanford University, reviewed a large case volume, with 780 unique patients undergoing surgery during the study period. The annual number of new patients remained relatively stable for the first 15 years, then significantly increased. Nearly 40% of patients had undergone surgery before being referred to LPCH, a trend that has become more common in recent years. Complete repair was achieved in 704 patients (90%), with 521 (67%) achieving this outcome during their first surgery at LPCH.
The median right-ventricular-to-aortic-pressure ratio was 0.34 (25th, 75th percentiles: 0.28, 0.40). The cumulative incidence of mortality was 15% (95% CI: 12% to 19%) at 10 years, with no significant difference based on the era of surgery (p = 0.53). Multivariable Cox regression analysis identified Alagille syndrome (HR: 2.8; 95% CI: 1.4 to 5.7; p equal to 0.004), preoperative respiratory support (HR: 2.0; 95% CI: 1.2% to 3.3%; p = 0.008), and palliative first surgery at the center (HR: 3.5; 95% CI: 2.3% to 5.4%; p < 0.001) as factors associated with a higher risk of death. LPCH's pulmonary artery reconstruction program, with increasing surgical volumes and a rising number of patients who had previous surgeries, has shown continuous improvement in outcomes for patients with pulmonary atresia or stenosis and MAPCAs.
Complications
MAPCAs often coexist with other congenital cardiac diseases. However, considering their unique pathophysiology, the following list of complications can develop if left untreated:
- Pulmonary overcirculation: MAPCAs can result in excessive blood flow to certain lung areas, potentially leading to pulmonary congestion and pulmonary hypertension.
- Heart failure: Increased pulmonary vascular pressure can cause the heart to work harder to pump blood through abnormal vessels, potentially leading to heart failure due to strain on the heart.
- Ventilation-perfusion mismatch: MAPCAs do not uniformly distribute blood like normal pulmonary arteries. Some lung regions may receive too much blood flow while others get too little. This uneven distribution can produce hyperperfused and underperfused lung areas, eventually leading to lung damage and cyanosis.
- Increased risk of infections: Abnormal blood flow and lung congestion can increase the risk of respiratory infections.
- Hemoptysis: These fragile collateral arteries occasionally rupture and bleed into the lungs.
Deterrence and Patient Education
Parents may be overwhelmed with the fear that comes with a congenital heart diagnosis. Additionally, parents may be concerned about how intelligent or functional their children may be in the future. Results from a recent European study found that only 85.4% of children with congenital heart disease (CHD) were enrolled in regular schools, significantly lower than the 97% enrollment rate of children in the general Swiss population. This result is consistent with findings from Germany, where 83.4% of children with CHD attend regular schools, and a previous Swiss study reported 88% enrollment in a cohort born roughly 10 years earlier.
Additionally, the study demonstrated that even children with CHD attending regular schools often required extra educational support, such as grade retention or an additional kindergarten year. On a positive note, children with CHD actively participated in leisure activities. Most were engaged in sports, social activities like scouts, or playing musical instruments. While established norms do not exist for participation in social and musical activities, the sports participation rate is comparable to that of the general population of Swiss children.[22]
Enhancing Healthcare Team Outcomes
Congenital conditions with MAPCAs pose a significant challenge to clinicians and require prompt and accurate diagnosis and management. Timely intervention is critical to improve affected individuals' prognoses and prevent potential complications. As such, healthcare professionals must remain vigilant and knowledgeable about these severe yet rare congenital heart defects. Interprofessional care for MAPCAs involves collaboration among cardiologists, cardiac surgeons, pediatricians, geneticists, and specialized nursing staff to provide comprehensive treatment. The care plan must include preoperative assessment, surgical intervention, and postoperative management to optimize patient outcomes. Regular interprofessional meetings ensure coordinated care and ongoing evaluation of treatment efficacy.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
McElhinney DB, Asija R, Zhang Y, Jaggi A, Shek J, Peng LF, Boltz MG, Ma M, Martin E, Hanley FL. 20-Year Experience With Repair of Pulmonary Atresia or Stenosis and Major Aortopulmonary Collateral Arteries. Journal of the American College of Cardiology. 2023 Sep 19:82(12):1206-1222. doi: 10.1016/j.jacc.2023.06.041. Epub [PubMed PMID: 37704311]
Leonard H, Derrick G, O'Sullivan J, Wren C. Natural and unnatural history of pulmonary atresia. Heart (British Cardiac Society). 2000 Nov:84(5):499-503 [PubMed PMID: 11040008]
Hoang LX, Tuyen LK, Gia TM, Anh NK, Dan DB, Phuong PK, Tin DN, Hoa T, Duc NM. Large isolated major aortopulmonary collateral artery causing dilated left ventricle. Radiology case reports. 2023 Apr:18(4):1530-1535. doi: 10.1016/j.radcr.2023.01.063. Epub 2023 Feb 7 [PubMed PMID: 36815146]
Level 3 (low-level) evidenceRiggs KW, Mahajan S, Kholwadwala D, Meyer D, Parnel VA. Unusual case of isolated major aortopulmonary collateral artery perfusing entire functional left lower lobe of the lung. Annals of pediatric cardiology. 2021 Oct-Dec:14(4):547-549. doi: 10.4103/apc.APC_133_20. Epub 2022 Mar 25 [PubMed PMID: 35527763]
Level 3 (low-level) evidenceHarshith K, Anoop A, Jineesh V. A Rare Cause of Hemoptysis in West Syndrome-Isolated Aortopulmonary Collaterals in Structurally Normal Heart. The Indian journal of radiology & imaging. 2021 Jul:31(3):745-747. doi: 10.1055/s-0041-1735865. Epub 2021 Oct 6 [PubMed PMID: 34790328]
Liava'a M, Brizard CP, Konstantinov IE, Robertson T, Cheung MM, Weintraub R, d'Udekem Y. Pulmonary atresia, ventricular septal defect, and major aortopulmonary collaterals: neonatal pulmonary artery rehabilitation without unifocalization. The Annals of thoracic surgery. 2012 Jan:93(1):185-91. doi: 10.1016/j.athoracsur.2011.08.082. Epub 2011 Nov 25 [PubMed PMID: 22119120]
Matsuoka R, Takao A, Kimura M, Imamura S, Kondo C, Joh-o K, Ikeda K, Nishibatake M, Ando M, Momma K. Confirmation that the conotruncal anomaly face syndrome is associated with a deletion within 22q11.2. American journal of medical genetics. 1994 Nov 15:53(3):285-9 [PubMed PMID: 7856665]
Mainwaring RD, Sheikh AY, Punn R, Reddy VM, Hanley FL. Surgical outcomes for patients with pulmonary atresia/major aortopulmonary collaterals and Alagille syndrome. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Aug:42(2):235-40; discussion 240-1. doi: 10.1093/ejcts/ezr310. Epub 2012 Mar 7 [PubMed PMID: 22402453]
Hofbeck M, Rauch A, Buheitel G, Leipold G, von der Emde J, Pfeiffer R, Singer H. Monosomy 22q11 in patients with pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Heart (British Cardiac Society). 1998 Feb:79(2):180-5 [PubMed PMID: 9538313]
Li C, Chudley AE, Soni R, Divekar A. Pulmonary atresia with intact ventricular septum and major aortopulmonary collaterals: association with deletion 22q11.2. Pediatric cardiology. 2003 Nov-Dec:24(6):585-7 [PubMed PMID: 12881773]
Chessa M, Butera G, Bonhoeffer P, Iserin L, Kachaner J, Lyonnet S, Munnich A, Sidi D, Bonnet D. Relation of genotype 22q11 deletion to phenotype of pulmonary vessels in tetralogy of Fallot and pulmonary atresia-ventricular septal defect. Heart (British Cardiac Society). 1998 Feb:79(2):186-90 [PubMed PMID: 9538314]
Sumitomo NF, Kodo K, Inoue T, Oyanagi T, Yamagishi H. Clinical Characteristics of Coronary-to-Pulmonary Artery Fistula in Patients with Pulmonary Atresia and Ventricular Septal Defect. Journal of cardiovascular development and disease. 2023 Jan 3:10(1):. doi: 10.3390/jcdd10010017. Epub 2023 Jan 3 [PubMed PMID: 36661912]
Verma M, Pandey NN, Yadav S, Kumar S. Coronary-to-pulmonary collateral artery from a superdominant morphologically right coronary artery. Journal of cardiac surgery. 2022 Mar:37(3):682-684. doi: 10.1111/jocs.16231. Epub 2022 Jan 14 [PubMed PMID: 35028980]
Ballal P, Menon S, Babu S, Dharan BS, Kiran M, Baruah SD, Ramanan SV, Karunakaran J. Pulmonary Atresia with Ventricular Septal Defect: Rare Presentation with Coronary-to-Pulmonary Artery Collaterals from Both Right and Left Coronaries. World journal for pediatric & congenital heart surgery. 2020 Jul:11(4):NP226-NP228. doi: 10.1177/2150135118825158. Epub 2019 Mar 27 [PubMed PMID: 30917743]
Mainwaring RD, Adamson G, Hanley FL. To Unifocalize or Not to Unifocalize?: A Comparison of Retroesophageal Versus Anterior Collaterals. The Annals of thoracic surgery. 2022 Mar:113(3):875-882. doi: 10.1016/j.athoracsur.2021.02.017. Epub 2021 Feb 22 [PubMed PMID: 33631151]
Uygur L, Demirci O, Yücel IK. Pulmonary atresia and ventricular septal defect: How accurate is the fetal echocardiography, and do the major aortopulmonary collateral arteries matter? Echocardiography (Mount Kisco, N.Y.). 2023 Nov:40(11):1259-1268. doi: 10.1111/echo.15706. Epub 2023 Oct 25 [PubMed PMID: 37878331]
Kawel N, Valsangiacomo-Buechel E, Hoop R, Kellenberger CJ. Preoperative evaluation of pulmonary artery morphology and pulmonary circulation in neonates with pulmonary atresia--usefulness of MR angiography in clinical routine. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2010 Sep 15:12(1):52. doi: 10.1186/1532-429X-12-52. Epub 2010 Sep 15 [PubMed PMID: 20843357]
Carotti A, Albanese SB, Filippelli S, Ravà L, Guccione P, Pongiglione G, Di Donato RM. Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. The Journal of thoracic and cardiovascular surgery. 2010 Nov:140(5):1092-103. doi: 10.1016/j.jtcvs.2010.07.087. Epub 2010 Sep 17 [PubMed PMID: 20850144]
Cho JM, Puga FJ, Danielson GK, Dearani JA, Mair DD, Hagler DJ, Julsrud PR, Ilstrup DM. Early and long-term results of the surgical treatment of tetralogy of Fallot with pulmonary atresia, with or without major aortopulmonary collateral arteries. The Journal of thoracic and cardiovascular surgery. 2002 Jul:124(1):70-81 [PubMed PMID: 12091811]
Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ, Schleck CD, Ilstrup DM. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. The Annals of thoracic surgery. 2003 Feb:75(2):399-410; discussion 410-1 [PubMed PMID: 12607647]
Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M, Ando M, Takao A. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. The Journal of thoracic and cardiovascular surgery. 1984 Oct:88(4):610-9 [PubMed PMID: 6482493]
Level 2 (mid-level) evidenceSpillmann R, Polentarutti S, Ehrler M, Kretschmar O, Wehrle FM, Latal B. Congenital heart disease in school-aged children: Cognition, education, and participation in leisure activities. Pediatric research. 2023 Oct:94(4):1523-1529. doi: 10.1038/s41390-021-01853-4. Epub 2021 Dec 1 [PubMed PMID: 34853428]