Introduction
Acute flaccid myelitis (AFM) is a rare disabling condition that primarily targets the anterior horns of the spinal cord and, less commonly, the gray matter of the brainstem. This condition occurs mostly in patients younger than 21 years and, occasionally, in immunocompromised adults.[1][2] The first AFM case was reported in California in 2012. AFM is now recognized worldwide.[3][4][5][6][7][8][9][10][11][12][13][14][15] AFM cases commonly emerge in geographical clusters marked by a seasonal biennial pattern.[16] The exact cause of AFM is not fully understood, but the link to certain viruses (eg, enterovirus D68, enterovirus A71, West Nile virus, adenovirus) has been observed.[17][18][19]
AFM clinically presents with acute asymmetric lower motor neuron paralysis with spared sensation. The involvement of oculomotor, facial palsy, and/or bulbar musculature is observed on rare occasions. The onset of symptoms in most patients follows after a 1- to 10-day period of upper respiratory tract infection. The classic symptoms of AFM comprise muscle weakness in the arms and/or legs, decreased or loss of muscle tone, and decreased or absent reflexes. In severe cases, respiratory insufficiency due to paralysis of respiratory muscles requires intubation and mechanical ventilation.
AFM is diagnosed based on a combination of a distinctive clinical presentation and diagnostic tests. Cerebrospinal fluid (CSF) analysis findings demonstrate lymphocytic pleocytosis and elevated protein levels.[2] The radiological hallmark of AFM includes focal lesions of the anterior horns of the spinal cord.[2] The long-term prognosis for AFM is still being studied. Rehabilitation, physical therapy, and psychological support play an indispensable role in maximizing recovery for patients with AFM. Vigilant surveillance and reporting of suspected cases are essential for better understanding the epidemiology and pathogenesis of AFM, thereby guiding public health interventions. Collective efforts to combat AFM and mitigate its impact on affected individuals and communities are enhanced by fostering collaboration and knowledge-sharing among healthcare professionals, researchers, and public health officials.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The exact cause of AFM remains elusive. AFM has been associated with various viral infections, particularly non-polio enteroviruses D68 and A71. Other viruses like adenovirus and West Nile virus have also been implicated.[17][18][19] Evidence supporting the causative role of enterovirus D68 in AFM in animal models includes loss of spinal cord motor neurons in affected limbs, virus detection in motor neurons of the spinal cord, and development of paralysis by injecting the virus-containing serum from affected mice into healthy ones, fulfilling Koch postulates, and preventing AFM by administering serum with enterovirus D68 antibodies from previously infected mice.[20] Further research is needed to address limitations and strengthen the evidence base.
Epidemiology
AFM predominantly occurs in patients younger than 21, with a slight predominance in those of the male sex. AFM affects individuals without underlying conditions and, occasionally, those who are immunocompromised and patients with asthma.[21][22][23] No common environmental or travel-related exposures were detected. No ethnic or racial predispositions were identified.[24]
AFM has gained increased attention due to several outbreaks in 2014, 2016, 2018, and 2020; a biennial seasonal pattern has been observed, with peaks in late summer and early fall (see Image. Histogram of Acute Flaccid Myelitis Cases).[25] The precise mechanisms of this phenomenon are not fully understood. This bi-yearly pattern is assumed to reflect a decrease in herd immunity and increased susceptibility to viruses.[26]
Pathophysiology
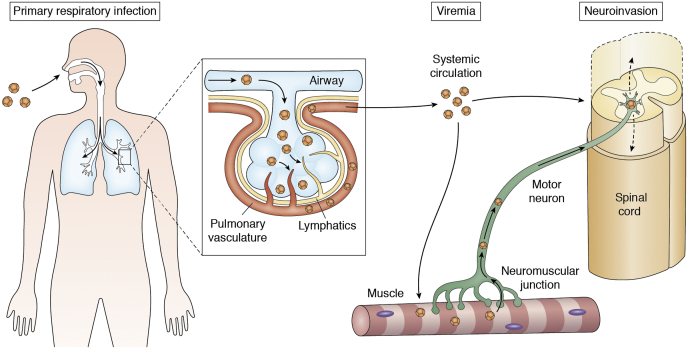
The pathophysiology of AFM involves a complex interplay of viral infections and immune responses that primarily target the spinal cord's anterior horn cells. Enteroviruses, especially enterovirus D68, are frequently implicated, suggesting a viral etiology. The infection triggers an inflammatory response, leading to neuronal damage and subsequent muscle weakness or paralysis. The destruction of motor neurons disrupts the transmission of nerve signals to muscles, resulting in the characteristic flaccid paralysis (see Image. Hypothesized Mechanisms of Neuroinvasion of Enterovirus D68). This damage can be extensive and irreversible, leading to varying degrees of long-term disability. Understanding the precise mechanisms of viral invasion and immune-mediated injury remains a focus of ongoing research, crucial for developing effective treatments and preventive measures.
History and Physical
A prodrome with fever and respiratory symptoms precedes AFM in most cases.[25] Gastrointestinal symptoms have also been reported.[27] The development of a neurological deficit usually emerges closely after the complete resolution of viral prodrome. The onset of symptoms can be associated with meningism, headache, fever recurrence, pain in the affected limbs, or lower back pain. AFM typically progresses rapidly over a few hours or days.
Symptoms of AFM include muscle weakness, loss or decreased muscle tone, hyporeflexia, respiratory failure, and oculomotor, facial, and/or bulbar muscle weakness.[24][28][4] Muscle weakness is prone to affect proximal muscles of the upper extremities and be asymmetric with differences up to 2 or more scores by the Medical Research Council (MRC) for muscle strength scale. The severity of paresis varies and can be severe enough to cause respiratory failure. Occasionally, motor cranial nerve deficits, neuropathic pain, and mild nuchal rigidity may be present. The presence of encephalopathy and sensory deficit are inconsistent with the diagnosis of AFM.[1]
Evaluation
The clinical diagnosis of AFM relies on clinical, imaging, and laboratory criteria and is divided into different levels of certainty (see Table. Diagnostic Criteria for Acute Flaccid Myelitis).[2] Blood, nasopharyngeal, oropharyngeal, CSF, and stool samples should be obtained without delay for laboratory evaluation. Detection of enterovirus D68, A71, or other viruses via reverse transcriptase-polymerase chain reaction testing is more likely to be positive early in the clinical course. The viral identification for enterovirus D68 is evident in respiratory samples and for enterovirus A71 in rectal or stool samples.[1] Changes in the CSF parameters in patients with AFM include lymphocytic pleocytosis and mild elevation of protein level.
A contrast magnetic resonance imaging (MRI) of the spinal cord typically demonstrates an extensive contrast-negative T2 hyperintense lesion of the anterior horns of the spinal cord, spreading to one or more vertebral segments.[7] In the vast majority of patients, signal abnormalities are observed throughout the entire length of the spinal cord (CDC. Acute flaccid myelitis). In the acute phase, perifocal edema can be observed. Most AFM patients display electrophysiological characteristics resembling those in either pure motor neuronopathy or axonal neuropathy.[29]
Table. Diagnostic Criteria for Acute Flaccid Myelitis
| Diagnostic Items | Definite | Probable | Possible | Uncertain |
| Acute onset of limb(s) weakness (period from onset to nadir: hours to 10 days) | P | P | P | P |
| Prodromal fever or illness | P/A | P/A | P/A | P |
| Weakness involving 1 or more limbs, neck, face, or cranial nerves | P | P | P | P |
| Decreased muscle tone in at least 1 weak limb | P | P | P/A | P |
| Decreased or absent deep tendon reflexes in at least 1 weak limb | P | P | P/A | P |
| MRI: spinal cord lesion with predominant gray matter involvement, with or without nerve root enhancement | P | P | P | NP |
| CSF: pleocytosis (white cell count > 5 cells/L) | P | A or NP | P/A or NP | P/A or NP |
A, diagnostic feature is absent; CSF, cerebral spinal fluid; MRI, magnetic resonance imaging; ND, testing was not performed; P, diagnostic parameter is present; P/A, presence of this diagnostic item is supportive but not required; ND, testing was not performed
Clinical characteristics favoring an alternative diagnosis include encephalopathy unexplained by fever, illness, respiratory distress, metabolic abnormalities, or medications; paralysis primarily affecting the legs and predominantly distal; hyperreflexia; spasticity; and the presence of a sensory level.[2] Adjunctive tests should be performed to exclude possible infectious, neoplastic, vascular, metabolic, and demyelinating disorders. Viral stool culture for poliovirus is indicated in some regions.[1] Serological testing for specific antibodies to myelin oligodendrocyte glycoprotein (MOG)-IgG and aquaporin-4 are necessary to identify potentially treatable MOG antibody disease (MOGAD) and neuromyelitis-optica spectrum disorder (NMSOD), respectively.[1] Detection of anti-ganglioside antibodies in serum may support an alternative diagnosis of Guillain-Barré syndrome despite its incomplete specificity.[1] The presence of a low titer of antibodies to gangliosides occurs in some neuropathies and in AFM, which does not exclude the diagnosis of AFM.[1] Computed tomography (CT) spinal angiography should be obtained if there is suspicion of spontaneous spinal cord infarction.
Treatment / Management
According to the clinical guidance for the acute treatment of AFM provided by the Centers for Disease Control and Prevention, no drugs are approved by the Food and Drug Administration for preventing or treating AFM. Possible management considerations, such as high-dose steroids, intravenous immunoglobulin, plasma exchange, antiviral agents, interferon, fluoxetine, and rituximab, have not shown efficacy in AFM treatment. The treatment of AFM is mainly supportive, including cardiovascular support, autonomic and bowel disturbances management, pain control, thromboembolism, and pressure ulcer prophylaxis. Nerve transfer surgery is a promising option for patients with acute flaccid myelitis with incomplete functional recovery.[30]
Differential Diagnosis
When evaluating a patient with symptoms suggestive of AFM, it is crucial to consider a broad differential diagnosis. Several other conditions can mimic AFM; distinguishing these is essential for accurate diagnosis and appropriate treatment. This section will explore key alternative diagnoses and their distinguishing features.
Guillain-Barré syndrome
Guillain-Barré syndrome (GBS) should be considered in the differential diagnosis of AFM. GBS typically presents with a length-dependent ascending pattern of progressive limb weakness over several days or weeks. This condition exhibits a symmetrical progression of weakness, sensory deficits, involvement of cranial nerves, especially bilateral facial muscle weakness, autonomic dysfunction, and neuropathic pain. Two-thirds of adult patients experience symptoms of a respiratory or gastrointestinal tract infection preceding the onset of weakness.
CSF analysis typically reveals albuminocytological dissociation. Contrast enhancement of anterior spinal nerve roots is suggestive of GBS.[31] Nerve conduction studies in patients with GBS indicate decreased conduction velocities, reduced sensory and motor-evoked amplitudes, and partial motor conduction blocks.[32]
Poliomyelitis
Poliomyelitis is caused by a highly infectious enterovirus with the main reservoir of infection in the human digestive tract.[33] The routes of infection are oro-oral and feco-oral. The highest degree of infectivity occurs in families with small children and can reach 100%. The virus can be isolated from the nasopharynx within 5 days and from stool up to 5 weeks after the onset of symptoms.
In the prodromal phase of the disease, patients experience high fever, pharyngitis, nausea and vomiting, myalgia, as well as headache, and nuchal rigidity. In non-paralytic disease, symptoms usually resolve within 1 or 2 weeks. An estimated 95% of all infections are asymptomatic or self-limiting influenza-like illnesses. In paralytic poliomyelitis, most patients develop a spinal form of poliomyelitis, in which severe muscle pain occurs, often with cramps, and then weakness and fasciculations develop. Weakness is usually asymmetrical, predominantly affecting the lower extremities, while sensation is spared. The maximum degree of paralysis develops within 48 hours, especially in children. CSF examination shows increased protein and pleocytosis (neutrophils in the first few days, then lymphocytes) with normal sugar levels.[33] Poliomyelitis is under control nowadays due to worldwide vaccination.
Botulism
Botulism is a rare, life-threatening disease caused by Clostridium botulinum.[34] Patients with botulism usually present with a descending pattern of paralysis with cranial nerve involvement, respiratory muscle paralysis, and distinctive dysautonomia with prior gastrointestinal symptoms. Possible mechanisms of botulism infection are ingestion of toxin-contaminated food, wound and intestine bacterial contamination, high-concentration cosmetic or therapeutic injections, and bioterrorism.[34] Treatment includes supportive care, mechanical ventilation if needed, and administration of botulinum antitoxin.[34]
Acute demyelinating encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is an immune-mediated disease affecting the white matter of the central nervous system.[35] This condition can occur at any age but tends to develop in early childhood. ADEM typically manifests between 2 days to 4 weeks after an antigenic attack. Approximately 70% to 77% of patients report a prior infection or vaccination within the preceding few weeks.
ADEM is often preceded by prodromal symptoms such as fever, malaise, irritability, drowsiness, headache, nausea, and vomiting. Typical symptoms of ADEM include the sudden onset of encephalopathy, accompanied by a multifocal neurological deficit. The clinical course is typically monophasic and rapidly progressive, with maximum deficits occurring within 2 to 5 days.[35] Neurological signs may include pyramidal signs, ataxia, acute hemiparesis, optic neuritis or other cranial nerve deficits, seizures, spinal cord syndrome, and speech impairment. Seizures can progress to status epilepticus. Rarely, respiratory failure may occur if the brainstem is involved.
MRI findings in ADEM typically show multiple T2 hyperintense lesions that are bilateral, asymmetric, heterogeneous, and poorly demarcated, often with perifocal edema. The cortical and subcortical white matter, cortical gray-white matter junction, thalamus, basal ganglia, cerebellum, and brainstem are typically involved. In about one-third of patients, spinal cord involvement is described, with large confluent lesions extending over several segments and associated edema. Contrast enhancement is detected in about 30% of patients.[35]
Neuromyelitis-optica spectrum disorder
A classical clinical manifestation of neuromyelitis-optica spectrum disorder (NMOSD) is a combination of optic neuritis and transverse myelitis. Symptoms may include vision loss in 1 or both eyes, eye pain, upper motor neuron muscle weakness, spasticity, hypesthesia or anesthesia with a sensory level, imperative frequent urge to urinate or urinary retention, and sudden shock-like pain in the neck and back.
NMOSD typically follows a relapsing-remitting pattern, with relapses progressing over days to weeks. Lesions in the optic nerves, brainstem, corpus callosum, and spinal cord typically show contrast enhancement on the MRI due to blood-brain barrier disruption.[36] The highly specific diagnostic marker, serum anti-aquaporin-4 antibodies, is included in the diagnostic criteria for NMOSD.[36]
Spontaneous spinal cord infarction
The clinical manifestation of spinal cord infarction (SCI) usually occurs suddenly with rapid progression over minutes to hours.[37] Neurological deficits depend on the location of the infarction and the degree of arterial occlusion. Most often, SCI is observed in the anterior spinal artery and manifests itself in the form of symmetric motor paralysis, loss of pain, temperature and tactile sensitivity below the level of the lesion, gross autonomic dysfunction, and bowel organ dysfunction.[37] Further spasticity and hyperreflexia are characteristic.[37] Occlusion of the vessels supplying blood to the segments of the spinal cord at the level of C3 to C5 leads to respiratory dysfunction.[37]
Prognosis
The prognosis of AFM varies widely from complete recovery of function to lethal outcomes among affected individuals. While some patients experience significant recovery, most have persistent and severe neurological deficits.[26] Early and aggressive rehabilitation can improve outcomes, but the extent of recovery is often limited by the degree of initial neuronal damage.
Factors influencing prognosis include the severity and rapidity of symptom onset, the specific areas of the spinal cord involved, and the patient's age and overall health. Long-term follow-up is typically required, as residual weakness, paralysis, and other complications can persist, necessitating ongoing medical and supportive care. Advances in understanding and treating AFM are essential to improving the long-term outlook for affected patients.
Complications
Complications in patients with AFM can be grouped into neurological, musculoskeletal, and psychological categories and include the following:
- Neurological consequences: respiratory impairment leading to ventilator assistance, artificial nutrition and hydration dependence, neuropathic pain, and chronic constipation
- Musculoskeletal complications: muscle atrophy, joint contractures, chest deformities, limb length discrepancies, dislocations, subluxations of joints, and scoliosis
- Psychological complications: anxiety and depression [38][39][40]
Consultations
Patients with AFM require a multidisciplinary approach, necessitating consultations with various specialists to optimize care and improve outcomes. Neurologists play a central role in diagnosing and managing AFM, providing expertise in neuroimaging, electrophysiological studies, and therapeutic strategies. Infectious disease specialists are essential for identifying and managing potential viral etiologies and advising on appropriate antiviral treatments or vaccinations. Physical and occupational therapists are crucial for developing and implementing rehabilitation plans to enhance mobility and function, aiming to minimize long-term disability.
Additionally, pulmonologists may be needed for patients experiencing respiratory involvement, ensuring adequate respiratory support and management. Psychologists or psychiatrists can provide support for patients and families coping with the emotional and psychological impact of AFM. Coordination with primary care providers is also vital to ensure continuity of care, monitor overall health, and address any comorbid conditions
Deterrence and Patient Education
Deterrence and patient education play pivotal roles in managing AFM. Educating patients and caregivers about preventive measures is essential for reducing the risk of infection and subsequent AFM development. Key strategies include frequent handwashing with soap and water, covering the mouth and nose when coughing or sneezing, and avoiding close contact with individuals exhibiting respiratory symptoms. Regularly disinfecting frequently touched surfaces further minimizes viral transmission. Emphasizing the importance of up-to-date vaccinations can help prevent viruses linked to AFM.
Parents should be aware of AFM signs, such as sudden limb weakness or facial drooping, to ensure they seek prompt medical attention. Immediate medical evaluation is critical for early intervention and improved outcomes. Providing resources, specialist connections, and information on support organizations can aid families in navigating AFM challenges. Additionally, educating on mosquito bite prevention is vital, as some AFM cases are associated with mosquito-borne viruses like West Nile.
Pearls and Other Issues
Understanding AFM requires familiarity with key clinical pearls to aid in early recognition, accurate diagnosis, and effective management. These pearls encompass essential aspects such as diagnostic criteria, therapeutic approaches, and long-term care strategies that collectively enhance patient outcomes and care quality and include the following:
-
Early recognition: Timely identification of sudden onset limb weakness or paralysis, particularly in children, is critical for early intervention.
-
Distinguishing features: Look for asymmetrical weakness and consider AFM in the differential diagnosis when respiratory illness or fever precedes neurological symptoms.
-
Diagnostic imaging: MRI of the spinal cord is a key diagnostic tool, often revealing lesions in the gray matter of the spinal cord.
-
Cerebrospinal fluid analysis: CSF typically shows pleocytosis, helping differentiate AFM from other conditions with similar presentations.
-
Viral testing: Investigate potential viral etiologies, especially enteroviruses, using polymerase chain reaction testing of respiratory and stool samples.
-
Multidisciplinary approach: Involve neurologists, infectious disease specialists, and rehabilitation experts early in the management process.
-
Acute management: Consider early use of intravenous immunoglobulin or corticosteroids, although their efficacy is not well-established and remains under investigation.
-
Rehabilitation: Intensive physical and occupational therapy is essential to maximize functional recovery and minimize long-term disability.
-
Respiratory monitoring: Monitor for respiratory complications, as diaphragmatic weakness may require ventilatory support.
-
Long-term follow-up: Regular follow-up is necessary to manage ongoing neurological deficits and adjust rehabilitation strategies.
-
Patient and family education: Educate families on the importance of early symptom recognition and prompt medical evaluation to improve outcomes.
-
Preventive measures: Encourage good hygiene practices, such as frequent handwashing, and have up-to-date vaccinations to reduce the risk of infections linked to AFM.
-
Research participation: Encourage eligible patients to participate in clinical studies to help advance understanding and treatment of AFM.
-
Support networks: Connect families with support organizations and resources to assist with the emotional and practical challenges of managing AFM.
Enhancing Healthcare Team Outcomes
AFM is a rare, disabling condition that requires a collaborative interprofessional approach among healthcare professionals.[41] Emergency medicine physicians, pediatricians, primary care physicians, and advanced care practitioners play a crucial role in recognizing the initial symptoms and promptly referring patients to specialized care to mitigate long-term complications. Nurses play a critical role in monitoring patient status, providing supportive care, and educating families about the disease and care procedures. Pharmacists ensure appropriate medication management, monitor for potential drug interactions, and provide guidance on drug therapy.
Case managers and social workers can assist in coordinating appointments, therapy sessions, and community resources. Seamless coordination among clinicians is vital for managing the complex care needs of patients with AFM, including physical therapy, occupational therapy, and long-term rehabilitation. The interprofessional healthcare team educates patients and caregivers about AFM, including its symptoms, potential complications, and strategies for managing the condition. This education empowers patients and caregivers to actively participate in their care and enhance compliance with the treatment. Effective communication among team members is critical in the patient's pathway from diagnosis through treatment and rehabilitation.
By focusing on patient-centered care, enhancing communication, and continuously improving care processes, healthcare professionals can significantly improve outcomes and safety for patients with AFM. Advocating for collaborative research initiatives and data sharing is crucial to uncovering the underlying mechanisms of AFM and identifying preventive measures. These innovations will lead to more targeted and personalized care, ultimately improving the prognosis for those affected by AFM.
Media
(Click Image to Enlarge)
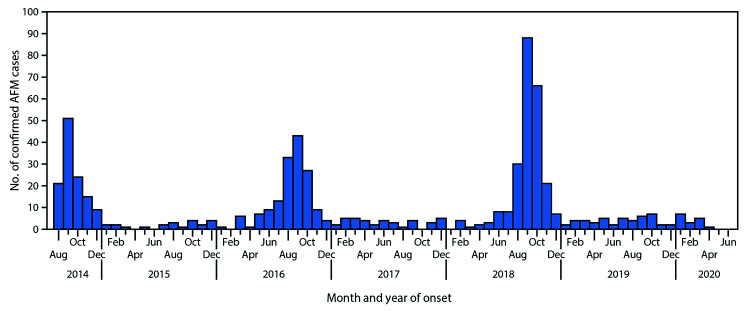
Histogram of Acute Flaccid Myelitis Cases. According to the Centers for Disease Control, the histogram shows 633 registered acute flaccid myelitis (AFM) cases in the United States. Outbreaks of AFM occurred every 2 years, with the first clusters reported in 2014.
MMWR Series, Centers for Disease Control and Prevention
(Click Image to Enlarge)
References
Murphy OC, Messacar K, Benson L, Bove R, Carpenter JL, Crawford T, Dean J, DeBiasi R, Desai J, Elrick MJ, Farias-Moeller R, Gombolay GY, Greenberg B, Harmelink M, Hong S, Hopkins SE, Oleszek J, Otten C, Sadowsky CL, Schreiner TL, Thakur KT, Van Haren K, Carballo CM, Chong PF, Fall A, Gowda VK, Helfferich J, Kira R, Lim M, Lopez EL, Wells EM, Yeh EA, Pardo CA, AFM working group. Acute flaccid myelitis: cause, diagnosis, and management. Lancet (London, England). 2021 Jan 23:397(10271):334-346. doi: 10.1016/S0140-6736(20)32723-9. Epub 2020 Dec 23 [PubMed PMID: 33357469]
Helfferich J, Neuteboom RF, de Lange MMA, Benschop KSM, Van Leer-Buter CC, Meijer A, Bakker DP, de Bie E, Braakman HMH, Brandsma R, Niks EH, Niermeijer JM, Roelfsema V, Schoenmaker N, Sie LT, Niesters HG, Te Wierik MJM, Jacobs BC, Brouwer OF. Pediatric acute flaccid myelitis: Evaluation of diagnostic criteria and differentiation from other causes of acute flaccid paralysis. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2023 May:44():28-36. doi: 10.1016/j.ejpn.2023.03.002. Epub 2023 Mar 22 [PubMed PMID: 36996587]
Van Haren K, Ayscue P, Waubant E, Clayton A, Sheriff H, Yagi S, Glenn-Finer R, Padilla T, Strober JB, Aldrovandi G, Wadford DA, Chiu CY, Xia D, Harriman K, Watt JP, Glaser CA. Acute Flaccid Myelitis of Unknown Etiology in California, 2012-2015. JAMA. 2015 Dec 22-29:314(24):2663-71. doi: 10.1001/jama.2015.17275. Epub [PubMed PMID: 26720027]
Messacar K, Schreiner TL, Maloney JA, Wallace A, Ludke J, Oberste MS, Nix WA, Robinson CC, Glodé MP, Abzug MJ, Dominguez SR. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet (London, England). 2015 Apr 25:385(9978):1662-71. doi: 10.1016/S0140-6736(14)62457-0. Epub 2015 Jan 29 [PubMed PMID: 25638662]
Knoester M, Helfferich J, Poelman R, Van Leer-Buter C, Brouwer OF, Niesters HGM, 2016 EV-D68 AFM Working Group. Twenty-nine Cases of Enterovirus-D68-associated Acute Flaccid Myelitis in Europe 2016: A Case Series and Epidemiologic Overview. The Pediatric infectious disease journal. 2019 Jan:38(1):16-21. doi: 10.1097/INF.0000000000002188. Epub [PubMed PMID: 30234793]
Level 2 (mid-level) evidenceUnited Kingdom Acute Flaccid Paralysis (AFP) Task Force. An increase in reports of acute flaccid paralysis (AFP) in the United Kingdom, 1 January 2018-21 January 2019: early findings. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2019 Feb:24(6):. doi: 10.2807/1560-7917.ES.2019.24.6.1900093. Epub [PubMed PMID: 30755296]
Chong PF, Kira R, Mori H, Okumura A, Torisu H, Yasumoto S, Shimizu H, Fujimoto T, Hanaoka N, Kusunoki S, Takahashi T, Oishi K, Tanaka-Taya K, Acute Flaccid Myelitis Collaborative Study Investigators. Clinical Features of Acute Flaccid Myelitis Temporally Associated With an Enterovirus D68 Outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August-December 2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Feb 10:66(5):653-664. doi: 10.1093/cid/cix860. Epub [PubMed PMID: 29028962]
Level 3 (low-level) evidenceSarmast SN, Gowda VK, Ahmed M, Gv B, Saini J, Benakappa A. Acute flaccid myelitis-Clustering of polio-like illness in the tertiary care centre in Southern India. Journal of tropical pediatrics. 2019 Aug 1:65(4):309-314. doi: 10.1093/tropej/fmy052. Epub [PubMed PMID: 30169722]
Chen IJ, Hu SC, Hung KL, Lo CW. Acute flaccid myelitis associated with enterovirus D68 infection: A case report. Medicine. 2018 Sep:97(36):e11831. doi: 10.1097/MD.0000000000011831. Epub [PubMed PMID: 30200066]
Level 3 (low-level) evidenceAndersen EW, Kornberg AJ, Freeman JL, Leventer RJ, Ryan MM. Acute flaccid myelitis in childhood: a retrospective cohort study. European journal of neurology. 2017 Aug:24(8):1077-1083. doi: 10.1111/ene.13345. Epub 2017 Jun 22 [PubMed PMID: 28639345]
Level 2 (mid-level) evidenceFall A, Ndiaye N, Jallow MM, Barry MA, Touré CSB, Kebe O, Kiori DE, Sy S, Dia M, Goudiaby D, Ndiaye K, Niang MN, Dia N. Enterovirus D68 Subclade B3 Circulation in Senegal, 2016: Detection from Influenza-like Illness and Acute Flaccid Paralysis Surveillance. Scientific reports. 2019 Sep 25:9(1):13881. doi: 10.1038/s41598-019-50470-z. Epub 2019 Sep 25 [PubMed PMID: 31554908]
Crone M, Tellier R, Wei XC, Kuhn S, Vanderkooi OG, Kim J, Mah JK, Mineyko A. Polio-Like Illness Associated With Outbreak of Upper Respiratory Tract Infection in Children. Journal of child neurology. 2016 Mar:31(4):409-14. doi: 10.1177/0883073815596613. Epub 2015 Jul 27 [PubMed PMID: 26215391]
Ruggieri V, Paz MI, Peretti MG, Rugilo C, Bologna R, Freire C, Vergel S, Savransky A. Enterovirus D68 infection in a cluster of children with acute flaccid myelitis, Buenos Aires, Argentina, 2016. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2017 Nov:21(6):884-890. doi: 10.1016/j.ejpn.2017.07.008. Epub 2017 Jul 19 [PubMed PMID: 28747261]
Carballo CM, Erro MG, Sordelli N, Vazquez G, Mistchenko AS, Cejas C, Rodriguez M, Cisterna DM, Freire MC, Contrini MM, Lopez EL. Acute Flaccid Myelitis Associated with Enterovirus D68 in Children, Argentina, 2016. Emerging infectious diseases. 2019 Mar:25(3):573-576. doi: 10.3201/eid2503.170897. Epub 2019 Mar 17 [PubMed PMID: 30602120]
Rodesch M, Sculier C, Lolli V, Remiche G, Delpire I, Fricx C, Vermeulen F, Christiaens F. A First Case of Acute Flaccid Myelitis Related to Enterovirus D68 in Belgium: Case Report. Case reports in neurology. 2024 Jan-Dec:16(1):41-47. doi: 10.1159/000535316. Epub 2024 Jan 31 [PubMed PMID: 38405019]
Level 3 (low-level) evidenceMcLaren N, Lopez A, Kidd S, Zhang JX, Nix WA, Link-Gelles R, Lee A, Routh JA. Characteristics of Patients with Acute Flaccid Myelitis, United States, 2015-2018. Emerging infectious diseases. 2020 Feb:26(2):212-9. doi: 10.3201/eid2602.191453. Epub [PubMed PMID: 31961305]
Sejvar JJ, Lopez AS, Cortese MM, Leshem E, Pastula DM, Miller L, Glaser C, Kambhampati A, Shioda K, Aliabadi N, Fischer M, Gregoricus N, Lanciotti R, Nix WA, Sakthivel SK, Schmid DS, Seward JF, Tong S, Oberste MS, Pallansch M, Feikin D. Acute Flaccid Myelitis in the United States, August-December 2014: Results of Nationwide Surveillance. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Sep 15:63(6):737-745. doi: 10.1093/cid/ciw372. Epub 2016 Jun 17 [PubMed PMID: 27318332]
Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Ewing D, Mazowiecki M, Pavot PV, Schmitt J, Pape J, Biggerstaff BJ, Petersen LR. West Nile virus-associated flaccid paralysis. Emerging infectious diseases. 2005 Jul:11(7):1021-7 [PubMed PMID: 16022775]
Bitnun A, Yeh EA. Acute Flaccid Paralysis and Enteroviral Infections. Current infectious disease reports. 2018 Jun 29:20(9):34. doi: 10.1007/s11908-018-0641-x. Epub 2018 Jun 29 [PubMed PMID: 29959591]
Hixon AM, Yu G, Leser JS, Yagi S, Clarke P, Chiu CY, Tyler KL. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS pathogens. 2017 Feb:13(2):e1006199. doi: 10.1371/journal.ppat.1006199. Epub 2017 Feb 23 [PubMed PMID: 28231269]
Wali RK, Lee AH, Kam JC, Jonsson J, Thatcher A, Poretz D, Ambardar S, Piper J, Lynch C, Kulkarni S, Cochran J, Djurkovic S. Acute Neurological Illness in a Kidney Transplant Recipient Following Infection With Enterovirus-D68: An Emerging Infection? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Dec:15(12):3224-8. doi: 10.1111/ajt.13398. Epub 2015 Jul 30 [PubMed PMID: 26228743]
Mauri E, Mastrangelo A, Testa S, Pellegrinelli L, Pariani E, Binda S, Triulzi F, Barbieri S, Bana C, Montini G, Dilena R. Acute flaccid paralysis due to Echovirus 30 in an immunosuppressed transplant recipient. Journal of neurovirology. 2020 Apr:26(2):284-288. doi: 10.1007/s13365-019-00812-4. Epub 2019 Oct 22 [PubMed PMID: 31642013]
Division of Viral Diseases, National Centers for Immunization and Respiratory Diseases, CDC; Division of Vector-Borne Diseases, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Children's Hospital Colorado; Council of State and Territorial Epidemiologists. Notes from the field: acute flaccid myelitis among persons aged ≤21 years - United States, August 1-November 13, 2014. MMWR. Morbidity and mortality weekly report. 2015 Jan 9:63(53):1243-4 [PubMed PMID: 25577990]
Messacar K, Schreiner TL, Van Haren K, Yang M, Glaser CA, Tyler KL, Dominguez SR. Acute flaccid myelitis: A clinical review of US cases 2012-2015. Annals of neurology. 2016 Sep:80(3):326-38. doi: 10.1002/ana.24730. Epub 2016 Aug 4 [PubMed PMID: 27422805]
Level 3 (low-level) evidenceKidd S, Lopez A, Nix WA, Anyalechi G, Itoh M, Yee E, Oberste MS, Routh J. Vital Signs: Clinical Characteristics of Patients with Confirmed Acute Flaccid Myelitis, United States, 2018. MMWR. Morbidity and mortality weekly report. 2020 Aug 7:69(31):1031-1038. doi: 10.15585/mmwr.mm6931e3. Epub 2020 Aug 7 [PubMed PMID: 32759919]
Dinov D, Donowitz JR. Acute flaccid myelitis a review of the literature. Frontiers in neurology. 2022:13():1034607. doi: 10.3389/fneur.2022.1034607. Epub 2022 Dec 20 [PubMed PMID: 36605787]
Ayers T, Lopez A, Lee A, Kambhampati A, Nix WA, Henderson E, Rogers S, Weldon WC, Oberste MS, Sejvar J, Hopkins SE, Pallansch MA, Routh JA, Patel M. Acute Flaccid Myelitis in the United States: 2015-2017. Pediatrics. 2019 Nov:144(5):. pii: e20191619. doi: 10.1542/peds.2019-1619. Epub 2019 Oct 7 [PubMed PMID: 31591135]
Helfferich J, Knoester M, Van Leer-Buter CC, Neuteboom RF, Meiners LC, Niesters HG, Brouwer OF. Acute flaccid myelitis and enterovirus D68: lessons from the past and present. European journal of pediatrics. 2019 Sep:178(9):1305-1315. doi: 10.1007/s00431-019-03435-3. Epub 2019 Jul 23 [PubMed PMID: 31338675]
Chong PF, Torisu H, Yasumoto S, Okumura A, Mori H, Sato T, Kimura J, Ohga S, Tanaka-Taya K, Kira R, Acute Flaccid Myelitis Collaborative Study Investigators. Clinical and electrophysiological features of acute flaccid myelitis: A national cohort study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2021 Oct:132(10):2456-2463. doi: 10.1016/j.clinph.2021.07.013. Epub 2021 Jul 27 [PubMed PMID: 34454273]
Rivera GS, Stokum JA, Dean J, Sadowsky CL, Belzberg AJ, Elrick MJ. Nerve Transfer Surgery in Acute Flaccid Myelitis: Prognostic Factors, Long-Term Outcomes, Comparison With Natural History. Pediatric neurology. 2024 Jan:150():74-81. doi: 10.1016/j.pediatrneurol.2023.10.019. Epub 2023 Oct 31 [PubMed PMID: 37981447]
Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet (London, England). 2016 Aug 13:388(10045):717-27. doi: 10.1016/S0140-6736(16)00339-1. Epub 2016 Mar 2 [PubMed PMID: 26948435]
Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, van Doorn PA, Dourado ME, Hughes RAC, Islam B, Kusunoki S, Pardo CA, Reisin R, Sejvar JJ, Shahrizaila N, Soares C, Umapathi T, Wang Y, Yiu EM, Willison HJ, Jacobs BC. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nature reviews. Neurology. 2019 Nov:15(11):671-683. doi: 10.1038/s41582-019-0250-9. Epub 2019 Sep 20 [PubMed PMID: 31541214]
Kidd D, Williams AJ, Howard RS. Poliomyelitis. Postgraduate medical journal. 1996 Nov:72(853):641-7 [PubMed PMID: 8944203]
Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2021 May 7:70(2):1-30. doi: 10.15585/mmwr.rr7002a1. Epub 2021 May 7 [PubMed PMID: 33956777]
Wang CX. Assessment and Management of Acute Disseminated Encephalomyelitis (ADEM) in the Pediatric Patient. Paediatric drugs. 2021 May:23(3):213-221. doi: 10.1007/s40272-021-00441-7. Epub 2021 Apr 8 [PubMed PMID: 33830467]
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG, International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015 Jul 14:85(2):177-89. doi: 10.1212/WNL.0000000000001729. Epub 2015 Jun 19 [PubMed PMID: 26092914]
Level 3 (low-level) evidenceVargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO, Dietemann JL. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR. American journal of neuroradiology. 2015 May:36(5):825-30. doi: 10.3174/ajnr.A4118. Epub 2014 Oct 16 [PubMed PMID: 25324492]
Bove R, Rowles W, Carleton M, Olivera E, Sheehan M, Werdal HP, Scott R, Axton L, Benson L. Unmet Needs in the Evaluation, Treatment, and Recovery for 167 Children Affected by Acute Flaccid Myelitis Reported by Parents Through Social Media. Pediatric neurology. 2020 Jan:102():20-27. doi: 10.1016/j.pediatrneurol.2019.08.009. Epub 2019 Aug 24 [PubMed PMID: 31630913]
Melicosta ME, Dean J, Hagen K, Oppenheimer K, Porter C, Rybczynski S, Salorio C, Sadowsky C. Acute flaccid myelitis: Rehabilitation challenges and outcomes in a pediatric cohort. Journal of pediatric rehabilitation medicine. 2019:12(3):245-253. doi: 10.3233/PRM-180549. Epub [PubMed PMID: 31476175]
Martin JA, Messacar K, Yang ML, Maloney JA, Lindwall J, Carry T, Kenyon P, Sillau SH, Oleszek J, Tyler KL, Dominguez SR, Schreiner TL. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017 Jul 11:89(2):129-137. doi: 10.1212/WNL.0000000000004081. Epub 2017 Jun 14 [PubMed PMID: 28615421]
Vawter-Lee M, Peariso K, Frey M, Bolikal P, Schaffzin JK, Schwentker A, O'Brien WT Sr, Zamor R, Kerrey BT. Acute Flaccid Myelitis: A Multidisciplinary Protocol to Optimize Diagnosis and Evaluation. Journal of child neurology. 2021 May:36(6):421-431. doi: 10.1177/0883073820975230. Epub 2020 Dec 1 [PubMed PMID: 33258719]