Introduction
Extracorporeal membrane oxygenation (ECMO) is a specialized technique used primarily in critical care settings for patients experiencing severe cardiac and respiratory failure who remain unresponsive to conventional treatment methods. The ECMO circuit consists of several key components, including a centrifugal pump, an efficient oxygenator, and specialized tubing. Since its introduction in 1972 for adult patients, ECMO technology has undergone significant advancement with enhanced circuit components, improved cannulae, oxygenators, new centrifugal pumps, and compact, simplified systems, thereby increasing both efficacy and safety. Venous blood is extracted through cannulas inserted into major vessels, and transported through biocompatible tubing towards the oxygenator and heat exchanger. Gas exchange occurs through diffusion, and oxygenated blood is pumped back to the patient. The monitoring systems that track vital metrics such as pressure and flow are critical to the functionality of ECMO. The system is equipped with a gas blender for precise control of oxygen concentration and a flow meter to regulate the flow rate of the gas mixture accurately. Primarily, ECMO is a temporary mechanical support system for circulation and oxygenation, bridging to definitive therapeutic interventions or recovery (see Image. ECMO in an ICU Patient).[1]
The ECMO circuit initiates with a venous cannula for accessing the patient's blood, connected through biocompatible tubing to the inlet line of a centrifugal pump, which is an update from older roller pumps. Some centers use a reservoir before the pump to avert continuous suction risks during line occlusions, such as from kinking, hypovolemia, or coughing. The pump circulates blood continuously, operating at 2000 to 6000 rpm. However, concerns regarding hemolysis and blood stagnation are associated with the pump's mechanism. Blood then flows into the oxygenator, comprising efficient hollow fibers of polymethyl pentene for gas exchange. Attached to the oxygenator is a gas blender, typically set to 100% oxygen. The oxygenator includes a thin, gas-permeable polymer membrane, facilitating gas exchange between the blood and oxygen from the blender. The sweep rate, controlling this gas flow, is adjustable for optimal carbon dioxide removal. A heat exchanger in the oxygenator warms the blood to about 37 °C to enhance gas exchange efficiency. Once the patient's blood has undergone oxygenation, the blood is directed back to the patient through a return cannula, with the flow rate monitored using a flowmeter. The placement of the return cannula varies depending on the ECMO modality. The canula may be reinserted into a vein for veno-venous ECMO or an artery for venoarterial ECMO. Additional circuit components of the circuit include pressure and oxygen saturation monitors, air bubble detectors, temperature sensors, hemoconcentrators, and safety alarms. These readings are integrated into a central console, allowing precise ECMO adjustments for comprehensive monitoring. Meticulous management of the circuit is essential to ensure appropriate cardiac and respiratory support for the patient. Furthermore, monitoring of anticoagulation is crucial for maintaining a balance between clotting and bleeding.[2]
Clinical Significance
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Clinical Significance
Types of Extracorporeal Membrane Oxygenation
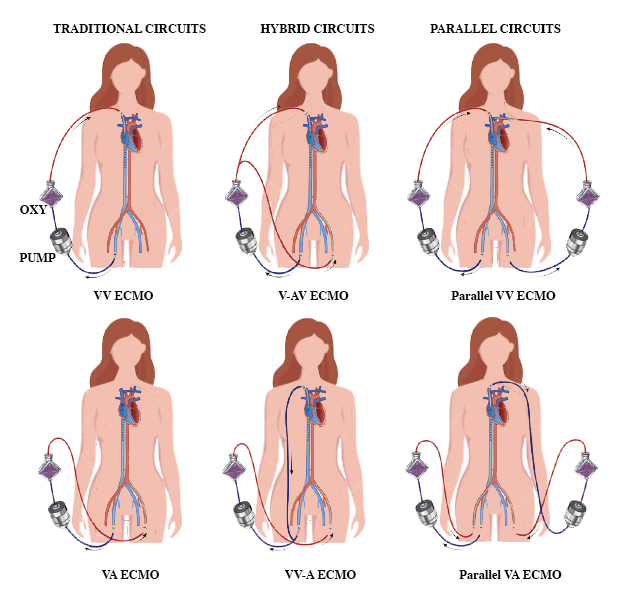
ECMO is primarily categorized into 2 types based on the method of circulation—veno-venous (VV) ECMO and venoarterial (VA) ECMO. Each type has a distinct purpose based on the patient's clinical condition (see Image. Types of ECMO Circuits).
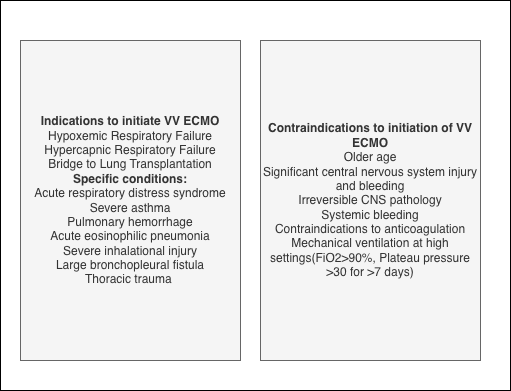
Veno-venous extracorporeal membrane oxygenation: This type is indicated for severe but potentially reversible respiratory failure encompassing conditions such as hypoxemia, hypercarbia, or a combination of both). VV ECMO facilitates oxygenation and the elimination of CO2, avoiding the need for harmful mechanical ventilation. Blood is withdrawn from a large vein, such as the femoral, jugular, or subclavian vein, oxygenated, and returned to another vein. The main indications include acute respiratory distress syndrome, severe pneumonia, aspiration, barotrauma, and interstitial pneumonitis. In some cases, such as end-stage pulmonary disease, ECMO can be utilized as a bridge to lung transplantation. Contraindications for VV ECMO are significant and must be carefully considered, which include central nervous system (CNS) hemorrhage, major CNS injuries or pathologies, systemic bleeding, contraindications to anticoagulation, severe immunosuppression, and prolonged mechanical ventilation under high settings that could have already caused extensive lung damage. In addition, older age is considered a relative contraindication, as the risk of death increases with age, although no specific age threshold is set. Veno-venous ECMO does not provide direct hemodynamic support but aids in gas exchange. Ventilator settings are selected to minimize ventilator-induced lung injury (see Image. Indications and Contraindications of VV ECMO).[3]
Venoarterial extracorporeal membrane oxygenation: VA ECMO is a critical intervention in treating severe cardiac conditions, often coexisting with respiratory failure. The primary role is in extracorporeal blood oxygenation, typically drawn from major veins, such as the femoral or jugular vein, and reinfused into an artery, such as the femoral, axillary or subclavian, or carotid artery. This advanced therapeutic intervention is pivotal in conditions such as cardiogenic shock, biventricular failure, and profound hypoxemia, especially when conventional therapies fail. This intervention is a bridge, sustaining end-organ perfusion and reducing inotrope-induced damage, while clinicians work toward a definitive solution for the underlying cardiac condition. Cannulation methods for venoarterial ECMO encompass both central and peripheral approaches.[4]
- Central cannulation: Central cannulation is often used in post-cardiotomy patients who cannot be weaned off cardiopulmonary bypass. The venous cannula is placed into the right atrium or a central vein, and the arterial cannula into the ascending aorta or another central arterial location. This method is ideal for direct cardiac and pulmonary support during surgeries or complex clinical encounters.
- Peripheral cannulation: Peripheral cannulation is initiated percutaneously or through surgical cut-down and involves inserting the venous cannula into a large peripheral vein, such as the femoral vein, and the arterial cannula into a peripheral artery, such as the femoral artery. Preferred for rapid initiation, peripheral cannulation is a key strategy in emergency settings or when central cannulation is impractical.
Indications and Contraindications
VV ECMO is indicated for a spectrum of severe cardiac conditions, including cardiogenic shock, biventricular heart failure, refractory cardiac arrest, postcardiotomy syndrome, massive pulmonary embolism, profound hypoxemia, and as a bridge to decision-making or device implantation. The role of VV ECMO in extracorporeal cardiopulmonary resuscitation and toxin-induced cardiomyopathies is also significant. However, contraindications such as uncontrollable bleeding, severe peripheral arterial disease, aortic dissection, severe aortic insufficiency, and other irreversible conditions must be carefully weighed against potential benefits in a multidisciplinary setting.
Hemodynamic Management
VV ECMO alters cardiac dynamics by reducing right heart volume and pressure while increasing left ventricular afterload, thus challenging poor contractility. Cannula sizes typically range from 19F to 25F for venous cannulas and 15F to 24F for arterial cannulas.[5] Anticoagulation, typically with unfractionated heparin monitored through anti-Xa assay, is essential to prevent clot formation. The target blood flow for patients with minimal cardiac function is 60 mL/kg/min. Adjustments are made based on the patient's cardiac function and metabolic demands. Maintaining lower flow rates of 3 to 4 L/min is often suitable, especially in patients who are obese with recovering cardiac function. ECMO flow rates must be adjusted based on blood lactate concentration, a marker for end-organ perfusion. Targeting a mean arterial pressure of 65 to 80 mm Hg is recommended for most patients. Higher mean arterial pressure may be needed for some patients, but it increases the risk of thrombosis. The oxygen concentration should not be reduced below 100% to prevent hypoxic blood delivery. Target arterial pH is achieved by adjusting the sweep gas flow rate. The aim should be to normalize the patient’s arterial pH, particularly using samples from the right radial artery.
Complications and Troubleshooting of Venoarterial Extracorporeal Membrane Oxygenation
- Low circuit blood flow: ECMO circuit tubing and cannulas should be checked for kinks and chattering. Chattering is due to excessive negative suction on the side of the venous cannula. Common causes of chattering include hypovolemia, patient straining, or high-peak airway pressures. For hypovolemia, reducing pump speed and administering a fluid bolus are recommended. Increasing sedation or considering neuromuscular blockade is advised if the patient is straining. Echocardiography might show right atrial involution in patients with hypovolemia. Other medical reasons for reduced venous return include intra-abdominal hypertension and cardiac tamponade.
- Oxygenator thrombosis: Oxygenator thrombosis is indicated by a pressure drop ≥ 100 mm Hg across the oxygenator, which should be replaced, if necessary, with a plan to support the patient's hemodynamics.
- Differential hypoxemia: Differential hypoxemia, also known as Harlequin syndrome or North-South Syndrome, is a unique phenomenon in peripheral VV ECMO, particularly affecting patients with compromised pulmonary function.[6] This condition arises when deoxygenated blood from the heart mixes with oxygenated ECMO blood in the descending thoracic aorta, leading to the upper body receiving deoxygenated blood and the lower body receiving oxygenated blood. This differential in oxygenation can result in complications such as myocardial or cerebral ischemia. Monitoring typically involves assessing arterial blood gases or Sp02 from the right upper extremity and utilizing cerebral oximetry. Patients show normal Sp02 in the lower extremities but less than 90% in the upper extremities, with cerebral tissue saturation lower compared to that in the leg. Management strategies include enhancing venous drainage, increasing ECMO pump speed, reducing left ventricular ejection, optimizing ventilation parameters, and considering a switch to veno-arteriovenous or central VV ECMO when refractory cases do not respond to medical treatment.
- Left ventricular distention: VV ECMO increases left ventricular end-diastolic pressure and volume, which exacerbates pulmonary edema, raises myocardial oxygen consumption, and elevates atrial pressure. Management strategies include enhancing venous drainage, providing inotropic support, administering diuretic therapy, and utilizing intra-aortic balloon pumps, which improve coronary blood flow but may have variable effects on pulmonary capillary wedge pressure. More direct and controlled left ventricular unloading can be achieved through advanced methods such as left atrial venting, surgical or percutaneous left ventricular venting, and inserting percutaneous transaortic ventricular assist devices such as the Impella. These advanced methods relieve left ventricular pressure, support antegrade flow, and may assist in ECMO weaning.
- Airway hemorrhage: Patients can present with hemoptysis, which is common in up to 10% of patients. Management strategies include withholding anticoagulation, bronchoscopic interventions, and treating coagulopathy.
- Intracardiac thrombosis: Transesophageal echocardiography can identify intracardiac and valvular thrombosis. To reduce risks, ensuring native cardiac ejection and avoiding hypertension is recommended.
- Limb ischemia: The risk of ischemia is present in the cannulated limb. Treatment strategies include the maintenance of anticoagulation and placement of distal perfusion cannulas.[7][8][9][10][11][12][13]
Weaning From Venoarterial Extracorporeal Membrane Oxygenation Complications
Assessing hepatic, pulmonary, and metabolic status is essential, along with hemodynamic evaluation. Improved cardiac filling pressures, improved arterial pulsatility, and minimal inotrope support are crucial to assess readiness for weaning. Echocardiography is performed to assess the myocardial recovery. Patients should maintain a mean arterial pressure of ≥65 mm Hg with minimal support and adequate ventricular function at low flow.[14]
Alternatives and Emerging Techniques
Recent advancements in ECMO focus on improving cardiac support and patient mobility. Percutaneous catheter-based microaxial transaortic ventricular assist devices for left ventricle venting during VV ECMO is a significant development. These devices help in left ventricle decompression and provide additional antegrade flow to the aortic root. New surgical methods for central VV ECMO, such as dual cannulation with a centrifugal flow pump and ambulatory VV ECMO setups, have also been explored. These innovations are transforming ECMO by enhancing therapeutic effectiveness, patient mobility, and overall quality of life.[15]
Enhancing Healthcare Team Outcomes
VV ECMO is a critical intervention for patients with severe cardiac conditions, requiring meticulous management and continuous assessment of the hemodynamic impacts. Advances in techniques, patient selection, and treatment strategies are expected to improve outcomes in patients requiring this life-saving intervention. Coordination with an interprofessional healthcare team improves patient care.
Media
(Click Image to Enlarge)
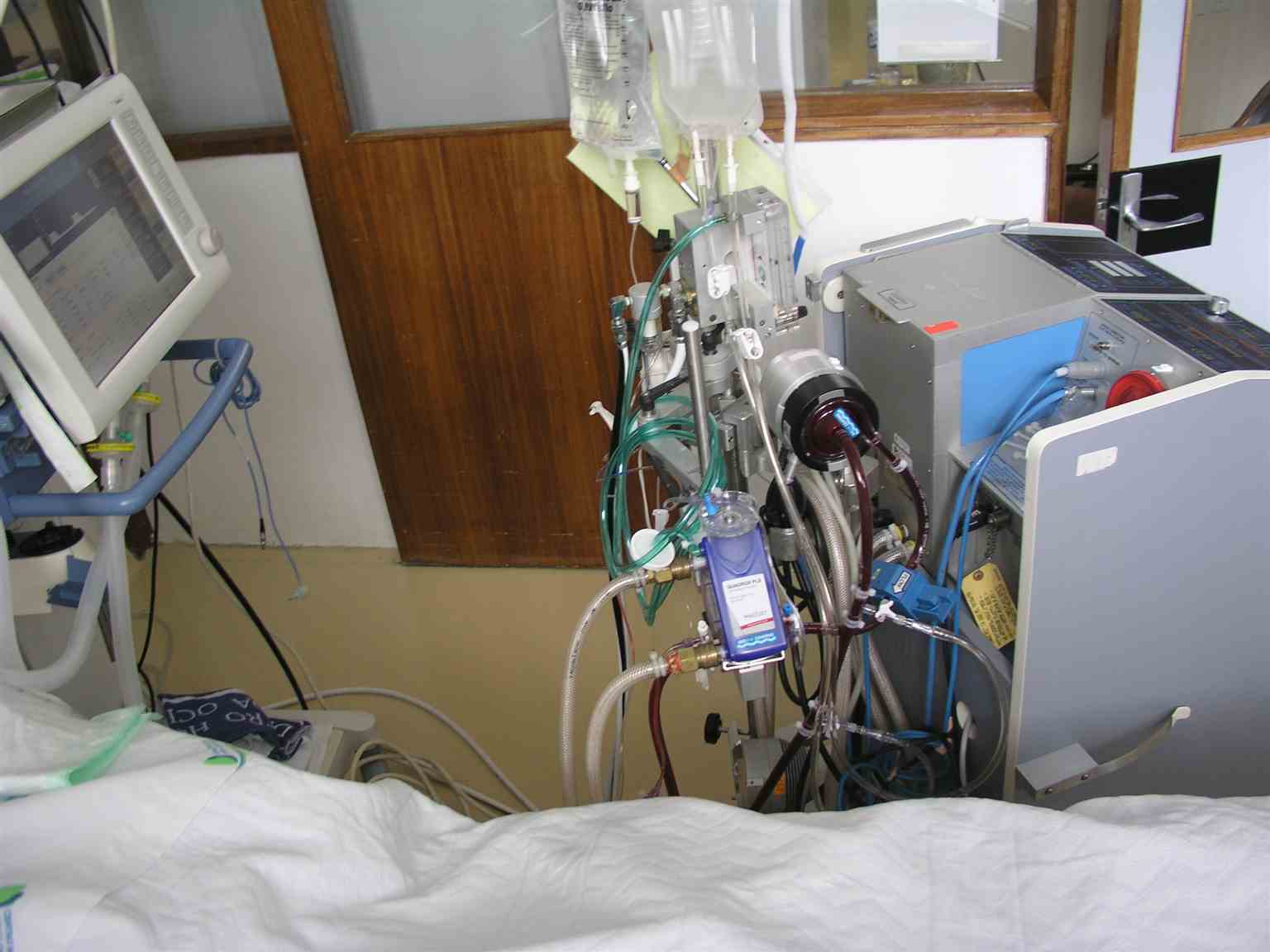
ECMO Machine for a Patient in the Intensive Care Unit. ECMO serves as a temporary mechanical support system for circulation and oxygenation that can be used as a bridge to definitive therapeutic interventions or recovery.
Cmenesesoliveira, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Mazzeffi MA, Rao VK, Dodd-O J, Del Rio JM, Hernandez A, Chung M, Bardia A, Bauer RM, Meltzer JS, Satyapriya S, Rector R, Ramsay JG, Gutsche J. Intraoperative Management of Adult Patients on Extracorporeal Membrane Oxygenation: An Expert Consensus Statement From the Society of Cardiovascular Anesthesiologists-Part I, Technical Aspects of Extracorporeal Membrane Oxygenation. Anesthesia and analgesia. 2021 Dec 1:133(6):1459-1477. doi: 10.1213/ANE.0000000000005738. Epub [PubMed PMID: 34559089]
Level 3 (low-level) evidenceLequier L, Horton SB, McMullan DM, Bartlett RH. Extracorporeal membrane oxygenation circuitry. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013 Jun:14(5 Suppl 1):S7-12. doi: 10.1097/PCC.0b013e318292dd10. Epub [PubMed PMID: 23735989]
Tonna JE, Abrams D, Brodie D, Greenwood JC, Rubio Mateo-Sidron JA, Usman A, Fan E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO journal (American Society for Artificial Internal Organs : 1992). 2021 Jun 1:67(6):601-610. doi: 10.1097/MAT.0000000000001432. Epub [PubMed PMID: 33965970]
Vyas A, Bishop MA. Extracorporeal Membrane Oxygenation in Adults. StatPearls. 2024 Jan:(): [PubMed PMID: 35015451]
Sy E, Sklar MC, Lequier L, Fan E, Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. Journal of critical care. 2017 Jun:39():87-96. doi: 10.1016/j.jcrc.2017.02.014. Epub 2017 Feb 12 [PubMed PMID: 28237895]
Level 1 (high-level) evidenceWilson J, Fisher R, Caetano F, Soliman-Aboumarie H, Patel B, Ledot S, Price S, Vandenbriele C. Managing Harlequin Syndrome in VA-ECMO - do not forget the right ventricle. Perfusion. 2022 Jul:37(5):526-529. doi: 10.1177/02676591211020895. Epub 2021 May 31 [PubMed PMID: 34053349]
Mazzeffi MA, Rao VK, Dodd-O J, Del Rio JM, Hernandez A, Chung M, Bardia A, Bauer RM, Meltzer JS, Satyapriya S, Rector R, Ramsay JG, Gutsche J. Intraoperative Management of Adult Patients on Extracorporeal Membrane Oxygenation: an Expert Consensus Statement From the Society of Cardiovascular Anesthesiologists- Part II, Intraoperative Management and Troubleshooting. Journal of cardiothoracic and vascular anesthesia. 2021 Dec:35(12):3513-3527. doi: 10.1053/j.jvca.2021.07.047. Epub 2021 Sep 28 [PubMed PMID: 34774253]
Level 3 (low-level) evidenceSidebotham D. Troubleshooting adult ECMO. The journal of extra-corporeal technology. 2011 Mar:43(1):P27-32 [PubMed PMID: 21449237]
Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart, lung & circulation. 2008:17 Suppl 4():S41-7. doi: 10.1016/j.hlc.2008.08.009. Epub 2008 Oct 29 [PubMed PMID: 18964254]
Ezad SM, Ryan M, Donker DW, Pappalardo F, Barrett N, Camporota L, Price S, Kapur NK, Perera D. Unloading the Left Ventricle in Venoarterial ECMO: In Whom, When, and How? Circulation. 2023 Apr 18:147(16):1237-1250. doi: 10.1161/CIRCULATIONAHA.122.062371. Epub 2023 Apr 17 [PubMed PMID: 37068133]
Pitcher HT, Harrison MA, Shaw C, Cowan SW, Hirose H, Cavarocchi N. Management considerations of massive hemoptysis while on extracorporeal membrane oxygenation. Perfusion. 2016 Nov:31(8):653-658. doi: 10.1177/0267659116651484. Epub 2016 Jul 9 [PubMed PMID: 27229004]
Bhat AG, Golchin A, Pasupula DK, Hernandez-Montfort JA. Right Sided Intracardiac Thrombosis during Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report and Literature Review. Case reports in critical care. 2019:2019():8594681. doi: 10.1155/2019/8594681. Epub 2019 Jan 6 [PubMed PMID: 30723555]
Level 3 (low-level) evidenceKrasivskyi I, Großmann C, Dechow M, Djordjevic I, Ivanov B, Gerfer S, Bennour W, Kuhn E, Sabashnikov A, Rahmanian PB, Mader N, Eghbalzadeh K, Wahlers T. Acute Limb Ischaemia during ECMO Support: A 6-Year Experience. Life (Basel, Switzerland). 2023 Feb 10:13(2):. doi: 10.3390/life13020485. Epub 2023 Feb 10 [PubMed PMID: 36836842]
Bishop MA, Moore A. Extracorporeal Membrane Oxygenation Weaning. StatPearls. 2024 Jan:(): [PubMed PMID: 34033326]
Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circulation. Heart failure. 2018 Sep:11(9):e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905. Epub [PubMed PMID: 30354364]