Introduction
Congenital heart disease (CHD) is the most common form of congenital disability, affecting approximately 8 out of every 1000 live births.[1] In many developing countries, CHD often goes undiagnosed until after birth or even later in life, sometimes presenting during childhood or adulthood. About 25% of infants born with a heart defect have critical CHD, necessitating surgical intervention or other procedures within the first year of life.[2]
A commonly used palliative procedure for patients with CHD is the modified Blalock-Taussig-Thomas (mBTT) shunt. This procedure improves blood flow to the lungs, reduces cyanosis, promotes pulmonary artery growth, and helps maintain optimal cardiac preload, afterload, and coronary artery perfusion.[3] The mBTT shunt is commonly performed in developing countries as a precursor to more definitive corrective surgery but has become less prevalent in developed nations. Advances in surgical techniques, technology, and the availability of specialized expertise have enabled early corrective surgeries in these regions.[4]
Modern surgical decision-making relies on a blend of experience and careful consideration of competing performance goals. For example, the conduit must be sufficiently large to ensure adequate blood flow to the pulmonary vasculature for optimal oxygenation. However, excessive pulmonary blood flow can lead to complications such as pulmonary edema and heart failure. Additional risks associated with shunt hemodynamics include the potential for shunt stenosis or thrombosis, distortion of the pulmonary arteries, impaired coronary perfusion, and uneven growth of the pulmonary arteries.[5]
History
The mBTT shunt, a previous iteration of the classical Blalock-Taussig (BT) shunt, is a palliative surgical procedure designed to treat patients with cyanotic heart diseases that are characterized by decreased pulmonary artery flow. The first BT shunt was performed at the Johns Hopkins Hospital in 1944, resulting from the collaborative efforts of 3 individuals—pediatric cardiologist Dr Helen Taussig, cardiac surgeon Dr Alfred Blalock, and laboratory assistant Mr Vivien Thomas.[6] The term "Blalock-Taussig shunt" was first used by de Leval when reporting on a series of patients who underwent the procedure between 1975 and 1979.[7] In 2003, a request was made to include the eponym "Thomas" to acknowledge the significant contributions of Mr Vivien Thomas to the success of the procedure.[8]
The mBTT shunt is designed to provide adequate blood flow to the pulmonary artery, relieving cyanosis while avoiding pulmonary overcirculation. The classical BT shunt procedure involved a lateral thoracotomy, where the subclavian artery was divided and anastomosed to the pulmonary artery in an end-to-side manner. This original technique has undergone extensive modifications, evolving into the mBTT shunt, which uses an interposition polytetrafluoroethylene (PTFE) graft to create a systemic-to-pulmonary shunt without sacrificing the subclavian artery or any of the brachiocephalic tributaries.[9] In 1976, Gazzaniga and colleagues reported using a PTFE graft to construct an aortopulmonary shunt (see Image. Comparison of Classical Blalock-Taussig and Modified Blalock-Taussig-Thomas Shunts).[10]
The mBTT shunt provides several advantages over the original iteration. By utilizing an interposition PTFE graft, the takedown procedure is simplified, blood flow to the ipsilateral upper limb is preserved, and shunt flow can be more precisely regulated by adjusting the diameter and length of the graft and selecting the appropriate anastomotic site.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
In the United States, CHD affects 1% of all deliveries, with about 25% of these cases classified as cyanotic congenital heart lesions.[11][12]
Common cyanotic CHD anatomic lesions include, but are not limited to:
- Tetralogy of Fallot (TOF)
- Ebstein anomaly
- Hypoplastic left heart syndrome
- Total anomalous pulmonary venous return
- Transposition of great arteries
- Tricuspid atresia and other univentricular conditions
- Truncus arteriosus
Alternative physiological classification systems of cyanotic congenital heart lesions include:
- Right-to-left shunting
- Inadequate pulmonary blood flow
- Common mixing lesions
The mBTT shunt offers temporary pulmonary arterial flow for cyanotic infants who are not yet candidates for definitive cardiac repair. Other aortopulmonary shunts, such as the Potts and Waterston shunts, are now rarely used due to high complication rates and challenges with removal during final repair (see Image. Systemic-to-Pulmonary Shunts). The Sano shunt, a right ventricle-to-pulmonary artery conduit, is used during stage I palliation for hypoplastic left heart syndrome and was first introduced by Norwood and colleagues in 1981.[13]
Indications
Palliative shunt procedures are less common today due to advancements in extracorporeal circulatory support, neonatal intensive care units, and improved surgical techniques that enable early corrective interventions. However, certain neonates with specific cyanotic congenital heart defects still require initial palliative surgery, as many of these conditions are not immediately treatable with corrective interventions. These defects often cannot be fully repaired initially, necessitating augmented pulmonary blood flow to support pulmonary artery development.
Additionally, neonates with intracerebral bleeding or other contraindications to cardiopulmonary bypass may be suitable candidates for a palliative mBTT shunt. Severely premature neonates with cyanotic heart lesions may also receive an mBTT shunt until they are mature enough for definitive cardiac reconstructive surgery.
Examples of cardiac anomalies that prevent complete repair but may be treated with the mBTT shunt include:
- Hypoplastic left heart syndrome: An mBTT shunt is placed as part of the Norwood procedure during stage 1 of the repair.[14]
- Congenitally corrected transposition of the great arteries: An mBTT shunt with pulmonary artery banding is the principal palliative treatment when this anomaly cannot be corrected.
- Coronary artery anomalies: Approximately 5% of neonates with TOF demonstrate the left anterior descending coronary artery crossing the right ventricular outflow tract.[15][16][17]
The mBTT shunt is utilized in various congenital cardiac lesions, particularly univentricular conditions where bidirectional Glenn and Fontan shunts cannot be established until neonatal pulmonary vascular resistance decreases.[18] Other cardiac anomalies that necessitate augmented pulmonary blood flow and promote pulmonary artery development include:
- Pulmonary atresia.
- Ebstein anomaly with functional pulmonary atresia.
- Inadequate pulmonary arteries.
- TOF, with palliative shunts for symptomatic, low-birth-weight neonates who cannot thrive on medical management.
- Ventricular septal defect with hypoplastic major aortopulmonary collateral arteries, where an mBTT shunt may be an option alongside unifocalization.
- Right ventricular outflow tract obstruction, including tricuspid stenosis or atresia, which was the primary indication for the BT shunt as described in the 1945 seminal article.[19]
The modern approach to surgical decision-making is grounded in experience and requires balancing multiple, often conflicting, performance objectives. For example, while the conduit size must be adequate to ensure sufficient blood supply to the pulmonary vascular bed for proper oxygenation, an oversized conduit can cause excessive pulmonary blood flow, leading to complications like pulmonary edema and heart failure. Moreover, the hemodynamics of the shunt may result in additional complications, including shunt stenosis or thrombosis, distortion of the pulmonary arteries, compromised coronary perfusion, and uneven growth of the pulmonary arteries.
Contraindications
Pulmonary hypertension with elevated pulmonary vascular resistance is a relative contraindication for establishing an mBTT shunt.
Equipment
Cardiopulmonary bypass must be available if cardioplegia is required during mBTT shunt placement, although not all cases will necessitate its use. If the surgical approach involves a median sternotomy, an electric sternotomy saw and stainless steel closure wires are essential. In contrast, a minimally invasive thoracotomy requires a Finochietto or Tuffier rib spreader, along with long-shafted pediatric instruments and standard cardiac surgery tools. PTFE grafts with predetermined diameters are the preferred conduits, as they ensure a precise fit for efficient anastomosis and optimal blood flow.
Personnel
The personnel required for the placement of an mBTT shunt typically include:
- Pediatric cardiothoracic surgeon
- Surgical first assistant
- Cardiac anesthesiologist
- Surgical technician or operating room nurse
- Circulating or operating room nurse
- Perfusionist, if cardiopulmonary bypass is utilized
Each healthcare team member plays a vital role in ensuring the procedure's success and safety.
Preparation
Preoperative evaluation of a cyanotic neonate involves a thorough prenatal and postnatal medical history, along with a complete physical examination. Congenital cardiac defects may occur in isolation or as part of a syndrome, making it essential to identify all associated anomalies. A transthoracic echocardiogram, interpreted by an experienced pediatric cardiologist, should include a detailed evaluation of the ascending aorta, aortic arch, and subclavian vessels. In some cases, coronary angiography or computed tomography angiography may be necessary to complete the assessment.
When evaluating a cyanotic neonate, close attention should be given to any history of recurrent respiratory infections, which may signal abnormal pulmonary blood flow. Performing mBTT shunt placement on a neonate or infant with an active respiratory infection increases the risk of perioperative complications. Premature and term infants with a body weight of less than 3 kg are at heightened risk of perioperative complications and may require intraoperative and postoperative cardiopulmonary support.
The diameter of the PTFE interposition graft should be determined preoperatively using the Hagen–Poiseuille equation:
Q = Δpπd4/8Lμ
where,
- Q = Volumetric flow rate
- Δp = Pressure difference
- π (pi) = 3.14159...
- d = Diameter of the graft
- L = Length of the graft
- μ = Blood viscosity
A small change in shunt diameter can exponentially affect blood flow, whereas shunt length has a lesser impact. Neonates with a body weight of 2.5 to 4.0 kg typically receive adequate pulmonary blood flow with a PTFE conduit measuring 3.0 to 3.5 mm in diameter. For neonates with a body weight of less than 2.5 kg, a conduit smaller than 3.0 mm is recommended, while those with a body weight of more than 4.0 kg may benefit from a shunt with a 4.0 mm diameter.[20]
Technique or Treatment
Surgical Technique for Modified Blalock-Taussig-Thomas Shunt Placement
The mBTT shunt procedure is typically performed without cardiopulmonary bypass in most centers. While the classical BT shunt was performed via a thoracotomy on the side of the aortic arch, the mBTT shunt is now routinely placed through a median sternotomy. This surgical approach offers several advantages, including the flexibility to place the shunt on either side of the chest, enhanced surgical exposure, and easier access to urgent cannulation and cardiopulmonary support if needed. Moreover, median sternotomy reduces the risk of intraoperative injury to the recurrent laryngeal nerve and lowers the likelihood of postoperative Horner syndrome.
A median sternotomy also allows for the repair of concomitant congenital lesions, such as a patent ductus arteriosus. However, early use of this approach can hinder the development of collateral flow from the internal thoracic arteries, which may complicate and pose challenges for future cardiac interventional procedures.[21]
Regardless of the surgical approach, many surgeons ligate the azygos vein early during the repair of complex lesions to simplify future shunt takedown procedures. mBTT shunt placement also necessitates a subtotal thyroidectomy. Additionally, a laryngoscopic examination during intubation is performed before making the surgical incision.
A standard median sternotomy is performed with a skin incision slightly shorter than the length of the sternum. Mosquito forceps are used to detach the diaphragmatic attachments from the xiphoid process and the inferior sternum. After dividing the sternum, a subtotal thyroidectomy is performed on the side of the shunt placement, taking special care to avoid injury to the recurrent laryngeal nerve. The cervical extension of the thyroid may be preserved on the opposite side. An extensive thymectomy is conducted using electrocautery to excise thymic tissue from its capsule, particularly on the side planned for the shunt.
The pericardium is incised longitudinally at the center, and traction sutures are placed to facilitate access to the right atrium. Early in the procedure, the right atrium is exposed, and a pursestring suture is secured in preparation for emergency cannulation or the insertion of an Intracath® to monitor right atrial pressure.
The innominate artery is identified and dissected up to the subclavian-to-carotid artery bifurcation. The decision to place the proximal anastomosis on either the innominate or subclavian artery depends on the surgeon's preference and anatomical feasibility, ensuring that no distortion occurs. Retracting the aorta facilitates access to the right pulmonary artery, which should be dissected from its attachment to the main pulmonary artery on one end to the level of the lung hilum on the other.
The surrounding lymph nodes and lymphatics should be resected to ensure the PTFE conduit lies comfortably, eliminating any residual anastomotic tension at the end of the operation. The PTFE graft is beveled on the side intended for anastomosis with the subclavian artery, while the other end is directed toward the right pulmonary artery. Although the PTFE graft has proven to be a superior option, there has been renewed interest in using homografts from the femoral artery or saphenous vein.[22]
The proximal anastomosis is performed using an atraumatic C-shaped vascular clamp applied to the innominate or subclavian artery. A longitudinal incision is made, and the beveled end of the PTFE graft is sutured to the arteriotomy with monofilament nonabsorbable sutures, such as 6-0, 7-0, or 8-0 polypropylene, in a continuous manner. After completing the proximal anastomosis, heparinization is administered to minimize clot formation within the graft. The graft is cross-clamped, and the C-clamp is released to assess the proximal anastomosis for leaks and to ensure optimal graft position and length. Once confirmed, the distal anastomosis is performed on the right pulmonary artery.
The pulmonary artery is clamped with an atraumatic C-clamp, and an arteriotomy is made along the clamped area to prepare for anastomosis with the distal end of the PTFE conduit. Many surgeons prefer to test the patient's oxygen saturation and hemodynamics by temporarily clamping the pulmonary artery before creating the arteriotomy. The distal anastomosis is performed using the same materials and technique as the proximal connection to the innominate artery. Careful attention is required to ensure proper orientation of the distal anastomosis when the clamps are released. The mBTT shunt is then inspected at both ends for leakage and checked for fibrinous clots within the graft.
In patients who are duct-dependent, the patent ductus arteriosus should be ligated only after confirming the patency of the newly created systemic-to-pulmonary shunt. Shunt patency and flow can be evaluated using echocardiography or manual testing. Manual testing of the shunt involves clamping and releasing it while palpating for a thrill in the distal pulmonary artery and monitoring hemodynamic changes. A spontaneous rise in systemic oxygen saturation, detected via photoplethysmography, serves as a reliable indicator of a functional shunt.
Reversal of heparinization is not essential. Standard practices include routine chest closure with mediastinal drains, placement of atrioventricular pacing leads, and sternal approximation using stainless steel wires. Most patients tolerate the operation well, with minimal need for inotropic support. Upon completion of the procedure, patients require intensive care for at least 1 week, with some potentially needing postoperative extracorporeal membrane oxygenation.
Surgical Technique for Modified Blalock-Taussig-Thomas Shunt Takedown
The takedown of an mBTT shunt is an integral part of any intracardiac repair. Shunt takedown halts the mixing of systemic and pulmonary blood, preventing the superior tenting of the pulmonary artery as the child grows. Tenting has been recognized as a significant contributor to unbalanced pulmonary blood flow between the lungs.
Previous operative notes must be reviewed before the takedown procedure. Preoperative evaluation should include echocardiography, with angiography performed if needed. Following a median sternotomy, comprehensive dissection is often required to identify and mobilize the right pulmonary artery, which may be encased in a thick fibrous peel.
The cardiopulmonary bypass should not be established until the surgeon has full control of the systemic-to-pulmonary shunt, especially in patients with duct-dependent physiology. Once the cardiopulmonary bypass flow is initiated, the shunt may be ligated proximally and distally using hemostatic clips. The surgeon must ensure the PTFE conduit is completely disconnected to prevent arterial tenting and imbalanced pulmonary blood flow.
Transcatheter occlusion of the mBTT shunt has been reported in hybrid repair settings and isolated cases. However, the risk of embolization from a clot or device is higher with the transcatheter approach than with surgical takedown.[23]
Complications
Despite advancements in surgical techniques, enhanced intensive care services, and cutting-edge surgical materials, systemic-to-pulmonary artery shunt operations continue to exhibit high in-hospital mortality rates, ranging from 2.3% to 16%.[24] However, only 2 factors—body weight less than 4.25 kg and the timing of surgery (elective versus emergency)—have been found to significantly affect mortality following a BT shunt procedure. Specifically, a body weight of less than 4.25 kg increases the mortality risk by 20.8 times, while emergency surgery raises the risk by 3.5 times. Notably, no studies have specifically investigated the correlation between the timing of surgery and mortality risk.
The primary disadvantage of using PTFE as the conduit for the mBTT shunt is the sporadic occurrence of serous leakage through the synthetic polymer fabric. This leakage can lead to localized seroma formation and an extended requirement for tube thoracostomy.
The most significant complication of the mBTT shunt procedure is pulmonary overcirculation in the early postoperative period. This condition may arise from high systemic vascular resistance, low pulmonary vascular resistance, or an inappropriately large systemic-to-pulmonary shunting. Pulmonary overcirculation can result in systemic hypotension, ventricular overload, pulmonary edema, and acidosis. If left uncorrected, it may lead to cardiac arrest and death. The imbalance in blood shunting from the systemic to pulmonary circulation heightens the risk of heart failure and compromises systemic perfusion of vital organs, potentially leading to intestinal ischemia and necrotizing enterocolitis.
Contrarily, shunt distortion or thrombosis can result in pulmonary hypoperfusion, hypoxia, and cyanosis. To prevent thrombosis, prophylactic heparinization is initiated in the immediate postoperative period at a rate of 10 units/kg/h, with adjustments made based on subsequent coagulation profile results. Additionally, aspirin is started at a dosage of 3 to 5 mg/kg once daily, not exceeding a maximum of 75 mg/d, and is continued for the patient's lifetime.
Although technically demanding and associated with a risk of significant complications, transcatheter intervention can serve as a viable rescue approach for reestablishing patency in the event of thrombotic blockage in an mBTT shunt.[25] Continuous, vigilant monitoring of hemodynamics during early postoperative management is crucial, accompanied by a low threshold for surgical reintervention when necessary.
The functionality of an mBTT shunt can be clinically assessed through palpation and auscultation. A palpable thrill on the side of the shunt that radiates to the infraclavicular region, which was not present preoperatively, is a reassuring sign of shunt functionality and can be confirmed by auscultation over the area. A continuous murmur, best heard in the same subclavicular region, indicates that the systemic-to-pulmonary shunt functions effectively. Transthoracic echocardiography may be used to quantify shunted blood flow, while computed tomography angiography can provide a conclusive evaluation of shunt blood flow.
Clinical Significance
In a large retrospective study spanning 6 decades of research at the Johns Hopkins Hospital, Williams et al reported an overall decline in the use of the mBTT shunt.[18] However, However, they observed a relative increase in its application for treating univentricular heart lesions. Notably, over 75% of the shunts analyzed in this study were classical BT shunts, which limits the applicability of the findings to the mBTT shunt. The mBTT shunt is also considered safer than the Potts or Waterston shunt.[26]
The Single Ventricle Reconstruction trial revealed that patients who underwent the Norwood procedure with right ventricle-to-pulmonary artery shunts had higher survival rates at 12 months without the need for cardiac transplantation compared to those receiving mBTT shunt placement. However, long-term survival rates for both groups were similar, and patients in the Norwood procedure group experienced a significant decline in overall right ventricular function.[27] At 6 years, there were no significant differences between the 2 groups in the risks of death, transplant, or catheter-based interventions.[28][29] Another study revealed that patients with the mBTT shunt experienced a greater decline in weight-for-age z score compared to those with a Sano shunt.[30]
When comparing the mBTT shunt with newer, less invasive, and more cost-effective palliative techniques, such as ductal stenting, McMullan et al reported a higher percentage of procedure-related complications and distal branch pulmonary artery stenosis in the mBTT group, while freedom from intervention was similar in both groups.[31] Right ventricular outflow tract stenting is another less-invasive palliative option that has demonstrated greater safety while being equally effective as the mBTT shunt in neonates with complex TOF lesions.[32]
The classical BT shunt procedure, known as "the blue baby operation" in the 1940s, gained international fame and has saved the lives of millions of children worldwide. However, palliative procedures in congenital cardiac surgery are increasingly falling out of favor in contemporary clinical practice. The focus has shifted from palliating cardiac lesions to prioritizing the avoidance of complications associated with these interventions.
Enhancing Healthcare Team Outcomes
Modern prenatal care enables the in-utero diagnosis of many congenital cardiac lesions. Neonates requiring surgical repair for complex cardiac conditions benefit from delivery at a tertiary cardiac center when possible. Effective communication and collaborative planning among healthcare specialists, including obstetricians, maternal-fetal medicine specialists, neonatal intensive care teams, pediatric cardiologists, and pediatric cardiothoracic surgeons, during the antenatal period ensures well-coordinated care while the patient is still a fetus. These prearranged plans can then be implemented at delivery, ideally in a controlled environment, thereby optimizing outcomes.
All congenital abnormalities can be evaluated once the neonate is stabilized. Neonatal intensive care clinical staff and pharmacists have integral roles in caring for neonates with cyanosis from complex cardiac lesions. Additionally, family counselors and palliative care teams within many neonatal intensive care units provide necessary support to families navigating the complexities of the care system.
The decision to perform a palliative cardiac procedure requiring intraoperative and postoperative cardiopulmonary support involves the expertise of multiple interprofessional team members, including surgeons, anesthesiologists, perfusionists, cardiologists, pediatricians, echocardiographers, dietitians, nurses, and pharmacists. Care coordinators play a vital role in facilitating communication among these professionals to optimize outcomes for patients undergoing mBTT shunt placement.
Media
(Click Image to Enlarge)
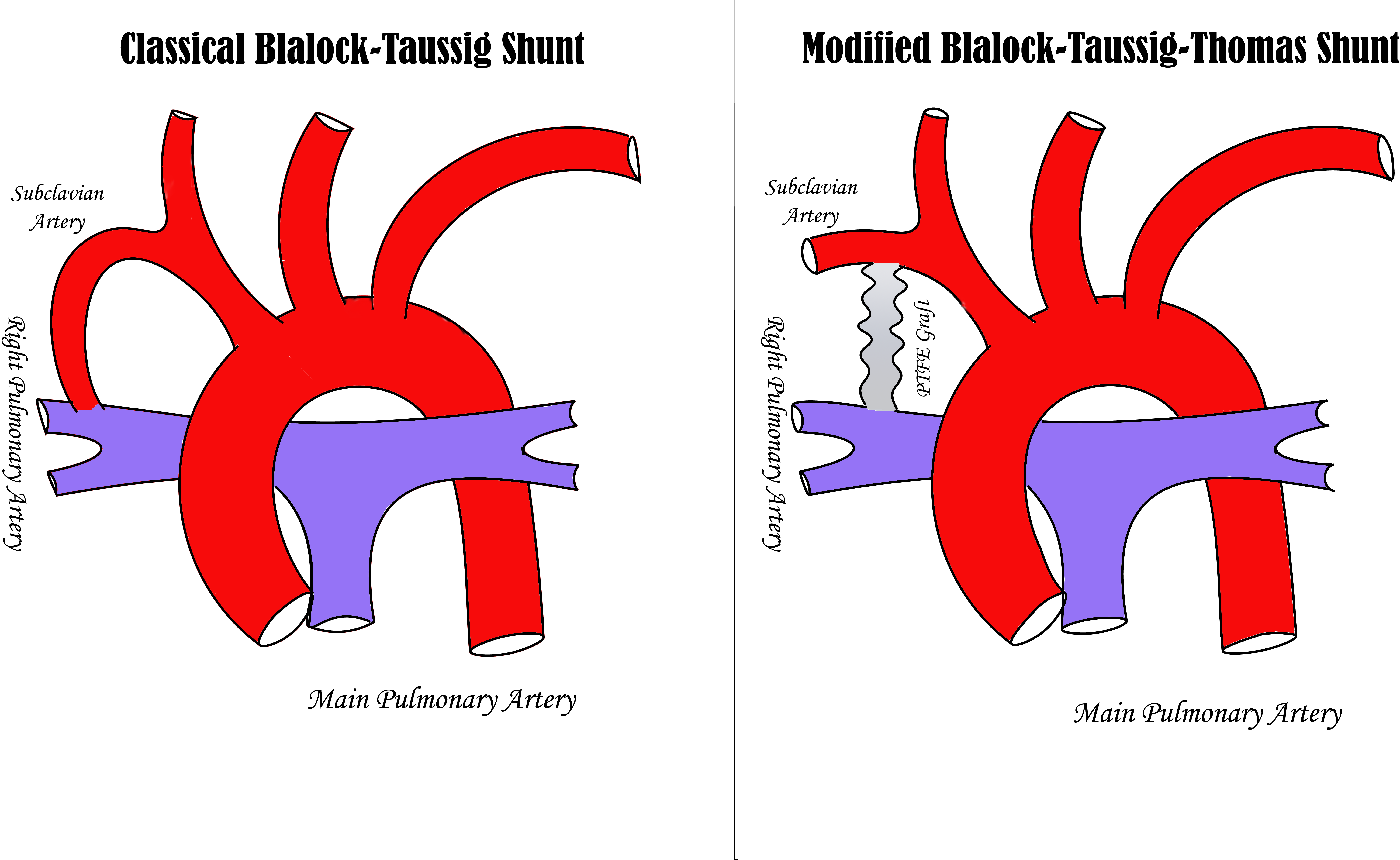
Comparison of Classical Blalock-Taussig and Modified Blalock-Taussig-Thomas Shunts. The image on the left illustrates the classical aortopulmonary Blalock-Taussig shunt, which uses a divided subclavian artery as the shunt conduit. In contrast, the image on the right depicts the modified Blalock-Taussig-Thomas shunt, which utilizes a polytetrafluoroethylene (PTFE) graft as the shunt conduit.
Contributed by MH Alahmadi, MD
(Click Image to Enlarge)
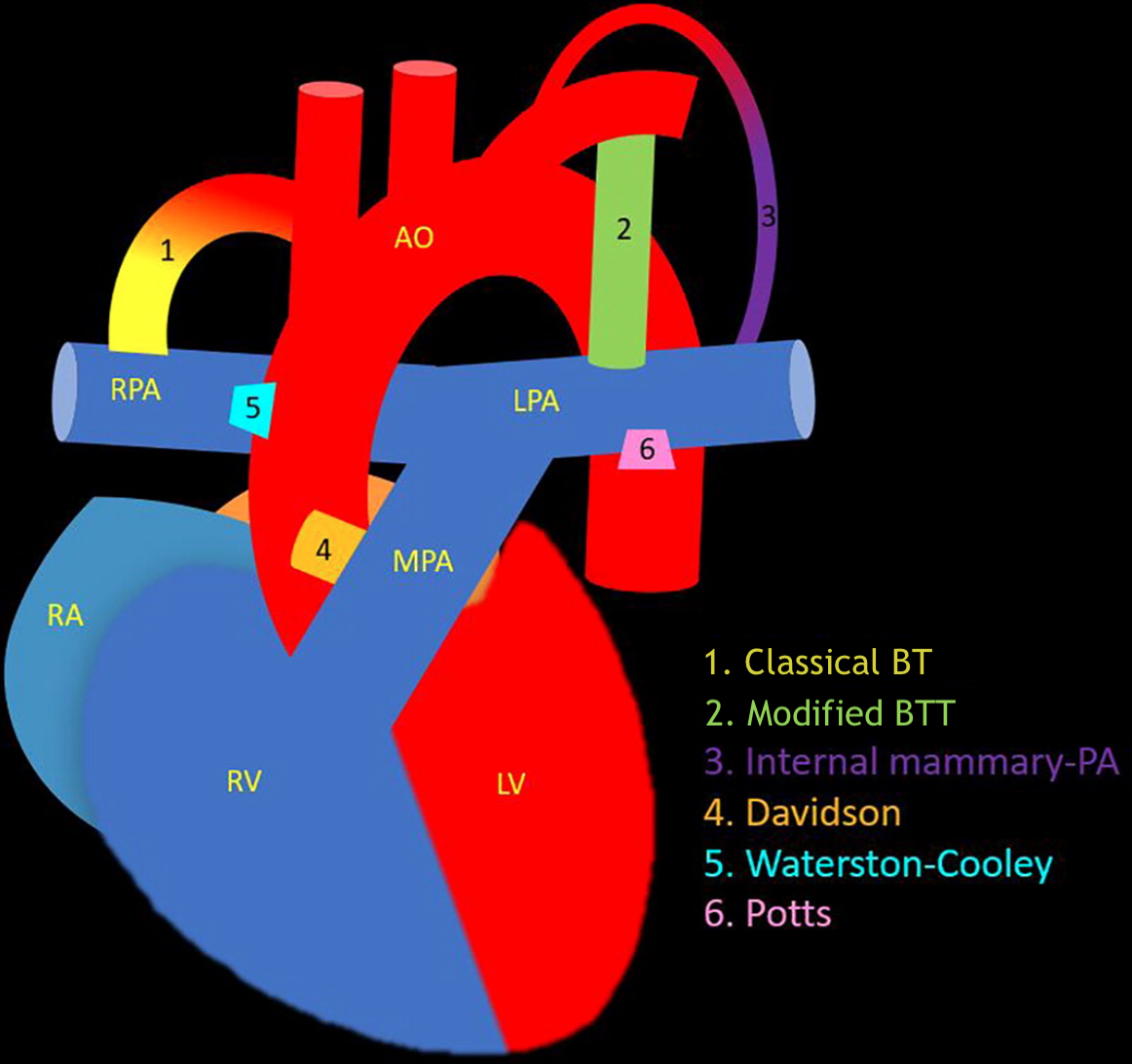
Systemic-Pumonary Shunts. This illustration demonstrates the differences among various systemic-pulmonary shunts, including the classical Blalock-Taussig (BT), modified Blalock-Taussig-Thomas (BTT), internal mammary artery to pulmonary artery (PA), Davidson, Waterston-Cooley, and Potts shunts.
Contributed by P Rajiah, MD
References
Sasikumar N, Hermuzi A, Fan CS, Lee KJ, Chaturvedi R, Hickey E, Honjo O, Van Arsdell GS, Caldarone CA, Agarwal A, Benson L. Outcomes of Blalock-Taussig shunts in current era: A single center experience. Congenital heart disease. 2017 Dec:12(6):808-814. doi: 10.1111/chd.12516. Epub 2017 Jul 24 [PubMed PMID: 28736841]
Jonas RA. Congenital heart surgery in developing countries. Seminars in thoracic and cardiovascular surgery. Pediatric cardiac surgery annual. 2008:():3-6. doi: 10.1053/j.pcsu.2007.12.001. Epub [PubMed PMID: 18396218]
Amelia P, Advani N, Pulungan AB, Djer MM, Hegar B, Prawira Y, Sukardi R. Predicting Factors for Mortality in Patients After the Modified Blalock-Taussig Shunt Procedure in Developing Countries: A Retrospective Study. International journal of general medicine. 2023:16():5291-5300. doi: 10.2147/IJGM.S432855. Epub 2023 Nov 15 [PubMed PMID: 38021062]
Level 2 (mid-level) evidenceRana JS, Ahmad KA, Shamim AS, Hassan SB, Ahmed MA. Blalock-Taussig shunt: experience from the developing world. Heart, lung & circulation. 2002:11(3):152-6 [PubMed PMID: 16352089]
Piskin S, Altin HF, Yildiz O, Bakir I, Pekkan K. Hemodynamics of patient-specific aorta-pulmonary shunt configurations. Journal of biomechanics. 2017 Jan 4:50():166-171. doi: 10.1016/j.jbiomech.2016.11.014. Epub 2016 Nov 11 [PubMed PMID: 27866675]
Mainwaring RD, Mainwaring S. The retirement years of Doctor Helen B. Taussig: an intersection of art and medicine. Cardiology in the young. 2024 Feb:34(2):334-347. doi: 10.1017/S1047951123001397. Epub 2023 Jul 10 [PubMed PMID: 37427599]
de Leval MR, McKay R, Jones M, Stark J, Macartney FJ. Modified Blalock-Taussig shunt. Use of subclavian artery orifice as flow regulator in prosthetic systemic-pulmonary artery shunts. The Journal of thoracic and cardiovascular surgery. 1981 Jan:81(1):112-9 [PubMed PMID: 6450303]
Brogan TV, Alfieris GM. Has the time come to rename the Blalock-Taussig shunt? Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003 Oct:4(4):450-3 [PubMed PMID: 14525641]
Yuan SM, Shinfeld A, Raanani E. The Blalock-Taussig shunt. Journal of cardiac surgery. 2009 Mar-Apr:24(2):101-8. doi: 10.1111/j.1540-8191.2008.00758.x. Epub 2008 Nov 7 [PubMed PMID: 19040408]
Elliott MP, Gazzaniga AB, Thomas JM, Haiduc NJ, Rosen SM. Use of expanded polytetrafluoroethylene grafts for vascular access in hemodialysis: laboratory and clinical evaluation. The American surgeon. 1977 Jul:43(7):455-9 [PubMed PMID: 879604]
Level 3 (low-level) evidenceHoffman JI, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002 Jun 19:39(12):1890-900 [PubMed PMID: 12084585]
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2011 Nov 15:58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. Epub [PubMed PMID: 22078432]
Level 1 (high-level) evidencePiber N, Ono M, Palm J, Kido T, Burri M, Röhlig C, Strbad M, Cleuziou J, Lemmer J, Dilber D, Klawonn F, Ewert P, Hager A, Hörer J. Influence of Shunt Type on Survival and Right Heart Function after the Norwood Procedure for Aortic Atresia. Seminars in thoracic and cardiovascular surgery. 2022 Winter:34(4):1300-1310. doi: 10.1053/j.semtcvs.2021.11.012. Epub 2021 Nov 25 [PubMed PMID: 34838954]
Goldberg CS, Gaynor JW, Mahle WT, Ravishankar C, Frommelt P, Ilardi D, Bellinger D, Paridon S, Taylor M, Hill KD, Minich LL, Schwartz S, Afton K, Lamberti M, Trachtenberg FL, Gongwer R, Atz A, Burns KM, Chowdhury S, Cnota J, Detterich J, Frommelt M, Jacobs JP, Miller TA, Ohye RG, Pizarro C, Shah A, Walters P, Newburger JW, Pediatric Heart Network Investigators. The pediatric heart network's study on long-term outcomes of children with HLHS and the impact of Norwood Shunt type in the single ventricle reconstruction trial cohort (SVRIII): Design and adaptations. American heart journal. 2022 Dec:254():216-227. doi: 10.1016/j.ahj.2022.09.005. Epub 2022 Sep 15 [PubMed PMID: 36115392]
Hurwitz RA, Smith W, King H, Girod DA, Caldwell RL. Tetralogy of Fallot with abnormal coronary artery: 1967 to 1977. The Journal of thoracic and cardiovascular surgery. 1980 Jul:80(1):129-34 [PubMed PMID: 7382526]
Need LR, Powell AJ, del Nido P, Geva T. Coronary echocardiography in tetralogy of fallot: diagnostic accuracy, resource utilization and surgical implications over 13 years. Journal of the American College of Cardiology. 2000 Oct:36(4):1371-7 [PubMed PMID: 11028497]
Level 2 (mid-level) evidenceFellows KE, Freed MD, Keane JF, Praagh R, Bernhard WF, Castaneda AC. Results of routine preoperative coronary angiography in tetralogy of Fallot. Circulation. 1975 Mar:51(3):561-6 [PubMed PMID: 1139765]
Williams JA, Bansal AK, Kim BJ, Nwakanma LU, Patel ND, Seth AK, Alejo DE, Gott VL, Vricella LA, Baumgartner WA, Cameron DE. Two thousand Blalock-Taussig shunts: a six-decade experience. The Annals of thoracic surgery. 2007 Dec:84(6):2070-5; discussion 2070-5 [PubMed PMID: 18036938]
Level 2 (mid-level) evidenceBlalock A, Taussig HB. Landmark article May 19, 1945: The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock and Helen B. Taussig. JAMA. 1984 Apr 27:251(16):2123-38 [PubMed PMID: 6368878]
Bove EL, Migliavacca F, de Leval MR, Balossino R, Pennati G, Lloyd TR, Khambadkone S, Hsia TY, Dubini G. Use of mathematic modeling to compare and predict hemodynamic effects of the modified Blalock-Taussig and right ventricle-pulmonary artery shunts for hypoplastic left heart syndrome. The Journal of thoracic and cardiovascular surgery. 2008 Aug:136(2):312-320.e2. doi: 10.1016/j.jtcvs.2007.04.078. Epub [PubMed PMID: 18692636]
Chandrasekaran A, Mathew T, Menon S, Dharan BS, Karunakaran J, Kapilamoorthy TR. Sternotomy after classic Blalock-Taussig shunt: a unique challenge. The Annals of thoracic surgery. 2014 Sep:98(3):1114. doi: 10.1016/j.athoracsur.2014.05.018. Epub [PubMed PMID: 25193204]
Level 3 (low-level) evidenceKaur R, Bhurtel D, Bielefeld MR, Morales JM, Durham LA 3rd. Cryopreserved Saphenous Vein Compared With PTFE Graft for Use as Modified Blalock-Taussig or Central Shunt in Cyanotic Congenital Heart Disease. World journal for pediatric & congenital heart surgery. 2018 Sep:9(5):509-512. doi: 10.1177/2150135118776616. Epub [PubMed PMID: 30157727]
Baspinar O, Sahin DA, Sulu A, Gokaslan G. Interventions Involving the Use of Covered Coronary Artery Stents for Pseudoaneurysms of Blalock-Taussig Shunts. World journal for pediatric & congenital heart surgery. 2016 Jul:7(4):494-7. doi: 10.1177/2150135115603332. Epub 2016 Feb 6 [PubMed PMID: 26852366]
Yilmaz M, Turkcan BS, Ecevit AN, Şahan YÖ, Atalay A. Comparative Analysis of Modified BT Shunt and Central Shunt in Pediatric Patients. Brazilian journal of cardiovascular surgery. 2024 May 15:39(3):e20230376. doi: 10.21470/1678-9741-2023-0376. Epub 2024 May 15 [PubMed PMID: 38748885]
Level 2 (mid-level) evidenceBonnet M, Petit J, Lambert V, Brenot P, Riou JY, Angel CY, Belli E, Baruteau AE. Catheter-based interventions for modified Blalock-Taussig shunt obstruction: a 20-year experience. Pediatric cardiology. 2015 Apr:36(4):835-41. doi: 10.1007/s00246-014-1086-0. Epub 2015 Jan 6 [PubMed PMID: 25560736]
Marbarger JP Jr, Sandza JG Jr, Hartmann AF Jr, Weldon CS. Blalock-taussig anastomosis: the preferred shunt in infants and newborns. Circulation. 1978 Sep:58(3 Pt 2):I73-7 [PubMed PMID: 14740682]
Hill GD, Frommelt PC, Stelter J, Campbell MJ, Cohen MS, Kharouf R, Lai WW, Levine JC, Lu JC, Menon SC, Slesnick TC, Wong PC, Saudek DE. Impact of initial norwood shunt type on right ventricular deformation: the single ventricle reconstruction trial. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015 May:28(5):517-21. doi: 10.1016/j.echo.2015.01.018. Epub 2015 Feb 15 [PubMed PMID: 25690998]
Level 2 (mid-level) evidenceNewburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, Chen S, Cnota J, Dunbar-Masterson C, Ghanayem NS, Goldberg CS, Jacobs JP, Lewis AB, Mital S, Pizarro C, Eckhauser A, Stark P, Ohye RG, Pediatric Heart Network Investigators. Transplant-Free Survival and Interventions at 6 Years in the SVR Trial. Circulation. 2018 May 22:137(21):2246-2253. doi: 10.1161/CIRCULATIONAHA.117.029375. Epub 2018 Feb 1 [PubMed PMID: 29437119]
Marx GR, Shirali G, Levine JC, Guey LT, Cnota JF, Baffa JM, Border WL, Colan S, Ensing G, Friedberg MK, Goldberg DJ, Idriss SF, John JB, Lai WW, Lu M, Menon SC, Ohye RG, Saudek D, Wong PC, Pearson GD, Pediatric Heart Network Investigators. Multicenter study comparing shunt type in the norwood procedure for single-ventricle lesions: three-dimensional echocardiographic analysis. Circulation. Cardiovascular imaging. 2013 Nov:6(6):934-42. doi: 10.1161/CIRCIMAGING.113.000304. Epub 2013 Oct 4 [PubMed PMID: 24097422]
Level 1 (high-level) evidenceBurch PT, Gerstenberger E, Ravishankar C, Hehir DA, Davies RR, Colan SD, Sleeper LA, Newburger JW, Clabby ML, Williams IA, Li JS, Uzark K, Cooper DS, Lambert LM, Pemberton VL, Pike NA, Anderson JB, Dunbar-Masterson C, Khaikin S, Zyblewski SC, Minich LL, Pediatric Heart Network Investigators. Longitudinal assessment of growth in hypoplastic left heart syndrome: results from the single ventricle reconstruction trial. Journal of the American Heart Association. 2014 Jun 23:3(3):e000079. doi: 10.1161/JAHA.114.000079. Epub 2014 Jun 23 [PubMed PMID: 24958780]
Level 1 (high-level) evidenceMcMullan DM, Permut LC, Jones TK, Johnston TA, Rubio AE. Modified Blalock-Taussig shunt versus ductal stenting for palliation of cardiac lesions with inadequate pulmonary blood flow. The Journal of thoracic and cardiovascular surgery. 2014 Jan:147(1):397-401. doi: 10.1016/j.jtcvs.2013.07.052. Epub 2013 Sep 23 [PubMed PMID: 24071469]
Level 2 (mid-level) evidenceBigdelian H, Ghaderian M, Sedighi M. Surgical repair of Tetralogy of Fallot following primary palliation: Right ventricular outflow track stenting versus modified Blalock-Taussig shunt. Indian heart journal. 2018 Dec:70 Suppl 3(Suppl 3):S394-S398. doi: 10.1016/j.ihj.2018.06.020. Epub 2018 Jun 24 [PubMed PMID: 30595296]