Electrophysiology Study and Ablation of Atrial Tachycardia
Electrophysiology Study and Ablation of Atrial Tachycardia
Introduction
Paroxysmal supraventricular tachycardias (PSVTs) affect approximately 36 out of 100,000 individuals annually in the United States, with women being twice as likely as men to develop the condition. The risk of PSVT increases significantly with age, with older individuals being 5 times more likely to experience the arrhythmia compared to younger people.[1] PSVT accounts for 10% to 15% of cases in adults undergoing catheter ablation, while in children, it accounts for 10% to 20% of cases.
Focal atrial tachycardia (AT) is a supraventricular arrhythmia with rapid heart rates originating from ectopic atrial foci independent of the sinus or atrioventricular (AV) nodes, and it is a common mechanism of PSVT. Presenting as either unifocal or multifocal, AT has distinct pathophysiological mechanisms. Unifocal AT arises from enhanced automaticity, triggered activity, or micro-reentry mechanisms, resulting in a single, consistent P-wave morphology with an atrial rate of 150 to 250 bpm. Typically benign and paroxysmal, unifocal AT is often nonsustained. However, incessant tachycardia may cause tachycardia-induced cardiomyopathy.
On the other hand, multifocal atrial tachycardia (MAT) involves three or more distinct P-wave morphologies and an irregular atrial rate exceeding 100 bpm.[2] Enhanced automaticity is the primary mechanism, with triggered activity also playing a role. MAT is frequently associated with underlying conditions such as pulmonary disease, metabolic imbalances, or drug effects.[3]
Electrophysiology (EP) studies and catheter ablation are crucial for diagnosing and managing AT. Successful elimination of unifocal AT is frequently achieved through catheter ablation with low complication rates, while the management of MAT focuses on treating underlying causes.[4][5] Advances in mapping technologies and ablation techniques have greatly enhanced outcomes for patients undergoing these procedures, making ablation a cornerstone in focal AT treatment. This review explores the mechanisms, diagnostic strategies, and therapeutic approaches, emphasizing the essential role of EP studies and ablation in contemporary cardiac care.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Understanding the anatomy and physiology of AT involves examining the structural and electrical components of the atria and the mechanisms driving these abnormal rhythms.
Anatomy
The atria consist of two chambers—the right atrium (RA) and the left atrium (LA)—the upper portions of the heart responsible for receiving blood from the body and lungs, respectively. Several anatomical structures within the atria are key to the development of tachycardia:
- Sinoatrial (SA) node
- This is the heart's natural pacemaker, located in the upper part of the RA. Normally, it initiates electrical impulses that set the heart rate.
- Atrial myocardium
- This muscular tissue of the atria can generate and conduct electrical signals.
- Pulmonary veins
- The junction between the pulmonary veins and the LA is a common site for focal ATs, especially in patients with atrial fibrillation.
- Tricuspid and mitral valve annuli
- These fibrous rings surrounding the valves separating the atria from the ventricles can become regions for abnormal electrical reentry circuits, leading to AT.
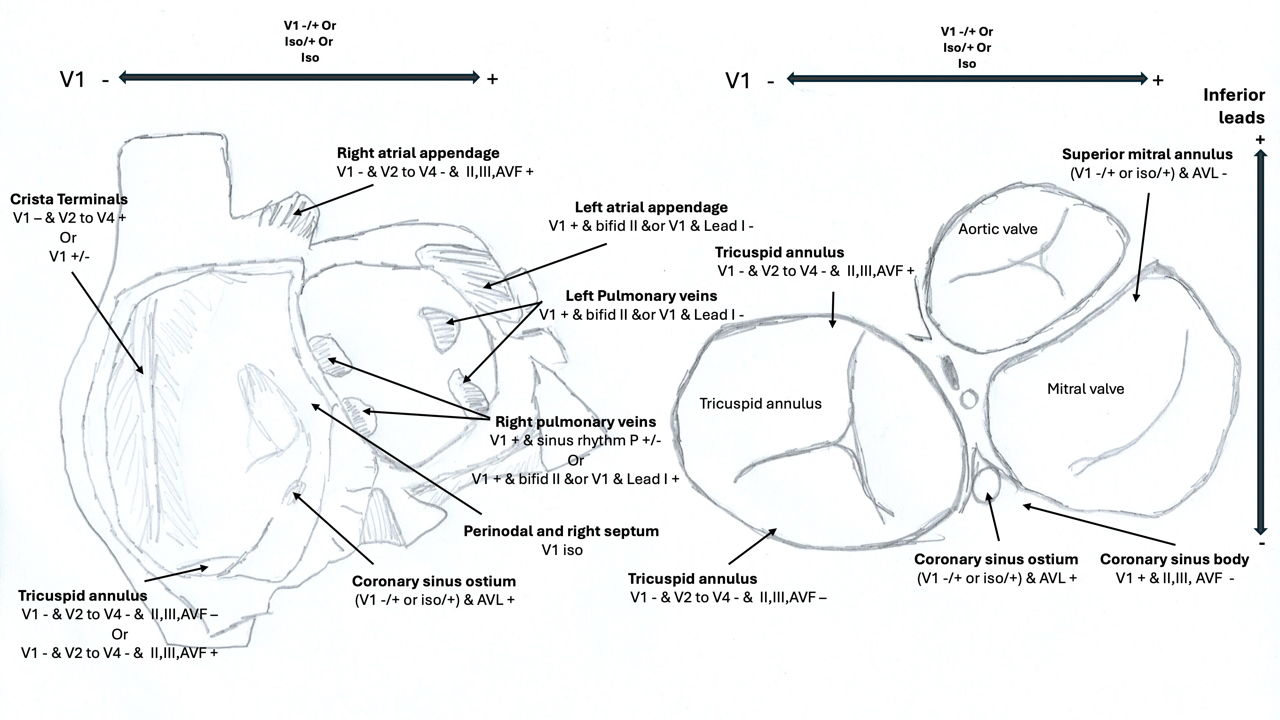
Focal AT predominantly originates from the RA, accounting for approximately 80% of cases. The most common RA site is along the crista terminalis, where about 37% of focal ATs are observed. Other notable RA sites include the posteroseptal area and coronary sinus ostium (14%), anteroseptal region (9%), and the tricuspid annulus (7%). In contrast, LA foci are less common, with the pulmonary veins accounting for around 10% of cases. Other LA sites include the mitral annulus and LA appendage, each contributing approximately 4%.[6]
Gender differences are noted in the distribution of focal AT. Women more commonly exhibit tachycardias along the crista terminalis, whereas men tend to have foci along the tricuspid annulus and the RA appendage. Other focal AT locations are distributed similarly across genders.
Physiology of Atrial Tachycardias
ATs are driven by abnormal electrical activity that bypasses the usual pacemaker function of the SA node. The physiological mechanisms of AT include:
- Enhanced automaticity: atrial cells outside the SA node spontaneously depolarize faster than the SA node, overriding its pacemaking function. This automatic firing can lead to focal AT (unifocal AT), where electrical impulses arise from a single location.
- Triggered activity: abnormal calcium handling within atrial cells can cause delayed afterdepolarizations, leading to rapid, triggered electrical discharges. This process occurs after an action potential, particularly when cells are exposed to excessive sympathetic stimulation or ischemia, creating bursts of rapid atrial beats.
- Reentry: a critical mechanism of AT that involves a self-sustaining loop of electrical activity around an anatomical or functional obstacle within the atria. Microreentry circuits, where electrical impulses circulate within a small area of atrial tissue, are common in unifocal AT. In contrast, macro reentry circuits, often seen in atrial flutter, involve larger tissue areas, leading to sustained rapid atrial rates.
During ablation procedures, ATs occur spontaneously in 27% of patients, while 37% require programmed stimulation for induction. Isoproterenol is used in 22% of cases to induce tachycardia, and atropine is needed in 6%. In 17% of patients, concomitant arrhythmias are induced, including atrial fibrillation, atrial flutter, and AV-nodal reentrant tachycardia (AVNRT).[7]
Focal ATs exhibit sensitivity to adenosine, and their response varies based on the underlying mechanism. Adenosine terminates ATs of triggered activity and transiently suppresses those due to enhanced automaticity but has no effect on reentrant ATs.[8] Adenosine's action is mediated through the activation of IK-Ado (adenosine increases the outward potassium current), leading to hyperpolarization and decreased diastolic depolarization, affecting repolarization duration.[9]
Types of Atrial Tachycardia
The 2 types of AT are unifocal and multifocal AT.
- Unifocal AT: characterized by a single focus of automatic or triggered electrical activity and typically presents with a regular atrial rhythm and a consistent P-wave morphology on the electrocardiogram (ECG) with an isoelectric baseline between the P waves.
- MAT: multiple sites within the atria independently generate electrical impulses, leading to multiple P-wave morphologies and an irregular atrial rhythm. MAT is often associated with underlying conditions like lung disease or critical illness.
Impact on Cardiac Physiology
ATs can disrupt normal hemodynamics by reducing the efficiency of atrial contraction. If persistent, this can lead to increased atrial pressure, poor ventricular filling, and eventual heart failure. In severe cases, sustained AT can lead to tachycardia-induced cardiomyopathy, where the heart's structure and function deteriorate due to prolonged rapid heart rates.
Indications
EP studies and catheter ablation are indicated for AT in several clinical scenarios, particularly when medical therapy is insufficient or poorly tolerated. The primary indications include:
Symptomatic AT: EP studies and ablation are recommended for patients with recurrent, symptomatic AT that significantly impacts quality of life. Symptoms may include palpitations, dizziness, shortness of breath, or fatigue. When these symptoms persist despite antiarrhythmic medications or when medications cause intolerable side effects, ablation becomes a preferred treatment option.
Incessant AT: In cases of incessant or persistent AT, which can lead to tachycardia-induced cardiomyopathy, catheter ablation is indicated to prevent long-term cardiac dysfunction, whether symptomatic or asymptomatic.[10] Incessant AT, though uncommon, can lead to progressive heart failure if left untreated.
Tachycardia-induced cardiomyopathy: Patients with AT who develop cardiomyopathy due to rapid or prolonged tachycardia are candidates for EP studies and ablation. By eliminating the arrhythmia, ablation helps restore normal cardiac function and reverse or prevent further deterioration.
Drug-refractory AT: When pharmacological management fails to control the arrhythmia or when patients experience significant side effects from antiarrhythmic drugs, EP studies, and ablation offer an effective and curative option. Ablation eliminates the focus of the arrhythmia, avoiding the need for long-term medication use.
Patients with structural heart disease: While ablation is generally more challenging in patients with underlying structural heart disease, such as congenital heart defects, hypertrophic cardiomyopathy, or prior cardiac surgery, it remains an important therapeutic option when AT causes significant hemodynamic compromise or symptoms.[11][12]
Young patients and athletes: Ablation is often preferred in younger patients or athletes who wish to avoid lifelong dependence on antiarrhythmic drugs, as it offers a potential cure for focal AT with minimal long-term complications.
MAT: While MAT is typically managed by addressing underlying conditions such as pulmonary disease or metabolic imbalances, EP studies, and ablation may be considered in selected cases, particularly if the arrhythmia is resistant to medical treatment or symptomatic.[13]
In all reentrant and most focal ATs, catheter ablation should be offered as an initial choice to patients after explaining the potential risks and benefits in detail. When possible, with ATs occurring after atrial fibrillation ablation, focal or macroreentrant, ablation should be deferred for >3 months.
Contraindications
AT ablation is an effective treatment with specific absolute and relative contraindications that must be considered.
Absolute Contraindications
Known LA thrombus: LA ablation should never be performed in the presence of an atrial thrombus due to the significant risk of dislodging the clot and causing a stroke or systemic embolism.[14]
Relative Contraindications
Pregnancy: Pregnant patients should avoid fluoroscopy due to the risks of radiation exposure to the fetus. However, advancements in technology have led to the development of zero-fluoroscopy catheter ablation techniques, which offer an effective and safe alternative for pregnant patients.[15]
Vascular access issues: Venous thrombosis or a venous filtering device may pose challenges for venous access, while conditions like peripheral artery disease, aortic dissection, or an aortic valve prosthesis can complicate arterial access in procedures requiring a retrograde approach.
Severe coagulopathy or bleeding disorders: Patients with significant clotting abnormalities or bleeding tendencies may face increased procedural risks.
Known sensitivity to heparin: Alternative anticoagulation strategies are required for patients with heparin-induced thrombocytopenia or other sensitivities to heparin, which can complicate ablation.
Each relative contraindication requires careful evaluation and, where possible, mitigation strategies to ensure the safety and success of the ablation procedure.
Equipment
Successful EP study and ablation of AT require specialized equipment to monitor and map the heart's electrical activity and deliver therapeutic ablation energy. This equipment includes:
Multichannel Physiologic Recorders
This data acquisition system records the electrical signals from the heart. Signals pass from electrode pins in catheters to an amplifier, which filters and amplifies them before sending them to the multichannel recording system. High-pass (30-40 Hz) and low-pass (400-500 Hz) filters are used to isolate relevant signals, and a notch filter can be employed to reduce 60-cycle noise, though it may result in some information loss. Bipolar electrograms are recorded by closely spaced electrode pairs (2-5 mm apart), providing highly filtered signals for accurate mapping of the origin of focal tachycardia. Simultaneously, unipolar electrograms with a QS signal confirm the catheter’s position at the signal's origin.
Stimulators
The stimulator is a device that paces the heart by delivering a constant current output through 1 or more output channels. This device is capable of delivering at least 4 coupled extrastimuli. The stimulator output is generally connected to the recording system, where the computer controls electrode pairs for pacing. Stimulators are designed to deliver variable currents ranging from 0.1 mA to 10 mA. With the catheters positioned satisfactorily, current thresholds below 2 mA (with 2 ms pulse width) can usually be achieved in both the atrium and ventricle.
Catheters
Electrode catheters are essential tools in EP testing that record and pace the heart's electrical activity. These catheters consist of insulated wires with electrodes at the distal end for recording and a plug at the proximal end for external connection. They are typically made of materials like woven Dacron, which provides stiffness for maintaining shape while remaining flexible at body temperature to form loops. Alternatively, synthetic materials like polyurethane are less expensive but offer reduced maneuverability. Catheters come in various sizes, ranging from 3 to 8 F, with 5 to 7 F being most commonly utilized in adults. The recordings can be either unipolar or bipolar, with interelectrode distances ranging from 1 to 10 mm or more.
Multipolar electrode catheters, such as the Halo catheter, are specifically used to map atrial electrical activity during AT. These catheters are often positioned within the coronary sinus or along the crista terminalis. Ablation catheters, with tip electrodes ranging from 4 to 10 mm, are crucial for delivering targeted therapy during catheter ablation procedures.
Fluoroscopy
Fluoroscopy aids in guiding catheters to the correct position within the heart. Though some operators now use zero-fluoroscopy techniques, fluoroscopy remains a backup in some instances to ensure proper catheter placement.
Energy Sources for Ablation
Radiofrequency (RF) generators are used for catheter ablation procedures. RF energy involves delivering alternating current at 300 to 1000 kHz from the small tip of the ablation catheter to a large patch applied to the patient's skin. The RF current heats the tissue in proximity (1-2 mm) to the electrode by resistive heating. This heat is then conducted to deeper tissue where thermal injury occurs. The lesion size increases with the current density, the surface area of the electrode, the degree of contact pressure between the tissue and the catheter, and the temperature at the catheter tip-tissue interface. If the temperature exceeds 100 °C, boiling occurs, resulting in a sudden rise in electrical impedance. Steam pops also occur, which may result in tissue injury. RF generators come with safety features that terminate energy delivery if there's a sudden rise in impedance. The power delivered can also be reduced not to exceed the maximum temperature at the catheter tip, typically 50 to 60 °C.
RF ablation for cardiac arrhythmias is associated with a 70% incidence of coagulum formation on the catheter tip during ablation and a 10% incidence of thromboembolic events. Catheter tip thrombus can impede RF energy to the tissue, reducing efficacy and increasing procedure times.[16] To decrease coagulum formation on the catheter tip during ablation, power output, contact force, and duration of energy delivery should be reduced as much as possible, and the catheter tip surface area should be increased as much as possible; using an irrigated catheter tip also decreases coagulum formation.
Cardioverter/Defibrillator
Defibrillation pads are attached to the patient throughout the procedure to allow for immediate cardioversion or defibrillation in the event of atrial fibrillation, ventricular tachycardia, or other life-threatening arrhythmias. This equipment ensures patient safety during ablation procedures.
Personnel
An EP study and ablation procedure for AT requires a multidisciplinary team with specialized roles to ensure both safety and procedural success. This team typically includes:
- Cardiac electrophysiologist: a highly trained cardiologist specializing in cardiac electrical activity who is responsible for diagnosing AT and performing the ablation. They interpret complex intracardiac signals and direct catheter positioning and energy delivery during ablation.
- Cardiac EP laboratory technician: assists with operating and maintaining specialized EP equipment, including multichannel recorders, catheters, and RF generators. They are key in monitoring the patient’s physiological data and assisting the electrophysiologist in technical tasks.
- Nursing staff: provides patient care before, during, and after the procedure. They monitor vital signs, administer medications, and assist in patient preparation and postprocedure recovery, ensuring the patient remains stable.
- Anesthesiology personnel: required for cases that involve deep or general anesthesia, particularly for patients with higher Mallampati classes (III & IV) who may have difficult airway management.
- Fluoroscopy technician: in cases where fluoroscopy is used, the radiographer operates the imaging equipment, assisting with the visualization of catheter placement. They also help minimize radiation exposure for the patient and the medical team.
- Backup surgical staff: although rare, emergencies such as cardiac perforation or vascular complications may require surgical intervention. Having a cardiac surgeon and surgical team on standby ensures rapid response in case an immediate surgical procedure becomes necessary.
Preparation
Preparation for an EP study and ablation of AT involves several important steps to ensure patient safety and procedural success.
Patient Counseling and Explanation
The patient must be fully informed about the procedure. The physician explains the steps involved in the EP study and ablation, outlining the potential benefits and possible complications, including bleeding, infection, or injury to the heart structures. Risks associated with radiation exposure or anesthesia should also be discussed. This helps the patient provide informed consent and sets appropriate expectations.
Preprocedural Laboratory Tests
Essential lab work includes a complete blood count to assess for anemia or infection, renal function tests to ensure proper metabolism of contrast agents and medications, a coagulation profile to check for bleeding risks, and liver function tests to assess any metabolic concerns. Abnormal results may require modification of the procedural approach or delay in the procedure until corrected.
Medication Adjustments
Antiarrhythmic drugs should be stopped before the procedure. Stopping these medications allows for better provocation of the arrhythmia during the EP study, increasing the chances of successful ablation.
Pregnancy Test
For female patients, especially those of childbearing age, a pregnancy test is mandatory if there is uncertainty about their pregnancy status. Radiation exposure during the procedure can be harmful to a developing fetus, particularly during the first trimester, and must be avoided.
Vascular Access Site Preparation
The patient is draped in a sterile fashion, and the site for vascular access (usually the femoral or subclavian vein) is cleaned thoroughly with antiseptic agents. This step mirrors the preparation for other cardiac catheterization procedures to minimize the risk of infection.
Technique or Treatment
Origin of Focal Atrial Tachycardias
Focal ATs originate from distinct areas within the atrial myocardium and are commonly identified through electroanatomic mapping. This technique helps pinpoint the precise site of earliest electrical activation, which typically precedes the onset of the P-wave on surface ECG by at least 30 ms. A unipolar electrogram from the site of origin should display a QS configuration, with a sharp downward deflection occurring before the P-wave. Ablation of the tachycardia can be performed using RF energy. Standard 4-mm electrodes, irrigated RF catheters, or large-tip electrodes are typically employed in the RA. Irrigated catheters are preferred for LA ablation to minimize the risk of thrombus formation.
Surface ECG provides valuable clues regarding the anatomical location of focal AT before the procedure, aiding in localization and procedural planning (see Image. Anatomical Distribution of Focal Atrial Tachycardia).[6][17] Clinically, focal AT often presents as long RP tachycardia (supraventricular tachycardia [SVT] characterized by an RP interval longer than the PR interval on an ECG), sometimes associated with AV block. This is found in 10% to 15% of SVTs in adults and accounts for 10% to 20% of SVTs in children undergoing ablation. These characteristics emphasize the importance of accurate mapping and targeted ablation to manage focal AT successfully.
Techniques for Diagnosing Atrial Tachycardia
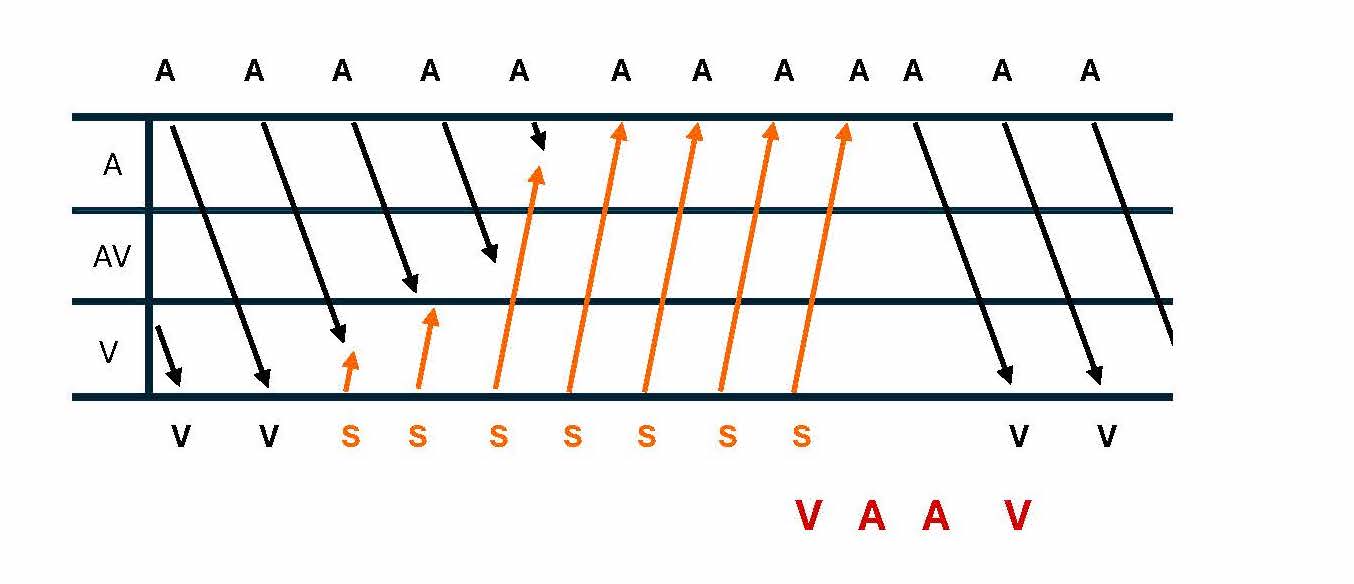
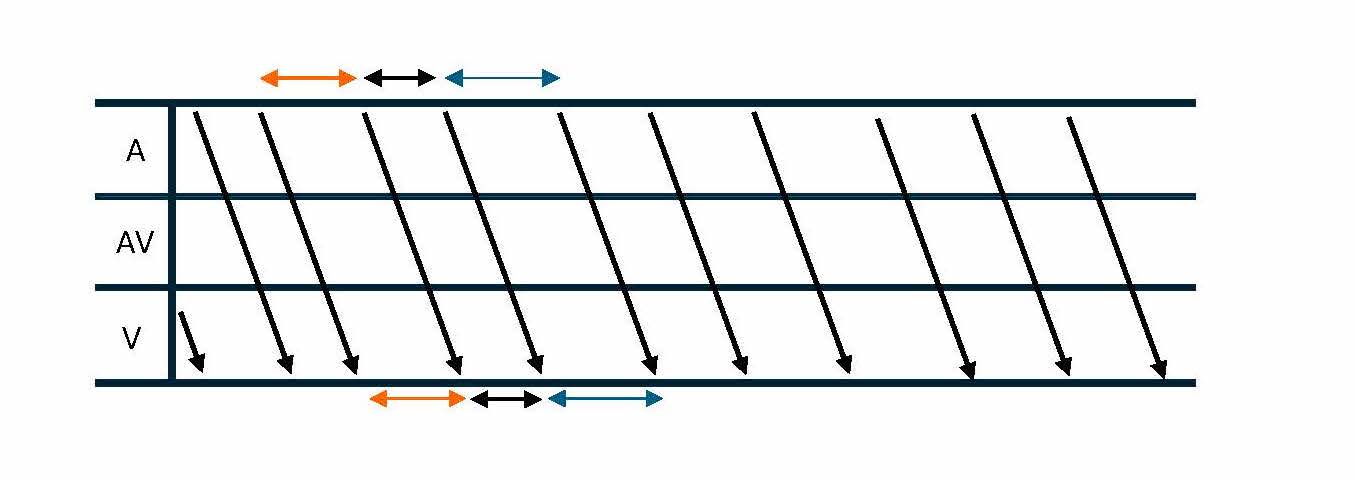
Diagnosing AT in the EP laboratory involves several techniques that help differentiate AT from other forms of SVT. One method is recognizing the A-A-V electrogram response following cessation of ventricular pacing, especially during paroxysmal SVT with 1:1 ventriculoatrial (VA) conduction (see Image. Ladder Diagram of VAAV Response).[18] AT can be excluded as the underlying mechanism of burst pacing consistently terminates the tachycardia without affecting atrial activation.[19] Variability in tachycardia cycle length, particularly changes of 15 ms or more that predict ventricular cycle length changes, may indicate AT or atypical AVNRT (see Image. Ladder Diagram of Atrial Tachycardia Cycle Length Variability).[20] Many ATs are due to reentry through large "macroreentrant" circuits, which can be difficult to define by catheter mapping of the activation sequence. Entrainment techniques are crucial for mapping macroreentrant circuits, allowing the identification of sites within the reentrant loop compared to bystander sites outside the circuit.[21]
Macroreentrant ATs, aside from typical cavotricuspid isthmus (CTI)-dependent atrial flutter, frequently occur in patients with underlying atrial disease, including cardiomyopathies, previous atrial ablations, or prior cardiac surgeries. With the increasing use of ablation for atrial fibrillation, macroreentrant ATs have become the most common form of AT observed after such procedures.[22] A hallmark of macro reentry is the ability to entrain the tachycardia with fusion; this property is inconsistent with a truly focal source. Another feature of macroreentry is the identification of activation throughout the tachycardia cycle length with adjacent zones of ‘early’ and ‘late’ activation relative to a fiducial point. Macroreentrant tachycardias are consistently insensitive to adenosine, which results in AV block but does not interrupt the tachycardia.[8]
When a macroreentrant circuit is suspected, verifying that the entire cycle length is accounted for in the electroanatomical map is essential. The inability to annotate all segments of the tachycardia cycle length might indicate the presence of a focal AT. Entrainment confirms that a particular site participates in the tachycardia circuit when the post-pacing interval is within 30 msec of the tachycardia cycle length. Once the mechanism and dimensions of the macroreentrant AT are identified, ablation strategies focus on targeting critical components of the reentrant circuit. Linear lesions are created between non-excitable barriers like scar tissue or anatomical structures. Ultrahigh-density mapping has significantly improved the ability to visualize scar regions, conduction blocks, and zones of slow conduction, facilitating the identification of a practical isthmus within the circuit. Ablation of this narrowest part of the reentrant pathway is often more effective than creating extensive linear lesions, offering a more focused and efficient approach to treatment.[23]
Approach to Different Types of Atrial Tachycardia
Different types of AT require distinct mapping and ablation strategies to achieve successful outcomes. Examples include:
Para-Hisian atrial tachycardia
A stepwise approach may be employed to map para-Hisian ATs. Firstly, the tricuspid annulus and RA septum should be carefully mapped to identify early activation at a distance (>1 cm) from the His bundle. Once this is identified, ablation can be safely and successfully performed. The noncoronary cusp should be mapped and ablated if the His bundle region demonstrates the earliest RA activation. If this is unsuccessful, mapping the LA septum should be considered.[24]
Right atrium reentry tachycardia
The RA's most common form of macro reentry is free wall reentry, typically associated with scarring (usually from previous right atriotomies) but also as a spontaneous phenomenon in myopathic atria. A linear lesion between the preexisting scar and an anatomical boundary, such as the inferior vena cava, tricuspid annulus, or superior vena cava, is accomplished by ablation of lateral wall reentry in the RA.[25]
Left atrium reentry tachycardia
Perimitral reentry is a form of tachycardia that occurs around the mitral annulus. Linear ablation can be performed in the "mitral isthmus" or from the septal mitral annulus to the right superior pulmonary vein. Interrupting tachycardia in the lateral mitral isthmus may require additional ablation in the coronary sinus to target epicardial fibres or connections between the coronary sinus and LA, including the ligament of Marshall.[26] Another common form of LA macro reentry is reentry around a pair of ipsilateral pulmonary veins, known as 'roof-dependent LA reentry'. This type of reentry can be treated with linear ablation between the superior pulmonary veins.
Complications
EP studies and atrial ablation are generally safe for treating arrhythmias, including AT, but they can carry potential complications. These complications can be classified into immediate, short-term, and long-term categories. Their risk is influenced by factors such as patient comorbidities, anatomy, the complexity of the arrhythmia, and procedural details.
Immediate Complications
Vascular access complications
Hematoma: Bleeding at the catheter insertion site (usually the femoral vein or artery) is common. Hematomas are typically managed conservatively but may require intervention if severe.[27]
Pseudoaneurysm or arteriovenous fistula: Injury to adjacent arteries or veins can result in abnormal connections (fistulas) or pseudoaneurysms, which can potentially require surgical repair.
Perforation and cardiac tamponade
Catheter manipulation can lead to the heart chambers or the great vessels perforation. This can cause pericardial effusion, resulting in cardiac tamponade, a life-threatening condition requiring emergent pericardiocentesis or surgical drainage.
Arrhythmia induction or exacerbation
During EP studies and ablation, abnormal arrhythmias may be triggered, including atrial fibrillation or ventricular tachycardia. These can sometimes be life-threatening, requiring immediate treatment with medications, cardioversion, or defibrillation.
Air embolism
Accidental introduction of air into the bloodstream during catheter manipulation or transseptal puncture can cause an air embolism, leading to ischemia or stroke if it affects the coronary or cerebral circulation.
Short-Term Complications
Thromboembolism and stroke
Thrombus formation on the catheters or ablation sites can lead to stroke or systemic embolization.[27] Anticoagulation is critical during and after the procedure. Monitoring and minimizing coagulum formation during RF ablation are essential to prevent this.
Pulmonary vein stenosis
This complication is specific to LA ablation. Ablation near the pulmonary veins can cause scarring, leading to stenosis. This can result in symptoms like dyspnea and hemoptysis. Severe cases may require pulmonary vein angioplasty or stenting.
Phrenic nerve injury
Caution is required when ablating between the inferior pulmonary veins across the posterior wall to avoid esophageal injury and when ablating near the right pulmonary veins and superior vena cava to prevent phrenic nerve injury. During RA or septal ablation, the phrenic nerve may be damaged, leading to diaphragmatic paralysis. This can cause respiratory symptoms, especially in patients with preexisting lung disease.
Long-Term Complications
Recurrence of arrhythmia
Though ablation aims to cure AT, the arrhythmia can recur in a small percentage of patients. This may necessitate a repeat ablation or additional antiarrhythmic medications.
AV block
Ablation near the AV node may lead to an AV block, which could require a permanent pacemaker implant.[27] This is especially relevant when ablation is performed near critical conduction tissue in the septum.
Atrioesophageal fistula
This complication is specific to LA ablation. Although rare, ablation in the posterior left atrium near the esophagus can cause a serious complication known as gastroesophageal fistula. This medical emergency can result in air or food entering the heart chambers, causing sepsis and often requiring surgical intervention.
Coronary artery injury
In cases of ablation near the coronary arteries, especially during procedures involving the LA, injury to the arteries can occur, which may lead to myocardial ischemia.
Radiation Exposure
For procedures requiring extensive fluoroscopic guidance, prolonged radiation exposure can pose a risk of skin injury or increase the risk of cancer over time. Newer techniques, such as zero-fluoroscopy ablation, help mitigate this risk.[28]
Infection
Though rare, infection at the vascular access site, pneumonia, or endocarditis following ablation can occur.[27] This requires antibiotic therapy and, in some cases, surgical intervention.
Death
Death as a complication of EP studies and atrial ablation is extremely rare but remains a potential risk, especially in complex procedures or in patients with significant comorbidities.[27] The mortality rate is generally low, around 0.1%, but can increase in certain clinical scenarios.
Clinical Significance
EP studies and AT ablation are clinically significant in treating patients with cardiac arrhythmias. Understanding and treating these conditions have evolved significantly, improving patient outcomes and quality of life and reducing morbidity and mortality associated with arrhythmias. EP studies allow for accurate diagnosis and characterization of arrhythmias, crucial for effective treatment planning. By identifying the precise mechanisms and locations of arrhythmias, clinicians can tailor ablation strategies to eliminate or control abnormal electrical pathways, significantly improving patient outcomes.
Successful ablation procedures can relieve debilitating symptoms such as palpitations, dyspnea, and fatigue, enhancing patients' quality of life. After undergoing ablation, many patients report improved daily activities, exercise tolerance, and overall well-being. AT is often managed with antiarrhythmic medications, which can have side effects and may not be effective for all patients. Therefore, catheter ablation is recommended in patients with symptomatic focal AT as an alternative to pharmacological therapy. When the AT can be induced in the laboratory, acute success rates above 90% to 95% have consistently been reported, with a complication rate of less than 1% to 2%.[2][29] Although uncommon, focal AT-mediated cardiomyopathy should be recognized in patients presenting with heart failure, reduced ventricular function, and persistent tachycardia.
By effectively treating AT and preventing recurrences, ablation reduces the risk of complications associated with prolonged arrhythmias, including tachycardia-induced cardiomyopathy and thromboembolic events like stroke. Successful ablation can lower the incidence of stroke and other cardiovascular events, particularly in patients with atrial fibrillation. Many studies demonstrate favourable long-term success rates for AT ablation, particularly for unifocal ATs. The procedure has become increasingly effective with advancements in catheter technology and mapping techniques, yielding low recurrence rates, especially for idiopathic AT.
EP and AT ablation are typically performed using minimally invasive techniques, associated with shorter recovery times, reduced hospital stays, and lower rates of complications compared to open-heart surgery, making them preferred options for many patients. While the upfront costs of ablation procedures can be high, studies suggest that the long-term benefits—such as reduced healthcare utilization due to fewer hospitalizations, decreased need for ongoing medication, and improved quality of life—can lead to cost savings for patients and healthcare systems. As the understanding of arrhythmias deepens, the indications for EP studies and ablation are expanding to include more complex and challenging cases, such as those involving structural heart disease and patients with significant comorbid conditions. In summary, the clinical significance of EP and AT ablation extends beyond merely treating arrhythmias; it encompasses improved patient quality of life, reduced dependence on medications, decreased risk of complications, and cost-effectiveness, all contributing to a vital role in managing cardiac arrhythmias.
Enhancing Healthcare Team Outcomes
Effective care coordination and interprofessional communication are critical for optimizing patient outcomes, safety, and team performance in caring for patients undergoing electrophysiology (EP) studies and atrial tachycardia (AT) ablation. Physicians, particularly electrophysiologists, must collaborate closely with advanced practitioners, nurses, radiographers, pharmacists, and anesthesiologists to ensure that every aspect of the patient's care is addressed. Physicians and advanced practitioners lead the diagnostic and therapeutic processes, ensuring that the arrhythmia is identified correctly and ablation procedures are performed safely. They must recognize complex arrhythmic patterns, interpret mapping data, and respond to procedural complications quickly and precisely. Close collaboration with anesthesiologists ensures appropriate sedation or anesthesia, particularly in complex cases requiring deep sedation or general anesthesia. Pharmacists are critical in managing anticoagulation therapy, antiarrhythmic medications, and periprocedural medications, ensuring drug interactions, dosing, and timing are optimized for safety and efficacy.
Nurses and EP lab technicians are essential in preparing the patient for the procedure, ensuring sterile techniques, and monitoring the patient’s vital signs and arrhythmias during the ablation. They also provide post-procedure care and education, helping patients understand recovery expectations, medication regimens, and follow-up schedules. Open communication between the healthcare team enhances team performance and patient-centred care. Preprocedural planning involves coordinated decision-making, including evaluating risks like thromboembolic events or bleeding complications while ensuring the patient understands the procedure, potential outcomes, and postoperative expectations. Clear, consistent communication between all health professionals ensures early recognition and response to complications, such as vascular injury or arrhythmia recurrence. Ultimately, this level of interprofessional collaboration improves patient outcomes and enhances patient safety and the overall efficiency of the EP team, creating a well-integrated approach to patient-centred care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Orejarena LA, Vidaillet H Jr, DeStefano F, Nordstrom DL, Vierkant RA, Smith PN, Hayes JJ. Paroxysmal supraventricular tachycardia in the general population. Journal of the American College of Cardiology. 1998 Jan:31(1):150-7 [PubMed PMID: 9426034]
Ganz LI, Friedman PL. Supraventricular tachycardia. The New England journal of medicine. 1995 Jan 19:332(3):162-73 [PubMed PMID: 7800009]
Kastor JA. Multifocal atrial tachycardia. The New England journal of medicine. 1990 Jun 14:322(24):1713-7 [PubMed PMID: 2188131]
Chen SA, Tai CT, Chiang CE, Ding YA, Chang MS. Focal atrial tachycardia: reanalysis of the clinical and electrophysiologic characteristics and prediction of successful radiofrequency ablation. Journal of cardiovascular electrophysiology. 1998 Apr:9(4):355-65 [PubMed PMID: 9581952]
Lesh MD, Van Hare GF, Epstein LM, Fitzpatrick AP, Scheinman MM, Lee RJ, Kwasman MA, Grogin HR, Griffin JC. Radiofrequency catheter ablation of atrial arrhythmias. Results and mechanisms. Circulation. 1994 Mar:89(3):1074-89 [PubMed PMID: 8124793]
Kistler PM, Roberts-Thomson KC, Haqqani HM, Fynn SP, Singarayar S, Vohra JK, Morton JB, Sparks PB, Kalman JM. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. Journal of the American College of Cardiology. 2006 Sep 5:48(5):1010-7 [PubMed PMID: 16949495]
Turkmen Y, Insulander P, Bastani H, Drca N, Saluveer O, Tapanainen J, Bourke T, Kennebäck G, Schwieler J, Braunschweig F, Jensen-Urstad M. Focal atrial tachycardia-the localization differences between men and women: A study of 487 consecutive patients. Anatolian journal of cardiology. 2020 Dec:24(6):405-409. doi: 10.14744/AnatolJCardiol.2020.93024. Epub [PubMed PMID: 33253134]
Liu CF, Cheung JW, Ip JE, Thomas G, Yang H, Sharma S, Markowitz SM, Lerman BB. Unifying Algorithm for Mechanistic Diagnosis of Atrial Tachycardia. Circulation. Arrhythmia and electrophysiology. 2016 Aug:9(8):. pii: e004028. doi: 10.1161/CIRCEP.116.004028. Epub [PubMed PMID: 27516463]
Lerman BB, Markowitz SM, Cheung JW, Liu CF, Thomas G, Ip JE. Supraventricular Tachycardia: Mechanistic Insights Deduced From Adenosine. Circulation. Arrhythmia and electrophysiology. 2018 Dec:11(12):e006953. doi: 10.1161/CIRCEP.118.006953. Epub [PubMed PMID: 30562103]
Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, Gomez-Doblas JJ, Gorenek B, Grace A, Ho SY, Kaski JC, Kuck KH, Lambiase PD, Sacher F, Sarquella-Brugada G, Suwalski P, Zaza A, ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). European heart journal. 2020 Feb 1:41(5):655-720. doi: 10.1093/eurheartj/ehz467. Epub [PubMed PMID: 31504425]
Bessière F, Mondésert B, Chaix MA, Khairy P. Arrhythmias in adults with congenital heart disease and heart failure. Heart rhythm O2. 2021 Dec:2(6Part B):744-753. doi: 10.1016/j.hroo.2021.10.005. Epub 2021 Dec 17 [PubMed PMID: 34988526]
Combes N, Derval N, Hascoët S, Zhao A, Amet D, Le Bloa M, Maltret A, Heitz F, Thambo JB, Marijon E. Ablation of supraventricular arrhythmias in adult congenital heart disease: A contemporary review. Archives of cardiovascular diseases. 2017 May:110(5):334-345. doi: 10.1016/j.acvd.2017.01.007. Epub 2017 Mar 27 [PubMed PMID: 28359691]
Havranek S, Fingrova Z, Dusik M, Dytrych V, Ambroz D, Jansa P. Benefits from catheter ablation in patients with pulmonary hypertension: Recent advances. Kardiologia polska. 2024:82(6):602-608. doi: 10.33963/v.phj.101246. Epub [PubMed PMID: 38973418]
Level 3 (low-level) evidenceLubitz SA, Fischer A, Fuster V. Catheter ablation for atrial fibrillation. BMJ (Clinical research ed.). 2008 Apr 12:336(7648):819-26. doi: 10.1136/bmj.39513.555150.BE. Epub [PubMed PMID: 18403546]
Mladoniczky S, Nagy Z, Földesi C, Som Z, Bálint HO, Környei L, Ruzsa D, Fődi E, Simor T, Kardos A. Case series of catheter-based arrhythmia ablation in 13 pregnant women. Clinical cardiology. 2023 Aug:46(8):942-949. doi: 10.1002/clc.24072. Epub 2023 Jul 5 [PubMed PMID: 37408170]
Level 2 (mid-level) evidenceLim B, Venkatachalam KL, Henz BD, Johnson SB, Jahangir A, Asirvatham SJ. Prevention of Coagulum Formation With Simultaneous Charge Delivery in Radiofrequency Ablation: A Canine Model. JACC. Clinical electrophysiology. 2016 Apr:2(2):233-241. doi: 10.1016/j.jacep.2015.11.007. Epub 2016 Mar 23 [PubMed PMID: 29766876]
Kistler PM, Chieng D, Tonchev IR, Sugumar H, Voskoboinik A, Schwartz LA, McLellan AJ, Prabhu S, Ling LH, Al-Kaisey A, Parameswaran R, Anderson RD, Lee G, Kalman JM. P-Wave Morphology in Focal Atrial Tachycardia: An Updated Algorithm to Predict Site of Origin. JACC. Clinical electrophysiology. 2021 Dec:7(12):1547-1556. doi: 10.1016/j.jacep.2021.05.005. Epub 2021 Jun 30 [PubMed PMID: 34217661]
Knight BP, Zivin A, Souza J, Flemming M, Pelosi F, Goyal R, Man C, Strickberger SA, Morady F. A technique for the rapid diagnosis of atrial tachycardia in the electrophysiology laboratory. Journal of the American College of Cardiology. 1999 Mar:33(3):775-81 [PubMed PMID: 10080480]
Knight BP, Ebinger M, Oral H, Kim MH, Sticherling C, Pelosi F, Michaud GF, Strickberger SA, Morady F. Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. Journal of the American College of Cardiology. 2000 Aug:36(2):574-82 [PubMed PMID: 10933374]
Crawford TC, Mukerji S, Good E, Chugh A, Bogun F, Pelosi F Jr, Oral H, Morady F, Jongnarangsin K. Utility of atrial and ventricular cycle length variability in determining the mechanism of paroxysmal supraventricular tachycardia. Journal of cardiovascular electrophysiology. 2007 Jul:18(7):698-703 [PubMed PMID: 17537206]
Stevenson WG, Sager PT, Friedman PL. Entrainment techniques for mapping atrial and ventricular tachycardias. Journal of cardiovascular electrophysiology. 1995 Mar:6(3):201-16 [PubMed PMID: 7620645]
Markowitz SM, Thomas G, Liu CF, Cheung JW, Ip JE, Lerman BB. Atrial Tachycardias and Atypical Atrial Flutters: Mechanisms and Approaches to Ablation. Arrhythmia & electrophysiology review. 2019 May:8(2):131-137. doi: 10.15420/aer.2019.17.2. Epub [PubMed PMID: 31114688]
Takigawa M, Derval N, Frontera A, Martin R, Yamashita S, Cheniti G, Vlachos K, Thompson N, Kitamura T, Wolf M, Massoullie G, Martin CA, Al-Jefairi N, Amraoui S, Duchateau J, Klotz N, Pambrun T, Denis A, Sacher F, Cochet H, Hocini M, Haïssaguerre M, Jais P. Revisiting anatomic macroreentrant tachycardia after atrial fibrillation ablation using ultrahigh-resolution mapping: Implications for ablation. Heart rhythm. 2018 Mar:15(3):326-333. doi: 10.1016/j.hrthm.2017.10.029. Epub 2017 Nov 23 [PubMed PMID: 29081399]
Wang Z, Ouyang J, Liang Y, Jin Z, Yang G, Liang M, Li S, Yu H, Han Y. Focal atrial tachycardia surrounding the anterior septum: strategy for mapping and catheter ablation. Circulation. Arrhythmia and electrophysiology. 2015 Jun:8(3):575-82. doi: 10.1161/CIRCEP.114.002281. Epub 2015 Apr 23 [PubMed PMID: 25908691]
Kanagasundram AN, Baduashvili A, Liu CF, Cheung JW, Thomas G, Ip JE, Young SD, Lerman BB, Markowitz SM. A novel criterion for conduction block after catheter ablation of right atrial tachycardia after mitral valve surgery. Circulation. Arrhythmia and electrophysiology. 2013 Feb:6(1):39-47. doi: 10.1161/CIRCEP.112.976340. Epub 2012 Dec 16 [PubMed PMID: 23243191]
Jaïs P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clémenty J, Haïssaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004 Nov 9:110(19):2996-3002 [PubMed PMID: 15520313]
Steinbeck G, Sinner MF, Lutz M, Müller-Nurasyid M, Kääb S, Reinecke H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: a nationwide in-hospital analysis of administrative data for Germany in 2014. European heart journal. 2018 Dec 1:39(45):4020-4029. doi: 10.1093/eurheartj/ehy452. Epub [PubMed PMID: 30085086]
Piros K, Perge P, Salló Z, Herczeg S, Nagy VK, Osztheimer I, Merkely B, Gellér L, Szegedi N. Zero fluoroscopy ablation for atrioventricular nodal reentrant tachycardia and typical atrial flutter is equally safe and effective with EnSite NavX, Carto3, and Rhythmia mapping systems. Frontiers in cardiovascular medicine. 2023:10():1185187. doi: 10.3389/fcvm.2023.1185187. Epub 2023 Jul 25 [PubMed PMID: 37560116]
Ferrero de Loma-Osorio Á, Díaz-Infante E, Macías Gallego A, Spanish Catheter Ablation Registry Collaborators. Spanish Catheter Ablation Registry. 12th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2012). Revista espanola de cardiologia (English ed.). 2013 Dec:66(12):983-92. doi: 10.1016/j.rec.2013.08.002. Epub 2013 Nov 5 [PubMed PMID: 24774111]