Electrophysiology Study and Ablation of Atrioventricular Nodal Reentrant Tachycardia
Electrophysiology Study and Ablation of Atrioventricular Nodal Reentrant Tachycardia
Introduction
Atrioventricular nodal reentrant tachycardia (AVNRT) is the most common form of regular supraventricular tachycardia and is routinely treated with catheter ablation (CA). AVNRT most commonly affects young and middle-aged otherwise healthy adults; females are more frequently affected than males.[1][2]
AVNRT is characterized by a reentrant circuit involving fast and slow pathways near the compact AV node; the ventricles do not participate in the tachycardia circuit. Although the exact location of this circuit remains elusive, specific anatomical landmarks have been used successfully to guide arrhythmia treatment with CA over the past 25 years.[3]
Differentiating AVNRT from other supraventricular tachycardias can be challenging and often requires specific pacing maneuvers during an electrophysiology study.[4] This activity will address the main features of the electrophysiological diagnosis and ablation therapy of AVNRT.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Moe and colleagues were the first to describe 2 pathways with different conduction times and refractoriness within the atrioventricular (AV) node by delivering premature atrial stimuli in animal models.[5] The physiology of a similar dual AV node pathway was subsequently described in humans.[6] These initial findings supported the hypothesis of AV nodal functional dissociation as a potential arrhythmogenic substrate responsible for developing a specific form of paroxysmal reentrant tachycardia initially described as AV junctional tachycardia, now known as AVNRT.[7] The effective treatment of this type of supraventricular tachycardia with CA targeting the putative regions of the fast pathway above the His bundle and the slow pathway below it provided additional evidence for dual AV node physiology.[8]
Subsequent electrophysiological studies challenged the initial concept of AVNRT as a reentrant tachycardia limited to the AV node, revealing that the reentrant circuit of AVNRT extended beyond the compact AV node into the surrounding myocardium.[9] This finding could eventually explain the variants of tachycardia involving the potential presence of more than one slow pathway. It was suggested that a posterior extension of the AV node corresponded to the slow pathway, while the fast pathway was located within an anterior nodal extension. Autopsy studies confirmed the presence of lesions several millimeters below the compact AV node after a successful AVNRT ablation.[10]
Inoue and colleagues provided additional histological evidence of rightward and leftward posterior AV nodal extensions, while more recent histological studies described connections between the compact AV node and the atrial myocardium, most probably representing the fast pathway.[11][12] Recently, Katritsis et al, using histological and electrophysiological observations, proposed an updated AV nodal circuit model extending even beyond the borders of the rightward and leftward posterior nodal extensions and involving parts of the tricuspid and mitral vestibules.[13]
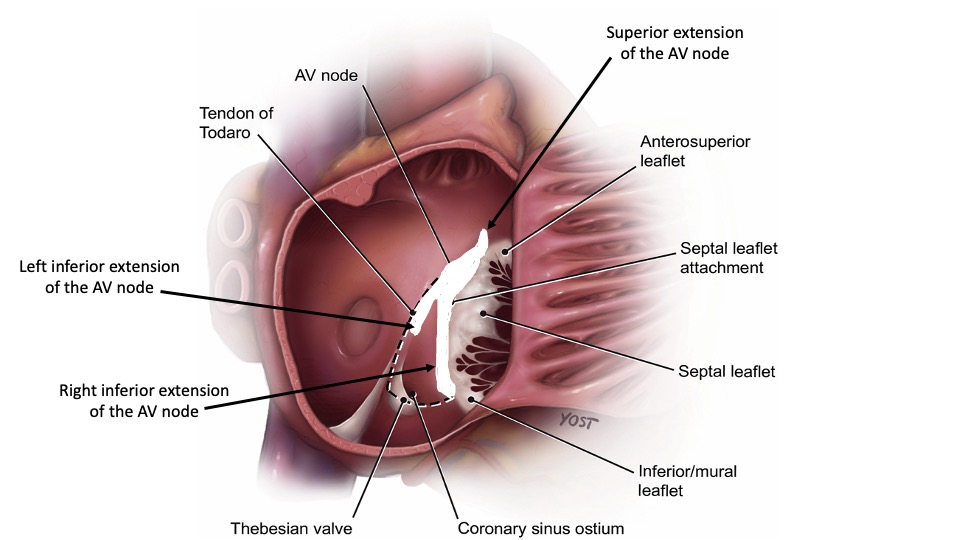
Although the exact anatomic substrate contributing to the development of AVNRT remains incompletely understood, electrophysiologists rely on the triangle of Koch as a guide for catheter placement during ablation procedures. This triangle is bounded by the ostium of the coronary sinus inferiorly, the septal leaflet of the tricuspid valve anteriorly, and the tendon of Todaro posterosuperiorly. While there have been some objections to this approach, it is still widely used in everyday clinical practice.[14][15] (see Image. Triangle of Koch)
AVNRT is a narrow QRS complex paroxysmal tachycardia with abrupt onset and termination. AVNRT may occasionally present with a QRS complex >120 ms when aberrant AV conduction occurs. The reentrant circuit is primarily confined to the AV nodal region and does not necessarily involve the atria and ventricles. Even in cases of AV dissociation, tachycardia can continue without interruption.[16] There are currently 2 recognized forms of AVNRT, typical and atypical.
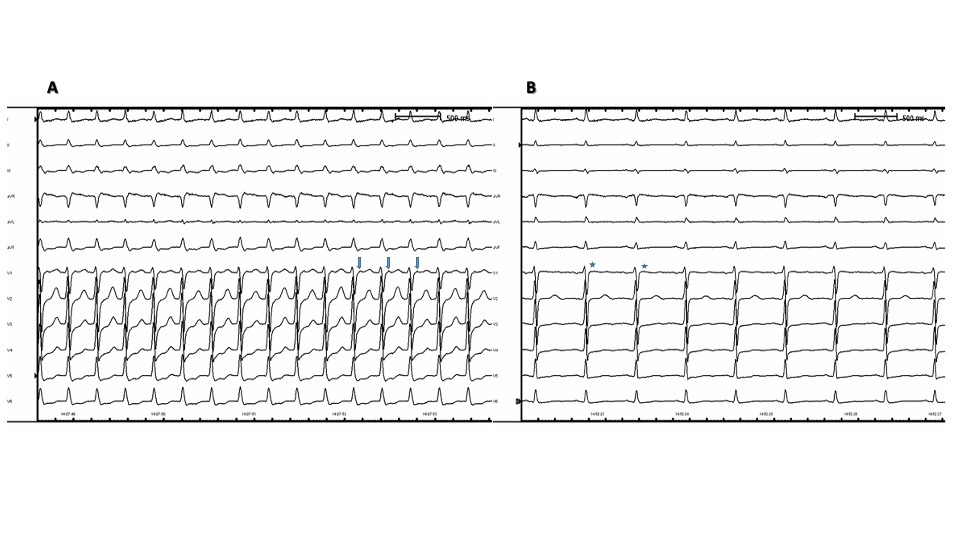
Typical AVNRT, also known as slow-fast AVNRT, is usually identified by the absence of distinguishable P waves, resulting in short RP tachycardia. Due to antegrade conduction through the slow pathway and retrograde conduction through the fast pathway of the circuit, atrial signals are either hidden or appear as a slight deflection at the start or end of the QRS complex. This can be seen as a pseudo-R in lead V1 or a pseudo-S in leads II, III, and aVF. (see Image. Typical Pseudo-R Waves) An RP interval of <70 msec is a well-known ECG criterion for the noninvasive diagnosis of typical AVNRT.[16] Typical AVNRT is encountered far more often than atypical AVNRT in clinical practice.
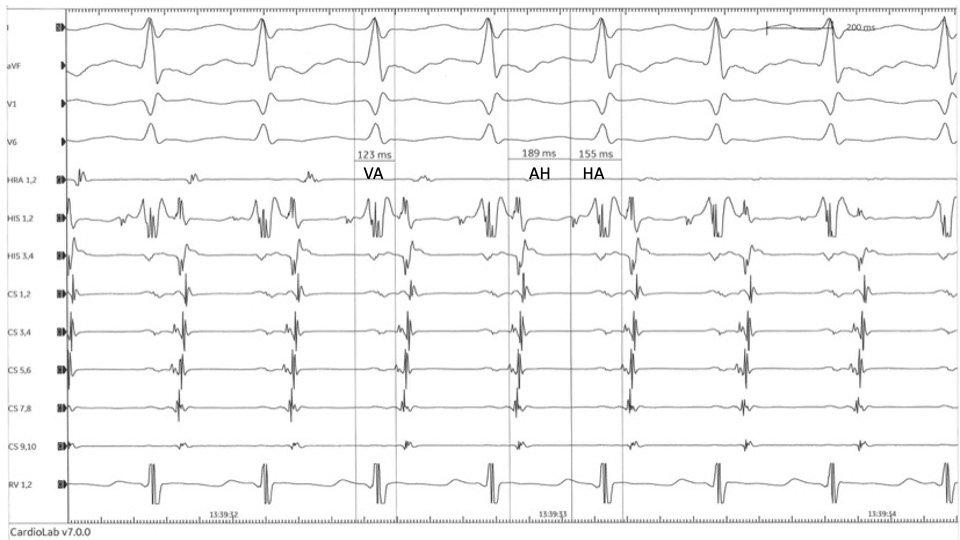
Atypical AVNRT exists in 2 variants. A fast antegrade limb and a slow retrograde limb characterize fast-slow AVNRT. In slow-slow AVNRT, where both antegrade and retrograde limbs are slow, P waves may be observed before the QRS due to the retrograde conduction through the slow pathway, resulting in a long RP tachycardia.[17] After an atrial extrasystole, a significant extension of the PR interval known as the A-H jump indicates the initial forward conduction through the slow pathway. (see Image. AVNRT AH Intervals)
Although clinical and ECG criteria are essential for the initial diagnostic approach to AVNRT, only electrophysiology studies can distinguish between different forms of regular supraventricular arrhythmias, confirm an AVNRT diagnosis, and guide treatment using CA.
Indications
AVNRT can significantly impact the quality of life.[18] Additionally, it may result in increased healthcare expenditures secondary to frequent hospital admissions and outpatient visits.[19] Data indicates that CA can improve quality of life and decrease healthcare expenses for patients with AVNRT; antiarrhythmic drugs are less effective at reducing hospitalizations.[18][20]
For symptomatic patients with recurrent episodes of AVNRT, CA is the treatment of choice for the long-term management of arrhythmia. According to recent contemporary clinical practice guidelines, CA is a Class I indication.[3]
Contraindications
There is no absolute contraindication to performing an electrophysiology study and CA in patients with AVNRT. It appears safe to pursue invasive treatment for supraventricular arrhythmias, even in elderly patients.[21] However, patients with preexisting PR prolongation may be at increased risk of developing a more severe AV block during or after the procedure.[22]
Equipment
Whether the electrophysiology laboratory is dedicated or also used for cardiac catheterization, it must include the following equipment and components.
- Single- or bi-plane fluoroscopic system, including C-arm, radiographic table, and image intensifier
- Electrophysiology data acquisition system, including computer workstation with data processing software, 12-lead ECG, at least 24 channels for intracardiac recordings, and monitors for displaying the electrograms
- Programmable electrical stimulator for delivering burst pacing and premature stimuli
- Radiofrequency ablation system and cryo-console for cryoablation
- Diagnostic and ablation catheters
- Hemodynamic monitoring system
- Emergency trays for pericaridiocentesis and thoracentesis.
- Transthoracic and transesophageal echocardiography to rule out cardiac tamponade or assist the transeptal puncture during a left atrial approach
The following equipment is helpful but not considered mandatory.
- A 3-dimensional electroanatomic mapping system ensures accurate catheter placement while minimizing radiation exposure[23]
- Intracardiac echocardiography for catheter navigation facilitates a zero-fluoroscopy technique[24]
- Vascular ultrasonography to obtain femoral venous access has been associated with fewer vascular complications[25]
Personnel
The minimum staff requirements for CA are:
- Certified electrophysiologist
- First assistant
- Cardiac nurse
- Electrophysiology technician or trained electrophysiology nurse
- Standby personnel, including anesthesiology, interventional cardiology, and cardiac surgery
Preparation
Patients with AVNRT undergoing CA must fast for at least 6 hours before the procedure; discontinuing antiarrhythmic therapy for at least 5 half-lives is required. Hypoglycemic treatment must be adjusted accordingly.
Upon arrival at the electrophysiology laboratory, the patient is connected to a 12-lead ECG, blood pressure measurement cuff, pulse oximeter, and ground pad electrode. If a 3-dimensional electroanatomic mapping system is used, those electrodes must be placed.
Femoral venous access is obtained after the administration of local anesthesia.
Diagnostic catheters are placed in the right atrium, right ventricle, coronary sinus, and area of the His bundle. A 4-mm radiofrequency (RF) ablation catheter or cryoablation catheter is used for mapping and ablation of AVNRT.[26]
Patient sedation is a matter of personal preference. Most electrophysiologists avoid sedation to facilitate the induction of tachycardia. However, conscious sedation is frequently utilized, especially among pediatric patients.
Technique or Treatment
Distinguishing AVNRT, particularly its atypical form, from other supraventricular tachycardias with comparable clinical and electrocardiographic manifestations can be challenging. The main diagnostic problem is differentiating AVNRT from a focal or micro-reentrant atrial tachycardia (AT) or an orthodromic atrioventricular tachycardia (AVRT) that uses the His-Purkinje system as an antegrade limb and a septal or right-sided accessory pathway (AP) as a retrograde limb.
Observations During Sinus Rhythm and Induction of Tachycardia
Preexcitation on surface ECG while in sinus rhythm or after rapid atrial pacing makes the diagnosis of AVRNT unlikely. However, the role of an accessory pathway (AP) as a simple bystander AV connection independent of the arrhythmia mechanism cannot be excluded.[27]
Concentric atrial activation after ventricular pacing, meaning coronary sinus signals with a proximal to distal configuration implying retrograde activation through the His-Purkinje system and not through the left lateral ventricular wall, excludes a retrograde conducting left AP. Ventriculoatrial (VA) block excludes any form of AP, ruling out the diagnosis of AVRT. However, the presence of concentric retrograde atrial activation does not rule out the existence of a septal or right-sided AP.
Dual AV nodal physiology is the sudden increase of AH time by >50 msec after a 10 msec decrement of the coupling interval of an extrasystole delivered through atrial pacing. This Atrial-His interval jump, or AH jump, represents a switch of the antegrade AV conduction from a pathway with a short conduction time and a long refractory period (fast pathway) to another pathway with a longer conduction time but a shorter refractory period (slow pathway).[1]
Dual AV nodal physiology has a predictive value of 86% for the diagnosis of AVNRT.[28] It is possible to detect the existence of dual AV nodal physiology in 85 to 95% of patients with documented AV nodal reentrant tachycardia; this phenomenon can also be present in 5 to 10% of subjects who have never exhibited such an arrhythmia. Accordingly, even if there is evidence of an AH jump, AVNRT may not necessarily be the clinical arrhythmia. It is also possible to demonstrate dual AV nodal physiology through the simultaneous transmission of an atrial-paced beat through both the fast and slow pathways, resulting in a 1:2 AV conduction with varying AH intervals.[29]
The cross-over maneuver is another helpful technique for confirming the diagnosis of dual AV nodal physiology. This maneuver is particularly useful when an AH jump has not been demonstrated, even after repeated pacing runs.[30] Gradual prolongation of the PR interval with incremental atrial pacing can result in a PR interval exceeding the RR interval, manifested as a paced atrial impulse not conducting to the immediately following QRS complex but to the next QRS complex. This exceptionally slow conduction is possible only in the presence of a slow pathway.
Retrograde dual AV conduction further supports the diagnosis of dual AV nodal physiology. Retrograde dual conduction can be demonstrated by delivering premature ventricular extrasystoles or incremental ventricular pacing. A retrograde VA jump or a 1:2 response can be observed. Retrograde VA conduction can also indicate the area of the earliest atrial activation, which is different for the fast and slow pathways. Traditionally, the earliest retrograde atrial activation through the fast pathway has been recorded at the apex of the Koch triangle. Conversely, the earliest retrograde atrial activation through the slow pathway has been recorded at the base of the Koch triangle. Recent studies have shown a substantial heterogeneity of this pattern, with approximately 8% of patients demonstrating a left-sided earliest atrial activation through the fast pathway.[31] Although supportive of a dual AV nodal physiology, a VA jump can also occur due to an infra-His block forcing the retrogradely conducted impulse to cross the septum and reach the atria through the contralateral bundle branch.
Typical AVNRT is usually initiated with the decremental delivery of premature atrial beats. At a critical coupling interval, the atrial impulse is blocked down the fast pathway and conducted through the still-excitable slow pathway. The prolonged conduction time permits the recovery of the fast pathway, and the retrograde conduction of the impulse generates an echo atrial beat. In most cases, this initial reentry circuit continues to produce tachycardia. The critical coupling interval for initiating tachycardia can vary due to changes in autonomic tone and be affected by medications like isoproterenol, which can facilitate the induction of tachycardia.
Ventricular pacing is less effective at inducing typical AVNRT but can usually induce atypical AVNRT. Two or more premature atrial impulses are required to induce the tachycardia in some patients with dual AV node physiology; occasionally, despite extensive efforts, the tachycardia cannot be induced. In this case, the decision to proceed with ablation therapy is challenging. A recent study showed that the induction of 2 consecutive echo beats could be used as a criterion to ablate.[32]
Observations During Tachycardia
The electrophysiology study performed during tachycardia can offer additional important information. Eccentric atrial activation occurring distal to the proximal configuration in coronary sinus signals during the tachycardia, although highly suggestive of orthodromic AVRT with the use of a left-sided AP, cannot be used as the sole criterion since retrograde atrial activation through a left inferior slow pathway extension can show a similar eccentric pattern in 1% of the patients with AVNRT.[33] A septal VA interval of less than 60 to 70 msec when measured from the initial deflection of the QRS on the surface ECG to the onset of the A wave at the His electrogram practically rules out AVRT and atypical AVNRT but does not rule in typical AVNRT; a focal junctional or septal AT can have a similar configuration.[34][28] In atypical AVNRT, where the VA interval is always >60 ms, the association between AH and HA intervals can discriminate between fast-slow and slow-slow forms of tachycardia. An AH:HA <1 indicates fast-slow AVNRT, while AH:HA >1 characterizes slow-slow AVNRT.[31] (see Image. Atypical AVNRT)
Termination of the tachycardia with an AV block is highly suggestive of the participation of the AV node in the tachycardia circuit, practically excluding the diagnosis of an AT. This is especially true if the termination occurs more than once. The coincidental termination of an AT and the development of an AV block can theoretically always occur, but the probability is extremely low. Termination of the tachycardia without VA conduction and the spontaneous initiation of the tachycardia with a sudden AH prolongation is highly predictive of typical AVNRT. Contrarily, a more than 20-second increase in the VA interval with the development of a functional bundle branch block confirms the diagnosis of AVRT.[28]
Pacing Maneuvers During Tachycardia
Using specific atrial and ventricular pacing protocols during tachycardia is an important step in the diagnostic algorithm. Pacing maneuvers can confirm or exclude the diagnosis of AVNRT and dictate the therapeutic strategy.
The most common initial step is a fast rule-in or rule-out of AT using ventricular overdrive pacing (VOP) from the right ventricular apex for 15 to 20 beats at a cycle length 10 to 30 msec shorter than the tachycardia cycle length (TCL). The capture of the ventricle, acceleration of the atrial cycle length to the pacing cycle length, and continuation of the tachycardia after pacing are required to make the test interpretable. A post-VOP ventricular-atrial-atrial-ventricular (V-A-A-V or V-A-A-His-V) response, as measured from the last-paced ventricular electrogram to the first after-pacing ventricular electrogram, rules in AT. This happens due to the refractoriness of the AV conduction system caused by the last retrogradely conducted paced impulse. Since the AV conduction system is still refractory, it does not permit further antegrade travel of this impulse back to the ventricle. The second atrial signal comes from the ectopic atrial focus, which continues to fire after pacing.
The only shortcoming of this maneuver is that frequently in AT, the paced ventricular impulse does not reach the atria; this is VA dissociation. Nevertheless, even this finding is helpful. VA dissociation excludes the presence of an accessory pathway, rules out AVRT, and suggests but does not prove the diagnosis of AT.[35] A post-VOP ventricular-atrial-ventricular response (V-A-V or V-A-His-V) rules out AT since the last-paced beat can echo back to the ventricle through an ongoing circuit that includes the AV node.
Although post-VOP responses cannot differentiate between AVNRT and AVRT, VOP can offer additional diagnostic information. The paced, or orthodromic, impulse continuously resets or entrains the tachycardia to the paced cycle length via the excitable gap of the tachycardia circuit. A proportion of the same paced impulse travels in the opposite direction of the circuit; this is the antidromic impulse. This antidromic impulse collides somewhere in the ventricle or the AV-His-Purkinje system with the tachycardia wavefront. A collision in the ventricular myocardium leads to manifest entrainment, a fusion morphology of the QRS complex, and confirms the diagnosis of AVRT.
Collision within the AV conduction system will not manifest as QRS complex fusion; thus, entrainment cannot be proven.[34] However, this phenomenon, known as concealed entrainment, can occur in AVNRT and AVRT. Therefore, the absence of fusion cannot distinguish between the 2 dysrhythmias. Pacing away from the His-Purkinje insertion to the ventricular myocardium but still close to the ventricular insertion of an AP may uncover the entrainment by permitting more myocardium to be depolarized from the orthodromic impulse before it collides with the antidromic one. This is the case for pacing from the right ventricular apex for right-sided and septal pathways and pacing from the left ventricular apex for left-sided pathways.
Additional measurements during and after VOP can further contribute to a final diagnosis. The distance the paced beat has to travel to penetrate the arrhythmia circuit and subsequently return to the pacing site is considerably longer in AVNRT when compared to AVRT. On the other hand, the circuit of AVNRT is significantly shorter. Accordingly, in AVNRT, the VA interval during pacing (StimA) will be long, while the VA interval during tachycardia will be short. A StimA-VA difference >85 ms favors the diagnosis of AVNRT.
For the same reason, if VOP interrupts the tachycardia, the post-pacing interval (PPI) is expected to be long in AVNRT, while in AVRT, it will be shorter due to the proximity of the ventricular insertion of the AP with the pacing site. Consequently, a PPI-TCL difference >115 ms suggests AVNRT as the most likely diagnosis.[36] A corrected formula can be used to compensate for a false prolongation of the PPI due to A-His interval prolongation of the first return beat caused by decremental conduction properties of the AV node. The corrected PPI-TCL (cPPI-TCL) difference can be calculated by subtracting the A-His difference of the first return beat from the A-His interval measured during the tachycardia. A cPPI-TCL >110 ms supports the diagnosis of AVNRT.[37] However, this finding can not exclude the presence of a slow-conducting septal AP.[38]
Atrial resetting during the transition zone of the VOP is an additional criterion with a high predictive value for AVRT. During the initiation of pacing, a fusion of the antegrade tachycardia impulse with the paced beat can lead to a transition zone or a progressive change in the QRS complex. This is followed by stabilization of the QRS morphology as constant fusion or fully paced. Due to the refractoriness of the His-Pukinje system from the tachycardia impulse, which activates the integrated part of the ventricle, it is impossible to reset the atrial activity during the transition zone unless a retrograde conducting AP coexists. For the same reason, the advancement of the atrium to the pacing cycle length happens unanimously late after the transition zone in AVNRT.
The role of the AP as a simple bystander cannot be excluded. However, studies have shown that atrial advancement or "atrial timing perturbation" and a fixed stimulus-to-atrial interval within the transition zone or in the first beat after have a high diagnostic yield for AVRT.[39] Additionally, termination of the tachycardia within the transition zone favors the diagnosis of AVRT.[40] A short burst of ventricular pacing can be beneficial in cases where VOP consistently terminates the tachycardia, making it challenging to identify the mentioned features.[41] This can induce VA dissociation, which excludes AVRT, or terminate the tachycardia without atrial conduction, ruling out AT.[28]
Additional pacing maneuvers when VOP is not diagnostic include the delivery of ventricular premature beats (VPB) during diastole, para-Hisian or differential pacing (apex vs. base), and atrial overdrive pacing (AOP). A single VPB delivered when the His bundle is refractory will affect atrial timing only in the presence of an AP, either as a participant in the arrhythmia mechanism or as a bystander; this is very rare. Accordingly, this finding strongly favors the diagnosis of AVRT. However, even the presence of an AP as a simple bystander is practically excluded if a VPB delivered during His bundle refractoriness terminates the tachycardia without conducting to the atria. This finding suggests that an accessory pathway connecting the atria and the ventricles is an active part of the arrhythmia mechanism.
Both para-Hisian and differential ventricular pacing during sinus rhythm can indicate the presence of a septal AP. A lengthening of the stimulus-to-atrial time with the loss of His bundle capture indicates AV nodal conduction alone. Similarly, in the presence of a septal AP, the VA conduction time is expected to be shorter during basal pacing than apical pacing. These maneuvers have limited diagnostic accuracy in the case of a slow-conducting AP due to the possibility of retrograde fusion over the conducting system and the accessory pathway.[42]
Finally, AOP can be beneficial in cases where VOP has been unsuccessful in distinguishing between AVNRT from AT. VA linking during AOP, where the VA interval of the first post-pacing beat is not greater than 10 ms from the VA interval of the tachycardia, favors the diagnosis of AVNRT.[28]
In summary, differentiating AVNRT, particularly the atypical form, from AVRT or AT can be complicated. A single 100% sensitive diagnostic criterion does not exist.[43] However, a combination of tachycardia features and pacing maneuvers can establish the diagnosis in the majority of patients. A thorough understanding of this diagnostic algorithm is crucial for planning the subsequent therapeutic strategy with catheter ablation.
Differentiating typical AVNRT from the rare focal junctional ectopic tachycardia (JET) can be challenging. However, this distinction is important since the ablation of JET can be associated with a significantly higher risk of AV block.[44] Overdrive ventricular pacing during tachycardia entrains AVNRT but suppresses JET. Consequently, at progressively shorter pacing cycle lengths, the return cycle length remains stable in AVNRT, while it is expected to increase in JET. Introducing a premature atrial beat or atrial burst pacing during tachycardia can also facilitate the diagnosis. An early-coupled premature atrial beat conducted to the ventricles through the fast pathway tends to terminate AVNRT due to the refractoriness of the fast pathway to the next retrograde tachycardia impulse. Conversely, it may advance the immediate JET beat, but the tachycardia will likely continue. Moreover, a late-coupled atrial premature beat introduced when the His bundle is refractory will not affect JET; it will perturb, advance, or delay the subsequent His electrogram through antegrade conduction via the slow pathway in AVNRT.[45]
Atrial overdrive pacing slightly faster than the tachycardia will suppress JET, and after cessation of pacing, the tachycardia will continue with a junctional beat starting with a His signal. This will produce an A-His-His-A response. The same pacing maneuver will entrain AVNRT. The paced beat will conduct antegrade via the slow pathway since the fast pathway will be refractory due to the retrograde tachycardia wavefront. However, the last-paced beat will conduct antegrade, producing both A and His signals, but will also echo back in the atrium, giving an additional A signal (A-His-A response). Accurate measurement of His-His intervals will help correctly identify the last-paced atrial beat and the immediate His signal in an AVNRT with delayed conduction through the slow pathway. This will avoid including the last before the cessation of pacing A and His signals in the measurement, which can lead to the false diagnosis of an A-His-His-A response.[46]
Catheter Ablation
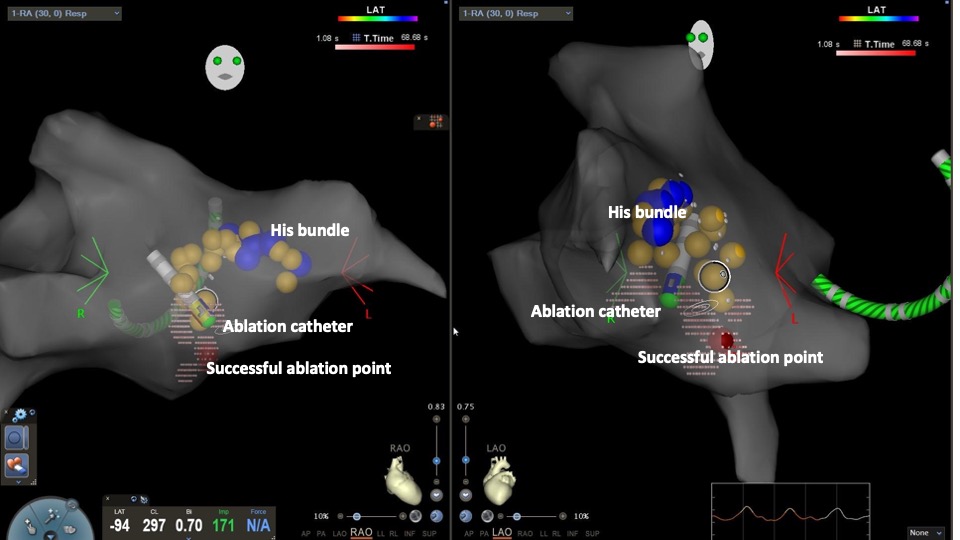
Catheter Ablation (CA) using RF energy (RF-ablation) or tissue freezing (cryoablation) is currently the treatment of choice for symptomatic patients. The slow pathway located in the right-sided inferior extension of the AV node (right inferoposterior septal region) is commonly the ablation target. The procedure is usually performed under fluoroscopic guidance. Recently, three-dimensional electroanatomic mapping has gained popularity due to the more accurate identification of the slow pathway; it also minimizes or eliminates radiation exposure, especially in the pediatric population.[47][48] (see Image. 3-dimensional Reconstruction of the Right Atrium With CARTO Mapping)
A combined electroanatomical approach is used in most electrophysiology labs.[49] A 4-mm nonirrigated-tip ablation catheter is advanced in the right ventricle under fluoroscopy in the right anterior oblique (RAO) or left anterior oblique (LAO) projection. The catheter is slowly withdrawn to reach the septal area between the septal leaflet of the tricuspid valve and the ostium of the coronary sinus. Keeping the catheter below the ostium of the coronary sinus practically eliminates the risk of AV block. (see Image. 3dimensional Reconstruction of the Right Atrium With CARTO Mapping)
A gentle clockwise rotation of the catheter in the LAO view facilitates contact with the interatrial septum. Occasionally, a long sheath can help to navigate a prominent Eustachian ridge, which tends to push the catheter away during systole. An A:V signal ratio <1 at the recording of the ablation catheter is sought. Additional catheter-guiding signals include a multicomponent low-potential atrial electrogram and an atrial electrogram that is delayed compared to the His catheter; this most likely represents the slow pathway potential.[50][51]
RF-ablation is performed at an electrical power of 30 to 50 W, targeting a temperature of 50 to 60°C for 20 to 30 sec until a junctional rhythm is elicited. If a junctional block is elicited, ablation continues. It is crucial to stop RF-current delivery immediately if an AV or a VA block develops.
The optimal ablation approach following an unsuccessful ablation procedure at the right lower septum remains controversial. Some operators would ablate at the right mid-septal area, perhaps using a cryoablation catheter, which lowers the risk of creating an AV block.[52] Others ablate inside the coronary sinus up to 40 mm from the ostium, targeting the muscular roof with an irrigated catheter.[53] Finally, a left-sided approach is the preferred second step for some electrophysiologists, who consider this approach the safest and most effective.[54] The left-sided approach targets the left inferior AV nodal extension, located at the inferoposterior mitral annulus. This area can be reached via a transseptal puncture or retrograde transaortic approach. The ablation catheter is positioned away from the area of the His bundle and below the ostium of the coronary sinus; this can be marked with an electrophysiology catheter positioned via the transaortic approach. At successful ablation sites, an A:V ratio <1 is recorded while the atrial signal is closer to the coronary sinus atrial electrograms in comparison to the atrial signal at the left His bundle recording.[55]
Cryoablation has emerged as a safe alternative method. Cryoablation is characterized by the reversibility of effect during the initial phase of ablation. In addition, freezing adheres the ablation catheter to the endocardium, ensuring a stable catheter position. A 6-mm cryoablation catheter is commonly used. Cryomapping with initial freezing to -30°C is performed. If no AV node conduction disturbances occur, cryoablation is performed via cryo-application up to 70 to 80°C for 4 to 6 min. A randomized trial showed that cryoablation was initially as effective as RF ablation. However, more arrhythmia recurrences were observed in the cryoablation arm during the 6-month follow-up.[56] Similarly, a retrospective study in a pediatric population showed a 3 times higher recurrence rate in the cryoablation group during the 14-month follow-up period.[57]
Recent studies have shown that the area of the slow pathway, either right or left posterior extensions, is also the appropriate ablation target for atypical AVNRT. In most cases, the arrhythmia can be ablated in the right posteroseptal area; only a few patients require a left-sided approach.[58] The conversion of typical AVNRT to atypical AVNRT after slow pathway ablation has been reported. In these cases, a slight anterior movement of the ablation catheter eliminated the arrhythmia.[59] These findings support the hypothesis that all types of AVNRT share a common slow conduction component, most likely located in the area of the posterior AV nodal extension.[60] However, a rare form of the slow-slow variant, known as the superior variant, has been reported, wherein retrograde conduction utilizes the superior extension of the AV node located above the compact AV node.[61] Although posteroseptal ablation may eliminate the arrhythmia even in these cases, a more superior procedure near the right perinodal area or even a left-sided transaortic approach from the noncoronary aortic cusp may be necessary.[62]
The development of an accelerated junctional rhythm is a sensitive but not specific marker of ablation success.[63] The duration of junctional rhythm has shown a positive association with a more favorable outcome.[64] The underlying mechanism is unclear; increased automaticity of the AV node due to thermal injury has been proposed. Contrarily, the development of a more rapid junctional rhythm has been unanimously considered a marker of direct injury to the His bundle and imminent AV block. However, this was not confirmed in a recent study.[65]
Complications
Although CA of AVNRT is considered a safe procedure, it carries a low but definite risk of AV block, which may require immediate or late implantation of a permanent pacemaker.[66] AV block can affect 1 to 2.3% of patients after slow pathway ablation.[67] Ablation of the fast pathway has been associated with a significantly higher risk of AV block; this procedure is reserved for patients with a PR prolongation suggestive of poor antegrade fast-pathway conduction.[68]
Other complications, including vascular access complications, cardiac perforation, tamponade, bleeding, or thromboembolic events, are rare.[69] A recent meta-analysis showed that all-cause mortality from ablation of supraventricular tachycardia is as low as 0.1%, while the rate of adverse events was 2.9%.[70]
Clinical Significance
Although non-inducibility of the arrhythmia and complete elimination of the conduction over the slow pathway without impairing either antegrade or retrograde fast pathway conduction is considered the optimal ablation endpoints, several studies have indicated that complete elimination of the slow pathway is not mandatory for clinical success. A single echo beat is still inducible in electrophysiology studies after ablation in many patients who remain arrhythmia-free.[71] In those patients, the electrophysiological properties of the slow pathway have been altered, but the slow pathway itself has not been wholly eradicated; this is termed slow pathway modification.
Slow pathway modification instead of slow pathway ablation may not be the appropriate treatment for patients who have undergone cryoablation. AH jump with a single echo has been associated with recurrences in these patients.[72] Accordingly, non-inducibility of the arrhythmia remains the single most credible end point of successful AVNRT ablation.[73] The long-term success rate of CA is high. Several studies have shown that the chance of recurrence is as low as 1.5%.[74][75][76]
Enhancing Healthcare Team Outcomes
Expertise in arrhythmia management is essential for accurately diagnosing and effectively treating AVNRT. Primary care practitioners, clinical cardiologists, and electrophysiologists play critical roles in decision-making before and after the procedure. Team involvement of allied healthcare professionals is equally important. Registered nurses, advanced practice providers, and cardiovascular technicians should receive training in managing complex arrhythmias.
The primary electrophysiologist performing the diagnostic and therapeutic procedures must possess the qualifications and skills to ensure safe and efficient treatment. The electrophysiologist is also accountable for educating junior clinicians involved in the procedure, dedicating adequate time to coordinating teamwork, and effectively communicating important information about procedural strategy and preprocedural and postprocedural care. Informed consent must be obtained, and all team members are responsible for effective communication within the team and with the patient.
The involvement of an anesthesiologist in AVNRT ablation is typically minimal, as conscious sedation or general anesthesia is rarely required. However, the anesthesia team must be readily available and provide educational support to the clinical staff administering sedative agents.
Advanced practice providers actively participate in patient care before and after CA. During the procedure, the clinical nursing staff diligently monitors the patient and promptly responds to any emergencies that may arise.
Electrophysiology technicians ensure equipment function, resolve technical issues, and potentially operate the recording system and stimulator.
Primary care practitioners play a crucial role in providing the electrophysiology team with feedback on the long-term effects of the CA, possible recurrences of AVNRT, and advancements in the quality of life of the patient.
A multidisciplinary team approach is essential to ensure procedural success and patient safety. Consistent training and adaptation to evolving technology, effective communication, strategic planning, and quality evaluation are crucial for achieving this objective.[77] [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
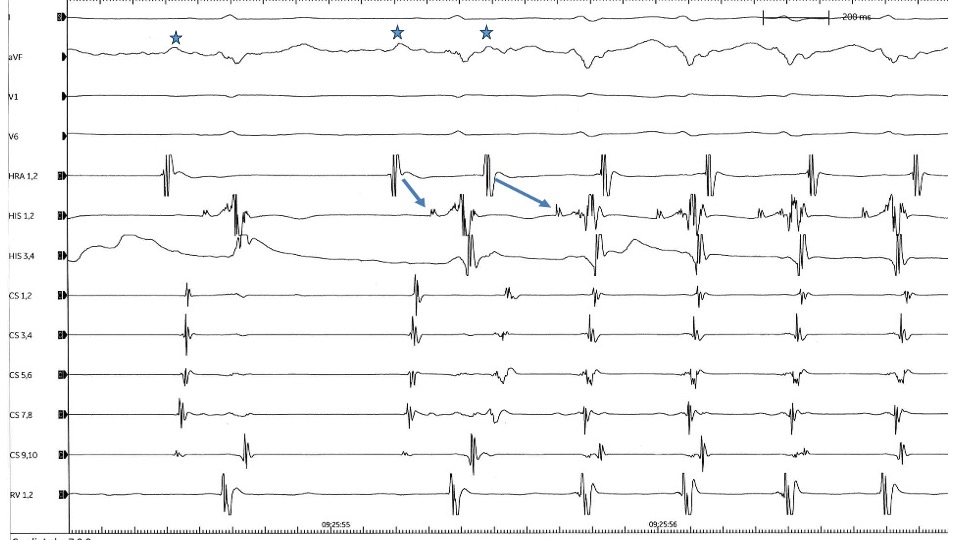
AVNRT AH Intervals. A premature atrial beat conducts to the ventricle with marked prolongation of PR and AH intervals indicating the presence of double atrioventricular nodal physiology. Conduction through the slow pathway initiates a typical atrioventricular nodal reentrant tachycardia (AVNRT). P waves are marked with stars. Contributed by Spyridon Koulouris, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Katritsis DG, Camm AJ. Atrioventricular nodal reentrant tachycardia. Circulation. 2010 Aug 24:122(8):831-40. doi: 10.1161/CIRCULATIONAHA.110.936591. Epub [PubMed PMID: 20733110]
Porter MJ, Morton JB, Denman R, Lin AC, Tierney S, Santucci PA, Cai JJ, Madsen N, Wilber DJ. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart rhythm. 2004 Oct:1(4):393-6 [PubMed PMID: 15851189]
Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, Gomez-Doblas JJ, Gorenek B, Grace A, Ho SY, Kaski JC, Kuck KH, Lambiase PD, Sacher F, Sarquella-Brugada G, Suwalski P, Zaza A, ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). European heart journal. 2020 Feb 1:41(5):655-720. doi: 10.1093/eurheartj/ehz467. Epub [PubMed PMID: 31504425]
Katritsis DG, Josephson ME. Differential diagnosis of regular, narrow-QRS tachycardias. Heart rhythm. 2015 Jul:12(7):1667-76. doi: 10.1016/j.hrthm.2015.03.046. Epub 2015 Mar 28 [PubMed PMID: 25828600]
MOE GK, PRESTON JB, BURLINGTON H. Physiologic evidence for a dual A-V transmission system. Circulation research. 1956 Jul:4(4):357-75 [PubMed PMID: 13330177]
Denes P, Wu D, Dhingra RC, Chuquimia R, Rosen KM. Demonstration of dual A-V nodal pathways in patients with paroxysmal supraventricular tachycardia. Circulation. 1973 Sep:48(3):549-55 [PubMed PMID: 4726237]
Scheinman MM, Yang Y. The history of AV nodal reentry. Pacing and clinical electrophysiology : PACE. 2005 Nov:28(11):1232-7 [PubMed PMID: 16359294]
Jazayeri MR, Hempe SL, Sra JS, Dhala AA, Blanck Z, Deshpande SS, Avitall B, Krum DP, Gilbert CJ, Akhtar M. Selective transcatheter ablation of the fast and slow pathways using radiofrequency energy in patients with atrioventricular nodal reentrant tachycardia. Circulation. 1992 Apr:85(4):1318-28 [PubMed PMID: 1555276]
McGuire MA, Bourke JP, Robotin MC, Johnson DC, Meldrum-Hanna W, Nunn GR, Uther JB, Ross DL. High resolution mapping of Koch's triangle using sixty electrodes in humans with atrioventricular junctional (AV nodal) reentrant tachycardia. Circulation. 1993 Nov:88(5 Pt 1):2315-28 [PubMed PMID: 8222125]
Olgin JE, Ursell P, Kao AK, Lesh MD. Pathological findings following slow pathway ablation for AV nodal reentrant tachycardia. Journal of cardiovascular electrophysiology. 1996 Jul:7(7):625-31 [PubMed PMID: 8807408]
Level 3 (low-level) evidenceInoue S, Becker AE. Posterior extensions of the human compact atrioventricular node: a neglected anatomic feature of potential clinical significance. Circulation. 1998 Jan 20:97(2):188-93 [PubMed PMID: 9445172]
Level 2 (mid-level) evidenceAnderson RH, Sanchez-Quintana D, Mori S, Cabrera JA, Back Sternick E. Re-evaluation of the structure of the atrioventricular node and its connections with the atrium. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2020 May 1:22(5):821-830. doi: 10.1093/europace/euaa031. Epub [PubMed PMID: 32304217]
Katritsis DG, Calkins H, Anderson RH. The Specialized Atrioventricular Ring Tissues Participate in the Circuit of Atrioventricular Nodal Reentrant Tachycardia. Journal of the American Heart Association. 2021 Nov 16:10(22):e022811. doi: 10.1161/JAHA.121.022811. Epub 2021 Oct 30 [PubMed PMID: 34719243]
James TN. The tendons of Todaro and the "triangle of Koch": lessons from eponymous hagiolatry. Journal of cardiovascular electrophysiology. 1999 Nov:10(11):1478-96 [PubMed PMID: 10571368]
Klimek-Piotrowska W, Holda MK, Koziej M, Salapa K, Piatek K, Holda J. Geometry of Koch's triangle. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017 Mar 1:19(3):452-457. doi: 10.1093/europace/euw022. Epub [PubMed PMID: 27247009]
González-Torrecilla E, Arenal A, Atienza F, Datino T, Atea LF, Calvo D, Pachón M, Miracle A, Fernández-Avilés F. EGC diagnosis of paroxysmal supraventricular tachycardias in patients without preexcitation. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2011 Jan:16(1):85-95. doi: 10.1111/j.1542-474X.2010.00399.x. Epub [PubMed PMID: 21251139]
Katritsis DG, Josephson ME. Classification of electrophysiological types of atrioventricular nodal re-entrant tachycardia: a reappraisal. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013 Sep:15(9):1231-40. doi: 10.1093/europace/eut100. Epub 2013 Apr 23 [PubMed PMID: 23612728]
Walfridsson U, Walfridsson H, Arestedt K, Strömberg A. Impact of radiofrequency ablation on health-related quality of life in patients with paroxysmal supraventricular tachycardia compared with a norm population one year after treatment. Heart & lung : the journal of critical care. 2011 Sep-Oct:40(5):405-11. doi: 10.1016/j.hrtlng.2010.09.004. Epub 2011 Mar 21 [PubMed PMID: 21419492]
Level 2 (mid-level) evidenceChew DS, Sacks NC, Emden MR, Preib MT, Cyr PL, Wood D, Pokorney SD. Trends in health care resource use and expenditures in patients with newly diagnosed paroxysmal supraventricular tachycardia in the United States. American heart journal. 2021 Mar:233():132-140. doi: 10.1016/j.ahj.2020.12.012. Epub 2021 Jan 15 [PubMed PMID: 33359780]
Katritsis DG, Zografos T, Katritsis GD, Giazitzoglou E, Vachliotis V, Paxinos G, Camm AJ, Josephson ME. Catheter ablation vs. antiarrhythmic drug therapy in patients with symptomatic atrioventricular nodal re-entrant tachycardia: a randomized, controlled trial. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017 Apr 1:19(4):602-606. doi: 10.1093/europace/euw064. Epub [PubMed PMID: 28431060]
Level 1 (high-level) evidenceWilly K, Frommeyer G, Dechering DG, Wasmer K, Höwel D, Welle SS, Bögeholz N, Ellermann C, Wolfes J, Rath B, Leitz PR, Köbe J, Lange PS, Müller P, Reinke F, Eckardt L. Outcome of catheter ablation in the very elderly-insights from a large matched analysis. Clinical cardiology. 2020 Dec:43(12):1423-1427. doi: 10.1002/clc.23455. Epub 2020 Aug 31 [PubMed PMID: 32865252]
Reithmann C, Remp T, Oversohl N, Steinbeck G. Ablation for atrioventricular nodal reentrant tachycardia with a prolonged PR interval during sinus rhythm: the risk of delayed higher-degree atrioventricular block. Journal of cardiovascular electrophysiology. 2006 Sep:17(9):973-9 [PubMed PMID: 16800857]
Level 2 (mid-level) evidenceBlockhaus C, Gülker JE, Bufe A, Seyfarth M, Koektuerk B, Shin DI. Reduction of Radiation Exposure in Atrioventricular Nodal Reentrant Tachycardia Ablations Using an Electroanatomical Mapping System With Fluoroscopy Integration Module. Frontiers in cardiovascular medicine. 2021:8():728422. doi: 10.3389/fcvm.2021.728422. Epub 2021 Oct 20 [PubMed PMID: 34746250]
Luani B, Rauwolf T, Genz C, Schmeißer A, Wiemer M, Braun-Dullaeus RC. Intracardiac echocardiography versus fluoroscopy for endovascular and endocardial catheter navigation during cryo-ablation of the slow pathway in AVNRT patients. Cardiovascular ultrasound. 2019 Jun 11:17(1):12. doi: 10.1186/s12947-019-0162-2. Epub 2019 Jun 11 [PubMed PMID: 31186001]
Wiles BM, Child N, Roberts PR. How to achieve ultrasound-guided femoral venous access: the new standard of care in the electrophysiology laboratory. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2017 Jun:49(1):3-9. doi: 10.1007/s10840-017-0227-9. Epub 2017 Feb 7 [PubMed PMID: 28168447]
Strickberger SA, Kalbfleisch SJ, Williamson B, Man KC, Vorperian V, Hummel JD, Langberg JJ, Morady F. Radiofrequency catheter ablation of atypical atrioventricular nodal reentrant tachycardia. Journal of cardiovascular electrophysiology. 1993 Oct:4(5):526-32 [PubMed PMID: 8269319]
Leonelli FM, De Ponti R, Bagliani G. Arrhythmias with Bystander Accessory Pathways. Cardiac electrophysiology clinics. 2020 Dec:12(4):495-503. doi: 10.1016/j.ccep.2020.08.009. Epub 2020 Oct 12 [PubMed PMID: 33161998]
Knight BP, Ebinger M, Oral H, Kim MH, Sticherling C, Pelosi F, Michaud GF, Strickberger SA, Morady F. Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. Journal of the American College of Cardiology. 2000 Aug:36(2):574-82 [PubMed PMID: 10933374]
Hanumanthu BK, Krummerman A, Grushko M. Simultaneous dual AV node antegrade conduction (2 for 1) and lower common pathway (2-1) block illustrate AVNRT physiology. Indian pacing and electrophysiology journal. 2020 Mar-Apr:20(2):70-72. doi: 10.1016/j.ipej.2019.12.007. Epub 2019 Dec 16 [PubMed PMID: 31857211]
Shah B, Saidullah S, Awan ZA. Cross Over: A Reliable Maneuver In The Confirmation Of Atrioventricular Nodal Reentrant Tachycardia Ablation. Journal of Ayub Medical College, Abbottabad : JAMC. 2017 Jul-Sep:29(3):408-411 [PubMed PMID: 29076671]
Katritsis DG, Josephson ME. Classification, Electrophysiological Features and Therapy of Atrioventricular Nodal Reentrant Tachycardia. Arrhythmia & electrophysiology review. 2016 Aug:5(2):130-5. doi: 10.15420/AER.2016.18.2. Epub [PubMed PMID: 27617092]
Wegner FK, Silvano M, Bögeholz N, Leitz PR, Frommeyer G, Dechering DG, Zellerhoff S, Kochhäuser S, Lange PS, Köbe J, Wasmer K, Mönnig G, Eckardt L, Pott C. Slow pathway modification in patients presenting with only two consecutive AV nodal echo beats. Journal of cardiology. 2017 Feb:69(2):471-475. doi: 10.1016/j.jjcc.2016.02.011. Epub 2016 Mar 25 [PubMed PMID: 27021469]
Green J, Aziz Z, Nayak HM, Upadhyay GA, Moss JD, Tung R. "Left ventricular" AV nodal reentrant tachycardia: Case report and review of the literature. HeartRhythm case reports. 2016 Sep:2(5):367-371. doi: 10.1016/j.hrcr.2016.03.008. Epub 2016 Jul 22 [PubMed PMID: 28491712]
Level 3 (low-level) evidenceVeenhuyzen GD, Quinn FR, Wilton SB, Clegg R, Mitchell LB. Diagnostic pacing maneuvers for supraventricular tachycardia: part 1. Pacing and clinical electrophysiology : PACE. 2011 Jun:34(6):767-82. doi: 10.1111/j.1540-8159.2011.03076.x. Epub 2011 Mar 25 [PubMed PMID: 21438892]
Maruyama M, Kobayashi Y, Miyauchi Y, Ino T, Atarashi H, Katoh T, Mizuno K. The VA relationship after differential atrial overdrive pacing: a novel tool for the diagnosis of atrial tachycardia in the electrophysiologic laboratory. Journal of cardiovascular electrophysiology. 2007 Nov:18(11):1127-33 [PubMed PMID: 17711437]
Michaud GF, Tada H, Chough S, Baker R, Wasmer K, Sticherling C, Oral H, Pelosi F Jr, Knight BP, Strickberger SA, Morady F. Differentiation of atypical atrioventricular node re-entrant tachycardia from orthodromic reciprocating tachycardia using a septal accessory pathway by the response to ventricular pacing. Journal of the American College of Cardiology. 2001 Oct:38(4):1163-7 [PubMed PMID: 11583898]
González-Torrecilla E, Arenal A, Atienza F, Osca J, García-Fernández J, Puchol A, Sánchez A, Almendral J. First postpacing interval after tachycardia entrainment with correction for atrioventricular node delay: a simple maneuver for differential diagnosis of atrioventricular nodal reentrant tachycardias versus orthodromic reciprocating tachycardias. Heart rhythm. 2006 Jun:3(6):674-9 [PubMed PMID: 16731468]
Bennett MT, Leong-Sit P, Gula LJ, Skanes AC, Yee R, Krahn AD, Hogg EC, Klein GJ. Entrainment for distinguishing atypical atrioventricular node reentrant tachycardia from atrioventricular reentrant tachycardia over septal accessory pathways with long-RP [corrected] tachycardia. Circulation. Arrhythmia and electrophysiology. 2011 Aug:4(4):506-9. doi: 10.1161/CIRCEP.111.961987. Epub 2011 Jun 2 [PubMed PMID: 21636810]
Level 2 (mid-level) evidenceAlMahameed ST, Buxton AE, Michaud GF. New criteria during right ventricular pacing to determine the mechanism of supraventricular tachycardia. Circulation. Arrhythmia and electrophysiology. 2010 Dec:3(6):578-84. doi: 10.1161/CIRCEP.109.931311. Epub 2010 Oct 22 [PubMed PMID: 20971759]
Level 2 (mid-level) evidenceMaruyama M, Uetake S, Miyauchi Y, Seino Y, Shimizu W. Analyses of the Mode of Termination During Diagnostic Ventricular Pacing to Differentiate the Mechanisms of Supraventricular Tachycardias. JACC. Clinical electrophysiology. 2017 Nov:3(11):1252-1261. doi: 10.1016/j.jacep.2017.05.014. Epub 2017 Sep 13 [PubMed PMID: 29759621]
Rosman JZ, John RM, Stevenson WG, Epstein LM, Tedrow UB, Koplan BA, Albert CM, Michaud GF. Resetting criteria during ventricular overdrive pacing successfully differentiate orthodromic reentrant tachycardia from atrioventricular nodal reentrant tachycardia despite interobserver disagreement concerning QRS fusion. Heart rhythm. 2011 Jan:8(1):2-7. doi: 10.1016/j.hrthm.2010.09.089. Epub 2010 Oct 7 [PubMed PMID: 20933101]
Level 2 (mid-level) evidenceVeenhuyzen GD, Quinn FR, Wilton SB, Clegg R, Mitchell LB. Diagnostic pacing maneuvers for supraventricular tachycardias: part 2. Pacing and clinical electrophysiology : PACE. 2012 Jun:35(6):757-69. doi: 10.1111/j.1540-8159.2012.03352.x. Epub 2012 Mar 4 [PubMed PMID: 22385228]
Kupó P, Tutuianu CI, Kaninski G, Gingl Z, Sághy L, Pap R. Limitations of ventricular pacing maneuvers to differentiate orthodromic reciprocating tachycardia from atrioventricular nodal reentry tachycardia. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2022 Mar:63(2):323-331. doi: 10.1007/s10840-021-00993-1. Epub 2021 Apr 19 [PubMed PMID: 33871788]
Hamdan M, Van Hare GF, Fisher W, Gonzalez R, Dorostkar P, Lee R, Lesh M, Saxon L, Kalman J, Scheinman M. Selective catheter ablation of the tachycardia focus in patients with nonreentrant junctional tachycardia. The American journal of cardiology. 1996 Dec 1:78(11):1292-7 [PubMed PMID: 8960595]
Level 2 (mid-level) evidenceAlasti M, Mirzaee S, Machado C, Healy S, Bittinger L, Adam D, Kotschet E, Krafchek J, Alison J. Junctional ectopic tachycardia (JET). Journal of arrhythmia. 2020 Oct:36(5):837-844. doi: 10.1002/joa3.12410. Epub 2020 Jul 27 [PubMed PMID: 33024461]
Fan R, Tardos JG, Almasry I, Barbera S, Rashba EJ, Iwai S. Novel use of atrial overdrive pacing to rapidly differentiate junctional tachycardia from atrioventricular nodal reentrant tachycardia. Heart rhythm. 2011 Jun:8(6):840-4. doi: 10.1016/j.hrthm.2011.01.011. Epub 2011 Jan 7 [PubMed PMID: 21220046]
Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing and clinical electrophysiology : PACE. 2007 Apr:30(4):510-8 [PubMed PMID: 17437575]
Level 2 (mid-level) evidenceKozluk E, Rodkiewicz D, Piątkowska A, Opolski G. Safety and efficacy of cryoablation without the use of fluoroscopy. Cardiology journal. 2018:25(3):327-332. doi: 10.5603/CJ.a2017.0065. Epub 2017 Jun 14 [PubMed PMID: 28612907]
Giazitzoglou E, Korovesis S, Kokladi M, Venetsanakos I, Paxinos G, Katritsis DG. Slow-pathway ablation for atrioventricular nodal re-entrant tachycardia with no risk of atrioventricular block. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2010 Sep-Oct:51(5):407-12 [PubMed PMID: 20876053]
Haissaguerre M, Gaita F, Fischer B, Commenges D, Montserrat P, d'Ivernois C, Lemetayer P, Warin JF. Elimination of atrioventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation. 1992 Jun:85(6):2162-75 [PubMed PMID: 1591833]
Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, Moulton KP, Twidale N, Hazlitt HA, Prior MI. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. The New England journal of medicine. 1992 Jul 30:327(5):313-8 [PubMed PMID: 1620170]
Kumar DS, Dewland TA, Balaji S, Henrikson CA. How to Approach Difficult Cases of AVNRT. Current treatment options in cardiovascular medicine. 2017 May:19(5):34. doi: 10.1007/s11936-017-0531-9. Epub [PubMed PMID: 28374333]
Level 3 (low-level) evidenceWang NC. Catheter ablation via the left atrium for atrioventricular nodal reentrant tachycardia: A narrative review. Heart rhythm O2. 2021 Apr:2(2):187-200. doi: 10.1016/j.hroo.2021.01.007. Epub 2021 Jan 29 [PubMed PMID: 34113921]
Level 3 (low-level) evidenceKatritsis DG, Becker A. The atrioventricular nodal reentrant tachycardia circuit: a proposal. Heart rhythm. 2007 Oct:4(10):1354-60 [PubMed PMID: 17905343]
Katritsis DG, John RM, Latchamsetty R, Muthalaly RG, Zografos T, Katritsis GD, Stevenson WG, Efimov IR, Morady F. Left Septal Slow Pathway Ablation for Atrioventricular Nodal Reentrant Tachycardia. Circulation. Arrhythmia and electrophysiology. 2018 Mar:11(3):e005907. doi: 10.1161/CIRCEP.117.005907. Epub [PubMed PMID: 29540373]
Deisenhofer I, Zrenner B, Yin YH, Pitschner HF, Kuniss M, Grossmann G, Stiller S, Luik A, Veltmann C, Frank J, Linner J, Estner HL, Pflaumer A, Wu J, von Bary C, Ucer E, Reents T, Tzeis S, Fichtner S, Kathan S, Karch MR, Jilek C, Ammar S, Kolb C, Liu ZC, Haller B, Schmitt C, Hessling G. Cryoablation versus radiofrequency energy for the ablation of atrioventricular nodal reentrant tachycardia (the CYRANO Study): results from a large multicenter prospective randomized trial. Circulation. 2010 Nov 30:122(22):2239-45. doi: 10.1161/CIRCULATIONAHA.110.970350. Epub 2010 Nov 15 [PubMed PMID: 21098435]
Level 1 (high-level) evidencePapagiannis J, Papadopoulou K, Rammos S, Katritsis D. Cryoablation versus radiofrequency ablation for atrioventricular nodal reentrant tachycardia in children: long-term results. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2010 Mar-Apr:51(2):122-6 [PubMed PMID: 20378513]
Level 2 (mid-level) evidenceKatritsis DG, Marine JE, Contreras FM, Fujii A, Latchamsetty R, Siontis KC, Katritsis GD, Zografos T, John RM, Epstein LM, Michaud GF, Anter E, Sepahpour A, Rowland E, Buxton AE, Calkins H, Morady F, Stevenson WG, Josephson ME. Catheter Ablation of Atypical Atrioventricular Nodal Reentrant Tachycardia. Circulation. 2016 Nov 22:134(21):1655-1663 [PubMed PMID: 27754882]
Fujiki A, Usui M, Mizumaki K, Hayashi H, Nagasawa H, Inoue H. Electrophysiological mechanisms of conversion of typical to atypical atrioventricular nodal reentrant tachycardia occurring after radiofrequency catheter ablation of the slow pathway. Japanese circulation journal. 1999 Dec:63(12):999-1001 [PubMed PMID: 10614848]
Level 3 (low-level) evidenceKatritsis DG. A unified theory for the circuit of atrioventricular nodal re-entrant tachycardia. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2020 Dec 23:22(12):1763-1767. doi: 10.1093/europace/euaa196. Epub [PubMed PMID: 32978626]
Kaneko Y, Nakajima T, Iizuka T, Tamura S, Hasegawa H, Kurabayashi M. Atypical Slow-Slow Atrioventricular Nodal Reentrant Tachycardia with Use of a Superior Slow Pathway. International heart journal. 2020 Mar 28:61(2):380-383. doi: 10.1536/ihj.19-082. Epub 2019 Dec 26 [PubMed PMID: 31875615]
Kaneko Y, Naito S, Okishige K, Morishima I, Tobiume T, Nakajima T, Irie T, Ota M, Iijima T, Iizuka T, Tamura M, Tamura S, Saito A, Igawa O, Kato R, Matsumoto K, Suzuki F, Kurabayashi M. Atypical Fast-Slow Atrioventricular Nodal Reentrant Tachycardia Incorporating a "Superior" Slow Pathway: A Distinct Supraventricular Tachyarrhythmia. Circulation. 2016 Jan 12:133(2):114-23. doi: 10.1161/CIRCULATIONAHA.115.018443. Epub 2015 Nov 5 [PubMed PMID: 26541829]
McGavigan AD, Rae AP, Cobbe SM, Rankin AC. Junctional rhythm--a suitable surrogate endpoint in catheter ablation of atrioventricular nodal reentry tachycardia? Pacing and clinical electrophysiology : PACE. 2005 Oct:28(10):1052-4 [PubMed PMID: 16221262]
Level 2 (mid-level) evidenceNikoo MH, Emkanjoo Z, Jorat MV, Kharazi A, Alizadeh A, Fazelifar AF, Sadr-Ameli MA. Can successful radiofrequency ablation of atrioventricular nodal reentrant tachycardia be predicted by pattern of junctional ectopy? Journal of electrocardiology. 2008 Jan-Feb:41(1):39-43 [PubMed PMID: 17884078]
Level 1 (high-level) evidenceSugumar H, Chieng D, Prabhu S, Voskoboinik A, Anderson RD, Al-Kaisey A, Lee G, McLellan AJ, Morton JB, Taylor AJ, Ling LH, Kalman JM, Kistler PM. A prospective evaluation of the impact of individual RF applications for slow pathway ablation for AVNRT: Markers of acute success. Journal of cardiovascular electrophysiology. 2021 Jul:32(7):1886-1893. doi: 10.1111/jce.15045. Epub 2021 May 3 [PubMed PMID: 33855753]
Sim MG, Chan SP, Kojodjojo P, Tan ESJ. Late pacemaker implantation after atrioventricular nodal reentrant tachycardia ablation: A systematic review and meta-analysis. Journal of cardiovascular electrophysiology. 2022 Nov:33(11):2297-2304. doi: 10.1111/jce.15680. Epub 2022 Sep 30 [PubMed PMID: 36124400]
Level 1 (high-level) evidenceChen H, Shehata M, Ma W, Xu J, Cao J, Cingolani E, Swerdlow C, Chen M, Chugh SS, Wang X. Atrioventricular block during slow pathway ablation: entirely preventable? Circulation. Arrhythmia and electrophysiology. 2015 Jun:8(3):739-44. doi: 10.1161/CIRCEP.114.002498. Epub [PubMed PMID: 26082530]
Tuohy S, Trulock KM, Wiggins NB, Bassiouny M, Ono M, Kiehl EL, Cantillon D, Tarakji K, Tanaka C, Dresing T, Saliba W, Varma N, Tchou P. Should fast pathway ablation be reconsidered in typical atrioventricular nodal re-entrant tachycardia? Journal of cardiovascular electrophysiology. 2019 Sep:30(9):1569-1577. doi: 10.1111/jce.14012. Epub 2019 Jul 2 [PubMed PMID: 31187543]
Eitel C, Ince H, Brachmann J, Kuck KH, Willems S, Spitzer SG, Tebbenjohanns J, Iden L, Straube F, Hochadel M, Senges J, Tilz RR. Catheter ablation of supraventricular tachycardia in patients with and without structural heart disease: insights from the German ablation registry. Clinical research in cardiology : official journal of the German Cardiac Society. 2022 May:111(5):522-529. doi: 10.1007/s00392-021-01878-z. Epub 2021 Jun 9 [PubMed PMID: 34106323]
Spector P, Reynolds MR, Calkins H, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. The American journal of cardiology. 2009 Sep 1:104(5):671-7. doi: 10.1016/j.amjcard.2009.04.040. Epub [PubMed PMID: 19699343]
Level 1 (high-level) evidenceNikoo MH, Attar A, Pourmontaseri M, Jorat MV, Kafi M. Atrioventricular nodal echoes over a wide echo window as a therapeutic end point for the catheter-guided radiofrequency ablation of atrioventricular nodal reentrant tachycardia: a prospective study. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018 Apr 1:20(4):659-664. doi: 10.1093/europace/euw434. Epub [PubMed PMID: 28340121]
De Sisti A, Tonet J, Amara W, Raguin D, Aouate P, Gueffaf F, Touil F, Hidden-Lucet F. Correlations between long-term results after cryoablation for atrioventricular nodal reentry tachycardia and a residual jump associated or not with a single echo. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012 Feb:14(2):261-6. doi: 10.1093/europace/eur297. Epub 2011 Sep 13 [PubMed PMID: 21920912]
Katritsis DG, Zografos T, Siontis KC, Giannopoulos G, Muthalaly RG, Liu Q, Latchamsetty R, Varga Z, Deftereos S, Swerdlow C, Callans DJ, Miller JM, Morady F, John RM, Stevenson WG. Endpoints for Successful Slow Pathway Catheter Ablation in Typical and Atypical Atrioventricular Nodal Re-Entrant Tachycardia: A Contemporary, Multicenter Study. JACC. Clinical electrophysiology. 2019 Jan:5(1):113-119. doi: 10.1016/j.jacep.2018.09.012. Epub 2018 Nov 28 [PubMed PMID: 30678775]
Level 2 (mid-level) evidenceEstner HL, Ndrepepa G, Dong J, Deisenhofer I, Schreieck J, Schneider M, Plewan A, Karch M, Weyerbrock S, Wade D, Zrenner B, Schmitt C. Acute and long-term results of slow pathway ablation in patients with atrioventricular nodal reentrant tachycardia--an analysis of the predictive factors for arrhythmia recurrence. Pacing and clinical electrophysiology : PACE. 2005 Feb:28(2):102-10 [PubMed PMID: 15679639]
Feldman A, Voskoboinik A, Kumar S, Spence S, Morton JB, Kistler PM, Sparks PB, Vohra JK, Kalman JM. Predictors of acute and long-term success of slow pathway ablation for atrioventricular nodal reentrant tachycardia: a single center series of 1,419 consecutive patients. Pacing and clinical electrophysiology : PACE. 2011 Aug:34(8):927-33. doi: 10.1111/j.1540-8159.2011.03092.x. Epub 2011 May 13 [PubMed PMID: 21569056]
Level 2 (mid-level) evidenceClague JR, Dagres N, Kottkamp H, Breithardt G, Borggrefe M. Targeting the slow pathway for atrioventricular nodal reentrant tachycardia: initial results and long-term follow-up in 379 consecutive patients. European heart journal. 2001 Jan:22(1):82-8 [PubMed PMID: 11133213]
Haines DE, Beheiry S, Akar JG, Baker JL, Beinborn D, Beshai JF, Brysiewicz N, Chiu-Man C, Collins KK, Dare M, Fetterly K, Fisher JD, Hongo R, Irefin S, Lopez J, Miller JM, Perry JC, Slotwiner DJ, Tomassoni GF, Weiss E. Heart Rythm Society expert consensus statement on electrophysiology laboratory standards: process, protocols, equipment, personnel, and safety. Heart rhythm. 2014 Aug:11(8):e9-51. doi: 10.1016/j.hrthm.2014.03.042. Epub 2014 May 7 [PubMed PMID: 24814989]
Level 3 (low-level) evidence