Urinary Crystals Identification and Analysis
Urinary Crystals Identification and Analysis
Introduction
Urinary crystal formation is a marker of urinary supersaturation with various substances. Crystals can occur secondary to inherited diseases, metabolic disorders, and medications. The presence of crystals in the urine is not always pathognomic for an abnormal metabolic or renal condition, as uric acid, calcium oxalate, calcium phosphate (CaP), and drug-induced crystals can also be observed under normal physiological conditions. The diagnosis of various monogenetic diseases, such as cystinuria, primary hyperoxaluria, and adenine phosphoribosyl transferase deficiency, can be suggested by examining the urinary sediment and correct identification of the associated urinary crystals.
Urinary crystal formation always precedes renal stone formation but does not always lead to nephrolithiasis. When kidney stones are formed, they have the potential to cause acute kidney injury, obstructive uropathy, hydronephrosis, renal colic, pyonephrosis, and urosepsis. Urinary crystals can also cause renal crystal deposition disease, resulting in acute kidney injury, progressive chronic kidney disease, and end-stage kidney disease.
Urine sediment examination can be considered a liquid biopsy of the kidney because it provides valuable information about the events occurring in an individual's kidney. However, with the increased utilization of automated systems, urine sediment examination is becoming a lost art. Nonetheless, it remains an excellent source of clinical information to help appropriately diagnose and manage patients with various diseases associated with urinary crystal formation. Please see StatPearls' companion resource, "Calcium Deposition and Other Renal Crystal Diseases," for more information.
Etiology and Epidemiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology and Epidemiology
Metabolic factors, inherited diseases, medications, dietary habits, dehydration, and anatomical abnormalities are the common etiologies contributing to urinary crystal formation. Kidney stone formation is a known risk factor for developing diabetes, cardiovascular disease, fractures, and chronic renal disease. Conversely, these conditions also increase the risk of nephrolithiasis.
Kidney stone disease is a polygenetic and multifactorial health problem worldwide that affects between 1% and 13% of the global population and almost 11% of the Western population.[1] Over the past 30 years, the prevalence of kidney stones has been rising across all ages, genders, racial and ethnic groups globally. Approximately 10% of the United States population experience nephrolithiasis in their lifetime.[2][3]
Estimating the overall prevalence of urinary crystalluria is challenging, as this condition is underdiagnosed and underreported. Although scant literature is available on the prevalence of urinary crystalluria, several studies showed crystalluria in approximately 8% of urine collections. Calcium oxalate, uric acid, and amorphous urate crystals accounted for most of the crystals encountered. Brushite, ammonium biurate, and cystine crystals were rare.[4][5][6]
Pathophysiology
Urine contains both promoters and inhibitors of crystal formation. Crystallization occurs when the concentration of crystal promoters in the glomerular filtrate exceeds the kidney's ability to keep them as soluble molecules. Stone inhibitors include citrate; uromodulin, also known as Tamm-Horsfall protein; magnesium; glycosaminoglycans; and pyrophosphate. Some chemical mediators decrease the urinary crystal's growth, aggregation, and adhesion to the tubular epithelium and the tubular cell; examples are osteopontin, matrix Gla protein, Tamm-Horsfall protein, and urinary fragment 1 of prothrombin.[7][8]
Calcium, oxalate, urate, and phosphate ions are the leading promoters of crystal formation. Physiological factors influencing kidney stone formation include high urinary osmolarity or low urine volume, bacteria in the urinary tract, and urine pH. Structural factors influencing nephrolithiasis include urinary reflux, instrumentation, and genetic abnormalities of the urinary tract.[8] Please see StatPearls' companion resource, "Renal Calculi, Nephrolithiasis," for more information.
Specimen Requirements and Procedure
A first-morning urine sample (void after awakening) is ideal for crystal analysis as it tends to be the most concentrated for crystal formation. The urine sample should be brought to the lab and processed within 2 hours of collection. Uric acid and phosphorous crystallization can occur if the urine stays stagnant for longer than 2 hours. Refrigeration of the urine specimen is necessary after 2 hours, and examination within 8 hours of collection is recommended, even when refrigerated.[6][9]
Urine analysis through automated and manual methods can provide significant clues to the type and characteristics of urinary crystals. When examining the sediment, urine pH, specific gravity, and a dipstick analysis should always be recorded. Of note, casts degrade quickly in alkaline urine. Red blood cells (RBCs) and white blood cells (WBCs) can swell in low osmolality and shrink in high osmolality.[10]
Urine pH plays a key role in crystallization, as CaP, struvite, and ciprofloxacin crystals precipitate in alkaline urine. In contrast, uric acid, cystine, and other drug-induced crystals tend to precipitate in acidic urine. These characteristics assist clinicians in accurately identifying urinary crystals.[6]
For microscopic examination, 10 mL of urine should be centrifuged at 1500 to 3000 revolutions per minute for at least 5 min. The sediment is obtained through a pipette after discarding the top 9.5 cc of supernatant and gentle manual agitation of the test tube. A single drop of urine sediment is placed on the glass slide and covered with a cover slip for direct observation. The sediment is examined using a standard brightfield or phase contrast microscope at low and high magnification. A minimum of 10 fields should be reviewed at each power. The specimen is then examined under polarization to determine birefringence and identify other detailed characteristics.[6]
Automated urinary analyzers also often miss crystals when compared to manual microscopic evaluation.[11] Off note, a recent study found significant interoperator variability identifying urinary sediment components, even among experienced nephrologists. Therefore, when possible, a personal examination of urine sediment is preferable.[12]
Diagnostic Tests
A comprehensive workup is essential for diagnosing the etiology of urinary crystals. This approach includes obtaining a detailed history and performing a thorough physical examination, and may also include x-ray KUB, renal or abdominal ultrasound, and plain computed tomography (CT) of the abdomen and pelvis to help localize the renal stones. Simple urinalysis and analysis of the urinary crystals can help understand a stone's chemical composition. A chemical analysis of a passed stone can give a definitive answer.[7][13]
Crystalluria indicates supersaturation of the components in the urine, leading to precipitation of the crystals. However, supersaturation alone is insufficient to cause nephrolithiasis; other inciting factors are also typically present. The process can be related to metabolic disorders, inherited diseases, drug use, or toxins. The initial diagnostic test is generally a 24-hour urine collection evaluating the following parameters—urinary volume, pH, calcium, citrate, creatinine, magnesium, phosphate, uric acid, sodium, serum calcium, oxalate, and uric acid levels. The collection should ideally be performed in nonacute conditions at the patient's home with their normal diet and fluid intake for optimal information. Additional workup is typically guided by the type of crystal identified and patient history.[7][8][10][13] Please see StatPearls' companion resources, "24-Hour Urine Collection," and "24-Hour Urine Testing for Nephrolithiasis: Interpretation and Treatment Guidelines," for more information.
Further Diagnostic Testing Based on Stone Composition
Calcium oxalate: Calcium oxalate stones comprise up to 80% of all nephrolithiasis cases. Calcium oxalate and CaP often coexist in stones, but 1 component is typically primary. Patients with calcium oxalate crystals should be considered for primary and secondary causes of hyperoxaluria. Please see StatPearls' companion resource, "Calcium Deposition and Other Renal Crystal Diseases," for more information. Calcium oxalate dihydrate (COD) crystals are also highly associated with hypercalcemia, which should also be considered. Calcium oxalate crystalluria is not necessarily pathologic, as these crystals can sometimes be found in patients with high oxalate diets.[6]
Hyperoxaluria of >75 mg/d in a 24-hour urine collection suggests possible hereditary hyperoxaluria (types I, II, or III).
Calcium phosphate: CaP nephrolithiasis is the second most common type, representing about 10% to 20% of kidney stones. The presence of these stones should prompt evaluation for conditions causing hypercalciuria, hyperphosphaturia, and alkaline urine formation. These crystals can also be found under normal physiological conditions.
Struvite: Struvite comprises ammonium, magnesium, and calcium binding with phosphate (triple phosphate). The presence of struvite stones, even in lower quantities, is almost always associated with infection by urease-producing microorganisms, such as Proteus mirabilis, Klebsiella pneumonia, Staphylococcus aureus, Pseudomonas aeruginosa, Providencia stuartii, Serratia, and Morganella morganii.[8]
Uric acid: Uric acid dihydrate crystals primarily depend on low urine pH, whereas the amorphous ones are mainly related to high urinary urate concentration. Uric acid crystals are only found when the urinary pH is acidic, as uric acid becomes much more soluble as the pH increases.[8][9][14] The presence of uric acid dihydrate crystals should prompt the workup for conditions causing impaired renal ammoniogenesis, such as metabolic syndrome and type 2 diabetes.[10]
Xanthine: Xanthine crystals are found in xanthinuria, a rare genetic disorder with autosomal recessive pattern inheritance caused by the deficiency of the enzyme xanthine dehydrogenase. Not all laboratories can test for xanthine and hypoxanthine, nor are these part of a routine urine collection. If this rare disorder is suspected, this test must be ordered specifically. Biopsy of the gastrointestinal tract to quantify xanthine dehydrogenase is rarely performed.[15] An allopurinol loading test can also be used to detect a lack of xanthine oxidase, as allopurinol is abnormally metabolized in patients with hereditary xanthinuria; however, these measurements may not be available in all laboratories.[16] Xanthinuria is also associated with hypouricemia and hypouricosuria.[17]
Patients with 2,8-dihydroxyadenine (DHA) crystalluria, cystinuria, and suspected primary hyperoxaluria can also be genetically tested to confirm the diagnosis.[13][18]
Please see StatPearls' companion resources, "Calcium Deposition and Other Renal Crystal Diseases," "Hyperoxaluria," "Uric Acid Nephrolithiasis," "Struvite and Triple Phosphate Renal Calculi," and "Cystinuria," for more information.
Testing Procedures
Effective testing procedures for urine crystal identification and analysis are crucial for accurate diagnosis and patient care. Techniques commonly used for crystal analysis in clinical practice include the following:
- Standard light microscopy
- Optical polarization microscopy
- Automated microscopy
- Flow cytometry
- Digitalized microscopy
- Scanning electron microscopy
- Spectroscopy
Standard Light Microscopy
Manual direct microscopic examination is the gold standard technique used in clinics to identify various urinary crystals based on their morphology. When sufficiently trained and equipped, nephrologists can acquire important diagnostic information through direct microscopy. Brightfield microscopy is more commonly used compared to phase contrast microscopy, but phase contrast microscopy may be more beneficial for analyzing urine sediment.[6][19]
Optical Polarization Microscopy
Polarized light microscopy is used to observe the color, morphology, and birefringence of crystals for identification. The color, refraction of the light, and double refraction are the parameters used in mineral identification. However, contaminants can sometimes distort or interfere with the interpretations of results.[10]
Automated Microscopy
Automated microscopy is a method used in laboratory practice that does not require urine centrifugation or standardized microscope measuring parameters. These machines are based on the Colter principle and became popular in hematology laboratory procedures in 1954 for the rapid and accurate enumeration of cells in the blood. They have been used for urine microscopy since the 1990s. Automated microscopy has been expanded to laser-based flow cytometry for better cell characterization.
This technique can utilize a large sample size, reducing the laboratories' workload. Automated microscopy can deliver a complete urine analysis result if urinary biochemical data are combined and integrated with the microscopy analysis. The practical approach is to use automated and manual machines to survey many specimens and expert microscopic examination for selected specimens. Automated systems have significant advantages over manual microscopy, as the specimens do not need centrifugation. They are also highly effective in diagnostic screening for blood, bacteria, and WBCs. However, automated systems significantly underperform in recognizing urinary casts and crystals compared to manual examinations performed by experienced lab technicians, pathologists, and nephrologists.[11] The 2 major types of automated microscopy techniques are as follows:
Flow cytometry:
- These machines use laser-based flow cytometry with fluorescent dyes. Flow cytometry can reduce the need for microscopy or culture and is more useful in screening bacteriuria, pyuria, and hematuria, although sometimes microscopy is still required.
- This machine produces scattergrams, not actual images. Microscopy is required to differentiate similar particles, such as various types of crystals, dysmorphic or isomorphic RBCs, and different types of epithelial cells. The advantage of this machine is that minimum expertise is required to operate it, and approximately 100 samples can be tested in 1 hour.[10][20]
Digitalized microscopy:
- The digitalized microscopy technique uses computerized software to analyze digitalized monochrome images of urine particles. Two different instruments that supply digitized images are available. One is based on automated intelligent microscopy, which shows particles in the samples by categories such as RBCs and WBCs. The other uses cuvette-based microscopy, which displays particles in the same way they appear in the microscopic field during manual examination.
- A significant advantage of this technique is that it can be used for many samples and selective individual specimens. The images can be stored on a computer and sent to different sites, including other laboratories and clinics, for review by pathologists or nephrologists. In addition, this method is relatively cheaper compared to flow cytometry.[10][20]
Scanning Electron Microscopy
Scanning electron microscopy produces high-resolution images of the crystal surface, aiding in studying the morphology and texture of urinary calculi. This method can analyze the characteristics of stones ranging from 1 mm to 5 mm without changing the morphology of their components, and it does not destroy the stone sample. This method is also a proposed tool for diagnosing primary hyperoxaluria.
Spectroscopy
Spectroscopy identifies the molecular structure of a sample by investigating its interaction with electromagnetic radiation. In this technique, infrared light passes through the sample, and the detector analyzes the intensity of the transmitted light. Vibrational spectroscopy identifies a sample's composition and molecular structure through its vibrational characteristics when interacting with a beam source. This technique is analyzed based on the sample's vibrational characteristics when interacting with electromagnetic waves. Other spectroscopy methods, such as Raman and Fourier transform-infrared spectroscopy, are increasingly used in clinical practice.[14]
These rapid, nondestructive, and relatively inexpensive methods have been increasingly used in recent years to identify the chemical nature of urinary crystals and detect organic matter. Infrared spectroscopy is extremely useful in identifying calcium oxalate crystals.[21]
Overall, clinicians should be able to identify most crystals by checking urine pH and urinalysis, then examining the urine sediment under bright field, phase contrast, and polarizing light microscopy. Fourier transform-infrared and Raman spectroscopy and solubility testing can be additional resources for unclear crystal composition.[20][22]
Results, Reporting, and Critical Findings
The presence of urinary crystals associated with active urinary sediment, acute kidney injury, or kidney stones suggests a pathogenic process. Identifying and characterizing urinary crystals are essential for diagnosing the type of stone, especially when the actual stone is not available for analysis (see Table 1. Characteristics of Commonly Observed Urinary Crystals). The results of urinary crystal analysis are reported after identifying various characteristics of urinalysis and microscopic examination of the urine sediment.
Commonly Reported Urinary Crystals
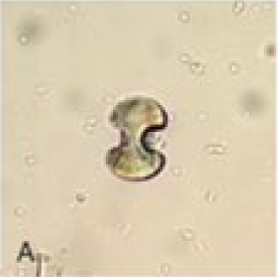
Calcium oxalate crystals: There are 2 significant types of calcium oxalate crystals—monohydrate and dihydrate. The calcium oxalate monohydrate (COM) crystals are typically colorless on light microscopy. Their shape varies from oval, biconvex, and dumbbells to elongated rods (see Image. Calcium Oxalate Monohydrate Crystals). These crystals are strongly birefringent on polarized microscopy.
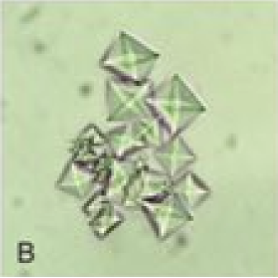
COD crystals are colorless on light microscopy and are bipyramidal or envelope-shaped, and their size is variable (see Image. Calcium Oxalate Dihydrate Crystals). These crystals are non-birefringent on polarization. Calcium oxalate crystallization is commonly observed in the urine pH range of 5.0 to 6.5 and is rarely observed when the urine pH increases to >7.0. A rare third form of calcium oxalate crystals, calcium oxalate trihydrate or caoxite, is hexagonal and seldom found in urine.[9][14]
Calcium phosphate crystals: There are calcium orthophosphate crystals, mainly amorphous carbonated CaP and carbapatite, whereas brushite is dicalcium phosphate dihydrate.
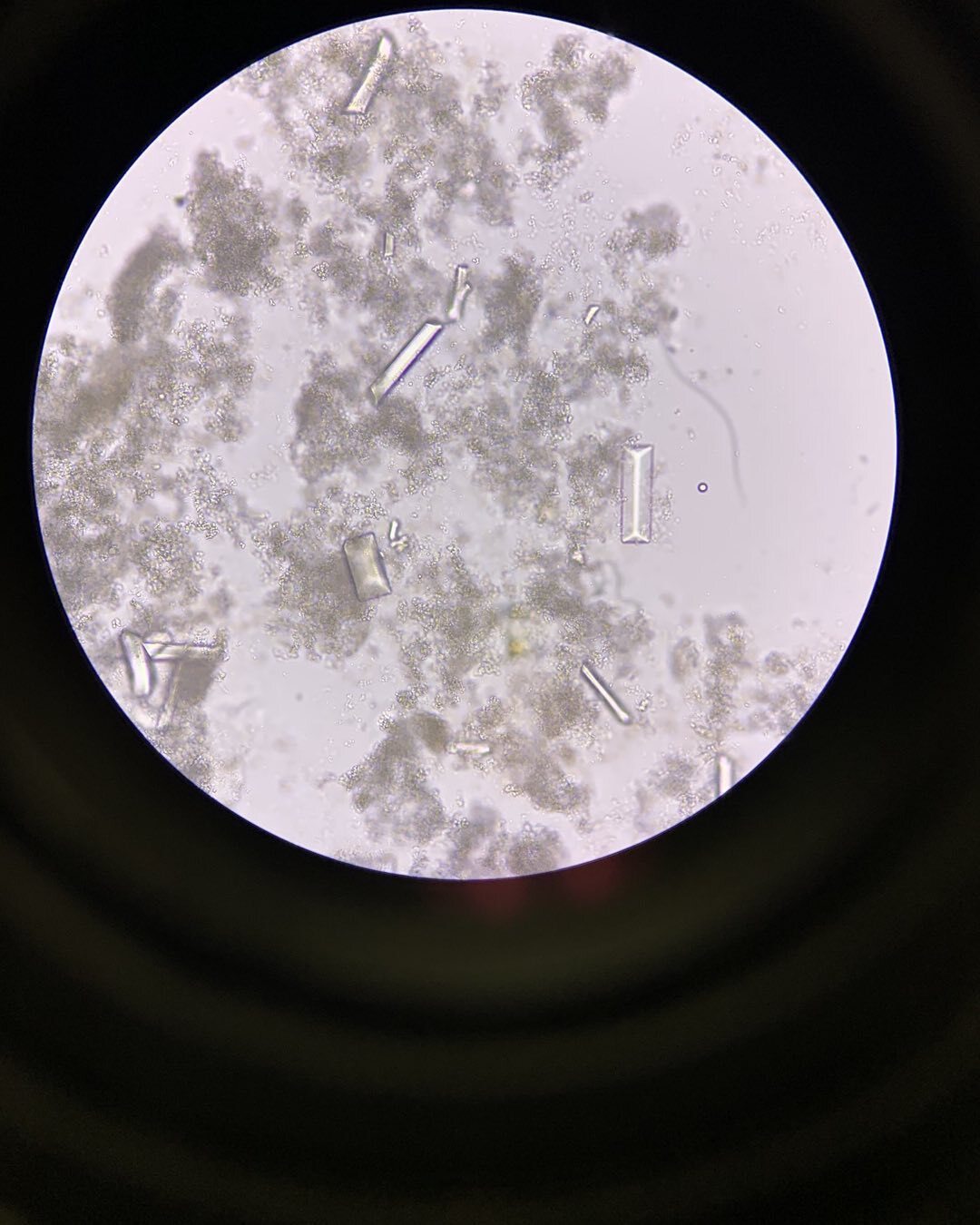
CaP crystals are colorless on light microscopy. Calcium orthophosphate crystals are amorphous crystals that appear as small granulations with or without large plates and have no polarization (see Image. Amorphous Phosphate and Triple Phosphate Crystals in the Urine). Brushite crystals have a broad spectrum of shapes, such as prisms, rosettes, starts, needle-like or splinter-like, or sticks or rods (see Image. Calcium Phosphate Crystals). These crystals are strongly birefringent on polarized microscopy and precipitate in alkaline urine.[9][14]
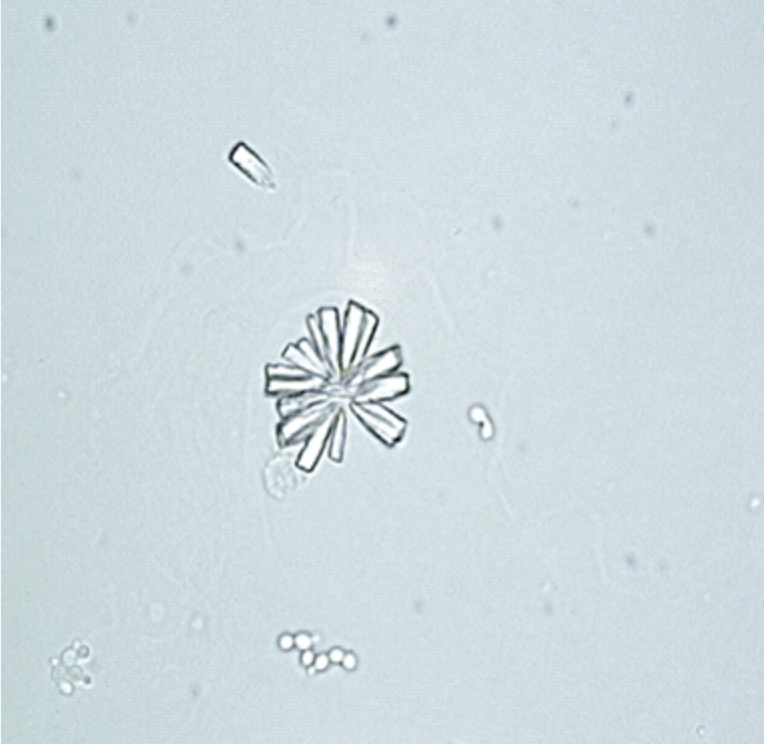
Struvite or triple phosphate crystals: These are radioopaque magnesium ammonium phosphate hexahydrate or triple phosphate crystals. Struvite crystals are colorless under light microscopy. The most common shape observed is a coffin lid; the other shapes are feather-like structures, elongated prisms, and trapezoids. These crystals are weak to strongly birefringent on polarization and precipitate in alkaline urine (see Images. Triple Phosphate Crystals; and Amorphous Phosphate and Triple Phosphate Crystals in the Urine).[9][14]
Cystine crystals: Cystine crystals are colorless on light microscopy and are either found alone or heaped onto each other, resembling perfect hexagonal plates. These crystals show weak birefringence on polarized microscopy and precipitate in urine with an acidic pH (see Image. Cystine Crystals in the Urinary Sediment).[18] Please see StatPearls' companion resource, "Cystinuria," for more information.
Uric acid and urate crystals: There are 4 types of uric acid crystals in urine—amorphous, anhydrous uric acid (uricite), monohydrate, and dihydrate crystals. Among these, uric acid dihydrate and amorphous uric acid crystals are most commonly observed.[9][14]
Uric acid crystals are often amber-colored on light microscopy and have various shapes, such as rho boards, barrels, rosettes, 4-sided plates, needles, rounds, or parallelograms. These crystals show a very strong polychromatic birefringence on polarization (see Images. Uric Acid Crystals, Barrel-Shaped; Uric Acid Crystals, Rosette-Shaped; and Uric Acid Crystals, Birefringent on Polarized Light). These crystals precipitate in urine with an acidic pH.[9][14]
Xanthine crystals: Xanthine crystals are nonspecific in appearance and can be observed as polarized granules or sticks without a characteristic aspect. Crystal identification requires infrared spectroscopy, high-pressure liquid chromatography, or chemical analysis of a xanthine stone. These crystals precipitate in urine with an acidic pH.[9][14]
Dihydroxyadenine crystals: DHA crystals are somewhat brown or reddish-brown with a dark perimeter and central spicules that are round in shape on light microscopy. These crystals turn yellow and may demonstrate a characteristic birefringent Maltese-cross pattern on polarized microscopy. In suspected 2,8-DHA crystalluria, it is mandatory to investigate the crystals using infrared spectroscopy on a centrifuged pellet. These crystals precipitate in urine with an acidic or alkaline pH (4.8 to 9.0) and are relatively insoluble at any urinary pH.[9][14]
Table 1. Characteristics of Commonly Observed Urinary Crystals
|
Crystal |
Morphology |
Birefringence |
Average pH (ranges) |
Dependence on Urine pH |
|
Calcium oxalate monohydrate |
Colorless dumbbells, ovoids, rods Elongated hexagonal shape (ethylene glycol poisoning) |
Strong |
5.9 (4.8-7.5) |
Low |
|
Calcium oxalate dihydrate |
Colorless bipyramidal, rarely octahedral and dodecahedral |
Weak |
5.8 (4.8-7.5) |
Low |
|
Calcium phosphate (orthophosphates) |
Amorphous round granulations |
Weak |
6.7 (6.0-8.5) |
High |
|
Calcium phosphate (brushite) |
Needles, sticks, rosettes, prisms |
Strong |
6.5 (5.9-8.0) |
High |
|
Struvite |
Coffin lids, prisms, trapezoids |
Strong |
7.7 (6.8-9.0) |
High |
|
Amorphous uric acid |
Amorphous, small, yellow, or red granulations |
Strong |
5.5 (4.7-6.5) |
Moderate |
|
Uric acid dihydrate |
Rhomboids, barrels, rosettes, needles, 6-sided plates |
Strong polychromatic |
5.2 (4.7-6.3) |
High |
|
Xanthine |
Granules or sticks |
Strong |
5.6 (4.8-7.2) |
Moderate |
|
2,8-Dihydroxyadenine |
Reddish-brown spherical |
Strong Maltese cross |
6.2 (4.8-9.0) |
Very low |
|
Cystine |
Hexagonal plates |
Weak |
6.8 (5.0-7.8) |
Moderate |
Drug-Induced Crystals
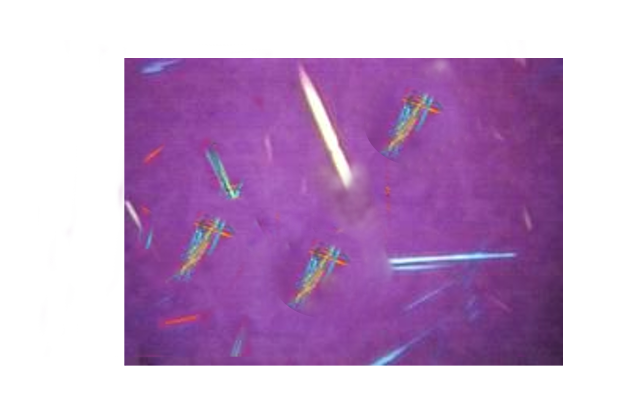
Drug-induced urinary crystals are variable in size, shape, and other characteristics depending on the specific medication (see Table 2. Characteristics of Various Drug-Induced Crystals). These rare crystals comprise only 1% to 2% of all nephrolithiasis (see Images. Sulfamethoxazole Crystals; Sulfadiazine Crystals; and Acyclovir Crystals).[7]
Table 2. Characteristics of Various Drug-Induced Crystals
|
Drug Name |
pH |
Color |
Shape |
Polarization |
|
Sulfonamide |
Acidic |
Colorless |
Hexagon or ovoid |
None |
|
Amoxicillin |
Acidic |
Colorless |
Thin needles or broom or brush-like |
Strongly birefringent |
|
Ciprofloxacin |
Alkaline |
Colorless |
Needles, star fans, or sheaves |
Strongly birefringent |
|
Triamterene |
Acidic |
Brown, green, orange, or red |
Spheres or unusual pleomorphism |
Maltese cross birefringence |
|
Sulfadiazine |
Acidic |
Amber |
Shocks or sheaves of wheat shells |
Strongly birefringent |
|
Methotrexate |
Acidic |
Yellow-Brown |
Thin needles |
Strongly birefringent |
|
Acyclovir |
Acidic |
Colorless |
Thin needles with sharp or blunt ends |
Strongly birefringent |
|
Atazanavir |
Acidic |
Colorless |
Needles in isolation or as aggregates |
Strongly birefringent |
Rare Crystals
Tyrosine crystals are colorless, thin, and needle-shaped, whereas leucine crystals are oval or round with circular striations. Both these crystals are found in acidic urine and are associated with aminoaciduria secondary to liver disease. Bilirubin crystals, which are clumped needles or yellow granules, are highly birefringent on polarization, and they are observed in conditions associated with hyperbilirubinemia. Cholesterol crystals are thin, transparent plates with cut edges. They are highly birefringent on polarization, typically observed in association with nephrotic syndrome.[9][14]
Solubility Testing
The solubility test involves exposing the crystals to 10% KOH, 30% acetic acid, 30% HCL, and chloroform. The solubility of crystals in these fluids provides additional clues that aid in crystal identification (see Table 3. Solubility Characteristics of Crystals). This test is beneficial in identifying crystals with similar shapes.[9][14]
Table 3. Solubility Characteristics of Crystals
|
Crystal Type |
Solubility in 10% KOH |
Solubility in 30% Acetic Acid |
Solubility in 30% HCL |
Solubility in Chloroform |
|
Calcium oxalate |
No |
No |
Yes |
No |
|
Calcium phosphate |
No |
Yes |
Yes |
No |
|
Struvite |
No |
Yes |
Yes |
No |
|
Uric acid |
Yes |
No |
No |
No |
|
Bilirubin |
Yes |
Yes |
Yes |
Yes |
|
Cholesterol |
No |
No |
No |
Yes |
|
Cystine |
Yes |
No |
Yes |
No |
|
2,8-Dihydroxyadenine |
Yes |
No |
No |
No |
|
Hippuric acid |
No data |
No |
No data |
No data |
|
Leucine |
Yes |
Yes |
Yes |
No |
|
Tyrosine |
Yes |
No |
Yes |
No |
|
Xanthine |
Yes |
No |
No |
No data |
Interfering Factors
Identifying urinary crystals can be challenging due to similarities in their shapes. Calcium sulfate, CaP, and sodium urate crystals can be needle-shaped or rectangle-shaped with blunt ends. COM, hippuric acid, uric acid, and ammonium biurate crystals can be rectangles with pointed ends. Amorphous urate and phosphate granules can have similar minor granular appearances. Solubility tests can help differentiate urinary crystals with similar shapes.[14] Key considerations include:
- Free hemosiderin granules are yellow-brown coarse granules that can be confused with amorphous phosphate and urate granules.
- 2,8-DHA crystals can be confused with ammonium hydrogen urate as both have a small spherical shape.
- Starch granules are oval or round and highly refractive. Their irregular central indentation can resemble molar tooth grooves.
- Under polarized light, starch granules can appear similar to a Maltese cross and be confused with 2,8-DHA crystals.
- Cholesterol and leucine crystals can also exhibit a typical Maltese-cross appearance under polarized light.
Clinical Significance
Renal stone formation is always preceded by crystalluria, although the presence of urinary crystals does not always result in nephrolithiasis. Investigating crystalluria is crucial for diagnosing and managing acute and chronic renal failure due to crystal precipitations associated with various inherited and acquired diseases. Identifying the type of urinary crystals is also helpful in offering specific targeted treatment for nephrolithiasis.
Calcium Oxalate Crystals
Calcium oxalate stones comprise up to 80% of all nephrolithiasis. Patients with calcium oxalate crystals should be considered for primary and secondary causes of hyperoxaluria. Please see StatPearls' companion resource, "Calcium Deposition and Other Renal Crystal Diseases," for more information. Calcium oxalate crystalluria is not necessarily pathologic, as these crystals can sometimes be found in those with high oxalate diets.[6] As an overview, calcium monohydrate crystals are generally associated with high urinary oxalate levels, calcium dihydrate crystals are generally associated with high urinary calcium, and the rare dodecahedral calcium dihydrate crystals are associated with very high urinary calcium.
Secondary hyperoxaluria is much more common compared to primary hyperoxaluria and is often related to 1 of 3 mechanisms as follows:
- Increased dietary intake of oxalate-containing foods, such as peanuts, cashews, almonds, spinach, rhubarb, star fruit, chaga mushroom powder, and black iced tea.
- Increased intestinal absorption of oxalate in a setting of fat malabsorption (enteric hyperoxaluria secondary to intestinal bypass surgery, short gut syndrome, Crohn disease, celiac disease, chronic pancreatitis, and orlistat).
- Increased intake of oxalate precursors, such as ethylene glycol and vitamin C, can develop increased gut oxalate absorption, leading to excess urinary oxalate excretion. These patients are also at high risk for developing calcium oxalate crystalluria, crystalline nephropathy, chronic kidney disease, and end-stage kidney disease. Ingestion of the toxic substance, ethylene glycol, results in severe hyperoxaluria, and a very peculiar kind of monohydrate crystals with elongated and narrow hexagonal shapes similar to diamonds are found in the urine.[9]
COD crystals are typically associated with hypercalciuria, such as hyperparathyroidism; hyperthyroidism; multiple myeloma; paraneoplastic syndrome; bone metastasis; vitamin D toxicity; particular drug use, including loop diuretics and vitamin-D supplements; and milk-alkali syndrome.
According to Daudon et al, over 94% of urine specimens containing calcium oxalate crystals with a calcium-to-oxalate ratio of <5 show either monohydrate crystals alone or along with dihydrate crystals. In contrast, over 90% of urine specimens containing calcium oxalate crystals with a calcium-to-oxalate ratio >14 contain dihydrate crystals alone. Dodecahedral COD crystals typically indicate severe hypercalciuria, >7 mmol/L. All types of calcium-containing crystals are radioopaque.[8][9]
Calcium Phosphate Crystals
Carbapatite crystals are typically observed in patients with hypercalciuria on a background urine pH of 5.8 to 6.5. In contrast, amorphous CaP crystals are typically observed in patients with near-normal calcium and phosphate concentrations with a pH >6.6.[14]
Amorphous CaP crystals suggest a possible environment created by urinary tract infections or metabolic disorders keeping the urine alkaline. When associated with the calcium monohydrate crystals, they indicate the presence of medullary sponge kidneys and other causes related to urinary stasis. When carbapatite crystals are associated with struvite crystals, they indicate the presence of urea-splitting organisms causing urinary tract infections.
Brushite crystals are made of dicalcium phosphate dihydrate ions. These crystals are mainly observed in conditions associated with marked hypercalciuria and hyperphosphaturia and are generally favored by low concentrations of citrates. The presence of >500/mL brushite crystals should prompt an evaluation for primary hyperparathyroidism. CaP crystals are radioopaque.[8][9][14]
Struvite Crystals
Struvite crystals are associated with high ammonium content and precipitate in alkaline urine. These crystals are always associated with urinary tract infections by urea-splitting bacteria, where the bacteria hydrolyze the urea, producing ammonia and leading to alkaline urine. These bacteria are Klebsiella, Pseudomonas, Proteus, Providencia, and some species of Staphylococcus. These are radioopaque, sometimes large enough to be called staghorn stones.[8][9][14]
Cystine Crystals
Cystine crystals are associated with hereditary cystinuria, in which a proximal tubular dysfunction leads to increased excretion of dibasic amino acids such as cystine, ornithine, lysine, and arginine. Cystinuria is an autosomal recessive Mendelian disorder, and there are reports of acquired cystinuria post-renal transplant. Cystine forms radiolucent stones, which are not detected on x-rays but can be detected by ultrasound if the stones are large enough. CT scan is the gold standard for diagnosing renal stones and can detect cystine stones. Cystine becomes more soluble as the pH increases. The therapeutic urinary pH level to maximize solubility is ≥7.5, typically higher compared to uric acid.[23]
Uric Acid Crystals
Uric acid crystals are associated with medical conditions leading to increased uric acid production or altered uric acid metabolism, such as gout, Lesch-Nyhan syndrome, tumor lysis, chemotherapy, or conditions associated with decreased urine pH, such as chronic diarrhea, metabolic syndrome, and diabetes mellitus. However, over half of patients with uric acid stones do not have hyperuricemia. These crystals form radiolucent renal stones that are not detectable on x-rays but can be visualized on CT and ultrasound.[6]
Xanthine Stones
Xanthine crystals are found in xanthinuria, a rare genetic disorder with autosomal recessive pattern inheritance caused by the deficiency of the enzyme xanthine dehydrogenase. This disorder leads to xanthine accumulation and highly insoluble xanthine excretion. Xanthinuria is associated with hypouricemia and hypouricosuria.[17] Rarely, xanthinuria can occur related to allopurinol use for Lesch-Nyan syndrome or tumor lysis syndrome.[24] More recently, the xanthine oxidase inhibitor, febuxostat, has also been noted to cause xanthinuria when used for tumor lysis syndrome.[25]
2,8-Dihydroxyadenine Crystals
2,8-DHA crystalluria is secondary to adenine phosphoribosyltransferase (APRT) deficiency, an autosomal recessive condition leading to excess DHA production, 2,8-DHA crystalluria, and kidney stones. The condition is underdiagnosed in the United States, with fewer than 300 cases reported in the medical literature. The worldwide prevalence is 1/5000 to 1/100,000 population. A significant number of patients remain asymptomatic, whereas 90% of patients develop urinary crystals and radiolucent renal stones. In addition, 10% of patients with 2,8-DHA crystalluria progress to end-stage renal failure. The diagnosis is confirmed by kidney stone analysis, histologic findings of crystal nephropathy, genetic testing, or lack of APRT activity in RBC lysate. Treatment is recommended with allopurinol or febuxostat, which blocks the conversion of adenosine to DHA. The dosage should be titrated to eliminate 2,8-DHA crystalluria.[26][27]
Drug-Induced Crystals
Drug-induced crystals are formed in association with the use of various drugs, as previously described. These crystals precipitate in the urine when used at a high dose, for long durations, or both. They form radiolucent renal stones when they contain no calcium or other radioopaque constituents. Patients experiencing signs and symptoms of nephrolithiasis while using these drugs should prompt the search for renal stones by ultrasound or contrast-enhanced CT imaging for diagnosis. Analyzing these crystals is crucial for identifying at-risk patients and clarifying the drugs or medications involved.[28]
Follow-Up Monitoring
The complete disappearance of crystalluria is the best indicator of improved lithogenic activity in CaP, uric acid, and other common forms of nephrolithiasis. However, high-risk patients, such as those with cystinuria and PH1, can have active urinary crystallization even when the disease is under reasonable control.
Global crystal volume (GCV) helps determine lithogenicity in patients with crystalluria. GCV is the product of the number of crystals/mm3 multiplied by the average size of crystals (in μm) and by a numerical factor given for each crystal species (the numerical number is provided based on the geometrical form of a crystal). GCV of <500 μm3/mm3 is associated with the lowest risk for tubular obstruction and crystalline nephropathy in patients with PH1. In patients with cystinuria, a GCV of >3000 μm3/mm3 is associated with an increased risk for recurrent nephrolithiasis.[9]
Enhancing Healthcare Team Outcomes
Clinical outcomes can be significantly improved by emphasizing the importance of urine sediment examination and ensuring that medical students, residents, physician assistants, nurse practitioners, laboratory technicians, and other healthcare professionals receive comprehensive training on its routine application.
The preanalytical phase is the most vulnerable part of the evaluation, accounting for up to 75% of all laboratory errors. Outcomes can be improved by following appropriate protocol-based patient preparation, obtaining mid-stream urine samples, rapid sample transportation, and preparation techniques. Nursing, lab processing, and transporting staff are crucial in these collection steps. Physicians must often collect samples shortly before examination for the optimal specimens. Effective communication among team members is paramount. Conscientious laboratory staff are paramount in the accurate processing and calibration of microscopic and analytic equipment. Clear and open communication facilitates rapid diagnosis and treatment decisions, preventing errors and ensuring a coordinated interprofessional team response.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Uric Acid Crystals, Rosette-Shaped. The image shows rosette-shaped uric acid crystals on light microscopy.
Iqbal Osman, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
Sulfamethoxazole Crystals. Urine sediment without staining ×400. Left: bright field light microscopy; right: polarized light microscopy.
Seltzer J, Velez JC, Buchkremer F, Poloni JAT. Urine sediment of the month: drugs & crystalluria. Renal Fellow Network Web site. https://www.renalfellow.org/2020/05/28/urine-sediment-of-the-month-drugs-crystalluria/. Published May 28, 2020. Accessed May 20, 2024.
(Click Image to Enlarge)
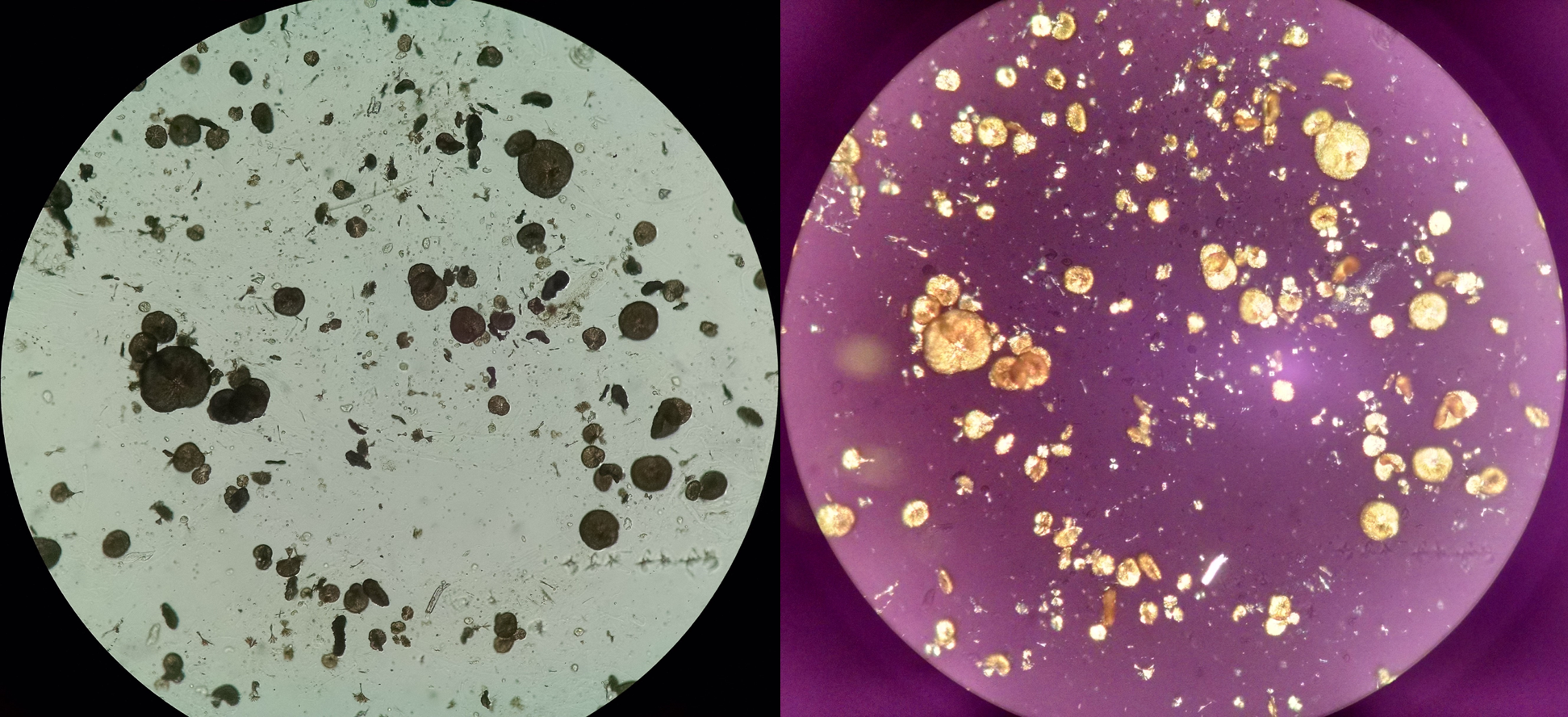
Sulfadiazine Crystals. Unstained urine sediment ×100. Left: bright field light microscopy; right: polarized light microscopy.
Seltzer J, Velez JC, Buchkremer F, Poloni JAT. Urine sediment of the month: drugs & crystalluria. Renal Fellow Network Web site. https://www.renalfellow.org/2020/05/28/urine-sediment-of-the-month-drugs-crystalluria/. Published May 28, 2020. Accessed May 20, 2024.
(Click Image to Enlarge)
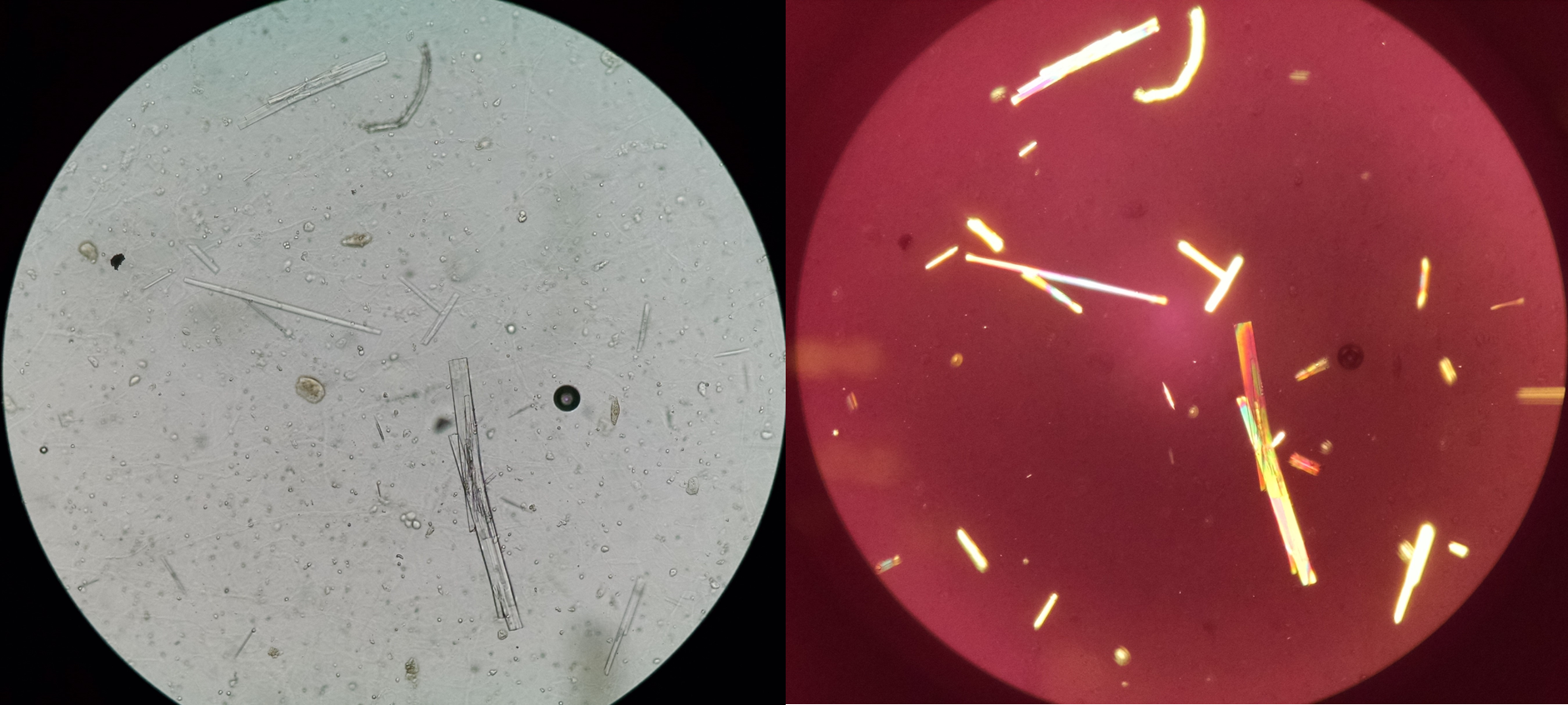
Acyclovir Crystals. Left: light microscopy unstained; right: polarized light with birefringence.
Seltzer J, Velez JC, Buchkremer F, Poloni JAT. Urine sediment of the month: drugs & crystalluria. Renal Fellow Network Web site. https://www.renalfellow.org/2020/05/28/urine-sediment-of-the-month-drugs-crystalluria/. Published May 28, 2020. Accessed May 20, 2024.
References
Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World journal of urology. 2017 Sep:35(9):1301-1320. doi: 10.1007/s00345-017-2008-6. Epub 2017 Feb 17 [PubMed PMID: 28213860]
Lang J, Narendrula A, El-Zawahry A, Sindhwani P, Ekwenna O. Global Trends in Incidence and Burden of Urolithiasis from 1990 to 2019: An Analysis of Global Burden of Disease Study Data. European urology open science. 2022 Jan:35():37-46. doi: 10.1016/j.euros.2021.10.008. Epub 2022 Jan 3 [PubMed PMID: 35024630]
Chewcharat A, Curhan G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis. 2021 Feb:49(1):27-39. doi: 10.1007/s00240-020-01210-w. Epub 2020 Sep 1 [PubMed PMID: 32870387]
Couto J, Dos Santos LP, Alves JC, López R, Maldonado C. Amoxicillin Crystalluria: A Rare Side-Effect of a Commonly Prescribed Antibiotic. European journal of case reports in internal medicine. 2017:4(10):000736. doi: 10.12890/2017_000736. Epub 2017 Oct 23 [PubMed PMID: 30755915]
Level 3 (low-level) evidenceVerdesca S, Fogazzi GB, Garigali G, Messa P, Daudon M. Crystalluria: prevalence, different types of crystals and the role of infrared spectroscopy. Clinical chemistry and laboratory medicine. 2011 Mar:49(3):515-20. doi: 10.1515/CCLM.2011.078. Epub 2010 Dec 14 [PubMed PMID: 21143023]
Cavanaugh C, Perazella MA. Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2019 Feb:73(2):258-272. doi: 10.1053/j.ajkd.2018.07.012. Epub 2018 Sep 21 [PubMed PMID: 30249419]
Shastri S, Patel J, Sambandam KK, Lederer ED. Kidney Stone Pathophysiology, Evaluation and Management: Core Curriculum 2023. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2023 Nov:82(5):617-634. doi: 10.1053/j.ajkd.2023.03.017. Epub 2023 Aug 9 [PubMed PMID: 37565942]
Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation (Review). International journal of molecular medicine. 2021 Aug:48(2):. pii: 149. doi: 10.3892/ijmm.2021.4982. Epub 2021 Jun 16 [PubMed PMID: 34132361]
Level 3 (low-level) evidenceFrochot V, Daudon M. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. International journal of surgery (London, England). 2016 Dec:36(Pt D):624-632. doi: 10.1016/j.ijsu.2016.11.023. Epub 2016 Nov 12 [PubMed PMID: 27847293]
Fogazzi GB. Crystalluria: a neglected aspect of urinary sediment analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996 Feb:11(2):379-87 [PubMed PMID: 8671802]
İnce FD, Ellidağ HY, Koseoğlu M, Şimşek N, Yalçın H, Zengin MO. The comparison of automated urine analyzers with manual microscopic examination for urinalysis automated urine analyzers and manual urinalysis. Practical laboratory medicine. 2016 Aug 1:5():14-20. doi: 10.1016/j.plabm.2016.03.002. Epub 2016 Mar 11 [PubMed PMID: 28856199]
Palsson R, Colona MR, Hoenig MP, Lundquist AL, Novak JE, Perazella MA, Waikar SS. Assessment of Interobserver Reliability of Nephrologist Examination of Urine Sediment. JAMA network open. 2020 Aug 3:3(8):e2013959. doi: 10.1001/jamanetworkopen.2020.13959. Epub 2020 Aug 3 [PubMed PMID: 32821922]
Letavernier E, Bazin D, Daudon M. Description of Stone Morphology and Crystalluria Improve Diagnosis and Care of Kidney Stone Formers. Healthcare (Basel, Switzerland). 2022 Dec 20:11(1):. doi: 10.3390/healthcare11010002. Epub 2022 Dec 20 [PubMed PMID: 36611462]
Lee AJ, Yoo EH, Bae YC, Jung SB, Jeon CH. Differential identification of urine crystals with morphologic characteristics and solubility test. Journal of clinical laboratory analysis. 2022 Nov:36(11):e24707. doi: 10.1002/jcla.24707. Epub 2022 Sep 26 [PubMed PMID: 36164743]
Nagae A, Murakami E, Hiwada K, Sato Y, Kawachi M, Kono N. Asymptomatic hereditary xanthinuria: a case report. Japanese journal of medicine. 1990 May-Jun:29(3):287-91 [PubMed PMID: 2273608]
Level 3 (low-level) evidenceIchida K, Amaya Y, Okamoto K, Nishino T. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. International journal of molecular sciences. 2012 Nov 21:13(11):15475-95. doi: 10.3390/ijms131115475. Epub 2012 Nov 21 [PubMed PMID: 23203137]
Grases F, Costa-Bauza A, Roig J, Rodriguez A. Xanthine urolithiasis: Inhibitors of xanthine crystallization. PloS one. 2018:13(8):e0198881. doi: 10.1371/journal.pone.0198881. Epub 2018 Aug 29 [PubMed PMID: 30157195]
Sadiq S, Cil O. Cystinuria: An Overview of Diagnosis and Medical Management. Turkish archives of pediatrics. 2022 Jul:57(4):377-384. doi: 10.5152/TurkArchPediatr.2022.22105. Epub [PubMed PMID: 35822468]
Level 3 (low-level) evidenceFogazzi GB, Delanghe J. Microscopic examination of urine sediment: Phase contrast versus bright field. Clinica chimica acta; international journal of clinical chemistry. 2018 Dec:487():168-173. doi: 10.1016/j.cca.2018.09.036. Epub 2018 Oct 1 [PubMed PMID: 30287257]
Singh VK,Rai PK, Kidney stone analysis techniques and the role of major and trace elements on their pathogenesis: a review. Biophysical reviews. 2014 Dec; [PubMed PMID: 28510032]
Steenbeke M, De Bruyne S, Boelens J, Oyaert M, Glorieux G, Van Biesen W, Linjala J, Delanghe JR, Speeckaert MM. Exploring the possibilities of infrared spectroscopy for urine sediment examination and detection of pathogenic bacteria in urinary tract infections. Clinical chemistry and laboratory medicine. 2020 Sep 25:58(10):1759-1767. doi: 10.1515/cclm-2020-0524. Epub [PubMed PMID: 32649292]
Khan AH, Imran S, Talati J, Jafri L. Fourier transform infrared spectroscopy for analysis of kidney stones. Investigative and clinical urology. 2018 Jan:59(1):32-37. doi: 10.4111/icu.2018.59.1.32. Epub 2018 Jan 3 [PubMed PMID: 29333512]
Simhadri PK, Vaitla PK, Marathi R, Khan A. Donor-transmitted cystinuria in a renal transplant recipient. Journal of nephrology. 2024 Mar 21:():. doi: 10.1007/s40620-023-01877-5. Epub 2024 Mar 21 [PubMed PMID: 38512367]
LaRosa C, McMullen L, Bakdash S, Ellis D, Krishnamurti L, Wu HY, Moritz ML. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatric nephrology (Berlin, Germany). 2007 Jan:22(1):132-5 [PubMed PMID: 17039332]
Ito S, Fujiwara SI, Yoshizawa T, Hayatsu K, Sekiguchi K, Murahashi R, Nakashima H, Matsuoka S, Ikeda T, Toda Y, Kawaguchi S, Nagayama T, Umino K, Minakata D, Nakano H, Morita K, Yamasaki R, Ashizawa M, Yamamoto C, Hatano K, Sato K, Ohmine K, Kanda Y. Urine Xanthine Crystals in Hematologic Malignancies with Tumor Lysis Syndrome. Internal medicine (Tokyo, Japan). 2022 Nov 1:61(21):3271-3275. doi: 10.2169/internalmedicine.9332-22. Epub 2022 Apr 2 [PubMed PMID: 35370238]
Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO. Kidney Disease in Adenine Phosphoribosyltransferase Deficiency. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016 Mar:67(3):431-8. doi: 10.1053/j.ajkd.2015.10.023. Epub 2015 Dec 25 [PubMed PMID: 26724837]
Nasr SH, Sethi S, Cornell LD, Milliner DS, Boelkins M, Broviac J, Fidler ME. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010 Jun:25(6):1909-15. doi: 10.1093/ndt/gfp711. Epub 2010 Jan 11 [PubMed PMID: 20064951]
Perazella MA, Herlitz LC. The Crystalline Nephropathies. Kidney international reports. 2021 Dec:6(12):2942-2957. doi: 10.1016/j.ekir.2021.09.003. Epub 2021 Sep 17 [PubMed PMID: 34901567]