Introduction
From 1961 to 1971, during the Vietnam War, the chemical herbicide known as "Agent Orange" was sprayed in massive quantities across Vietnam, Laos, and Cambodia to clear foliage and crops benefitting the Viet Cong as part of the United States Air Force's Operation Ranch Hand and Army Chemical Corps operations.[1][2] The name Agent Orange originates from the orange stripe on the storage barrels. Agent Orange was one of several "Rainbow Herbicides" used during these operations.
Studies examining the link between Agent Orange and developmental disorders led to the official discontinuation of its use in 1970.[2] Health concerns in returning Vietnam veterans eventually led to the 1991 Agent Orange Act, tasking the National Academy of Medicine to investigate the health effects of Agent Orange exposure.[1] This activity reviews the potential health impacts of Agent Orange exposure in at-risk populations and an approach to their care. This activity includes a discussion of herbicides and dioxin-related compounds similar to Agent Orange; for clarity, subsequent mentions of "Agent Orange" refer to all dioxin-contaminated herbicides used throughout the Vietnam War.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Agent Orange comprises equal parts of the chlorophenoxy herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). The obligatory byproduct of 2,4,5-T production is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a highly toxic carcinogen. TCDD contaminated many herbicides containing 2,4,5-T used in Vietnam from 1961 to 1971. The 6 herbicides used during this timeframe were collectively called the "Rainbow Herbicides." Of this group, Agent Blue was the only herbicide that lacked 2,4,5-T.[1]
Approximately 77 million liters of TCDD-containing herbicides were dispersed during the war, 95% of which was released via aerial application by Operation Ranch Hand fixed-wing aircraft. The remaining 5% was spread by ACC operations using ground sprayers near troop encampments or by helicopters, similar to Operation Ranch Hand missions.[3] Agent Orange exposure may have occurred during its handling, distribution, or from secondary contacts.
The planning and execution of herbicide spraying missions during Operation Ranch Hand were strict; spray missions did not occur with friendly forces in the area and were often deep in hostile territory. Antipersonnel ordinance from escort aircraft was used in targeted areas to suppress hostile ground fire, and friendly units were prohibited from entering areas immediately after spraying due to possible unexploded ordinance.[4] Multiple safeguards and strict adherence to protocols made direct spraying of United States and Allied forces, or their passage through recently sprayed areas, very unlikely.[5]
In contrast, handlers of the chemicals took minimal protective precautions due to the historical presumption that the herbicides were not a human health hazard. Direct exposure routes included indirect and direct cutaneous contact (from contaminated clothing and foliage), inhalation, and ingestion.[1] The half-life of TCDD in the environment varies with many factors, including sun exposure; the half-life ranges from hours to days on foliage and up to several years if absorbed into soil.[3]
Epidemiology
Agent Orange was utilized primarily from 1961 to 1971 in Vietnam and from 1967 to 1971 in the Korean Demilitarized Zone (DMZ). The United States (US) Army Chemical Corps (ACC) herbicide sprayers operated from ground or helicopter-mounted chemical sprayers, including near installations in the Korean DMZ.
Operation Ranch Hand personnel may be risk-stratified by job classification, which is predictive of the degree of exposure.[6] Groups at the highest risk of significant exposure during these periods include:
- US ACC herbicide sprayers and handlers
- US Air Force Operation Ranch Hand personnel
- Higher risk: sprayer-console operators and flight engineers
- Moderate risk: aircraft mechanics, crew chiefs, and support personnel
- Lower risk: pilots and navigators
- Local populations of contaminated regions in Vietnam, Cambodia, and Laos
In addition to the above higher-risk groups, military units deployed near heavily sprayed areas had an increased chance of potential exposure. These units include:
- Units stationed in the "III Corps Military Region" of Vietnam [3]
- The Republic of Korea 9th and Capital Divisions [7]
- Veterans from other US-allied combat units, including Australia, New Zealand, and Thailand
- Personnel stationed in US or Royal Thai military bases in Thailand from 1962 to 1975
These veterans who were potentially exposed to Agent Orange due to their proximity to heavily sprayed areas constitute a tremendous number of individuals with difficult-to-assess exposures, which is a controversial issue. For example, in 1988, the Centers for Disease Control Veterans Health Studies attempted to use military records and self-reported exposure to identify US Army veterans possibly exposed to Agent Orange and, similar to other studies, found nearly identical serum TCDD levels in veterans who served in heavily-sprayed areas compared to non Vietnam veterans.[3] This supports the prevailing belief that most military personnel who served in Vietnam were not heavily exposed to Agent Orange or TCDD, except for those who directly handled or sprayed herbicides.[3][4]
Determining the impact of Agent Orange on local populations is also highly controversial, as controlling for confounding effects from decades of war on these populations is incredibly difficult, if not impossible. However, the soil where Agent Orange was stored or loaded onto aircraft was contaminated with TCDD.[8] Over time, residents may have been exposed to TCDD in soil and water entering the food supply through contaminated animal and plant life.[9][10] Ten US Air Force bases handled most of the Agent Orange entering the country and were located in the following cities, from north to south:
- Da Nang
- Pleiku
- Phu Cat
- Tuy Hoa
- Nha Trang
- Cam Ranh
- Phan Rang
- Bien Hoa
- Tan San Nhut
- Binh Thuy
An additional population who may be adversely affected by Agent Orange includes the children of those directly exposed to Agent Orange, in whom birth and developmental defects were a subject of concern. The generational impact of these exposures is a topic of continued study.[1][2] Given that the contaminant, TCDD, is the most likely component of Agent Orange to cause consequential adverse health events, other comparable populations include known exposures from dioxin-like chemicals or similar herbicides. These populations include industrial chemical workers with occupational exposure, those in British Malaya exposed to "triaxone" in 1952, and individuals subjected to environmental contamination in West Virginia (1949), Missouri (1971), and Seveso, Italy (1976).[1][11][12]
Identifying veterans exposed to Agent Orange outside of direct occupational exposure presents several challenges. Serum TCDD levels remotely measured after exposure may not be a reliable predictor of exposure.[3][7] The Korean Veterans Health Study employed a self-reported perceived exposure index and an objective measure of exposure based on the proximity of military units to herbicide-sprayed areas. The self-reported exposures in this and other studies were less reliable than if an objective measure of exposure was used.[7] For example, reports of troops directly sprayed by aircraft were likely insecticides for mosquito control during a similar period but without the same strict controls as herbicide spray missions.[4] This example highlights the limitations of relying solely on self-reported data due to recall bias, especially when combined with self-reported health outcomes.[1][3] However, veterans self-reporting personally spraying or handling herbicides during service was a reasonably reliable indicator of exposure.[3][13]
Pathophysiology
Agent Orange comprises 2,4-D and 2,4,5-T plus the contaminant TCDD. Most research on Agent Orange pertains to the effects of TCDD due to its consistent contamination of 2,4,5-T and the profoundly toxic nature of dioxins. 2,4-D had longstanding use as an herbicide before Agent Orange, and its toxicity is well-described within its class of chlorophenoxy herbicides.
2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic Acid (2,4-D) is a chlorophenoxy herbicide marketed since the 1940s to kill broadleaf weeds. 2,4-D has generally low toxicity, and poisoning is rare. Severe toxicity requires substantial acute ingestion or chronic exposure to high herbicide concentrations. Acutely, 2,4-D is a corrosive substance that uncouples cellular oxidative phosphorylation. Poisoning classically causes gastrointestinal, muscular, and neurotoxicity. These toxic manifestations include but are not limited to acute gastritis, upper gastrointestinal bleeding, acute kidney injury, rhabdomyolysis, ataxia, hypotonia, seizures, and coma.[14][15][16][17][18] Cutaneous and inhalational exposure may cause mild gastrointestinal symptoms and progressive mixed sensory-motor peripheral neuropathy.[15][17]
Chronic exposure to high concentrations of 2,4-D may induce hepatic, renal, and neurologic injury. Despite this, 2,4-D is not clearly carcinogenic.[19] One study of a population exposed to 2,4-D observed genotoxic damage in the lymphocytes of smokers compared to nonsmokers and provided results that suggested a potentiation of the genotoxic effects of known carcinogens.[20] Results from another study suggested a possible association with non-Hodgkin lymphoma.[21]
2,4,5-Trichlorophenoxyacetic Acid and 2,3,7,8-Tetrachlorodibenzo-p-dioxin
Agent Orange exposure is associated with various diseases, differing from nonTCDD-contaminated herbicides. Health complications related to 2,3,7; TCDD; and Agent Orange exposure remain controversial and are a subject of continued study. The National Academy of Medicine originally published a comprehensive consensus study report on the health impacts of Agent Orange on United States veterans in 1994; the 11th biennial update of the report was issued in 2018.[1]
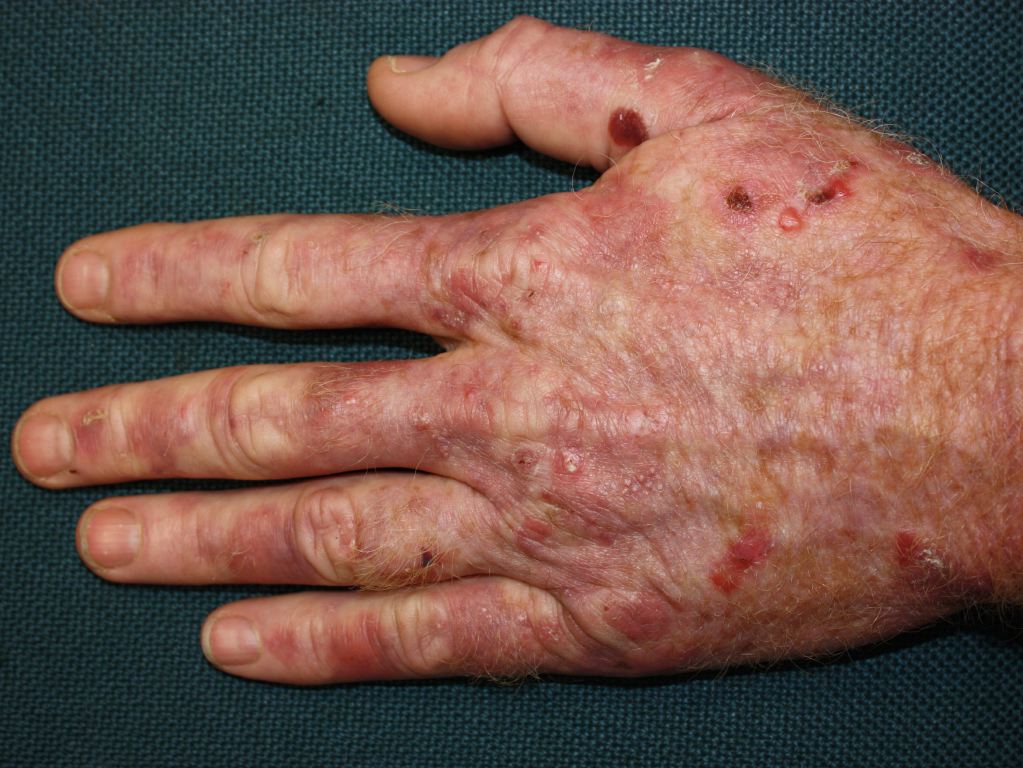
The effects of acute exposure to Agent Orange are due to the contained chlorophenoxy herbicides 2,4-D and 2,4,5-T, as well as TCDD. These effects include acute liver injury, acute pancreatitis, amenorrhea, chloracne, porphyria cutanea tarda, and wasting syndrome.[11][22][23][24][25] See Image. Porphyria Cutanea Tarda.
The predominant biological mechanism of TCDD toxicity has been connected with the high affinity of TCDD to the cytosolic aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor.[26][27] Dioxins bind to the AhR and activate it, inducing translocation to the nucleus to induce or suppress a diverse library of genes. This process and its consequences are not fully understood due to the complex pathways involving the AhR, though numerous studies and reviews examine the AhR and its function.[28]
Some of the cellular functions and pathways altered by TCDD activation of AhR include cell cycle progression, cellular adhesion and migration, the function of receptor tyrosine kinases, and the expression and function of nuclear hormone receptors, growth factors, and growth factor receptors. Additionally, epigenetic changes result from dioxin toxicity. Several in vitro and animal study results have demonstrated altered expression of deoxyribonucleic acid methyltransferases and subsequent changes in gene expression linked with congenital malformations after maternal exposure.[29][30] Other epigenetic mechanisms include changes in noncoding ribonucleic acids and histones.[31][32][33]
The potential consequences of these changes are a subject of debate and ongoing study. The diseases reportedly associated with Agent Orange exposure vary significantly among sources, as does the strength of the evidence itself. These associations rely heavily on occupational and environmental data from other herbicide and dioxin-like exposures to extrapolate the potential health impacts of Agent Orange.
Toxicokinetics
Agent Orange components are well-absorbed via gastrointestinal and respiratory routes. Dermal absorption of 2,4-D and 2,4,5-T is minimal, while TCDD is dose-dependent, with lower absorption at higher doses.[1][34] Chlorophenoxy herbicides such as 2,4-D and 2,4,5-T are water-soluble with a wide volume of distribution in the body. 2,4-D and 2,4,5-T are primarily eliminated unchanged in the urine, with trace amounts of toxic metabolites 2,4-dichlorophenol and 2,4,5-trichlorophenol.[35] The estimated half-life of 2,4-D and 2,4,5-T is 18 to 23 hours and decreases with higher urine pH.[18]
TCDD is a lipophilic dioxin that is rapidly absorbed but slowly eliminated.[36][37] TCDD is stored in adipose tissue and the liver, becoming an inducer of the cytochrome P450 enzyme CYP1A2. CYP1A2 is involved in the metabolism of drugs, including caffeine and neuroleptics such as clozapine, mirtazapine, and olanzapine.[38] TCDD is excreted in stool with a half-life of elimination dependent on factors such as body fat, breastfeeding, age, and other inducers of cytochrome P450 enzymes, including cigarette smoke. One study's results showed a 30% reduction in TCDD half-life in individuals who smoke cigarettes.[39] Contrarily, increasing body mass index was associated with increasing half-life.[40][41] The half-life of TCDD ranges from 7.2 to 11.3 years in adults and is approximately 0.4 years in children younger than 2.
History and Physical
When evaluating patients with suspected exposure to Agent Orange, a comprehensive medical history should be obtained, and a physical examination should be performed. To assess the risk of exposure to Agent Orange in military veterans, obtain specific details of their service history, including service in Operation Ranch Hand or the ACC and handling or spraying of herbicides during service in Vietnam, the Korean DMZ, or Thai military bases. Veterans should also be questioned regarding signs or symptoms of acute dioxin exposure, such as chloracne.
Vietnam veterans who did not serve in the ACC or Operation Ranch Hand and did not personally handle herbicides are at significantly lower risk of substantial exposure to Agent Orange. However, they may have been exposed during service in highly sprayed areas. The "III Corps" combat region of South Vietnam encompasses the southwestern territory surrounding Saigon, where considerable US military presence and many Operation Ranch Hand herbicide missions occurred. These veterans may report traveling through or drinking fresh water from recently sprayed areas. The use of the herbicides stopped in 1971; any military service after that is not at risk of Agent Orange exposure.
The herbicides' effects continue to be studied in populations living within Agent Orange-impacted countries of Southeast Asia. Cities near US Air Force bases where the herbicides were stored may have a higher risk of exposure due to the persistence of TCDD within the environment and food chain.[9][42][43] Individuals living in these areas from the late 1960s on may have the potential for exposure.
A thorough review of systems should be obtained. Neurologic, cardiovascular, and general or constitutional questions may warrant increased focus. Patients with undiagnosed malignancy or endocrinopathy may report unintentional weight changes, fatigue, depression, or night sweats. Questions should cover potential cognitive slowing, neuropathy, or movement disorder. When obtaining a social history, environmental or occupational exposures that may increase the risk of neurologic complications or malignancy should be queried, in addition to standard items such as tobacco, alcohol, and drug use. The family medical history should be updated regularly, documenting any malignancies in family members and the health of any offspring, with particular attention to congenital malformations or genetic syndromes. Clinicians should perform a thorough head-to-toe physical examination annually in patients with suspected exposure to Agent Orange. This examination should include a complete neurologic exam, diabetic foot exam, and careful examination for lymphadenopathy.
Evaluation
Primary care practitioners should employ a patient-centered approach to health screenings in patients with possible exposure to Agent Orange. Expanded screening should be performed on patients with a high risk of exposure, particularly if they handled herbicides or experienced symptoms of acute dioxin exposure. Consideration of expanded screening in others who report Agent Orange exposure is reasonable, though they may be reassured of the overall low likelihood of related health complications.
The expanded screening laboratory evaluation for patients with suspected exposure to Agent Orange monitors for exposure-associated conditions in addition to standard preventative health screenings. Annual laboratory testing with a complete blood count (CBC), prostate-specific antigen (PSA), and urinalysis (UA) allows for screening for the hematologic and genitourinary malignancies for which these patients may be at greater risk. Conditions or testing not discussed herein should follow standard society guidelines, and diligent evaluation of abnormalities revealed during the medical history, physical examination, or laboratory testing is appropriate.
Laboratory Testing
- CBC with differential
- Comprehensive metabolic panel (CMP)
- PSA
- Thyroid-stimulating hormone (TSH)
- UA with microscopy
- Lipid or cholesterol panel and hemoglobin A1C (every 3 years)
Hematologic Malignancies
Annual screening with a CBC may allow for early detection of hematologic malignancies presenting with depressed or elevated cell counts. An abnormal protein gap, defined as a difference between serum albumin and total protein greater than 4, can signify a pathologic increase in immunoglobulins or paraproteins. Elevated serum calcium and creatinine may be seen in monoclonal gammopathies, which may be further evaluated with free light chain serum or urine protein electrophoresis.
Lung Cancer
Lung cancer screening with an annual low-dose computed tomography of the chest is recommended for patients between ages 50 and 80 with at least a 20-pack-year smoking history who are currently actively smoking or have quit in the previous 15 years. There are no consensus guidelines specifying modifications based on Agent Orange exposure. In exposed patients with other significant risk factors for lung cancer or a substantial smoking history that does not meet the threshold for screening, it is reasonable to engage in joint decision-making with the patient and discuss the risks, benefits, and alternatives to lung cancer screening.
Prostate and Bladder Cancer
Patients exposed to Agent Orange or dioxins may be at higher risk for bladder and prostate cancer. Society guidelines generally do not recommend routine screening for these conditions due to the risk of harm and limited evidence of mortality benefit. For high-risk populations, obtaining a PSA and UA annually may be considered for screening for prostate and bladder cancer, respectively.
Treatment / Management
The treatment of acute herbicide toxicity, including 2,4-D, is generally supportive. Decontamination and removal of soiled clothing can prevent further exposure, though most cases are due to intentional or accidental ingestion.[18] Chlorophenoxy herbicides are largely excreted by the kidney, and urine alkalization, in addition to ensuring high urine output, may be considered to prevent complications in significant poisonings.[44][45] Renal replacement therapy with hemodialysis has been used successfully for patients who are critically ill, oliguric, or anuric.[14][46](B3)
Routine monitoring for complications potentially related to Agent Orange exposure is recommended for timely diagnosis and treatment as appropriate. This monitoring should ideally be performed by an appropriate health agency such as the Veterans Affairs Environmental Health Clinic. Given the increased risk of malignancies and cardiovascular disease in individuals exposed to Agent Orange, additional risk factors or positive findings revealed by routine monitoring warrant prompt evaluation and referral to the appropriate specialist.
Differential Diagnosis
Patients with a substantial history of malignancy or conditions associated with exposure to TCDD or dioxins should undergo a thorough review of their family and social history, risk factors, and potential exposures. If the history does not reveal a family history of malignancy, the patient does not have a known genetic syndrome that predisposes to malignancy, and there is no likely history of exposure to Agent Orange, other predisposing historical or clinical conditions must be considered. These conditions include exposure to other environmental or occupational carcinogens, including other dioxins, a history of heavy cigarette smoking, and hereditary cancer syndromes, including Cowden, Li-Fraumeni, and hereditary nonpolyposis colorectal cancer syndromes.
Prognosis
The prognosis of Agent Orange exposure is overall very good. Most Vietnam veterans were unlikely to experience significant Agent Orange exposure during service if they were not involved with herbicide-spraying operations. The current understanding of the potential health complications of Agent Orange is highly reliant on other dioxin-like exposures and long-term occupational exposure to herbicides. Population studies focusing solely on Vietnam veterans are often conflicting and do not provide adequate evidence of associations with most of the health complications in question. Ultimately, the prognosis depends on the severity and duration of exposure, which carries the risk of developing the associated conditions.
Complications
Agent Orange exposure may lead to long-term health outcomes associated with TCDD or dioxin exposure. The current understanding of Agent Orange or dioxin-related complications is primarily through epidemiological, observational, and retrospective studies of the rare exposures in history. These studies are supported by related occupational exposures and animal or in vitro studies to support biological mechanisms for disease development. The level of evidence for each association varies and is challenging to research given the scarcity of events and indeterminate degrees of exposure in the impacted populations.
The connection between actual Agent Orange exposure in veterans and these health outcomes remains controversial. Most Vietnam veterans experienced no or negligible exposure to Agent Orange. Associations between these herbicides and health complications rely on data from long-term occupational exposures, where the degree and duration of exposure over many years are responsible for the increased risk of disease.
The following clinical conditions demonstrated a statistically significant association with one or more components of Agent Orange or dioxin-like compounds; however, the power, confounding, and bias from study to study vary significantly. An adequate lack of bias or confounding and biological plausibility further supports the connection and inclusion by the National Academy of Medicine committee as an associated condition. This is noted below with ¹ or ² for strong and limited or suggestive evidence, respectively. The exception is type 2 diabetes mellitus, about which the committee could not agree between strong or limited evidence of an association.[1]
Neurological System
1Strong Evidence, 2Limited or Suggestive Evidence:
- Parkinson disease and parkinsonianism¹ [47][48][49]
- Peripheral neuropathy² [50][51][52]
- Stroke² [53][54]
- Mild cognitive impairment and dementia [48][55][56][57]
Veterans with known exposure to Agent Orange demonstrate nearly double the incidence of dementia compared to veterans without. Specifically, Parkinson disease and Parkinson-like syndromes demonstrated a stronger association with exposure.[55][57] Other considerations for cognitive impairment and dementia progression included vascular effects due to the increased prevalence of the cardiovascular risk factors diabetes and hypertension.[23]
Upper and Lower Respiratory Tract
1Strong Evidence, 2Limited or Suggestive Evidence:
Some occupational studies suggest an increased risk of laryngeal cancer; these studies were small and did not control for smoking. The risk appeared to be more pronounced in patients who developed chloracne, suggesting a dose-dependent risk relationship. Similar to laryngeal cancer, studies examining other respiratory tract malignancies were often limited by a lack of assessment of the degree of exposure. Despite this, several veteran and occupational studies have demonstrated an increased lung cancer risk. Results from a 2022 epidemiologic study reviewing Veterans Health Administration data found a potential relationship with idiopathic pulmonary fibrosis. Furthermore, there was overall increased mortality from respiratory disease in Army Chemical Corp Vietnam veterans compared to other veterans.[64]
Cardiovascular System
1Strong Evidence, 2Limited or Suggestive Evidence:
The development of hypertension and atherosclerotic disease appear to be associated with Agent Orange exposure.[69][70] In vitro and animal studies on TCDD and dioxins support this mechanistically from impacts on lipid and glucose metabolism, inflammation, and vascular endothelial function via interactions with AhR pathways.[71][72][73][74]
Hepatobiliary and Gastrointestinal Systems
- Liver disease and cirrhosis
The components of Agent Orange have been shown to cause acute liver injury, and dioxin-related hepatotoxicity can lead to steatosis and cirrhosis.[11][75][76][77][78] However, there has not been a consistent association between exposure to the herbicide and these health outcomes.[1][12][13][79][80][81]
Renal System
Studies examining effects on renal function and rates of renal malignancies did not find evidence of an association with Agent Orange exposure.[59] However, some study results have suggested an association between chronic, high dioxin exposure in endemic populations with decreased eGFR and an increased incidence of chronic kidney disease.[82][83][84]
Hematologic System
1Strong Evidence, 2Limited or Suggestive Evidence:
- AL amyloidosis² [59]
- Chronic lymphocytic leukemia¹ [85][86][87][88]
- Hodgkin lymphoma¹ [63][85][86]
- Non-Hodgkin lymphoma¹ [63][85][86][89]
- Myeloproliferative neoplasms [90][91][92]
- Monoclonal gammopathy of undetermined significance (MGUS)¹ [90][93]
- Multiple myeloma² [90][93][94]
TCDD and dioxins profoundly impact multiple hematologic cell lines due to interactions with the AhR pathways.[95][96][97][98][99] An increased risk was found in the results of most studies examining Hodgkin and non-Hodgkin lymphomas, with an apparent predilection towards lymphoid malignancies. The association of TCDD exposure with myeloid malignancies is not well-established and was recommended for further study in the 2016 update to the National Academy of Medicine consensus report.[1][92][100]
Immunologic System
A 2015 New Zealand Vietnam veteran study of hospital discharge records from 1988 to 2009 observed an increased risk of rheumatoid arthritis in exposed populations and may have underestimated the prevalence due to a lack of outpatient data.[80] Some environmental and occupational studies support this correlation, albeit with inconsistent results.[67][79][102] The AhR has been implicated with increased inflammatory cytokines and autoimmune disease, and it may be a target for future therapeutics.[102][103][104]
Genitourinary System
1Strong Evidence, 2Limited or Suggestive Evidence:
The results of several studies examining prostate cancer and Agent Orange exposure generally demonstrate some association but are limited by an increased incidence that is not statistically significant.[114][115][116] The strongest evidence favoring an association between Agent Orange exposure and prostate cancer is among highly-exposed Vietnamese populations, similar to the risk of other cancers.[117][118][119][120]
The association between herbicides and bladder cancer is obfuscated by limited controlling for confounding, notably smoking, in veteran populations. Occupational studies of the chlorophenoxy herbicides provided further support; this includes results from the Agricultural Health Study, a large prospective cohort of US pesticide and herbicide sprayers, which showed a higher risk of bladder cancer in individuals who never smoked with higher uses of 2,4,5-T and 2,4-D.[1][111]
Endocrine System
1Strong Evidence, 2Limited or Suggestive Evidence:
- Type 2 diabetes¹,² [1][67][68][59][121][122]
- Hypothyroidism² [57][123]
- Thyroid cancer [124][125][126]
Evidence supporting the association between Agent Orange exposure and diabetes exists in results from a variety of studies. The weakness in the evidence stems from mildly elevated relative risks reported in some cohorts and confounding from compounds in addition to dioxins in occupational studies. A 2022 meta-analysis identified women in exposed populations as having a higher relative risk of developing diabetes; women may not be represented in studies of veterans.[122] TCDD causes thyroid hormone disruption via overstimulation of TSH receptors and increased thyroid hormone metabolism.[127][128] Data on thyroid cancer is limited; an increased risk of developing thyroid cancer was observed in the results from some studies of Vietnam veterans but not in studies of the Seveso chemical factory accident or similar exposures.
Reproductive System
Studies addressing reproductive health in populations exposed to Agent Orange have been limited and inconclusive in establishing a clear association. Men may experience alterations in sperm morphology, though studies largely demonstrated no statistically significant associations. These associations include concerns about altered sex ratios in offspring and gynecological issues of endometriosis and polycystic ovarian syndrome.[1][135][136] The impact of Agent Orange exposure on a woman's fertility is better supported, with data from the Seveso Women's Health Study demonstrating increased time to pregnancy correlated with increasing serum TCDD.[12]
Developmental Adverse Effects
- Congenital abnormalities and malformations [33][137][138][139][140][141][142]
- Specific congenital disorders
- Neurodevelopmental issues [148][149][150][151][152][153]
Agent Orange exposure leading to adverse events on offspring is a highly controversial topic with widespread potential political implications. The US Department of Veterans Affairs recognizes and compensates only 2 groups of affected children, individuals with spina bifida and children with congenital anomalies who are the offspring of veteran women.[154][155] In their biennial reviews of the literature conducted since 1994, the National Academy of Medicine repeatedly found inadequate evidence of any association, citing the relative rarity of specific congenital disorders and mixed results in population-based studies as weaknesses in establishing a link.[1] Major congenital malformations and disorders suspected to be related to Agent Orange exposure include spina bifida, cleft palate, limb deformities, structural heart disease, and hypothyroidism.[137] An additional area of study with increasing evidence is developmental issues in offspring, notably emotional and cognitive development in children.
Other Oncologic Conditions
1Strong Evidence, 2Limited or Suggestive Evidence:
The evidence supporting the association between Agent Orange exposure and soft tissue sarcomas is robust, both from the chlorophenoxy herbicides and dioxins. This stands in contrast to efforts to establish a link with other solid cancers, which are less represented in the literature.[159][160] Studies examining overall cancer risk have demonstrated some increased cancer mortality but not increased incidence, suggesting mechanistic differences in carcinogenesis in identified malignancies or a role for future research.[7][12][7][59][63][80][119][161][162][163][164][165]
Deterrence and Patient Education
Patients exposed to Agent Orange should be counseled on the potential long-term complications and to contact the appropriate health agency for continued monitoring with specialists familiar with the complications of Agent Orange exposure. Patients with concerns about acute occupational or environmental dioxin exposure should contact a public health agency or regulatory body if the accidental release of dioxin-like compounds poses a worker or public health hazard.
Enhancing Healthcare Team Outcomes
Environmental and occupational exposure to Agent Orange is rare. Monitoring patients for the potential complications of exposure to Agent Orange requires multidisciplinary coordination and long-term follow-up with appropriate specialists. Primary care providers managing patients exposed to Agent Orange or dioxins should:
- Perform a thorough head-to-toe examination and review of symptoms annually
- Review the medical history, including social and family history, to identify other risk factors for malignancy
- Routine hematologic, renal, and hepatic monitoring with laboratory testing
- Perform age-appropriate cancer-related and routine screenings
- Consider expanded screening protocols for conditions such as bladder, prostate, and respiratory tract malignancies
- Counsel patients on the broad array of potential complications, including associations with developmental issues in children
- Refer the patient to the appropriate health agency, such as the Department of Veteran Affairs, for Agent Orange-specific care
- Ensure access to a breadth of necessary specialty care as needed
Media
(Click Image to Enlarge)
References
National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Eleventh Biennial Update). Veterans and Agent Orange: Update 11 (2018). 2018 Nov 15:(): [PubMed PMID: 30629395]
Stellman JM, Stellman SD, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003 Apr 17:422(6933):681-7 [PubMed PMID: 12700752]
. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin levels in US Army Vietnam-era veterans. The Centers for Disease Control Veterans Health Studies. JAMA. 1988 Sep 2:260(9):1249-54 [PubMed PMID: 2841506]
Young AL, Cecil PF Sr, Guilmartin JF Jr. Assessing possible exposures of ground troops to Agent Orange during the Vietnam War: the use of contemporary military records. Environmental science and pollution research international. 2004:11(6):349-58 [PubMed PMID: 15603523]
Level 2 (mid-level) evidenceYoung AL, Giesy JP, Jones PD, Newton M. Environmental fate and bioavailability of Agent Orange and its associated dioxin during the Vietnam War. Environmental science and pollution research international. 2004:11(6):359-70 [PubMed PMID: 15603524]
Michalek JE, Wolfe WH, Miner JC, Papa TM, Pirkle JL. Indices of TCDD exposure and TCDD body burden in veterans of Operation Ranch Hand. Journal of exposure analysis and environmental epidemiology. 1995 Apr-Jun:5(2):209-23 [PubMed PMID: 7492907]
Yi SW, Ryu SY, Ohrr H, Hong JS. Agent Orange exposure and risk of death in Korean Vietnam veterans: Korean Veterans Health Study. International journal of epidemiology. 2014 Dec:43(6):1825-34. doi: 10.1093/ije/dyu183. Epub 2014 Sep 2 [PubMed PMID: 25186308]
Level 2 (mid-level) evidenceYoung AL, Giesy JP, Jones P, Newton M, Guilmartin JF Jr, Cecil PF Sr. Assessment of potential exposure to Agent Orange and its associated TCDD. Environmental science and pollution research international. 2004:11(6):347-8 [PubMed PMID: 15603522]
Schecter A, Quynh HT, Pavuk M, Päpke O, Malisch R, Constable JD. Food as a source of dioxin exposure in the residents of Bien Hoa City, Vietnam. Journal of occupational and environmental medicine. 2003 Aug:45(8):781-8 [PubMed PMID: 12915779]
Level 3 (low-level) evidenceSchecter A, Dai LC, Päpke O, Prange J, Constable JD, Matsuda M, Thao VD, Piskac AL. Recent dioxin contamination from Agent Orange in residents of a southern Vietnam city. Journal of occupational and environmental medicine. 2001 May:43(5):435-43 [PubMed PMID: 11382178]
Level 3 (low-level) evidenceSweeney MH, Mocarelli P. Human health effects after exposure to 2,3,7,8-TCDD. Food additives and contaminants. 2000 Apr:17(4):303-16 [PubMed PMID: 10912244]
Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The Seveso studies on early and long-term effects of dioxin exposure: a review. Environmental health perspectives. 1998 Apr:106 Suppl 2(Suppl 2):625-33 [PubMed PMID: 9599710]
Level 2 (mid-level) evidenceKang HK, Dalager NA, Needham LL, Patterson DG Jr, Lees PS, Yates K, Matanoski GM. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. American journal of industrial medicine. 2006 Nov:49(11):875-84 [PubMed PMID: 17006952]
Level 2 (mid-level) evidenceBradberry SM, Proudfoot AT, Vale JA. Poisoning due to chlorophenoxy herbicides. Toxicological reviews. 2004:23(2):65-73 [PubMed PMID: 15578861]
Level 3 (low-level) evidenceBradberry SM, Watt BE, Proudfoot AT, Vale JA. Mechanisms of toxicity, clinical features, and management of acute chlorophenoxy herbicide poisoning: a review. Journal of toxicology. Clinical toxicology. 2000:38(2):111-22 [PubMed PMID: 10778907]
Level 3 (low-level) evidenceBeasley VR, Arnold EK, Lovell RA, Parker AJ. 2,4-D toxicosis. I: A pilot study of 2,4-dichlorophenoxyacetic acid- and dicamba-induced myotonia in experimental dogs. Veterinary and human toxicology. 1991 Oct:33(5):435-40 [PubMed PMID: 1746132]
Level 3 (low-level) evidenceDESI J, SOS J. [Effect of 2,4-dichlorophenoxyacetic acid and tri-orthocresyl-phosphate on the activity of the higher segments of the central nervous system. (Bioelectrical activity and conditioned reflex reactions)]. Gigiena i sanitariia. 1962 Dec:27():38-46 [PubMed PMID: 14027258]
Rajendran A, Mahalingam S, Ramesh Babu G, Rajeshwari Rajendra K, Nathan B. 2,4-Dichlorophenoxyacetic Acid Poisoning Mimicking as Organophosphorus Poisoning. Cureus. 2021 Jan 22:13(1):e12852. doi: 10.7759/cureus.12852. Epub 2021 Jan 22 [PubMed PMID: 33633885]
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. DDT, Lindane, and 2,4-D. 2018:(): [PubMed PMID: 29912510]
Andreotti G, Hoppin JA, Hou L, Koutros S, Gadalla SM, Savage SA, Lubin J, Blair A, Hoxha M, Baccarelli A, Sandler D, Alavanja M, Beane Freeman LE. Pesticide Use and Relative Leukocyte Telomere Length in the Agricultural Health Study. PloS one. 2015:10(7):e0133382. doi: 10.1371/journal.pone.0133382. Epub 2015 Jul 21 [PubMed PMID: 26196902]
McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. Non-Hodgkin's lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001 Nov:10(11):1155-63 [PubMed PMID: 11700263]
Level 2 (mid-level) evidenceGeusau A, Abraham K, Geissler K, Sator MO, Stingl G, Tschachler E. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: clinical and laboratory effects. Environmental health perspectives. 2001 Aug:109(8):865-9 [PubMed PMID: 11564625]
Level 3 (low-level) evidenceEskenazi B, Warner M, Brambilla P, Signorini S, Ames J, Mocarelli P. The Seveso accident: A look at 40 years of health research and beyond. Environment international. 2018 Dec:121(Pt 1):71-84. doi: 10.1016/j.envint.2018.08.051. Epub 2018 Sep 1 [PubMed PMID: 30179766]
Calvert GM, Sweeney MH, Fingerhut MA, Hornung RW, Halperin WE. Evaluation of porphyria cutanea tarda in U.S. workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. American journal of industrial medicine. 1994 Apr:25(4):559-71 [PubMed PMID: 7912041]
Level 2 (mid-level) evidencePoland AP, Smith D, Metter G, Possick P. A health survey of workers in a 2,4-D and 2,4,5-T plan with special attention to chloracne, porphyria cutanea tarda, and psychologic parameters. Archives of environmental health. 1971 Mar:22(3):316-27 [PubMed PMID: 4250712]
Level 3 (low-level) evidenceDenison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences : an official journal of the Society of Toxicology. 2011 Nov:124(1):1-22. doi: 10.1093/toxsci/kfr218. Epub 2011 Sep 9 [PubMed PMID: 21908767]
Level 3 (low-level) evidenceChopra M, Schrenk D. Dioxin toxicity, aryl hydrocarbon receptor signaling, and apoptosis-persistent pollutants affect programmed cell death. Critical reviews in toxicology. 2011 Apr:41(4):292-320. doi: 10.3109/10408444.2010.524635. Epub 2011 Feb 17 [PubMed PMID: 21323611]
Level 3 (low-level) evidenceSorg O. AhR signalling and dioxin toxicity. Toxicology letters. 2014 Oct 15:230(2):225-33. doi: 10.1016/j.toxlet.2013.10.039. Epub 2013 Nov 12 [PubMed PMID: 24239782]
Level 3 (low-level) evidencePatrizi B, Siciliani de Cumis M. TCDD Toxicity Mediated by Epigenetic Mechanisms. International journal of molecular sciences. 2018 Dec 18:19(12):. doi: 10.3390/ijms19124101. Epub 2018 Dec 18 [PubMed PMID: 30567322]
Gaspari L, Paris F, Kalfa N, Soyer-Gobillard MO, Sultan C, Hamamah S. Experimental Evidence of 2,3,7,8-Tetrachlordibenzo-p-Dioxin (TCDD) Transgenerational Effects on Reproductive Health. International journal of molecular sciences. 2021 Aug 23:22(16):. doi: 10.3390/ijms22169091. Epub 2021 Aug 23 [PubMed PMID: 34445797]
Wang C, Yuan XG, Liu CP, Zhai SN, Zhang DW, Fu YX. Preliminary research on DNA methylation changes during murine palatogenesis induced by TCDD. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2017 May:45(5):678-684. doi: 10.1016/j.jcms.2017.02.004. Epub 2017 Feb 12 [PubMed PMID: 28336320]
Beedanagari SR, Taylor RT, Bui P, Wang F, Nickerson DW, Hankinson O. Role of epigenetic mechanisms in differential regulation of the dioxin-inducible human CYP1A1 and CYP1B1 genes. Molecular pharmacology. 2010 Oct:78(4):608-16. doi: 10.1124/mol.110.064899. Epub 2010 Jul 14 [PubMed PMID: 20631054]
Yoshioka W, Tohyama C. Mechanisms of Developmental Toxicity of Dioxins and Related Compounds. International journal of molecular sciences. 2019 Jan 31:20(3):. doi: 10.3390/ijms20030617. Epub 2019 Jan 31 [PubMed PMID: 30708991]
Banks YB, Birnbaum LS. Absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) after low dose dermal exposure. Toxicology and applied pharmacology. 1991 Feb:107(2):302-10 [PubMed PMID: 1994512]
Level 3 (low-level) evidenceBukowska B. Effects of 2,4-D and its metabolite 2,4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2003 Aug:135(4):435-41 [PubMed PMID: 12965188]
Level 2 (mid-level) evidenceEmond C, Michalek JE, Birnbaum LS, DeVito MJ. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environmental health perspectives. 2005 Dec:113(12):1666-8 [PubMed PMID: 16330344]
Level 2 (mid-level) evidenceMichalek JE, Pirkle JL, Needham LL, Patterson DG Jr, Caudill SP, Tripathi RC, Mocarelli P. Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso adults and veterans of operation Ranch Hand. Journal of exposure analysis and environmental epidemiology. 2002 Jan-Feb:12(1):44-53 [PubMed PMID: 11859432]
Guo J, Zhu X, Badawy S, Ihsan A, Liu Z, Xie C, Wang X. Metabolism and Mechanism of Human Cytochrome P450 Enzyme 1A2. Current drug metabolism. 2021:22(1):40-49. doi: 10.2174/1389200221999210101233135. Epub [PubMed PMID: 33397254]
Flesch-Janys D, Becher H, Gurn P, Jung D, Konietzko J, Manz A, Päpke O. Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. Journal of toxicology and environmental health. 1996 Mar:47(4):363-78 [PubMed PMID: 8600289]
Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environmental health perspectives. 2009 Mar:117(3):417-25. doi: 10.1289/ehp.11781. Epub 2008 Oct 3 [PubMed PMID: 19337517]
Level 1 (high-level) evidenceWolfe WH, Michalek JE, Miner JC, Pirkle JL, Caudill SP, Patterson DG Jr, Needham LL. Determinants of TCDD half-life in veterans of operation ranch hand. Journal of toxicology and environmental health. 1994 Apr:41(4):481-8 [PubMed PMID: 8145287]
Armitage JM, Ginevan ME, Hewitt A, Ross JH, Watkins DK, Solomon KR. Environmental fate and dietary exposures of humans to TCDD as a result of the spraying of Agent Orange in upland forests of Vietnam. The Science of the total environment. 2015 Feb 15:506-507():621-30. doi: 10.1016/j.scitotenv.2014.11.026. Epub 2014 Nov 26 [PubMed PMID: 25433383]
Scialli AR, Watkins DK, Ginevan ME. Agent Orange Exposure and 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) in Human Milk. Birth defects research. Part B, Developmental and reproductive toxicology. 2015 Jun:104(3):129-39. doi: 10.1002/bdrb.21145. Epub 2015 Jul 20 [PubMed PMID: 26195119]
Pannu AK, Saroch A, Agrawal J, Sharma N. 2,4-D poisoning: a review with illustration of two cases. Tropical doctor. 2018 Oct:48(4):366-368. doi: 10.1177/0049475518786834. Epub 2018 Jul 17 [PubMed PMID: 30012080]
Level 3 (low-level) evidenceBadu AB, Cempakadewi AA, Budihardja BM, Ake A. Alkaline Diuresis as Treatment for 2,4-D Dimethylamine Herbicide Intoxication. European journal of case reports in internal medicine. 2022:9(1):003126. doi: 10.12890/2022_003126. Epub 2022 Jan 19 [PubMed PMID: 35169579]
Level 3 (low-level) evidenceDurakovic Z, Durakovic A, Durakovic S, Ivanovic D. Poisoning with 2,4-dichlorophenoxyacetic acid treated by hemodialysis. Archives of toxicology. 1992:66(7):518-21 [PubMed PMID: 1444815]
Level 3 (low-level) evidenceMcKnight S, Hack N. Toxin-Induced Parkinsonism. Neurologic clinics. 2020 Nov:38(4):853-865. doi: 10.1016/j.ncl.2020.08.003. Epub 2020 Sep 9 [PubMed PMID: 33040865]
Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014 Jun:10(3 Suppl):S213-25. doi: 10.1016/j.jalz.2014.04.014. Epub [PubMed PMID: 24924672]
Level 3 (low-level) evidenceYang Y, Cheon M, Kwak YT. Is Parkinson's Disease with History of Agent Orange Exposure Different from Idiopathic Parkinson's Disease? Dementia and neurocognitive disorders. 2016 Sep:15(3):75-81. doi: 10.12779/dnd.2016.15.3.75. Epub 2016 Sep 30 [PubMed PMID: 30906346]
Ames J, Warner M, Brambilla P, Mocarelli P, Satariano WA, Eskenazi B. Neurocognitive and physical functioning in the Seveso Women's Health Study. Environmental research. 2018 Apr:162():55-62. doi: 10.1016/j.envres.2017.12.005. Epub 2017 Dec 26 [PubMed PMID: 29287180]
Urban P, Pelclová D, Lukás E, Kupka K, Preiss J, Fenclová Z, Smerhovský Z. Neurological and neurophysiological examinations on workers with chronic poisoning by 2,3,7,8-TCDD: follow-up 35 years after exposure. European journal of neurology. 2007 Feb:14(2):213-8 [PubMed PMID: 17250732]
Pelclova D, Urban P, Fenclova Z, Vlckova S, Ridzon P, Kupka K, Meckova Z, Bezdicek O, Navratil T, Rosmus J, Zakharov S. Neurological and Neurophysiological Findings in Workers with Chronic 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Intoxication 50 Years After Exposure. Basic & clinical pharmacology & toxicology. 2018 Feb:122(2):271-277. doi: 10.1111/bcpt.12899. Epub 2017 Sep 27 [PubMed PMID: 28862800]
Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, Matsumura F, Vogel CF. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arteriosclerosis, thrombosis, and vascular biology. 2011 Jun:31(6):1260-7. doi: 10.1161/ATVBAHA.110.220202. Epub 2011 Mar 24 [PubMed PMID: 21441140]
Level 3 (low-level) evidenceHan S, Hwang I, Kim SM, Yang YS, Ha S, Han JH, Park TH. Differences in the clinical manifestations and short-term prognosis of acute cerebral infarction after exposure to Agent Orange. Annals of occupational and environmental medicine. 2016:28():66 [PubMed PMID: 27891240]
Martinez S, Yaffe K, Li Y, Byers AL, Peltz CB, Barnes DE. Agent Orange Exposure and Dementia Diagnosis in US Veterans of the Vietnam Era. JAMA neurology. 2021 Apr 1:78(4):473-477. doi: 10.1001/jamaneurol.2020.5011. Epub [PubMed PMID: 33492338]
Barrett DH, Morris RD, Akhtar FZ, Michalek JE. Serum dioxin and cognitive functioning among veterans of Operation Ranch Hand. Neurotoxicology. 2001 Aug:22(4):491-502 [PubMed PMID: 11577806]
Level 2 (mid-level) evidenceYi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environmental research. 2014 Aug:133():56-65. doi: 10.1016/j.envres.2014.04.027. Epub 2014 Jun 4 [PubMed PMID: 24906069]
Kaul B, Lee JS, Glidden DV, Blanc PD, Zhang N, Collard HR, Whooley MA. Agent Orange Exposure and Risk of Idiopathic Pulmonary Fibrosis among U.S. Veterans. American journal of respiratory and critical care medicine. 2022 Sep 15:206(6):750-757. doi: 10.1164/rccm.202112-2724OC. Epub [PubMed PMID: 35559726]
Yi SW, Ohrr H. Agent Orange exposure and cancer incidence in Korean Vietnam veterans: a prospective cohort study. Cancer. 2014 Dec 1:120(23):3699-706. doi: 10.1002/cncr.28961. Epub 2014 Aug 7 [PubMed PMID: 25103108]
Watanabe KK, Kang HK. Mortality patterns among Vietnam veterans: a 24-year retrospective analysis. Journal of occupational and environmental medicine. 1996 Mar:38(3):272-8 [PubMed PMID: 8882099]
Level 2 (mid-level) evidencePesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. Cancer incidence in the population exposed to dioxin after the "Seveso accident": twenty years of follow-up. Environmental health : a global access science source. 2009 Sep 15:8():39. doi: 10.1186/1476-069X-8-39. Epub 2009 Sep 15 [PubMed PMID: 19754930]
Bonner MR, Freeman LE, Hoppin JA, Koutros S, Sandler DP, Lynch CF, Hines CJ, Thomas K, Blair A, Alavanja MC. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environmental health perspectives. 2017 Apr:125(4):544-551. doi: 10.1289/EHP456. Epub 2016 Jul 6 [PubMed PMID: 27384818]
Level 3 (low-level) evidenceVuong TP. Research on the Relationship between Exposure to Dioxins and Cancer Incidence in Vietnam. Toxics. 2022 Jul 11:10(7):. doi: 10.3390/toxics10070384. Epub 2022 Jul 11 [PubMed PMID: 35878289]
Cypel Y, Kang H. Mortality patterns of Army Chemical Corps veterans who were occupationally exposed to herbicides in Vietnam. Annals of epidemiology. 2010 May:20(5):339-46. doi: 10.1016/j.annepidem.2010.02.003. Epub [PubMed PMID: 20382334]
Level 2 (mid-level) evidenceBrulport A, Le Corre L, Chagnon MC. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology. 2017 Sep 1:390():43-52. doi: 10.1016/j.tox.2017.07.017. Epub 2017 Aug 1 [PubMed PMID: 28774668]
Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide Exposure, Vietnam Service, and Hypertension Risk in Army Chemical Corps Veterans. Journal of occupational and environmental medicine. 2016 Nov:58(11):1127-1136 [PubMed PMID: 27820763]
Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. Journal of occupational medicine and toxicology (London, England). 2016:11():7. doi: 10.1186/s12995-016-0095-8. Epub 2016 Feb 20 [PubMed PMID: 26900394]
Yamamoto K, Kudo M, Arito H, Ogawa Y, Takata T. A cross-sectional analysis of dioxins and health effects in municipal and private waste incinerator workers in Japan. Industrial health. 2015:53(5):465-79. doi: 10.2486/indhealth.2015-0006. Epub 2015 Jul 23 [PubMed PMID: 26212412]
Level 2 (mid-level) evidenceZhu K, Meng Q, Zhang Z, Yi T, He Y, Zheng J, Lei W. Aryl hydrocarbon receptor pathway: Role, regulation and intervention in atherosclerosis therapy (Review). Molecular medicine reports. 2019 Dec:20(6):4763-4773. doi: 10.3892/mmr.2019.10748. Epub 2019 Oct 16 [PubMed PMID: 31638212]
Polonikov AV, Bushueva OY, Bulgakova IV, Freidin MB, Churnosov MI, Solodilova MA, Shvetsov YD, Ivanov VP. A comprehensive contribution of genes for aryl hydrocarbon receptor signaling pathway to hypertension susceptibility. Pharmacogenetics and genomics. 2017 Feb:27(2):57-69. doi: 10.1097/FPC.0000000000000261. Epub [PubMed PMID: 27977510]
Yu Y, Qin J, Chen D, Wang H, Wang J, Yu Y. Chronic cardiovascular disease-associated gene network analysis in human umbilical vein endothelial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cardiovascular toxicology. 2015 Apr:15(2):157-71. doi: 10.1007/s12012-014-9279-6. Epub [PubMed PMID: 25216946]
Level 3 (low-level) evidenceKopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicology and applied pharmacology. 2010 May 15:245(1):91-9. doi: 10.1016/j.taap.2010.02.007. Epub 2010 Feb 19 [PubMed PMID: 20171976]
Petriello MC, Brandon JA, Hoffman J, Wang C, Tripathi H, Abdel-Latif A, Ye X, Li X, Yang L, Lee E, Soman S, Barney J, Wahlang B, Hennig B, Morris AJ. Dioxin-like PCB 126 Increases Systemic Inflammation and Accelerates Atherosclerosis in Lean LDL Receptor-Deficient Mice. Toxicological sciences : an official journal of the Society of Toxicology. 2018 Apr 1:162(2):548-558. doi: 10.1093/toxsci/kfx275. Epub [PubMed PMID: 29216392]
Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicological sciences : an official journal of the Society of Toxicology. 2010 Oct:117(2):537-46. doi: 10.1093/toxsci/kfq218. Epub 2010 Jul 15 [PubMed PMID: 20634294]
Level 3 (low-level) evidenceYan J, Tung HC, Li S, Niu Y, Garbacz WG, Lu P, Bi Y, Li Y, He J, Xu M, Ren S, Monga SP, Schwabe RF, Yang D, Xie W. Aryl Hydrocarbon Receptor Signaling Prevents Activation of Hepatic Stellate Cells and Liver Fibrogenesis in Mice. Gastroenterology. 2019 Sep:157(3):793-806.e14. doi: 10.1053/j.gastro.2019.05.066. Epub 2019 Jun 3 [PubMed PMID: 31170413]
Zheng S, Yang Y, Wen C, Liu W, Cao L, Feng X, Chen J, Wang H, Tang Y, Tian L, Wang X, Yang F. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environment international. 2021 Sep:154():106555. doi: 10.1016/j.envint.2021.106555. Epub 2021 Apr 12 [PubMed PMID: 33857709]
Jin J, Wahlang B, Shi H, Hardesty JE, Falkner KC, Head KZ, Srivastava S, Merchant ML, Rai SN, Cave MC, Prough RA. Dioxin-like and non-dioxin-like PCBs differentially regulate the hepatic proteome and modify diet-induced nonalcoholic fatty liver disease severity. Medicinal chemistry research : an international journal for rapid communications on design and mechanisms of action of biologically active agents. 2020 Jul:29():1247-1263. doi: 10.1007/s00044-020-02581-w. Epub 2020 Jun 7 [PubMed PMID: 32831531]
Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, Cave MC. Toxicant-associated steatohepatitis. Toxicologic pathology. 2013 Feb:41(2):343-60. doi: 10.1177/0192623312468517. Epub 2012 Dec 21 [PubMed PMID: 23262638]
Level 3 (low-level) evidence't Mannetje A, Eng A, Walls C, Dryson E, Douwes J, Bertazzi P, Ryder-Lewis S, Scott D, Brooks C, McLean D, Cheng S, Pearce N. Morbidity in New Zealand pesticide producers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Environment international. 2018 Jan:110():22-31. doi: 10.1016/j.envint.2017.09.018. Epub 2017 Oct 12 [PubMed PMID: 29031942]
Cox B, McBride D, Broughton J, Tong D. Health conditions in a cohort of New Zealand Vietnam veterans: hospital admissions between 1988 and 2009. BMJ open. 2015 Dec 9:5(12):e008409. doi: 10.1136/bmjopen-2015-008409. Epub 2015 Dec 9 [PubMed PMID: 26656012]
Pham PQ, Nguyen VB, Pham TT, Duong NX, Nguyen HT, Ha QV, Nguyen TD, Hoang TM, Dinh DT, Tran QTN, Bui LK, Vu TT, Phan MV, Luong TM, Nguyen K, Vu DA, Pham TN. Histopathological Alterations in the Livers of Chronic Hepatitis Patients Exposed to Agent Orange/Dioxin in Vietnam. Toxics. 2022 Jun 10:10(6):. doi: 10.3390/toxics10060315. Epub 2022 Jun 10 [PubMed PMID: 35736923]
Huang CY, Wu CL, Wu JS, Chang JW, Cheng YY, Kuo YC, Yang YC, Lee CC, Guo HR. Association between Blood Dioxin Level and Chronic Kidney Disease in an Endemic Area of Exposure. PloS one. 2016:11(3):e0150248. doi: 10.1371/journal.pone.0150248. Epub 2016 Mar 10 [PubMed PMID: 26963719]
Chang JW, Ou HY, Chen HL, Su HJ, Lee CC. Hyperuricemia after exposure to polychlorinated dibenzo-p-dioxins and dibenzofurans near a highly contaminated area. Epidemiology (Cambridge, Mass.). 2013 Jul:24(4):582-9. doi: 10.1097/EDE.0b013e318294ef68. Epub [PubMed PMID: 23676268]
Level 2 (mid-level) evidenceKataria A, Trasande L, Trachtman H. The effects of environmental chemicals on renal function. Nature reviews. Nephrology. 2015 Oct:11(10):610-25. doi: 10.1038/nrneph.2015.94. Epub 2015 Jun 23 [PubMed PMID: 26100504]
von Stackelberg K. A Systematic Review of Carcinogenic Outcomes and Potential Mechanisms from Exposure to 2,4-D and MCPA in the Environment. Journal of toxicology. 2013:2013():371610. doi: 10.1155/2013/371610. Epub 2013 Feb 26 [PubMed PMID: 23533401]
Level 1 (high-level) evidenceCoggon D, Ntani G, Harris EC, Jayakody N, Palmer KT. Soft tissue sarcoma, non-Hodgkin's lymphoma and chronic lymphocytic leukaemia in workers exposed to phenoxy herbicides: extended follow-up of a UK cohort. Occupational and environmental medicine. 2015 Jun:72(6):435-41. doi: 10.1136/oemed-2014-102654. Epub 2015 Feb 18 [PubMed PMID: 25694496]
Level 2 (mid-level) evidenceBaumann Kreuziger LM, Tarchand G, Morrison VA. The impact of Agent Orange exposure on presentation and prognosis of patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2014 Jan:55(1):63-6. doi: 10.3109/10428194.2013.794267. Epub 2013 May 15 [PubMed PMID: 23573826]
Level 2 (mid-level) evidenceMescher C, Gilbertson D, Randall NM, Tarchand G, Tomaska J, Baumann Kreuziger L, Morrison VA. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leukemia & lymphoma. 2018 Jun:59(6):1348-1355. doi: 10.1080/10428194.2017.1375109. Epub 2017 Sep 14 [PubMed PMID: 28905668]
Hardell L, Eriksson M. A case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer. 1999 Mar 15:85(6):1353-60 [PubMed PMID: 10189142]
Level 2 (mid-level) evidenceLandgren O, Shim YK, Michalek J, Costello R, Burton D, Ketchum N, Calvo KR, Caporaso N, Raveche E, Middleton D, Marti G, Vogt RF Jr. Agent Orange Exposure and Monoclonal Gammopathy of Undetermined Significance: An Operation Ranch Hand Veteran Cohort Study. JAMA oncology. 2015 Nov:1(8):1061-8. doi: 10.1001/jamaoncol.2015.2938. Epub [PubMed PMID: 26335650]
Sperling AS, Leventhal M, Gibson CJ, Ebert BL, Steensma DP. Myelodysplastic syndromes (MDS) occurring in Agent Orange exposed individuals carry a mutational spectrum similar to that of de novo MDS. Leukemia & lymphoma. 2020 Mar:61(3):728-731. doi: 10.1080/10428194.2019.1689394. Epub 2019 Nov 12 [PubMed PMID: 31714164]
Shallis RM, Gore SD. Agent Orange and dioxin-induced myeloid leukemia: a weaponized vehicle of leukemogenesis. Leukemia & lymphoma. 2022 Jul:63(7):1534-1543. doi: 10.1080/10428194.2022.2034156. Epub 2022 Feb 2 [PubMed PMID: 35105250]
Dodlapati J, Hall JA, Kulkarni P, Reely KB, Nangrani AA, Copeland LA. Agent Orange Exposure, Transformation From MGUS to Multiple Myeloma, and Outcomes in Veterans. Federal practitioner : for the health care professionals of the VA, DoD, and PHS. 2022 Aug:39(Suppl 3):S23-S29a. doi: 10.12788/fp.0303. Epub 2022 Aug 15 [PubMed PMID: 36426111]
Wang L, Kumar M, Deng Q, Wang X, Liu M, Gong Z, Zhang S, Ma X, Xu-Monette ZY, Xiao M, Yi Q, Young KH, Ramos KS, Li Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces peripheral blood abnormalities and plasma cell neoplasms resembling multiple myeloma in mice. Cancer letters. 2019 Jan:440-441():135-144. doi: 10.1016/j.canlet.2018.10.009. Epub 2018 Oct 19 [PubMed PMID: 30343114]
Level 3 (low-level) evidenceZhang L, Zhao R, Ye SQ, Zhou L, Wu YN, Zeng Y. Synergistic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and N-nitrosodiethylamine on cell malignant transformation. Biomedical and environmental sciences : BES. 2013 May:26(5):323-30. doi: 10.3967/0895-3988.2013.05.001. Epub [PubMed PMID: 23611125]
Level 3 (low-level) evidenceAngelos MG, Kaufman DS. Advances in the role of the aryl hydrocarbon receptor to regulate early hematopoietic development. Current opinion in hematology. 2018 Jul:25(4):273-278. doi: 10.1097/MOH.0000000000000432. Epub [PubMed PMID: 29697485]
Level 3 (low-level) evidenceGasiewicz TA, Singh KP, Bennett JA. The Ah receptor in stem cell cycling, regulation, and quiescence. Annals of the New York Academy of Sciences. 2014 Mar:1310():44-50. doi: 10.1111/nyas.12361. Epub 2014 Feb 3 [PubMed PMID: 24495120]
Level 3 (low-level) evidenceSingh KP, Bennett JA, Casado FL, Walrath JL, Welle SL, Gasiewicz TA. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem cells and development. 2014 Jan 15:23(2):95-106. doi: 10.1089/scd.2013.0346. Epub 2013 Oct 19 [PubMed PMID: 24138668]
Level 3 (low-level) evidenceFracchiolla NS, Annaloro C, Guidotti F, Fattizzo B, Cortelezzi A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) role in hematopoiesis and in hematologic diseases: A critical review. Toxicology. 2016 Dec 30:374():60-68. doi: 10.1016/j.tox.2016.10.007. Epub 2016 Oct 17 [PubMed PMID: 27765685]
Saberi Hosnijeh F, Christopher Y, Peeters P, Romieu I, Xun W, Riboli E, Raaschou-Nielsen O, Tjønneland A, Becker N, Nieters A, Trichopoulou A, Bamia C, Orfanos P, Oddone E, Luján-Barroso L, Dorronsoro M, Navarro C, Barricarte A, Molina-Montes E, Wareham N, Vineis P, Vermeulen R. Occupation and risk of lymphoid and myeloid leukaemia in the European Prospective Investigation into Cancer and Nutrition (EPIC). Occupational and environmental medicine. 2013 Jul:70(7):464-70. doi: 10.1136/oemed-2012-101135. Epub 2013 Apr 10 [PubMed PMID: 23576671]
Chittrakul J, Sapbamrer R, Sirikul W. Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics. 2022 Apr 21:10(5):. doi: 10.3390/toxics10050207. Epub 2022 Apr 21 [PubMed PMID: 35622621]
Level 1 (high-level) evidenceNguyen CH, Nakahama T, Dang TT, Chu HH, Van Hoang L, Kishimoto T, Nguyen NT. Expression of aryl hydrocarbon receptor, inflammatory cytokines, and incidence of rheumatoid arthritis in Vietnamese dioxin-exposed people. Journal of immunotoxicology. 2017 Dec:14(1):196-203. doi: 10.1080/1547691X.2017.1377323. Epub [PubMed PMID: 29096558]
Ebel AV, Lutt G, Poole JA, Thiele GM, Baker JF, Cannon GW, Gaffo A, Kerr GS, Reimold A, Schwab P, Singh N, Richards JS, Ascherman DP, Mikuls TR, England BR. Association of Agricultural, Occupational, and Military Inhalants With Autoantibodies and Disease Features in US Veterans With Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, N.J.). 2021 Mar:73(3):392-400. doi: 10.1002/art.41559. Epub 2021 Jan 29 [PubMed PMID: 33058561]
Hui W, Dai Y. Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic & clinical pharmacology & toxicology. 2020 Jun:126(6):469-474. doi: 10.1111/bcpt.13372. Epub 2019 Dec 19 [PubMed PMID: 31811747]
Chang C, Benson M, Fam MM. A review of Agent Orange and its associated oncologic risk of genitourinary cancers. Urologic oncology. 2017 Nov:35(11):633-639. doi: 10.1016/j.urolonc.2017.08.029. Epub 2017 Sep 22 [PubMed PMID: 28947305]
Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer. 2013 Jul 1:119(13):2399-404. doi: 10.1002/cncr.27941. Epub 2013 May 13 [PubMed PMID: 23670242]
Giri VN, Cassidy AE, Beebe-Dimmer J, Ellis L, Smith DC, Bock CH, Cooney KA. Association between Agent Orange and prostate cancer: a pilot case-control study. Urology. 2004 Apr:63(4):757-60; discussion 760-1 [PubMed PMID: 15072895]
Level 3 (low-level) evidenceChamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008 Nov 1:113(9):2464-70. doi: 10.1002/cncr.23695. Epub [PubMed PMID: 18666213]
Level 2 (mid-level) evidenceKabir A, Zendehdel R, Tayefeh-Rahimian R. Dioxin Exposure in the Manufacture of Pesticide Production as a Risk Factor for Death from Prostate Cancer: A Meta-analysis. Iranian journal of public health. 2018 Feb:47(2):148-155 [PubMed PMID: 29445624]
Level 1 (high-level) evidenceMossanen M, Kibel AS, Goldman RH. Exploring exposure to Agent Orange and increased mortality due to bladder cancer. Urologic oncology. 2017 Nov:35(11):627-632. doi: 10.1016/j.urolonc.2017.07.030. Epub 2017 Aug 18 [PubMed PMID: 28826703]
Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, Lynch CF, Sandler DP, Blair A, Beane Freeman LE. Occupational exposure to pesticides and bladder cancer risk. International journal of epidemiology. 2016 Jun:45(3):792-805. doi: 10.1093/ije/dyv195. Epub 2015 Sep 27 [PubMed PMID: 26411407]
Boers D, Portengen L, Bueno-de-Mesquita HB, Heederik D, Vermeulen R. Cause-specific mortality of Dutch chlorophenoxy herbicide manufacturing workers. Occupational and environmental medicine. 2010 Jan:67(1):24-31. doi: 10.1136/oem.2008.044222. Epub 2009 Sep 6 [PubMed PMID: 19736176]
Level 2 (mid-level) evidenceManuwald U, Velasco Garrido M, Berger J, Manz A, Baur X. Mortality study of chemical workers exposed to dioxins: follow-up 23 years after chemical plant closure. Occupational and environmental medicine. 2012 Sep:69(9):636-42. doi: 10.1136/oemed-2012-100682. Epub 2012 Jul 5 [PubMed PMID: 22767868]
Zafar MB, Terris MK. Prostate cancer detection in veterans with a history of Agent Orange exposure. The Journal of urology. 2001 Jul:166(1):100-3 [PubMed PMID: 11435832]
Level 2 (mid-level) evidenceSun XL, Kido T, Honma S, Okamoto R, Manh HD, Maruzeni S, Nishijo M, Nakagawa H, Nakano T, Koh E, Takasuga T, Nhu DD, Hung NN, Son le K. Influence of dioxin exposure upon levels of prostate-specific antigen and steroid hormones in Vietnamese men. Environmental science and pollution research international. 2016 Apr:23(8):7807-13. doi: 10.1007/s11356-015-5931-3. Epub 2016 Jan 12 [PubMed PMID: 26758301]
Etheridge T, Liou JI, Downs TM, Abel EJ, Jarrard DF, Richards KA. The Impact of Agent Orange Exposure on Prostate Cancer Outcomes. The Journal of urology. 2019 Apr:201(4):742-750. doi: 10.1016/j.juro.2018.10.005. Epub [PubMed PMID: 30321553]
Koutros S, Langseth H, Grimsrud TK, Barr DB, Vermeulen R, Portengen L, Wacholder S, Freeman LE, Blair A, Hayes RB, Rothman N, Engel LS. Prediagnostic Serum Organochlorine Concentrations and Metastatic Prostate Cancer: A Nested Case-Control Study in the Norwegian Janus Serum Bank Cohort. Environmental health perspectives. 2015 Sep:123(9):867-72. doi: 10.1289/ehp.1408245. Epub 2015 Mar 3 [PubMed PMID: 25734605]
Level 2 (mid-level) evidenceLi Q, Lan L, Klaassen Z, Shah SR, Moses KA, Terris MK. High level of dioxin-TEQ in tissue is associated with Agent Orange exposure but not with biochemical recurrence after radical prostatectomy. Prostate cancer and prostatic diseases. 2013 Dec:16(4):376-81. doi: 10.1038/pcan.2013.33. Epub 2013 Sep 10 [PubMed PMID: 24018710]
Collins JJ, Bodner KM, Aylward LL, Bender TJ, Anteau S, Wilken M, Bodnar CM. Mortality risk among workers with exposure to dioxins. Occupational medicine (Oxford, England). 2016 Dec:66(9):706-712 [PubMed PMID: 27932487]
Chang ET, Boffetta P, Adami HO, Cole P, Mandel JS. A critical review of the epidemiology of Agent Orange/TCDD and prostate cancer. European journal of epidemiology. 2014 Oct:29(10):667-723. doi: 10.1007/s10654-014-9931-2. Epub 2014 Jul 27 [PubMed PMID: 25064616]
Level 2 (mid-level) evidenceHuang CY, Wu CL, Yang YC, Chang JW, Kuo YC, Cheng YY, Wu JS, Lee CC, Guo HR. Association between Dioxin and Diabetes Mellitus in an Endemic Area of Exposure in Taiwan: A Population-Based Study. Medicine. 2015 Oct:94(42):e1730. doi: 10.1097/MD.0000000000001730. Epub [PubMed PMID: 26496286]
Gang N, Van Allen K, Villeneuve PJ, MacDonald H, Bruin JE. Sex-specific Associations Between Type 2 Diabetes Incidence and Exposure to Dioxin and Dioxin-like Pollutants: A Meta-analysis. Frontiers in toxicology. 2021:3():685840. doi: 10.3389/ftox.2021.685840. Epub 2022 Feb 23 [PubMed PMID: 35295132]
Level 1 (high-level) evidencePavuk M, Schecter AJ, Akhtar FZ, Michalek JE. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in Air Force veterans of the Vietnam War. Annals of epidemiology. 2003 May:13(5):335-43 [PubMed PMID: 12821272]
Level 2 (mid-level) evidenceLe KT, Sawicki MP, Wang MB, Hershman JM, Leung AM. HIGH PREVALENCE OF AGENT ORANGE EXPOSURE AMONG THYROID CANCER PATIENTS IN THE NATIONAL VA HEALTHCARE SYSTEM. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Jun:22(6):699-702. doi: 10.4158/EP151108.OR. Epub 2016 Jan 27 [PubMed PMID: 27176142]
Lee S, Lim Y, Kang Y, Jung K, Jee S. The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. International journal of environmental research and public health. 2022 Jul 18:19(14):. doi: 10.3390/ijerph19148745. Epub 2022 Jul 18 [PubMed PMID: 35886598]
van Gerwen M, Vasan V, Genden E, Saul SR. Human 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure and thyroid cancer risk. Toxicology. 2023 Apr:488():153474. doi: 10.1016/j.tox.2023.153474. Epub 2023 Mar 2 [PubMed PMID: 36868552]
Yang JM, Salmon AG, Marty MA. Development of TEFs for PCB congeners by using an alternative biomarker--thyroid hormone levels. Regulatory toxicology and pharmacology : RTP. 2010 Mar:56(2):225-36. doi: 10.1016/j.yrtph.2009.12.008. Epub 2010 Jan 1 [PubMed PMID: 20043972]
Level 3 (low-level) evidenceBloom MS, Vena JE, Olson JR, Kostyniak PJ. Assessment of polychlorinated biphenyl congeners, thyroid stimulating hormone, and free thyroxine among New York state anglers. International journal of hygiene and environmental health. 2009 Nov:212(6):599-611. doi: 10.1016/j.ijheh.2009.04.005. Epub 2009 Jun 2 [PubMed PMID: 19493696]
Level 2 (mid-level) evidenceGalimova EF, Amirova ZK, Galimov ShN. Dioxins in the semen of men with infertility. Environmental science and pollution research international. 2015 Oct:22(19):14566-9. doi: 10.1007/s11356-014-3109-z. Epub 2014 Jun 5 [PubMed PMID: 24894758]
Mínguez-Alarcón L, Sergeyev O, Burns JS, Williams PL, Lee MM, Korrick SA, Smigulina L, Revich B, Hauser R. A Longitudinal Study of Peripubertal Serum Organochlorine Concentrations and Semen Parameters in Young Men: The Russian Children's Study. Environmental health perspectives. 2017 Mar:125(3):460-466. doi: 10.1289/EHP25. Epub 2016 Oct 7 [PubMed PMID: 27713107]
Level 3 (low-level) evidencePaul R, Moltó J, Ortuño N, Romero A, Bezos C, Aizpurua J, Gómez-Torres MJ. Relationship between serum dioxin-like polychlorinated biphenyls and post-testicular maturation in human sperm. Reproductive toxicology (Elmsford, N.Y.). 2017 Oct:73():312-321. doi: 10.1016/j.reprotox.2017.07.004. Epub 2017 Jul 10 [PubMed PMID: 28705580]
Eskenazi B, Ames J, Rauch S, Signorini S, Brambilla P, Mocarelli P, Siracusa C, Holland N, Warner M. Dioxin exposure associated with fecundability and infertility in mothers and daughters of Seveso, Italy. Human reproduction (Oxford, England). 2021 Feb 18:36(3):794-807. doi: 10.1093/humrep/deaa324. Epub [PubMed PMID: 33367671]
Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA. Exposure to endocrine disruptors during adulthood: consequences for female fertility. The Journal of endocrinology. 2017 Jun:233(3):R109-R129. doi: 10.1530/JOE-17-0023. Epub 2017 Mar 29 [PubMed PMID: 28356401]
Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology (Cambridge, Mass.). 2010 Mar:21(2):224-31. doi: 10.1097/EDE.0b013e3181cb8b95. Epub [PubMed PMID: 20124903]
Level 2 (mid-level) evidenceTan Z, Zhou J, Chen H, Zou Q, Weng S, Luo T, Tang Y. Toxic effects of 2,4-dichlorophenoxyacetic acid on human sperm function in vitro. The Journal of toxicological sciences. 2016:41(4):543-9. doi: 10.2131/jts.41.543. Epub [PubMed PMID: 27432240]
Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, Cox B, Nawrot TS, Van Larebeke N, D'Hooghe T. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environment international. 2015 Nov:84():154-60. doi: 10.1016/j.envint.2015.07.017. Epub 2015 Aug 24 [PubMed PMID: 26292060]
Level 2 (mid-level) evidenceNgo AD, Taylor R, Roberts CL, Nguyen TV. Association between Agent Orange and birth defects: systematic review and meta-analysis. International journal of epidemiology. 2006 Oct:35(5):1220-30 [PubMed PMID: 16543362]
Level 1 (high-level) evidenceKang HK, Mahan CM, Lee KY, Magee CA, Mather SH, Matanoski G. Pregnancy outcomes among U.S. women Vietnam veterans. American journal of industrial medicine. 2000 Oct:38(4):447-54 [PubMed PMID: 10982986]
Level 2 (mid-level) evidenceKobayashi S, Sata F, Miyashita C, Sasaki S, Ban S, Araki A, Goudarzi H, Kajiwara J, Todaka T, Kishi R. Dioxin-metabolizing genes in relation to effects of prenatal dioxin levels and reduced birth size: The Hokkaido study. Reproductive toxicology (Elmsford, N.Y.). 2017 Jan:67():111-116. doi: 10.1016/j.reprotox.2016.12.002. Epub 2016 Dec 7 [PubMed PMID: 27939992]
Troudi A, Soudani N, Mahjoubi Samet A, Ben Amara I, Zeghal N. 2,4-Dichlorophenoxyacetic acid effects on nephrotoxicity in rats during late pregnancy and early postnatal periods. Ecotoxicology and environmental safety. 2011 Nov:74(8):2316-23. doi: 10.1016/j.ecoenv.2011.07.032. Epub 2011 Aug 11 [PubMed PMID: 21835467]
Level 3 (low-level) evidenceUeker ME, Silva VM, Moi GP, Pignati WA, Mattos IE, Silva AMC. Parenteral exposure to pesticides and occurence of congenital malformations: hospital-based case-control study. BMC pediatrics. 2016 Aug 12:16(1):125. doi: 10.1186/s12887-016-0667-x. Epub 2016 Aug 12 [PubMed PMID: 27520287]
Level 2 (mid-level) evidenceNoel JK, Namazi S, Haddock RL. Disparities in Infant Mortality Due to Congenital Anomalies on Guam. Hawai'i journal of medicine & public health : a journal of Asia Pacific Medicine & Public Health. 2015 Dec:74(12):397-402 [PubMed PMID: 26668770]
Warner M, Rauch S, Ames J, Mocarelli P, Brambilla P, Signorini S, Eskenazi B. Prenatal dioxin exposure and thyroid hormone levels in the Seveso second generation study. Environmental research. 2020 Apr:183():109280. doi: 10.1016/j.envres.2020.109280. Epub 2020 Feb 21 [PubMed PMID: 32311913]
Reale C, Porreca I, Russo F, Marotta M, Roberto L, Russo NA, Carchia E, Mallardo M, De Felice M, Ambrosino C. Genetic background and window of exposure contribute to thyroid dysfunction promoted by low-dose exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Scientific reports. 2018 Nov 5:8(1):16324. doi: 10.1038/s41598-018-34427-2. Epub 2018 Nov 5 [PubMed PMID: 30397221]
Baccarelli A, Giacomini SM, Corbetta C, Landi MT, Bonzini M, Consonni D, Grillo P, Patterson DG, Pesatori AC, Bertazzi PA. Neonatal thyroid function in Seveso 25 years after maternal exposure to dioxin. PLoS medicine. 2008 Jul 29:5(7):e161. doi: 10.1371/journal.pmed.0050161. Epub [PubMed PMID: 18666825]
Giang HTN, Hai TT, Nguyen H, Vuong TK, Morton LW, Culbertson CB. Elevated congenital heart disease birth prevalence rates found in Central Vietnam and dioxin TCDD residuals from the use of 2, 4, 5-T herbicides (Agent Orange) in the Da Nang region. PLOS global public health. 2022:2(10):e0001050. doi: 10.1371/journal.pgph.0001050. Epub 2022 Oct 12 [PubMed PMID: 36962560]
Rocheleau CM, Bertke SJ, Lawson CC, Romitti PA, Sanderson WT, Malik S, Lupo PJ, Desrosiers TA, Bell E, Druschel C, Correa A, Reefhuis J, National Birth Defects Prevention Study. Maternal occupational pesticide exposure and risk of congenital heart defects in the National Birth Defects Prevention Study. Birth defects research. Part A, Clinical and molecular teratology. 2015 Oct:103(10):823-33. doi: 10.1002/bdra.23351. Epub 2015 Jun 2 [PubMed PMID: 26033688]
Tran NN, Pham-The T, Pham TN, Vu HT, Luong KN, Nishijo M. Neurodevelopmental Effects of Perinatal TCDD Exposure Differ from Those of Other PCDD/Fs in Vietnamese Children Living near the Former US Air Base in Da Nang, Vietnam. Toxics. 2023 Jan 21:11(2):. doi: 10.3390/toxics11020103. Epub 2023 Jan 21 [PubMed PMID: 36850978]
Pham NT, Nishijo M, Pham TT, Tran NN, Le VQ, Tran HA, Phan HAV, Nishino Y, Nishijo H. Perinatal dioxin exposure and neurodevelopment of 2-year-old Vietnamese children in the most contaminated area from Agent Orange in Vietnam. The Science of the total environment. 2019 Aug 15:678():217-226. doi: 10.1016/j.scitotenv.2019.04.425. Epub 2019 Apr 30 [PubMed PMID: 31075589]
Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Schölmerich A. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PloS one. 2015:10(6):e0129906. doi: 10.1371/journal.pone.0129906. Epub 2015 Jun 12 [PubMed PMID: 26066795]
Pham TT, Nishijo M, Nguyen ATN, Tran NN, Hoang LV, Tran AH, Nguyen TV, Nishijo H. Perinatal dioxin exposure and the neurodevelopment of Vietnamese toddlers at 1 year of age. The Science of the total environment. 2015 Dec 1:536():575-581. doi: 10.1016/j.scitotenv.2015.07.055. Epub 2015 Aug 4 [PubMed PMID: 26247686]
Tai PT, Nishijo M, Nghi TN, Nakagawa H, Van Luong H, Anh TH, Nishijo H. Effects of Perinatal Dioxin Exposure on Development of Children during the First 3 Years of Life. The Journal of pediatrics. 2016 Aug:175():159-166.e2. doi: 10.1016/j.jpeds.2016.04.064. Epub 2016 May 14 [PubMed PMID: 27189679]
Tran NN, Pham TT, Ozawa K, Nishijo M, Nguyen AT, Tran TQ, Hoang LV, Tran AH, Phan VH, Nakai A, Nishino Y, Nishijo H. Impacts of Perinatal Dioxin Exposure on Motor Coordination and Higher Cognitive Development in Vietnamese Preschool Children: A Five-Year Follow-Up. PloS one. 2016:11(1):e0147655. doi: 10.1371/journal.pone.0147655. Epub 2016 Jan 29 [PubMed PMID: 26824471]
Makelarski JA, Romitti PA, Rocheleau CM, Burns TL, Stewart PA, Waters MA, Lawson CC, Bell EM, Lin S, Shaw GM, Olney RS, National Birth Defects Prevention Study. Maternal periconceptional occupational pesticide exposure and neural tube defects. Birth defects research. Part A, Clinical and molecular teratology. 2014 Nov:100(11):877-86. doi: 10.1002/bdra.23293. Epub 2014 Aug 15 [PubMed PMID: 25124525]
Level 2 (mid-level) evidencePettigrew SM, Bell EM, Van Zutphen AR, Rocheleau CM, Shaw GM, Romitti PA, Olshan A, Lupo PJ, Soim A, Makelarski JA, Michalski AM, Sanderson W, and the National Birth Defects Prevention Study. Paternal and joint parental occupational pesticide exposure and spina bifida in the National Birth Defects Prevention Study, 1997 to 2002. Birth defects research. Part A, Clinical and molecular teratology. 2016 Nov:106(11):963-971. doi: 10.1002/bdra.23551. Epub [PubMed PMID: 27891778]
Cormier JN, Pollock RE. Soft tissue sarcomas. CA: a cancer journal for clinicians. 2004 Mar-Apr:54(2):94-109 [PubMed PMID: 15061599]
Wingren G, Fredrikson M, Brage HN, Nordenskjöld B, Axelson O. Soft tissue sarcoma and occupational exposures. Cancer. 1990 Aug 15:66(4):806-11 [PubMed PMID: 2386907]
Level 2 (mid-level) evidenceEdwards D, Voronina A, Attwood K, Grand'Maison A. Association between occupational exposures and sarcoma incidence and mortality: systematic review and meta-analysis. Systematic reviews. 2021 Aug 13:10(1):231. doi: 10.1186/s13643-021-01769-4. Epub 2021 Aug 13 [PubMed PMID: 34389054]
Level 1 (high-level) evidenceRhee J, Medgyesi DN, Fisher JA, White AJ, Sampson JN, Sandler DP, Ward MH, Jones RR. Residential proximity to dioxin emissions and risk of breast cancer in the sister study cohort. Environmental research. 2023 Apr 1:222():115297. doi: 10.1016/j.envres.2023.115297. Epub 2023 Jan 13 [PubMed PMID: 36642125]
Krishnamurthy P, Hazratjee N, Opris D, Agrawal S, Markert R. Is exposure to Agent Orange a risk factor for hepatocellular cancer?-A single-center retrospective study in the U.S. veteran population. Journal of gastrointestinal oncology. 2016 Jun:7(3):426-32. doi: 10.21037/jgo.2016.01.09. Epub [PubMed PMID: 27284476]
Level 2 (mid-level) evidenceBaek K, Park JT, Kwak K. Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies. Epidemiology and health. 2022:44():e2022070. doi: 10.4178/epih.e2022070. Epub 2022 Sep 1 [PubMed PMID: 36097807]
Level 1 (high-level) evidenceXu J, Ye Y, Huang F, Chen H, Wu H, Huang J, Hu J, Xia D, Wu Y. Association between dioxin and cancer incidence and mortality: a meta-analysis. Scientific reports. 2016 Nov 29:6():38012. doi: 10.1038/srep38012. Epub 2016 Nov 29 [PubMed PMID: 27897234]
Level 1 (high-level) evidenceBoffetta P, Mundt KA, Adami HO, Cole P, Mandel JS. TCDD and cancer: a critical review of epidemiologic studies. Critical reviews in toxicology. 2011 Aug:41(7):622-36. doi: 10.3109/10408444.2011.560141. Epub 2011 Jul 1 [PubMed PMID: 21718216]
Level 3 (low-level) evidenceVoPham T, Bertrand KA, Jones RR, Deziel NC, DuPré NC, James P, Liu Y, Vieira VM, Tamimi RM, Hart JE, Ward MH, Laden F. Dioxin exposure and breast cancer risk in a prospective cohort study. Environmental research. 2020 Jul:186():109516. doi: 10.1016/j.envres.2020.109516. Epub 2020 Apr 13 [PubMed PMID: 32305677]
Kogevinas M, Saracci R, Winkelmann R, Johnson ES, Bertazzi PA, Bueno de Mesquita BH, Kauppinen T, Littorin M, Lynge E, Neuberger M. Cancer incidence and mortality in women occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins. Cancer causes & control : CCC. 1993 Nov:4(6):547-53 [PubMed PMID: 8280832]
Level 3 (low-level) evidence