Introduction
As the utilization of the robotic platform gains momentum in managing various urologic pathologies, its application to bladder surgery continues to evolve. Traditionally, open radical cystectomy with bilateral pelvic node dissection and urinary diversion has been the standard of care for managing muscle-invasive and select high-risk nonmuscle-invasive bladder cancer.[1][2] However, robotic radical cystectomy has emerged as a minimally invasive surgical technique for managing these conditions and reducing surgical morbidity for advanced localized bladder cancer without compromising oncologic outcomes.
Since the first documented case series on robotic cystectomy in 2003, the utilization of this approach has been gradually gaining popularity.[3][4] Despite its technical challenges, long learning curve, and longer operating times, robotic radical cystectomy has been associated with several benefits compared to open radical cystectomy. These include less intraoperative blood loss, reduced need for transfusions, fewer major postoperative complications, a lower rate of positive surgical margins, 40% more lymph nodes recovered on average, and earlier hospital discharge.[5][6][7][8][9][10][11][12] In addition, robotic surgery also offers a reduced risk of wound-related complications and thromboembolic events.[8][13]
Quality of life scores are generally similar between the robotic and open surgical approaches, but when the robotic intracorporeal urinary diversion was added, quality of life scores improved.[7][8] Comparative studies have demonstrated that oncological outcomes are comparable between open and robotic surgical approaches to radical cystectomy over 10 years with regard to recurrence-free, progression-free, and overall survival.[14][15][16][17][18] Given its potential value, long learning curve, high technical complexity, and limitations, the role of minimally invasive major robotic bladder surgery continues to evolve and is the subject of ongoing investigation and study.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The primary histological type of bladder cancer is urothelial carcinoma, accounting for approximately 90% of all bladder tumors. Less common types include schistosoma-related squamous cell carcinoma, which makes up about 5% of bladder tumors and is more prevalent in the Middle East and Africa, and adenocarcinoma, which accounts for about 2% of bladder tumors and may develop from the urachus.[19]
Robotic radical cystectomy in males involves the removal of the bladder, distal ureters, prostate, and vas deferens. In females, the procedure classically involves anterior pelvic exenteration, removing the bladder, urethra, uterus, cervix, and anterior vaginal wall, although pelvic organ-sparing cystectomy may be performed in selected patients. When performing bilateral pelvic lymphadenectomy, surgeons should remove, at a minimum, the external and internal iliac nodes as well as the obturator lymph nodes (standard lymphadenectomy).
Indications
Indications for robotic radical cystectomy are similar to those for open cystectomy. These include the absence of metastatic urothelial lesions and the patient's willingness and ability to undergo a major surgical intervention.
Indications for cystectomy surgery include muscle-invasive bladder cancer or non-muscle–invasive cancer with at least 1 of the following criteria:[20][21][22][23][24][25]
- High-risk patients with persistent high-grade T1 disease on repeat transurethral resection or recurrent disease.
- High-risk patients with persistent or recurrent carcinoma in situ within 1 year after 2 cycles of Bacillus Calmette-Guerin (BCG) induction therapy or while on maintenance BCG treatment.
- High-risk patients with persistent or recurrent T1 tumors associated with lymphovascular invasion or variant bladder cancer histology, as well as low glomerular filtration rate (GFR) and larger tumor size (>3 cm) who are not responding to BCG.
- T1 disease associated with carcinoma in situ.
Special considerations arise with the robotic approach, particularly regarding patient positioning, tolerance of pneumoperitoneum, and abdominal access. The combination of the steep Trendelenburg position and pneumoperitoneum used during this procedure can lead to cardiopulmonary consequences, such as decreased lung compliance, reduced functional residual capacity, increased ventilation/perfusion mismatch, and heightened risk of hypercarbia/metabolic acidosis. Previous intrabdominal or pelvic surgery, as well as radiation therapy, may complicate robotic access to the abdomen.[26] Therefore, it is crucial to thoroughly evaluate and discuss these critical preoperative considerations on an individual basis with the entire care team before proceeding with robotic radical cystectomy.
Patients with indications for a radical cystectomy but are unwilling or medically unfit to undergo surgery are best addressed with trimodal therapy. This approach typically involves maximal transurethral bladder tumor resection followed by chemotherapy and external beam radiation treatment.[27][28][29]
Contraindications
Robotic radical cystectomy is a major operative procedure that can carry significant perioperative morbidity and mortality risks.[30] Therefore, patients with localized disease being considered for this surgery must undergo a comprehensive preoperative medical evaluation of their functional status. This evaluation aims to identify any significant or unexpected medical comorbidities, such as bleeding diathesis, poor pulmonary function, or cardiac issues, which could potentially prevent them from undergoing surgery or lead to preventable major complications.
Specific contraindications for robotic radical cystectomy surgery include disease that extends into the pelvis or surrounding structures with bladder fixation, as well as an uncorrected bleeding diathesis.[31]
Morbid obesity, prior abdominal intestinal or vascular surgery, previous radiation therapy to the abdomen, locally advanced disease, and advanced age of older patients may pose technical challenges for robotic cystectomy surgery. However, they are not absolute contraindications, as good outcomes can still be achieved with careful surgical planning and management.[32][33][34][35]
Various contraindications may exist for specific types of urinary diversion, including:
- Patients with short bowel syndrome, inflammatory small bowel disease, and a history of extensive radiation to the ileum should not undergo an ileal conduit diversion.
- Continent cutaneous diversions may have relative contraindications, such as poor renal function and severe hepatic dysfunction, due to the higher risk of metabolic complications associated with these techniques.
- Mental impairment, disability, or extreme frailty are other potential contraindications for continent cutaneous diversions, as they require the ability to self-catheterize postoperatively.[36]
Equipment
The techniques and equipment used can vary. The described technique for robotic radical cystectomy involves the use of 6 ports, as outlined in the "Technique or Treatment" section below.
Standard robotic instruments utilized in this procedure include the permanent cautery hook on the right arm, Maryland bipolar forceps, SynchroSeal, or Vessel Sealer on the left arm, the Tip-Up Fenestrated grasper, and the 60-mm robotic stapler on the fourth arm. Large needle drivers are utilized for suturing during the urinary diversion.
The assistant's primary instruments include a laparoscopic grasper, a long suction-irrigator, and laparoscopic scissors.
Personnel
Performing and caring for a patient undergoing robotic cystectomy of the bladder requires a multidisciplinary team of specialized personnel. This includes a complete surgical team comprising the urologic surgeon, anesthesiologist, circulating nurse, scrub technician, bedside assistant, and other operating room personnel essential for performing this operation.
Postoperatively, the post-anesthesia recovery nurses, intensive care unit nurses, floor nurses, stoma nurses, and case managers play vital roles in aftercare. A dedicated team comprising physical therapists, dietitians, and oncologists also contributes to the patient's rehabilitation and long-term care, ensuring a comprehensive approach to recovery and overall health management.
Preparation
Preparation for this procedure includes administering a high carbohydrate diet to the patient 2 to 3 days before surgery, along with participation in a preoperative education class. Clear liquids are started the day before surgery, followed by nothing-by-mouth starting at midnight. Routine use of laxatives or mechanical bowel preparation before surgery is avoided, as they have not been shown to reduce rates of anastomotic leaks or wound infections.[37]
In the preoperative holding area, the patient receives a single dose of broad-spectrum antimicrobial prophylaxis, deep venous thrombosis prophylaxis, and a μ-opioid receptor antagonist (alvimopan 12 mg) to promote early return of bowel function postoperatively.[38] Alvimopan can be continued postoperatively at 12 mg BID.[39][40]
A stoma therapist will mark the site if the patient is expected to receive an ileal conduit. This practice is often beneficial, even if another type of urinary diversion is planned, due to potential unforeseen developments during surgery.
Technique or Treatment
Radical cystectomy surgery for urothelial cancer should be preceded by neoadjuvant cisplatin-based chemotherapy.[23][41][42] Robotic radical cystectomy, especially when combined with intracorporeal urinary diversion, presents significant challenges and complexities. Yet, it has become the predominant surgical approach for invasive bladder cancer in tertiary care centers.[8] Notably, it is estimated that over 130 procedures are required for surgeons to achieve a stable level of maximum proficiency with minimal 90-day postoperative complication rates.[43] Patients requiring this advanced type of robotic surgery are recommended to be referred to high-volume centers of excellence (performing more than 50 such cases per year) to optimize outcomes and minimize complications.[44][45]
Treatment Procedures for Robotic Radical Cystectomy
Patient positioning and equipment: The patient is initially placed in a supine position on an anti-skid foam pad to prevent sliding and pressure injuries. Female patients requiring vaginal access are positioned in a low lithotomy position. The patient's arms are wrapped in foam padding and tucked at their sides, ensuring padding for the hands and elbows. A chest and leg straps may also be utilized to secure the patient further. A warming blanket and intermittent compression hose are applied for comfort and circulation support.
Once appropriately positioned, it is crucial to test the patient in steep Trendelenburg to confirm stability and minimize movement. A Foley catheter is then inserted and allowed to drain under gravity. Best practice typically involves temporarily reducing the steep Trendelenburg position after about 4 hours to mitigate the risk of positional injuries.
The robot is positioned on the patient's left side, with the primary assistant and surgical technician seated on the right side. While different positioning setups are acceptable, it is preferable for the assistant to sit on the side where bowel work is conducted, opposite the robotic stapler. Alternatively, the robot may be placed and secured between the patient's legs.[46][47][48][49]
Port placement and robotic equipment: A Veress needle is inserted through a periumbilical puncture to insufflate the peritoneum for port placement. Notably, 6 ports are utilized, positioned similarly to a robotic radical prostatectomy but more cephalad.[50] This strategic port placement is crucial for executing the urinary diversion and extended lymph node dissection (see Image. Port Placement for Robotic Radical Cystectomy).
- The robotic arm and camera ports are positioned approximately 5 to 7 cm cephalad from the umbilicus to facilitate bowel work on the ileum. This also permits a cephalad dissection of lymph nodes if necessary.
- An 8-mm supraumbilical camera port is initially placed, and a 30° camera is utilized. Subsequently, the patient is then placed in a steep Trendelenburg position.
- Under direct vision, a 12-mm assistant port is placed 3 fingerbreadths cranial to the right iliac crest in a location anterior to the cecum.
- An 8-mm right robotic instrument port is positioned 1 handbreadth (10-12 cm) lateral to the camera port.
- A 5-mm assistant port is then placed between the camera port and the right robotic instrument port.
- Another 8-mm robotic instrument port is positioned on the left side of the abdomen to replicate the placement of the right robotic instrument port.
- A 12-mm robotic port is placed in the left lower quadrant, approximately 2 fingerbreadths (4 cm) off of the left anterior superior iliac spine.
This positioning is designed to enhance the maneuverability of the fourth robotic arm, facilitating easier placement of the stapler during the creation of the urinary diversion.
Alternative approaches include adding a 15-mm port to facilitate easier manipulation of the GIA stapler by the assistant.[46] This additional port also facilitates the placement of an intracorporeal specimen bag.[46] Another option involves adding a 12-mm suprapubic port after placing the bladder in the specimen bag.[51] This modification enhances access for side-to-side bowel anastomoses and helps prevent torsion.[51]
The pneumoperitoneum is generally set at 10 mm Hg throughout the procedure unless there is limited visualization necessitating adjustment.
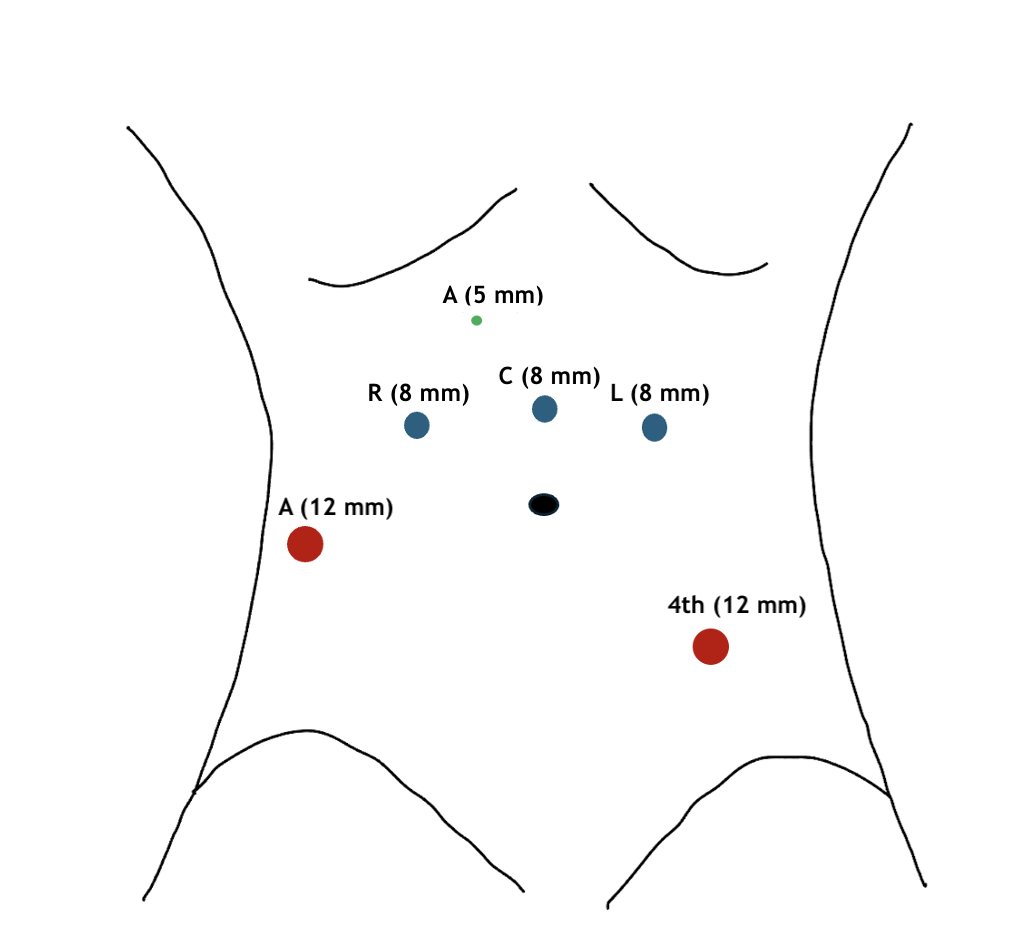
Bowel mobilization and ureteral dissection: The procedure begins by releasing the white line of Toldt adjacent to the ascending and descending colon to maximize exposure of the deep pelvis. The sigmoid peritoneal attachments are then released, facilitating the retraction of the large bowel from the pelvis. This mobilization is crucial for exposing the pelvis and accessing and mobilizing the small bowel to create the urinary diversion. Once the retroperitoneum is exposed, the right ureteral dissection is performed (see Image. Right Ureteral Identification, Dissection, and Transection). The right ureter is identified at approximately the level of the right iliac artery bifurcation. The ureter is then circumferentially dissected distally toward the ureterovesical junction.
If an intracorporeal urinary diversion is planned, the ureter should not be dissected deeply close to the bladder, as much of the distal ureter will be discarded. Thus, it is important to utilize an atraumatic technique during ureteral dissection and to preserve a healthy margin of surrounding fat and periureteral vasculature.
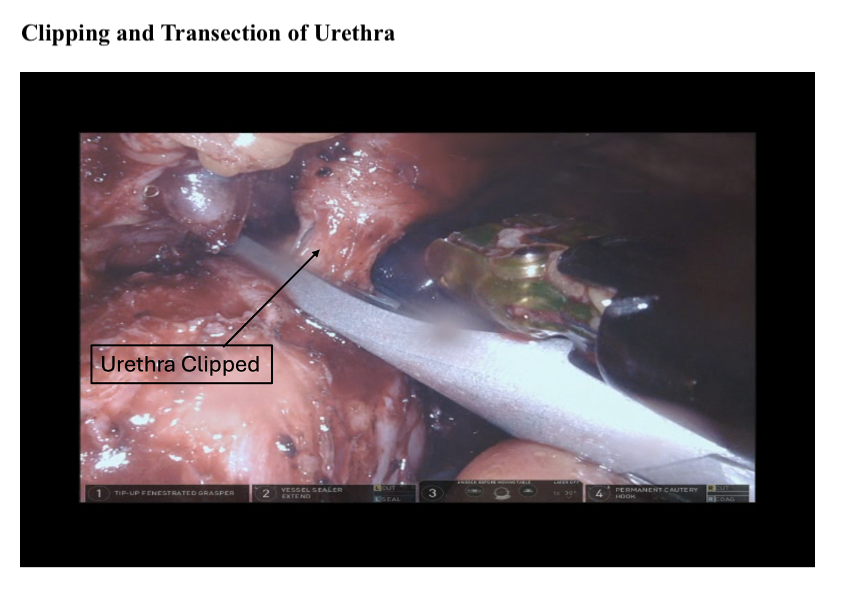
Once the distal portion of the right ureter is dissected, the ureter is transected sharply. A section from the distal right ureter is sent for frozen section analysis to rule out malignancy. The distal end of the proximal ureter should be clipped to prevent urine from dripping into the peritoneum and to induce proximal hydronephrosis and mild ureteral distension. This distension will be helpful when creating the ureteral-intestinal anastomoses for urinary diversion (see Image. Clipping and Transection of Urethra).
The left ureteral dissection is performed similarly but must be continued distally to account for its passage under the sigmoid colon. A sample from the distal edge of the left ureter is sent for frozen section analysis to ensure a negative surgical margin.
Posterior dissection: The posterior dissection is performed before entry into the space of Retzius, providing anterior traction for exposure of the posterior plane and dissection of the vascular pedicles.
The bladder is retracted proximally and anteriorly using the fourth robotic arm. A transverse incision is made through the peritoneum at the rectovesical cul-de-sac. The seminal vesicles and vas deferens are identified bilaterally and dissected.
The dissection plane is carried posteriorly between the seminal vesicles and the rectum until Denonvilliers fascia is reached and incised. This allows the posterior dissection to be carried bluntly distally toward the prostatic apex, taking care to avoid injury to the rectum, which lies immediately inferior. To aid exposure, the fourth arm and/or an assistant are used to help retract the seminal vesicles cephalad.
Control of the vascular pedicles: An incision is made in the parietal peritoneum just lateral to the medial umbilical ligament bilaterally to separate the bladder from the pelvic side wall and pelvic vasculature. The vas deferens are clipped and divided.
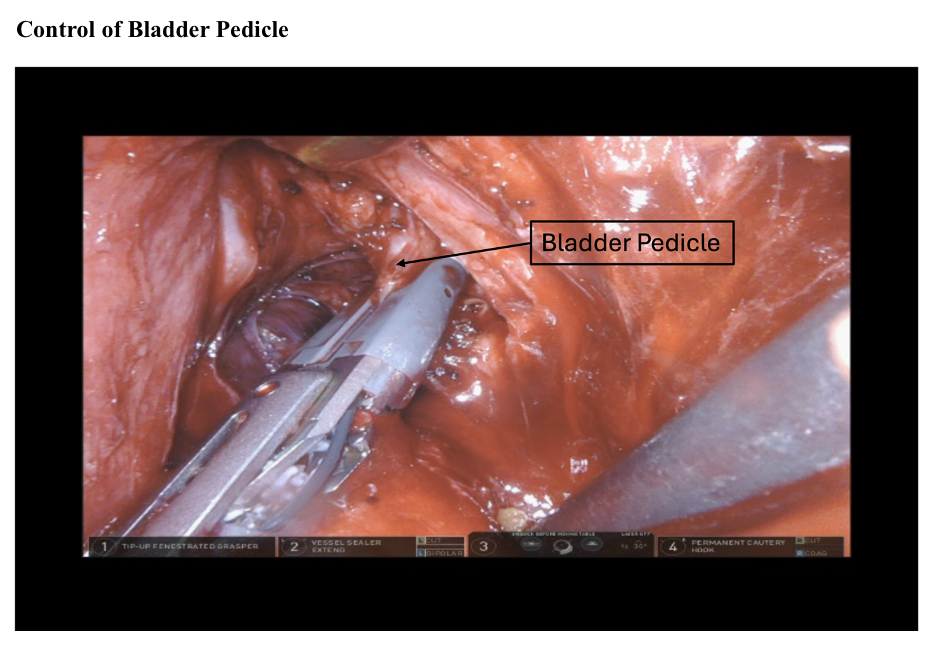
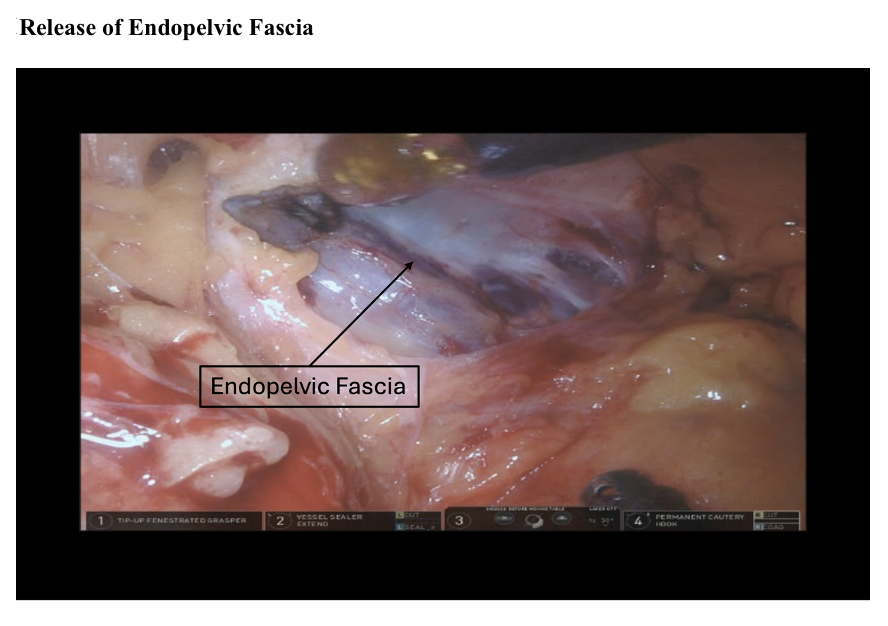
Blunt dissection is continued caudally toward the endopelvic fascia, exposing the superior vesical pedicle. The superior vesical artery is ligated bilaterally at the origin of the hypogastric artery using clips, a SynchroSeal, or a Vessel Sealer. The exposed endopelvic fascia is then opened to define the lateral plane of the prostatic pedicles (see Image. Release of Endopelvic Fascia).
Dissection between the pelvic side wall and the bladder can be facilitated by medial retraction of the bladder using the fourth robotic arm. This maneuver ensures clearance from the obturator nerve and external iliac vessels and enhances the exposure of the bladder's lateral pedicles.
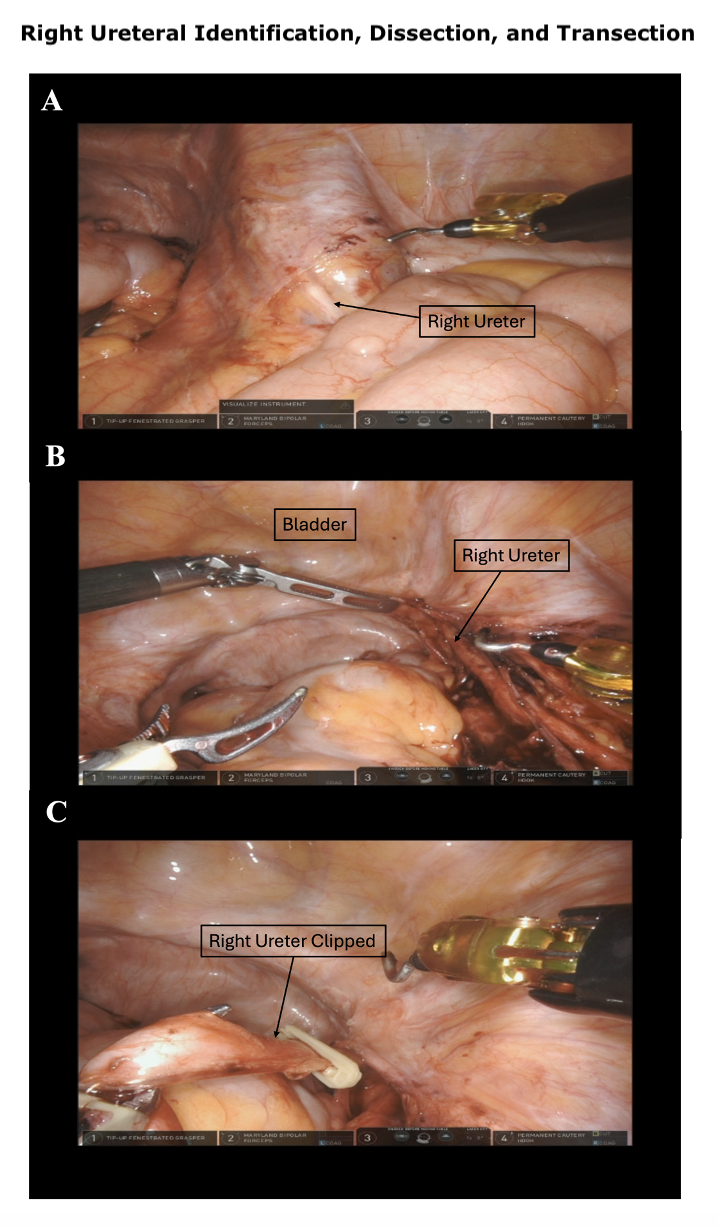
- Non-nerve–sparing procedures: In non-nerve-sparing procedures, anterior-cranial traction is applied by the assistant or robotic arm. The lateral pedicles of the bladder can then be managed, ligated, and divided using 60-mm laparoscopic vascular staplers, clips, or similar devices (see Image. Control of Bladder Pedicle).
- Nerve-sparing procedures: The neurovascular bundles may be spared similarly to nerve-sparing techniques used during radical prostatectomy in appropriately selected patients. In a nerve-sparing approach, the neurovascular bundles located on the posterior-lateral aspects of the prostate can be released along the surface of the prostate or vagina.
The dissection begins at the seminal vesicles and proceeds toward the prostate. The neurovascular bundles are meticulously separated from the bladder and the posterolateral prostate by gently peeling them off in a superolateral direction. In such cases, it is typically preferable to clip the inferior vesical and prostate pedicles rather than dividing them sharply.
Anterior dissection: Anterior dissection involves retracting the urachus cranially with the fourth robotic arm to facilitate transection of the urachus and medial umbilical ligaments. This maneuver exposes the space of Retzius, allowing the bladder to descend from the anterior abdominal wall. The avascular plane between the anterior abdominal wall and bladder is then carefully extended toward the pubic bone, with blunt dissection in the soft areolar tissue anterior to the prostate and bladder.
The levator ani muscles can be dissected after the endopelvic fascia has been incised. The lateral pelvic fascia is then incised above the level of the neurovascular bundles, allowing them to fall posterolaterally, which is crucial for nerve-sparing procedures.
The fourth robotic arm and assistant provide cephalad traction on the bladder to expose the dorsal venous complex (DVC). The puboprostatic ligaments and DVC are identified and then transected using monopolar cautery or cold scissors. Optionally, the transected DVC complex may be oversewn with a running suture ligature to minimize bleeding.
The prostatic apex and urethra are circumferentially dissected. Once the urethral stump is isolated, the Foley catheter is removed, and a clip is placed proximally on the urethra to prevent spillage. Alternatively, the apical prostate can be oversewn. The urethra is transected proximal to the verumontanum, and the freed specimen is then fully released and placed into an intracorporeal specimen bag. A specimen of the urethral margin from the apical prostatic urethra is sent to pathology for a frozen section.
A urethrectomy should be performed if high-grade malignant tissue is identified by a frozen or permanent section of the surgical margin of the apical prostatic urethra after its surgical removal.[23][52] In women, a urethrectomy should be considered if they are not planning to undergo orthotopic neobladder reconstruction, aiming to reduce the risk of recurrence or inadvertent positive surgical margins.[23]
Bilateral pelvic lymph node dissections (standard and extended): Bilateral pelvic lymph node dissection involves a standard bilateral lymphadenectomy, which includes the removal of the external iliac, obturator, and internal iliac (hypogastric) nodes.[53][54][55] More than 12 nodes should be resected for adequate staging.[23] A larger number of identifiable lymph nodes is also considered a positive indicator of the quality of the surgery.[22][56]
The anatomic boundaries of the standard dissection template typically include the genitofemoral nerves laterally, the internal iliac artery medially, the node of Cloquet inferiorly, and the common iliac artery superiorly. Careful attention is crucial during lymphadenectomy to prevent injury to critical nerves and vasculature.
Optionally, the lymphadenectomy can be expanded to encompass the common iliac and presacral lymph nodes (extended lymph node dissection) using a "split and roll" technique. However, an extended lymph node dissection adds considerable time to an already lengthy procedure, heightens the risk of complications and inadvertent injury to surrounding structures, and increases the likelihood of lymphocele formation. Importantly, large-scale clinical trials have not shown significant survival benefits compared to standard lymph node dissection.[57]
A few important tips for pelvic lymph node dissections include:
- All major lymphatic channels should be clipped to avoid chylous ascites and the development of lymphoceles.
- Nodes should not be cut to avoid the possibility of tumor spillage.
- All lymph nodes collected should be placed in separate packets based on the anatomical region of their dissection.
Pelvic lymph node dissections usually commence at the node of Cloquet on the right side and proceed superiorly up to the aortic bifurcation. Proximal freeing of the right ureter may be necessary to avoid inadvertent injury during this process.
The external iliac vessels can be gently reflected to facilitate the removal of the entire Triangle of Marcille lymph node packet at the origin of the obturator nerve, approached laterally. Lateral retraction of the sigmoid colon provides access to the presacral lymph nodes, enabling their dissection and retrieval.
On the left side, a small opening can be made posterior to the sigmoid colon, which will later facilitate the movement of the left ureter to the right side during the creation of the urinary diversion. The sigmoid is then retracted medially, allowing for a left-sided lymph node dissection performed in a manner similar to the right side.
- Earlier studies suggested that more extensive lymph node dissections, including up to the aortic bifurcation, might lead to lower overall and urothelial cancer-specific mortality rates. However, these findings have been questioned due to methodological issues, variations in surgical techniques, and inconsistent results across studies.[58][59][60][61][62] More recent studies indicate minimal or no significant benefit from extended lymph node dissections in terms of overall survival.[63][64][65][66]
- A recent systematic review and meta-analysis indicated that a moderately extended lymph node dissection may offer optimal benefits by improving recurrence-free survival without the additional time or morbidity associated with superextended dissections (above the aortic bifurcation).[67] However, the review found that overall survival rates were comparable to those achieved with the more limited standard dissection template.[67]
- Many surgeons opt for the extended lymph node dissection template based on studies suggesting improvements in recurrence-free and cancer-specific survival; however, this approach remains optional.[67][68][69] Evidence does not support the use of the superextended dissection, as it does not provide any oncological or survival advantage; hence, it is not recommended.[67][70][71]
- The current guidelines from the American Urological Association (AUA) and European Urological Association (EUA) recommend only a standard bilateral lymph node dissection as the minimal requirement. This recommendation is based on insufficient evidence to justify the routine use of the extended template at this time.[57][72][73]
Female radical cystectomy and anterior exenteration: In female patients, radical cystectomy typically involves a total anterior pelvic exenteration, which includes removal of the bladder, urethra, anterior vagina, uterus, and cervix. After completing ureteral dissection and controlling the vessels, the next step is to proceed with the dissection of the uterus and vagina.
A vaginal vault manipulator, such as a sponge stick, is inserted into the vagina to facilitate anterior traction and expose the posterior vaginal fornix, which is then incised. Subsequently, the infundibulopelvic ligaments and uterine vessels are transected and ligated using GIA staplers. The pouch of Douglas is also incised during this step.
Dissection of the bladder and transection of the pedicles, urachus, and DVC are performed as previously described, allowing the bladder to fall posteriorly. The dissection plane of the posterior vaginal canal is then extended anteriorly toward the urethra. The presence of the Foley catheter facilitates anatomical identification. The urethra is transected as described previously. This facilitates the removal of the specimen, which includes a small strip of the anterior vaginal wall along with the bladder, adnexa, and uterus.
Using a sponge stick, the uterus is directed posteriorly, while the fourth robotic arm provides anterior traction to the bladder. Posterior dissection of the bladder proceeds toward the vaginal canal. Laterally to the cervix, the ureter is identified, running posteriorly to the cardinal ligament, which houses the uterine artery. The ureter is dissected free, preserving the uterine pedicle. A urethrectomy can now be performed if an orthotopic urinary diversion is not planned.
An ongoing discussion regarding the potential role of organ-sparing and sexual function-sparing cystectomy exists in select patients, which emphasizes the preservation of reproductive organs, including the vagina, uterus, fallopian tubes, and ovaries. To optimize postoperative sexual and urinary function while maintaining oncologic control, organ-sparing radical cystectomy may be considered in female patients who are young, sexually active with early, unifocal, organ-confined disease, and in whom an orthotopic neobladder may be planned.[74][75] Women with a family history of breast or ovarian cancer should undergo bilateral salpingo-oophorectomy.
Patients with evidence of malignancy at the bladder neck and posterior bladder are generally considered poor candidates for vaginal sparing cystectomy due to concern for positive margins. In most cases, the entire specimen can be extracted through the vaginal canal, thereby forgoing a large extraction site incision on the abdomen. A sponge is used to pack the vaginal canal to preserve the pneumoperitoneum until the vaginal canal is closed using a 2-0 suture.
Urinary diversion: Urinary diversion options, based on preoperative patient characteristics, may now include intracorporeal techniques. These techniques commonly involve intracorporeal ileal conduit, orthotopic neobladder, or continent cutaneous diversion.[8][76][77]
Robotic radical cystectomy with bilateral lymph node dissection and intracorporeal urinary diversion has become the preferred approach for bladder cancer patients in high-volume tertiary care centers.[8][43][77] The technique of robotic radical cystectomy and orthotopic intracorporeal ileal neobladder is well described by Abreu, Simone, Truong, and others.[74][78][79][80][81][82][83][84]
The AUA maintains a series of instructional videos on robotic radical cystectomy and intracorporeal urinary diversions, accessible to members via their website. Additional educational resources on robotic radical cystectomy and intracorporeal urinary diversion can be found on YouTube, featuring videos by experts such as Steven Chang from Brigham and Women's Hospital, Raj Pruthi MD from the University of California, San Francisco (UCSF), Mark Tyson from the Mayo Clinic, Joan Palau, Richard Gaston from the European Congress on Robotics, among others.
Complications
Robotic radical cystectomy is a complex procedure involving both the genitourinary and gastrointestinal tracts, which may lead to frequent morbidity and high complication rates. The most common complications include gastrointestinal (up to 20%), infectious (up to 17%), and genitourinary complications (up to 10%), such as urinary tract infections, wound infections, ileus formation, and anemia requiring transfusion.[85] Despite an overall high 30-day complication rate (up to 48%), most complications are low-grade. Furthermore, the complication rate is similar to the corresponding open surgical technique.[85][86][87] Efforts have been focused on perioperative care to reduce the risk of complications following surgery.
Multimodal, interdisciplinary Enhanced Recovery After Surgery (ERAS) protocols have been established to shorten hospital stays, accelerate recovery of bowel function, and decrease overall morbidity following robotic cystectomy.[88][89][90][90][91][92][93] These protocols typically emphasize early ambulation, goal-directed fluid management, multimodal postoperative analgesia, and early enteral feeding.[94][95][88]
Clinical Significance
The surgical management of muscle-invasive bladder cancer has seen increasing adoption of robotic cystectomy. This approach offers comparable oncologic outcomes and quality of life scores to the open approach, albeit with longer operating times and higher costs. However, robotic cystectomy also demonstrates advantages such as reduced operative blood loss, lower transfusion rates, decreased incidence of positive margins, fewer major complications, increased lymph node yields, and shorter length of hospital stays.[46] As robotic training advances and expands, robotic radical cystectomy is expected to become more commonplace in the urological and surgical armamentarium.
Enhancing Healthcare Team Outcomes
Providing patient-centered care for individuals undergoing robotic cystectomy requires a collaborative approach among healthcare professionals. Bladder cancer can be a life-altering diagnosis for patients, which requires high-quality, integrated, multidisciplinary care throughout the perioperative period.
Before undergoing robotic cystectomy, patients will need comprehensive multidisciplinary education and counseling. This includes information about the benefits and risks of surgery and anesthesia, preoperative nutrition, future self-care for a stoma (if applicable), and expectations for recovery.
During the intraoperative phase, each member of the surgical team must fulfill their designated roles to ensure an efficient and safe procedure. Postoperatively, efforts are directed at reducing the risk of complications and optimizing the restoration of normal physiological functions. This involves a coordinated effort involving the entire interprofessional healthcare team, including, but not limited to, bedside nurses, physical therapists, occupational therapists, stoma nurses, dieticians, pharmacists, and physicians. Ultimately, each member of the healthcare team plays a crucial role in ensuring a safe and enhanced recovery for patients undergoing robotic cystectomy.
Nursing, Allied Health, and Interprofessional Team Interventions
Postoperative interprofessional care is essential for a successful outcome after robotic radical cystectomy. Regarding nursing care, key focuses in the immediate postoperative period include postoperative analgesia, early ambulation, and an emphasis on the return of bowel function.[96][97]
Healthcare professionals should regularly assess patients' pain levels and treat them using a multimodal approach. Early ambulation should be encouraged to reduce the risk of cardiovascular and pulmonary complications. Frequent assessments of gastrointestinal function should be made to determine the appropriate timing for enteral feeding. Interdisciplinary ERAS protocols should be implemented. Postoperatively, patients often have many externalized tubes (such as drains, ureteral stents, urostomy, and catheters), which require strict monitoring to assess fluid balance and ensure optimal recovery.
Stoma nurses play a pivotal role in the comprehensive aftercare of patients following conduit diversion. They may offer personalized guidance on stoma care techniques, including proper cleaning, appliance fitting, and troubleshooting potential problems.[96] Before discharge from the hospital, the interprofessional healthcare team should educate patients and their family members on dietary adjustments, physical activities, and lifestyle modifications essential for optimal recovery.
Media
(Click Image to Enlarge)
References
Aminoltejari K, Black PC. Radical cystectomy: a review of techniques, developments and controversies. Translational andrology and urology. 2020 Dec:9(6):3073-3081. doi: 10.21037/tau.2020.03.23. Epub [PubMed PMID: 33457280]
Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, Adams JL, Saigal CS, Urologic Diseases in America Project. Use of radical cystectomy for patients with invasive bladder cancer. Journal of the National Cancer Institute. 2010 Jun 2:102(11):802-11. doi: 10.1093/jnci/djq121. Epub 2010 Apr 16 [PubMed PMID: 20400716]
Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, Shaaban A, Abol-Enein H, Ghoneim MA. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU international. 2003 Aug:92(3):232-6 [PubMed PMID: 12887473]
Pak JS, Lee JJ, Bilal K, Finkelstein M, Palese MA. Utilization Trends and Short-term Outcomes of Robotic Versus Open Radical Cystectomy for Bladder Cancer. Urology. 2017 May:103():117-123. doi: 10.1016/j.urology.2016.10.067. Epub 2017 Feb 8 [PubMed PMID: 28189553]
Sathianathen NJ, Kalapara A, Frydenberg M, Lawrentschuk N, Weight CJ, Parekh D, Konety BR. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. The Journal of urology. 2019 Apr:201(4):715-720. doi: 10.1016/j.juro.2018.10.006. Epub [PubMed PMID: 30321551]
Level 1 (high-level) evidenceKowalewski KF, Wieland VLS, Kriegmair MC, Uysal D, Sicker T, Stolzenburg JU, Michel MS, Haney CM. Robotic-assisted Versus Laparoscopic Versus Open Radical Cystectomy-A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. European urology focus. 2023 May:9(3):480-490. doi: 10.1016/j.euf.2022.12.001. Epub 2022 Dec 16 [PubMed PMID: 36529645]
Level 1 (high-level) evidenceRai BP, Bondad J, Vasdev N, Adshead J, Lane T, Ahmed K, Khan MS, Dasgupta P, Guru K, Chlosta PL, Aboumarzouk OM. Robotic versus open radical cystectomy for bladder cancer in adults. The Cochrane database of systematic reviews. 2019 Apr 24:4(4):CD011903. doi: 10.1002/14651858.CD011903.pub2. Epub 2019 Apr 24 [PubMed PMID: 31016718]
Level 1 (high-level) evidenceHan JH, Ku JH. Robot-assisted radical cystectomy: Where we are in 2023. Investigative and clinical urology. 2023 Mar:64(2):107-117. doi: 10.4111/icu.20220384. Epub [PubMed PMID: 36882169]
Feng D, Liu S, Tang Y, Yang Y, Wei W, Han P. Comparison of perioperative and oncologic outcomes between robot-assisted and laparoscopic radical cystectomy for bladder cancer: a systematic review and updated meta-analysis. International urology and nephrology. 2020 Jul:52(7):1243-1254. doi: 10.1007/s11255-020-02406-0. Epub 2020 Feb 20 [PubMed PMID: 32078116]
Level 1 (high-level) evidenceMortezavi A, Crippa A, Kotopouli MI, Akre O, Wiklund P, Hosseini A. Association of Open vs Robot-Assisted Radical Cystectomy With Mortality and Perioperative Outcomes Among Patients With Bladder Cancer in Sweden. JAMA network open. 2022 Apr 1:5(4):e228959. doi: 10.1001/jamanetworkopen.2022.8959. Epub 2022 Apr 1 [PubMed PMID: 35482309]
Zhou N, Tian F, Feng Y, Zhao K, Chen L, Fan R, Lu W, Gu C. Perioperative outcomes of intracorporeal robot-assisted radical cystectomy versus open radical cystectomy: A systematic review and meta-analysis of comparative studies. International journal of surgery (London, England). 2021 Oct:94():106137. doi: 10.1016/j.ijsu.2021.106137. Epub 2021 Sep 30 [PubMed PMID: 34600124]
Level 1 (high-level) evidenceMurthy PB, Lone Z, Munoz Lopez C, Ericson JZK, Thomas L, Caveney M, Gerber D, Khanna A, Abouassaly R, Haber GP, Lee BH. Comparison of Oncologic Outcomes Following Open and Robotic-assisted Radical Cystectomy with both Extracorporeal and Intracorporeal Urinary Diversion. Urology. 2021 Aug:154():184-190. doi: 10.1016/j.urology.2021.03.041. Epub 2021 Apr 21 [PubMed PMID: 33891929]
Catto JWF, Khetrapal P, Ricciardi F, Ambler G, Williams NR, Al-Hammouri T, Khan MS, Thurairaja R, Nair R, Feber A, Dixon S, Nathan S, Briggs T, Sridhar A, Ahmad I, Bhatt J, Charlesworth P, Blick C, Cumberbatch MG, Hussain SA, Kotwal S, Koupparis A, McGrath J, Noon AP, Rowe E, Vasdev N, Hanchanale V, Hagan D, Brew-Graves C, Kelly JD, iROC Study Team. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. JAMA. 2022 Jun 7:327(21):2092-2103. doi: 10.1001/jama.2022.7393. Epub [PubMed PMID: 35569079]
Level 1 (high-level) evidenceVenkatramani V, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Kibel AS, Pruthi RS, Montgomery JS, Weight CJ, Sharp DS, Chang SS, Cookson MS, Gupta GN, Gorbonos A, Uchio EM, Skinner E, Soodana-Prakash N, Becerra MF, Swain S, Kendrick K, Smith JA Jr, Thompson IM, Parekh DJ. Predictors of Recurrence, and Progression-Free and Overall Survival following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year Followup. The Journal of urology. 2020 Mar:203(3):522-529. doi: 10.1097/JU.0000000000000565. Epub 2019 Sep 24 [PubMed PMID: 31549935]
Hussein AA, Elsayed AS, Aldhaam NA, Jing Z, Osei J, Kaouk J, Redorta JP, Menon M, Peabody J, Dasgupta P, Khan MS, Mottrie A, Stöckle M, Hemal A, Richstone L, Hosseini A, Wiklund P, Schanne F, Kim E, Ho Rha K, Guru KA. Ten-Year Oncologic Outcomes Following Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. The Journal of urology. 2019 Nov:202(5):927-935. doi: 10.1097/JU.0000000000000386. Epub 2019 Oct 9 [PubMed PMID: 31188729]
Ip KL, Javier-DesLoges JF, Leung C, Nie J, Khajir G, Nawaf CB, Syed J, Rosoff JS, Martin TV, Hesse DG. Comparison of long-term outcomes in a 10-year experience of robotic cystectomy vs. open cystectomy. Journal of robotic surgery. 2021 Oct:15(5):773-780. doi: 10.1007/s11701-020-01175-3. Epub 2020 Nov 23 [PubMed PMID: 33226567]
Elsayed AS, Gibson S, Jing Z, Wijburg C, Wagner AA, Mottrie A, Dasgupta P, Peabody J, Hussein AA, Guru KA. Rates and Patterns of Recurrences and Survival Outcomes after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. The Journal of urology. 2021 Feb:205(2):407-413. doi: 10.1097/JU.0000000000001380. Epub 2020 Sep 18 [PubMed PMID: 32945729]
Zennami K, Sumitomo M, Takahara K, Nukaya T, Takenaka M, Fukaya K, Ichino M, Fukami N, Sasaki H, Kusaka M, Shiroki R. Intra-corporeal robot-assisted versus open radical cystectomy: a propensity score-matched analysis comparing perioperative and long-term survival outcomes and recurrence patterns. International journal of clinical oncology. 2021 Aug:26(8):1514-1523. doi: 10.1007/s10147-021-01939-3. Epub 2021 May 19 [PubMed PMID: 34009486]
Mori K, Abufaraj M, Mostafaei H, Quhal F, Karakiewicz PI, Briganti A, Kimura S, Egawa S, Shariat SF. A Systematic Review and Meta-Analysis of Variant Histology in Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. The Journal of urology. 2020 Dec:204(6):1129-1140. doi: 10.1097/JU.0000000000001305. Epub 2020 Jul 27 [PubMed PMID: 32716694]
Level 1 (high-level) evidenceChang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, Skinner EC, Smith ND, McKiernan JM. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. The Journal of urology. 2016 Oct:196(4):1021-9. doi: 10.1016/j.juro.2016.06.049. Epub 2016 Jun 16 [PubMed PMID: 27317986]
Power NE, Izawa J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE). Bladder cancer (Amsterdam, Netherlands). 2016 Jan 7:2(1):27-36 [PubMed PMID: 27376122]
Holzbeierlein JM, Bixler BR, Buckley DI, Chang SS, Holmes R, James AC, Kirkby E, McKiernan JM, Schuckman AK. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. The Journal of urology. 2024 Apr:211(4):533-538. doi: 10.1097/JU.0000000000003846. Epub 2024 Jan 24 [PubMed PMID: 38265030]
Holzbeierlein J, Bixler BR, Buckley DI, Chang SS, Holmes RS, James AC, Kirkby E, McKiernan JM, Schuckman A. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/SUO Guideline (2017; Amended 2020, 2024). The Journal of urology. 2024 Jul:212(1):3-10. doi: 10.1097/JU.0000000000003981. Epub 2024 Apr 25 [PubMed PMID: 38661067]
McFadden J, Tachibana I, Adra N, Collins K, Cary C, Koch M, Kaimakliotis H, Masterson TA, Rice KR. Impact of variant histology on upstaging and survival in patients with nonmuscle invasive bladder cancer undergoing radical cystectomy. Urologic oncology. 2024 Mar:42(3):69.e11-69.e16. doi: 10.1016/j.urolonc.2023.12.008. Epub 2024 Jan 23 [PubMed PMID: 38267301]
Iida K, Miyake M, Murakami K, Komiyama M, Okajima E, Sazuka T, Nishiyama N, Yasumoto H, Kimura T, Ito A, Shiga K, Yamagishi A, Kikuchi H, Sugimoto M, Taoka R, Kobayashi T, Kojima T, Kitamura H, Nishiyama H, Fujimoto K. Bacillus Calmette-Guérin-unresponsive non-muscle invasive bladder cancer outcomes in patients without radical cystectomy. International journal of clinical oncology. 2021 Nov:26(11):2104-2112. doi: 10.1007/s10147-021-01988-8. Epub 2021 Jul 27 [PubMed PMID: 34313904]
Hsu RL, Kaye AD, Urman RD. Anesthetic Challenges in Robotic-assisted Urologic Surgery. Reviews in urology. 2013:15(4):178-84 [PubMed PMID: 24659914]
Sell A, Jakobsen A, Nerstrøm B, Sørensen BL, Steven K, Barlebo H. Treatment of advanced bladder cancer category T2 T3 and T4a. A randomized multicenter study of preoperative irradiation and cystectomy versus radical irradiation and early salvage cystectomy for residual tumor. DAVECA protocol 8201. Danish Vesical Cancer Group. Scandinavian journal of urology and nephrology. Supplementum. 1991:138():193-201 [PubMed PMID: 1785004]
Level 1 (high-level) evidenceZlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, Drumm M, Mari A, Thio E, Fleshner NE, Kulkarni GS, Jewett MAS, Bristow RG, Catton C, Berlin A, Sridhar SS, Schuckman A, Feldman AS, Wszolek M, Dahl DM, Lee RJ, Saylor PJ, Michaelson MD, Miyamoto DT, Zietman A, Shipley W, Chung P, Daneshmand S, Efstathiou JA. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. The Lancet. Oncology. 2023 Jun:24(6):669-681. doi: 10.1016/S1470-2045(23)00170-5. Epub 2023 May 12 [PubMed PMID: 37187202]
Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 1:32(34):3801-9. doi: 10.1200/JCO.2014.57.5548. Epub 2014 Nov 3 [PubMed PMID: 25366678]
Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA Jr, Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. The Journal of urology. 2008 Apr:179(4):1313-8; discussion 1318. doi: 10.1016/j.juro.2007.11.084. Epub 2008 Mar 4 [PubMed PMID: 18289578]
Abreu AL, Chopra S, Azhar RA, Berger AK, Miranda G, Cai J, Gill IS, Aron M, Desai MM. Robotic radical cystectomy and intracorporeal urinary diversion: The USC technique. Indian journal of urology : IJU : journal of the Urological Society of India. 2014 Jul:30(3):300-6. doi: 10.4103/0970-1591.135673. Epub [PubMed PMID: 25097317]
Al-Daghmin A, Aboumohamed A, Din R, Khan A, Raza SJ, Sztorc J, Mehedint D, Sharif M, Shi Y, Wilding G, Guru KA. Readmission after robot-assisted radical cystectomy: outcomes and predictors at 90-day follow-up. Urology. 2014 Feb:83(2):350-6. doi: 10.1016/j.urology.2013.09.056. Epub [PubMed PMID: 24468509]
Al-Daghmin A, Kauffman EC, Shi Y, Badani K, Balbay MD, Canda E, Dasgupta P, Ghavamian R, Grubb R 3rd, Hemal A, Kaouk J, Kibel AS, Maatman T, Menon M, Mottrie A, Nepple K, Pattaras JG, Peabody JO, Poulakis V, Pruthi R, Palou Redorta J, Rha KH, Richstone L, Schanne F, Scherr DS, Siemer S, Stöckle M, Wallen EM, Weizer A, Wiklund P, Wilson T, Wilding G, Woods M, Guru KA. Efficacy of robot-assisted radical cystectomy (RARC) in advanced bladder cancer: results from the International Radical Cystectomy Consortium (IRCC). BJU international. 2014 Jul:114(1):98-103. doi: 10.1111/bju.12569. Epub 2014 May 22 [PubMed PMID: 24219170]
Lee DJ, Rothberg MB, McKiernan JM, Benson MC, Badani KK. Robot-assisted radical cystoprostatectomy in complex surgical patients: single institution report. The Canadian journal of urology. 2009 Jun:16(3):4664-9 [PubMed PMID: 19497175]
Phillips EA, Uberoi V, Tuerk IA. Robot-assisted radical cystectomy in octogenarians. Journal of endourology. 2014 Feb:28(2):219-23. doi: 10.1089/end.2013.0159. Epub 2013 Dec 26 [PubMed PMID: 24074288]
Lee DJ, Tyson MD, Chang SS. Conduit Urinary Diversion. The Urologic clinics of North America. 2018 Feb:45(1):25-36. doi: 10.1016/j.ucl.2017.09.006. Epub [PubMed PMID: 29169448]
Hashad MM, Atta M, Elabbady A, Elfiky S, Khattab A, Kotb A. Safety of no bowel preparation before ileal urinary diversion. BJU international. 2012 Dec:110(11 Pt C):E1109-13. doi: 10.1111/j.1464-410X.2012.11415.x. Epub 2012 Nov 20 [PubMed PMID: 23167296]
Kauf TL, Svatek RS, Amiel G, Beard TL, Chang SS, Fergany A, Karnes RJ, Koch M, O'Hara J, Lee CT, Sexton WJ, Slaton JW, Steinberg GD, Wilson SS, Techner L, Martin C, Moreno J, Kamat AM. Alvimopan, a peripherally acting μ-opioid receptor antagonist, is associated with reduced costs after radical cystectomy: economic analysis of a phase 4 randomized, controlled trial. The Journal of urology. 2014 Jun:191(6):1721-7. doi: 10.1016/j.juro.2013.12.015. Epub 2013 Dec 14 [PubMed PMID: 24342144]
Level 1 (high-level) evidenceReichert M, Willis F, Post S, Schneider M, Vilz T, Willis M, Hecker A. Pharmacologic prevention and therapy of postoperative paralytic ileus after gastrointestinal cancer surgery - systematic review and meta-analysis. International journal of surgery (London, England). 2024 Mar 25:():. doi: 10.1097/JS9.0000000000001393. Epub 2024 Mar 25 [PubMed PMID: 38526522]
Level 1 (high-level) evidenceHanna P, Regmi S, Kalapara A, Mulpuri KS, Zabell J, Albersheim J, Wahr J, Randle D, Kaizer A, Patten L, Konety B, Weight C. Alvimopan as part of the Enhanced Recovery After Surgery protocol following radical cystectomy is associated with decreased hospital stay. International journal of urology : official journal of the Japanese Urological Association. 2021 Jun:28(6):696-701. doi: 10.1111/iju.14546. Epub 2021 Mar 26 [PubMed PMID: 33769634]
International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, Australian Bladder Cancer Study Group, National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, Club Urologico Espanol de Tratamiento Oncologico Group, Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jun 1:29(16):2171-7. doi: 10.1200/JCO.2010.32.3139. Epub 2011 Apr 18 [PubMed PMID: 21502557]
Level 1 (high-level) evidenceGrossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England journal of medicine. 2003 Aug 28:349(9):859-66 [PubMed PMID: 12944571]
Wijburg CJ, Hannink G, Michels CTJ, Weijerman PC, Issa R, Tay A, Decaestecker K, Wiklund P, Hosseini A, Sridhar A, Kelly J, d'Hondt F, Mottrie A, Klaver S, Edeling S, Dell'Oglio P, Montorsi F, Rovers MM, Witjes JA. Learning Curve Analysis for Intracorporeal Robot-assisted Radical Cystectomy: Results from the EAU Robotic Urology Section Scientific Working Group. European urology open science. 2022 May:39():55-61. doi: 10.1016/j.euros.2022.03.004. Epub 2022 Apr 2 [PubMed PMID: 35528784]
Arora S, Keeley J, Patel A, Eleswarapu SV, Bronkema C, Alanee S, Menon M. Defining a "High Volume" Radical Cystectomy Hospital: Where Do We Draw the Line? European urology focus. 2020 Sep 15:6(5):975-981. doi: 10.1016/j.euf.2019.02.001. Epub 2019 Feb 14 [PubMed PMID: 30772360]
Pyrgidis N, Volz Y, Ebner B, Kazmierczak PM, Enzinger B, Hermans J, Buchner A, Stief C, Schulz GB. The effect of hospital caseload on perioperative mortality, morbidity and costs in bladder cancer patients undergoing radical cystectomy: results of the German nationwide inpatient data. World journal of urology. 2024 Jan 10:42(1):19. doi: 10.1007/s00345-023-04742-z. Epub 2024 Jan 10 [PubMed PMID: 38197902]
Level 3 (low-level) evidenceMartin AS, Corcoran AT. Contemporary techniques and outcomes of robotic assisted radical cystectomy with intracorporeal urinary diversion. Translational andrology and urology. 2021 May:10(5):2216-2232. doi: 10.21037/tau.2019.09.45. Epub [PubMed PMID: 34159105]
Chan KG, Guru K, Wiklund P, Catto J, Yuh B, Novara G, Murphy DG, Al-Tartir T, Collins JW, Zhumkhawala A, Wilson TG, Pasadena Consensus Panel. Robot-assisted radical cystectomy and urinary diversion: technical recommendations from the Pasadena Consensus Panel. European urology. 2015 Mar:67(3):423-31. doi: 10.1016/j.eururo.2014.12.027. Epub 2015 Jan 14 [PubMed PMID: 25595099]
Level 3 (low-level) evidenceCollins JW, Tyritzis S, Nyberg T, Schumacher M, Laurin O, Khazaeli D, Adding C, Jonsson MN, Hosseini A, Wiklund NP. Robot-assisted radical cystectomy: description of an evolved approach to radical cystectomy. European urology. 2013 Oct:64(4):654-63. doi: 10.1016/j.eururo.2013.05.020. Epub 2013 May 27 [PubMed PMID: 23769588]
Hussein AA, Ahmed YE, Kozlowski JD, May PR, Nyquist J, Sexton S, Curtin L, Peabody JO, Abol-Enein H, Guru KA. Robot-assisted approach to 'W'-configuration urinary diversion: a step-by-step technique. BJU international. 2017 Jul:120(1):152-157. doi: 10.1111/bju.13824. Epub 2017 Mar 22 [PubMed PMID: 28220593]
Abreu AL, Chopra S, Berger AK, Leslie S, Desai MM, Gill IS, Aron M. Management of large median and lateral intravesical lobes during robot-assisted radical prostatectomy. Journal of endourology. 2013 Nov:27(11):1389-92. doi: 10.1089/end.2013.0302. Epub 2013 Aug 24 [PubMed PMID: 23859125]
Azzouni FS, Din R, Rehman S, Khan A, Shi Y, Stegemann A, Sharif M, Wilding GE, Guru KA. The first 100 consecutive, robot-assisted, intracorporeal ileal conduits: evolution of technique and 90-day outcomes. European urology. 2013 Apr:63(4):637-43. doi: 10.1016/j.eururo.2012.11.055. Epub 2012 Dec 8 [PubMed PMID: 23265384]
Laukhtina E, Rajwa P, Mori K, Moschini M, D'Andrea D, Abufaraj M, Soria F, Mari A, Krajewski W, Albisinni S, Teoh JY, Quhal F, Sari Motlagh R, Mostafaei H, Katayama S, Grossmann NC, Enikeev D, Zimmermann K, Fajkovic H, Glybochko P, Shariat SF, Pradere B, European Association of Urology Young Academic Urologists Urothelial Carcinoma Working Group (EAU YAU). Accuracy of Frozen Section Analysis of Urethral and Ureteral Margins During Radical Cystectomy for Bladder Cancer: A Systematic Review and Diagnostic Meta-Analysis. European urology focus. 2022 May:8(3):752-760. doi: 10.1016/j.euf.2021.05.010. Epub 2021 Jun 12 [PubMed PMID: 34127436]
Level 1 (high-level) evidencePackiam VT, Tsivian M, Boorjian SA. The evolving role of lymphadenectomy for bladder cancer: why, when, and how. Translational andrology and urology. 2020 Dec:9(6):3082-3093. doi: 10.21037/tau.2019.06.01. Epub [PubMed PMID: 33457281]
Herr H, Lee C, Chang S, Lerner S, Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. The Journal of urology. 2004 May:171(5):1823-8; discussion 1827-8 [PubMed PMID: 15076285]
Abol-Enein H, Tilki D, Mosbah A, El-Baz M, Shokeir A, Nabeeh A, Ghoneim MA. Does the extent of lymphadenectomy in radical cystectomy for bladder cancer influence disease-free survival? A prospective single-center study. European urology. 2011 Sep:60(3):572-7. doi: 10.1016/j.eururo.2011.05.062. Epub 2011 Jun 12 [PubMed PMID: 21684070]
Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. The Journal of urology. 2004 Oct:172(4 Pt 1):1286-90 [PubMed PMID: 15371825]
Gschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, Frohneberg D, de Geeter P, Heidenreich A, Kälble T, Stöckle M, Schnöller T, Stenzl A, Müller M, Truss M, Roth S, Liehr UB, Leißner J, Bregenzer T, Retz M. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. European urology. 2019 Apr:75(4):604-611. doi: 10.1016/j.eururo.2018.09.047. Epub 2018 Oct 15 [PubMed PMID: 30337060]
Level 1 (high-level) evidenceHerr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. The Journal of urology. 2002 Mar:167(3):1295-8 [PubMed PMID: 11832716]
Shirotake S, Kikuchi E, Matsumoto K, Yazawa S, Kosaka T, Miyajima A, Nakagawa K, Oya M. Role of pelvic lymph node dissection in lymph node-negative patients with invasive bladder cancer. Japanese journal of clinical oncology. 2010 Mar:40(3):247-51. doi: 10.1093/jjco/hyp147. Epub 2009 Nov 3 [PubMed PMID: 19887521]
Brunocilla E, Pernetti R, Schiavina R, Borghesi M, Vagnoni V, Rocca GC, Borgatti F, Concetti S, Martorana G. The number of nodes removed as well as the template of the dissection is independently correlated to cancer-specific survival after radical cystectomy for muscle-invasive bladder cancer. International urology and nephrology. 2013 Jun:45(3):711-9. doi: 10.1007/s11255-013-0461-8. Epub 2013 May 12 [PubMed PMID: 23666588]
Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, Miranda G, Birkhäuser F, Stein J, Burkhard FC, Daneshmand S, Thalmann GN, Gill IS, Skinner DG. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. The Journal of urology. 2011 Oct:186(4):1261-8. doi: 10.1016/j.juro.2011.06.004. Epub 2011 Aug 17 [PubMed PMID: 21849183]
Level 2 (mid-level) evidenceSimone G, Papalia R, Ferriero M, Guaglianone S, Castelli E, Collura D, Muto G, Gallucci M. Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. International journal of urology : official journal of the Japanese Urological Association. 2013 Apr:20(4):390-7. doi: 10.1111/j.1442-2042.2012.03148.x. Epub 2012 Sep 12 [PubMed PMID: 22970939]
Kosiba M, Stolzenbach LF, Collà Ruvolo C, Nocera L, Mansour M, Tian Z, Roos FC, Becker A, Kluth LA, Tilki D, Shariat SF, Saad F, Chun FKH, Karakiewicz PI. Contemporary Trends and Efficacy of Pelvic Lymph Node Dissection at Radical Cystectomy for Urothelial and Variant Histology Carcinoma of the Urinary Bladder. Clinical genitourinary cancer. 2022 Apr:20(2):195.e1-195.e8. doi: 10.1016/j.clgc.2021.10.010. Epub 2021 Nov 5 [PubMed PMID: 34906434]
von Deimling M, Furrer M, Mertens LS, Mari A, van Ginkel N, Bacchiani M, Maas M, Pichler R, Li R, Moschini M, Bianchi A, Vetterlein MW, Lonati C, Crocetto F, Taylor J, Tully KH, Afferi L, Soria F, Del Giudice F, Longoni M, Laukhtina E, Antonelli A, Rink M, Fisch M, Lotan Y, Spiess PE, Black PC, Kiss B, Pradere B, Shariat SF, CLIPOLY Study Group Collaborators. Impact of the extent of lymph node dissection on survival outcomes in clinically lymph node-positive bladder cancer. BJU international. 2024 Mar:133(3):341-350. doi: 10.1111/bju.16210. Epub 2023 Nov 15 [PubMed PMID: 37904652]
Crocerossa F, Autorino R, Carbonara U, Cantiello F, Damiano R, Mir MC. Extent of lymph node dissection and impact on survival in radical cystectomy for advanced bladder cancer. Current opinion in urology. 2022 Nov 1:32(6):607-613. doi: 10.1097/MOU.0000000000001035. Epub 2022 Sep 13 [PubMed PMID: 36101521]
Level 3 (low-level) evidenceHeck MM, Gschwend JE. Extended Lymph Node Dissection for Bladder Cancer: Do Clinical Trials Rule Out a Benefit? European urology focus. 2020 Jul 15:6(4):617-619. doi: 10.1016/j.euf.2019.08.003. Epub 2019 Aug 20 [PubMed PMID: 31439506]
Qi W, Zhong M, Jiang N, Zhou Y, Lv G, Li R, Shi B, Chen S. Which lymph node dissection template is optimal for radical cystectomy? A systematic review and Bayesian network meta-analysis. Frontiers in oncology. 2022:12():986150. doi: 10.3389/fonc.2022.986150. Epub 2022 Nov 25 [PubMed PMID: 36505883]
Level 1 (high-level) evidencePerera M, McGrath S, Sengupta S, Crozier J, Bolton D, Lawrentschuk N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nature reviews. Urology. 2018 Nov:15(11):686-692. doi: 10.1038/s41585-018-0066-1. Epub [PubMed PMID: 30104615]
Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, Stewart F, Lam TB, N'Dow J, van der Heijden AG, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Sherif A, Witjes JA. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. European urology. 2014 Dec:66(6):1065-77. doi: 10.1016/j.eururo.2014.05.031. Epub 2014 Jul 26 [PubMed PMID: 25074764]
Level 1 (high-level) evidenceMuilwijk T, Akand M, Gevaert T, Joniau S. No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial? Translational andrology and urology. 2019 Mar:8(Suppl 1):S112-S115. doi: 10.21037/tau.2018.12.09. Epub [PubMed PMID: 31143684]
Level 1 (high-level) evidenceMøller MK, Høyer S, Jensen JB. Extended versus superextended lymph-node dissection in radical cystectomy: subgroup analysis of possible recurrence-free survival benefit. Scandinavian journal of urology. 2016 Jun:50(3):175-80. doi: 10.3109/21681805.2015.1132473. Epub 2016 Apr 6 [PubMed PMID: 27049808]
Soria F, Gontero P. Re: Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. European urology. 2019 Sep:76(3):408-409. doi: 10.1016/j.eururo.2019.03.042. Epub 2019 Apr 12 [PubMed PMID: 30987842]
Level 1 (high-level) evidenceAlfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A, Martini A, Mertens LS, Meijer RP, Milowsky MI, Neuzillet Y, Panebianco V, Redlef J, Rink M, Rouanne M, Thalmann GN, Sæbjørnsen S, Veskimäe E, van der Heijden AG. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. European urology. 2024 Jan:85(1):17-31. doi: 10.1016/j.eururo.2023.08.016. Epub 2023 Oct 17 [PubMed PMID: 37858453]
Truong H, Maxon V, Goh AC. Robotic Female Radical Cystectomy. Journal of endourology. 2021 Sep:35(S2):S106-S115. doi: 10.1089/end.2020.1190. Epub [PubMed PMID: 34499552]
Niver BE, Daneshmand S, Satkunasivam R. Female reproductive organ-sparing radical cystectomy: contemporary indications, techniques and outcomes. Current opinion in urology. 2015 Mar:25(2):105-10. doi: 10.1097/MOU.0000000000000146. Epub [PubMed PMID: 25581538]
Level 3 (low-level) evidenceChopra S, Abreu AL, Gill IS. Robotic urinary diversion: the range of options. Current opinion in urology. 2016 Jan:26(1):107-13. doi: 10.1097/MOU.0000000000000248. Epub [PubMed PMID: 26574875]
Level 3 (low-level) evidenceThress TM, Cookson MS, Patel S. Robotic Cystectomy with Intracorporeal Urinary Diversion: Review of Current Techniques and Outcomes. The Urologic clinics of North America. 2018 Feb:45(1):67-77. doi: 10.1016/j.ucl.2017.09.009. Epub [PubMed PMID: 29169452]
Simone G, Papalia R, Misuraca L, Tuderti G, Minisola F, Ferriero M, Vallati G, Guaglianone S, Gallucci M. Robotic Intracorporeal Padua Ileal Bladder: Surgical Technique, Perioperative, Oncologic and Functional Outcomes. European urology. 2018 Jun:73(6):934-940. doi: 10.1016/j.eururo.2016.10.018. Epub 2016 Oct 22 [PubMed PMID: 27780643]
Hussein AA, Li Q, Guru KA. Robot-assisted radical cystectomy: surgical technique, perioperative and oncologic outcomes. Current opinion in urology. 2022 Jan 1:32(1):116-122. doi: 10.1097/MOU.0000000000000953. Epub [PubMed PMID: 34798640]
Level 3 (low-level) evidenceElsayed AS, Aldhaam NA, Nitsche L, Siam A, Jing Z, Hussein AA, Shigemura K, Fujisawa M, Guru KA. Robot-assisted radical cystectomy: Review of surgical technique, and perioperative, oncological and functional outcomes. International journal of urology : official journal of the Japanese Urological Association. 2020 Mar:27(3):194-205. doi: 10.1111/iju.14178. Epub 2020 Jan 25 [PubMed PMID: 31981379]
Wiklund NP, Poulakis V. Robotic neobladder. BJU international. 2011 May:107(9):1514-37. doi: 10.1111/j.1464-410X.2011.10307.x. Epub [PubMed PMID: 21518236]
Chen AB, Polotti CF, Zhang M, Yip W, Desai M. Robotic Intracorporeal Ileal Conduit Urinary Diversion Technique. Journal of endourology. 2021 Sep:35(S2):S116-S121. doi: 10.1089/end.2020.1079. Epub [PubMed PMID: 34499542]
Murthy PB, Campbell RA, Lee BH. Intracorporeal Urinary Diversion in Robotic Radical Cystectomy. The Urologic clinics of North America. 2021 Feb:48(1):51-70. doi: 10.1016/j.ucl.2020.09.005. Epub 2020 Nov 5 [PubMed PMID: 33218594]
Tan WS, Lamb BW, Sridhar A, Briggs TP, Kelly JD. A comprehensive guide to perioperative management and operative technique for robotic cystectomy with intracorporeal urinary diversion. Urologia. 2017 Apr 28:84(2):71-78. doi: 10.5301/uj.5000224. Epub 2017 Feb 28 [PubMed PMID: 28256704]
Katsimperis S, Tzelves L, Tandogdu Z, Ta A, Geraghty R, Bellos T, Manolitsis I, Pyrgidis N, Schulz GB, Sridhar A, Shaw G, Kelly J, Skolarikos A. Complications After Radical Cystectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials with a Meta-regression Analysis. European urology focus. 2023 Nov:9(6):920-929. doi: 10.1016/j.euf.2023.05.002. Epub 2023 May 26 [PubMed PMID: 37246124]
Level 1 (high-level) evidenceJohar RS, Hayn MH, Stegemann AP, Ahmed K, Agarwal P, Balbay MD, Hemal A, Kibel AS, Muhletaler F, Nepple K, Pattaras JG, Peabody JO, Palou Redorta J, Rha KH, Richstone L, Saar M, Schanne F, Scherr DS, Siemer S, Stökle M, Weizer A, Wiklund P, Wilson T, Woods M, Yuh B, Guru KA. Complications after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European urology. 2013 Jul:64(1):52-7. doi: 10.1016/j.eururo.2013.01.010. Epub 2013 Jan 16 [PubMed PMID: 23380164]
Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, Motttrie A, Peabody JO, Skinner EC, Wiklund PN, Guru KA, Yuh B. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. European urology. 2015 Mar:67(3):376-401. doi: 10.1016/j.eururo.2014.12.007. Epub 2015 Jan 2 [PubMed PMID: 25560798]
Level 1 (high-level) evidenceIvan SJ, Roebuck EH, Sinks AL, Robinson MM, Clark PE, Gaston KE, Matulay JT, Riggs SB. It's complicated: The relationship of non-narcotic medications and postoperative opioid use in radical cystectomy patients. Urologic oncology. 2024 May 11:():. pii: S1078-1439(24)00363-6. doi: 10.1016/j.urolonc.2024.03.007. Epub 2024 May 11 [PubMed PMID: 38735799]
Çetin B, Çilesiz NC, Ozkan A, Onuk Ö, Kır G, Balci MBC, Özdemir E. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Length of Hospital Stay in Radical Cystectomy: A Prospective Randomized Controlled Study. Cureus. 2024 Mar:16(3):e55460. doi: 10.7759/cureus.55460. Epub 2024 Mar 3 [PubMed PMID: 38571847]
Level 1 (high-level) evidenceZennami K, Kusaka M, Tomozawa S, Toda F, Ito K, Kawai A, Nakamura W, Muto Y, Saruta M, Motonaga T, Takahara K, Sumitomo M, Shiroki R. Impact of an enhanced recovery protocol in frail patients after intracorporeal urinary diversion. BJU international. 2024 Mar 19:():. doi: 10.1111/bju.16340. Epub 2024 Mar 19 [PubMed PMID: 38500447]
Grilo N, Crettenand F, Bohner P, Rodrigues Dias SC, Cerantola Y, Lucca I. Impact of Enhanced Recovery after Surgery(®) Protocol Compliance on Length of Stay, Bowel Recovery and Complications after Radical Cystectomy. Diagnostics (Basel, Switzerland). 2024 Jan 25:14(3):. doi: 10.3390/diagnostics14030264. Epub 2024 Jan 25 [PubMed PMID: 38337779]
Yanada BA, Dias BH, Corcoran NM, Zargar H, Bishop C, Wallace S, Hayes D, Huang JG. Implementation of the enhanced recovery after surgery protocol for radical cystectomy patients: A single centre experience. Investigative and clinical urology. 2024 Jan:65(1):32-39. doi: 10.4111/icu.20230282. Epub [PubMed PMID: 38197749]
Lee G, Patel HV, Srivastava A, Ghodoussipour S. Updates on enhanced recovery after surgery for radical cystectomy. Therapeutic advances in urology. 2022 Jan-Dec:14():17562872221109022. doi: 10.1177/17562872221109022. Epub 2022 Jul 12 [PubMed PMID: 35844831]
Level 3 (low-level) evidenceWilliams SB, Cumberbatch MGK, Kamat AM, Jubber I, Kerr PS, McGrath JS, Djaladat H, Collins JW, Packiam VT, Steinberg GD, Lee E, Kassouf W, Black PC, Cerantola Y, Catto JWF, Daneshmand S. Reporting Radical Cystectomy Outcomes Following Implementation of Enhanced Recovery After Surgery Protocols: A Systematic Review and Individual Patient Data Meta-analysis. European urology. 2020 Nov:78(5):719-730. doi: 10.1016/j.eururo.2020.06.039. Epub 2020 Jul 2 [PubMed PMID: 32624275]
Level 1 (high-level) evidenceCollins JW, Patel H, Adding C, Annerstedt M, Dasgupta P, Khan SM, Artibani W, Gaston R, Piechaud T, Catto JW, Koupparis A, Rowe E, Perry M, Issa R, McGrath J, Kelly J, Schumacher M, Wijburg C, Canda AE, Balbay MD, Decaestecker K, Schwentner C, Stenzl A, Edeling S, Pokupić S, Stockle M, Siemer S, Sanchez-Salas R, Cathelineau X, Weston R, Johnson M, D'Hondt F, Mottrie A, Hosseini A, Wiklund PN. Enhanced Recovery After Robot-assisted Radical Cystectomy: EAU Robotic Urology Section Scientific Working Group Consensus View. European urology. 2016 Oct:70(4):649-660. doi: 10.1016/j.eururo.2016.05.020. Epub 2016 May 24 [PubMed PMID: 27234997]
Jensen BT, Retinger NL, Lauridsen SV. From fast-track to enhanced recovery after surgery in radical cystectomy pathways: A nursing perspective. Asia-Pacific journal of oncology nursing. 2022 Jul:9(7):100048. doi: 10.1016/j.apjon.2022.02.010. Epub 2022 Mar 12 [PubMed PMID: 35647225]
Level 3 (low-level) evidenceOverstreet DL, Sims TW. Care of the patient undergoing radical cystectomy with a robotic approach. Urologic nursing. 2006 Apr:26(2):117-22 [PubMed PMID: 16703919]