Introduction
Amyloidosis is an uncommon spectrum of heterogeneous disease patterns with local to systemic effects which may involve the cardiovascular, pulmonary, neurologic, gastrointestinal, genitourinary, musculoskeletal, and integumentary tissues. The deposition of misfolded and insoluble proteins secreted into the extracellular space defines the pathophysiology of amyloidosis. Amyloid fibrils are derived from soluble precursors, such as serum amyloid P component (SAP) and apolipoprotein E (ApoE), which aggregate into insoluble antiparallel configurations in the form of beta-pleated sheets, and are resistant to catabolic proteolysis.[1][2]
Organ involvement and degree of disease is a function of amyloid protein subtype, plaque burden, and organ-specific toxic effects that leads to inflammation, swelling, end-organ dysfunction, and eventual failure.[3][4] Several amyloidosis subtypes are associated with long-term dialysis dependence, plasma cell dyscrasia, malignancy, and certain hereditary conditions.[5]
While there are around 40 identified protein subtypes of amyloidosis, the most common amyloid subtypes that involve the musculoskeletal system include the immunoglobulin light chain (AL), the beta-2 microglobulin (AB2M), and the transthyretin (ATTR) proteins--the latter having genetic and senescent variants.[6] The diagnostic gold standard for the diagnosis of amyloidosis is tissue biopsy.[5] Classic histologic findings include positive Congo Red staining patterns and apple-green birefringence under polarized light.[1]
The musculoskeletal tissues most commonly involved in amyloidosis include osseous, articular, ligamentous, cartilaginous, synovial tissues, and intervertebral discs.[7] Patients experiencing symptoms of musculoskeletal amyloidosis may report fatigue, weakness, and osseous pain; other specific symptoms depend on the site of involvement. When a primary disease such as multiple myeloma exists, the signs and symptoms of secondary amyloidosis may be obfuscated.[8]
The etiologic pattern of musculoskeletal amyloidosis is variable and can mimic other disease processes, such as rheumatoid arthritis, multiple spondyloarthropathies, myopathy, and chondrosarcoma.[3] Furthermore, certain primary disease states convey a higher risk of developing amyloidosis. Approximately 10 to 15% of patients diagnosed with multiple myeloma will develop amyloidosis. In contrast, nearly 100% of dialysis-dependent patients will develop amyloidosis between the first to second decade following the initiation of treatment.[9][10]
A separate, primary disease process combined with the nonspecific symptoms and imaging appearance of amyloidosis necessitates a careful differential consideration—especially when initial treatment for a primary diagnosis is ineffective. Underdiagnosis of amyloidosis is common and contributes to nearly 50% of patients expiring within the first two years of diagnosis. Several treatment options are available for most amyloid subtypes--however, efficacy is inversely proportional to the degree of end-organ damage.[4]
The radiologic features of amyloidosis are problematically diverse and nonspecific.[11] Although pathologic confirmation is the most reliable diagnostic method, the need for invasive tissue sampling may be obviated in the context of specific imaging patterns and anatomic involvement. Moreover, the imaging findings of amyloidosis may precede the clinical manifestations, providing important diagnostic and prognostic information.[12]
Advancements in radiologic imaging have improved the specificity of MRI and nuclear medicine modalities for detecting amyloidosis. Common imaging patterns seen in various amyloid subtypes include the calcification of involved tissues and infiltration into adjacent soft tissues.[5] The most common imaging modalities used in the imaging of amyloidosis include the radiograph, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), and nuclear medicine (NM) imaging techniques such as fluorodeoxyglucose positron emission tomography (FDG-PET).
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The most common joints involved in musculoskeletal amyloidosis include the shoulder and pelvic girdles, elbow, and wrist.[8] Soft tissue infiltration of amyloid into the shoulder myofibrillar tissues may manifest as hypertrophy of musculature with concomitant weakness and pain in an elderly patient with chronic illness. The cross-sectional imaging features of shoulder involvement are summarized by the "shoulder-pad" sign, which describes the counterintuitive finding of muscular hypertrophy in the setting of functional loss in this patient demographic.[12]
One of the most common presentations of musculoskeletal amyloidosis manifests as a late onset of carpal tunnel syndrome. Amyloid-related carpal tunnel syndrome is typically associated with the senescent type ATTR protein, which reflects systemic involvement, and can be a prognosticator of future myocardial involvement.[5] This presentation can also be seen in AB2M dialysis-related amyloidosis, which is seen in 31% of those undergoing long-term treatment.[13][7]
The flexor tendons of the hand, wrist, and adjacent synovium become hypertrophied as amyloid is deposited into the myofibrillar tissues and may subsequently infiltrate into surrounding structures. The median nerve may appear compressed on cross-sectional imaging, and bony erosions are common. Paradoxical T2 signal loss of the median nerve may be seen on MRI, which is usually increased in signal in other forms of carpal tunnel syndrome.[5] These findings are seen in the radiographic images for patient 4.
Dialysis-related musculoskeletal amyloidosis is seen in approximately 30% of older patients undergoing long-term treatment, where the duration of dialysis dependence and prevalence of amyloidosis appear to be positively correlated.[5][9][14] This form of amyloidosis is typically associated with the AB2M subtype and manifests as soft tissue calcifications, extensive osseous destruction, and bilateral polyarthropathy, which may mimic various spondyloarthropathies.[10]
Anatomic involvement includes the shoulder and hip girdles, as well as the carpal bones. Radiographic imaging may demonstrate lucent osseous lesions with peripheral sclerosis. CT imaging helps evaluate the degree of osseous erosion and the extent of soft tissue calcifications. MRI evaluation of the joints may show low T1 and T2 signals and minimal enhancement. Typical ultrasound appearance includes discontinuous hypertrophy of the tendons and adjacent synovium.[5]
Plain Films
The plain film is critical for the initial evaluation of any musculoskeletal complaint, especially in a patient with suspected or known amyloidosis. Plain film appearances of musculoskeletal amyloidosis include osseous erosions, osteopenia, well-defined lytic lesions, bone marrow infiltration, and pathologic fracture, as seen in the plain film images for patients 2 and 3. Affected joints may display subchondral cysts and calcification of adjacent soft tissues, highlighted in the plain film images for patients 3 through 5.[8][14]
Computed Tomography
The most common CT manifestations of amyloidosis involving bony structures include osteopenia, osseous erosions, well-defined lytic lesions, and bone marrow infiltration--all of which can lead to pathologic fractures--as seen in the CT images for patients 3 and 5. Affected joints may exhibit subchondral cysts, adjacent hypertrophy of soft tissues, and osteopenia.[8]
Soft tissues may also demonstrate fine calcification, as seen in the CT images for patient 2. The "shoulder pad" sign can be in hypertrophied shoulder musculature, secondary to myofibrillar amyloid deposition, which is more commonly seen in AL amyloidosis. A CT-guided biopsy is useful for tissue sampling of suspected osseous amyloid. Imaging surveillance of suspected amyloid deposits is also a useful strategy, as seen in the CT images for patient 1. Dual-energy CT is infrequently used to assess for crystal deposition diseases, such as monosodium urate and calcium pyrophosphate, as seen in the CT images for patient 5.[15][16]
Magnetic Resonance
Magnetic resonance imaging (MRI) is useful for evaluating the structure and soft tissue characteristics of nerves, soft tissues, articular surfaces, and tendons.[5] A frequently encountered soft tissue manifestation of periarticular musculoskeletal amyloidosis seen on MRI is that of a tenosynovial giant cell tumor mimic (formerly PVNS) without the accompanying susceptibility artifacts to suggest a recurrent hemorrhagic process. These mimics appear as T1 and T2 hypointense lesions with mild to moderate contrast enhancement without significant blooming on susceptibility-weighted imaging.[12]
It is an important consideration that both tenosynovial giant cell tumor and amyloidosis can coexist simultaneously, as seen in the MRI images for patient 5. The "shoulder pad" sign is also visible in hypertrophied shoulder musculature due to myofibrillar amyloid deposition. In amyloid-related carpal tunnel syndrome, paradoxical T2 signal loss of the median nerve may be seen on MRI, which is expected to demonstrate increased signal with other forms of carpal tunnel syndrome. The MRI manifestations of amyloid-related carpal tunnel syndrome can be seen in the MRI images for patient 4.[5]
Imaging manifestations of tenosynovitis appear as an increased fluid signal on T2 and STIR sequences. Further examples of musculoskeletal amyloid MR imaging manifestations can be seen in the MRI images for patient images 2, 3, and 5.
Ultrasonography
Ultrasound can be used to evaluate musculoskeletal complaints in a patient with suspected or known amyloidosis. Ultrasound is useful as a screening tool for the presence of a mass, can provide additional information regarding mass composition, such as fluid or microcalcification, and may evaluate the integrity of structures, such as nerves and tendons, with both static and dynamic acquisition. Soft tissue infiltration, calcification, and joint effusions may be visible.[5]
Ultrasound can also monitor known or new soft tissue lesions, as seen in the ultrasound images for patient 2. Ultrasound-guided biopsy is a valuable intervention for tissue sampling of suspected soft tissue amyloidomas.
Nuclear Medicine
Fluorodeoxyglucose positron emission tomography is the primary scintigraphic imaging technique. It is useful in the detection, localization, assessment of treatment response, and surveillance of amyloid plaques, as seen in the FDG PET/CT images for patient 2. Some common areas of uptake include the shoulder and pelvic girdles, the elbow, and the wrist. Myofibrillar amyloid deposition within the shoulder musculature will demonstrate avid uptake, complementing the "shoulder pad" sign, which may also be seen on other cross-sectional modalities. Soft tissue amyloid plaques will also demonstrate uptake.[9]
Although less frequently used, radionuclide bone scintigraphy using technetium-99m methylene diphosphonate may demonstrate uptake into affected osseous structures.[14]
Patient Positioning
There are no specific patient positioning requirements to optimize the imaging of amyloidosis. Positioning that maximizes patient comfort coupled with using standardized views is considered sufficient. Minimizing unnecessary radiation dosage to the patient can be achieved by reducing photon energy and quantity, optimizing the source-to-object distance, maximizing collimation and filtration, and ensuring the patient is within the isocenter of the field of view.[17][18]
Radiographic positioning should correspond to the standard imaging plane view types associated with the specific area of interest, such as anterior-posterior and lateral views of the tibia and fibula.[19] The optimal patient positioning for cross-sectional imaging (such as CT, MRI, and FDG-PET) corresponds to the supine positioning of the patient on the gantry isocenter.[17][18]
Optimization of patient positioning during the use of ultrasound prioritizes patient comfort while positioning the area of interest in such a way as to accommodate the probe and planned sonographic window.[20]
Clinical Significance
Musculoskeletal amyloidosis (MA) is a complex disease process that tends to be systemic but sporadic in involvement and frequently poses a diagnostic challenge. MA usually manifests as a secondary disease process but can also present as a primary process. The most common primary disease states predisposing a patient to MA include long-term dialysis treatment, multiple myeloma, insulin dependence, and occasionally rheumatoid arthritis. Disease processes such as rheumatoid arthritis, myopathy, chondrosarcoma, and several spondyloarthropathies may mimic the signs and symptoms of MA.
The imaging characteristics of MA are frequently nonspecific and must be appropriately contextualized with the patient's medical history. Many imaging findings related to MA may appear quite similar to other musculoskeletal pathologies, such as tenosynovial giant cell tumors, idiopathic carpal tunnel syndrome, and bone mineral loss. Although specific imaging characteristics (in conjunction with anatomical involvement and patient history) may obviate the need for tissue sampling, pathologic confirmation remains the gold standard for diagnosing musculoskeletal amyloidosis. Despite the associated diagnostic challenge, amyloidosis must be identified and treated as soon as possible to preserve the patient's quality of life; therefore, strong attention to detail, a high index of suspicion, and effective interprofessional cooperation are required.
One of the earliest signs of amyloidosis is declining renal function. The internal medicine physician and nephrologist are essential in correlating the patient's current functional renal status to their medical history. The nephrologist is also critical in the context of long-term dialysis treatment, considering the onset of beta-2 microglobulin amyloidosis approaches near certainty by the second decade of dialysis treatment. The family medicine physician and endocrinologist can provide helpful guidance and surveillance for those patients requiring injectable insulin to ensure that injection site rotation mitigates the risk of insulin-derived amyloidosis and its mimics. The diagnosis of transthyretin amyloidosis prognosticates cardiac involvement, which necessitates cardiologic intervention.
A consultation with the rheumatologic physician will provide diagnostic clarity regarding arthritides and spondyloarthropathies that mimic amyloidosis. Frequent coexisting anemia and the need for therapeutic intervention with chemotherapy and plasmapheresis will require the expertise of the Hematology/Oncology physician in conjunction with the pharmacist. The involvement of surgical specialties, such as orthopedic surgery and general surgery, is critical in the identification and surgical palliation of the various manifestations and complications of musculoskeletal amyloidosis. Nursing specialties can assist in patient monitoring, provide education, and ensure patient comfort in inpatient and outpatient settings.
Finally, the radiologist plays a pivotal role in reducing the diagnostic uncertainty of musculoskeletal amyloidosis by correlating the imaging findings and extent of the disease with the patient's medical history and obtaining tissue samples for diagnosis and monitoring disease response following an intervention. Early involvement of the radiologist regarding the most appropriate imaging study for the clinical scenario will increase diagnostic efficiency while reducing the patient's exposure to unnecessary radiation. As such, the ACR appropriateness criteria provides ordering guidance for the healthcare provider and represents a meta-analysis of over 6,200 articles and 8,800 recommendations of the most suitable study for the given clinical scenario.[21] [Level 1]
Although musculoskeletal amyloidosis frequently presents a diagnostic dilemma for the treating physician, rapid diagnosis and treatment will improve patient outcomes and quality of life. A multidisciplinary treatment strategy to address the systemic nature of amyloidosis is recommended.
The views expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, the Department of Defense, nor any agencies under the U.S. Government.
Media
(Click Image to Enlarge)

Patient 1: A 59-year-old male with type-2 insulin-dependent diabetes mellitus, chronic kidney disease, and suspected amyloidosis presents for a 4-year follow-up of painful, enlarging ventral abdominal masses corresponding to his insulin injection sites. Despite maximal pharmacologic intervention, his lab values demonstrate severe insulin resistance. A soft tissue ultrasound of the area of concern reveals bilateral, avascular, edematous subcutaneous lesions with fine echogenic calcifications and posterior acoustic shadowing. The right mass measures 1.4 x 5.1 cm (AP x TR) and the left mass measures 1.3 x 5.3 cm. Contributed by Tylor Connor, DO
(Click Image to Enlarge)
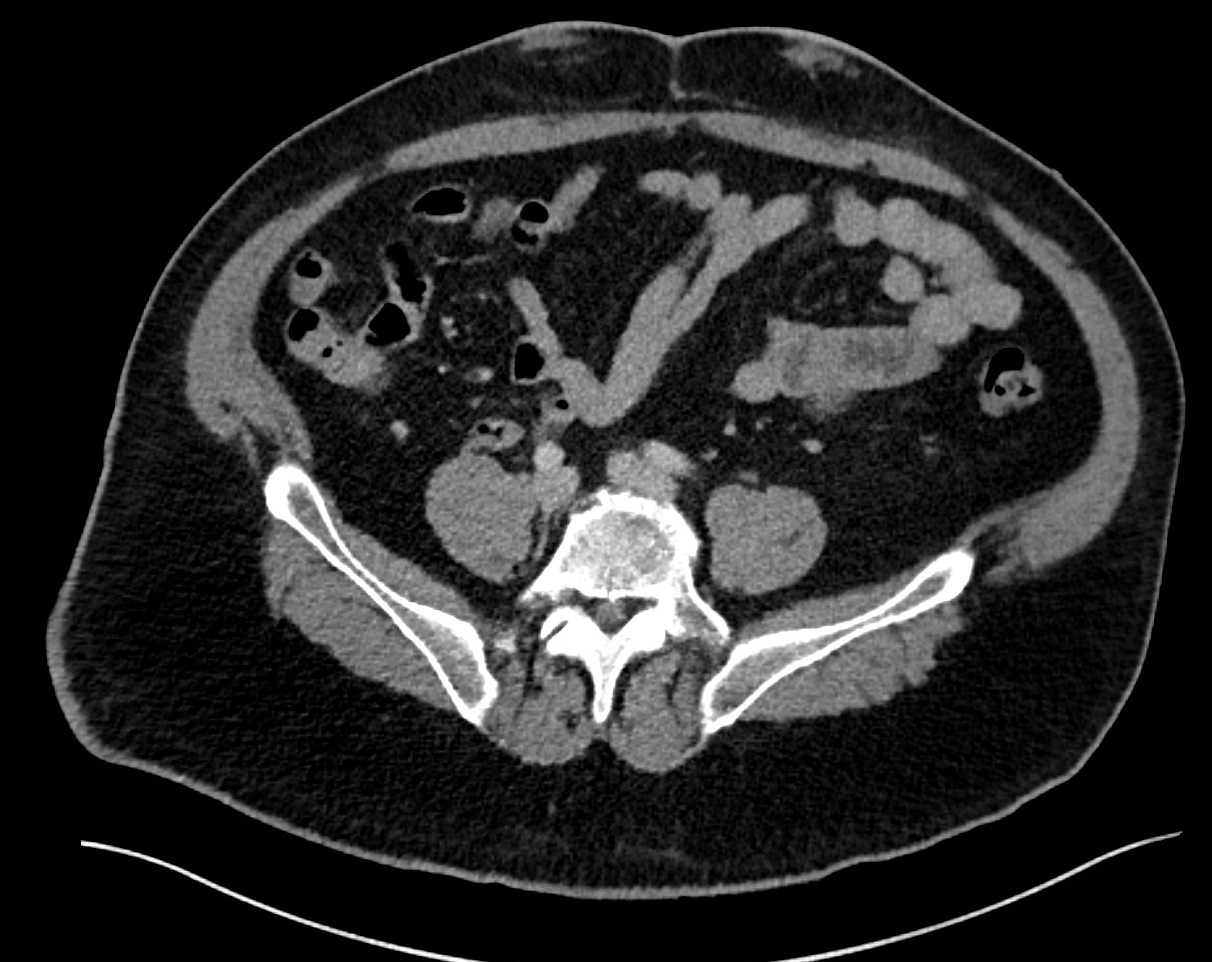
Patient 1: A 55-year-old male with type-2 insulin-dependent diabetes mellitus, chronic kidney disease, and suspected amyloidosis presents with painful, enlarging ventral abdominal masses corresponding to insulin injection sites. Relevant lab values include elevated fasting blood glucose and HbA1C despite aggressive pharmacologic intervention. Initial CT of the abdomen and pelvis demonstrates small, nearly symmetric periumbilical subcutaneous masses with soft tissue attenuation and lack of encapsulation. The right mass measures 0.7 x 2.2 cm (AP x TR) and the left mass measures 1.1 x 2.1 cm. Contributed by Tylor Connor, DO
(Click Image to Enlarge)

Patient 1: A 59-year-old male with type-2 insulin-dependent diabetes mellitus, chronic kidney disease, and suspected amyloidosis presents for a 4-year follow-up of painful, enlarging ventral abdominal masses corresponding to insulin injection sites. Despite maximal pharmacologic intervention, lab values demonstrate severe insulin resistance. Follow-up CT of the abdomen and pelvis demonstrates an interval enlargement of the periumbilical subcutaneous masses. The right mass now measures 1.6 x 4.8 cm (AP x TR) and the left mass now measures 1.9 x 4.1 cm. Contributed by Tylor Connor, DO
(Click Image to Enlarge)

Patient 2: A 70-year-old male with pathology-proven Amyloid Light Chain (AL) amyloidosis, now in remission following chemotherapeutic intervention two years prior, presents for follow-up of enlarging and painful lower extremity nodules. A follow-up CT of his known left lower extremity amyloidoma demonstrates a soft tissue mass showing stippled calcification adjacent to the tibia, similar in size to pre-treatment imaging. Contributed by Tylor Connor, DO
(Click Image to Enlarge)

Patient 2: A 68-year-old male presents with an enlarging and painful left lower extremity nodule overlying the tibia. An MRI of his left lower extremity was performed. STIR sequence demonstrates a non-circumscribed, heterogeneously isointense mass extending across tissue plans to invade anterior compartment musculature. There is generalized fluid signal intensity throughout the surrounding soft tissues. Bone signal is normal. Contributed by Tylor Connor, DO
References
Hazenberg BP. Amyloidosis: a clinical overview. Rheumatic diseases clinics of North America. 2013 May:39(2):323-45. doi: 10.1016/j.rdc.2013.02.012. Epub 2013 Mar 13 [PubMed PMID: 23597967]
Level 3 (low-level) evidencePepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Noble GE, Hutchinson WL, Hawkins PN, Nelson SR, Gallimore JR, Herbert J. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proceedings of the National Academy of Sciences of the United States of America. 1994 Jun 7:91(12):5602-6 [PubMed PMID: 8202534]
Level 3 (low-level) evidenceNguyen TX, Naqvi A, Thompson TL, Wilson RH. Musculoskeletal Manifestations of Amyloidosis: A Focused Review. Journal of surgical orthopaedic advances. 2018 Spring:27(1):1-5 [PubMed PMID: 29762107]
Level 3 (low-level) evidenceGertz MA, Dispenzieri A. Systemic Amyloidosis Recognition, Prognosis, and Therapy: A Systematic Review. JAMA. 2020 Jul 7:324(1):79-89. doi: 10.1001/jama.2020.5493. Epub [PubMed PMID: 32633805]
Level 1 (high-level) evidenceSugi MD, Kawashima A, Salomao MA, Bhalla S, Venkatesh SK, Pickhardt PJ. Amyloidosis: Multisystem Spectrum of Disease with Pathologic Correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2021 Sep-Oct:41(5):1454-1474. doi: 10.1148/rg.2021210006. Epub 2021 Aug 6 [PubMed PMID: 34357805]
M'bappé P, Grateau G. Osteo-articular manifestations of amyloidosis. Best practice & research. Clinical rheumatology. 2012 Aug:26(4):459-75. doi: 10.1016/j.berh.2012.07.003. Epub [PubMed PMID: 23040361]
Chang CY, Rosenthal DI, Mitchell DM, Handa A, Kattapuram SV, Huang AJ. Imaging Findings of Metabolic Bone Disease. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016 Oct:36(6):1871-1887 [PubMed PMID: 27726750]
Urban BA, Fishman EK, Goldman SM, Scott WW Jr, Jones B, Humphrey RL, Hruban RH. CT evaluation of amyloidosis: spectrum of disease. Radiographics : a review publication of the Radiological Society of North America, Inc. 1993 Nov:13(6):1295-308 [PubMed PMID: 8290725]
Hotta M, Minamimoto R, Kaneko H, Yamashita H. Fluorodeoxyglucose PET/CT of Arthritis in Rheumatic Diseases: A Pictorial Review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2020 Jan-Feb:40(1):223-240. doi: 10.1148/rg.2020190047. Epub [PubMed PMID: 31917663]
Huang M, Schweitzer ME. The role of radiology in the evolution of the understanding of articular disease. Radiology. 2014 Nov:273(2 Suppl):S1-22. doi: 10.1148/radiol.14140270. Epub [PubMed PMID: 25340431]
Level 3 (low-level) evidenceCzeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S. Amyloidosis: Modern Cross-sectional Imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015 Sep-Oct:35(5):1381-92. doi: 10.1148/rg.2015140179. Epub 2015 Jul 31 [PubMed PMID: 26230754]
Level 2 (mid-level) evidenceGeorgiades CS, Neyman EG, Barish MA, Fishman EK. Amyloidosis: review and CT manifestations. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Mar-Apr:24(2):405-16 [PubMed PMID: 15026589]
Hatano M, Kitajima I, Yamamoto S, Nakamura M, Isawa K, Suwabe T, Hoshino J, Sawa N, Ubara Y. Dialysis-related carpal tunnel syndrome in the past 40 years. Clinical and experimental nephrology. 2022 Jan:26(1):68-74. doi: 10.1007/s10157-021-02122-8. Epub 2021 Aug 20 [PubMed PMID: 34415463]
Ross LV, Ross GJ, Mesgarzadeh M, Edmonds PR, Bonakdarpour A. Hemodialysis-related amyloidomas of bone. Radiology. 1991 Jan:178(1):263-5 [PubMed PMID: 1984316]
Level 3 (low-level) evidenceChou H, Chin TY, Peh WC. Dual-energy CT in gout - A review of current concepts and applications. Journal of medical radiation sciences. 2017 Mar:64(1):41-51. doi: 10.1002/jmrs.223. Epub 2017 Feb 26 [PubMed PMID: 28238226]
Kravchenko D, Karakostas P, Kuetting D, Meyer C, Brossart P, Behning C, Schäfer VS. The role of dual energy computed tomography in the differentiation of acute gout flares and acute calcium pyrophosphate crystal arthritis. Clinical rheumatology. 2022 Jan:41(1):223-233. doi: 10.1007/s10067-021-05949-4. Epub 2021 Oct 9 [PubMed PMID: 34626261]
Nakashima J, Duong H. Radiology, Image Production and Evaluation. StatPearls. 2024 Jan:(): [PubMed PMID: 31985938]
Al-Hayek Y, Zheng X, Hayre C, Spuur K. The influence of patient positioning on radiation dose in CT imaging: A narrative review. Journal of medical imaging and radiation sciences. 2022 Dec:53(4):737-747. doi: 10.1016/j.jmir.2022.09.027. Epub 2022 Oct 21 [PubMed PMID: 36280573]
Level 3 (low-level) evidencePazanin A, Skrk D, O'Driscoll JC, McEntee MF, Mekis N. OPTIMAL COLLIMATION SIGNIFICANTLY IMPROVES LUMBAR SPINE RADIOGRAPHY. Radiation protection dosimetry. 2020 Jul 24:189(4):420-427. doi: 10.1093/rpd/ncaa057. Epub [PubMed PMID: 32363403]
Strakowski JA, Visco CJ. Diagnostic and therapeutic musculoskeletal ultrasound applications of the shoulder. Muscle & nerve. 2019 Jul:60(1):1-6. doi: 10.1002/mus.26505. Epub 2019 May 11 [PubMed PMID: 31054148]
Kurth DA, Karmazyn BK, Waldrip CA, Chatfield M, Lockhart ME. ACR Appropriateness Criteria® Methodology. Journal of the American College of Radiology : JACR. 2021 Nov:18(11S):S240-S250. doi: 10.1016/j.jacr.2021.03.021. Epub 2021 Jul 9 [PubMed PMID: 34794586]