Introduction
Chromatography is an analytical technique used to separate a given mixture into its components. The technique is based on the principle that when a mixture and a mobile phase are allowed to flow over a stationary phase, the separation occurs based on the differential affinities of the components for these 2 phases.[1] Chromatography is a commonly used technique in clinical laboratories for diagnosing inborn carbohydrate, protein, and lipid metabolism errors.[2] The parameters quantified are vitamins, hormones, metabolites, tumor markers, and drugs in body fluids. In pharmacology, chromatography is used to estimate the purity and potency of drugs. Chromatography can analyze environmental samples for drugs, toxins, and pollutants and help discover many new biomolecules, providing insights into disease mechanisms and biomarker discovery.[1][3]
The chromatography phases refer to the stationary and mobile phases involved in the separation process.
Here are the main phases of chromatography:
Mobile Phase
The mobile phase is a liquid or gas that carries the sample and propels the compounds through the stationary phase, resulting in separation. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The mobile phase can be isocratic or gradient, polar or nonpolar, based on the nature of the analyte.[4]
Stationary Phase
The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Solid, liquid, or gas can be held over a supporting medium. The stationary phase interacts with various mixture components based on the polarity, affinity, size, and charge. Thus, different analytes have varying degrees of interaction with the stationary phase, leading to differential retention times and elution profiles.[1]
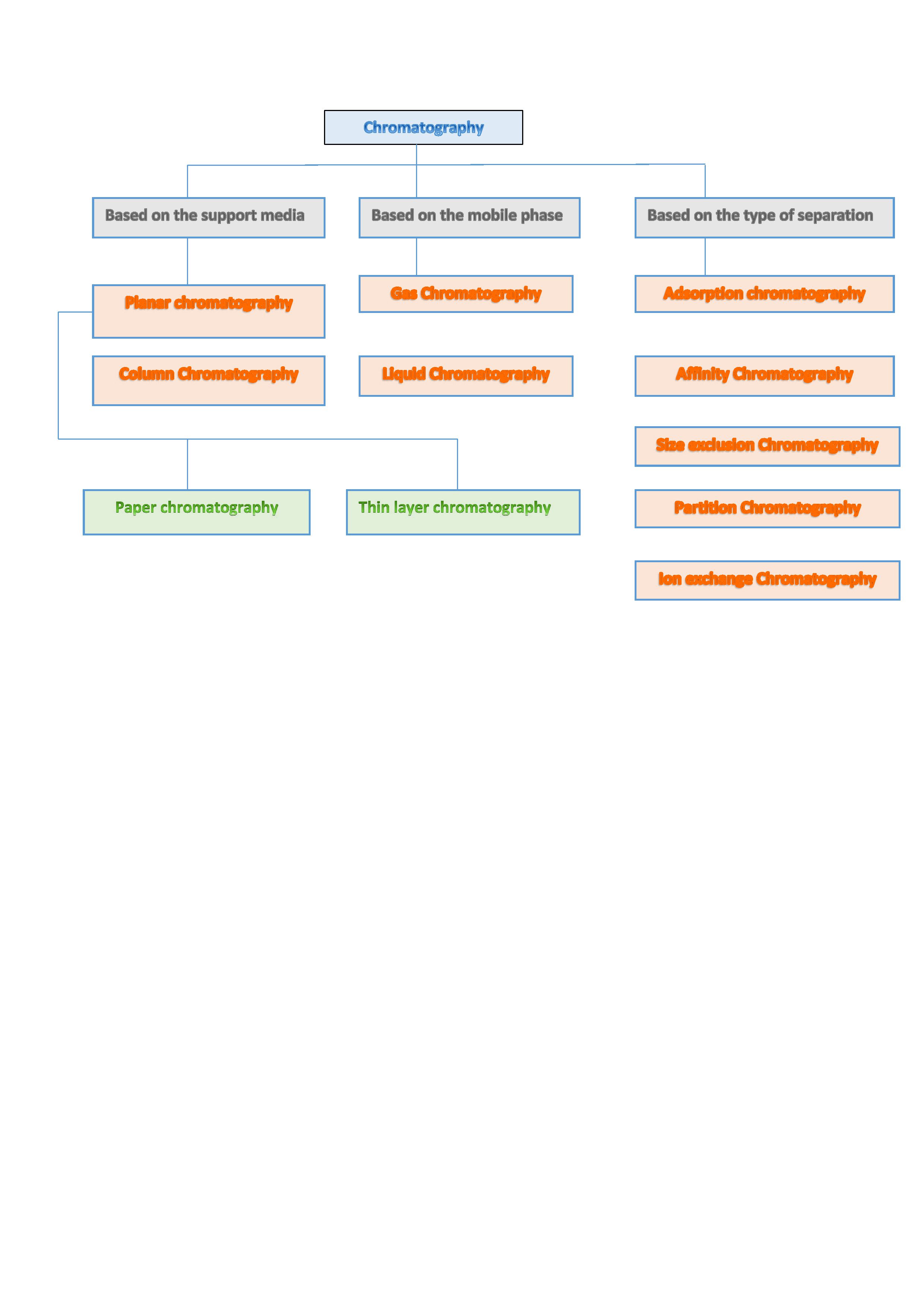
Different types of chromatography use combinations of stationary and mobile phases to achieve separation. Refer to image 1 for the classification of chromatography.
Planar Chromatography
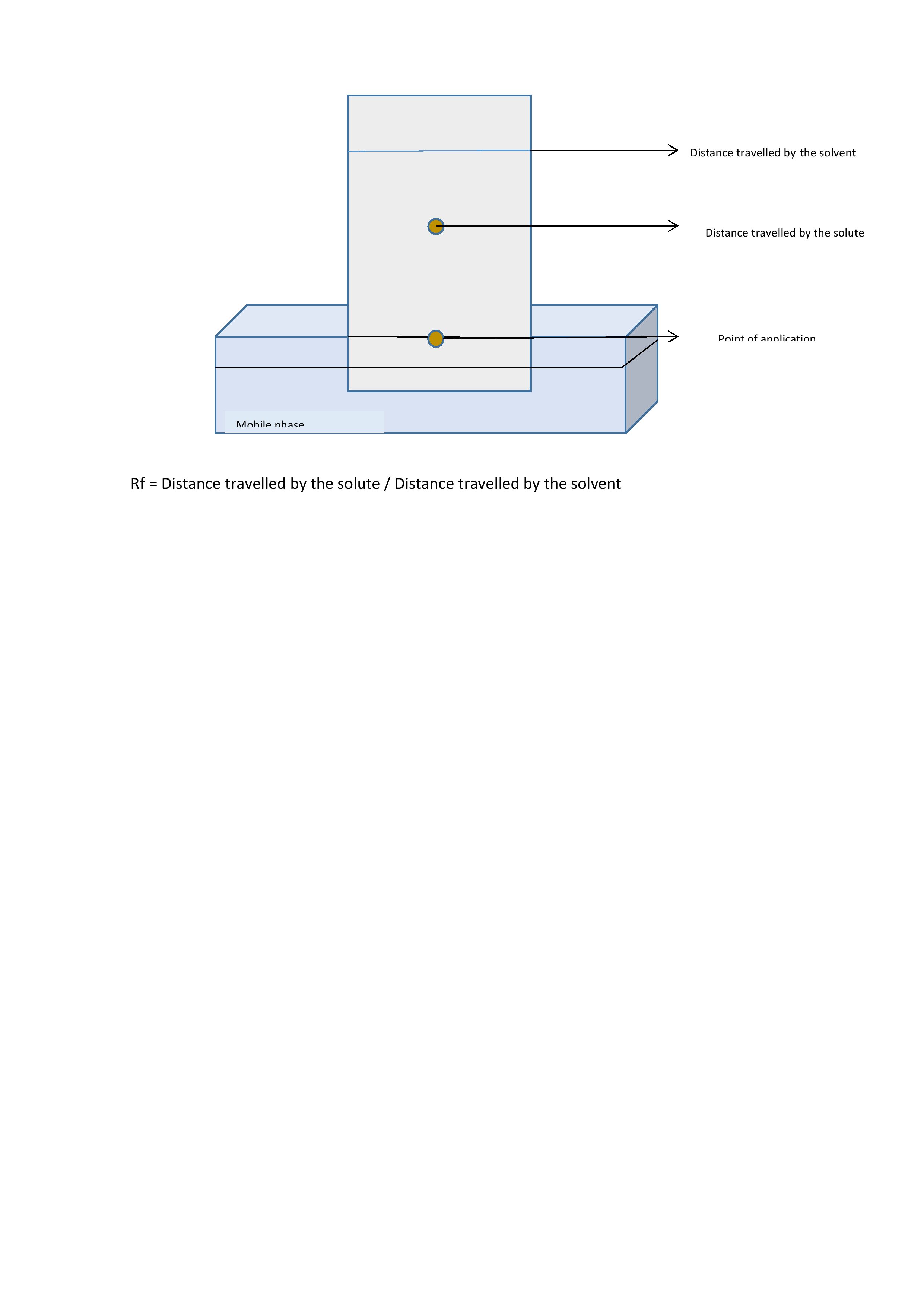
The stationary phase is spread as a thin layer on a flat surface. The sample is added as a small spot or a band over it, and the entire plane is kept in a slanting position over a liquid mobile phase. The mobile phase moves over the stationary phase due to capillary action carrying the sample, resulting in separation. When a sheet of paper is used, it is called paper chromatography. If a thin layer of absorbent material like silica, aluminum, or cellulose coated over a glass plate is used, it is called thin-layer chromatography.[5] After separation, the colorless compounds are identified using fluorescence, radioactivity, or by producing visible color. The position of each molecule is identified, and the distance traveled is measured. The retention factor or Rf value for each of the molecules is expressed, and the identification of the molecules is compared with the standard Rf. The Rf value is calculated by dividing the distance a solute travels by the distance the solvent travels. A number between 0 and 1 represents the Rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent, the temperature, and the solvent concentration.[6] Refer to image 2 (planar chromatography).
Column Chromatography
This is the most commonly used type, where a column of fiberglass or steel filled with silica particles acts as a stationary phase.[1] The mobile phase is gas or liquid. In normal-phase column chromatography, a polar stationary phase separates non-polar compounds; in reversed-phase chromatography, the nonpolar stationary phase and the polar mobile phase are used.[7]
Adsorption Chromatography
Here, the separation is based on the differential adsorption of the analytes onto the solid stationary phase. Silica, charcoal, and calcium hydroxyapatite are the common absorbents packed as stationary phases onto the columns.[8] The interactions between the compounds and stationary phase can be Van der Waals, hydrogen bonding, dipole-dipole, and hydrophobic interactions. These interactions cause analytes to be retained on the stationary phase to varying degrees.[9]
Partition Chromatography
Separation is based on differences in partition coefficients, allowing selective elution and separation of compounds based on their affinity for the 2 phases.[1]
Size Exclusion Chromatography
Also known as gel permeation chromatography, the separation is based on the size. The column is packed with gels of controlled pore size, which acts like a molecular sieve, and by steric effects, the compounds are classified based on molecular size. This is useful for separating proteins, viruses, and nucleic acids.[1]
Ion Exchange Chromatography
This relies on the reversible interaction between charged analytes (ions) and oppositely charged groups immobilized on a solid stationary phase. This interaction allows ions to be selectively retained and then eluted from the stationary phase by manipulating the composition of the mobile phase. Cation and anion exchange resins are used in the stationary phase to isolate anionic and cationic substances, respectively.[10]
Affinity Chromatography
The separation is based on the interaction of the proteins and the ligands. The gel with bound ligands interacts with the proteins and retains them, enabling the separation of desired proteins, which can be eluted. This separates enzymes, vitamins, hormones, antibodies, etc.[11]
Gas Chromatography
Here, inert gasses like nitrogen, helium, or argon or a low-mass gas such as hydrogen are used in the mobile phase to separate volatile compounds or substances that can become volatile after derivatization. The separation occurs based on the differences in vapor pressure after converting them into volatile compounds.[12] Gas chromatography (GC) estimates lipids, drugs, and vitamins.[13] There are several ways of classifying GC methods based on the type of stationary phase present. These categories include gas-solid chromatography (GSC), gas-liquid chromatography (GLC), and bonded phase gas chromatography.[1]
Liquid Chromatography
In liquid chromatography (LC), the mobile phase is a liquid in which the sample with ions and molecules is dissolved. The 2 most common forms of LC used for analysis are liquid chromatography-mass spectrometry (LC-MS) and high-performance liquid chromatography (HPLC). Liquid chromatography (LC), when combined with MS, is highly efficient with low detection limits.[14] MS further sorts and identifies the compounds eluted from LC in the electric and magnetic fields according to the charge mass ratio.
High-Performance Liquid Chromatography (HPLC)
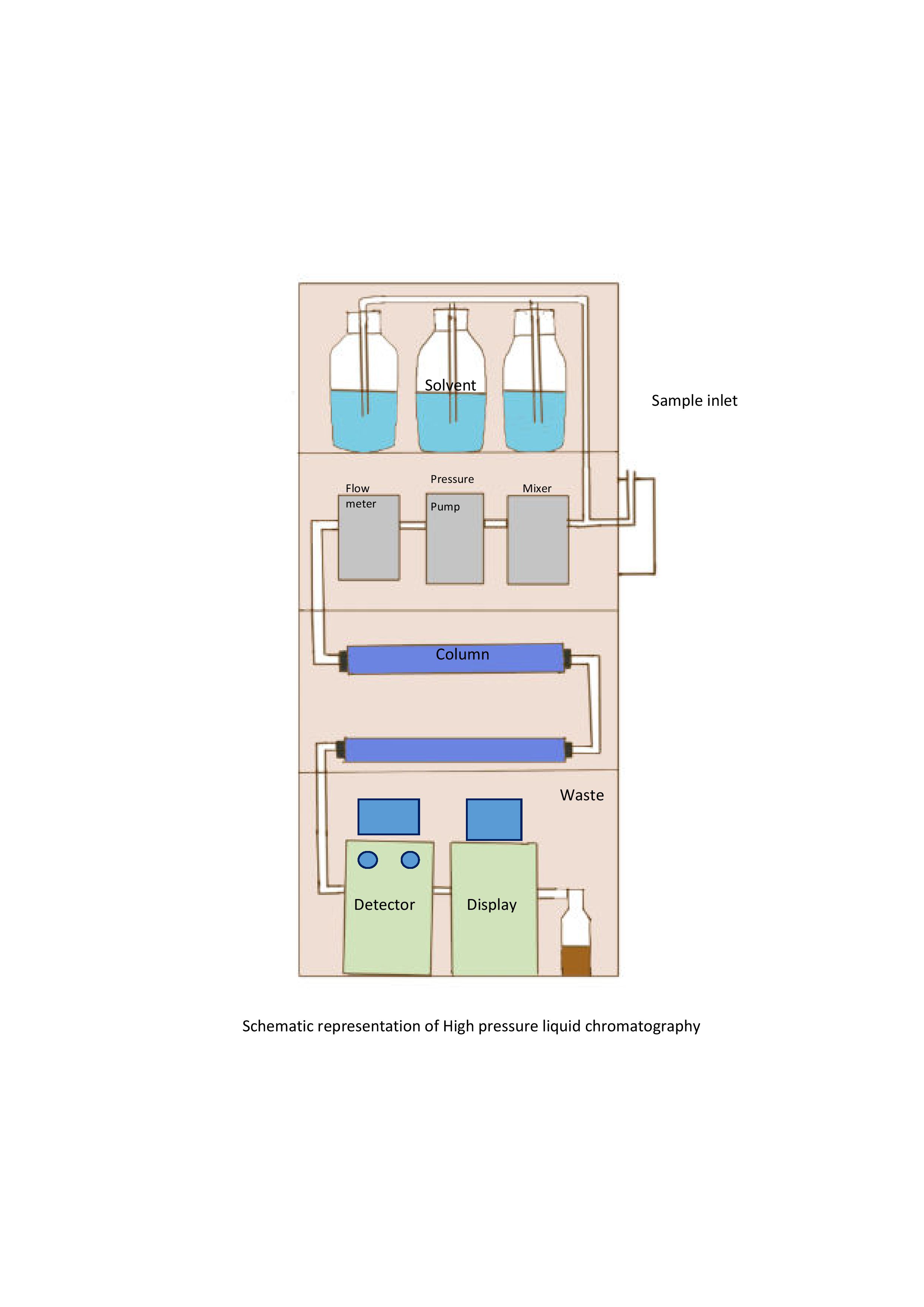
This is a highly sensitive and efficient technique among different chromatography techniques, also known as high-pressure liquid chromatography.[1] Here, the solvent mixture can pass through columns containing stationary phases under high atmospheric pressure of 10 Pa to 400 Pa. This high pressure creates a high flow rate in a sample of 0.1 cm/sec to 5 cm/sec, allowing separation in a few minutes. High pressure ensures high resolution and better separation of closely related compounds; it also enables using MS as detectors, which require high flow rates.[4] The eluents are detected using UV absorption and fluorescence. The basic instrumentation setup is given in image 3.
Specimen Requirements and Procedure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Specimen Requirements and Procedure
Specimen requirements and procedures for clinical samples may vary depending on the type of chromatography and the analyte being assayed. Serum, plasma, whole blood, saliva, and urine samples are commonly used specimens.[15] All the clinical samples should be collected using an aseptic technique in appropriate containers. Clean-catch urine is obtained for urine samples. The urine sample should be free from any contamination, including blood. Similarly, serum or whole blood is collected in appropriate gel or anticoagulant tubes.[16] Samples should be handled carefully to avoid contamination or degradation. Processing should be carried out based on the chromatography technique used. This may involve extraction, derivitization, or dilution.[17]
Dried blood spots (DBS) are collected for newborn screening from infants between 24 to 48 hours of age.[18] A heel prick specimen is preferred rather than from fingers or ear lobes.
Blood, tissue, or saliva is obtained to isolate DNA for nucleic acid assay. After the standard extraction technique, concentration and purity are measured by UV spectrometry or fluorometry.[19] Sample preparation steps like reverse transcription (for RNA) or polymerase chain reaction (PCR) amplification are performed before sample assay.
Diagnostic Tests
M. Tswett was the first to perform chromatography on plant pigments in 1906 using calcium carbonate as a stationary phase. Over time, thin-layer chromatography, gas chromatography, and HPLC were applied. Now, ultra HPLC, two-dimensional GC with full automation, and an advanced detector system are in use.[4] Since then, chromatography has evolved and found its application in many fields, such as analytical chemistry, clinical diagnostics, forensics, food science, pharmacology, and molecular biology.
| Classification | Analyte |
|
Newborn screening Amino acids Acyl carnitines Organic acids Lipids |
Alanine, arginine, citrulline, leucine, lysine, methionine, ornithine, phenylalanine, proline, serine, tyrosine. Free carnitine, acetylcarnitine, propionyl carnitine, butyryl carnitine, and isovalerylcarnitine. Methylmalonic acid, propionic acid, isovaleric acid, glutaric acid. Free fatty acids, very long-chain fatty acids, and phytanic acid.[20] |
| Other metabolites |
Serum lactate Pyruvate Urate Ketone bodies |
| Hormones |
Cortisol, cortisone, corticosterone. Testosterone, estradiol, progesterone. Dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulphate (DHEA-S), androstenedione.[21] |
| Hemoglobin |
Normal and abnormal Hb like Hb A, Hb A2, Hb F, HbA1C, and Hb variants.[22] Porphyrins like uroporphyrinogen, coproporphyrinogen, and porphobilinogen. Bilirubin |
| Vitamins |
A, E, K Vitamin D and its metabolites like 24,25-dihydroxy cholecalciferol.[21] |
| Tumor marker |
Catecholamines such as epinephrine, nor-epinephrine, dopamine, and serotonin and their metabolites like homovanillic acid (HVA), vanillylmandelic acid (VMA), and 5-hydroxy indole acetic acid (5-HIAA).[21] |
| Markers of neurodegenerative disorders | Aβ40, Aβ42 peptides, total tau, p-tau proteins. |
| Infections |
Anaerobic infections Tuberculosis[23] |
| Drugs and toxins |
Analgesics like morphine, hydromorphone and fentanyl. Antidepressants like tricyclic antidepressants and selective serotonin reuptake inhibitors. Antipsychotics Antiepileptics Immunosuppresants like tacrolimus, sirolimus, evalolimus etc Narcotics such as cocaine, cannabinoids, and amphetamines. Anticancer drugs like methotrexate, imatinib, tamoxifen, and hydroxyurea.[14] |
Testing Procedures
The instrument used to perform a separation in chromatography is known as a chromatograph. For instance, in GC, the instrument is a gas chromatograph, and in LC, the instrument used is a liquid chromatograph. These instruments can respond to the amount of a compound that is exiting or eluting from the column as a function of the elution time or the volume of the mobile phase that has passed through the system.[1]
The following are the basic steps of chromatography.
1. Sample preparation: Sample preparation involves the extraction, purification, derivitization, and concentration of the analyte from the sample matrix. The preparation steps reduce any interferences during the assay.[16] Solid-phase extraction, liquid-liquid extraction, and protein precipitation are common. Filtration, centrifugation, and derivatization are followed after extraction for improved chromatographic separation.
2. Sample injection into the device: In modern HPLC systems, an autosampler is commonly used for automated and precise sample injection.[24] The autosampler can handle multiple samples, perform injections with minimal user intervention, and ensure consistent results.
3. Separation of analyte: Separation is based on the different chromatography types. In gas chromatography, the analytes are vaporized and carried by the heated column, where they are separated based on their volatility and interaction with the stationary phase. The analytes are separated in liquid chromatography based on their size, charge, and polarity. In HPLC, the separation is similar to LC but at a higher pressure, leading to faster and higher separation resolution.[1]
4. Elution of the separated substance: Elution is one of the important steps in chromatography that involves the collection of the compounds for analysis or further separation. The compounds elute from the columns at different times and concentrations. Extraction using solvents may be required.
5. Detection and interpretation: In column chromatography, as the solute migrates through the entire system and emerges from the column, the detectors continuously monitor the emerging mobile phase called the eluate and transmit signals in the form of voltage recorded on the chromatograph. Some of the detectors used in chromatography are mass spectrometry, refractive index, electrochemical, ultraviolet, flame ionization, electron capture, and fluorescence detectors.[25] Among these, ultraviolet detectors are the most commonly used in clinical laboratories.
Interfering Factors
Interfering factors during different stages of chromatography can affect the assay. Contaminants in the patient sample or during the sample preparation step might introduce unwanted contents and affect the detection. The presence of moisture or air bubbles in the samples might alter the retention time. Sample degradation after preparation and exposure to light, improper storage, handling, or extraction can alter the composition of analytes, affecting separation.[26] Overloading a GC machine with too much sample volume can cause the mixture to separate poorly. The column must be allowed to fully elute its contents before injecting the following sample.
Columns can be contaminated by precipitated proteins or by solvents. This will interfere with chromatogram results by causing distinct peaks or smearing to appear from previous elutions. Proper cleaning with solvents that dissolve the suspected contaminant is necessary to avoid this.[27] When making a column, the choice of adsorbent is key. The matrix of the column must be a loose, porous network permitting the entry and exit of macromolecules. Usually, silica is used, but this can vary.[28]
Changes in the pH of the buffer can affect the overall analysis. The pH changes after the sample injection when the sample and the mobile phase mix, mainly if the sample’s pH significantly differs from the mobile phase’s. Additionally, when air is exposed, a mobile phase can become acidic due to carbon dioxide absorption. Alterations in pH can affect the retention time (RT). Even a minor change of 0.1 pH units can change RT by as much as 10% with ionizable or ionic samples.[29] Buffer quality is also important for accurate results. Bacterial contamination in the buffer, precipitants, color change, and turbidity in the buffers can affect the run.
When similar drug molecules or metabolites are tested, the antibodies may bind to these related substances in the patient sample in a non-specific manner, causing erroneous results. The structural similarities among steroid hormones, including differences in keto groups, double bonds, and OH groups, can lead to interference in affinity chromatography analysis, particularly when hormones with high serum concentrations (eg, cortisol) interfere with hormones of lower concentration but similar structures (eg,11-deoxycortisol). Similarly, high concentrations of dihydroxy epiandrosterone sulfate can interfere with estradiol, 17-OH progesterone, and testosterone. Furthermore, steroid metabolites contribute to the spectrum of potential interfering molecules.[30]
Results, Reporting, and Critical Findings
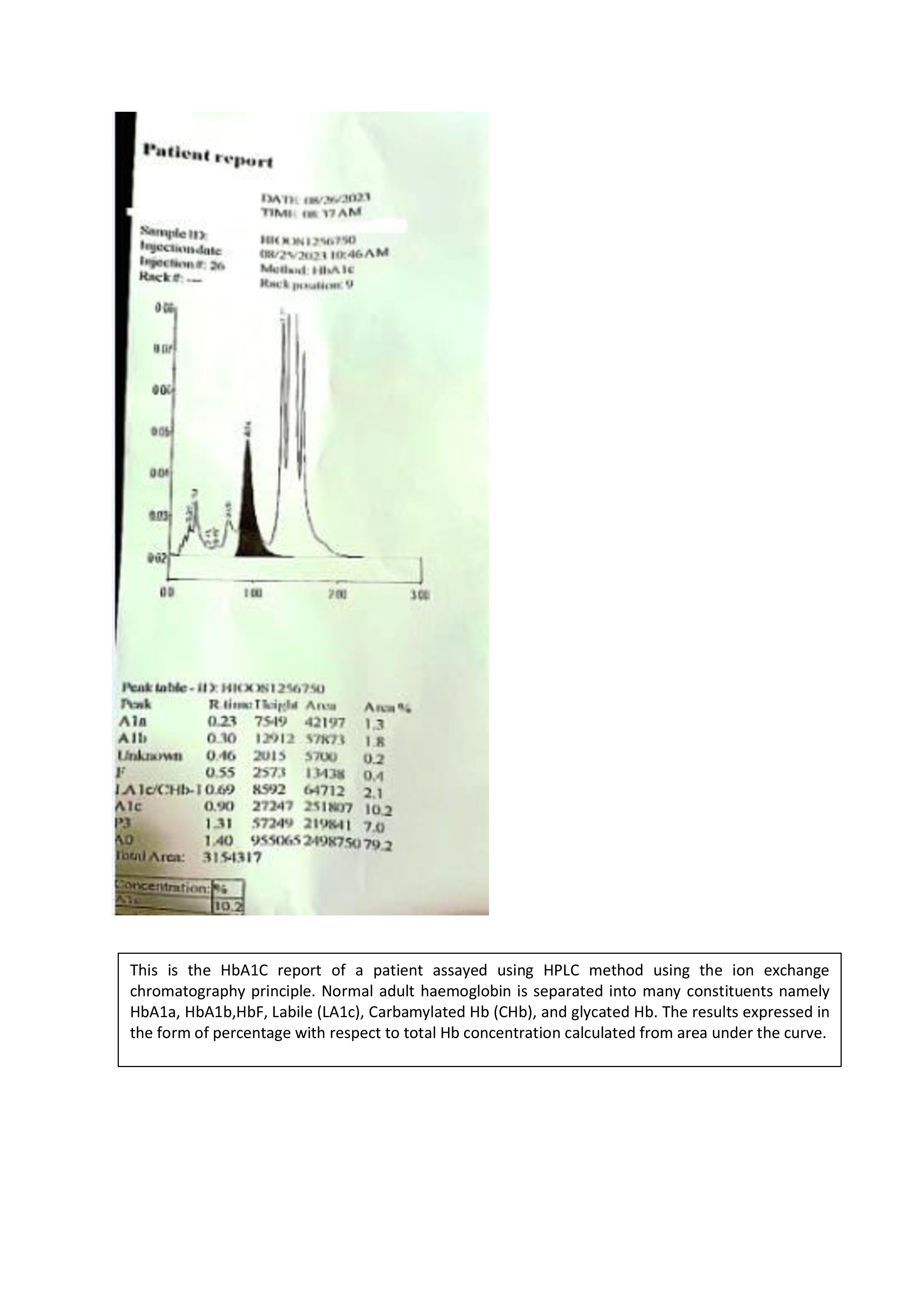
The chromatography results are interpreted from a chromatogram, a printout given by the detector. These results display the intensity of the detected signal (eg, absorbance, fluorescence, or mass spectrometry response) as a function of time or volume as the composites elute from the chromatographic column or stationary phase. Refer to Image 4.
The x-axis represents time (or volume) as the compounds elute from the column. The y-axis represents the signal intensity. The height of the peaks corresponds to the concentration of the separated compounds. The peaks represent the elution of the individual compounds. Each peak's shape, width, and area provide information about the compound's retention time, concentration, and purity.[31] Peak areas tend to provide a more precise means for measuring an analyte, whereas peak heights are easier to use if there is no complete resolution between the analyte and its neighboring peaks.[32]
Retention time (RT) is the interval between the sample injection and the maximum peak. Each molecule has a characteristic RT under standard chromatographic techniques. The time taken for the specific component to elute is a characteristic feature. The baseline indicates the background signal and helps determine the noise level.[33] Peak width represents the efficiency of separation; the lesser the width, the more efficient the separation. The area under a peak represents the quantity of a compound eluted. Good resolution is indicated by better separation between 2 adjacent peaks. Higher resolution indicates better separation and distinct peaks. Retention volume is calculated from RT multiplied by the mobile phase's flow rate.[34] The compound can be identified by running reference material or control under the same operating conditions. Matching the retention time and the component peak with a standard or control identifies the unknown component in the sample.
Clinical Significance
Chromatography helps accurately separate, identify, and quantify compounds in patient samples, enabling diagnostic, therapeutic, and research applications in healthcare.
Newborn Screening of Metabolic Disorders
Newborn screening using heel prick dried blood spots is a valuable tool for detecting various inborn errors of metabolism using chromatography. Thin layer chromatography, HPLC, LC-MS, and GC-MS-based techniques are commonly employed to assay various analytes. Commonly screened disorders include phenylketonuria, maple syrup urine disease, homocystinuria, tyrosinemia, methyl malonyl acidemia, homocystinuria, citrullinemia, tyrosinemia type I, carnitine deficiency, fatty acid oxidation disorders, and lysosomal storage disorders, etc. Most countries have implemented these screening programs, but the adoption and extent may vary from country to country.[35] This allows for the early detection of certain genetic and metabolic disorders, enabling timely intervention and treatment before disability.
Diagnosis of Hemoglobinopathies, Thalassemia, and Porphyrias
HPLC offers high sensitivity and specificity compared to electrophoresis for diagnosing hemoglobinopathies and thalassemias. Cation exchange HPLC is the method of choice that can separate nearly 45 different hemoglobin variants and accurately quantify normal hemoglobin.[22] It is also used for the newborn screening of sickle cell anemia. Denaturing HPLC is also used to detect alpha and beta globin chain mutations in thalassemia in chorionic villi samples. Analysis of porphyrins like 5-aminolevulinic acid (ALA) and porphobilinogen (PBG) in physiological liquids can be performed by LC/MS to diagnose the type of porphyrias.
Diabetes Mellitus and Other Metabolic Disorders
The hemoglobin A1c (HbA1c) is one of the gold standard tests for diagnosing diabetes mellitus and is measured using high-performance liquid chromatography (HPLC). Among the National Glycohemoglobin Standardization Program (NGSP) recommended methods for HbA1c, HPLC offers the least interference, high accuracy, and traceability. In the pediatric age group, it is used to quantify different steroids and aids in the diagnosis of congenital adrenal hyperplasia, hyperaldosteronism, Cushing's disease, and Addison's disease. LC-MS is more accurate than conventional immunoassay for diagnosing the above conditions.[36]
Cancers
Denaturing HPLC detects BRCA1 and BRCA2 gene mutations, guiding risk assessment and management for hereditary breast cancer.[37] Clinically important biogenic amines are bioactive endogenous compounds that play a significant physiological role. Serotonin, dopamine, noradrenaline, adrenaline and their metabolites, vanillylmandelic acid, and 5-hydroxy indoleacetic acid (5-HIAA) acid are some of the biogenic amines that are biomarkers for the diagnosis, prognosis, therapy, and follow-up of several neuroendocrine and cardiovascular diseases.[38]
Therapeutic Drug Monitoring
This involves quantitatively measuring the drug in biological fluids to manage drug therapy. It also involves assessing the efficacy and safety of a drug in the treatment. Mass spectrometric methods for many classes, such as immunosuppressive drugs, antivirals, antiepileptic drugs, chemotherapeutic drugs, antibiotics, antidepressants, and antipsychotics, are employed along with chromatography for drug monitoring.[14]
Genetic Analysis
Denaturing HPLC is a sensitive and comprehensive technology for studying gene expressions and detecting many gene mutations and polymorphisms. Single-stranded DNA is subjected to varying temperatures in a reversed-phase HPLC column, which will melt or denature at different temperatures based on the nucleotide sequence.[19] The main advantage is that up to 1.5 kb fragment can be analyzed in 5 minutes. Some applications are detecting mutations in the CFTR gene for cystic fibrosis, globin gene mutation in thalassemia, mutations related to phenylketonuria, and other metabolic disorders.
Toxicology
Liquid chromatography-mass spectrometry is used in the characterization of urine samples in patients with suspected benzodiazepine use disorder. Chromatography can be utilized to confirm the diagnosis of anabolic steroid use disorder through pulverization of nail clippings and urine samples at a level of pg/mL.[39] Thus, chromatography is an extremely sensitive technique that can potentially be used to investigate athletes' doping.[40]
Quality Control and Lab Safety
Quality control and laboratory safety are paramount when running chromatography for medical diagnostics.[41] Good laboratory practice and quality assurance systems ISO 9001 and ISO 17025 have established many rules to install and maintain a quality system in laboratory diagnostics.[42] The initial step in quality control involves using a validated method to meet analytical requirements. At the same time, routine analysis necessitates assessing performance based on relevant tests such as calibration injections and peak shape observations to ensure instrument functionality and proper separation.
Analytical accuracy is typically assessed through reference materials, internal reference samples, and participation in proficiency testing to ensure quality performance. Whether the sample is urine or blood, an internal standard or a substance similar to the substance of interest (analyte) must be set in the GC-MS. For example, if morphine is the analyte, nalorphine may be used as an internal standard.[43] Regular calibration of instruments, proper sample handling, and meticulous record-keeping are essential to maintain precision.
Chromatography involves the usage of potentially caustic skin irritants such as ethanol or NaOH. Care must be taken in advance to ensure minimal exposure to biological substances and irritation.[44]
A material safety data sheet (MSDS) is a catalog of chemicals and their potential risks of use.[45] At a minimum, healthcare facilities must have an MSDS that notes the physical risks, symptoms of exposure, safe handling instructions, suggested personal protective equipment, the names of all hazardous ingredients, characteristics of the reagents, routes of entry, exposure time limits, emergency and first aid instructions, the contact information of the manufacturers, as well as dates of MSDS preparation, per Occupational Safety and Health Administration (OSHA) guidelines.[46] When onboarding new healthcare professionals to a laboratory environment, they should be allocated adequate time to orient themselves to the chemicals they will utilize.
Whether it is a blood or urine sample, samples are exchanged between the hands of assistants and laboratory staff, which merits the utmost caution in preventing exposure and contamination.[44] Healthcare staff is encouraged to hold each other accountable for maintaining aseptic techniques to achieve clean samples.[47] Standard safety precautions should be followed when placing and securing the gas cylinder or mobile phase source used for gas chromatography (GC). If hydrogen is used as the carrier gas, extra precautions should be taken in training laboratory personnel in the handling and use of this combustible and potentially explosive gas. Proper ventilation facilities should be available in the work area to deal with carrier gas that has passed through the GC system.[48] All samples, reagents, and solutions used during a GC analysis should also be handled and stored using appropriate laboratory procedures.[49]
Enhancing Healthcare Team Outcomes
Healthcare team outcomes can be enhanced when using chromatography for medical diagnostics by ensuring effective interdisciplinary communication and proper training for all team members and implementing strict quality control measures. In the preanalytical phase, clinicians outside the laboratory, especially the medical assistants, residents, and nurses, must work with laboratory scientists to obtain appropriate samples, correctly handle the samples, record patient information, and maintain specimen integrity. Effective communication between healthcare professionals and the laboratory team ensures a clear understanding of sample requirements and any considerations. Reporting adverse events or issues during sample collection helps the laboratory assess the impact on results.
Technicians should be proficient in handling chromatography techniques and instrument operation in the laboratory. They should be backed by professionals with a background in inherited metabolic diseases and drug kinetics to evaluate the results, confirm the diagnosis, and advise on further action plans. All the lab professionals should be involved in the comprehensive plan of selecting the appropriate chromatographic methods, deciding on the sampling requirements, quality control, and reporting. International guidelines for assessing laboratory performance, such as ERNDIM and CDC, are available. Patient confidentiality, informed consent, and accurate reporting of results should be ensured throughout the process. By integrating these aspects, healthcare professionals can ensure that chromatography in medical diagnostics contributes effectively to patient-centered care, improved outcomes, and enhanced safety.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Coskun O. Separation techniques: Chromatography. Northern clinics of Istanbul. 2016:3(2):156-160. doi: 10.14744/nci.2016.32757. Epub 2016 Nov 11 [PubMed PMID: 28058406]
Shetty PP, Jacob P, Shenoy RP, Nalini K. Use of single solvent thin layer chromatography to diagnose different organic acidurias. The Indian journal of medical research. 2021 Jul:154(1):150-153. doi: 10.4103/ijmr.IJMR_1402_18. Epub [PubMed PMID: 34782541]
Neverova I, Van Eyk JE. Role of chromatographic techniques in proteomic analysis. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2005 Feb 5:815(1-2):51-63 [PubMed PMID: 15652798]
Sahu PK, Ramisetti NR, Cecchi T, Swain S, Patro CS, Panda J. An overview of experimental designs in HPLC method development and validation. Journal of pharmaceutical and biomedical analysis. 2018 Jan 5:147():590-611. doi: 10.1016/j.jpba.2017.05.006. Epub 2017 May 7 [PubMed PMID: 28579052]
Level 1 (high-level) evidenceSantiago M, Strobel S. Thin layer chromatography. Methods in enzymology. 2013:533():303-24. doi: 10.1016/B978-0-12-420067-8.00024-6. Epub [PubMed PMID: 24182936]
Escuder-Gilabert L, Bermúdez-Saldaña JM, Villanueva-Camañas RM, Medina-Hernández MJ, Sagrado S. Reliability of the retention factor estimations in liquid chromatography. Journal of chromatography. A. 2004 Apr 16:1033(2):247-55 [PubMed PMID: 15088745]
Jandera P, Hájek T. Dual-mode hydrophilic interaction normal phase and reversed phase liquid chromatography of polar compounds on a single column. Journal of separation science. 2020 Jan:43(1):70-86. doi: 10.1002/jssc.201900920. Epub 2019 Nov 4 [PubMed PMID: 31630481]
Meyers CL. Column chromatography. Current protocols in nucleic acid chemistry. 2001 May:Appendix 3():Appendix 3E. doi: 10.1002/0471142700.nca03es03. Epub [PubMed PMID: 18428814]
Buszewski B, Žuvela P, Sagandykova G, Walczak-Skierska J, Pomastowski P, David J, Wong MW. Mechanistic Chromatographic Column Characterization for the Analysis of Flavonoids Using Quantitative Structure-Retention Relationships Based on Density Functional Theory. International journal of molecular sciences. 2020 Mar 17:21(6):. doi: 10.3390/ijms21062053. Epub 2020 Mar 17 [PubMed PMID: 32192096]
Cummins PM, Rochfort KD, O'Connor BF. Ion-Exchange Chromatography: Basic Principles and Application. Methods in molecular biology (Clifton, N.J.). 2017:1485():209-223 [PubMed PMID: 27730555]
Urh M, Simpson D, Zhao K. Affinity chromatography: general methods. Methods in enzymology. 2009:463():417-38. doi: 10.1016/S0076-6879(09)63026-3. Epub [PubMed PMID: 19892186]
Level 3 (low-level) evidenceDorman FL, Whiting JJ, Cochran JW, Gardea-Torresdey J. Gas chromatography. Analytical chemistry. 2010 Jun 15:82(12):4775-85. doi: 10.1021/ac101156h. Epub [PubMed PMID: 20504041]
Level 3 (low-level) evidenceEiceman GA, Gardea-Torresdey J, Overton E, Carney K, Dorman F. Gas chromatography. Analytical chemistry. 2004 Jun 15:76(12):3387-94 [PubMed PMID: 15193115]
Tuzimski T, Petruczynik A. Review of Chromatographic Methods Coupled with Modern Detection Techniques Applied in the Therapeutic Drugs Monitoring (TDM). Molecules (Basel, Switzerland). 2020 Sep 3:25(17):. doi: 10.3390/molecules25174026. Epub 2020 Sep 3 [PubMed PMID: 32899296]
Yin P, Zhou L, Zhao X, Xu G. Sample collection and preparation of biofluids and extracts for liquid chromatography-mass spectrometry. Methods in molecular biology (Clifton, N.J.). 2015:1277():51-9. doi: 10.1007/978-1-4939-2377-9_5. Epub [PubMed PMID: 25677146]
Emwas AH, Al-Talla ZA, Kharbatia NM. Sample collection and preparation of biofluids and extracts for gas chromatography-mass spectrometry. Methods in molecular biology (Clifton, N.J.). 2015:1277():75-90. doi: 10.1007/978-1-4939-2377-9_7. Epub [PubMed PMID: 25677148]
Level 3 (low-level) evidencePitt JJ. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. The Clinical biochemist. Reviews. 2009 Feb:30(1):19-34 [PubMed PMID: 19224008]
George RS, Moat SJ. Effect of Dried Blood Spot Quality on Newborn Screening Analyte Concentrations and Recommendations for Minimum Acceptance Criteria for Sample Analysis. Clinical chemistry. 2016 Mar:62(3):466-75. doi: 10.1373/clinchem.2015.247668. Epub 2015 Dec 8 [PubMed PMID: 26647314]
Level 2 (mid-level) evidenceFackenthal DL, Chen PX, Howe T, Das S. Denaturing high-performance liquid chromatography for mutation detection and genotyping. Methods in molecular biology (Clifton, N.J.). 2013:1015():25-54. doi: 10.1007/978-1-62703-435-7_2. Epub [PubMed PMID: 23824847]
Woontner M, Goodman SI. Chromatographic analysis of amino and organic acids in physiological fluids to detect inborn errors of metabolism. Current protocols in human genetics. 2006 Nov:Chapter 17():Unit 17.2. doi: 10.1002/0471142905.hg1702s51. Epub [PubMed PMID: 18428392]
Grebe SK, Singh RJ. LC-MS/MS in the Clinical Laboratory - Where to From Here? The Clinical biochemist. Reviews. 2011 Feb:32(1):5-31 [PubMed PMID: 21451775]
Baig MA, Swamy KB, Baksh AD, Bahashwan A, Moshrif Y, Al Sawat A, Al Mutairi N, Alharbi N. Evaluation of role of HPLC (Merits & Pitfalls), in the diagnosis of various hemoglobinopathies & thalassemic syndromes. Indian journal of pathology & microbiology. 2021 Jul-Sep:64(3):518-523. doi: 10.4103/IJPM.IJPM_709_20. Epub [PubMed PMID: 34341263]
Toka Özer T, Yula E, Doğan M, Baskın H. Determination of antibiotic resistance and high-performance liquid chromatography profiles for Mycobacterium species. Journal of clinical laboratory analysis. 2018 Sep:32(7):e22459. doi: 10.1002/jcla.22459. Epub 2018 Apr 27 [PubMed PMID: 29701251]
Mehl A, Schwack W, Morlock GE. On-surface autosampling for liquid chromatography-mass spectrometry. Journal of chromatography. A. 2021 Aug 16:1651():462334. doi: 10.1016/j.chroma.2021.462334. Epub 2021 Jun 8 [PubMed PMID: 34153734]
Nikolin B, Imamović B, Medanhodzić-Vuk S, Sober M. High perfomance liquid chromatography in pharmaceutical analyses. Bosnian journal of basic medical sciences. 2004 May:4(2):5-9 [PubMed PMID: 15629016]
Wilson ID. High-performance liquid chromatography-mass spectrometry (HPLC-MS)-based drug metabolite profiling. Methods in molecular biology (Clifton, N.J.). 2011:708():173-90. doi: 10.1007/978-1-61737-985-7_10. Epub [PubMed PMID: 21207290]
Wang L, Dembecki J, Jaffe NE, O'Mara BW, Cai H, Sparks CN, Zhang J, Laino SG, Russell RJ, Wang M. A safe, effective, and facility compatible cleaning in place procedure for affinity resin in large-scale monoclonal antibody purification. Journal of chromatography. A. 2013 Sep 20:1308():86-95. doi: 10.1016/j.chroma.2013.07.096. Epub 2013 Aug 1 [PubMed PMID: 23953712]
Level 3 (low-level) evidenceCuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proceedings of the National Academy of Sciences of the United States of America. 1968 Oct:61(2):636-43 [PubMed PMID: 4971842]
Kaliszan R, Wiczling P, Markuszewski MJ. pH gradient high-performance liquid chromatography: theory and applications. Journal of chromatography. A. 2004 Dec 10:1060(1-2):165-75 [PubMed PMID: 15628159]
French D. Clinical utility of laboratory developed mass spectrometry assays for steroid hormone testing. Journal of mass spectrometry and advances in the clinical lab. 2023 Apr:28():13-19. doi: 10.1016/j.jmsacl.2023.01.006. Epub 2023 Jan 28 [PubMed PMID: 36756146]
Level 3 (low-level) evidencePápai Z, Pap TL. Analysis of peak asymmetry in chromatography. Journal of chromatography. A. 2002 Apr 12:953(1-2):31-8 [PubMed PMID: 12058945]
Li J. Comparison of the capability of peak functions in describing real chromatographic peaks. Journal of chromatography. A. 2002 Apr 5:952(1-2):63-70 [PubMed PMID: 12064546]
Katajamaa M, Oresic M. Processing methods for differential analysis of LC/MS profile data. BMC bioinformatics. 2005 Jul 18:6():179 [PubMed PMID: 16026613]
Beyaza A, Fana W, Carr PW, Schellinger AP. Instrument parameters controlling retention precision in gradient elution reversed-phase liquid. Journal of chromatography. A. 2014 Dec 5:1371():90-105 [PubMed PMID: 25459648]
Powell CM. What is Newborn Screening? North Carolina medical journal. 2019 Jan-Feb:80(1):32-36. doi: 10.18043/ncm.80.1.32. Epub [PubMed PMID: 30622202]
Kulle AE, Welzel M, Holterhus PM, Riepe FG. Principles and clinical applications of liquid chromatography - tandem mass spectrometry for the determination of adrenal and gonadal steroid hormones. Journal of endocrinological investigation. 2011 Oct:34(9):702-8. doi: 10.3275/7843. Epub 2011 Jul 5 [PubMed PMID: 21738000]
Wagner T, Stoppa-Lyonnet D, Fleischmann E, Muhr D, Pagès S, Sandberg T, Caux V, Moeslinger R, Langbauer G, Borg A, Oefner P. Denaturing high-performance liquid chromatography detects reliably BRCA1 and BRCA2 mutations. Genomics. 1999 Dec 15:62(3):369-76 [PubMed PMID: 10644434]
Plenis A, Olędzka I, Kowalski P, Miękus N, Bączek T. Recent Trends in the Quantification of Biogenic Amines in Biofluids as Biomarkers of Various Disorders: A Review. Journal of clinical medicine. 2019 May 9:8(5):. doi: 10.3390/jcm8050640. Epub 2019 May 9 [PubMed PMID: 31075927]
Kintz P, Gheddar L, Raul JS. Testing for anabolic steroids in human nail clippings. Journal of forensic sciences. 2021 Jul:66(4):1577-1582. doi: 10.1111/1556-4029.14729. Epub 2021 Apr 22 [PubMed PMID: 33885144]
Hand RA, Bassindale T, Turner N, Morgan G. Application of comprehensive 2D chromatography in the anti-doping field: Sample identification and quantification. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2021 Jul 15:1178():122584. doi: 10.1016/j.jchromb.2021.122584. Epub 2021 Feb 21 [PubMed PMID: 34224963]
Westgard JO. Perspectives on quality control, risk management, and analytical quality management. Clinics in laboratory medicine. 2013 Mar:33(1):1-14. doi: 10.1016/j.cll.2012.10.003. Epub 2012 Dec 20 [PubMed PMID: 23331725]
Level 2 (mid-level) evidenceWadhwa V, Rai S, Thukral T, Chopra M. Laboratory quality management system: road to accreditation and beyond. Indian journal of medical microbiology. 2012 Apr-Jun:30(2):131-40. doi: 10.4103/0255-0857.96647. Epub [PubMed PMID: 22664426]
Level 2 (mid-level) evidenceMeatherall R. GC-MS confirmation of codeine, morphine, 6-acetylmorphine, hydrocodone, hydromorphone, oxycodone, and oxymorphone in urine. Journal of analytical toxicology. 1999 May-Jun:23(3):177-86 [PubMed PMID: 10369327]
Borgatta L, Fisher M, Robbins N. Hand protection and protection from hands: hand-washing, germicides and gloves. Women & health. 1989:15(4):77-92 [PubMed PMID: 2515660]
Allen LV Jr. Quality Control: (Material) Safety Data Sheets. International journal of pharmaceutical compounding. 2017 Mar-Apr:21(2):118-124 [PubMed PMID: 28346207]
Level 2 (mid-level) evidenceBernstein JA. Material safety data sheets: are they reliable in identifying human hazards? The Journal of allergy and clinical immunology. 2002 Jul:110(1):35-8 [PubMed PMID: 12110815]
Preston RM. Aseptic technique: evidence-based approach for patient safety. British journal of nursing (Mark Allen Publishing). 2005 May 26-Jun 8:14(10):540-2, 544-6 [PubMed PMID: 15928570]
Muñoz-Guerra JA, Prado P, García-Tenorio SV. Use of hydrogen as a carrier gas for the analysis of steroids with anabolic activity by gas chromatography-mass spectrometry. Journal of chromatography. A. 2011 Oct 14:1218(41):7365-70. doi: 10.1016/j.chroma.2011.08.009. Epub 2011 Aug 24 [PubMed PMID: 21907993]
Meisenhelder J, Bursik S, Lunn G, Strober W. Laboratory safety. Current protocols in human genetics. 2008 Apr:Appendix 2():Appendix 2A. doi: 10.1002/0471142905.hga02as57. Epub [PubMed PMID: 18428418]