Introduction
Thin basement membrane nephropathy (TBMN), also known as thin basement membrane disease, is one of the most prevalent yet underrecognized causes of glomerular bleeding in both children and adults.[1] TBMN is a genetic and often familial disorder caused by mutations in the genes encoding various chains of type IV collagen, which is the primary component of the glomerular basement membrane (GBM).[2]
TBMN was previously known as benign familial hematuria, but this term is no longer favored due to an improved understanding of the genetic etiologies and variations in the clinical presentation of the condition.[3][4] Other previously used nomenclature no longer favored include "benign familial hematuria," "benign persistent hematuria," and "benign essential hematuria."
TBMN is characterized by diffuse thinning of the GBM on renal biopsy and clinically presents as isolated microscopic hematuria in most cases.[5][6] However, other findings, such as proteinuria, hypertension, and varying degrees of kidney function impairment, may also be present.[3][7][8] TBMN shares overlapping features with Alport syndrome, as both conditions are caused by mutations in type IV collagen genes.[3][9] However, unlike Alport syndrome, particularly its most common X-linked variant, TBMN does not have extrarenal features. Please see StatPearls' companion resource, "Alport Syndrome," for further information.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Mutations in COL4A3 and COL4A4 cause TBMN. These genes are located on chromosome 2 at 2q35-37 and encode for 2 different chains of type IV collagen.[2][3][10] The mutations are heterozygous, involving a single allele, and can include various types such as splice variants, missense mutations, or frameshift mutations.[2]
One-half to two-thirds of patients with TBMN have an identifiable family history, typically inherited in an autosomal dominant pattern.[3][9] Patients without an identifiable family history may have a de novo mutation. The disease can also exhibit incomplete penetrance, which might explain a negative family history in some cases.[1][10]
Epidemiology
TBMN is a common cause of glomerular hematuria in both children and adults, with age at presentation varying widely. The median age at diagnosis in adults is 37, while in children, it is 7.[1] Most case series indicate that TBMN is more common in females and shows no predilection for any particular ethnicity.[1][11]
The exact prevalence of TBMN is difficult to determine due to its variable clinical presentation, with many cases likely going unrecognized. However, estimates from one biopsy series of kidneys that were transplanted indicated the incidence of thin basement membrane morphology in about 5% to 9% of the general population.[12] More conservative estimates place the incidence of TBMN between 1% and 2%.[1][10][13]
Pathophysiology
The GBM is the acellular middle layer of the glomerular filtration barrier, situated between the 2 cellular layers—the endothelial cells on one side and the epithelial foot processes on the other.[14][15] The GBM comprises extracellular matrix proteins, with type IV collagen being the most abundant.[14][15]
Type IV collagen in the GBM of adults is composed of a complex assembly of 3 chains—α-3, α-4, and α-5, which form a triple helix.[2][16] Each α-chain is encoded by a distinct gene—the α-5 chain is encoded by COL4A5 on the X chromosome, while the α-3 and α-4 chains are encoded by COL4A3 and COL4A4 on chromosome 2, respectively.[2][16] The α-1 and α-2 chains of type IV collagen are typically present only during embryonic development.[2][16]
Heterozygous mutations in COL4A3 and COL4A4 are the most commonly reported genetic abnormalities in TBMN.[1][3][9] These mutations lead to abnormal folding and assembly of the collagen chains, resulting in their early degradation. This degradation interferes with the normal developmental transition from the α-1 and α-2 chains to the α-3, α-4, and α-5 chains.[2] The embryonic forms of type IV collagen chains are more susceptible to degradation, which causes structural changes and thinning of the GBM.[2]
The thin GBM is fragile and sensitive to disruption by proteases, causing transient, localized ruptures of the membrane. These protease-induced ruptures of the glomerular filtration barrier allow red blood cells (RBCs) to escape from the capillary space into the Bowman space of the glomerulus. Once in the Bowman space, the RBCs appear in the urine as hematuria.[17][18]
A similar pathogenic process is observed in Alport syndrome, which is most commonly caused by mutations in COL4A5 on the X chromosome.[3] However, Alport syndrome can also result from mutations in COL4A3 and COL4A4, inherited in either autosomal dominant or autosomal recessive patterns.[3][10][19] Please see StatPearls' companion resource, "Alport Syndrome," for further information. Therefore, distinguishing between TBMN and Alport syndrome can be challenging due to significant overlap in clinical presentation. Both conditions are now considered part of the spectrum of type IV collagen disorders.[3][20]
Histopathology
The pathognomonic finding of TBMN is uniform thinning of the GBM, which is revealed by electron microscopy of a renal biopsy.[4][5][6]
Light Microscopy
In cases of TBMN, light microscopy typically does not reveal any specific findings. The glomeruli may appear normal or show mild mesangial cell proliferation or mild mesangial matrix expansion. In rare instances, slight attenuation of the GBM, indicative of thinning, can be observed using Jones methenamine silver or periodic acid-Schiff staining (see Image. Light Microscopy of Renal Parenchyma in Thin Basement Membrane Nephropathy).[4]
Global or focal segmental glomerulosclerosis is not typically associated with TBMN. If present, it may suggest additional pathologies or nonspecific manifestations of kidney disease progression. Similarly, interstitial fibrosis and tubular atrophy are nonspecific indicators of advancing kidney dysfunction.[1][4][5]
Immunofluorescence
Immunofluorescence is usually negative in TBMN. However, nonspecific staining of the mesangium for immunoglobulin M (IgM) and C3 can occasionally be observed, and, more rarely, staining for IgG or IgA may be positive.[1][4][6]
Unlike X-linked Alport syndrome, immunohistochemistry for collagen staining is not always helpful in diagnosing TBMN. In X-linked Alport syndrome, biopsies consistently lack staining for the α-5 chain. However, a normal staining pattern may be observed depending on the specific mutations in the autosomal forms of type IV collagen disorders involving the α-3 and α-4 chains.[5]
Electron Microscopy
The pathognomonic feature of TBMN is the uniform and extensive (>50%) thinning of the GBM, which can be visualized using electron microscopy (see Image. Electron Microscopy of Renal Parenchyma in Thin Basement Membrane Nephropathy).[1][21] Importantly, unlike in Alport syndrome, the electron microscopy features of TBMN do not show progressive irregularity in the thickness of the GBM, nor do they exhibit the splitting and layering characteristic of Alport syndrome.[5][22][23] Please see StatPearls' companion resource, "Alport Syndrome," for further information.
The thickness of the GBM normally varies with age. At birth, the GBM is approximately 150 nm thick and continues to increase throughout childhood. Around the age of 11, the thickness of the GBM approaches adult measurements. The mean normal GBM thickness in adult males is 370 ± 50 nm, while in adult females, it is 320 ± 50 nm.[4][21] The World Health Organization (WHO) has proposed the most commonly used threshold for determining a thin basement membrane, which is 250 nm for adults and 180 nm for children between the ages of 2 and 11.[4][21]
History and Physical
Most cases of TBMN initially present with isolated, asymptomatic microscopic hematuria that is often identified incidentally. The medical history and physical examination of these patients are typically otherwise unremarkable, and a family history of hematuria may or may not be present.
Gross hematuria, or "cola-colored" urine, which is frequently seen in other glomerulonephropathies, is less common in TBMN, occurring in only 5% to 22% of cases, often following infection or exercise. When gross hematuria does occur, it is more common in children with TBMN.[1][4] Proteinuria is uncommon but becomes more likely with advancing age. When present, proteinuria is typically subnephrotic, defined as less than 3000 mg of protein per 24 hours.[1][4] The primary extrarenal manifestation of TBMN is hypertension.[1][4][21]
The medical history and physical examination should concentrate on identifying any potential or obvious causes of microscopic hematuria. The medical history must focus on excluding other common causes, such as urological conditions, nephrolithiasis, and IgA nephropathy. A comprehensive review of systems is essential to rule out systemic manifestations of autoimmune diseases, such as systemic lupus erythematosus, which can also present with microscopic hematuria.
Most patients with TBMN will report a family history of hematuria without progressing to end-stage renal disease (ESRD). A family history of hematuria combined with a negative urological workup strongly suggests TBMN. Additionally, a specific history of hearing loss and ocular lesions can help distinguish TBMN from Alport syndrome.[3] Please see StatPearls' companion resource, "Alport Syndrome," for further information.
The family medical history is crucial in evaluating TBMN as it helps identify potential familial cases, differentiate TBMN from X-linked Alport syndrome, and establish prognosis.[3] Key questions should address instances of microscopic or gross hematuria, hearing or vision loss, and, most importantly, any history of progressive kidney disease or dialysis dependence in other family members. The physical examination should focus on measuring blood pressure, assessing for edema indicative of proteinuria, and conducting a careful evaluation of vision and hearing. Additionally, it should check for other systemic signs, such as rash or joint involvement, which could suggest alternative causes of glomerular hematuria.
Notably, geographic clusters of TBMN patients can exhibit variable clinical manifestations. For instance, a cohort in Cyprus was found to have a progressive variant of TBMN, with 14% of 127 patients developing ESRD. These geographic clusters should be considered when evaluating and managing TBMN.[4][21]
Evaluation
Initial Diagnostic Evaluation
The initial diagnostic evaluation for suspected TBMN includes urinalysis, microscopic examination of urinary sediment, and laboratory assessment of kidney function.
In patients with TBMN, dipstick urinalysis typically reveals microscopic hematuria without proteinuria.[1][4][21] If proteinuria is detected on dipstick testing, it must be quantified. Urine protein quantification is most commonly performed using a spot urine sample to measure the urine protein-to-creatinine ratio. A ratio of less than 0.2 is considered normal, while a ratio of 0.3 or higher is abnormal. Although a 24-hour urine protein quantification is the gold standard for assessing proteinuria, it is less practical and more cumbersome.[24] Please see StatPearls' companion resource, "Proteinuria," for more information.
Microscopic examination of urine sediment can help identify dysmorphic red blood cells (RBCs) or RBC casts, which are indicative of glomerular hematuria.[25] Dysmorphic RBCs are thought to result from passage through gaps in the GBM, while RBC casts form when RBCs enter the tubular lumen and become trapped in the tubular protein matrix.[26] However, the absence of dysmorphic RBCs or RBC casts does not exclude glomerular hematuria.
Kidney function is assessed through measurements of serum creatinine and blood urea nitrogen. Serum albumin levels should also be measured to rule out nephrotic syndrome if proteinuria is present.
Renal Biopsy
Beyond the initial investigations, the primary decision to evaluate TBMN is whether to proceed with a kidney biopsy. When the presentation and course of the disease are benign, characterized by isolated microscopic hematuria without proteinuria, hypertension, or laboratory evidence of kidney dysfunction, and there is no family history of progressive kidney dysfunction or ESRD, a biopsy is typically not recommended. In such cases, the diagnosis is inferred clinically.[1][4] However, a kidney biopsy can be considered in the following circumstances:
- The presence of additional features, such as laboratory evidence of kidney injury, proteinuria, or hypertension, particularly in individuals aged 50 or younger.
- The presence of extrarenal manifestations such as hearing and ocular defects or family history suggestive of kidney disease such as Alport syndrome, particularly X-linked Alport syndrome.
- If the individual is considering a kidney donation.
Genetic Testing
Genetic testing is generally not required to diagnose TBMN.[9] The genes involved in the pathogenesis of TBMN are COL4A3 and COL4A4 on chromosome 2.[1][2] The rate of detection of mutations is low in TBMN in patients with only microscopic hematuria as the clinical manifestation.[1] The likelihood of detecting a pathogenic variant is higher if there is kidney function impairment or an associated family history.[27]
In cases where biopsy-proven thin GBM is present along with kidney dysfunction and family history suggestive of X-linked inheritance, screening for COL4A5 mutations to rule out X-linked Alport syndrome becomes more pertinent.[9][27]
Treatment / Management
A specific, evidence-based management strategy does not exist for TBMN.[1][9] Most patients with isolated microscopic hematuria have a benign clinical course and do not require any specific therapy.[4]
Once diagnosed, management involves regular clinical monitoring for the development of hypertension, including routine blood pressure measurements. Laboratory studies should include serum creatinine and blood urea nitrogen tests, as well as urinalyses with urine protein-to-creatinine ratio assessments to monitor for renal impairment and proteinuria. These routine evaluations can be conducted by the primary care practitioner, with referrals made as necessary.
If proteinuria or hypertension is noted, management strategies for TBMN include:
- Blood pressure management, with a goal of less than 130/80 mm Hg, which is in line with the 2017 American College of Cardiology/American Heart Association Guidelines for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.[28]
- Salt intake minimization, with a target sodium intake of less than 2000 mg daily.
- Moderate-intensity physical activity for a total duration of at least 150 min/week.
- Pharmacological therapy, involving the use of the maximum tolerated doses of renin-angiotensin-system inhibitors, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs).[1][9][21] (A1)
Immunosuppression is not indicated in the management of TBMN.
Once treatment for hypertension or proteinuria is initiated, or if kidney dysfunction is noted, more frequent monitoring and management of associated complications—such as progression of chronic kidney disease (CKD), anemia due to CKD, CKD-related mineral and bone disorder, and ESRD—are necessary. The frequency of monitoring depends on blood pressure control, the degree of proteinuria, and the level of kidney dysfunction or stage of CKD, which is best managed under the guidance of a nephrologist.
Genetic Counseling
Counseling for patients with TBMN and their families is recommended. Counseling should include the diagnosis, the likely inherited nature of the disease, and the need for monitoring for microscopic hematuria, along with proteinuria, hypertension, and kidney function impairment.[9][27]
Renal Transplant Donation
Historically, potential renal transplant donors with TBMN were considered acceptable, as some recipients with TBMN have maintained kidney function without significant issues or complications.[29] However, with an increased understanding of the overlap between TBMN and autosomal forms of Alport syndrome and recognizing the higher risk of progressive kidney disease, it is now recommended that individuals with a heterozygous mutation in COL4A3 or COL4A4 should not be considered kidney donors.[27]
Differential Diagnosis
Alport Syndrome
The condition most closely related to TBMN that requires differentiation is Alport syndrome. Differentiating between these 2 conditions is crucial due to differences in prognosis.[3][20][22][23][30] Please see StatPearls' companion resource, "Alport Syndrome," for further information.
The salient differences between the 2 conditions include:
- Mutations and inheritance: The most well-recognized form of Alport syndrome is inherited in an X-linked fashion and is caused by mutations in COL4A5. However, Alport syndrome can also follow autosomal recessive or autosomal dominant inheritance patterns due to homozygous or heterozygous mutations in COL4A3 and COL4A4. In contrast, TBMN is most commonly inherited in an autosomal dominant pattern, typically due to heterozygous mutations in COL4A3 or COL4A4.
- Histopathology: The characteristic finding of TBMN on renal biopsy is uniform thinning of the GBM, observed in more than 50% of the membrane on electron microscopy.[17][31][32] In contrast, the pathognomonic biopsy feature of Alport syndrome includes progressive irregularity and uneven thickness of the GBM, with electron microscopy revealing splitting, layering, and small, dense "breadcrumb" inclusions between the layers.[33][34][35] A combination of electron microscopy and immunohistology for α-3 and α-5 collagen subtypes can facilitate a definitive diagnosis from a renal biopsy, even in challenging cases.[23]
- Presentation: X-linked Alport syndrome is more commonly seen in males and typically presents at a young age with hearing loss, vision changes, and a high risk of progression to ESRD. Female carriers of X-linked Alport syndrome generally have a milder clinical presentation and a lower risk of progression to ESRD. The autosomal dominant forms of Alport syndrome and TBMN present similarly, usually without extra-renal features, and the risk of progression to ESRD depends on the presence of proteinuria and a positive family history of renal disease.
- Management: Neither Alport syndrome nor TBMN are curable. The only approved pharmacological therapies for either condition are ACE inhibitors or ARBs when proteinuria is present. There is no role for immunosuppression in either condition. Novel agents currently under evaluation for Alport syndrome but not yet approved include bardoxolone methyl, a semi-synthetic triterpenoid molecule that activates the transcription factor nuclear factor erythroid 2-related factor 2 (CARDINAL trial); sparsentan, a dual endothelin type A receptor and angiotensin II type 1 receptor antagonist (EPPIK trial); and atrasentan, an endothelin type-A receptor antagonist (AFFINITY trial).[36] Currently, no agents are being studied for the treatment of TBMN.
- Prognosis: The X-linked form of Alport syndrome carries a poor prognosis, with approximately 50% of affected males reaching ESRD by age 30 and about 90% by age 40. Females with X-linked Alport syndrome have a better prognosis, with a 25% lifetime risk of progression. Autosomal recessive forms of Alport syndrome also have a poor prognosis, with most patients reaching ESRD by age 30. In contrast, autosomal dominant forms of Alport syndrome and TBMN nephropathy share a similar prognosis, with less than a 1% estimated risk of progression to ESRD, unless proteinuria and significant family history are present, which can increase the risk for progression to ESRD to up to 20%.
In summary, due to their shared molecular pathophysiological basis and overlapping clinical presentation, it has been proposed that both Alport syndrome and TBMN be classified as forms of type IV collagen disorders.[3]
Other Conditions
Additional conditions that should be considered in the differential diagnosis of TBMN include:
- IgA nephropathy (Please see StatPearls' companion resource, "IgA Nephropathy (Berger Disease)," for further information.)
- Postinfectious glomerulonephritis (Please see StatPearls' companion resources, "Poststreptococcal Glomerulonephritis" and "Glomerulonephritis," for further information.)
- Lupus nephritis (Please see StatPearls' companion resource, "Lupus Nephritis," for further information.)
- Medullary sponge kidney (Please see StatPearls' companion resource, "Medullary Sponge Kidney," for further information.)
Urological conditions, including malignancies and nephrolithiasis, should always be considered in the differential diagnosis of isolated microscopic hematuria. For patients aged 50 or younger with asymptomatic microscopic hematuria and no identified urological cause, a nephrology consultation is advisable. This is particularly important if the patient shows persistent proteinuria, abnormal serum creatinine, red cell casts or dysmorphic RBCs on urinalysis, worsening hypertension, increasing edema, or has a family history of kidney disease.
Prognosis
The long-term prognosis for most patients with TBMN is generally favorable.[1][21] The condition usually does not lead to the progression of kidney disease or ESRD. However, as the genetic basis of TBMN and its overlap with other type IV collagen disorders involving mutations in COL4A3 and COL4A4—such as Alport syndrome, variants of IgA nephropathy, and focal segmental glomerulosclerosis—have become clearer, TBMN is no longer considered a universally benign condition requiring no monitoring.[3][20] Therefore, the term "benign familial hematuria" is no longer recommended for this condition, and "thin basement membrane disease/nephropathy" or "TBMN" are preferred.[3]
The estimated risk of progression to ESRD in individuals with heterozygous mutations in COL4A3 or COL4A4, inherited in an autosomal dominant fashion as seen in TBMN, is less than 1% in the absence of associated risk factors.[3] However, in those with risk factors such as proteinuria, evidence of renal dysfunction, specific biopsy features (eg, focal segmental glomerulosclerosis, GBM thickening, and lamellation), and a family history of kidney disease progression, the risk of ESRD can increase up to 20%.[3]
Complications
The complications of TBMN include hypertension, proteinuria, and ESRD, although these are rare. Equally important are the psychosocial impacts, such as anxiety related to the diagnosis, especially in young individuals, and the potential issues arising from genetic testing without appropriate counseling. Additionally, complications related to investigations, such as kidney biopsy, should be considered.
Mitigating these complications involves obtaining a comprehensive medical history, including family history, providing genetic counseling, and carefully selecting patients for genetic testing and biopsy.
Deterrence and Patient Education
TBMN is a genetic condition; therefore, preventive measures are absent for its management. Patient education must include information regarding the genetic basis of the condition and its autosomal dominant inheritance while also acknowledging the rare occurrence of de novo cases.
Education should also emphasize the importance of monitoring for hypertension, proteinuria, and worsening kidney function. Genetic counseling is crucial for TBMN management. While it is important to reassure patients about the generally benign nature of the disease to avoid unnecessary anxiety, it is equally vital to inform them about the risk factors for progression to ESRD and the need for possible dialysis.
Pearls and Other Issues
Patients with unexplained persistent microscopic hematuria after a urological evaluation should be referred to a nephrologist if they also exhibit signs of possible renal disease, such as abnormal serum creatinine, red cell casts, dysmorphic RBCs on urinalysis, proteinuria, progressive hypertension, edema, or a family history of kidney disease. The absence of dysmorphic RBCs or RBC casts on urinalysis does not rule out a glomerular source for hematuria, even if the urological workup, including computed tomography (CT) urogram and cystoscopy, is completely negative.
Although TBMN is usually benign and does not require treatment, management with ACE inhibitors or ARBs is recommended if it is associated with features such as proteinuria or hypertension. Differentiating TBMN from Alport syndrome, which involves progressive renal function decline, is crucial. This differentiation may require a renal biopsy with electron microscopy.[22][23][30]
Enhancing Healthcare Team Outcomes
TBMN is a genetic condition affecting the kidneys without extra-renal manifestations. Nephrologists, both adult and pediatric, play a crucial role in managing TBMN, as it can present in children and young adults. Patients with microscopic hematuria are often first evaluated by urologists to rule out common urological causes. Coordination between urologists and nephrologists is essential for the ongoing evaluation of patients, especially those aged 50 or younger with asymptomatic microscopic hematuria and negative urological workups. However, comprehensive treatment of patients with TBMN requires a multidisciplinary team of interprofessional healthcare practitioners. This team includes primary care providers for initial evaluation of microscopic hematuria and ongoing monitoring of risk factors such as proteinuria, hypertension, or kidney dysfunction; geneticists for appropriate counseling and testing of the patient and their family members; and pathologists for accurate identification of histopathology.
If progression to ESRD is anticipated, patients require additional education and preparation for kidney replacement therapy. During this phase, interprofessional team members, including nurses, dietitians, and social workers, are crucial in managing TBMN. For individuals with TBMN considering kidney donation, the transplant nephrologist and the multidisciplinary living donation team must provide counseling about the risks and ongoing monitoring. Therefore, collaboration among the interprofessional healthcare team is crucial for managing TBMN. Although TBMN is a common cause of glomerular hematuria that may be mistakenly considered entirely benign, it requires ongoing monitoring to identify patients at risk for progression to ESRD.[3][20]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
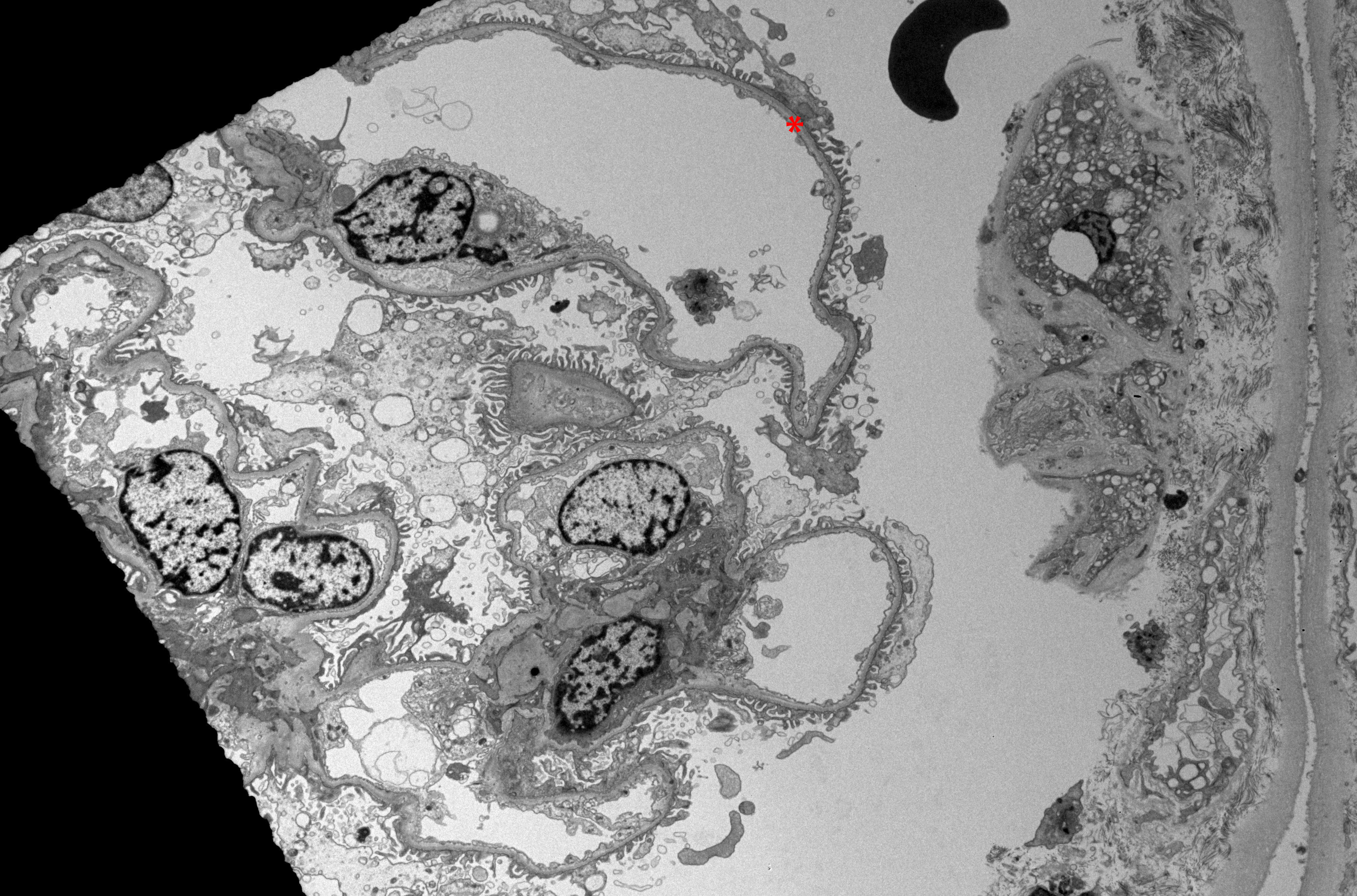
Electron Microscopy of Renal Parenchyma in Thin Basement Membrane Nephropathy. Electron microscopy examination of renal parenchyma in thin basement membrane nephropathy reveals a uniformly thin glomerular basement membrane in a renal biopsy from a patient with this condition.
Contributed by D Zhang, MD
References
Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY. Thin basement membrane nephropathy. Kidney international. 2003 Oct:64(4):1169-78 [PubMed PMID: 12969134]
Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. The New England journal of medicine. 2003 Jun 19:348(25):2543-56 [PubMed PMID: 12815141]
Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, Nozu K, Renieri A, Rheault M, Wang F, Gross O. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney international. 2018 May:93(5):1045-1051. doi: 10.1016/j.kint.2017.12.018. Epub 2018 Mar 16 [PubMed PMID: 29551517]
Uzzo M, Moroni G, Ponticelli C. Thin Basement Membrane: An Underrated Cause of End-Stage Renal Disease. Nephron. 2023:147(7):383-391. doi: 10.1159/000528243. Epub 2023 Mar 7 [PubMed PMID: 36882005]
Lusco MA, Fogo AB. Hereditary Nephritis and Thin Glomerular Basement Membrane Lesion. Glomerular diseases. 2021 Aug:1(3):135-144. doi: 10.1159/000516744. Epub 2021 Jun 15 [PubMed PMID: 36751492]
Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Thin Basement Membrane Lesion. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016 Oct:68(4):e17-e18. doi: 10.1053/j.ajkd.2016.08.005. Epub [PubMed PMID: 27664478]
Dische FE, Weston MJ, Parsons V. Abnormally thin glomerular basement membranes associated with hematuria, proteinuria or renal failure in adults. American journal of nephrology. 1985:5(2):103-9 [PubMed PMID: 3887920]
Matthaiou A, Poulli T, Deltas C. Prevalence of clinical, pathological and molecular features of glomerular basement membrane nephropathy caused by COL4A3 or COL4A4 mutations: a systematic review. Clinical kidney journal. 2020 Dec:13(6):1025-1036. doi: 10.1093/ckj/sfz176. Epub 2020 Feb 10 [PubMed PMID: 33391746]
Level 1 (high-level) evidenceSavige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. Journal of the American Society of Nephrology : JASN. 2013 Feb:24(3):364-75. doi: 10.1681/ASN.2012020148. Epub 2013 Jan 24 [PubMed PMID: 23349312]
Torra R, Tazón-Vega B, Ars E, Ballarín J. Collagen type IV (alpha3-alpha4) nephropathy: from isolated haematuria to renal failure. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004 Oct:19(10):2429-32 [PubMed PMID: 15280517]
Gregory MC. The clinical features of thin basement membrane nephropathy. Seminars in nephrology. 2005 May:25(3):140-5 [PubMed PMID: 15880323]
Dische FE, Anderson VE, Keane SJ, Taube D, Bewick M, Parsons V. Incidence of thin membrane nephropathy: morphometric investigation of a population sample. Journal of clinical pathology. 1990 Jun:43(6):457-60 [PubMed PMID: 2380394]
Haas M. Thin glomerular basement membrane nephropathy: incidence in 3471 consecutive renal biopsies examined by electron microscopy. Archives of pathology & laboratory medicine. 2006 May:130(5):699-706 [PubMed PMID: 16683888]
Miner JH. The glomerular basement membrane. Experimental cell research. 2012 May 15:318(9):973-8. doi: 10.1016/j.yexcr.2012.02.031. Epub 2012 Mar 5 [PubMed PMID: 22410250]
Level 3 (low-level) evidenceSuh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nature reviews. Nephrology. 2013 Aug:9(8):470-7. doi: 10.1038/nrneph.2013.109. Epub 2013 Jun 18 [PubMed PMID: 23774818]
Level 3 (low-level) evidenceKhoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microscopy research and technique. 2008 May:71(5):357-70. doi: 10.1002/jemt.20564. Epub [PubMed PMID: 18219669]
Level 3 (low-level) evidenceCollar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney international. 2001 Jun:59(6):2069-72 [PubMed PMID: 11380808]
Yuste C, Gutierrez E, Sevillano AM, Rubio-Navarro A, Amaro-Villalobos JM, Ortiz A, Egido J, Praga M, Moreno JA. Pathogenesis of glomerular haematuria. World journal of nephrology. 2015 May 6:4(2):185-95. doi: 10.5527/wjn.v4.i2.185. Epub [PubMed PMID: 25949932]
Quinlan C, Rheault MN. Genetic Basis of Type IV Collagen Disorders of the Kidney. Clinical journal of the American Society of Nephrology : CJASN. 2021 Jul:16(7):1101-1109. doi: 10.2215/CJN.19171220. Epub 2021 Apr 13 [PubMed PMID: 33849932]
Warady BA, Agarwal R, Bangalore S, Chapman A, Levin A, Stenvinkel P, Toto RD, Chertow GM. Alport Syndrome Classification and Management. Kidney medicine. 2020 Sep-Oct:2(5):639-649. doi: 10.1016/j.xkme.2020.05.014. Epub 2020 Aug 7 [PubMed PMID: 33094278]
Tryggvason K, Patrakka J. Thin basement membrane nephropathy. Journal of the American Society of Nephrology : JASN. 2006 Mar:17(3):813-22 [PubMed PMID: 16467446]
Żurawski J, Burchardt P, Seget M, Moczko J, Woźniak A, Grochowalski M, Salwa-Żurawska W. Difficulties in differentiating thin basement membrane disease from Alport syndrome. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2016:67(4):357-363. doi: 10.5114/pjp.2016.65869. Epub [PubMed PMID: 28547963]
Haas M. Alport syndrome and thin glomerular basement membrane nephropathy: a practical approach to diagnosis. Archives of pathology & laboratory medicine. 2009 Feb:133(2):224-32 [PubMed PMID: 19195966]
Imam AA, Saadeh SA. Evaluation of Proteinuria and Hematuria in Ambulatory Setting. Pediatric clinics of North America. 2022 Dec:69(6):1037-1049. doi: 10.1016/j.pcl.2022.07.002. Epub 2022 Oct 29 [PubMed PMID: 36880921]
Saha MK, Massicotte-Azarniouch D, Reynolds ML, Mottl AK, Falk RJ, Jennette JC, Derebail VK. Glomerular Hematuria and the Utility of Urine Microscopy: A Review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2022 Sep:80(3):383-392. doi: 10.1053/j.ajkd.2022.02.022. Epub 2022 Jun 29 [PubMed PMID: 35777984]
Hebert LA, Parikh S, Prosek J, Nadasdy T, Rovin BH. Differential diagnosis of glomerular disease: a systematic and inclusive approach. American journal of nephrology. 2013:38(3):253-66. doi: 10.1159/000354390. Epub 2013 Sep 13 [PubMed PMID: 24052039]
Level 1 (high-level) evidenceSavige J, Lipska-Zietkiewicz BS, Watson E, Hertz JM, Deltas C, Mari F, Hilbert P, Plevova P, Byers P, Cerkauskaite A, Gregory M, Cerkauskiene R, Ljubanovic DG, Becherucci F, Errichiello C, Massella L, Aiello V, Lennon R, Hopkinson L, Koziell A, Lungu A, Rothe HM, Hoefele J, Zacchia M, Martic TN, Gupta A, van Eerde A, Gear S, Landini S, Palazzo V, Al-Rabadi L, Claes K, Corveleyn A, Van Hoof E, van Geel M, Williams M, Ashton E, Belge H, Ars E, Bierzynska A, Gangemi C, Renieri A, Storey H, Flinter F. Guidelines for Genetic Testing and Management of Alport Syndrome. Clinical journal of the American Society of Nephrology : CJASN. 2022 Jan:17(1):143-154. doi: 10.2215/CJN.04230321. Epub 2021 Dec 20 [PubMed PMID: 34930753]
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex. : 1979). 2018 Jun:71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. Epub 2017 Nov 13 [PubMed PMID: 29133354]
Level 1 (high-level) evidenceChoi C, Ahn S, Min SK, Ha J, Ahn C, Kim Y, Lee H, Min SI. Midterm Outcome of Kidney Transplantation From Donors With Thin Basement Membrane Nephropathy. Transplantation. 2018 Apr:102(4):e180-e184. doi: 10.1097/TP.0000000000002089. Epub [PubMed PMID: 29334529]
Puapatanakul P, Miner JH. Alport syndrome and Alport kidney diseases - elucidating the disease spectrum. Current opinion in nephrology and hypertension. 2024 May 1:33(3):283-290. doi: 10.1097/MNH.0000000000000983. Epub 2024 Mar 13 [PubMed PMID: 38477333]
Level 3 (low-level) evidenceMandache E, Gherghiceanu M. Ultrastructural defects of the glomerular basement membranes associated with primary glomerular nephropathies. Ultrastructural pathology. 2004 Mar-Apr:28(2):103-8 [PubMed PMID: 15205110]
Liapis H, Foster K, Miner JH. Red cell traverse through thin glomerular basement membrane. Kidney international. 2002 Feb:61(2):762-3 [PubMed PMID: 11849422]
Level 3 (low-level) evidenceAntonovych TT, Deasy PF, Tina LU, D'Albora JB, Hollerman CE, Calcagno PL. Hereditary nephritis. Early clinical, functional, and morphological studies. Pediatric research. 1969 Nov:3(6):545-56 [PubMed PMID: 5361693]
Spear GS, Slusser RJ. Alport's syndrome. Emphasizing electron microscopic studies of the glomerulus. The American journal of pathology. 1972 Nov:69(2):213-24 [PubMed PMID: 4343992]
Liapis H, Gökden N, Hmiel P, Miner JH. Histopathology, ultrastructure, and clinical phenotypes in thin glomerular basement membrane disease variants. Human pathology. 2002 Aug:33(8):836-45 [PubMed PMID: 12203217]
Chavez E, Rodriguez J, Drexler Y, Fornoni A. Novel Therapies for Alport Syndrome. Frontiers in medicine. 2022:9():848389. doi: 10.3389/fmed.2022.848389. Epub 2022 Apr 25 [PubMed PMID: 35547199]
