Artificial Urinary Sphincters and Adjustable Dual-Balloon Continence Therapy in Men
Artificial Urinary Sphincters and Adjustable Dual-Balloon Continence Therapy in Men
Introduction
Guidelines from the American Urological Association (AUA) indicate that patients with persistent or severe urinary incontinence may benefit from surgical intervention.[1] Continence surgery should only be offered if the urinary incontinence is sufficiently bothersome despite conservative therapy. Post-prostatectomy incontinence is the most common cause of severe, intractable urinary leakage in men, with a reported occurrence of 4% to 69%, depending on the definition used.[2]
The primary etiology of post-prostatectomy urinary incontinence is damage or injury to the external urethral sphincter with resulting intrinsic sphincteric deficiency.[3] Incontinence symptoms can improve with postoperative pelvic floor physical therapy and lifestyle changes; most improvements are seen within 1 to 2 years following surgery.[4][5][6] A study reported that 8.4% of men still experienced severe incontinence 18 months after surgery.[7]
The current surgical standard for post-prostatectomy urinary incontinence is an artificial urinary sphincter (AUS), but other options, such as a urethral sling or dual-balloon adjustable continence therapy (DBACT), are available. Surgical intervention may be considered as early as 6 months after prostatectomy if the incontinence is severe and should be offered no sooner than 1 year after prostate surgery if the incontinence is moderate.[1]
Artificial Urinary Sphincter
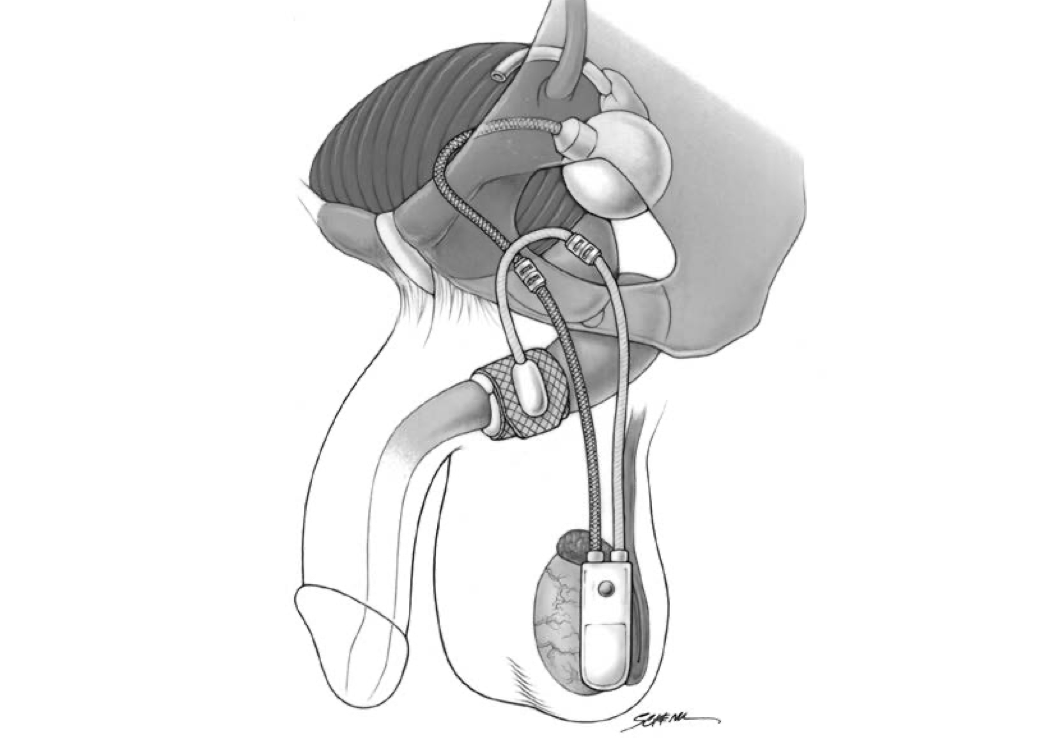
The artificial urinary sphincter (AUS) has been considered the gold standard treatment for stress urinary incontinence since it was first approved in the United States in 1972.[8][9][10] The AUS is an active system comprising an occlusive constricting cuff, a control pump, and a pressure-regulating balloon reservoir.[9][10] These parts are connected by kink-resistant tubing, creating a closed system.
The AUS promotes urinary continence via circumferential compression of the urethra. A fluid-filled silicone-elastomer cuff achieves dryness through urethral constriction, permitting micturition through a manually operated scrotal implanted pump that transfers fluid from the compression cuff into the pressure-regulating balloon reservoir.[11] After voiding, the compression cuff automatically refills within 3 to 5 minutes to restore cuff compression and continence.
The AUS is available worldwide to treat severe, intractable urinary incontinence in women and is approved for that purpose in the United States; that discussion is beyond the scope of this activity. AUS placement for severe, intractable intrinsic sphincter deficiency in women is infrequently performed in the US but more commonly so in Europe. The surgical approach is usually transabdominal, and the cuff is placed at the bladder neck; good long-term success rates are reported. The average life expectancy of an AUS is 10 years.
AUS devices are compatible with magnetic resonance imaging (MRI); image quality around the device may be compromised. Currently, AUS devices are made and marketed by only one manufacturer in the United States: Boston Scientific Corp., formerly American Medical Systems. AUS devices sold in the United States and most Western countries have an antibiotic coating of rifampicin and minocycline, offering substantial gram-positive bactericidal activity.
Dual-Balloon Adjustable Continence Therapy
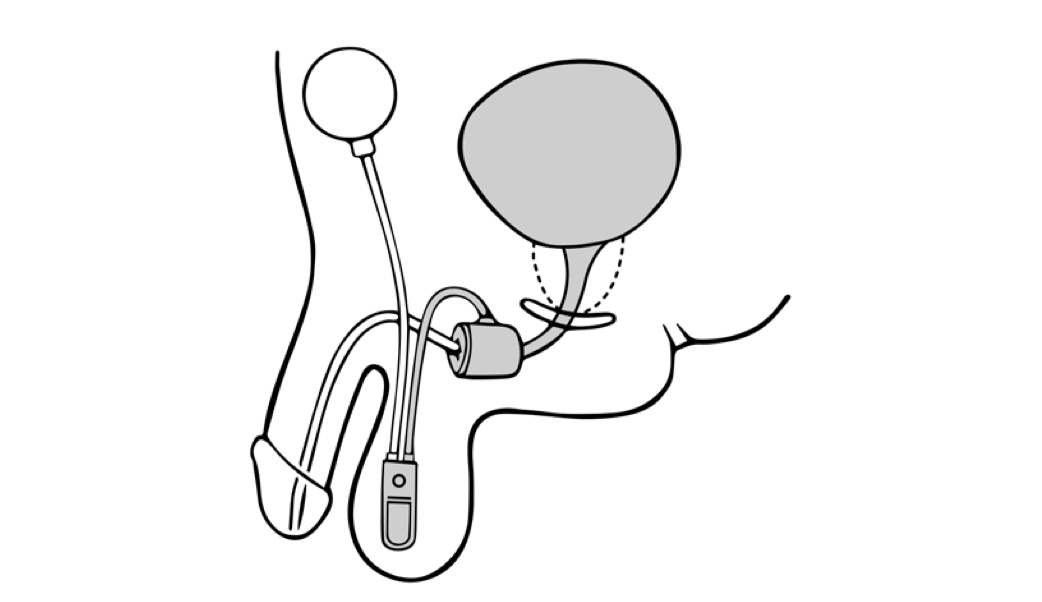
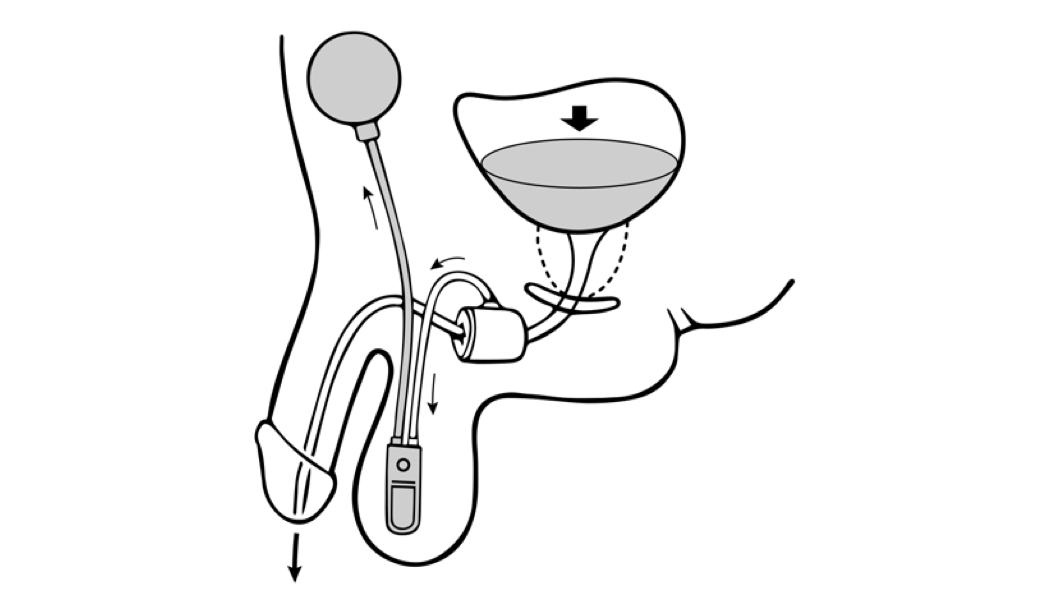
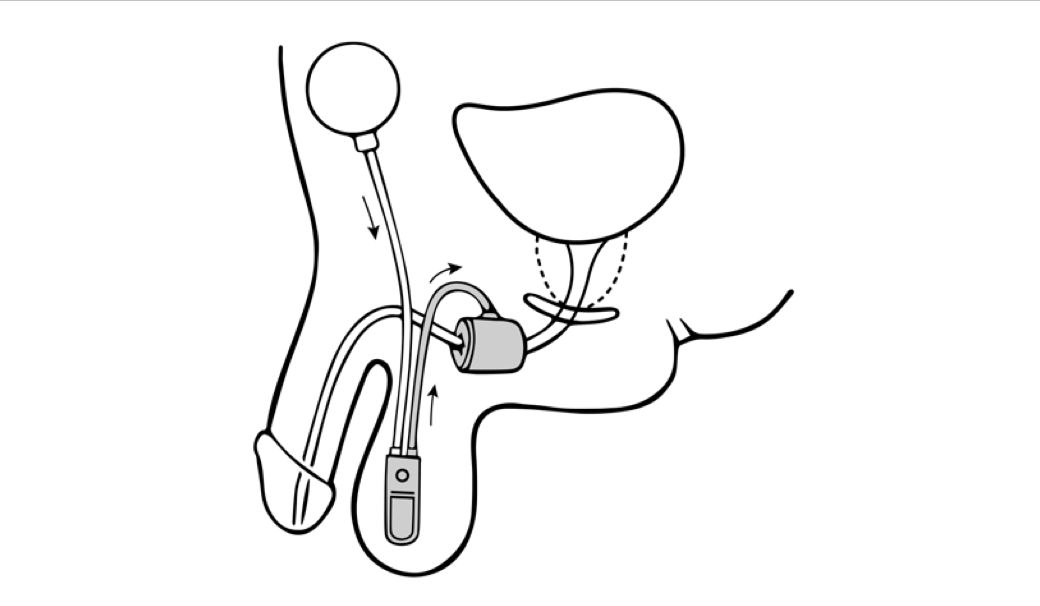
Dual-balloon adjustable continence therapy (DBACT) utilizes 2 periurethral silicon balloons implanted percutaneously on either side of the urethra just distal to the bladder neck.[12] These balloons increase passive urethral compression and improve bladder outlet resistance.[11] A titanium port connected to the balloons via a silicon conduit in the scrotum allows office-based volume adjustments to the implanted balloons as needed.[12]
DBACT placement is considered minimally invasive surgery and is easily reversible; the devices can be removed in the clinic with simple surgical instruments using only a local anesthetic. DBACT is MRI-compatible and is currently made by one manufacturer in the United States: UroMedica, Inc. While DBACT is available in Europe and South America for the treatment of stress urinary incontinence in women and men, the device is not approved for use in women in the United States.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Urinary continence in phenotypic males is achieved through 2 smooth muscle sphincters, the bladder neck or internal sphincter and passive prostatic sphincter, and 3 striated muscle sphincters: the prostatic, membranous urethral, and periurethral striated sphincters.[13] The prostate helps maintain the shape of the bladder neck and the internal sphincter for urinary continence.
The periurethral striated sphincter allows for voluntary urinary control and is somatically innervated. While the pathophysiology of post-prostatectomy urinary incontinence is multifactorial, a commonly attributed etiology is sustained damage to the external urethral sphincter during the dissection and division of the distal prostatic urethra, resulting in an intrinsic sphincteric deficiency.[13][14][15]
Intraoperative factors during radical prostatectomy that increase the risk of developing postoperative urinary incontinence are extensive surgical dissection and damage to the neurovascular bundle. Male urinary continence is maintained by the actions of the detrusor muscle, proximal intrinsic sphincter, rhabdosphincter, and pubourethral ligaments.
Radical prostatectomy removes the internal urethral sphincter, pubourethral ligaments, and part of the proximal urethral sphincter. The periurethral striated sphincter is mainly responsible for maintaining continence after prostatectomy. However, the action of the periurethral striated sphincter is also compromised by radical prostatectomy as the procedure damages the supplying neurovascular bundle.[16] The change in anatomic structure, interruption of innervation, and removal of supportive structures that contribute to preoperative urinary continence are all factors in post-prostatectomy urinary incontinence. The development of postoperative fibrosis is also a contributing factor.
Patient-related factors influencing post-prostatectomy urinary incontinence include age, diabetes mellitus, abnormal preoperative bladder function, and previously existing urinary incontinence.[11] Body mass index and patient lifestyle factors are currently being investigated as potential preoperative risk factors for developing post-prostatectomy urinary incontinence.[11]
Radiation therapy can negatively impact urinary continence, as the bladder and rectum often fall within the treatment field. Radiation damage results in chronic tissue inflammation, vascular insults, scar tissue formation, abnormal cell proliferation, and radiation cystitis. These changes can result in detrusor overactivity and urge urinary incontinence.[17] While prior radiation exposure can contribute to post-prostatectomy urinary incontinence, current prosthetic interventions are not intended to treat radiation-induced incontinence specifically.[18] Any use of these devices in patients with post-radiation urinary incontinence is considered off-label therapy; the implantation of these devices was not studied or approved for post-radiation incontinence.
Patients who undergo pelvic radiation therapy after prostatectomy have substantially lower continence rates with an AUS than similar patients who did not get radiotherapy.[19] Studies have demonstrated that radiotherapy was associated with significantly lower complete continence rates in all AUS patients, particularly primary AUS patients, and repeat implantation of an AUS was associated with poorer continence outcomes than primary AUS implantation.[18][19] Patients with a history of radiation therapy had similar rates of AUS device survival compared to nonirradiated patients if the final cuff size was at least 3.5 cm.[20]
Indications
The indication for an AUS is significant and bothersome urinary incontinence due to intrinsic sphincteric deficiency, often following prostate surgery, in patients who have failed to respond adequately to more conservative measures.[21][22] Careful patient selection is critical to the ultimate success of the AUS. Patients must be sufficiently motivated and capable of performing intermittent self-catheterization if required. Patients must also be willing, motivated, mentally competent, and physically able to find and operate the pump mechanism reliably. Advanced age is not a contraindication to AUS placement; patients more than 80 years of age have higher rates of infection and erosion but are still reasonable candidates for AUS implantation.[23]
The indication for a DBACT is stress incontinence arising from intrinsic sphincteric deficiency of at least 12 months duration following radical prostatectomy or transurethral resection of the prostate in patients who have failed to respond adequately to conservative therapy.[24]
Other causes of significant intrinsic sphincteric deficiency sufficient to justify surgical continence device implantation may include pelvic fractures, spinal cord injuries, posturethral reconstructions, neurogenic bladders with incompetent sphincter function, and failure of multiple previous continence procedures.
Contraindications
Absolute contraindications for AUS and DBACT therapy include patients who cannot tolerate anesthesia or possible surgical complications. A previously placed urethral sling is not a contraindication for either procedure.
The major contraindication to the AUS is an inability to operate the mechanical components due to cognitive dysfunction or poor manual dexterity.[1][25] Minor contraindications include patients with incontinence due to lower urinary tract obstruction, detrusor hyperreflexia, or bladder instability. The AUS is also contraindicated in patients with allergies to antibiotics, where the urinary tract cannot be made sterile, with active urinary tract infections, recurrent symptomatic or progressive urethral strictures, high post-void residual urine volumes, and a small bladder capacity.[22]
Significant vesicoureteral reflux should be surgically corrected before AUS placement to avoid reflux exacerbation. Recurrent nephrolithiasis and urothelial bladder tumors where regular cystoscopic instrumentation is expected are considered a relative contraindication as they increase the risk of cuff erosions in AUS patients.[22] Bladder neck contractures should be repaired and cystoscopically verified as permanently corrected at least 3 months post-correction before proceeding to AUS placement.[22]
Relative contraindications to DBACT include severe incontinence, a history of previous radiation therapy or planned radiation within 6 months of DBACT placement, and neurogenic stress urinary incontinence.[18][24] Other contraindications include an active urinary tract infection, incontinence due to detrusor instability or overactivity, reduced bladder compliance, post-void residual urine volumes >100 mL, significant urge incontinence, or uncorrected bleeding disorders.
Equipment
Artificial Urinary Sphincter
The equipment for AUS placement typically includes:[26]
- 3-0 nonabsorbable polypropylene ties
- 10- and 30-mL disposable syringes
- 500- and 1000-mL graduated containers
- Absorbable suture for closure
- Antibiotic solution
- Artificial urinary sphincter kit with quick connect assembly tool and tubing passers
- Babcock clamps
- Catheters
- Contrast solution
- Dry skin dressings
- Electrocautery (monopolar or bipolar)
- Hegar dilators
- Mayo and Metzembaum scissors
- Penrose drain or vessel loop
- Retractors
- Right-angle clamps
- Rubber-shod hemostats for clamping tubing
- Sponge bowl and basin
- Sterile normal saline
- Umbilical tape
Dual-Balloon Adjustable Continence Therapy
The equipment for DBACT placement typically includes:[27]
- 3-0 polyglycol absorbable suture
- 4-0 poliglecaprone skin suture
- 10-mL syringe
- 15- or 11-blade scalpel
- 50-mL Luer lock syringe
- Adjustable continence device kit
- Adson forceps
- Allis clamp
- Antibiotic irrigation
- C-arm fluoroscopy system
- Dry skin dressings
- Metzenbaum scissors
- Rigid cystoscopy set
- Rubber-shod forceps
- Solution of contrast and sterile water
- Sterile bin or basin
- Sterile lubricating gel
- Sterile normal saline
- Sterile water
- Urology surgical instrument set
Personnel
Personnel for incontinence procedures typically include:
- Primary urologic surgeon
- Anesthesia provider
- Circulator or operating room nurse
- Surgical technologist or operating room nurse
- Radiology technician (if image capture is required)
Preparation
Before the surgical intervention, all patients should undergo a thorough investigation of their urinary incontinence. This investigation should include a comprehensive medical history, physical examination, completion of voiding diaries, and any other necessary testing, such as urodynamics or video-urodynamics.[1] A preoperative urine culture should be performed before DBACT and AUS placement. If the culture is positive, preoperative antibiotic treatment and complete eradication of the infection is imperative.
For AUS and DBACT placement, patients should undergo cystoscopy to evaluate the urethra and rule out strictures, bladder neck contractures, or any other anatomic abnormality. All patients should receive prophylactic antibiotics before the procedure.
Silicone readily attracts dust and lint; all device components should be submerged in basins with sterile saline until used. Extra care should be taken to rinse surgical gloves of any particulates or powder before use.
Artificial Urinary Sphincter
AUS placement through the perineum is the preferred approach and the method described here.[28][29][30][31] However, some studies suggest equivalent outcomes with the technically easier penoscrotal approach.[32][33][34][35]
Implantation is typically performed at the bulbar urethra but can also be done at the bladder neck, albeit with some added difficulty. The bladder neck is theoretically the superior location for a compression cuff as there is thick, muscular, well-vascularized tissue in this area. However, following prostate surgery or radiation, the area surrounding the bladder neck becomes scarred, fibrous, and fixed to the surrounding structures, with a somewhat compromised blood supply leading to poorer healing.[9][10] Therefore, the bulbous urethra is usually chosen for cuff placement in men.[10]
Bladder neck implantation using a robotic-assisted laparoscopic approach to eliminate the morbidity associated with laparotomy has been reported for men and women. This approach is preferred in women where bladder neck placement of the AUS is required.[36][37][38][39][40][41][42]
The patient should be placed in the dorsal lithotomy position with appropriate padding of bony prominences and pressure points. The dorsal lithotomy position is preferred for AUS placement at the proximal bulbar urethra; this is the most common placement in cases of post-prostatectomy incontinence. Hair should be removed from the perineum to the infra-umbilical abdomen, and this area should be cleansed with chlorhexidine for 10 minutes with or without the addition of alcohol-based chlorhexidine.[43][44][45]
A 14F catheter should be placed for urine drainage and urethra identification. The scrotum should be elevated for ease of perineal access. The anus should be excluded from the field by draping. Sterile draping should allow for cystoscopy using a one-step cystoscopy draping set.
To prepare the device, place both tubing sets in a saline basin or basin of diluted contrast as per surgeon preference while holding the control pump. Using isotonic contrast fluid to facilitate identifying device leakage has been suggested and does not appear to affect device function, malfunction rate, the incidence of fluid leaks, or the incidence of reoperations.[46] Contrast should not be used in patients with a known allergy. The chosen fluid should be iso-osmolar to avoid fluid shifts. Fill the pump with fluid by holding it at a 45° angle and cycling through squeezing and releasing until all contained air has been replaced with fluid. While submerged, lightly clamp the tubing 4 to 5 cm from its end using a rubber-shod hemostat. Place the control pump in an empty sterile basin.
To prepare the pressure-regulating balloon, prepare a 30-mL syringe with 25 mL of filling solution and attach a 15-gauge blunt needle. Manually deflate the balloon and then fill it with 20 mL of the filling solution via the balloon tubing. Ensure all bubbles have been removed, clamp with a rubber-shod hemostat, and place into the antibiotic basin.
Once the cuff size is known (see Technique below), the cuff is similarly prepared with 5 to 10 mL of filling solution in the syringe; the size of the cuff selected dictates the total volume. Remove all bubbles, lightly clamp the tubing with a rubber-shod clamp, and place it in a dry sterile bin.[26] Do not place the rubber-shod clamp at the tip of the tubing; allow enough length for future placement of the metal tunneling device.
Dual-Balloon Adjustable Continence Therapy
The patient should be placed in the dorsal lithotomy position with appropriate padding of bony prominences and pressure points. Hair should be removed from the perineum. The perineum, penis, and surrounding area should be cleansed with chlorhexidine scrub. Although the instructions for use provided by the manufacturer state to use betadine, betadine is not the current standard of skin preparation for prosthetic urologic surgery, and this instruction should be ignored.[43][44][45] Contrast should not be used for balloon filling in patients with a known allergy.
Sterile draping should accommodate cystoscopy followed by device placement. The scrotum should be elevated to facilitate access to the perineum, and the anus should be excluded from the field with sterile towels.
Technique or Treatment
Artificial Urinary Sphincter Placement
Perform a vertical midline perineal incision with a scalpel into the skin overlying the bulbospongiosus muscle. Using a combination of blunt and sharp dissection and electrocautery, dissect the subcutaneous fat and tissues surrounding the urethra. Use handheld or self-retaining retractors to aid in dissection.
After exposing the spongiosum muscle overlying the bulbar urethra, identify the corporal bodies to assist urethral dissection. Utilize sharp dissection to divide the spongiosum muscle vertically, exposing the bulbar urethra. Continue to use retractors to expose the urethra. Once the urethra is thoroughly dissected, use a right-angle clamp to pass a Penrose drain or a vessel loop around the urethra. The vessel loop is used while mobilizing the urethra so that a space large enough for the cuff can be created as close to the crura as possible. For reference, the cuffs are approximately 1.8 cm when deflated.
The cuff sizer, when placed, should lay flat around the urethra, confirming adequate dissection. Wrap the cuff sizer around the urethra and measure the circumference. Sizes range from 3.5 to 11 cm in half-centimeter increments. The 4 cm cuff is used most often in men. A cuff measuring 6 to 8 cm is most commonly selected for women and is placed at the bladder neck.
Prepare the appropriate size cuff as described above. Grasp the tab of the cuff with a right-angle clamp. The implant should be placed with the mesh backing facing the outside and the inflatable side facing the urethra. Pass the cuff tubing through the hole in the mesh with a right-angle clamp, locking the system around the urethra and ensuring that the mesh locking mechanism is seated securely until the tab can be pulled over the tubing adapter.
Balloon reservoirs are available in 5 ranges of water pressure measured in centimeters of water (cm H2O). Available ranges are 41 to 50, 51 to 60, 61 to 70, 71 to 80, and 81 to 90 cm H2O. Selection of the reservoir pressure is based on the lowest pressure necessary for urethral closure; this is most commonly either 51 to 60 or 61 to 70 cm H2O.
The pressure-regulating balloon is most commonly placed on the same side as the pump through a separate transverse inguinal incision above the pubic bone lateral to the midline. Dissect down to fascia with sharp dissection and electrocautery with the help of handheld retractors. Identify the external oblique or rectus fascia. Place 2 transverse 2-0 polydioxanone sutures, one placed superiorly on the fascia and the other inferiorly to delineate where the transverse incision will be and to use for future incision closure. Make a small transverse incision between the two sutures with a 15-blade scalpel.
Bluntly spread the muscle to create a finger-sized space large enough for the balloon in the preperitoneal or retropubic space. Seat the balloon in the pocket using your finger. Fill the balloon using the blunt-tipped syringe according to the cuff size to reach the desired cuff pressure based on manufacturing guidelines. Connect the tubing using the rubber-shod hemostats. Close the fascia with the previously placed polydioxanone sutures.
The scrotal pump can be inserted through a scrotal incision or tunneled to the scrotum from the abdomen. Make a pocket in the subdartos area using blunt dissection and ring forceps. This dissection can be facilitated by manually inverting the scrotum into the incision and creating the pocket over the finger. This pocket should be away from the testis and easily palpable as well as accessible for the patient.
Once an adequate pocket has been created, place the pump into the pocket with the deactivation button facing the skin and seat it into the scrotum. At this point, 2 tubing sets should come out of the transverse incision, one for the pump and one for the pressure-regulating balloon reservoir.
There should also be a set of tubing coming out of the perineal incision with a shod clamp on it that is connected to the cuff. Connect the metal tunneling device to the tubing going to the cuff and tunnel subcutaneously from the perineal incision to the transverse incision.
The connection tubing should now be placed close to the pubic bone but deep enough in the subcutaneous tissue to minimize patient discomfort. Once the tunneling is complete, re-shod the tubing that goes to the cuff and remove the perineal tubing shod. All shods should be placed proximal enough so there is room for fingers and the stainless steel quick-connect assembly tool.
The pump has two sets of tubing emerging from it; one clear and the other black. The black tubing connects to the balloon reservoir, and the clear tubing connects to the cuff. Once the connections have been estimated, trim any excess tubing to maximize patient comfort. To connect the tubing, place the collet holder into the tubing; the collet holder has several collets on it.
Slide a collet onto the tubing with the teeth of the collet facing toward the open tubing end. Repeat this action twice for each side that will be connected. Using a 22-gauge needle attached to a 10-mL syringe filled with filing solution, flush the connector and tubing to remove air and particulate matter. Push the end of the tubing to the middle wall of the connector and check for appropriate placement through the connector window.
Push the other end of the tubing into the middle wall. Recheck the connector window to ensure that both tubing ends are still touching the middle walls of the connector. Slide the collets towards the connector until the collet teeth touch the connector. Place the connection in the jaw of the quick connect assembly tool and squeeze the tool handles until the closure stop touches the opposite handle. The tool is used once for the straight connector and twice for the right-angle connector, once on each end.
After all tubing has been connected, cycle the device to ensure proper functioning and deactivate it. Be certain there is some fluid in the pump mechanism, or it can become much more difficult to activate later.
All incisions should be irrigated copiously with saline or antibiotic irrigation solution. Close the perineal and abdominal incisions in several layers with absorbable sutures, then close the skin and dress with a dry sterile dressing.[26][27]
Dual-Balloon Adjustable Continence Therapy Device Placement
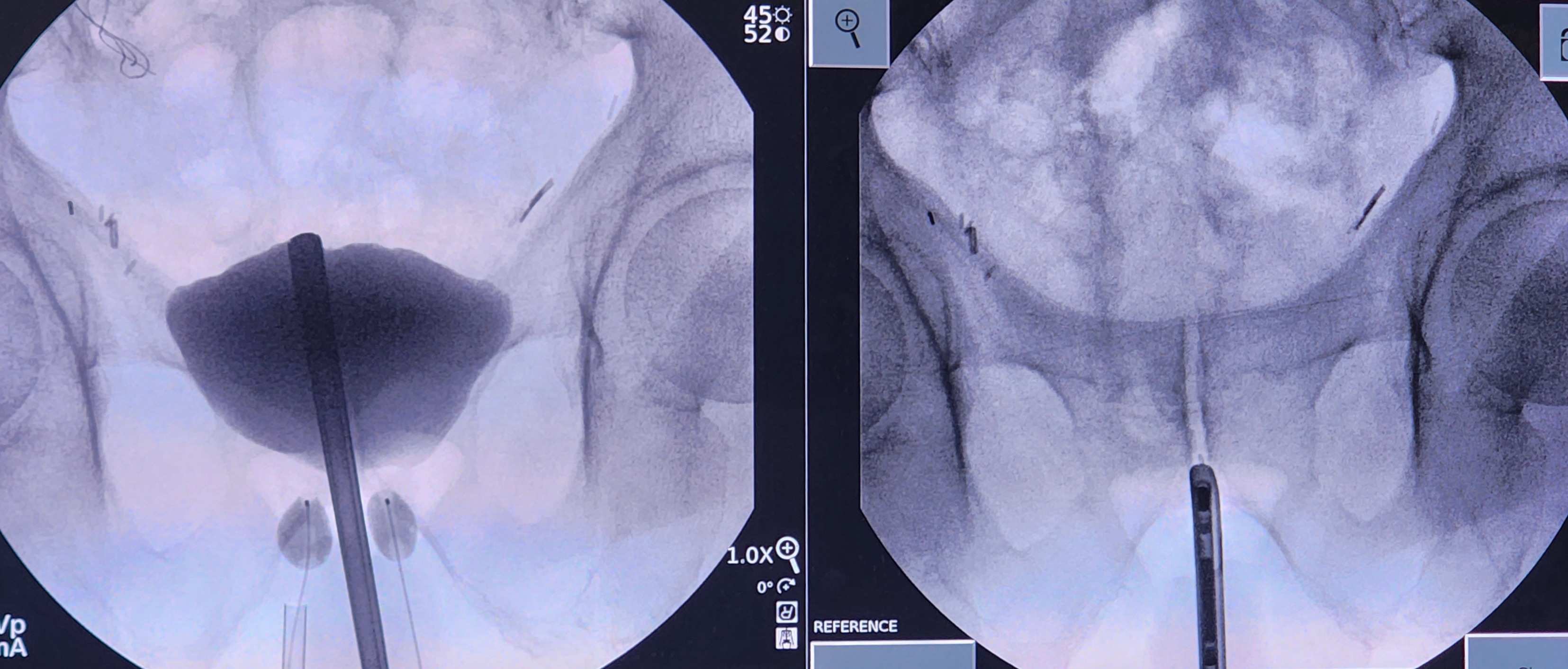
Perform a cystoscopy to ensure there are no anatomic abnormalities. If the decision is made to proceed with the procedure, retract the distal end of the cystoscope back to the bladder neck. Capture a fluoroscopic image to reference the location of the bladder neck during the procedure.
Remove the lens and bridge of the cystoscope and replace them with a blind obturator. Instill 50 mL of contrast through the cystoscope. Capture another image to identify how the contrast fills the bladder in relation to the bladder neck. This is important as the bladder neck can sometimes appear to be inside the bladder itself.
Once the anatomy has been identified, make a small transverse incision using a 15- or 11-blade scalpel at the level of the inferior pelvic ramus, typically 1 cm lateral to the midline raphe and 1.5 cm superior to the anus. Dissect the underlying tissue towards the inferior pubic ramus with a Kelly clamp. Palpate the ramus with the Kelly clamp under fluoroscopy to confirm the location is lateral to the urethra, which is delineated by the cystoscope.
The Kelly clamp should be parallel to the floor. It is imperative to confirm the dissection path before using the sharp trocar inside the U-shaped cannula. Under fluoroscopy, with the trocar introducer inside the U-shaped cannula and the open U facing the ceiling, place the trocar onto the bone at the junction of the angle of the inferior pubic ramus and the inferior portion of the pubic symphysis. The trocar should be parallel to the floor. Walk the trocar posteriorly off the bone and push the trocar through the urogenital diaphragm, all while hugging the anterior ramus inferiorly and staying parallel to the floor.
There should be a slight "popping" sensation when traversing the urogenital diaphragm; this may require twisting the trocar back and forth with gentle pressure. Care must be taken not to push the trocar too far into the bladder; space between the urogenital diaphragm and the bladder is minimal. After confirming complete penetration of the urogenital diaphragm and ideal trocar placement with fluoroscopic imaging, remove the sharp trocar and replace it with the blunt trocar.
Under live fluoroscopy, push on the bladder with the blunt trocar inside of the U-shaped cannula. A focal indentation of the bladder should be visualized. If there is movement of the entire bladder, left and right sides together, this is a sign that the urogenital diaphragm has not been perforated. Move the trocar side-to-side to see if the cystoscope moves. If the cystoscope does move, that denotes a location in the appropriate anterior-posterior plane. If no movement is appreciated or the trocar goes under the cystoscope, the location is too posterior, and a more anterior tract must be developed. Hugging the pelvic ramus anteriorly with the trocar helps prevent the posterior placement of the tract and balloon.
Once in the proper anterior-posterior plane and through the urogenital diaphragm, position the trocar lateral to the urethra and distal to the bladder neck. Implantation of the balloon can proceed if the bladder has not been perforated. To assess for bladder perforation, remove the blunt trocar while keeping the U-shaped cannula in position. If contrast or clear fluid comes out of the U-cannula, this could indicate that a bladder perforation has occurred. If a bladder perforation is suspected, evaluate for bladder decompression and extravasation of contrast under fluoroscopy. If a perforation is confirmed, abort the procedure on that side and place a Foley catheter for temporary bladder decompression.
If the bladder is uninjured, take the prepared balloon on the guidewire and place one of the three wings of the lambda-shaped deflated balloon into the groove of the U-shaped cannula. Slide the balloon into the incision with intermittent fluoroscopy. Once the radiopaque marker is at the end of the cannula, stabilize the balloon in its position while retracting the U-shaped cannula approximately 1 to 1.5 cm. Begin filling the balloon with isotonic contrast, typically to a volume of 0.5 mL.
A round balloon should be visualized during fluoroscopy. There may also be a flattened edge along the boundary of the urethra. If the balloon shape resembles a snowman or hourglass, this may indicate that it is not entirely through the urogenital diaphragm. If this is the case, an attempt should be made to perforate the diaphragm with the trocar fully. Take care not to inflate the balloon while it is in the cannula.
Once the balloon has been inflated, remove the U-cannula. In patients without a history of pelvic irradiation, increase the balloon volume to 1.5 mL. In patients with a history of irradiation or scarring, limit the initial volume to 0.5 mL to reduce early erosion of the balloon into the urethra or bladder.
Under fluoroscopic guidance, assess balloon placement by moving the cystoscope from right to left; the balloon should move with the cystoscope. If the balloon does not move, this could indicate an improper delivery of the balloon in the anterior-posterior plane.
Repeat these steps on the other side. Once this is complete, use fluoroscopy to visualize the balloons. They should be positioned lateral to the urethra but across from one another. Balloons can be offset in the superior-inferior plane and still be efficacious but may require more outpatient adjustments.
Remove the obturator from the cystoscope and replace it with a lens and bridge. Perform a cystourethroscopy to ensure no bladder, bladder neck, or urethral injury. If no injury is identified, retract the cystoscope to the bladder neck and pull on the balloon ports to visualize movement at the bladder neck in reference to the cystoscope. Sometimes, it is possible to visualize the balloons protruding into the urethra. Once the negative cystoscopy is complete, drain the bladder and remove the cystoscope.
Next, create a scrotal pocket using Metzenbaum scissors to create the subdartos scrotal pocket for the titanium ports. To accomplish this, estimate the placement by placing the ports over the scrotum and marking where the tip lands on the scrotal skin. This signifies the length of tunneling that needs to be performed. Have an assistant lift the scrotum to the ceiling for counter traction.
Using Adson forceps, Metzenbaum scissors, and blunt dissection, tunnel subcutaneously while staying superficial enough to facilitate office-based port access. Remove the internal wire and deliver the titanium port carefully into the created pocket to the previously marked level with an Allis clamp, Kelly clamp, or fingers.
Close the subcutaneous tissues with a 3-0 polyglycol interrupted stitch on each side. The skin should be closed using a 4-0 poliglecaprone suture with a single interrupted stitch. Cover the sites with dry, sterile dressings. Repeat on the other side. Use copious amounts of irrigation before and frequently throughout the closure. Some surgeons prefer to use a 0.05% chlorhexidine gluconate solution.[27]
Postoperative Care
Artificial Urinary Sphincter (AUS): Traditionally, patients are admitted overnight with a urethral catheter in place, but same-day discharge has become more common.[8] AUA guidelines recommend no more than 24 hours of IV antibiotics postoperatively.[47] Oral antibiotics, such as trimethoprim-sulfamethoxazole, are optionally used for 1 or 2 weeks postoperatively; such antibiotic therapy has not been definitively shown to affect the incidence of postoperative infections or cuff erosions.[48][49]
The device is left deactivated immediately after surgery and is not activated until 4 to 6 weeks postoperatively. During this period, incontinence must be managed with pads, intermittent self-catheterization, external condoms, a McGuire urinal, a Cunningham clamp, or some combination thereof.
Some suggest that the sphincter always be left deactivated overnight to allow a period where the urethra is not under compression by the cuff, possibly improving urethral blood flow and overall tissue health while reducing the risk of atrophy and erosions. Others believe this deactivation period is ineffective and serves only to make the patient incontinent overnight without proven benefit. There is no definitive data to support either method; further study is required.[50]
Patients should be carefully and repeatedly instructed that placement of a Foley catheter should only be attempted when the AUS is deactivated and the compression cuff is completely open. If a patient seeks care in an emergency department or healthcare facility, all personnel they encounter must be informed of this constraint. Any attempt to pass a Foley catheter without cuff deflation and deactivation could result in significant urethral injury or sphincter damage. Patients should be informed that most healthcare personnel will be unfamiliar with the AUS device and that they must know how to deactivate the device. The manufacturer provides a card for patients to carry with them at all times to identify them as having an artificial urinary sphincter that may require special attention. It is recommended that patients inform their medical power of attorney or healthcare proxy of the presence of the AUS should they be unable to relay that information themselves if incapacitated.
Dual Balloon Adjustable Continence Therapy: Overnight admission is not required. Placement of a Foley catheter is unnecessary unless the patient cannot void or other circumstances require it. Oral antibiotics can be used postoperatively per surgeon preference. The author uses trimethoprim-sulfamethoxazole for 1 week postoperatively. The patient should be seen in the clinic 2 weeks after the procedure for routine postoperative care. Adjustments to the device may begin 6 weeks postoperatively and monthly after that.
Special Situations
If the urethra is injured during the procedure to place an AUS, the injury should be repaired and the case aborted. A Foley catheter should be placed after the repair. A pressure-regulating balloon placed before the urethral injury may remain in place if the tubing is capped with the stainless steel tubing plug and the tubing is buried. The stainless steel tubing plug is available in the deactivation package.
If the urethra is injured during DBACT placement, the injury should be repaired and the case aborted. A Foley catheter should be placed after the repair. If the bladder is injured, place a Foley catheter for bladder decompression and abort the case. Patients with a history of pelvic radiation should have the initial balloon volumes limited to 0.5 mL, but the placement of DBACT in irradiated patients is considered off-label use. When performing this procedure in such patients, place the trocars slightly more lateral to mitigate the risk of future erosion.
Office-Based Adjustments of the Dual-Balloon Adjustable Continence Therapy Device
Office-based adjustment of balloon volumes requires the following materials: a 10-mL bottle of normal saline, a 23- and 18-gauge needles, a 3-mL syringe, alcohol wipes, and sterile gloves. This procedure should be treated similarly to obtaining port access.
Prepare the skin with alcohol wipes. In a sterile fashion, fill the syringe with 2 mL of normal saline using the 18-gauge needle and exchange this for the 23-gauge needle. The performing practitioner should stand on the side of the patient opposite their dominant hand. Hold the port with the non-dominant hand and deliver the needle through the scrotal skin with the dominant hand to access the port. Assistance may be required to retract the scrotal skin. Only 23-gauge needles should be used for device filling adjustments.
Depending on the severity of urinary leakage, 0.5 to 1.0 mL per balloon can be added at one time. If the patient experiences severe pain, do not add more volume. Volume adjustments should be based on patient tolerance. Patients with a history of radiation may experience more pain.
Complications
Artificial Urinary Sphincter
The most common intraoperative complication of AUS placement is urethral injury. While some surgeons choose to repair the injury and proceed with AUS placement immediately, it is generally recommended to repair the urethra and abort the AUS procedure to allow for urethral healing. Intraoperative urethral injuries are most likely to occur at the 12 o'clock position, where the urethra is fixed to the corpus cavernosum. Injuries can occur from direct contact, crushing injury, instrumental perforation, or thermal damage from cautery. If such an injury is not recognized, early cuff erosion and urethral tissue necrosis are likely. If intraoperative urethral injury occurs, another attempt at AUS implantation can be made at a later date; the recommended waiting period is 3 months.
Previous pelvic surgery or radiation, scarring, and adhesions will all increase the risk of bladder perforation. This risk is minimized by continuous intraoperative drainage with a Foley catheter. An initial transcorporal implantation has been suggested for use in these higher-risk patients to minimize complications.[51] Concomitant radical prostatectomy and radiation therapy significantly increase the risks of erosions, infections, persistent urinary incontinence, and urethral atrophy.[52]
Inadvertent peritoneal entry and bowel injuries have been reported. A bowel perforation would require an immediate repair and abandoning the artificial sphincter implantation procedure.
A critical point in female AUS placement is creating the proper plane between the bladder neck and vagina, as an injury may otherwise occur to immediately adjacent structures. Cystoscopy and a Cutter clamp have been used to assist in the dissection, and some surgeons will even open the bladder for better visualization of the ureteral orifices and bladder neck. An inadvertent vaginal perforation can be immediately closed, but a rectal injury requires abandonment of the procedure.
The most common postoperative complications of AUS placement are loss of fluid from the device, urethral tissue atrophy, erosion, infection, the need for additional surgery, no change in the quality of life, and continued lower urinary tract symptoms, such as stress or urge urinary incontinence, frequency, and nocturia.[14][53] Gross hematuria, dysuria, and difficulty urinating are all potential symptoms of infection, device erosion, or malfunction.
Urinary retention in the first 24 hours after AUS placement can be managed with a Foley catheter. If the retention lasts more than 48 hours, a suprapubic catheter can be placed to facilitate bladder drainage. If the retention persists, the urethral cuff may be too small and require revision.
Postoperative infection rates vary between 2% and 3% and are increased in patients with a history of pelvic radiation. The most common pathogens are Staphylococcus aureus and Streptococcus epidermidis. The AUA recommends prophylactic antibiotic therapy with vancomycin to mitigate this risk. If patients develop signs of infection, immediate removal of the device is required. Signs of infection include pain at the pump site, erythema, edema, and purulent discharge.
Urethral tissue atrophy is the most common cause of recurrent incontinence due to the loss of cuff compression functionality requiring surgical revision. This atrophy is generally from chronic tissue compression and ischemia, resulting in urethral thinning with a loss of mucosal coaptation and subsequent leakage. These processes occur over a long period, and patients report that the sphincter works properly but no longer provides continence. Tissue atrophy should be suspected in this clinical scenario.
Using a narrowed outer band to the cuff has helped reduce the occurrence of urethral tissue atrophy to less than 10%. Deactivation of the AUS device overnight has also been suggested to minimize compressive ischemia of the urethra, but there is no current evidence of any definitive benefit. The obligatory nighttime incontinence with cuff deactivation is bothersome to patients, and the adoption of this technique remains controversial.[50]
Treatment of urethral atrophy includes the use of tandem dual cuff implants, transcorporal cuff placement, transposition or movement of the cuff to an alternate urethral site, urethral bulking with fibrin-coated collagen fleece, changing to a higher pressure reservoir, and cuff downsizing.[54][55][56][57][58][59][60] Using a higher pressure will help temporarily, but this is likely to lead to further tissue atrophy and a recurrence of incontinence. Switching to a smaller cuff size can also be helpful, but as most men already use a small size, this may not be possible.[61] Moving the cuff to a healthier urethral location or using dual tandem cuffs are usually the best corrective choices.[58][59][60] However, the initial placement of dual tandem cuffs is not recommended as it extends operative time and increases the complication rate fourfold.[62][63]
If device erosion occurs, infection of the device is assumed. A Foley catheter should be placed, and the device should be removed. A rest and recovery period of at least 3 months is recommended before device replacement. The urethra should be carefully evaluated before a second AUS placement. While there is a significant risk of urethral stricture development following device erosion, this is usually associated with a prior history of radiation therapy; cuff erosion is approximately 50% more common in men with a history of pelvic radiation.[64] Signs of cuff erosion include obstructive urinary symptoms, worsening incontinence, and scrotal inflammation characterized by redness, swelling, and tenderness.[65] There is evidence that patients with low testosterone levels have a threefold risk of AUS erosions.[66][67]
Overall, the long-term AUS reoperation rate approaches 20%.
Troubleshooting the Artificial Urinary Sphincter
If the patient finds that the device is not working after placement, a physical examination is required; imaging may be necessary. It is recommended to work through the following steps when troubleshooting an AUS. If the patient never achieves continence after AUS activation, the most common causes are either the implanted cuff is too large or the reservoir has insufficient pressure.
The first recommended step is an assessment of the scrotal pump. If palpation of the scrotal pump reveals a dimple in the pump, the pump is deactivated in the open position. Resolve this by giving the pump a deliberate compression to open the valve mechanism within the pump itself. If the maneuver is successful, the pump will activate, and the sphincter will fill. The pump should inflate, and the palpable dimple should disappear. If the patient reports they cannot pump the device and there is no dimple, the pump may be inactivated in the closed position. The solution to this scenario requires the same maneuver: deliberately squeeze the pump, and the pump should automatically cycle.
If the patient cannot pump the device and physical examination reveals the pump is entirely flat, this could be due to one of two scenarios: either the pump is completely deactivated in the closed position with all of the fluid in the pressure-regulating balloon or there is no fluid in the system due to a leak. If the patient has difficulty pumping the device, it may have become deactivated. If the number of pump compressions required to allow voiding increases, urethral tissue atrophy or a fluid leak is likely.
There are two options to resolve this situation. The first option is to press down on the deactivation button for a few minutes to allow some fluid to leak from the pressure-regulating balloon into the pump and allow for a switch of the valve into the open position. The second option is to use a very narrow instrument, such as the tip of a hemostat or the back of a cotton-tipped applicator, to manually push the piston open on the exact opposite side of the deactivation button. To accomplish this, bring the pump up against the scrotal skin. Patients may require a local anesthetic due to the sensitivity of this area.
If the device remains nonoperational after troubleshooting, a cystoscopy is recommended to assess the cuff. If properly implanted, a closed cuff will have a starfish-shaped appearance. If the cuff appears closed, more fluid needs to go into the pump, or the piston needs to be opened. If the cuff is open, there is most likely a leak in the system. In the case of a leak in the system, the entire system needs to be replaced.
If the patient reports difficulty urinating despite being able to open the cuff, has recurrent urinary tract infections, or physical examination reveals a new thickening over the pump, cystoscopic evaluation is required to evaluate for possible urethral erosion. This cystoscopy should be performed with the cuff in the open and deactivated position. If the cuff is eroded, a Foley catheter should be placed until the mucosa has healed.
The AUS is made of a silicone elastomer that is somewhat permeable and tends to deteriorate slowly over time. It is expected that the cuff will gradually lose closing pressure as fluid escapes. To minimize osmotic fluid shifts into or out of the AUS device, only iso-osmolar filling solutions should be used. The reservoir typically holds 20 to 22 mL of filling solution. If the volume falls below 14 mL, the pressure drops dramatically. In this scenario, the patient would require more pump squeezes to open the cuff. Balloon leaks have been reported to occur in up to 13% of patients. However, the cuff may also thin and lose fluid from usage. This is most often noted on the lower cuff surface. Starting in 1983, additional reinforcement of fluorosilicone gel was added to the lower cuff surface, dramatically decreasing the cuff leak rate to a reported 1.3%.
A tube length that is too short can pull apart connections or cause pump migrations. However, a tube that is too long may develop kinks that can impair regular device operation. Using kinkproof tubing has dramatically reduced this complication, especially after 3 months postimplantation of the device.
The pump mechanism is small, which can make its operation more difficult. The pump mechanism may also rotate, twist, or migrate into the groin, further complicating its usage. The locking mechanism does not have tactile feedback, and it can be challenging to determine if the mechanism is open or locked. Some fluid must be in the pump to activate or unlock the device. Compression on both sides of the locking button will allow some fluid to return to the pump and bypass the delayed-refill resistor mechanism.
Radiographic studies can be a useful diagnostic tool if contrast was initially used as a filling fluid. When inactive or open, contrast should be visible in the pump and the reservoir but not in the cuff. When active or closed, a ring of contrast should be visible at the cuff site. If no contrast is visible, there is a leak.
A urethral pressure profile can be done with the cuff in active and inactive positions. A minimal change in the readings indicates a sphincteric malfunction. Retrograde perfusion sphincterometry with cystoscopy and electrical conductance testing can also help determine if the device is leaking.
Dual-Balloon Adjustable Continence Therapy Device
Bladder or urethral perforation is the most common intraoperative complication of DBACT placement, although other urethral injuries may occur. Acute urinary retention may be encountered and is managed by removing fluid volume from implanted balloons, with or without urethral catheterization. Device migration or erosion can also occur. Migration of the device can be due to poor initial positioning or excessive initial inflation.[24][68] Device removal usually resolves all long-term complications. Patients may undergo device reimplantation or receive more invasive treatment at a later date.[24]
Troubleshooting the Dual-Balloon Adjustable Continence Therapy Device
If the patient is suddenly unable to urinate, after either being able to urinate or having continued incontinence with the DBACT in place, a cystoscopy is required to assess for the erosion of one or both balloons into the urethra. If erosion is present, the eroded balloon(s) should be deflated and removed. This can be done in the office by administering appropriate analgesia over the port, deflating the balloon, and pulling the device out. A Foley catheter should be placed for several weeks to allow mucosal healing.
In situations of continued incontinence, a 5 mL adjustment in balloon volume should be made. If the incontinence does not improve, imaging is required. Pelvic radiography or computed tomography should be performed to assess balloon position and volume, as there may be leakage. The balloons should appear identical in position and inflation. If this is not the case, a balloon may be leaking, moved out of position, or the original placement may not have been optimal.
If the balloon position is adequate, more fluid volume can be added. If there is urethral displacement towards only one side, volume needs to be added to only one balloon. However, if the balloons are completely misaligned, the balloons can be removed in the office and replaced later in the operating room.
Clinical Significance
Both AUS and DBACT facilitate recovering urinary continence after prostate surgery in patients not responding to conservative measures. The AUS is regarded as an "active system" because it requires manipulation of the pump mechanism to operate the cuff. Adjustable continence therapy, like the DBACT, is considered a "passive system."
The AUS has demonstrated significantly improved long-term outcomes in patients; 50% of patients report complete continence 5 years postoperatively, and another 40% require 1 pad or fewer per day 5 years postoperatively.[27][69] The expected life of the AUS device is approximately 10 years. Overall patient satisfaction rates in men are reportedly 85% or greater; satisfaction rates are similar in women.[70][71][72] According to the manufacturer, more than 94000 devices have been implanted.
DBACT placement is a minimally invasive procedure, with 30% to 67% of patients achieving complete continence and an additional 22% to 38% achieving at least a 50% improvement in leakage.[24][68] In one study of 26 patients, 96% indicated either significant improvement or complete continence, but in a larger study of 240 patients, only 67% were improved, and 33% were considered "failures."[68][73] Data collected 5 years after the procedure revealed the improvement was long-lasting and durable, with similar success rates.[73] The complication rate was 15% to 32%, with the most commonly reported problem consistently being balloon failure, usually successfully treated with balloon replacement.[68][73]
The most recent meta-analysis of 1570 DBACT patients from 18 studies with a mean follow-up of 34.7 months demonstrated a mean dryness rate of 55%, an overall complication rate of 32%, and a 23% reoperation rate.[74] Proper patient selection, surgical experience with the DBACT device, and the lack of prior pelvic radiation appear to be important factors in reducing complications and improving dryness. The reported success rate decreased by more than one-third in previously irradiated patients; this is why DBACT is not recommended in this group.[75]
Although not approved in the United States, the global results in women using DBACT are similar, with about two-thirds indicating either dryness or improvement.[76][77][78][79] However, the most recent systematic review suggested that the overall success rate was relatively modest, with a significant complication and explantation rate.[77][80]
Device Selection
For patients with limited manual strength or dexterity, who desire a minimally invasive procedure, or who would otherwise find it difficult to operate an AUS for any reason, the DBACT would be the preferred selection. Patients who prefer an option with more long-term outcome data or desire a more "natural" functionality should probably receive the AUS device. Assuming a patient is a potential candidate for either device, shared decision-making incorporating surgeon experience and patient preference will provide the best outcome. However, due to the similar indications of AUS and DBACT, individual patient factors or preferences may be the determining factor in choosing one intervention over the other.
Many patients will expect perfect continence after surgery. Perfect continence is not impossible but may be unrealistic. Setting a more realistic expectation of improvement or "social continence," defined as needing no more than one incontinence pad daily, significantly improves postoperative satisfaction rates.
Future Developments
The AMS 800 is the only approved AUS device available in the United States and is considered the gold standard. However, the device has not been substantially updated since 1983 and would benefit from improvements. Complicated preoperative device preparation, difficult patient operation, awkward activation and deactivation, a relatively high rate of urethral atrophy, a relatively limited number of cuff sizes, and complex surgical insertion procedures are some targeted areas for improvement. Beneficial improvements would also include allowing adjustability without complete device replacement, decreasing the incidence of cuff erosions, decreasing fluid leakage from connections and the semipermeable tubing, and incorporating transmission of intraabdominal peak pressures to the cuff for compensatory closure pressure.[81][82]
Adding an implanted stress reservoir in selected patients with stress incontinence who already have an AUS is minimally invasive and effectively improves the functionality of AUS devices. Still, more studies are needed to verify the efficacy, long-term durability, and clinical viability of this additional procedure.[83]
Newer devices currently under development or available outside the United States have been designed to address some of these issues.[81] The Rigicon AUS device is designed to be easier to activate/deactivate, automatically adjust to changes in intraabdominal pressure, has a hydrophilic coating, and is available in more cuff sizes than the standard AMS product.[84] The Rigicon AUS device is being tested for possible approval in the United States; the device has been available in Europe since 2020.[81]
The Victo AUS from Austria is a preconnected system that is adjustable through a self-sealing percutaneous port and contains an additional stress balloon designed for preperitoneal placement.[85][86] This modification permits peak intraabdominal pressures to be immediately transmitted to the urethral cuff for additional closure pressure.[86] The Victo AUS is currently available in many countries, including all of Europe, but not in the United States.[81]
The Zephyr is a one-piece preconnected AUS device designed in Switzerland that does not have an abdominal reservoir, instead using a pressure-regulating reservoir and pump placed in the scrotum.[87] Both the cuff and pressure regulator are adjustable.[82] The Zephyr AUS device is currently available in many countries, including all of Europe, but not in the United States.[81]
Enhancing Healthcare Team Outcomes
Artificial urinary sphincters and dual-balloon adjustable continence therapy are surgical treatment options employing prosthetic implants for significant stress urinary incontinence due to intrinsic sphincter deficiency, not amenable or responsive to alternative treatments. Patients must be appropriately counseled on the indications, risks, and potential reasonable surgery outcomes. Patients should be informed that prophylactic antibiotics will be needed for future dental and other surgical procedures due to the implanted device. Communication between healthcare team members helps with the multifaceted approach to care from the preoperative to the postoperative setting and improves patient outcomes.[88]
Many patients undergoing surgical treatment for urinary incontinence utilize medications for symptom mitigation at some point during their treatment, have nurse visits in the clinic, and require preoperative counseling from anesthesiology providers. Collaboration on shared goals with nurses, pharmacists, surgeons, and other team members contributes to optimal patient outcomes.
When a patient seeks care in an emergency department or healthcare facility for any reason, all personnel they encounter must be informed of the presence of the prosthetic implant. This is particularly true for the AUS. Any attempt to pass a Foley catheter without cuff deflation and deactivation could result in significant urethral injury or sphincter damage.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sandhu JS, Breyer B, Comiter C, Eastham JA, Gomez C, Kirages DJ, Kittle C, Lucioni A, Nitti VW, Stoffel JT, Westney OL, Murad MH, McCammon K. Incontinence after Prostate Treatment: AUA/SUFU Guideline. The Journal of urology. 2019 Aug:202(2):369-378. doi: 10.1097/JU.0000000000000314. Epub 2019 Jul 8 [PubMed PMID: 31059663]
Radadia KD, Farber NJ, Shinder B, Polotti CF, Milas LJ, Tunuguntla HSGR. Management of Postradical Prostatectomy Urinary Incontinence: A Review. Urology. 2018 Mar:113():13-19. doi: 10.1016/j.urology.2017.09.025. Epub 2017 Oct 12 [PubMed PMID: 29031841]
Markland AD, Goode PS, Redden DT, Borrud LG, Burgio KL. Prevalence of urinary incontinence in men: results from the national health and nutrition examination survey. The Journal of urology. 2010 Sep:184(3):1022-7. doi: 10.1016/j.juro.2010.05.025. Epub [PubMed PMID: 20643440]
Level 2 (mid-level) evidenceNelson M, Dornbier R, Kirshenbaum E, Eguia E, Sweigert P, Baker M, Farooq A, McVary KT, Gonzalez CM, Gupta G, Bresler L. Use of Surgery for Post-Prostatectomy Incontinence. The Journal of urology. 2020 Apr:203(4):786-791. doi: 10.1097/JU.0000000000000618. Epub 2019 Oct 23 [PubMed PMID: 31642741]
Burnett AL. Patient-Reported Urinary Continence and Sexual Function After Anatomic Radical Prostatectomy. Urology. 2020 Nov:145():334. doi: 10.1016/j.urology.2020.04.022. Epub 2020 Apr 16 [PubMed PMID: 32304678]
Park JJ, Kwon A, Park JY, Shim SR, Kim JH. Efficacy of Pelvic Floor Exercise for Post-prostatectomy Incontinence: Systematic Review and Meta-analysis. Urology. 2022 Oct:168():175-182. doi: 10.1016/j.urology.2022.04.023. Epub 2022 May 5 [PubMed PMID: 35526757]
Level 1 (high-level) evidenceStanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, Albertsen PC, Harlan LC, Potosky AL. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000 Jan 19:283(3):354-60 [PubMed PMID: 10647798]
Shelton TM, Brimley SC, Nguyen HMT, Voznesensky I, Khalil MI, Machado B, Bhandari NR, Payakachat N, Davis R, Kamel MH, Raheem OA, Benson CR. Changing Trends in Management Following Artificial Urinary Sphincter Surgery for Male Stress Urinary Incontinence: An Analysis of the National Surgical Quality Improvement Program Database. Urology. 2021 Jan:147():287-293. doi: 10.1016/j.urology.2020.07.090. Epub 2020 Oct 16 [PubMed PMID: 33075382]
Level 2 (mid-level) evidenceKhouri RK Jr, Ortiz NM, Baumgarten AS, Ward EE, VanDyke ME, Hudak SJ, Morey AF. Artificial Urinary Sphincter Outperforms Sling for Moderate Male Stress Urinary Incontinence. Urology. 2020 Jul:141():168-172. doi: 10.1016/j.urology.2020.03.028. Epub 2020 Apr 11 [PubMed PMID: 32289365]
Elliott DS, Barrett DM. Current Indications for the Use of The Artificial Genitourinary Sphincter and Management of It's Complications. TheScientificWorldJournal. 2004:4():114-127. doi: 10.1100/tsw.2004.57. Epub 2004 Jun 7 [PubMed PMID: 29861669]
Gacci M, De Nunzio C, Sakalis V, Rieken M, Cornu JN, Gravas S. Latest Evidence on Post-Prostatectomy Urinary Incontinence. Journal of clinical medicine. 2023 Feb 2:12(3):. doi: 10.3390/jcm12031190. Epub 2023 Feb 2 [PubMed PMID: 36769855]
Hübner WA, Schlarp OM. Adjustable continence therapy (ProACT): evolution of the surgical technique and comparison of the original 50 patients with the most recent 50 patients at a single centre. European urology. 2007 Sep:52(3):680-6 [PubMed PMID: 17097218]
Level 2 (mid-level) evidenceMichl U, Tennstedt P, Feldmeier L, Mandel P, Oh SJ, Ahyai S, Budäus L, Chun FKH, Haese A, Heinzer H, Salomon G, Schlomm T, Steuber T, Huland H, Graefen M, Tilki D. Nerve-sparing Surgery Technique, Not the Preservation of the Neurovascular Bundles, Leads to Improved Long-term Continence Rates After Radical Prostatectomy. European urology. 2016 Apr:69(4):584-589. doi: 10.1016/j.eururo.2015.07.037. Epub 2015 Aug 12 [PubMed PMID: 26277303]
Son HS, Kim JH. Lower Urinary Tract Symptoms are Common After Artificial Urinary Sphincter Implantation. Urology. 2022 Jul:165():343-350. doi: 10.1016/j.urology.2022.01.031. Epub 2022 Jan 31 [PubMed PMID: 35108593]
Patel MN, Hemal AK. Robot-assisted laparoscopic simple anatomic prostatectomy. The Urologic clinics of North America. 2014 Nov:41(4):485-92. doi: 10.1016/j.ucl.2014.07.003. Epub 2014 Aug 30 [PubMed PMID: 25306160]
Rahnama'i MS, Marcelissen T, Geavlete B, Tutolo M, Hüsch T. Current Management of Post-radical Prostatectomy Urinary Incontinence. Frontiers in surgery. 2021:8():647656. doi: 10.3389/fsurg.2021.647656. Epub 2021 Apr 9 [PubMed PMID: 33898508]
Zwaans BM, Nicolai HG, Chancellor MB, Lamb LE. Challenges and Opportunities in Radiation-induced Hemorrhagic Cystitis. Reviews in urology. 2016:18(2):57-65. doi: 10.3909/riu0700. Epub [PubMed PMID: 27601964]
Fuller TW, Ballon-Landa E, Gallo K, Smith TG 3rd, Ajay D, Westney OL, Elliott SP, Alsikafi NF, Breyer BN, Cohen AJ, Vanni AJ, Broghammer JA, Erickson BA, Myers JB, Voelzke BB, Zhao LC, Buckley JC. Outcomes and Risk Factors of Revision and Replacement Artificial Urinary Sphincter Implantation in Radiated and Nonradiated Cases. The Journal of urology. 2020 Jul:204(1):110-114. doi: 10.1097/JU.0000000000000749. Epub 2020 Jan 17 [PubMed PMID: 31951498]
Level 3 (low-level) evidenceGuillaumier S, Solomon E, Jenks J, Pakzad M, Hamid R, Ockrim J, Shah J, Greenwell T. Radiotherapy is associated with reduced continence outcomes following implantation of the artificial urinary sphincter in men with post-radical prostatectomy incontinence. Urology annals. 2017 Jul-Sep:9(3):253-256. doi: 10.4103/UA.UA_25_17. Epub [PubMed PMID: 28794592]
Rivera ME, Linder BJ, Ziegelmann MJ, Viers BR, Rangel LJ, Elliott DS. The Impact of Prior Radiation Therapy on Artificial Urinary Sphincter Device Survival. The Journal of urology. 2016 Apr:195(4 Pt 1):1033-7. doi: 10.1016/j.juro.2015.10.119. Epub 2015 Oct 27 [PubMed PMID: 26518111]
Comiter C. Surgery for postprostatectomy incontinence: which procedure for which patient? Nature reviews. Urology. 2015 Feb:12(2):91-9. doi: 10.1038/nrurol.2014.346. Epub 2015 Jan 6 [PubMed PMID: 25558839]
Biardeau X, Aharony S, AUS Consensus Group, Campeau L, Corcos J. Artificial Urinary Sphincter: Report of the 2015 Consensus Conference. Neurourology and urodynamics. 2016 Apr:35 Suppl 2():S8-24. doi: 10.1002/nau.22989. Epub [PubMed PMID: 27064055]
Level 3 (low-level) evidenceZiegelmann MJ, Linder BJ, Rivera ME, Viers BR, Rangel LJ, Elliott DS. Outcomes of artificial urinary sphincter placement in octogenarians. International journal of urology : official journal of the Japanese Urological Association. 2016 May:23(5):419-23. doi: 10.1111/iju.13062. Epub 2016 Feb 18 [PubMed PMID: 26890355]
Larson T, Jhaveri H, Yeung LL. Adjustable continence therapy (ProACT) for the treatment of male stress urinary incontinence: A systematic review and meta-analysis. Neurourology and urodynamics. 2019 Nov:38(8):2051-2059. doi: 10.1002/nau.24135. Epub 2019 Aug 20 [PubMed PMID: 31429982]
Level 1 (high-level) evidenceHampson LA, Suskind AM, Breyer BN, Lai L, Cooperberg MR, Sudore RL, Keyhani S, Allen IE, Walter LC. Understanding the Health Characteristics and Treatment Choices of Older Men with Stress Urinary Incontinence. Urology. 2021 Aug:154():281-287. doi: 10.1016/j.urology.2021.05.002. Epub 2021 May 15 [PubMed PMID: 34004214]
Level 3 (low-level) evidenceScott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. 1974. The Journal of urology. 2002 Feb:167(2 Pt 2):1125-9; discussion 1130 [PubMed PMID: 11905886]
Koch GE, Kaufman MR. Male Stress Urinary Incontinence. The Urologic clinics of North America. 2022 Aug:49(3):403-418. doi: 10.1016/j.ucl.2022.04.005. Epub 2022 Jun 27 [PubMed PMID: 35931433]
Henry GD, Graham SM, Cleves MA, Simmons CJ, Flynn B. Perineal approach for artificial urinary sphincter implantation appears to control male stress incontinence better than the transscrotal approach. The Journal of urology. 2008 Apr:179(4):1475-9; discussion 1479. doi: 10.1016/j.juro.2007.11.058. Epub 2008 Mar 4 [PubMed PMID: 18295275]
Level 2 (mid-level) evidenceHenry GD, Graham SM, Cornell RJ, Cleves MA, Simmons CJ, Vakalopoulos I, Flynn B. A multicenter study on the perineal versus penoscrotal approach for implantation of an artificial urinary sphincter: cuff size and control of male stress urinary incontinence. The Journal of urology. 2009 Nov:182(5):2404-9. doi: 10.1016/j.juro.2009.07.068. Epub 2009 Sep 16 [PubMed PMID: 19762042]
Level 2 (mid-level) evidenceAnusionwu I, Miles-Thomas J, Hernandez DJ, Wright EJ. Anatomical and manometric comparison of perineal and transscrotal approaches to artificial urinary sphincter placement. The Journal of urology. 2012 Nov:188(5):1834-6. doi: 10.1016/j.juro.2012.07.032. Epub 2012 Sep 19 [PubMed PMID: 22999695]
Punjani N, Chan E, Chan G, Abed H, Campbell J, Brock G. Single perineal incision for artificial urinary sphincter: analysis of technique, outcomes, and experience. Translational andrology and urology. 2020 Oct:9(5):1912-1919. doi: 10.21037/tau-20-508. Epub [PubMed PMID: 33209655]
Altaweel W, Almesned R, Seyam R. A comparison of the perineal and penoscrotal approaches in artificial urinary sphincter implantation for the control of male stress urinary incontinence. Annals of Saudi medicine. 2023 Jan-Feb:43(1):57-61. doi: 10.5144/0256-4947.2023.57. Epub 2023 Feb 2 [PubMed PMID: 36739496]
Jamaer C, De Bruyn H, Van Renterghem A, Baten E, Van Renterghem K. Penoscrotal Incision for the Primary Implantation of an Artificial Urinary Sphincter. Current urology. 2020 Jun:14(2):74-78. doi: 10.1159/000499256. Epub 2020 Jun 23 [PubMed PMID: 32774231]
Shen YC, Chiang PH. Is the penoscrotal approach inferior to the perineal approach for artificial sphincter implantation in male urinary incontinence? A preliminary experience. International journal of urology : official journal of the Japanese Urological Association. 2012 Aug:19(8):786-9. doi: 10.1111/j.1442-2042.2012.03013.x. Epub 2012 Apr 11 [PubMed PMID: 22494163]
Level 2 (mid-level) evidenceStaniorski CJ, Singal A, Nettey O, Yura E, Keeter MK, Kielb S, Hofer MD. Revisiting the penoscrotal approach to artificial urinary sphincter surgery: how does it compare to a perineal incision for initial implantation? World journal of urology. 2021 Mar:39(3):871-876. doi: 10.1007/s00345-020-03244-6. Epub 2020 May 21 [PubMed PMID: 32440696]
Yates DR, Phé V, Rouprêt M, Vaessen C, Parra J, Mozer P, Chartier-Kastler E. Robot-assisted laparoscopic artificial urinary sphincter insertion in men with neurogenic stress urinary incontinence. BJU international. 2013 Jun:111(7):1175-9. doi: 10.1111/bju.12072. Epub 2013 Apr 2 [PubMed PMID: 23551759]
Biardeau X, Rizk J, Marcelli F, Flamand V. Robot-assisted laparoscopic approach for artificial urinary sphincter implantation in 11 women with urinary stress incontinence: surgical technique and initial experience. European urology. 2015 May:67(5):937-42. doi: 10.1016/j.eururo.2014.12.041. Epub 2015 Jan 9 [PubMed PMID: 25582931]
Rouprêt M, Misraï V, Vaessen C, Cardot V, Cour F, Richard F, Chartier-Kastler E. Laparoscopic approach for artificial urinary sphincter implantation in women with intrinsic sphincter deficiency incontinence: a single-centre preliminary experience. European urology. 2010 Mar:57(3):499-504. doi: 10.1016/j.eururo.2009.03.045. Epub 2009 Mar 31 [PubMed PMID: 19346059]
Fournier G, Callerot P, Thoulouzan M, Valeri A, Perrouin-Verbe MA. Robotic-assisted laparoscopic implantation of artificial urinary sphincter in women with intrinsic sphincter deficiency incontinence: initial results. Urology. 2014 Nov:84(5):1094-8. doi: 10.1016/j.urology.2014.07.013. Epub 2014 Oct 24 [PubMed PMID: 25443911]
Chung E. Artificial urinary sphincter surgery in the special populations: neurological, revision, concurrent penile prosthesis and female stress urinary incontinence groups. Asian journal of andrology. 2020 Jan-Feb:22(1):45-50. doi: 10.4103/aja.aja_128_19. Epub [PubMed PMID: 31793444]
Peyronnet B, Gray G, Capon G, Cornu JN, Van Der Aa F. Robot-assisted artificial urinary sphincter implantation. Current opinion in urology. 2021 Jan:31(1):2-10. doi: 10.1097/MOU.0000000000000837. Epub [PubMed PMID: 33239514]
Level 3 (low-level) evidenceBazinet A, Vaessen C, Mozer P, Popelin MB, Rod X, Chartier-Kastler E. Robotic-assisted artificial urinary sphincter revisions for women suffering from non-neurogenic stress incontinence: a single center experience. World journal of urology. 2023 Jun:41(6):1691-1696. doi: 10.1007/s00345-023-04399-8. Epub 2023 Apr 13 [PubMed PMID: 37055589]
Yeung LL, Grewal S, Bullock A, Lai HH, Brandes SB. A comparison of chlorhexidine-alcohol versus povidone-iodine for eliminating skin flora before genitourinary prosthetic surgery: a randomized controlled trial. The Journal of urology. 2013 Jan:189(1):136-40. doi: 10.1016/j.juro.2012.08.086. Epub 2012 Nov 16 [PubMed PMID: 23164373]
Level 1 (high-level) evidenceMagera JS Jr, Inman BA, Elliott DS. Does preoperative topical antimicrobial scrub reduce positive surgical site culture rates in men undergoing artificial urinary sphincter placement? The Journal of urology. 2007 Oct:178(4 Pt 1):1328-32; discussion 1332 [PubMed PMID: 17698144]
Level 2 (mid-level) evidenceSeidelman JL, Mantyh CR, Anderson DJ. Surgical Site Infection Prevention: A Review. JAMA. 2023 Jan 17:329(3):244-252. doi: 10.1001/jama.2022.24075. Epub [PubMed PMID: 36648463]
Inouye BM, Boysen WR, Barton GJ, Peterson AC. Use of isotonic contrast solution in the artificial urinary sphincter does not impact device longevity. Neurourology and urodynamics. 2021 Apr:40(4):1056-1062. doi: 10.1002/nau.24668. Epub 2021 Apr 3 [PubMed PMID: 33811366]
Lightner DJ, Wymer K, Sanchez J, Kavoussi L. Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. The Journal of urology. 2020 Feb:203(2):351-356. doi: 10.1097/JU.0000000000000509. Epub 2019 Aug 23 [PubMed PMID: 31441676]
Dropkin BM, Dallmer JD, Chisholm LP, Johnsen NV, Dmochowski RR, Milam DF, Kaufman MR. Are postoperative antibiotics necessary after artificial urinary sphincter insertion? The Canadian journal of urology. 2020 Dec:27(6):10437-10442 [PubMed PMID: 33325344]
Adamsky MA, Boysen WR, Cohen AJ, Ham S, Dmochowski RR, Faris SF, Bales GT, Cohn JA. Evaluating the Role of Postoperative Oral Antibiotic Administration in Artificial Urinary Sphincter and Inflatable Penile Prosthesis Explantation: A Nationwide Analysis. Urology. 2018 Jan:111():92-98. doi: 10.1016/j.urology.2017.07.064. Epub 2017 Sep 28 [PubMed PMID: 28964819]
Elliott DS, Barrett DM, Gohma M, Boone TB. Does nocturnal deactivation of the artificial urinary sphincter lessen the risk of urethral atrophy? Urology. 2001 Jun:57(6):1051-4 [PubMed PMID: 11377302]
Level 2 (mid-level) evidenceLe Long E, Rebibo JD, Nouhaud FX, Grise P. Transcorporal artificial urinary sphincter in radiated and non - radiated compromised urethra. Assessment with a minimum 2 year follow-up. International braz j urol : official journal of the Brazilian Society of Urology. 2016 May-Jun:42(3):494-500. doi: 10.1590/S1677-5538.IBJU.2015.0329. Epub [PubMed PMID: 27286112]
Bates AS, Martin RM, Terry TR. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. BJU international. 2015 Oct:116(4):623-33. doi: 10.1111/bju.13048. Epub 2015 Mar 12 [PubMed PMID: 25601072]
Level 2 (mid-level) evidenceMoses RA, Broghammer JA, Breyer BN, Voelzke BB, Buckley JC, Erickson BA, Elliott S, Vanni AJ, Ramkumar N, Myers JB. Patient Risk Factors Associated with Reported Urinary Quality of Life Following Artificial Urinary Sphincter Placement: A Paired Pre and Postoperative Analysis. Urology. 2022 Nov:169():226-232. doi: 10.1016/j.urology.2022.07.023. Epub 2022 Jul 27 [PubMed PMID: 35905775]
Level 2 (mid-level) evidenceHeah NH, Tan RBW. Management of urethral atrophy after implantation of artificial urinary sphincter: what are the weaknesses? Asian journal of andrology. 2020 Jan-Feb:22(1):60-63. doi: 10.4103/aja.aja_110_19. Epub [PubMed PMID: 31736473]
Margreiter M, Farr A, Sharma V, Schauer I, Klingler HC. Urethral buttressing in patients undergoing artificial urinary sphincter surgery. The Journal of urology. 2013 May:189(5):1777-81. doi: 10.1016/j.juro.2012.11.152. Epub 2012 Nov 30 [PubMed PMID: 23206425]
Guralnick ML, Miller E, Toh KL, Webster GD. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. The Journal of urology. 2002 May:167(5):2075-8; discussion 2079 [PubMed PMID: 11956443]
Level 2 (mid-level) evidenceRothschild J, Chang Kit L, Seltz L, Wang L, Kaufman M, Dmochowski R, Milam DF. Difference between urethral circumference and artificial urinary sphincter cuff size, and its effect on postoperative incontinence. The Journal of urology. 2014 Jan:191(1):138-42. doi: 10.1016/j.juro.2013.06.052. Epub 2013 Jun 29 [PubMed PMID: 23820053]
Wiedemann L, Cornu JN, Haab E, Peyrat L, Beley S, Cathelineau X, Haab F. Transcorporal artificial urinary sphincter implantation as a salvage surgical procedure for challenging cases of male stress urinary incontinence: surgical technique and functional outcomes in a contemporary series. BJU international. 2013 Dec:112(8):1163-8. doi: 10.1111/bju.12386. Epub [PubMed PMID: 24053170]
Level 3 (low-level) evidenceKowalczyk JJ, Spicer DL, Mulcahy JJ. Long-term experience with the double-cuff AMS 800 artificial urinary sphincter. Urology. 1996 Jun:47(6):895-7 [PubMed PMID: 8677584]
Bell BB, Mulcahy JJ. Management of cuff erosion of the double cuff artificial urinary sphincter. The Journal of urology. 2000 Jan:163(1):85-6 [PubMed PMID: 10604320]
Weis S, Ludwig TA, Bahassan O, Gild P, Vetterlein MW, Fisch M, Dahlem R, Maurer V. Outcomes and Complication Rates of Cuff Downsizing in the Treatment of Worsening or Persistent Incontinence After Artificial Urinary Sphincter Implantation. International neurourology journal. 2023 Jun:27(2):139-145. doi: 10.5213/inj.2346030.015. Epub 2023 Jun 30 [PubMed PMID: 37401025]
Ahyai SA, Ludwig TA, Dahlem R, Soave A, Rosenbaum C, Chun FK, Fisch M, Schmid M, Kluth LA. Outcomes of single- vs double-cuff artificial urinary sphincter insertion in low- and high-risk profile male patients with severe stress urinary incontinence. BJU international. 2016 Oct:118(4):625-32. doi: 10.1111/bju.13449. Epub 2016 Mar 22 [PubMed PMID: 26917355]
O'Connor RC, Lyon MB, Guralnick ML, Bales GT. Long-term follow-up of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinence. Urology. 2008 Jan:71(1):90-3. doi: 10.1016/j.urology.2007.08.017. Epub [PubMed PMID: 18242372]
Krughoff K, Dvergsten T, Foreman JR, Peterson AC. Urethral Stricture Formation After Artificial Urinary Sphincter Cuff Erosion Is Uncommon in the Absence of Pelvic Radiation. The Journal of urology. 2023 Jul:210(1):136-142. doi: 10.1097/JU.0000000000003431. Epub 2023 Mar 22 [PubMed PMID: 36947796]
Diao L, Nealon SW, Carpinito GP, Badkhshan S, Wolfe AR, Dropkin BM, Sanders SC, Hudak SJ, Morey AF. Presenting signs and symptoms of artificial urinary sphincter cuff erosion. International braz j urol : official journal of the Brazilian Society of Urology. 2022 Jul-Aug:48(4):679-685. doi: 10.1590/S1677-5538.IBJU.2022.0089. Epub [PubMed PMID: 35503704]
Wolfe AR, Ortiz NM, Baumgarten AS, VanDyke ME, West ML, Dropkin BM, Joice GA, Sanders SC, Hudak SJ, Morey AF. Most men with artificial urinary sphincter cuff erosion have low serum testosterone levels. Neurourology and urodynamics. 2021 Apr:40(4):1035-1041. doi: 10.1002/nau.24663. Epub 2021 Apr 1 [PubMed PMID: 33792973]
Baaklini GT, Hofer MD. Are androgens important in the setting of stress urinary incontinence? Translational andrology and urology. 2023 May 31:12(5):949-951. doi: 10.21037/tau-22-688. Epub 2023 Mar 13 [PubMed PMID: 37305634]
Munier P, Nicolas M, Tricard T, Droupy S, Costa P, Saussine C. What if artificial urinary sphincter is not possible? Feasibility and effectiveness of ProACT for patients with persistent stress urinary incontinence after radical prostatectomy treated by sling. Neurourology and urodynamics. 2020 Jun:39(5):1417-1422. doi: 10.1002/nau.24355. Epub 2020 Apr 6 [PubMed PMID: 32249971]
Level 2 (mid-level) evidenceElliott DS, Barrett DM. The artificial urinary sphincter in the female: indications for use, surgical approach and results. International urogynecology journal and pelvic floor dysfunction. 1998:9(6):409-15 [PubMed PMID: 9891964]
Litwiller SE, Kim KB, Fone PD, White RW, Stone AR. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. The Journal of urology. 1996 Dec:156(6):1975-80 [PubMed PMID: 8911369]
Thomas K, Venn SN, Mundy AR. Outcome of the artificial urinary sphincter in female patients. The Journal of urology. 2002 Apr:167(4):1720-2 [PubMed PMID: 11912395]
Costa P, Poinas G, Ben Naoum K, Bouzoubaa K, Wagner L, Soustelle L, Boukaram M, Droupy S. Long-term results of artificial urinary sphincter for women with type III stress urinary incontinence. European urology. 2013 Apr:63(4):753-8. doi: 10.1016/j.eururo.2012.03.008. Epub 2012 Mar 16 [PubMed PMID: 22445222]
Finazzi Agrò E, Gregori A, Bianchi D, Carone R, Giammò A, Ammirati E, Giovannelli V, Romanò AL, Martino P, Saracino A, Volpe A, Favro M, Canepa G, Varca V, Pinto A, Farullo G. Efficacy and safety of adjustable balloons (Proact™) to treat male stress urinary incontinence after prostate surgery: Medium and long-term follow-up data of a national multicentric retrospective study. Neurourology and urodynamics. 2019 Sep:38(7):1979-1984. doi: 10.1002/nau.24103. Epub 2019 Jul 14 [PubMed PMID: 31302928]
Level 2 (mid-level) evidenceTricard T, Song QX, Munier P, Li JY, Leng J, Saussine C, Pan JH, Xue W. Adjustable continence therapy (proACT) for the treatment of male stress urinary incontinence post-prostatectomy: a systematic review and meta-analysis (2023 update). World journal of urology. 2023 Jul:41(7):1793-1802. doi: 10.1007/s00345-023-04452-6. Epub 2023 Jun 13 [PubMed PMID: 37311990]
Level 1 (high-level) evidenceRouprêt M, Misraï V, Gosseine PN, Bart S, Cour F, Chartier-Kastler E. Management of stress urinary incontinence following prostate surgery with minimally invasive adjustable continence balloon implants: functional results from a single center prospective study. The Journal of urology. 2011 Jul:186(1):198-203. doi: 10.1016/j.juro.2011.03.016. Epub 2011 May 14 [PubMed PMID: 21575974]
Demeestere A, de Guerry ML, Bergot C, de Hauteclocque A, Hascoet J, Gamé X, Bajeot AS, Peyronnet B, Capon G, Perrouin-Verbe MA, Biardeau X. Adjustable continence therapy (ACT®) balloons to treat neurogenic and non-neurogenic female urinary incontinence. Neurourology and urodynamics. 2022 Jan:41(1):313-322. doi: 10.1002/nau.24822. Epub 2021 Oct 11 [PubMed PMID: 34633672]
Phé V, Nguyen K, Rouprêt M, Cardot V, Parra J, Chartier-Kastler E. A systematic review of the treatment for female stress urinary incontinence by ACT® balloon placement (Uromedica, Irvine, CA, USA). World journal of urology. 2014 Apr:32(2):495-505. doi: 10.1007/s00345-013-1117-0. Epub 2013 Jun 20 [PubMed PMID: 23783882]
Level 1 (high-level) evidenceBillault C, Chartier-Kastler E, Rouprêt M, Robain G, Phé V. Functional outcomes of adjustable continence therapy (ACT™) balloons in women aged }80 years and suffering from stress urinary incontinence caused by intrinsic sphincter deficiency. World journal of urology. 2015 Nov:33(11):1897-903. doi: 10.1007/s00345-015-1520-9. Epub 2015 Feb 21 [PubMed PMID: 25701129]
Level 2 (mid-level) evidencede Guerry ML, Demeestere A, Bergot C, de Hauteclocque A, Hascoet J, Bajeot AS, Ternynck C, Gamé X, Peyronnet B, Capon G, Perrouin-Verbe MA, Biardeau X. Adjustable Continence Therapy (ACT®) balloons to treat female stress urinary incontinence: effectiveness, safety and risk factors of failure and complication. International urogynecology journal. 2023 Apr:34(4):877-883. doi: 10.1007/s00192-022-05275-6. Epub 2022 Jun 25 [PubMed PMID: 35751672]
Guérin S, Khene ZE, Peyronnet B. Adjustable Continence Therapy Balloons in Female Patients with Stress Urinary Incontinence: A Systematic Review. Urologia internationalis. 2023:107(7):653-665. doi: 10.1159/000529712. Epub 2023 Jun 2 [PubMed PMID: 37271125]
Level 1 (high-level) evidencePrebay ZJ, Foss HE, Wang KR, Chung PH. A narrative review on surgical treatment options for male stress urinary incontinence. Translational andrology and urology. 2023 May 31:12(5):874-886. doi: 10.21037/tau-22-629. Epub 2023 Mar 6 [PubMed PMID: 37305628]
Level 3 (low-level) evidenceOstrowski I, Golabek T, Ciechan J, Śledź E, Przydacz M, Dyś W, Blewniewski M, von Heyden B, Pottek T, Neugart F, Carrieri G, Selvaggio O, Iori F, Arjona MF, Foley S, Yang B, Llorens C, Różanski W, Chłosta PL. Preliminary outcomes of the European multicentre experience with the ZSI 375 artificial urinary sphincter for treatment of stress urinary incontinence in men. Central European journal of urology. 2019:72(3):263-269. doi: 10.5173/ceju.2019.1920. Epub 2019 Jul 12 [PubMed PMID: 31720028]
Ameli G, Weibl P, Rutkowski M, Huebner WA. Using a stress reservoir to improve urine leakage after artificial urinary sphincter implantation. International urology and nephrology. 2023 Dec:55(12):3089-3094. doi: 10.1007/s11255-023-03756-1. Epub 2023 Aug 27 [PubMed PMID: 37634237]
Wilson SK, Chung E, Langford B, Schlesinger R, Koca O, Simsek A, Persu C, Pottek T, Mulcahy J. First safety outcomes for rigicon conticlassic® artificial urinary sphincter. International journal of impotence research. 2023 Aug 5:():. doi: 10.1038/s41443-023-00748-8. Epub 2023 Aug 5 [PubMed PMID: 37543658]
Giammò A, Falcone M, Blecher G, Ammirati E, Geretto P, Manassero A, Bottero D, Lorusso V, Signorello D, Gontero P, Carone R. A Novel Artificial Urinary Sphincter (VICTO®) for the Management of Postprostatectomy Urinary Incontinence: Description of the Surgical Technique and Preliminary Results from a Multicenter Series. Urologia internationalis. 2021:105(5-6):414-420. doi: 10.1159/000512722. Epub 2021 Feb 19 [PubMed PMID: 33611317]
Weibl P, Hoelzel R, Rutkowski M, Huebner W. VICTO and VICTO-plus - novel alternative for the mangement of postprostatectomy incontinence. Early perioperative and postoperative experience. Central European journal of urology. 2018:71(2):248-249. doi: 10.5173/ceju.2018.1655. Epub 2018 Apr 19 [PubMed PMID: 30038818]
Kretschmer A, Hüsch T, Thomsen F, Kronlachner D, Pottek T, Obaje A, Anding R, Rose A, Olianas R, Friedl A, Hübner W, Homberg R, Pfitzenmaier J, Grein U, Queissert F, Naumann CM, Schweiger J, Wotzka C, Nyarangi-Dix JN, Hofmann T, Buchner A, Haferkamp A, Bauer RM, Debates On Male Incontinence (DOMINO)-Project. Efficacy and safety of the ZSI375 artificial urinary sphincter for male stress urinary incontinence: lessons learned. World journal of urology. 2016 Oct:34(10):1457-63. doi: 10.1007/s00345-016-1787-5. Epub 2016 Feb 25 [PubMed PMID: 26914816]
Reese AC, Ginzburg S. The past, present, and future of urological quality improvement collaboratives. Translational andrology and urology. 2021 May:10(5):2280-2288. doi: 10.21037/tau.2019.10.18. Epub [PubMed PMID: 34159110]
Level 2 (mid-level) evidence