Introduction
Corneal neurotization involves a set of new surgical techniques that directly address the loss of corneal nerves by transferring sensory nerves from nearby areas to the perilimbal region.[1][2] Samii et al first described using the sural nerve as an interposition graft between the transected ophthalmic nerve and the greater occipital nerve.[3] However, this procedure was lengthy and required a large frontal craniotomy, leading to its rare utilization. Terzis et al demonstrated a direct transfer technique in 2009, which proved more practical.[1]
Neurotrophic keratopathy results from permanent damage to the corneal nerves (see Image. Neurotrophic Cornea), leading to corneal hypoesthesia, with a prevalence of 1.6 to 11 per 10,000.[4] The most common causes of neurotrophic keratopathy are herpetic disease, diabetes, dry eye syndrome, tumors, chemical or surgical trauma, and iatrogenic medication. Corneal nerves play a vital role in maintaining the homeostasis of the ocular surface, including tear production and epithelial regeneration.[5] They secrete substance P and calcitonin gene-related factors involved in epithelial cell proliferation and wound healing.[6] Corneal epithelial cells also produce neurotrophin-like nerve growth factor and neurotrophin 3, promoting nerve survival.[7][8]
The corneal sensation is crucial for reflex blinking and tearing. Corneal hypoesthesia increases the risk of corneal microtrauma, epithelial breakdown, and delayed wound healing.[6][7][9] Traditional management strategies have been predominantly supportive but do not reverse the loss of corneal nerves (see Table 1. The Mackie Classification of Neurotrophic Keratopathy and Traditional Supportive Treatment Options). Serum tears are blood derivatives containing multiple growth factors that aid in epithelial healing and allow some improvement in corneal sensation.[10][11] External replacement of nerve growth factors, including topical insulin-derived growth factor and human recombinant nerve growth factor, aims to promote nerve regeneration. While topical nerve growth factors have improved corneal sensitivity in animal models, recent randomized clinical trials have not demonstrated the same effect in humans.[12][13]
Table 1. The Mackie Classification of Neurotrophic Keratopathy and Traditional Supportive Treatment Options
| Mackie Stage | Examination Findings | Supportive Treatment |
| 1 |
Punctate epithelial erosions Decreased tear breakup time Stromal haze Increased mucous viscosity Staining of conjunctiva (rose bengal) |
Preservative-free artificial tears Anti-inflammatories Contact lenses Autologous serum Correction of lid abnormalities |
| 2 |
Persistent epithelial defect with smooth and rolled edges Stromal edema with Descemet membrane folds Aqueous cells and flare |
Scleral lenses Recombinant human nerve growth factor Amniotic membrane transplant Upper lid Botox to induce ptosis Tarsorrhaphy |
| 3 |
Corneal ulcer (recurrent) Stromal lysis and melt Corneal perforation |
Recombinant human nerve growth factor Multilayer amniotic membrane transplant Cyanoacrylate tissue adhesive Fibrin glue Tectonic keratoplasty |
The treatment objectives include promoting reepithelialization and preventing the further progression of ocular surface disease and neurotrophic keratopathy. Preservative-free artificial tears and autologous serum can be considered in the initial phases. Topical recombinant human nerve growth factor can be effective in patients with Mackie stage 2 to 3 neurotrophic corneas, with low recurrence rates after epithelial defect healing up to 48 weeks after treatment. The aim is to replace nerve growth factors with external applications.
Medical treatment can be attempted before considering corneal neurotization. Topical insulin eye drops have improved nerve regeneration and corneal sensitivity in animal models. Still, in randomized studies, no significant improvement in corneal sensation was found in human patients. Amniotic membrane transplant can reduce ocular surface inflammation and vascularization. Contact lenses and autologous serum tears are part of the traditional supportive approach that does not address the root cause of neurotrophic keratopathy.
Severe damage may require more complex surgical planning and can affect the success of the neurotization procedure. Surgery should be considered an option when vision is compromised, in the presence of stromal thinning, or when medical therapy alone is not sufficient. The availability of suitable donor nerves is necessary for the procedure, but donor nerve selection and surgical technique can be adapted based on the case's specifics. Other considerations for donor nerve selection are the distance from the donor to the affected cornea, the nerve caliber, and axon count. Both the sural and the great auricular nerves are purely sensory.
Measuring Outcomes of Corneal Neurotization
High rates of corneal healing have been observed with direct and indirect corneal neurotization. Results from a recent meta-analysis have shown a significant improvement in healing, with the Mackie grade decreasing from an average of 2.46 ± 0.77 to 0.86 ± 0.79.[14][15][16] Corneal sensation, however, does not fully return after corneal neurotization, especially when compared to the opposite cornea.[17] Patients may begin feeling subjective sensations such as pain and discomfort several weeks after surgery, with objective improvements occurring several months later and continuing for up to a year.[1][18]
Corneal nerves are organized into bundles that divide before entering the corneal tissue, passing through various layers before finally ending in free nerve endings. The corneal tissue's density and number of nerves are related to its sensitivity.[19] The speed of corneal sensation recovery is linked to the distance between the injured and the distal nerve. The density and visualization of corneal nerves can be measured using in vivo confocal microscopy, starting from 3 months post-surgery, with improvement observed up to 6 months.[17] Unfortunately, the blink reflex is not restored after corneal neurotization. The density of nerves varies throughout the cornea, with thinner axons, higher numbers of myelinated fibers, and the characteristic subbasal plexus whorl pattern not being fully restored.[9]
Visual improvement depends on corneal scarring, amblyopia, and other eye-related conditions. There is a suggestion to consider corneal neurotization at earlier clinical stages before permanent scarring or amblyopia develops. Patients younger than 18 tend to recover more rapidly and completely than older patients.[20] Keratoplasty can be performed after or simultaneously with corneal neurotization to improve vision.[21][22][23]
Corneal Neurotization in Herpetic Disease
Special considerations are necessary when considering corneal neurotization for patients with herpetic disease due to the risks of reinfection and reactivation.[2] Preoperative and postoperative oral antiviral prophylaxis is recommended. Both direct and indirect methods with ipsilateral or contralateral donor nerves have been used with similar outcomes.[2][24][25] Lin et al used the ipsilateral supratrochlear nerve in a direct transfer technique, with 78% of cases (n= 13 eyes) showing resolution of presenting corneal pathology and a shift from 70% Mackie stage III to 53% Mackie stage I after surgery.[24]
In patients with shorter denervation time (less than 2 years), improvements in corneal sensation can be rapid and detectable at 3 months postoperatively.[2] Persistent epithelial defects usually occur in the first 6 months following corneal neurotization but resolve with conventional management.[2] There is no consensus regarding the timing of surgical intervention; the eye needs to be stable and free from reactivation. Other ocular comorbidities, corneal fibrosis, and scarring limit the final visual potential. Some treatments on the horizon, such as topical losartan, show promise in reversing stromal scarring fibrosis.[26]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Corneal nerves help maintain the ocular surface's balance by playing a role in tear production and epithelial regeneration. These nerves secrete substance P and calcitonin gene-related factors, aiding epithelial proliferation and wound healing.[6] Corneal epithelial cells also secrete neurotrophin 3, ciliary neurotrophic factor, and other growth factors that support corneal nerve survival.[5] Additionally, glial cell-derived neurotrophic factor, epidermal growth factor, brain-derived neurotrophic factor, neuropeptide Y, and neurotrophin-like nerve growth factor contribute to epithelial cell and nerve regeneration after injury.[6]
The corneal sensation is important for tearing and reflex blinking. Reduced or abnormal cornea innervation may lead to reduced tear production, compromised epithelial function, and decreased expression of neurotrophic growth factors and epitheliotropic mediators.[6] The reduced innervation can affect patients with chronic inflammation or diabetes, leading to an unstable tear film and reduced corneal sensation.
Human corneal nerves form a dense network, including the limbal plexus, stromal plexus, intraepithelial terminals, subepithelial plexuses, and subbasal plexuses beneath and above the Bowman membrane.[27][28][29] Most of the nerve fibers are sensory, with some contribution to the autonomic nervous system. The sensory nerves mainly originate from the ophthalmic branch of the trigeminal nerve, with some contribution from the maxillary branch.[30] The limbal plexus originates from the trigeminal ganglion, exiting as 40 nerve bundles that travel suprachoroidally and converge 1 mm from the corneal limbus.[31] These nerves can remain prominent in certain conditions, such as multiple endocrine neoplasia and keratoconus, although they can also appear in unaffected corneas.[32][33]
The density of free nerve endings within the suprabasal epithelial plexus is among the highest in the human body, with approximately 606 terminals per mm².[29] These nerve endings have overlapping receptive fields and higher central density than the peripheral cornea. Approximately 70% of the nerve receptors are polymodal and transmit heat, inflammation, chemical irritants, and pain sensations. The remaining 20% are mechanoreceptors, and 10% transmit cold sensations.[34]
Indications
The common reasons for neurotization are surgical nerve damage following intracranial surgery, such as removing an acoustic neuroma, and nerve issues linked to infections like herpes simplex or varicella-zoster virus. Additional reasons are listed below (see Table 2. Etiology and Indications for Corneal Neurotization).[35]
Table 2. Etiology and Indications for Corneal Neurotization
| Indication/Etiology of Corneal Neurotization | Diagnosis |
| Neurosurgery/ophthalmic surgery |
Acoustic neuroma Arteriovenous malformation Trigeminal neuroma Chondrosarcoma Trigeminal neuralgia Acute hemorrhage (pontine cavernoma) Microvascular decompression of trigeminal nerve Gamma knife surgery Long ciliary nerve damage after retinal detachment surgery Ptosis surgery (for congenital oculomotor nerve palsy) Laser-assisted in situ keratomileusis Keratoplasty |
| Viral |
Herpes simplex virus Herpes zoster virus |
| Congenital |
Cerebellar hypoplasia and bilateral corneal anesthesia Peripheral nerve dysfunction Hypoplasia of trigeminal nerve Agenesis of trigeminal nerve Idiopathic |
| Vascular |
Stroke Diabetes mellitus |
| Trauma |
Chemical and thermal injuries Long-term contact lens wear |
| Radiotherapy | After excision of acoustic neuroma/other head and neck tumor |
| Infection | Microbial keratitis |
| Other |
Vitamin A deficiency Leprosy Chronic ocular surface disease |
Contraindications
Corneal neurotization is contraindicated if patients have an active ocular surface infection or uncontrolled inflammatory disease, perineural malignancy near the donor nerve, absent healthy sensory donor nerves, corneal epithelial defects, or ongoing radiotherapy to the eye or orbit. Relative contraindications are paraesthesia in the donor nerve dermatome, extensive conjunctival scarring, poorly controlled diabetic mellitus, multiple systemic comorbidities, anticoagulation use, and unrealistic expectations.
Equipment
The equipment needed for performing corneal neurotization will depend on the specific surgical technique and surgeon preference. Like most surgical procedures, the equipment required for this complex procedure includes microsurgical instruments (eg, microscissors, microneedles for suturing and nerve manipulation, microforceps for handling nerves and other small tissues, microvascular clamps, and micro dissectors). Suturing materials (eg, nylon, prolene, or absorbable) are also needed to complete the procedure.
Surgical loupes or operating microscopes are fundamental for visualizing the fine structures of nerves and vessels during surgery. Nerve harvesting tools include nerve retractors, dissection scissors, and nerve conduits or nerve grafts. Electrocautery devices are needed to control bleeding from small vessels. Local anesthetics can be used for regional blocks, while general anesthesia equipment is required for patient sedation and monitoring during surgery. Postoperative care usually requires an eye shield, antibiotic drops, anti-inflammatory medication, and lubricating eye drops.
Personnel
An interprofessional healthcare team involved in the corneal neurotization procedure includes ophthalmic surgeons (including specialists in the cornea, anterior segment, and oculoplastics), maxillofacial surgeons, anesthetists, physicians, optometrists, ophthalmic nurses/assistants, operating room staff, orthoptists, imaging technicians, and medical assistants. This collaborative team is essential to ensure precise nerve grafting, manage perioperative care, and support postoperative recovery and monitoring.
Preparation
Neurotization (sensation) assessment should be performed during the preoperative protocol. This assessment should include all potential donor nerves, such as the supraorbital (providing sensation to the lateral forehead skin, anterior hair-bearing scalp, upper eyelid including conjunctiva), supratrochlear (providing sensation to the medial forehead, upper eyelid, and nasal bridge), and the infraorbital nerves (providing sensation to the cheek skin, upper lip, lateral nose, upper teeth, and gingiva).
Other areas to be assessed include the skin innervated by the ipsilateral greater auricular nerve (inferior skin to external auditory meatus, the mastoid process, and parotid gland regions). Considerations should also be given to the distance from the donor nerve to the affected cornea, the caliber, and the axon count of the nerve. For instance, the supraorbital nerve has approximately 6000 myelinated axons, while the supratrochlear nerve has approximately 2500 myelinated axons.[36]
Technique or Treatment
Corneal neurotization techniques involve taking a healthy sensory nerve from a donor and transferring it directly or indirectly to the affected neurotrophic cornea (see Table 3. Summary of the Different Direct and Indirect Techniques for Corneal Neurotization). Indirect methods use a nerve graft that can be taken from the patient's body (autograft) or a donor (decellularized allograft). There are various combinations of ipsilateral (on the same side) and contralateral (on the opposite side) donor nerves from which to choose. The choice of donor nerve depends on factors such as the donor site's availability, the donor nerve's size, the surgeon's preference and experience, and the distance between the donor nerve end and the affected cornea.
The nerve ends can be connected in 3 ways: end-to-end (ETE), side-to-side, or end-to-side (ETS). The donor and recipient nerves need to be of similar size for the ETE approach. If there is a significant difference in nerve size or if the function of the donor nerve is critical, the ETS approach can be used. The ETE approach has resulted in a higher nerve fiber count and density.[37] In cases where the neurotrophic cornea is due to a localized ocular defect, intact sensation may still be present in the ipsilateral V1 distribution. In such cases, the ipsilateral supraorbital or supratrochlear nerves can be considered. A contralateral donor nerve will be necessary if the cause is a central defect.[38]
Direct Corneal Neurotization
The direct corneal neurotization technique, first described by Terzis et al in 2009, involves transferring nerves from the supratrochlear and supraorbital nerves to the corneoscleral limbus of the affected neurotrophic cornea.[1] This technique previously required open dissection, a coronal incision, and isolating, transecting, and tunneling branches of the supratrochlear and supraorbital nerves across the nasal bridge. The externalized nerve ends were then sutured in the sub-Tenon perilimbal space with 10-0 nylon to avoid complications associated with large incisions.
An endoscopic and minimally invasive direct corneal neurotization technique has been developed to minimize the invasiveness, involving two 1 cm sagittal incisions in the scalp instead of a coronal incision.[38][39] Dissection is done with an endoscope until the superior orbital rim is visualized. Further dissection with the endoscope can be done to visualize the deep branch of the supraorbital nerve. Ipsilateral nerves can be accessed solely via the upper lid incision, followed by transection and passage to the affected cornea. The choice between using an ipsilateral or contralateral supraorbital nerve for the procedure is determined by preoperative sensory testing.[23][36]
Indirect Corneal Neurotization
Sometimes, the direct transfer of the donor nerves to the affected cornea is impossible, and interposition autografts are used to bridge the gap. Site locations include the sural nerve, great auricular nerve, and lateral antebrachial cutaneous nerve.[9][24][40][41][42] Another commonly used option is the contralateral supratrochlear nerve accessed via an upper eyelid crease or sub-brow incision. Alternatives to the contralateral supratrochlear nerve include the supraorbital or infraorbital nerves, with autografts ideally being 10 to 15 cm or longer to ensure tension-free anastomosis.[43] Securing the nerve fascicles includes suturing and corneoscleral tunneling with the overlying conjunctiva to reduce risks of inflammation, scarring, and desiccation.
Processed nerve allografts have been used as a replacement for autograft nerve tissue.[44][45] ETE and ETS coaptations have been described from the ipsilateral and contralateral supraorbital, supratrochlear, and infraorbital nerves.[45] Sweeney et al found similar recovery time course and sensation level return for processed nerve allografts compared with nerve autografts in corneal neurotization, between ETE and ETS, laterality, and location of the donor nerve.[45]
Table 3. Summary of the Different Direct and Indirect Techniques for Corneal Neurotization
| Direct Corneal Neurotization | Indirect Corneal Neurotization |
|
Contralateral supratrochlear and supraorbital nerve Contralateral supraorbital nerve Ipsilateral infraorbital nerve Ipsilateral supraorbital nerve Bilateral ipsilateral supraorbital nerve |
Ophthalmic-sural-major occipital anastomosis End-to-end sural to contralateral ST nerve anastomosis Greater auricular nerve to contralateral ST nerve anastomosis End-to-side sural to contralateral ST anastomosis End-to-end sural to contralateral SO nerve anastomosis Lateral antebrachial cutaneous to contralateral SO nerve anastomosis End-to-end sural to contralateral ST and SO Bilateral end-to-end sural to ipsilateral ST End-to-side sural to ipsilateral ST End-to-end sural to ipsilateral ST End-to-end sural to ipsilateral SO End-to-end sural to ipsilateral IO End-to-side sural to GAN |
GAN, greater auricular nerve; IO, infraorbital; LAC, lateral antebrachial cutaneous; SO, supraorbital; ST, supratrochlear
Direct corneal neurotization, eg, utilizing the supraorbital or supratrochlear nerves, often results in quicker restoration of corneal sensation than indirect methods. Direct techniques provide a more immediate and robust reconnection of sensory pathways. A rapid recovery is usually preferred when possible. Direct neurotization achieves a faster clinical response, while indirect neurotization requires new axons to grow down the length of the nerve graft to the cornea, which can take approximately 6 months.
Indirect corneal neurotization requires microneurovascular surgical experience, as the nerves must be connected or "coapted." Coaptations can be performed "end-to-end," which maximizes the axonal load for neurotization. However, this outcome is achieved in exchange for losing innervation to the original dermatome supplied by the nerve. Coaptation may also be performed "side-to-end," which diverts fewer axons to the cornea but may result in reduced loss of sensation of the coapted nerve. Complication rates do not differ based on the donor site between indirect and direct methods, as long as appropriate techniques and asepsis are followed.
The choice between direct and indirect corneal neurotization is significantly influenced by the surgeon's experience with each technique and their preference, as well as the clinical history and results of preoperative neurotization assessment. The great auricular nerve is a suitable donor nerve as it bypasses the trigeminal innervation, which can be advantageous in patients with bilateral facial anesthesia. Using the great auricular nerve as a donor also avoids the loss of facial sensation in the areas of the frontal or supratrochlear nerves. The great auricular nerve also has a higher axon count than the supratrochlear or supraorbital nerve, allowing more robust neurotization.
Complications
Procedural-specific complications include site infection, bleeding, significant scarring, hematoma formation, neuroma, and reactive tissue or bony overgrowth at the dissection site. The lack of sensation in the cornea increases the risk of microtrauma, breakdown of the corneal epithelium, and delayed wound healing.[5][6][9] Reduced tear production and compromised epithelial function also occur, leading to decreased expression of neurotrophic growth factors. For example, patients with diabetes may have an unstable tear film and reduced corneal sensitivity. As a result, the neurotrophic cornea can develop severe complications such as persistent epithelial defects, corneal thinning, infection, melt, and perforation. These complications can lead to permanent scarring and vascularization, ultimately limiting vision.
In indirect corneal neurotization, sensation may be transferred to the donor nerve site in the early postoperative period.[43][44] Numbness in the donor nerve region or secondary harvest site improved or proved tolerable by 6 months after surgery.[17][41] Hyperesthesia, neuropathic pain, and localized scarring have been rarely reported.[18][46][47] Corneal transplantation can be performed several years after neurotization to aid visual rehabilitation. Complications of corneal transplantation may include delayed re-epithelialization after surgery and microbial keratitis.[21][22]
Clinical Significance
Corneal nerves have an essential role in the growth and repair of the cornea. Neurotrophic corneas have poor healing ability and are challenging to manage with reduced visual outcomes. Traditional management focused on supportive treatments but did not address the root causes (loss of corneal nerves and growth factors). Corneal neurotization describes various surgical methods that directly address nerve loss by transferring nerves to the perilimbal region to stimulate new nerve growth. The choice of neurotization technique is individualized, considering the donor nerve sensory function, caliber and axon count of the nerves, proximity to the recipient cornea, surgical accessibility, and the surgeon's experience.
Both direct and indirect corneal neurotization have demonstrated high improvement rates in Mackie grading within the first 6 months.[9][14][45] Although topical recombinant human nerve growth factor can improve the ocular surface regarding epithelial healing, it does not restore corneal sensation compared with corneal neurotization. Patients may report pain and discomfort in the first few weeks after surgery, with objective improvement after 6 months.[18][21][48] Improvements have been reported to continue for about a year after surgery. Any improvement in vision depends on the presence of corneal scarring, amblyopia, and underlying ocular comorbidity. Long-term retainment in corneal sensation needs to be established. The optimal timing, reactivation risk, and antiviral regimen in patients with herpetic disease undergoing corneal neurotization remain unclear. The impact of human recombinant nerve growth factor post-neurotization requires further research.
Enhancing Healthcare Team Outcomes
Patients with moderate to severe neurotrophic keratopathy are at higher risk of corneal melt and perforation due to corneal anesthesia and persistent epithelial defects. Early identification and management of these conditions is important to reduce morbidity. Caring for patients with neurotrophic keratopathy requires a collaborative approach among healthcare professionals to ensure optimal patient-centered care and outcomes. Ophthalmologists, including cornea and oculoplastic specialists, plastic surgeons, maxillofacial surgeons, ear, nose, and throat surgeons, advanced clinicians, nurses, pharmacists, optometrists, and other healthcare professionals should have the essential clinical skills and knowledge to manage patients with this condition safely. They should recognize the clinical presentation and severity stages and understand the different surgical techniques of corneal neurotization.
Patient and caregiver education about medication, symptoms, and signs of surgical complications is essential to prevent morbidity due to corneal neurotization and the sequelae of neurotrophic keratopathy. The ethical considerations of this surgical method mostly revolve around ensuring that patients provide informed consent and that their autonomy is respected when making decisions regarding corneal neurotization treatment.
Efficient interprofessional communication facilitates optimal information sharing and cooperative decision-making among the interprofessional team. An essential component is a planned approach incorporating evidence-based tactics to maximize treatment efficacy and reduce adverse events. Effective care coordination is critical for managing the patient's progression from diagnosis to therapy and follow-up, reducing mistakes, and improving patient safety. Healthcare professionals can enhance team performance and improve patient outcomes by adopting the principles of skill, strategy, ethics, duties, interprofessional communication, and care coordination. This multifaceted, comprehensive approach allows for the optimal delivery of patient-focused care.
Media
(Click Image to Enlarge)
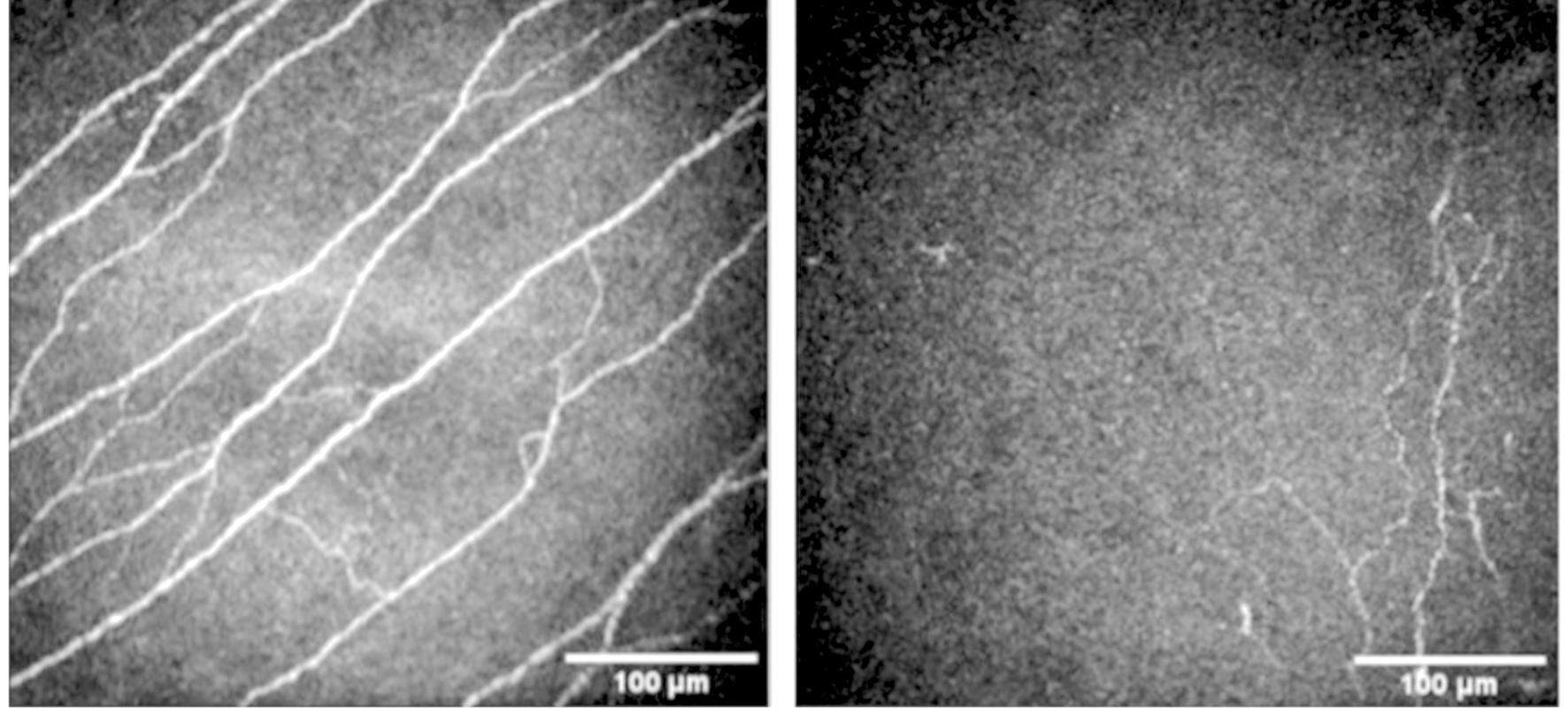
Neurotrophic Cornea. In vivo confocal microscopy (IVCM) examination (Heidelberg Retina Tomograph III with Rostock Cornea Module (HRT3/RCM), Heidelberg Engineering GmbH., Heidelberg, Germany) of a normal (right) and neurotrophic cornea (left). There is reduced density of corneal nerves in the neurotrophic cornea compared with the normal cornea.
Contributed by L Fu, MD
References
Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plastic and reconstructive surgery. 2009 Jan:123(1):112-120. doi: 10.1097/PRS.0b013e3181904d3a. Epub [PubMed PMID: 19116544]
Kim JS, Rafailov L, Leyngold IM. Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic plastic and reconstructive surgery. 2021 Jan-Feb 01:37(1):42-50. doi: 10.1097/IOP.0000000000001676. Epub [PubMed PMID: 32332687]
Level 2 (mid-level) evidenceSamii M. [Operative reconstruction of injured nerves]. Langenbecks Archiv fur Chirurgie. 1972:332():355-62 [PubMed PMID: 4345819]
Saad S, Abdelmassih Y, Saad R, Guindolet D, Khoury SE, Doan S, Cochereau I, Gabison EE. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. The ocular surface. 2020 Apr:18(2):231-236. doi: 10.1016/j.jtos.2019.11.008. Epub 2019 Nov 20 [PubMed PMID: 31759182]
Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye and brain. 2018:10():37-45. doi: 10.2147/EB.S117261. Epub 2018 Jun 28 [PubMed PMID: 29988739]
Ruiz-Lozano RE, Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A. The molecular basis of neurotrophic keratopathy: Diagnostic and therapeutic implications. A review. The ocular surface. 2021 Jan:19():224-240. doi: 10.1016/j.jtos.2020.09.007. Epub 2020 Oct 3 [PubMed PMID: 33022412]
Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (London, England). 2005 Dec:19(12):1276-9 [PubMed PMID: 15550934]
Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. Journal of cellular physiology. 2017 Apr:232(4):717-724. doi: 10.1002/jcp.25623. Epub 2016 Oct 17 [PubMed PMID: 27683068]
Level 3 (low-level) evidenceCatapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, Zuker RM, Borschel GH, Ali A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. The British journal of ophthalmology. 2019 Dec:103(12):1724-1731. doi: 10.1136/bjophthalmol-2018-313042. Epub 2019 Feb 15 [PubMed PMID: 30770356]
Level 2 (mid-level) evidenceTsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999 Oct:106(10):1984-9 [PubMed PMID: 10519596]
Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. The British journal of ophthalmology. 2016 Jan:100(1):22-7. doi: 10.1136/bjophthalmol-2015-306842. Epub 2015 Jul 15 [PubMed PMID: 26178904]
Bonini S, Lambiase A, Rama P, Filatori I, Allegretti M, Chao W, Mantelli F, REPARO Study Group. Phase I Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018 Sep:125(9):1468-1471. doi: 10.1016/j.ophtha.2018.03.004. Epub 2018 Apr 10 [PubMed PMID: 29653861]
Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, Mantelli F, REPARO Study Group. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018 Sep:125(9):1332-1343. doi: 10.1016/j.ophtha.2018.02.022. Epub 2018 Apr 10 [PubMed PMID: 29653858]
Level 1 (high-level) evidenceFogagnolo P, Giannaccare G, Bolognesi F, Digiuni M, Tranchina L, Rossetti L, Dipinto A, Allevi F, Lozza A, Rabbiosi D, Mariani S, Pellegrini M, Cazzola FE, Bagaglia S, Mazzotta C, Gabriele G, Gennaro P, Badiali G, Marchetti C, Campos EC, Biglioli F. Direct Versus Indirect Corneal Neurotization for the Treatment of Neurotrophic Keratopathy: A Multicenter Prospective Comparative Study. American journal of ophthalmology. 2020 Dec:220():203-214. doi: 10.1016/j.ajo.2020.07.003. Epub 2020 Jul 11 [PubMed PMID: 32659280]
Level 2 (mid-level) evidenceSwanson MA, Swanson RD, Kotha VS, Cai Y, Clark R, Jin A, Kumar AR, Davidson EH. Corneal Neurotization: A Meta-analysis of Outcomes and Patient Selection Factors. Annals of plastic surgery. 2022 Jun 1:88(6):687-694. doi: 10.1097/SAP.0000000000003117. Epub 2022 Apr 3 [PubMed PMID: 35502965]
Level 1 (high-level) evidenceWolkow N, Habib LA, Yoon MK, Freitag SK. Corneal Neurotization: Review of a New Surgical Approach and Its Developments. Seminars in ophthalmology. 2019:34(7-8):473-487. doi: 10.1080/08820538.2019.1648692. Epub 2019 Aug 1 [PubMed PMID: 31370735]
Benkhatar H, Levy O, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Corneal Neurotization With a Great Auricular Nerve Graft: Effective Reinnervation Demonstrated by In Vivo Confocal Microscopy. Cornea. 2018 May:37(5):647-650. doi: 10.1097/ICO.0000000000001549. Epub [PubMed PMID: 29474300]
Liu CY, Arteaga AC, Fung SE, Cortina MS, Leyngold IM, Aakalu VK. Corneal neurotization for neurotrophic keratopathy: Review of surgical techniques and outcomes. The ocular surface. 2021 Apr:20():163-172. doi: 10.1016/j.jtos.2021.02.010. Epub 2021 Feb 26 [PubMed PMID: 33647470]
Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Investigative ophthalmology & visual science. 2012 Jul 24:53(8):4926-31. doi: 10.1167/iovs.11-8708. Epub 2012 Jul 24 [PubMed PMID: 22695962]
Park JK, Charlson ES, Leyngold I, Kossler AL. Corneal Neurotization: A Review of Pathophysiology and Outcomes. Ophthalmic plastic and reconstructive surgery. 2020 Sep/Oct:36(5):431-437. doi: 10.1097/IOP.0000000000001583. Epub [PubMed PMID: 31923091]
Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, Allevi F, Cazzola FE, Ponzin D, Lozza A, Bovone C, Scorcia V, Busin M, Campos EC. In Vivo and Ex Vivo Comprehensive Evaluation of Corneal Reinnervation in Eyes Neurotized With Contralateral Supratrochlear and Supraorbital Nerves. Cornea. 2020 Feb:39(2):210-214. doi: 10.1097/ICO.0000000000002083. Epub [PubMed PMID: 31335523]
Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In Vivo Confocal Microscopy Reveals Corneal Reinnervation After Treatment of Neurotrophic Keratopathy With Corneal Neurotization. Cornea. 2018 Jan:37(1):109-112. doi: 10.1097/ICO.0000000000001315. Epub [PubMed PMID: 29053558]
Wisely CE, Rafailov L, Cypen S, Proia AD, Boehlke CS, Leyngold IM. Clinical and Morphologic Outcomes of Minimally Invasive Direct Corneal Neurotization. Ophthalmic plastic and reconstructive surgery. 2020 Sep/Oct:36(5):451-457. doi: 10.1097/IOP.0000000000001586. Epub [PubMed PMID: 32032169]
Lin CH, Lai LJ. Herpetic Corneal Keratopathy Management Using Ipsilateral Supratrochlear Nerve Transfer for Corneal Neurotization. Annals of plastic surgery. 2019 Nov:83(5):553-557. doi: 10.1097/SAP.0000000000002120. Epub [PubMed PMID: 31609805]
Weis E, Rubinov A, Al-Ghoul AR, Yau FM. Sural nerve graft for neurotrophic keratitis: early results. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Feb:53(1):24-29. doi: 10.1016/j.jcjo.2017.10.044. Epub 2018 Jan 10 [PubMed PMID: 29426435]
Wilson SE. Magic Bullets: The Coming Age of Meaningful Pharmacological Control of the Corneal Responses to Injury and Disease. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2022 Sep 26:38(9):594-606. doi: 10.1089/jop.2022.0088. Epub 2022 Sep 26 [PubMed PMID: 36161879]
Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Progress in retinal and eye research. 2019 Nov:73():100762. doi: 10.1016/j.preteyeres.2019.05.003. Epub 2019 May 7 [PubMed PMID: 31075321]
He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Experimental eye research. 2010 Oct:91(4):513-23. doi: 10.1016/j.exer.2010.07.007. Epub 2010 Jul 27 [PubMed PMID: 20650270]
Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Experimental eye research. 2010 Apr:90(4):478-92. doi: 10.1016/j.exer.2009.12.010. Epub 2009 Dec 29 [PubMed PMID: 20036654]
Marfurt C, Anokwute MC, Fetcko K, Mahony-Perez E, Farooq H, Ross E, Baumanis MM, Weinberg RL, McCarron ME, Mankowski JL. Comparative Anatomy of the Mammalian Corneal Subbasal Nerve Plexus. Investigative ophthalmology & visual science. 2019 Dec 2:60(15):4972-4984. doi: 10.1167/iovs.19-28519. Epub [PubMed PMID: 31790560]
Level 2 (mid-level) evidenceMüller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Experimental eye research. 2003 May:76(5):521-42 [PubMed PMID: 12697417]
Level 3 (low-level) evidenceKinoshita S, Tanaka F, Ohashi Y, Ikeda M, Takai S. Incidence of prominent corneal nerves in multiple endocrine neoplasia type 2A. American journal of ophthalmology. 1991 Mar 15:111(3):307-11 [PubMed PMID: 1672053]
Kriszt Á, Losonczy G, Berta A, Takács L. Presence of Fleischer ring and prominent corneal nerves in keratoconus relatives and normal controls. International journal of ophthalmology. 2015:8(5):922-7. doi: 10.3980/j.issn.2222-3959.2015.05.12. Epub 2015 Oct 18 [PubMed PMID: 26558202]
Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Experimental eye research. 2004 Mar:78(3):513-25 [PubMed PMID: 15106930]
Dragnea DC, Krolo I, Koppen C, Faris C, Van den Bogerd B, Ní Dhubhghaill S. Corneal Neurotization-Indications, Surgical Techniques and Outcomes. Journal of clinical medicine. 2023 Mar 13:12(6):. doi: 10.3390/jcm12062214. Epub 2023 Mar 13 [PubMed PMID: 36983215]
Gennaro P, Gabriele G, Aboh IV, Cascino F, Menicacci C, Mazzotta C, Bagaglia S. The Second Division of Trigeminal Nerve for Corneal Neurotization: A Novel One-Stage Technique in Combination With Facial Reanimation. The Journal of craniofacial surgery. 2019 Jun:30(4):1252-1254. doi: 10.1097/SCS.0000000000005483. Epub [PubMed PMID: 30908442]
Rönkkö H, Göransson H, Taskinen HS, Paavilainen P, Vahlberg T, Röyttä M. Comparison of Peripheral Nerve Regeneration with Side-to-side, End-to-side, and End-to-end Repairs: An Experimental Study. Plastic and reconstructive surgery. Global open. 2016 Dec:4(12):e1179. doi: 10.1097/GOX.0000000000001179. Epub 2016 Dec 22 [PubMed PMID: 28293523]
Leyngold I, Weller C, Leyngold M, Espana E, Black KD, Hall KL, Tabor M. Endoscopic Corneal Neurotization: Cadaver Feasibility Study. Ophthalmic plastic and reconstructive surgery. 2018 May/Jun:34(3):213-216. doi: 10.1097/IOP.0000000000000913. Epub [PubMed PMID: 28472009]
Level 2 (mid-level) evidenceLeyngold I, Weller C, Leyngold M, Tabor M. Endoscopic Corneal Neurotization: Technique and Initial Experience. Ophthalmic plastic and reconstructive surgery. 2018 Jan/Feb:34(1):82-85. doi: 10.1097/IOP.0000000000001023. Epub [PubMed PMID: 29194285]
Jowett N, Pineda Ii R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. The British journal of ophthalmology. 2019 Sep:103(9):1235-1238. doi: 10.1136/bjophthalmol-2018-312563. Epub 2018 Nov 23 [PubMed PMID: 30470713]
Bourcier T, Henrat C, Heitz A, Kremer SF, Labetoulle M, Liverneaux P. Lateral Antebrachial Cutaneous Nerve as Autologous Graft for Mini-Invasive Corneal Neurotization (MICORNE). Cornea. 2019 Aug:38(8):1029-1032. doi: 10.1097/ICO.0000000000002004. Epub [PubMed PMID: 31246678]
Lau N, Osborne SF, Vasquez-Perez A, Wilde CL, Manisali M, Jayaram R. Corneal Neurotization Using the Great Auricular Nerve for Bilateral Congenital Trigeminal Anesthesia. Cornea. 2022 May 1:41(5):654-657. doi: 10.1097/ICO.0000000000002951. Epub [PubMed PMID: 34839333]
Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA ophthalmology. 2014 Nov:132(11):1289-95. doi: 10.1001/jamaophthalmol.2014.2316. Epub [PubMed PMID: 25010775]
Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C. Minimally Invasive Corneal Neurotization With Acellular Nerve Allograft: Surgical Technique and Clinical Outcomes. Ophthalmic plastic and reconstructive surgery. 2019 Mar/Apr:35(2):133-140. doi: 10.1097/IOP.0000000000001181. Epub [PubMed PMID: 30059392]
Level 2 (mid-level) evidenceSweeney AR, Wang M, Weller CL, Burkat C, Kossler AL, Lee BW, Yen MT. Outcomes of corneal neurotisation using processed nerve allografts: a multicentre case series. The British journal of ophthalmology. 2022 Mar:106(3):326-330. doi: 10.1136/bjophthalmol-2020-317361. Epub 2020 Nov 16 [PubMed PMID: 33199302]
Level 2 (mid-level) evidenceVera-Duarte GR, Jimenez-Collado D, Kahuam-López N, Ramirez-Miranda A, Graue-Hernandez EO, Navas A, Rosenblatt MI. Neurotrophic keratopathy: General features and new therapies. Survey of ophthalmology. 2024 Sep-Oct:69(5):789-804. doi: 10.1016/j.survophthal.2024.04.004. Epub 2024 Apr 26 [PubMed PMID: 38679146]
Level 3 (low-level) evidenceAujla J, Tong JY, Curragh D, Caplash Y, Chehade M, Tumuluri K, Au A, Low N, Avisar I, Sagiv O, Barequet I, Ben Simon G, Selva D. Corneal Neurotization for Neurotrophic Keratopathy: A Multicenter Experience. Ophthalmic plastic and reconstructive surgery. 2024 Nov-Dec 01:40(6):655-660. doi: 10.1097/IOP.0000000000002684. Epub 2024 Apr 11 [PubMed PMID: 38624152]
Gross JN, Bhagat N, Tran K, Liu S, Boente CS, Ali A, Borschel GH. Minimally Invasive Corneal Neurotization: 10-Year Update in Technique Including Novel Donor Transfer of the Great Auricular Nerve. Plastic and reconstructive surgery. 2024 Oct 1:154(4):795e-798e. doi: 10.1097/PRS.0000000000011250. Epub 2023 Dec 22 [PubMed PMID: 38194587]