Introduction
Water vapor thermal ablation of the prostate (WVAP) is a minimally invasive surgical therapy for symptomatic benign prostatic hyperplasia (BPH). WVAP was introduced and approved by the United States Food and Drug Administration in 2015.[1] The incidence of BPH increases with age; 50% to 75% of men 50 years and older and 80% of men 70 years and older report clinically significant lower urinary tract symptoms due to BPH.[2] Lower urinary tract symptoms from BPH-induced extrinsic compression of the prostatic urethra include but are not limited to urinary urgency, frequency, hesitancy, intermittency, incomplete bladder emptying, nocturia, and a diminished urinary stream.[3][4] BPH is also associated with considerable morbidity characterized by urinary retention, recurrent urinary tract infections, urolithiasis, persistent hematuria, and renal insufficiency.[5]
Standard therapies for symptomatic BPH include continuous oral medications and various surgical procedures. The goal of WVAP is to relieve extrinsic compression of the prostatic urethra by inducing coagulative necrosis of hyperplastic prostatic tissue via the directed application of radiofrequency-generated thermal energy in the form of water vapor or steam.[3] Water vapor has a lower mass and density than prostatic tissue and moves easily through cellular interstitial spaces. However, water vapor cannot pass through natural barriers such as the tissue planes separating prostatic zones.[6] This inherent characteristic of water vapor promotes the specific targeting of the prostatic transition zone where most BPH occurs, minimizing the risk of necrosis to the bladder neck, striated urinary sphincter, prostatic capsule, seminal vesicles, prostatic vascular supply, and rectum.[7][8]
WVAP may be performed in the office or operating room. While the procedure requires an average of 10 minutes to perform, complete resorption of necrotic prostatic tissue may take up to 12 months. The resolution of lower urinary tract symptoms occurs progressively, and optimal results may not be achieved until tissue resorption is complete.[1][7]
The effectiveness of WVAP in alleviating clinically significant lower urinary tract symptoms has been reviewed in more than 20 published series and reports, including 5 prospective randomized studies and 2 randomized-controlled trials involving more than 2,000 patients followed for up to 5 years. Significant improvements were noted consistently in troubling symptoms, overall quality of life, post-void residual volumes, and peak urinary flow rates.[9] Additionally, WVAP is the only minimally invasive surgical therapy with proven efficacy for reducing lower urinary tract symptoms in patients with prostatic volumes of greater than 80 mL or obstructing median prostatic lobes.[10][11][12]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Anatomy of the Prostate Gland
The conically-shaped prostate gland is located inferior to the bladder, posterior to the pubic symphysis, and anterior to the rectum; this anatomic location makes the prostate clinically accessible via digital rectal examination.[13] The base of the prostate is at the bladder neck, and its apex is close to the external urethral sphincter. Prostate size varies widely among individuals and tends to increase with time; the average prostate weighs approximately 33 g.[14]
Lobes of the prostate gland
The prostate is clinically divided into 5 lobes: the anterior, median, posterior, and left and right lateral lobes. The prostate surrounds the prostatic urethra; as BPH progresses, the median and lateral lobes of the gland enlarge and encroach on the urethral lumen and bladder outlet, causing increasing degrees of obstruction to urine outflow, and symptoms result.[4]
Zones of the prostate gland
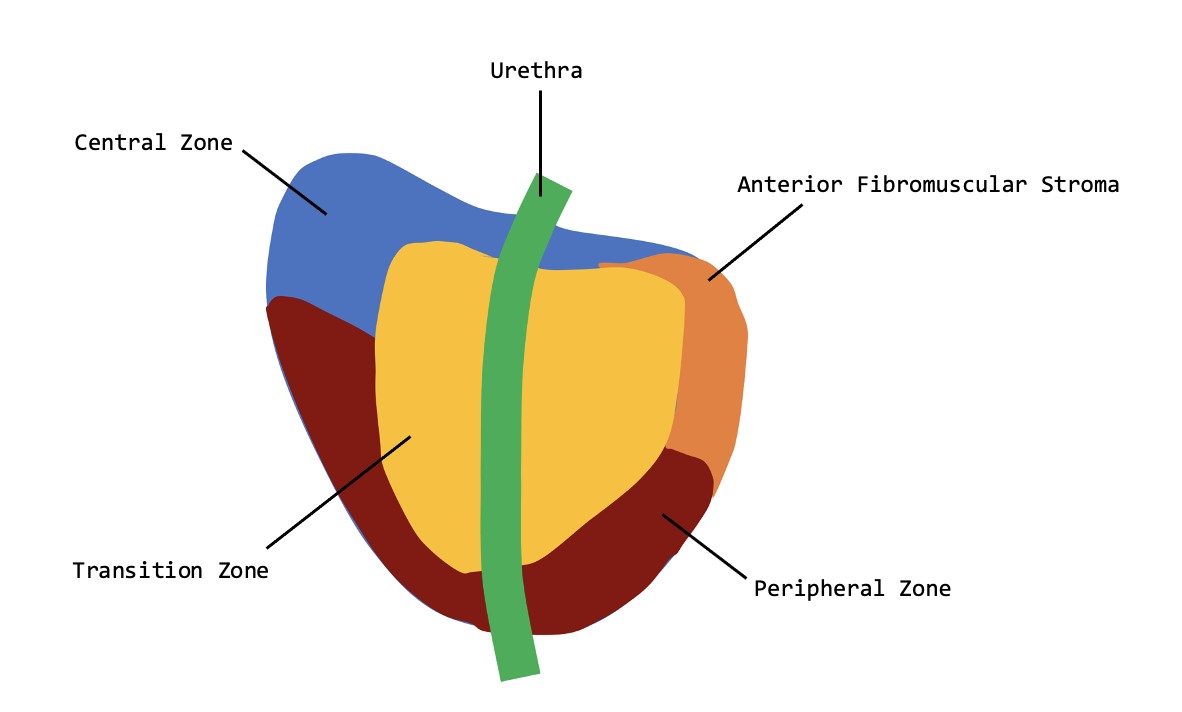
The human prostate is divided by function into major glandular regions or zones: the transitional, central, and peripheral zones (see Image. Prostate zones). A fourth region called the anterior fibromuscular stroma may also be considered a zone but is not glandular.[15]
The transitional zone surrounds the urethra between the bladder and the verumontanum. The transitional zone typically comprises only 5% of the volume of the normal prostate but is the principal site of the pathogenesis of BPH. When the transitional zone is enlarged and the nodules advance, the peripheral and central zones undergo compression and displacement towards the base of the prostate, where they may overlap the enlarged transitional zone.[16] Enlarged lateral lobes originate in the transitional zone of the prostate.
The central zone of the prostate is a wedge of tissue that constitutes most of the base of the prostate, is located between the peripheral and transitional zones, and surrounds the ejaculatory ducts. The central zone ducts run proximally to and closely follow the ejaculatory ducts.[16] The median lobe of the prostate is located between the ejaculatory ducts and the urethra within the central zone, extending proximally to the bladder neck and trigone. Median lobe enlargement may interfere with voiding, especially if an intravesical extension is large enough to create a ball-valve effect by blocking the bladder outlet during micturition. In such cases, this median lobar tissue must be eliminated to optimize voiding.[17]
The peripheral zone comprises the remainder of the prostate gland. The peripheral zone surrounds most of the central zone and extends caudally, partially surrounding the distal portion of the urethra. The peripheral zone ducts exit exactly laterally from the posterolateral recesses of the urethral wall. Most of the prostatic glandular tissue is found in the peripheral zone; this is the principal site of prostatitis and 70% of prostatic carcinomas.[18][19] The peripheral zone includes the proximal urethral segment of the prostate.[16]
The anterior fibromuscular stroma is a strip of fibromuscular tissue anterior to the transitional zone. This stroma is adjacent to the smooth muscle of the bladder superiorly and the skeletal muscle of the external sphincter inferiorly.[8][20]
Neurovascular supply to the prostate gland
The primary arterial vascular supply to the prostate gland is the prostatic arteries, the terminal branches of the inferior vesical arteries.[15] The inferior vesical arteries arise from various branches of the internal iliac artery, including the internal pudendal inferior vesical and middle rectal arteries.[15][21] The prostatic arteries are short and branch into the capsular and urethral groups of arteries.[21] The urethral branches of the prostatic arteries enter the prostate at the bladder neck at the 5- and 7-o’clock positions, running caudally along the urethra.[22]
The most common sites of arterial bleeds in other endoscopic procedures, such as transurethral resection of the prostate (TURP) and holmium laser enucleation of the prostate (HoLEP), originate from arteries derived from the capsular branch of the prostatic artery. These capsule branch derivative arteries enter the capsule and run along the outer prostate surface at the 2-, 5-, 7-, and 11-o’clock positions. The risk of bleeding in WVAP is lower than for TURP or HoLEP because these arteries are generally not encountered.[22]
The prostatic venous plexus surrounds the prostate gland and drains into the internal iliac vein via the inferior vesical vein. The prostatic venous plexus provides the primary venous drainage for the prostate gland.[15]
The neurovascular bundle of the prostate gland is necessary for processes involved in erection, ejaculation, and urinary continence. This bundle is within the space enclosed by the prostatic, lateral pelvic, and Denonvillier fascias.[23] Localized electrosurgical currents employed in procedures like TURP can sometimes cause damage to these nerves and contribute to postprocedural erectile dysfunction. This bundle is external to the prostate and is, therefore, unaffected by WAVP.
Important Surgical Landmarks
Surgical landmarks critical to WVAP include the location of the ureteric orifices, bladder neck, median and lateral prostatic lobes, verumontanum, and urinary sphincter. The anterior or ventral edge of the external sphincter muscle is more proximal than the posterior or dorsal edge; these locations should be noted, as should the distance from the bladder neck to the verumontanum.
Physiology of the Prostate Gland
The sole function of the prostate is to produce secretions for the seminal vesicles that nourish the sperm and promote fertility. The prostatic fluid contains citric acid, fibrolysin, acid phosphatase, and proteolytic enzymes, which improve the liquefaction of semen. These secretions also provide most of the immunoglobulin A in the seminal fluid. Prostatic secretions account for approximately 30% of the total semen volume.
Pathophysiology of Benign Prostatic Hyperplasia
The pathophysiology of BPH is not fully understood; testosterone has been implicated. Dihydrotestosterone is a potent biological derivative; androgen receptor signaling in the prostate is regulated by 5α-reductase isoenzymes that convert testosterone to dihydrotestosterone. It has been hypothesized that intraprostatic dihydrotestosterone levels increase with age despite falling serum testosterone levels. Metabolic or inflammatory insults may also initiate BPH.[24]
Symptomatic BPH typically involves the lateral lobes from the transitional zone but may also involve the median lobe from the central zone. The successful surgical treatment of symptomatic BPH may depend on effectively eliminating all obstructive tissue interfering with urine outflow; this includes the median lobe when it is involved in the hyperplastic process.
Indications
General Indications for the Treatment of Symptomatic Benign Prostatic Hyperplasia
The general indications for the treatment of symptomatic BPH include subjectively bothersome symptoms, such as a weak or intermittent urinary stream, incomplete bladder emptying, and nocturia, which significantly impact the quality of life. Treatment should be offered when patients have moderate-to-severe symptoms indicated by an International Prostate Symptom Score greater than 8 with age-stratified increases in prostate size and postvoid residual volume and a decrease in maximum urine flow rate (Qmax).
There are many options available to manage symptomatic BPH. Clinicians must understand each option's indications, distinctions, advantages, and disadvantages to counsel and guide patients toward optimal therapy properly. The initial management of symptomatic BPH is almost universally pharmacological with α-blockers, with or without adding 5α-reductase inhibitors.[25][26][27] However, when or if the patient experiences symptoms refractory to pharmacologic therapy, cannot tolerate the adverse effects of such therapy, or wishes to proceed to a more permanent or effective therapy, reasonable therapeutic options are available.
Therapeutic options beyond pharmacologic therapy can be classified as minimally invasive surgical treatments, traditional cavitating endoscopic procedures, simple prostatectomy, or prostatic artery embolization. A discussion of traditional cavitating endoscopic procedures and simple prostatectomy is beyond the scope of this activity.[17][28][29][30][31][32] Prostatic artery embolization is technically demanding, not widely available, offers marginal results with a retreatment rate of 21%, and is intended only for very select patients deemed unacceptable candidates for all other therapeutic options.[33][34]
Indications for Minimally Invasive Surgical Therapies for Symptomatic Benign Prostatic Hyperplasia
Minimally invasive surgical therapies include WVAP, prostatic urethral lift, Optilume® BPH, and the temporarily implanted nitinol device (iTind™).[35][36] All available minimally invasive surgical therapies preserve sexual function, provide measurable relief of lower urinary tract symptoms, have low perioperative morbidity with mild and transient adverse effects, and can generally be performed under a range of anesthetic options in either the clinic or operating room. The optimal therapeutic option is influenced by the prostatic size and morphology, the severity of lower urinary tract symptoms, patient preference and comorbidities, and the risk and benefit profile of each treatment. A comparison of these minimally invasive surgical therapies is shown in Table. A Comparison of Minimally Invasive Surgical Therapies for Symptomatic BPH.[35][37][38][39][40][41][42][37]
Table. A Comparison of Minimally Invasive Surgical Therapies for Symptomatic BPH.
| Water vapor thermal ablation | Prostatic urethral lift | Optilume® BPH | Temporarily implanted nitinol device (iTind™) | |
| Availability of published data | 5 years | 5 years | 12 months | 12 months |
| Recommended prostate size | 30 to 100 cm3 | 30 to 80 cm3 | 30 to 60 cm3 | 25 to 75 cm3 |
| Suitable for obstructive median lobes | Yes | No | No | No |
| Symptom relief | Improvement in 2 to 4 weeks; durable to 5 years | Improvement in 2 weeks; durable to 5 years | Improvement in 3 months; sustained at 12 months | Improvement in 3 months; sustained at 12 months |
| Retreatment rate | 4.4% at 5 years | 13.6% at 5 years | not available | 6.5 % at 2 years |
WVAP is recognized as an effective therapeutic option for symptomatic BPH by the American Urological Association.[43] WVAP appears to be the optimal minimally invasive therapeutic option in men with moderately enlarged prostates up to 100 cm3 in size who want to preserve sexual function and are willing to accept the potential risk of slightly reduced efficacy and longevity afforded by traditional cavitating procedures.[10][11][12] WVAP requires approximately 10 min of operative time and is ideal for patients in whom a prolonged anesthetic or a more invasive surgical option is deemed too risky. While TURP and hOLEP have retreatment rates of less than 3%, these traditional cavitating procedures are more aggressive, more invasive, require longer operative times, and carry increased anesthesia risks.
WVAP is proven effective in all prostate morphologies, including enlarged or obstructing median lobes; all other minimally invasive prostatic therapies are largely ineffectual or not recommended.[10][11][41] While symptom relief takes longer following WVAP and the prostatic urethral lift, WVAP has a superior long-term retreatment rate and does not increase the risk of infection or stone formation.
Contraindications
There are absolute and relative contraindications to WVAP as a treatment for symptomatic BPH. A thorough evaluation of the clinical history of potential surgical candidates is required to identify these contraindications and optimize procedural selection.
Absolute contraindications to WVAP include the presence of urinary sphincter or penile prosthetic implants and a personal history of prostatic radiotherapy. The WVAP procedure may damage sphincter or penile prostheses.[44] Prostatic radiotherapy induces interstitial scarring that limits the dispersion of water vapor.[45]
A comprehensive perioperative evaluation may minimize or eliminate many of the relative contraindications for WVAP. All patients considering any surgical intervention for symptomatic BPH should be evaluated for inadequate bladder contractility and urinary retention with urodynamic testing.[44][45]
WVAP does not produce tissue samples for histological analysis; patients with suspected prostate cancer should be thoroughly evaluated with traditional diagnostic methods before undergoing WVAP for symptomatic BPH. Additionally, all patients considering WVAP should be assessed for an active urinary tract infection, and sterilization of bacteriuria is required before the procedure is performed.[45] Limited data exists regarding the efficacy of WVAP in patients with a prostatic volume greater than 120 cm3; caution in this patient population is recommended.[1] Patients who have undergone a prostatic urethral lift will have implants and clips that can become infected and predispose to stone formation following WVAP; perioperative evaluation with rigid cystoscopy is recommended.[45]
Patients with stenosis of the bladder neck or Marion disease may not be optimal candidates for WVAP. WVAP acts in the lateral lobes of the transitional zone and median lobe of the central zone; it is ineffective at the bladder neck.[8][44] Some experts have suggested performing a bladder neck incision in selected patients with smaller prostates and high or tight bladder necks to minimize the need for retreatment later.[8][46]
Equipment
The equipment required to perform WVAP typically includes:
- Convective water vapor generator system and delivery device
- Bed to support lithotomy position
- Sterile skin preparation solution
- Sterile patient drapes
- Warmed normal saline and tubing with an IV pole
- Topical lidocaine gel
- 4-mm, 30-cm, 30-degree cystoscope lens with monitor and light source
- 2-way urinary catheter with drainage bag
- Transrectal ultrasound (optional; for local prostatic anesthesia)
- Local anesthetic (optional).
Personnel
When WVAP is performed in the clinic setting, only the primary surgeon and an assistant are required.
If WVAP is performed in an operating room, the personnel required typically include:
- Surgeon
- Surgical technician or operating room nurse
- Circulating or operating room nurse
- Anesthesia personnel.
Preparation
Medical History and Physical Examination
A comprehensive medical history must be obtained from all patients considering surgical intervention for lower urinary tract symptoms secondary to BPH. Symptoms of urinary frequency and urgency, incomplete bladder emptying, intermittency, straining, and nocturia should be fully explored; an AUA or IPSS can facilitate a discussion of these symptoms, promote an understanding of the patient's perception of these symptoms, and serve as a baseline for comparison following an intervention. Dysuria, hematuria, incontinence, and a history of urolithiasis must be clarified. Previous medical or surgical interventions for lower urinary tract symptoms should be identified.
Additionally, inquiries must be made about the medical and surgical history, specifically on anesthetic fitness, urological intervention, or prostatic radiotherapy. Erectile function should be scored with a measurable tool such as the International Index of Erectile Function-5 (IIEF-5). Risk factors for urological disease, such as a personal history of tobacco use, occupational exposures to aromatic amines, and a family history of BPH or prostate cancer, should be assessed.
A physical examination should include an inspection of the external genitalia with particular attention to the presence of metal stenosis, lichen sclerosis, and phimosis. A digital rectal examination to estimate prostate size and aid in prostate cancer screening is required.
Preprocedural Investigations
Laboratory testing: Urinalysis with microscopy, culture, and sensitivity analysis is required. The prostate-specific antigen (PSA) should be measured; if elevated, prostate cancer should be excluded.
Uroflowmetry and urodynamic testing: Uroflowmetry should be performed to determine the severity and etiology of lower urinary tract symptoms and should include the maximum urinary flow (Qmax), postvoid residual volume, and voiding pattern analysis. Urodynamic studies are not essential for all patients but are indispensable when alternate or coexisting pathologies like detrusor failure are suspected. Men who present with urinary retention should have urodynamic studies for this reason.[45]
Transrectal ultrasonography: Transrectal ultrasonography provides an accurate and objective prostatic size and morphology assessment. WVAP is most efficacious in men with a prostatic volume of 80 cm3 or less; the procedure can be offered to select men with prostatic volumes of no more than 120 cm3.[3][10]
Flexible cystoscopy: Flexible cystoscopy is recommended for all patients to assist in planning the number and position of WVAP injections; planning requires assessing the distance from the bladder neck to the verumontanum, the size of the lateral lobes, and the presence of an obstructing median lobe or high bladder neck. Flexible cystoscopy allows retroflexion to inspect the bladder neck, ureteric orifices, and intravesical protrusion of the prostate lobes. Finally, a preoperative flexible cystoscopy will help establish the pain tolerance of patients considering a procedure under local anesthesia.[8]
Shared Decision-Making and Patient Selection of Therapy
Clinicians should employ multiple methods of communication beyond verbal consultation, such as images, videos, and print materials, to ensure patients are thoroughly informed of the logistics, risks, benefits, and expected clinical course of WVAP. Attempts should be made to understand the therapeutic goals of the patient to the greatest extent possible. The patient should be counseled about reasonable alternative therapeutic options, including but not limited to watchful waiting, medical management, other minimally invasive surgical therapies, and endoscopic cavitating procedures.
Technique or Treatment
Mechanism of Action
The WVAP system comprises 2 primary components: a radiofrequency power supply generator and a single-use transurethral delivery device. The delivery device has a standard rigid cystoscope lens, permitting direct visualization during the procedure. Thermal energy is generated by applying radiofrequency current to an inductive coil, which heats the water and creates vapor. The device administers the heated water vapor at a consistent energy setting of approximately 208 J directly into prostatic tissues through a retractable needle.[3] WVAP employs the principle of convection, which is the transfer of heat by moving a gas or liquid within a space. Conversely, conductive energy transfer employs heat applied directly to a stationary object that moves through the object along a gradient from higher to lower temperature.[6] Thermodynamically, conductive heat transfer requires long treatment times and considerable energy deposition to affect thermal tissue destruction and ablation with an obligatory temperature gradient.[7]
Heated water vapor, injected directly into the prostate at 217.4° F (103 °C), rapidly and evenly disperses throughout the interstitium.[6] Heated water vapor condenses into liquid when it contacts cellular membranes, releases thermal energy to the tissue, and increases tissue temperatures to 158 to 176 °F (70 to 80 °C). This temperature increase causes instantaneous cell death and the formation of a spherical-shaped ablated lesion.[47]
Preoperative Protocols
A preprocedural timeout should be performed. The patient should be placed in the lithotomy position.[45] Ensure the space beneath the table is free of obstruction. The external genitalia and perineum should be thoroughly cleansed with a povidone-iodine solution.[48] Administer intravenous antibiotic prophylaxis based on preoperative urine culture results. If the preoperative urine is sterile, administer prophylactic antibiotics based on local guidelines for transurethral surgery; a first-generation cephalosporin is typical.[44][49] Alternative regimens, especially in patients with allergies to cephalosporins, include quinolones and gentamicin.[49]
Anesthesia and Pain Management
Convective water vapor thermal ablation is feasible and appropriate within the ambulatory setting. However, the subjective experiences of discomfort, pain, and anxiety, as well as institutional capabilities, may preempt an ambulatory procedure. The choice of anesthesia and pain management should be a collaborative decision involving the urologist, patient, and anesthetist.[45] Anesthetic options include general anesthesia, oral or intravenous sedation, or a local anesthetic prostate block with lidocaine or bupivacaine.[48] A prostate block should be performed with ultrasound guidance approximately 10 min before beginning the procedure.[48]
Operative Technique
Lateral lobes: Administer 10 mL of 2% lidocaine jelly into the urethra, irrespective of other anesthetic methods. Insert the treatment device with the rod lens into the urethra. Perform cystoscopy to assess the prostate size and contours, the presence of an obstructing median lobe, the bladder mucosa, and ureteric orifices.[8][45][50]
Position the delivery device 1 cm distal to the bladder neck at the bulk of the targeted tissue. Deploy the retractable needle, which deploys at 90°, measures 10.25 mm in length, and has 12 emitter holes to release the heated water vapor in a spherical array. Deploying the needle permits the activation of the injection mechanism. Each injection is a single treatment and takes 9 seconds to deliver completely. It is recommended to leave the needle in place for another 1 to 2 seconds to allow the water vapor to change phase and release its thermal energy completely.[7][45] Each injection creates a spherical thermal lesion of approximately 4 cm3
Administer additional injections every 0.5 to 1.0 cm from the initial injection site, progressing caudally down the length of the prostatic urethra until the proximal edge of the verumontanum is reached.[7] The goal is the creation of an approximate 1.5 to 2.0 cm lesion within the obstructing median and lateral lobes of the central and transitional zones of the prostate. The number of treatments for each lobe depends on the distance between the bladder neck and the verumontanum. This distance can be closely estimated; the visible length of the cystoscopic view through the delivery device is 0.5 cm.
An estimated distance of less than 2.0 cm typically requires 1 or 2 injections, distances between 2.0 and 3.0 cm typically require 2 or 3 injections, and distances greater than 3.0 cm may require 3 or 4 injections.[8] Injections should be performed to create contiguous, overlapping lesions aligned with the natural slope of the urethra.
The injections should be completed fully on one side of the gland before proceeding to the opposite side or median lobe. This sequential approach maximizes the latent heat from prior treatments on one side, enhancing the overall effectiveness of the procedure.
If treatment of the median lobe is not required, remove the device and place an indwelling Foley catheter.
Median lobe: Insert the needle at a 45-degree angle halfway between the base and apex of the median lobe. One treatment angled toward either side is sufficient in most patients requiring median lobe therapy.[45] However, if an intravesical protrusion is present, the treatment should be initiated 1.0 cm from the protrusion.[45] When the injection(s) are complete, remove the device and place an indwelling Foley catheter.
Postoperative Protocols
The Foley catheter typically should remain in situ for 2 to 14 days and will vary according to the number of injections; most patients can undergo catheter removal and a voiding trial on the third or fourth postoperative day.[8][10][45] A personal history of bladder hypotonicity, preoperative urinary retention, and higher preoperative postvoid residual urine volumes increases the risk of voiding trial failure.[51] Patients with preprocedural urinary retention should receive continuous bladder drainage with an indwelling catheter for 4 weeks to allow for completion of coagulative necrosis and tissue resorption.[45]
Patients should expect storage-type lower urinary tract symptoms for up to 4 weeks; a short course of nonsteroidal anti-inflammatories may benefit.[45] Patients should cease their oral BPH medications as voiding symptoms improve.[45] All patients should be evaluated at 1 to 3 months following WVAP.
Complications
Performing WVAP requires approximately 10 minutes of operative time, and intraoperative complication rates are low. A literature review of 17 studies evaluating 1481 patients undergoing WVAP described no intraoperative complications.[52]
Most postoperative complications of WVAP can be classified as Clavien Dindo Grade 1 or 2.[53] Necrotic prostatic tissue is eradicated through normal inflammatory processes, contributing to dysuria, urinary urgency, and hematuria. In patients with indwelling catheters, these processes also predispose to lower urinary tract infections.[41] There is a risk of urinary retention following WVAP; this risk is increased for men with preoperative urinary retention.[45] The resolution of lower urinary tract symptoms typically takes a minimum of 3 months; men with a history of prior surgical interventions for symptomatic BPH are at increased risk of persistent lower urinary tract symptoms following WVAP.[54]
Recurring lower urinary tract symptoms are the most significant long-term complication following WVAP; 4.4% of men undergoing WVAP require surgical retreatment within 5 years, and 11% of men require prostatic medication within 5 years.[40] Patients requiring surgical retreatment are more likely to have an obstructing or high bladder neck, lateral lobe asymmetry, a persistently obstructive median lobe, or unexpected prostatic cysts, cavities, or recesses.[46] Men with an obstructing or high bladder neck who require surgical retreatment tend to have smaller prostates and may benefit from a simple bladder neck incision.[46]
Long-term complications other than recurrent lower urinary tract symptoms are rare and include but are not limited to urethral strictures and bladder neck contractions.[55]
Clinical Significance
BPH is a commonly encountered pathologic process; the incidence increases with age. BPH can cause bothersome lower urinary tract symptoms and increase morbidity in affected men. WVAP is an elective minimally invasive surgical treatment for symptomatic BPH that delivers radiofrequency-generated thermal energy to the prostate to induce coagulative necrosis of hyperplastic tissue, thereby relieving obstruction of the prostatic lumen. Thermal ablation stays within the transitional and central zones with a very low risk of injury to the peripheral zone, prostatic capsule, bladder neck, urethral sphincter, or rectum. Electrosurgical stimulation, stimulation, and disruption of the bladder neck are avoided so ejaculatory and erectile functions are preserved. Additionally, the procedure takes approximately 10 minutes to complete and can be performed in various settings.
Compared to other minimally invasive therapies for symptomatic BPH, WVAP provides prolonged symptom relief and is efficacious across a wider range of prostatic volumes. Furthermore, WVAP is the only minimally invasive therapeutic option for symptomatic BPH with proven efficacy in patients with obstructing median prostatic lobes.[10][11][12]
While prostate cancer is not an absolute contraindication to WVAP treatment, the procedure is not a treatment for prostatic malignancies and is strictly for symptomatic outlet obstruction in suitable candidates. However, WVAP is especially useful in men with moderately enlarged prostates who want to preserve sexual function and wish to avoid more invasive surgical therapies. WVAP is also appropriate for patients at increased anesthetic risk and for whom medical therapy is ineffective or poorly tolerated.
Objective improvements in lower urinary tract parameters are routinely noted.[56][57][58] The median improvements are:
- Increase in peak urinary flow rate of 5 mL/sec
- Postvoid residual volumes decrease by 100 mL
- Bladder Outlet Obstruction Index decreases by 53.8
- Prostatic volume decreases by 40%, with the decrease preferentially occurring in areas of urinary flow obstruction
- IPSS symptom score is typically reduced by 50%.
Enhancing Healthcare Team Outcomes
The counseling and education of patients considering WVAP requires an interprofessional team approach. Urologists and advanced practice providers should possess the clinical expertise to effectively communicate the advantages, risks, limitations, and alternatives to WVAP to promote shared decision-making and establish realistic expectations regarding postoperative urinary control.
Nurses guide patients through procedural details, recovery expectations, and postoperative care. Pharmacists guide the appropriate dosages and regimens of oral BPH medications and prophylactic antibiotic therapy. Intraoperative communication between urologists, assistants, nurses, and anesthesia personnel ensures patient safety and reduces complication rates. Efficient communication and coordination among team members ensure consistent patient education and seamless care transitions before, during, and after WVAP. Regular postoperative appointments promote patient satisfaction and optimize outcomes.
Media
(Click Image to Enlarge)
References
Westwood J, Geraghty R, Jones P, Rai BP, Somani BK. Rezum: a new transurethral water vapour therapy for benign prostatic hyperplasia. Therapeutic advances in urology. 2018 Nov:10(11):327-333. doi: 10.1177/1756287218793084. Epub 2018 Aug 12 [PubMed PMID: 30344644]
Level 3 (low-level) evidenceEgan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. The Urologic clinics of North America. 2016 Aug:43(3):289-97. doi: 10.1016/j.ucl.2016.04.001. Epub [PubMed PMID: 27476122]
Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P. Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. The Cochrane database of systematic reviews. 2020 Mar 25:3(3):CD013251. doi: 10.1002/14651858.CD013251.pub2. Epub 2020 Mar 25 [PubMed PMID: 32212174]
Level 1 (high-level) evidenceNg M, Leslie SW, Baradhi KM. Benign Prostatic Hyperplasia. StatPearls. 2025 Jan:(): [PubMed PMID: 32644346]
Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, Lerner LB, Lightner DJ, Parsons JK, Roehrborn CG, Welliver C, Wilt TJ, McVary KT. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline. The Journal of urology. 2018 Sep:200(3):612-619. doi: 10.1016/j.juro.2018.05.048. Epub 2018 Jun 11 [PubMed PMID: 29775639]
Dixon CM, Rijo Cedano E, Mynderse LA, Larson TR. Transurethral convective water vapor as a treatment for lower urinary tract symptomatology due to benign prostatic hyperplasia using the Rezūm(®) system: evaluation of acute ablative capabilities in the human prostate. Research and reports in urology. 2015:7():13-8. doi: 10.2147/RRU.S74040. Epub 2015 Jan 30 [PubMed PMID: 25674555]
Mynderse LA, Hanson D, Robb RA, Pacik D, Vit V, Varga G, Wagrell L, Tornblom M, Cedano ER, Woodrum DA, Dixon CM, Larson TR. Rezūm System Water Vapor Treatment for Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia: Validation of Convective Thermal Energy Transfer and Characterization With Magnetic Resonance Imaging and 3-Dimensional Renderings. Urology. 2015 Jul:86(1):122-7. doi: 10.1016/j.urology.2015.03.021. Epub 2015 May 16 [PubMed PMID: 25987496]
Level 1 (high-level) evidenceWoo HH, Gonzalez RR. Perspective on the Rezūm(®) System: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Medical devices (Auckland, N.Z.). 2017:10():71-80. doi: 10.2147/MDER.S135378. Epub 2017 Apr 27 [PubMed PMID: 28490907]
Level 3 (low-level) evidenceÇakıroğlu B. Minimally invasive connective water vapor energy method for benign prostatic hyperplasia. Urologia. 2023 Dec 9:():3915603231216191. doi: 10.1177/03915603231216191. Epub 2023 Dec 9 [PubMed PMID: 38069654]
Elterman D, Bhojani N, Vannabouathong C, Chughtai B, Zorn KC. Rezūm therapy for ≥80-mL benign prostatic enlargement: a large, multicentre cohort study. BJU international. 2022 Oct:130(4):522-527. doi: 10.1111/bju.15753. Epub 2022 May 7 [PubMed PMID: 35466513]
Babar M, Loloi J, Tang K, Syed U, Ciatto M. Emerging outcomes of water vapor thermal therapy (Rezum) in a broad range of patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review. Lower urinary tract symptoms. 2022 May:14(3):140-154. doi: 10.1111/luts.12435. Epub 2022 Mar 1 [PubMed PMID: 35233955]
Level 1 (high-level) evidenceBole R, Gopalakrishna A, Kuang R, Alamiri J, Yang DY, Helo S, Ziegelmann MJ, Köhler TS. Comparative Postoperative Outcomes of Rezūm Prostate Ablation in Patients with Large Versus Small Glands. Journal of endourology. 2020 Jul:34(7):778-781. doi: 10.1089/end.2020.0177. Epub 2020 Jun 12 [PubMed PMID: 32408768]
Level 2 (mid-level) evidenceLee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinology and metabolism clinics of North America. 2011 Sep:40(3):565-75, viii-ix. doi: 10.1016/j.ecl.2011.05.012. Epub [PubMed PMID: 21889721]
Level 3 (low-level) evidenceBerry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of urology. 1984 Sep:132(3):474-9 [PubMed PMID: 6206240]
Singh O, Bolla SR. Anatomy, Abdomen and Pelvis, Prostate. StatPearls. 2023 Jan:(): [PubMed PMID: 31082031]
Aaron L, Franco OE, Hayward SW. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. The Urologic clinics of North America. 2016 Aug:43(3):279-88. doi: 10.1016/j.ucl.2016.04.012. Epub [PubMed PMID: 27476121]
Leslie SW, Chargui S, Stormont G. Transurethral Resection of the Prostate. StatPearls. 2025 Jan:(): [PubMed PMID: 32809719]
Hoeks CM, Hambrock T, Yakar D, Hulsbergen-van de Kaa CA, Feuth T, Witjes JA, Fütterer JJ, Barentsz JO. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology. 2013 Jan:266(1):207-17. doi: 10.1148/radiol.12120281. Epub 2012 Nov 9 [PubMed PMID: 23143029]
Huang G, Lebovic G, Vlachou PA. Diagnostic Value of CT in Detecting Peripheral Zone Prostate Cancer. AJR. American journal of roentgenology. 2019 Oct:213(4):831-835. doi: 10.2214/AJR.18.21013. Epub 2019 Jun 19 [PubMed PMID: 31216196]
Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A. Benign Conditions That Mimic Prostate Carcinoma: MR Imaging Features with Histopathologic Correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016 Jan-Feb:36(1):162-75. doi: 10.1148/rg.2016150030. Epub 2015 Nov 20 [PubMed PMID: 26587887]
Zaunbrecher N, Arbor TC, Samra NS. Anatomy, Abdomen and Pelvis: Internal Iliac Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 30725996]
Choo MS, Lee HE, Bae J, Cho SY, Oh SJ. Transurethral surgical anatomy of the arterial bleeder in the enucleated capsular plane of enlarged prostates during holmium laser enucleation of the prostate. International neurourology journal. 2014 Sep:18(3):138-44. doi: 10.5213/inj.2014.18.3.138. Epub 2014 Sep 24 [PubMed PMID: 25279241]
Kumar A, Patel VR, Panaiyadiyan S, Seetharam Bhat KR, Moschovas MC, Nayak B. Nerve-sparing robot-assisted radical prostatectomy: Current perspectives. Asian journal of urology. 2021 Jan:8(1):2-13. doi: 10.1016/j.ajur.2020.05.012. Epub 2020 Jun 11 [PubMed PMID: 33569267]
Level 3 (low-level) evidenceJarvis TR, Chughtai B, Kaplan SA. Testosterone and benign prostatic hyperplasia. Asian journal of andrology. 2015 Mar-Apr:17(2):212-6. doi: 10.4103/1008-682X.140966. Epub [PubMed PMID: 25337845]
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F, CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. European urology. 2010 Jan:57(1):123-31. doi: 10.1016/j.eururo.2009.09.035. Epub 2009 Sep 19 [PubMed PMID: 19825505]
Level 1 (high-level) evidenceNachawati D, Patel JB. Alpha-Blockers. StatPearls. 2024 Jan:(): [PubMed PMID: 32310526]
Salisbury BH, Tadi P. 5-Alpha-Reductase Inhibitors. StatPearls. 2024 Jan:(): [PubMed PMID: 32310390]
Mostafa MM, Khallaf A, Khalil M, Elgammal MA, Mahdy A. Efficacy and safety of TURP, HoLEP, and PVP in the management of OAB symptoms complicating BPH in patients with moderately enlarged prostates: A comparative study. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2023 Jan:17(1):E1-E7. doi: 10.5489/cuaj.7905. Epub [PubMed PMID: 36121889]
Level 2 (mid-level) evidenceLiu S, Liu H, Yao H, Sun F, Wu J, Zhou Z. A systematic review and meta-analysis of efficacy and safety comparing greenlight laser vaporization with transurethral resection of the prostate for benign prostatic hyperplasia with prostate volume less than 80 ml. Lasers in medical science. 2023 Jun 8:38(1):133. doi: 10.1007/s10103-023-03794-2. Epub 2023 Jun 8 [PubMed PMID: 37289405]
Level 1 (high-level) evidenceCastellani D, Pirola GM, Rubilotta E, Gubbiotti M, Scarcella S, Maggi M, Gauhar V, Teoh JY, Galosi AB. GreenLight Laser™ Photovaporization versus Transurethral Resection of the Prostate: A Systematic Review and Meta-Analysis. Research and reports in urology. 2021:13():263-271. doi: 10.2147/RRU.S277482. Epub 2021 May 20 [PubMed PMID: 34295844]
Level 1 (high-level) evidenceYilmaz M, Esser J, Suarez-Ibarrola R, Gratzke C, Miernik A. Safety and Efficacy of Laser Enucleation of the Prostate in Elderly Patients - A Narrative Review. Clinical interventions in aging. 2022:17():15-33. doi: 10.2147/CIA.S347698. Epub 2022 Jan 8 [PubMed PMID: 35035216]
Level 3 (low-level) evidenceFerretti M, Phillips J. Prostatectomy for benign prostate disease: open, laparoscopic and robotic techniques. The Canadian journal of urology. 2015 Oct:22 Suppl 1():60-6 [PubMed PMID: 26497345]
Malling B, Røder MA, Brasso K, Forman J, Taudorf M, Lönn L. Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis. European radiology. 2019 Jan:29(1):287-298. doi: 10.1007/s00330-018-5564-2. Epub 2018 Jun 14 [PubMed PMID: 29948079]
Level 1 (high-level) evidenceAbt D, Müllhaupt G, Hechelhammer L, Markart S, Güsewell S, Schmid HP, Mordasini L, Engeler DS. Prostatic Artery Embolisation Versus Transurethral Resection of the Prostate for Benign Prostatic Hyperplasia: 2-yr Outcomes of a Randomised, Open-label, Single-centre Trial. European urology. 2021 Jul:80(1):34-42. doi: 10.1016/j.eururo.2021.02.008. Epub 2021 Feb 19 [PubMed PMID: 33612376]
Level 1 (high-level) evidenceChughtai B, Elterman D, Shore N, Gittleman M, Motola J, Pike S, Hermann C, Terrens W, Kohan A, Gonzalez RR, Katz A, Schiff J, Goldfischer E, Grunberger I, Tu LM, Alshak MN, Kaminetzky J. The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial. Urology. 2021 Jul:153():270-276. doi: 10.1016/j.urology.2020.12.022. Epub 2020 Dec 26 [PubMed PMID: 33373708]
Level 1 (high-level) evidenceGarcia C, Chin P, Rashid P, Woo HH. Prostatic urethral lift: A minimally invasive treatment for benign prostatic hyperplasia. Prostate international. 2015 Mar:3(1):1-5. doi: 10.1016/j.prnil.2015.02.002. Epub 2015 Feb 13 [PubMed PMID: 26157759]
Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, Cantwell AL, McVary KT, Te AE, Gholami SS, Moseley WG, Chin PT, Dowling WT, Freedman SJ, Incze PF, Coffield KS, Herron S, Rashid P, Rukstalis DB. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. The Canadian journal of urology. 2017 Jun:24(3):8802-8813 [PubMed PMID: 28646935]
Level 1 (high-level) evidenceKaplan SA, Moss J, Freedman S, Coutinho K, Wu N, Efros M, Elterman D, D'Anna R, Padron O, Robertson KJ, Lawindy S, Mistry S, Shore N, Spier J, Kaminetsky J, Mazzarella B, Cahn D, Jalkut M, Te A. The PINNACLE Study: A Double-blind, Randomized, Sham-controlled Study Evaluating the Optilume BPH Catheter System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. The Journal of urology. 2023 Sep:210(3):500-509. doi: 10.1097/JU.0000000000003568. Epub 2023 Aug 9 [PubMed PMID: 37555604]
Level 1 (high-level) evidenceKadner G, Valerio M, Giannakis I, Manit A, Lumen N, Ho BSH, Alonso S, Schulman C, Barber N, Amparore D, Porpiglia F. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World journal of urology. 2020 Dec:38(12):3235-3244. doi: 10.1007/s00345-020-03140-z. Epub 2020 Mar 2 [PubMed PMID: 32124019]
McVary KT, Gittelman MC, Goldberg KA, Patel K, Shore ND, Levin RM, Pliskin M, Beahrs JR, Prall D, Kaminetsky J, Cowan BE, Cantrill CH, Mynderse LA, Ulchaker JC, Tadros NN, Gange SN, Roehrborn CG. Final 5-Year Outcomes of the Multicenter Randomized Sham-Controlled Trial of a Water Vapor Thermal Therapy for Treatment of Moderate to Severe Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. The Journal of urology. 2021 Sep:206(3):715-724. doi: 10.1097/JU.0000000000001778. Epub 2021 Apr 19 [PubMed PMID: 33872051]
Level 1 (high-level) evidenceMcVary KT, Rogers T, Roehrborn CG. Rezūm Water Vapor Thermal Therapy for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: 4-Year Results From Randomized Controlled Study. Urology. 2019 Apr:126():171-179. doi: 10.1016/j.urology.2018.12.041. Epub 2019 Jan 21 [PubMed PMID: 30677455]
Level 1 (high-level) evidenceShelton TM, Drake C, Vasquez R, Rivera M. Comparison of Contemporary Surgical Outcomes Between Holmium Laser Enucleation of the Prostate and Robotic-Assisted Simple Prostatectomy. Current urology reports. 2023 May:24(5):221-229. doi: 10.1007/s11934-023-01146-9. Epub 2023 Feb 17 [PubMed PMID: 36800115]
Sandhu JS, Bixler BR, Dahm P, Goueli R, Kirkby E, Stoffel JT, Wilt TJ. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia (BPH): AUA Guideline Amendment 2023. The Journal of urology. 2024 Jan:211(1):11-19. doi: 10.1097/JU.0000000000003698. Epub 2023 Sep 14 [PubMed PMID: 37706750]
Siena G, Cindolo L, Ferrari G, Maruzzi D, Fasolis G, Condorelli SV, Varvello F, Visalli F, Rabito S, Toso S, Caroassai S, Mari A, Viola L, Somani BK, Carini M. Water vapor therapy (Rezūm) for lower urinary tract symptoms related to benign prostatic hyperplasia: early results from the first Italian multicentric study. World journal of urology. 2021 Oct:39(10):3875-3880. doi: 10.1007/s00345-021-03642-4. Epub 2021 Mar 31 [PubMed PMID: 33787986]
Cantrill CH, Zorn KC, Elterman DS, Gonzalez RR. The Rezūm system - a minimally invasive water vapor thermal therapy for obstructive benign prostatic hyperplasia. The Canadian journal of urology. 2019 Jun:26(3):9787-9793 [PubMed PMID: 31180311]
Whiting D, Noureldin M, Abdelmotagly Y, Johnston MJ, Brittain J, Rajkumar G, Emara A, Hindley R. Real-world Early Outcomes and Retreatment Rates Following Water Vapour Ablative Therapy for Symptomatic Benign Prostatic Hyperplasia. European urology open science. 2022 May:39():72-78. doi: 10.1016/j.euros.2022.03.006. Epub 2022 Apr 7 [PubMed PMID: 35528787]
Bhowmick P, Coad JE, Bhowmick S, Pryor JL, Larson T, De La Rosette J, Bischof JC. In vitro assessment of the efficacy of thermal therapy in human benign prostatic hyperplasia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2004 Jun:20(4):421-39 [PubMed PMID: 15204522]
Issa MM, Ritenour C, Greenberger M, Hollabaugh R Jr, Steiner M. The prostate anesthetic block for outpatient prostate surgery. World journal of urology. 1998:16(6):378-83 [PubMed PMID: 9870283]
Lawson KA, Rudzinski JK, Vicas I, Carlson KV. Assessment of antibiotic prophylaxis prescribing patterns for TURP: A need for Canadian guidelines? Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2013 Jul-Aug:7(7-8):E530-6. doi: 10.5489/cuaj.205. Epub [PubMed PMID: 24032065]
Darson MF, Alexander EE, Schiffman ZJ, Lewitton M, Light RA, Sutton MA, Delgado-Rodriguez C, Gonzalez RR. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezūm system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Research and reports in urology. 2017:9():159-168. doi: 10.2147/RRU.S143679. Epub 2017 Aug 21 [PubMed PMID: 28861405]
Felice MD, Kim K, Janakiraman S, Pahouja G, Adams W, Fruth E, Farooq A, McVary KT. Risk factors for a failed trial without catheter following convective water vapor thermal therapy (CWVTT-Rezum). Lower urinary tract symptoms. 2023 Sep:15(5):158-164. doi: 10.1111/luts.12483. Epub 2023 May 26 [PubMed PMID: 37232068]
Cocci A, Bocchino AC, Cito G, Lisa A, Russo GI, Giudice AL, Sessa F, Viola L, Cindolo L, Somani BK, Siena G. Role of Rezum in the treatment of benign prostate hyperplasia: A review of the literature. Turkish journal of urology. 2021 Nov:47(6):452-460. doi: 10.5152/tud.2021.21128. Epub [PubMed PMID: 35118963]
Dixon C, Cedano ER, Pacik D, Vit V, Varga G, Wagrell L, Tornblom M, Mynderse L, Larson T. Efficacy and Safety of Rezūm System Water Vapor Treatment for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Urology. 2015 Nov:86(5):1042-7. doi: 10.1016/j.urology.2015.05.046. Epub 2015 Jul 26 [PubMed PMID: 26216644]
Janakiraman S, Felice M, Pahouja G, Adams W, Bsatee A, Farooq A, McVary KT. Risk Factors for Persistent Lower Urinary Tract Symptoms 1 Month Following Convective Water Vapor Thermal Therapy (CWVTT-Rezum). Urology. 2023 Sep:179():112-117. doi: 10.1016/j.urology.2023.06.005. Epub 2023 Jun 22 [PubMed PMID: 37353091]
Ottaiano N, Shelton T, Sanekommu G, Benson CR. Surgical Complications in the Management of Benign Prostatic Hyperplasia Treatment. Current urology reports. 2022 May:23(5):83-92. doi: 10.1007/s11934-022-01091-z. Epub 2022 Mar 9 [PubMed PMID: 35262855]
Martinelli E, Cindolo L, Grossi FS, Kuczyk MA, Siena G, Oelke M. Transurethral water vapor ablation of the prostate with the Rezūm system: Urodynamic findings. Neurourology and urodynamics. 2023 Jan:42(1):249-255. doi: 10.1002/nau.25076. Epub 2022 Nov 6 [PubMed PMID: 36335610]
Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU international. 1999 Jul:84(1):14-5 [PubMed PMID: 10444116]
Winkler T, von Klot CAJ, Madersbacher S, Kuczyk MA, Wolters M. Rezum water vapor thermal therapy for treatment of lower urinary tract symptoms: A retrospective single-centre analysis from a German high-volume centre. PloS one. 2023:18(1):e0279883. doi: 10.1371/journal.pone.0279883. Epub 2023 Jan 6 [PubMed PMID: 36607843]
Level 2 (mid-level) evidence