Introduction
Prostatic urethral lift (PUL) is a minimally invasive procedure approved by the United States Food and Drug Administration for the amelioration of benign prostatic hyperplasia (BPH) symptoms. PUL utilizes implantable medical devices to retract enlarged prostate tissue mechanically, thereby unobstructing the urethra and enhancing urinary flow.[1][2] The procedure is devoid of tissue heating, excision, or destruction. PUL can also be conducted in a single session under local anesthesia, with the potential to promptly alleviate BPH symptoms and reduce the dependence on chronic BPH medication.[3][4]
BPH is characterized by a histopathological condition that quantifiably increases the number of stromal and epithelial cells within the prostate. Cellular proliferation leads to a progressive narrowing of the prostatic urethra, culminating in the manifestation of lower urinary tract symptoms (LUTS). These symptoms encompass urgency, increased frequency of urination, nocturnal polyuria, impaired bladder evacuation, initial urinary hesitation, discontinuous urine flow, and a reduced force of the urinary stream.
Epidemiological data indicates that BPH-induced LUTS significantly impinges upon 50% to 75% of men advancing beyond the fifth decade of life, and prevalence escalates to 80% to 90% in the demographic exceeding age 70.[5] Although BPH-induced LUTS predominantly diminishes life quality, its morbidity spectrum extends to recurrent urinary tract infections, acute urinary retention, bladder stones, and hematuria.[5][6] Standard interventions for BPH-related symptoms involve surgical modalities such as transurethral resection or laser enucleation of the prostate, alongside pharmacological management through sustained oral medication regimens.[7] PUL provides a minimally invasive option in the arsenal of therapeutic modalities.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The prostate gland is a robust fibromuscular structure with an inverted cone shape; its base surrounds the bladder neck inferiorly, while its apex is at the external urethral sphincter.[8] Anterior to the prostate is the pubic symphysis, separated by a fatty pad and the prostatic venous plexus. Posteriorly, Denonvillier fascia delineates the boundary with the rectum. The external urethral sphincter below the gland plays a crucial role in urinary continence and facilitating ejaculatory flux. The levator ani muscles, situated laterally, are close to the prostate.[9]
Structurally, the prostate is partitioned into 5 distinct lobes: anterior, posterior, 2 lateral lobes, and a median lobe.[10] Regarding zonal anatomy, the central zone constitutes the base, enveloping the ejaculatory ducts, while the peripheral zone is the largest, accounting for 70% of the prostate's central area and partially encasing the distal prostatic urethra. The transition zone, albeit smaller, is glandular and surrounds the urethra from the bladder neck to the verumontanum. The anterior fibromuscular stroma, devoid of glandular tissue, enfolds the apex of the prostate. A fibrous capsule entirely encases the prostate, providing an anatomical boundary.[11]
BPH is characterized by nodular hyperplasia, primarily affecting the prostate's transition (lateral lobes) and central (median lobe) zones.[5] This hyperplasia often takes the form of multiple nodules, with those arising from the transition zone giving rise to the lateral lobes and those from the central zone forming the median lobe. When BPH results in urinary outflow obstruction, it is mainly due to the distortion of the urethral anatomy rather than direct compression of the bladder outlet.[12]
Indications
The primary modality for managing BPH is pharmacotherapy, typically commencing with alpha-adrenergic receptor antagonists, which may be prescribed singularly or in combination with 5 alpha-reductase inhibitors.[13] In instances where a patient presents with persistent symptomatology that is recalcitrant to initial pharmacological interventions, exhibits intolerance to the adverse effects of these medications, or elects to pursue therapy with greater durability and efficacy, alternative therapeutic strategies may be warranted.[5]
BPH involves the formation of nodular hyperplasia predominantly in the prostate gland's transition (lateral lobes) and central (median lobe) zones. This condition typically manifests as numerous nodules, which in the transition zone contribute to the enlargement of the lateral lobes and, in the central zone, lead to the growth of the median lobe. In cases where BPH obstructs urinary outflow, it is primarily due to the alteration of urethral anatomy instead of the outright compression of the bladder outlet.[5] The enlargement of the lateral lobes of the prostate is commonly implicated in the pathogenesis of LUTS, establishing a criterion for patient selection.[4]
Minimally Invasive Surgical Interventions for BPH
Surgical modalities that serve as potential therapeutic alternatives for relieving persistent LUTS include:
- Conventional endoscopic ablative methods such as transurethral resection of the prostate (TURP), GreenLight laser™ photovaporization (GLL-PVP), and holmium laser enucleation of the prostate (HoLEP) are options.
- Radical open surgical techniques like the simple (Millin) prostatectomy are potential therapies.
- Interventional radiology approaches encompass prostatic artery embolization (PAE) as an alternative therapy option.
- Minimally invasive interventions include PUL, water vapor thermal ablation of the prostate (WVAP), the urethral drug-coated balloon catheter BPH system, and the deployment of a temporary nitinol device.
Selecting the most suitable treatment strategy requires a thorough evaluation of multiple criteria: the patient's personal preferences and objectives, the comorbidities present and the overall risk profile of the patient, the dimensions and anatomical structure of the prostate, as well as a comprehensive analysis of the risks and potential advantages associated with each surgical option.[5]
PUL
PUL stands out as a targeted treatment modality for BPH, particularly in patients manifesting significant LUTS that adversely affect their quality of life.[4][14] Candidates for PUL ideally possess a normal bladder neck and exhibit only minimal enlargement of the prostate's median lobe. While historically, the presence of an obstructive median lobe was regarded as a contraindication to treatment, hence its role as a criterion for exclusion in the LIFT study, subsequent findings from the MedLift study have demonstrated that obstructive median lobes can be effectively addressed using the PUL technique, yielding comparable results.[15]
Recent investigations targeting patients with prominent median lobes undergoing TURP have indicated that individuals with a significant median lobe protrusion into the bladder of 10 mm or more may derive marked benefit from therapeutic interventions targeting this specific form of obstruction. The PUL method is efficacious independent of the overall prostatic volume or the extent of intravesical prostatic protrusion, with this efficacy sustained over a 2-year follow-up period.[16] Within 1 month postprocedure, 86% of patients reported significant alleviation of symptoms, alongside notable enhancements in maximum urinary flow rate (Qmax), postvoid residual (PVR) volume, international prostate symptom score (IPSS), and quality of life measures, with statistical significance (P <0.001).[16] While short-term IPSS improvements are evident, long-term data (>2 years) on targeted median lobe management is scarce. Clinicians should evaluate the efficacy of traditional cavitating procedures versus minimally invasive methods for enlarged median lobes rather than modified surgical techniques, especially if they are new to PUL.
Additionally, data from studies have been documented over a period extending to 5 years, indicating the procedure is optimal for individuals whose prostate volume measures between 20 to 100 cm3.[3][17] Residual volumes should ideally not be at significant extremities; however, it has been demonstrated to be effective in volumes between 44 to 488 mL with an average reduction of 139.7 mL.[18]
The preservation of sexual function is a paramount concern, especially for younger men who may be hesitant about treatments that potentially compromise erectile or ejaculatory capabilities. Encouragingly, results from studies, such as a notable investigation in Australia, have demonstrated that the PUL procedure does not detrimentally affect sexual function postoperatively.[2] Sexual and ejaculatory function was also maintained in individuals who had undergone PUL with an obstructive median lobe.[16] This benefit makes PUL a favorable option for those who are reluctant to commence or continue lifelong medication for LUTS or for patients whose symptoms are not adequately managed by medical therapy and who are seeking surgical intervention.
Inclusion criteria for PUL include the following:
- Age >50 years
- Prostate volume of 20 to 100 cm3
- IPSS >12
- Qmax <15 mL/s
- PVR <350 mL [19]
Obstructive median lobes were once considered a contraindication; however, recent evidence indicates that, with accurate surgical techniques, symptoms can be effectively alleviated for at least 2 years.[3] The preservation of erectile and ejaculatory functions has been noted.[16] The adverse events reported were generally mild and of a short-term nature. Symptomatic relief was observed as early as 2 weeks postprocedure and has been shown to persist for up to 5 years.[20][21] The retreatment rate is 6% annually among patients who have previously undergone such interventions. Of these patients, 51% underwent traditional endoscopic removal methods like TURP or laser enucleation of the prostate. In contrast, 32.7% received a secondary PUL, and 20% had a PUL device removal.[22]
Of note, the pilot trial comparing urolift and standard TURP ahead of radiotherapy (CO-STAR) is investigating whether PUL is as effective as TURP in the preradiotherapy setting for patients with concomitant LUTS and prostate cancer requiring intervention due to its rapid relief and minimal delay in treatment.[23] This trial has also proposed that the PUL implant can be effectively used as a surrogate for fiducial markers for definitive radiotherapy to the prostate for prostate cancer.[24][25]
WVAP
This procedure entails injecting heated water vapor at 103 °C into the prostatic tissue, which leads to immediate cellular death and creates an ablated lesion through apoptosis over 6 to 12 months.[26] Results from longitudinal studies have observed outcomes spanning up to 5 years. WVAP is applicable for prostatic volumes ranging from 30 to 100 cm3 and is effective across a spectrum of prostatic morphologies, including cases with median lobe enlargement.[26][27] The ejaculatory function remains unaltered postprocedure.[26][28] Patients report minimal and short-lived adverse events. Initial symptomatic improvement is observed within 4 weeks, with full benefits accruing up to 12 months and sustainability of up to 5 years.[29] The rate of retreatment at the 5-year mark stands at 4.4%.[29]
Urethral Drug-Coated Balloon Catheter System
The urethral drug-coated balloon catheter system uses a dual-lobe balloon for the mechanical expansion of the prostatic fossa and delivers paclitaxel to deter further tissue growth.[30] Data reflecting outcomes are accessible up to 2 years postprocedure.[30][31] The urethral drug-coated catheter system is designed for prostate sizes within the 20 to 80 cm3 range but is not recommended for cases with significant median lobe obstructions. The ejaculatory function is preserved. Side effects are typically mild and fleeting; symptom relief commences at 3 months, persisting for a year, as the published data supports.[32]
Temporarily Implanted Nitinol Device
The temporarily implanted nitinol device involves a temporary implant with nitinol struts that expand gradually over 5 to 7 days, exerting outward triangular force and inducing ischemia and subsequent necrosis for prostatic tissue remodeling.[33] Study findings are reported up to 2 years following implantation.[34] The advised prostate volume for this treatment is between 25 to 75 cm3, and it is not indicated for patients with median lobe enlargement, which was observed to have higher treatment failure rates.[34] The ejaculatory function is maintained. The intervention is associated with minor and transient adverse events. Relief from symptoms typically begins within 3 months, lasting up to a year. The retreatment rate at 2 years is 14.8%.[34]
Comparing PUL vs Alternatives
PUL is distinguished by its proven efficacy in symptom alleviation for up to 5 years, as recorded in the current literature. PUL can treat a broad range of prostatic sizes and is efficacious for volumes up to 100 cm3. Recent advancements in surgical techniques render median lobe enlargement a manageable condition rather than a contraindication for PUL. The procedure upholds normal ejaculatory functions. Symptom relief is instantaneous, surpassing other minimally invasive techniques such as WVAP. For patients with notable comorbidities and higher anesthetic risks, PUL offers a minimal risk profile and can be performed in an outpatient setting with local anesthesia within approximately 10 to 15 minutes; postoperative catheterization is less frequently necessitated with PUL.
PUL and WVAP are the most thoroughly researched and established minimally invasive techniques for managing symptomatic BPH. PUL, in particular, has been associated with more rapid symptom improvement and long-lasting effects. Comparatively, patient-reported outcomes post-PUL exhibit significantly better IPSS and quality-of-life metrics than that post-WVAP.[35] Notably, patients with PUL have a markedly lower catheterization rate by the third postoperative day than patients with WVAP; 7% of patients who underwent PUL were catheterized by postoperative day 3 compared to 55% of patients with WVAP.[35] Furthermore, patients with PUL report significantly higher sexual health inventory for men scores, indicative of better sexual function.[35] Thus, PUL is superior in several respects, including sexual health, reduced catheterization rates, greater patient satisfaction, and minimal disruption to daily activities. While PUL offers several advantages, including reduced catheterization rates, patient satisfaction, and minimal disruption to daily activities, WVAP is significantly cheaper, at half the cost of PUL, and provides a cost-effective, minimally invasive option for managing BPH.[36]
Choosing PUL for a Patient
The criteria for choosing PUL in patients is summarized by the following:
- Significant LUTS requiring surgical intervention
- Patient preference for retaining near baseline levels of erectile and ejaculatory function
- Prostate volume no larger than 100 mL
- Patients wanting rapid relief of symptoms
- Preference not to have an indwelling Foley catheter postoperatively
- All anesthetic risk profiles appropriate
- Acute urinary retention: does not preclude patient from having PUL
- Intravesical protrusion of the median lobe is managed
- Suspicion of prostate cancer (either present or at high risk for development, ie, strong family history)
If any of these considerations are questioned when evaluating a patient, more traditional cavitating procedures may benefit the patient. WVAP may also be considered in patients with increased anesthetic risks and with very large median lobes.
Contraindications
Based on scientific data, PUL is contraindicated in several specific situations and patient conditions. Individuals with a recent history of prostatitis within the past year or who have active urinary tract infections are not considered suitable candidates due to the potential for exacerbating inflammation and infection. Those with a history of urinary retention or who have undergone previous surgical interventions for benign BPH might have altered anatomy or scar tissue that could complicate the PUL procedure. Similar concerns apply to patients who have had prior pelvic surgery or irradiation, as these factors may alter pelvic anatomy or compromise tissue integrity, thereby increasing the risk of complications.[2][15][37][38] A prostate-specific antigen (PSA) level greater than 10 ng/mL is also a contraindication unless a negative biopsy has confirmed the absence of prostate cancer.[2][19][37][39]
In addition to these specific patient factors, the PUL system has limitations that might contraindicate its use in certain patients. Technical challenges may arise in individuals with a high bladder neck. PUL is also contraindicated for patients with significantly enlarged prostates (greater than 100 mL).[1][2][39][40] The lack of studies demonstrating long-term data and research on patients with multiple comorbidities further limits the procedure's suitability. Prospective patients should also be aware of the potentially high retreatment rate in the long term and the high cost per implant, which might influence their decision to proceed with PUL.[41]
The exclusionary criteria are summarized below:
- Acute urinary tract infection
- PSA >10 ng/mL (unless negative biopsy)
- Prostatitis within the past year
- History of urinary retention
- Previous BPH procedure
- Previous pelvic surgery or irradiation
Acute urinary retention was previously thought to be a contraindication to performing PUL in its early stages; however, much like the presence of a median lobe, it is no longer considered so. Results from the 2023 study of PUL for subjects in urinary retention (PULSAR) showed that 73% of individuals were catheter-independent and free from surgical reintervention at 12 months.[42] Patients with lower baseline PSA, PVR, and shorter prePUL catheter durations benefited most from remaining catheter-free.[42]
Equipment
The following equipment is essential for performing PUL:
- 2.9 mm 0° scope
- 20F sheath
- Visual obturator
- Cystoscopy camera and monitoring equipment
- Fluid irrigation system
- Standard endoscopic grasper kit
- Lidocaine infused lubrication
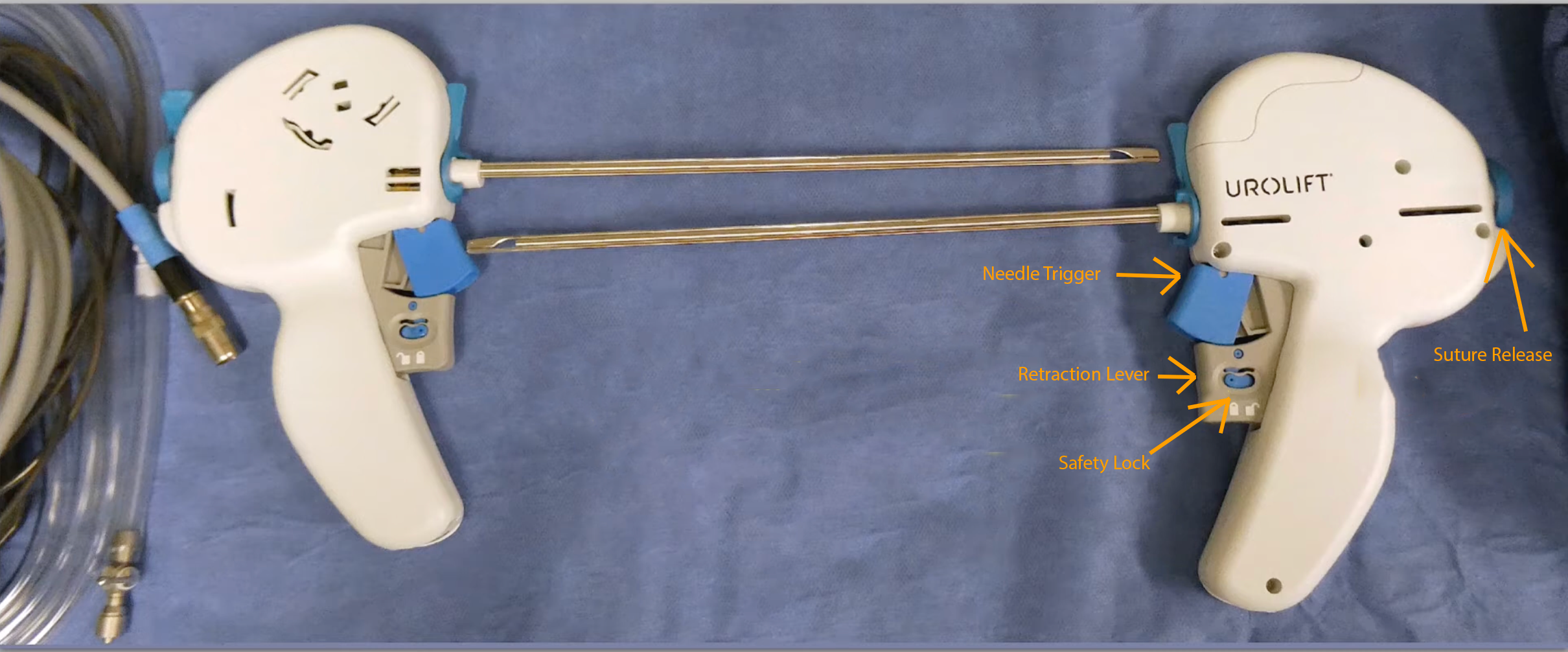
- Prostatic urethral device (see Image. Prostatic Urethral Device)
Keeping a grasper kit or a similar urological instrument designed for extracting foreign objects on hand is advised if removing or retrieving a part of the PUL implant during surgery becomes essential.
The following equipment is optional when performing PUL:
- Two-way Foley catheter and drainage bag
- Transrectal ultrasound for local prostatic anesthesia
- Local anesthetic and appropriate needle for injection
- A combination of short-acting and long-acting local anesthetics should generally be used to maximize patient comfort (eg, a 50:50 mixture of 1% lidocaine and 0.5% bupivacaine).
Personnel
The following personnel are needed to perform PUL:
- Surgeon
- Scrub nurse
- Circulating nurse
- Anesthesia team
Preparation
History
An exhaustive clinical history focused on the patient's LUTS is imperative, encompassing symptoms such as incomplete bladder emptying, frequency, urgency, intermittency, straining, and nocturia. Concomitant symptoms, including dysuria, hematuria, incontinence, and previous urinary calculi, must be meticulously documented. Prior medical or surgical interventions targeting LUTS, including alpha-blockers and 5-alpha reductase inhibitors, should be scrutinized.
A baseline International Prostate Symptom Score or American Urological Association (AUA) BPH symptom score is an invaluable benchmark.[43] The IPSS is an 8-item quantitative instrument designed for the evaluation, rapid detection, longitudinal monitoring, and stratification of treatment for BPH.[44] Comprised of 7 questions, it directly interrogates the severity of BPH-related LUTS and 1 additional item appraising the patient's perceived quality of life secondary to these symptoms. Responses to the symptom-specific questions are structured as a Likert scale, with the patient selecting from a gradation of responses indicating progressive symptom severity, scored from 0 (absent) to 5 (severe). The aggregate potential score spans from 0, denoting absence of symptoms, to 35, indicating extreme symptomatology.[44] The 7 urinary symptoms evaluated are incomplete bladder emptying, urinary frequency, intermittent urination, urgency, weak urinary stream, straining during urination, and nocturia.
The symptom score is aligned with that of the AUA symptom index, classifying symptoms into 3 categories based on the total score: mild (total symptom score ≤7), moderate (total symptom score range 8-19), and severe (total symptom score range 20–35). A comprehensive history ascertains the primary nature of LUTS (voiding, storage, or mixed) and may indicate an etiology other than BPH, such as a bladder dysfunction unrelated to the prostate.[45]
Additionally, an assessment of baseline health parameters is essential, emphasizing prior medical and surgical history, anesthetic assessments, prior urological procedures, or prostate radiation therapy. Erectile function should be quantified using tools like the International Index of Erectile Function-5 (IIEF-5).[46] Urological risk factors, including tobacco use, occupational exposures, and familial history of BPH or prostate cancer, warrant evaluation. Healthcare professionals must also clearly understand the patient's subjective symptom perception and treatment objectives. The clinician is responsible for providing the patient with shared decision-making and establishing reasonable expectations.
Physical Examination
The physical examination for LUTS should entail a digital rectal examination to approximate prostate size and screen for prostate malignancy.[47] Inspection of the external genitalia is vital for identifying pathologies such as meatal stenosis, lichen sclerosis, and phimosis, critical considerations before cystoscope insertion.
Preoperative Workup
The diagnostic workup for PUL mandates urinalysis with microscopy, culture, and sensitivity to exclude infection or unexplained hematuria. PSA measurement is a crucial screening modality preceding BPH interventions to mitigate the risk of delayed prostate cancer diagnosis. Should PSA levels be elevated, malignancy must be ruled out before performing a PUL.[48]
Uroflowmetric evaluation, including Qmax, PVR urine test, and voiding pattern analyses, are essential to ascertain symptom severity and etiology and to monitor therapeutic progress. Though not universally requisite, urodynamic studies are critical when alternative or concurrent pathologies such as detrusor underactivity are suspected, particularly in patients with urinary retention.[49] A renal tract ultrasound provides adequate prostate volume and morphology measurements; this will help identify patients who fit the selection criteria for PUL.[50]
Preoperative flexible cystoscopy is advised for strategically planning the PUL, evaluating the anatomy from the bladder neck to the verumontanum, the dimensions of the lateral lobes, and the presence of a median lobe or elevated bladder neck. Additionally, this procedure permits retroflexive evaluation of the bladder neck, ureteric orifices, and the degree of intravesical prostatic protrusion. Preoperative flexible cystoscopy can also gauge pain tolerance for those selecting local anesthesia.[51]
Preoperative Anticoagulation Guidelines
Blood thinners should ideally be stopped before the procedure; 3 days of preoperative cessation is suitable for new oral anticoagulants. Recommencement may also occur 2 to 3 days postoperatively.[52] For clopidogrel, 7 days is sufficient, provided the patient can temporarily cease the antiplatelet medication safely.[52] PUL can be performed while the patient continues on aspirin.
Technique or Treatment
The PUL system comprises a single-use, disposable endoscopic delivery instrument with a pistol-grip design for inserting a prostatic implant. This implant is structured as a pair of anchoring elements joined by a durable, nondegradable suture line. During surgical intervention, the surgeon advances the delivery instrument via the urethra, terminating at the prostatic segment. Deployment of the implant is achieved through the penetration of the prostatic tissue by a slender needle at the instrument's distal end, which then secures an anchor externally to the prostatic capsule. Upon retraction of the needle, tension is applied to the suture, culminating in the fixation of the second anchor within the urethral lumen. The clinical application typically requires approximately 3.5 implants, with the procedural approach adaptable to local or general anesthesia.[17] The patient's care setting for this procedure is flexible; inpatient or outpatient encounters are possible.
Traditional Surgical Technique
The PUL procedure aims to create anterolateral channels in the prostatic fossa above the verumontanum to relieve obstruction. This procedure can be performed using various anesthetic techniques, including local, spinal, or general anesthesia. Lidocaine-infused lubrication jelly is recommended as the most cost-efficient and effective local anesthetic, though other forms may be used. The patient is positioned in lithotomy, and a cystoscopy is performed to assess the bladder neck and the prostate's configuration, focusing on the median and lateral lobes. Ideally, this assessment is completed before the procedure using flexible cystoscopy.
Upon removal of the visual obturator, a 20Fr to 22Fr sheath remains in place. A 0° scope is inserted into the PUL device, and together, they are introduced through the cystoscope sheath. The device's implants are deployed at least 1.5 cm distal to the bladder neck, specifically at 10- or 2-o'clock positions, to prevent the needle from penetrating the bladder neck and base. A suitable gauge for the distal positioning of the implants can be the length measured from the extremity of the instrument to the head of the endoscopic camera.
The procedure involves compressing the lateral lobe at the targeted location by laterally angulating the scope to move the lateral lobe physically. The trigger is then unlocked, and the blue half of the double trigger is pulled in line with the grey trigger (half press), firing a hollow nitinol curved needle through the prostate tissue and its capsule. Care should be taken not to use the camera head as a lever to compress the lobe to avoid incomplete suture deployment. This action delivers a capsular anchor and nylon suture into the prostatic capsule. The operator then retracts the needle (full press), tensions the suture, and advances the instrument towards the bladder neck until a white line appears on the suture. This line indicates proper tension when visualized halfway between the device and the prostate lobe. The button on the back of the PUL device is depressed to deploy the urethral end of the implant and sever the suture. The instrument's tip is then moved to the midline to ensure the urethral anchor is disengaged from the device. Subsequently, the instrument is retracted into the bladder, and the procedure is repeated for both lateral lobes.
After the deployments, the sheath is left within the bladder, and a follow-up cystoscopy is performed to determine if additional implants are necessary to alleviate the obstruction adequately. Implants are distributed along the expanse of each lateral lobe of the prostate, positioning them at intervals of about 1 cm, with corresponding implants on the contralateral sides to achieve the requisite urethra dilation. On average, a patient generally receives 3 to 4 implants. The recommended guideline suggests placing no more than 10 implants per patient as the upper limit.
Routine PUL in ideal patients does not require catheterization following the procedure. Indications for immediate catheterization following a PUL are catheterization prior to the procedure, overnight stay due to anesthetic concerns, and significant adenomatous bleeding. This methodical approach optimizes the anatomical configuration for improved urinary flow while minimizing invasiveness and preserving erectile and ejaculatory functions.[3]
Updated MEDLIFT Technique for Obstructive Median Lobes
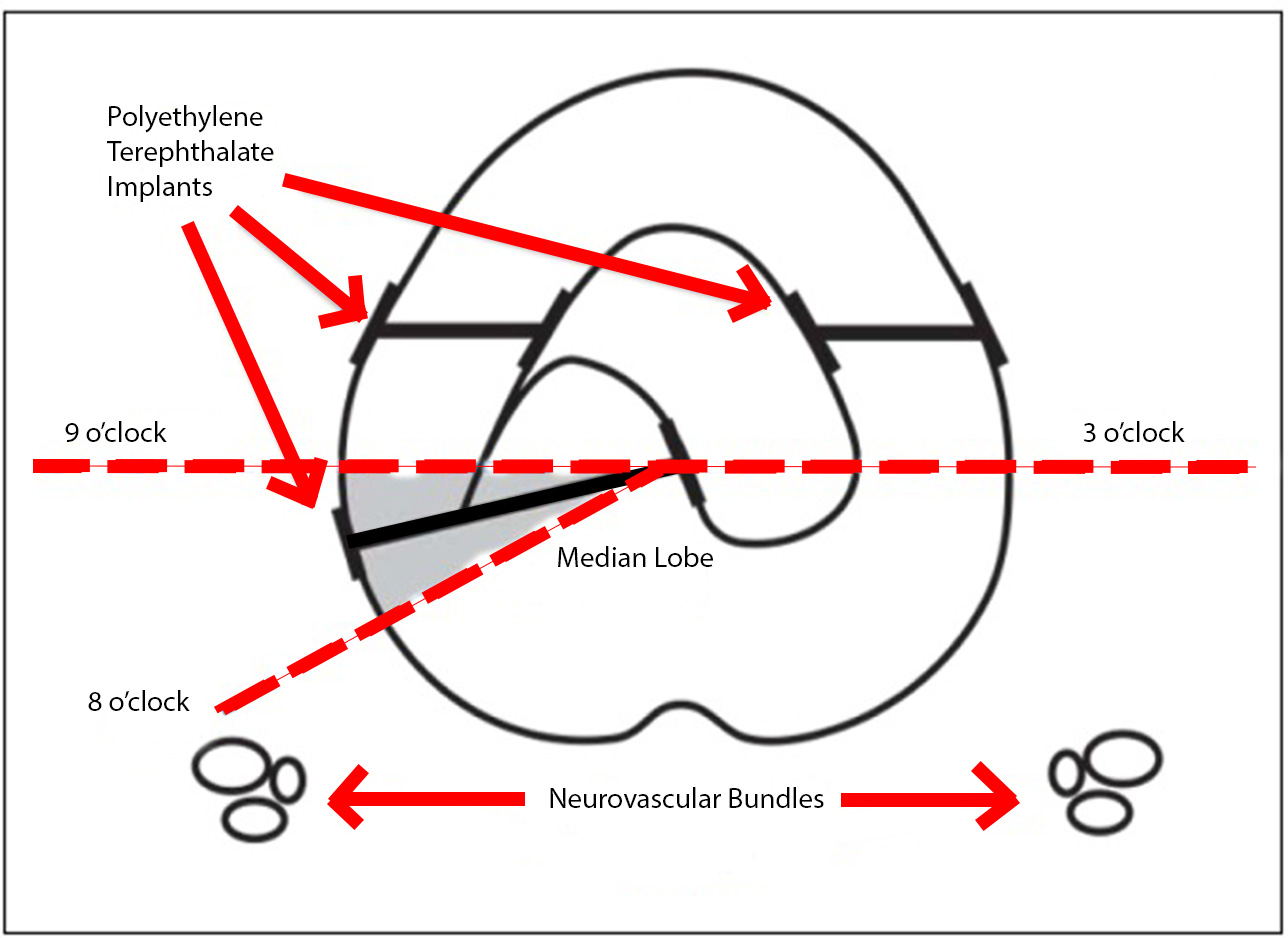
The initial aspect of this updated technique is largely unchanged from the traditional technique. For lateral lobe deployments, the system is angled laterally, 20° to 30°, usually at 10- and 2-o'clock, to compress the anterior third of the obstructive lobe. After addressing the lateral lobes, the obstructive middle lobe should be visualized and assessed with cystoscopy. Rukstalis et al found that, "For middle lobe deployments, the tissue that protrudes intravesically may be pulled into the prostatic fossa and affixed to either side of the urethra dependent on the individual's prostate anatomy. Notably, with large intravesical prostate protrusions, not all intravesical tissue needs to be retracted when creating the channel at the bladder neck. Additional tissue may remain intravesical, albeit not obstructing the prostatic fossa." They noted the importance of deploying the implant away from the neurovascular bundles, accomplished by maintaining a deployment trajectory anterior to the 4- and 8-o'clock position when viewing the transverse plane of the urethra as a clock face (see Image. Modified Median Lobe Technique).[15]
Explant of the PUL Implant
In the event of complications in deploying the PUL or postoperative complications, the implant may need to be removed. Several methods exist for endoscopic removal of the implant. In the short term, graspers can be used if the suture is still visible in the prostatic fossa. Monopolar transurethral resection is also effective for implant removal. Additionally, laser enucleation en bloc has been reported; however, during the morcellation of the adenoma, there have been instances of the equipment jamming with the implant.[53]
Complications
Complications
PUL is associated with a spectrum of potential postoperative complications ranging from minor to major.[54] Patients must be closely monitored postoperatively so complications can be managed promptly if they arise.
Minor
Minor complications are typically mild, transient, and resolve swiftly. These include:
- Dysuria
- Urinary urgency
- Hematuria
- General discomfort or pelvic pain
- Urinary tract infections [55]
Major
Major complications, while less common, can be more severe. They encompass:
- Clot retention
- This is the accumulation of blood clots within the bladder, which can obstruct urinary outflow.
- Acute urinary retention
- Urinary incontinence
- Prostatitis
- Epididymo-orchitis [55][56]
- Prostatic fossa stones [56]
- Breakage of the implant
- This is exceedingly rare, with an incidence of 0.004%.[20]
Device-Related
Complications can arise from the misplacement of the PUL device, which should be positioned at least 1 to 1.5 cm below the bladder neck. If placed too close to the bladder neck, reepithelialization over the nitinol suture may not occur. This can lead to severe irritative symptoms, stone formation adherent to the exposed suture (although stone formation rates are remarkably low at 0.006%), and recurrent urinary tract infections.[20]
Magnetic Resonance Imaging Considerations
Nonclinical evaluations have established that the PUL implant is magnetic resonance imaging (MRI)-compatible, though conditionally so. Individuals implanted with this device may undergo MRI without adverse effects under the stipulated criteria:
- The static magnetic field must be 3 Tesla or lower.
- Based on extrapolation, the spatial field gradient should not exceed 1500 Gauss/cm (or 15 Tesla/m).
- The MRI system's reported whole-body average specific absorption rate should be at or below 4 W/kg during 15 minutes of continuous scanning in first-level controlled operating mode.
With adherence to the above scanning parameters, the PUL implant is predicted to cause a maximum temperature increase of 2.4 °C following a 15-minute continuous scanning session. In nonclinical trials, the PUL implant has been shown to produce an image artifact that extends roughly 15 mm from the device, primarily when subjected to gradient echo pulse sequencing in a 3-Tesla MRI environment.
Compared to the apex, image quality deterioration is disproportionately higher in the transitional zone relative to the peripheral zone and in the base and middle regions. The phenomenon is pronounced in diffusion-weighted imaging when contrasted with T2-weighted and dynamic contrast-enhanced sequences. A prostate imaging quality score of 2 or less, indicative of suboptimal imaging, was recorded in 16% to 24% of examinations. The obscuration of the prostatic tissue by the UroLift artifact was significantly more pronounced in diffusion-weighted and dynamic contrast-enhanced imaging than in T2-weighted imaging.[57] Patients considering the PUL procedure should be informed about the potential effects of the implant on the quality of MRI images and the consequent ramifications for image-guided prostate cancer assessments.
Clinical Significance
PUL, a minimally invasive surgical procedure to treat LUTS due to BPH, carries significant clinical relevance.
Symptom Relief
PUL has been demonstrated to provide a swift amelioration of LUTS, significantly improving urinary flow and the patient's quality of life. Data from a systematic review involving approximately 600 patients indicated a pooled improvement in the IPSS ranging from 9.73 to 12.16 over 2 years of follow-up. Moreover, the Qmax exhibited an increase from 3.44 mL/s to 4.26 mL/s.[54] These improvements were observed early on and sustained throughout the 24-month observation period, suggesting the durability of PUL outcomes.[54]
However, it is important to note that these improvements, while significant, were less pronounced than those typically achieved with traditional resective procedures like TURP, GLL-PVP, and HoLEP, which have reported Qmax improvements in the range of 10 to 13 mL/s.[58] Results from a study comparing TURP and PUL demonstrated within the first month following the procedure that 82% of individuals who underwent PUL achieved a positive response on the BPH symptom endpoint, in stark contrast to the 53% response rate observed in patients who had undergone TURP.[59]
Sexual Function Preservation
PUL stands out for its conservative impact on sexual health, exhibiting minimal influence on sexual and ejaculatory functions postoperatively. Clinical investigations, including the evaluation of scores from the male sexual health questionnaire ejaculatory dysfunction short form, have noted improvements in sexual function after PUL, with pooled estimates of these enhancements ranging from 0.81 to 1.81 across various follow-up intervals.[54] Further research corroborates these findings, indicating a lack of sexual function decline following the procedure.
In comparative terms, Aquablation, a waterjet-based BPH treatment, has been associated with higher instances of ejaculatory dysfunction, reported in approximately 7.8% to 26.7% of cases.[60] Additionally, traditional TURP has been associated with a significant occurrence of retrograde ejaculation, affecting nearly 47.8% of sexually active patients after the procedure, highlighting the advantageous profile of PUL in maintaining sexual function.[61]
Reduced Recovery Time
Because it's less invasive, the recovery time for the PUL procedure is typically shorter compared to traditional surgical procedures such as TURP or open simple prostatectomy, allowing patients to return to normal activities sooner. Significant contrast was observed in postprocedure catheterization, with approximately 74% of patients with TURP requiring catheter use beyond 24 hours after surgery, compared to only 45% of those treated with PUL. The average hospital stay postprocedure is shorter for PUL, typically 1 day, compared to nearly 2 days for TURP recipients. Furthermore, the return to preoperative activity levels was more expedient for patients undergoing PUL, averaging 11 days. In contrast, those with a TURP reported an average of 17 days to reach the same activity level.[59]
Safety Profile
PUL has a favorable safety profile with a low risk of serious complications, making it a suitable option even for some patients who may not be candidates for more invasive procedures due to comorbid conditions.
Anesthetic Flexibility
The procedure can be performed under local, spinal, or general anesthesia, providing flexibility based on patient preference and comorbidities.
Reduced Medication Needs
PUL can reduce or eliminate the need for ongoing BPH medications, which may have side effects or be contraindicated in certain patients.
Outpatient Procedure
PUL can often be performed as an outpatient procedure, which is cost-effective and convenient for patients and healthcare systems.
Enhancing Healthcare Team Outcomes
Managing benign prostatic hyperplasia using the prostatic urethral lift system necessitates a synchronized interprofessional team effort to ensure patient-centered care and superior outcomes. Urologists, primary care physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals must collaborate in an informed and cohesive manner, each contributing their skills and expertise. The team's collective understanding of the criteria for PUL, the procedural details, and postoperative management strategies is critical for reducing the morbidity associated with BPH and enhancing patients' quality of life.
A strategic approach is essential, focusing on evidence-based methods to ensure the procedure's effectiveness and minimize complications. This includes comprehensive preoperative evaluations, meticulous execution of the procedure, and diligent postoperative supervision. Ethical considerations are paramount, and the team is responsible for ensuring that patients are fully informed about PUL's advantages and potential risks, thus facilitating informed decision-making. Each healthcare professional has distinct responsibilities: urologists lead the procedure, advanced practitioners and nurses focus on patient education and recovery management, and pharmacists manage medication reviews and postoperative care. Effective communication within the team is crucial for keeping all members updated on the patient's condition and treatment plan. Care coordination, from initial scheduling to follow-up care, is vital and may include additional services like physical therapy or specialist consultations. By integrating their expertise, maintaining open communication, and coordinating care, the healthcare team can significantly improve patient safety and outcomes, enhancing the overall management of BPH with the PUL system.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Woo HH, Bolton DM, Laborde E, Jack G, Chin PT, Rashid P, Thavaseelan J, McVary KT. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. The journal of sexual medicine. 2012 Feb:9(2):568-75. doi: 10.1111/j.1743-6109.2011.02568.x. Epub 2011 Dec 16 [PubMed PMID: 22172161]
Roehrborn CG, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, Brown BT, McVary KT, Te AE, Gholami SS, Rashid P, Moseley WG, Chin PT, Dowling WT, Freedman SJ, Incze PF, Coffield KS, Borges FD, Rukstalis DB. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. The Journal of urology. 2013 Dec:190(6):2161-7. doi: 10.1016/j.juro.2013.05.116. Epub 2013 Jun 11 [PubMed PMID: 23764081]
Woo HH, Chin PT, McNicholas TA, Gill HS, Plante MK, Bruskewitz RC, Roehrborn CG. Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU international. 2011 Jul:108(1):82-8. doi: 10.1111/j.1464-410X.2011.10342.x. Epub 2011 May 6 [PubMed PMID: 21554526]
Level 2 (mid-level) evidenceNg M, Leslie SW, Baradhi KM. Benign Prostatic Hyperplasia. StatPearls. 2025 Jan:(): [PubMed PMID: 32644346]
Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Lawrentschuk N, Ptasznik G, Ong S. Benign Prostate Disorders. Endotext. 2000:(): [PubMed PMID: 25905239]
Miernik A, Gratzke C. Current Treatment for Benign Prostatic Hyperplasia. Deutsches Arzteblatt international. 2020 Dec 4:117(49):843-854. doi: 10.3238/arztebl.2020.0843. Epub [PubMed PMID: 33593479]
McNeal JE. Anatomy of the prostate and morphogenesis of BPH. Progress in clinical and biological research. 1984:145():27-53 [PubMed PMID: 6201879]
Aaron L, Franco OE, Hayward SW. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. The Urologic clinics of North America. 2016 Aug:43(3):279-88. doi: 10.1016/j.ucl.2016.04.012. Epub [PubMed PMID: 27476121]
Garcia C, Chin P, Rashid P, Woo HH. Prostatic urethral lift: A minimally invasive treatment for benign prostatic hyperplasia. Prostate international. 2015 Mar:3(1):1-5. doi: 10.1016/j.prnil.2015.02.002. Epub 2015 Feb 13 [PubMed PMID: 26157759]
Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, Franco O, Baskin LS. Development of the human prostate. Differentiation; research in biological diversity. 2018 Sep-Oct:103():24-45. doi: 10.1016/j.diff.2018.08.005. Epub 2018 Sep 4 [PubMed PMID: 30224091]
Lee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinology and metabolism clinics of North America. 2011 Sep:40(3):565-75, viii-ix. doi: 10.1016/j.ecl.2011.05.012. Epub [PubMed PMID: 21889721]
Level 3 (low-level) evidenceFoo KT. Pathophysiology of clinical benign prostatic hyperplasia. Asian journal of urology. 2017 Jul:4(3):152-157. doi: 10.1016/j.ajur.2017.06.003. Epub 2017 Jun 13 [PubMed PMID: 29264224]
Zitoun OA, Farhat AM, Mohamed MA, Hamad MR, Aramini B, Haider KH. Management of benign prostate hyperplasia (BPH) by combinatorial approach using alpha-1-adrenergic antagonists and 5-alpha-reductase inhibitors. European journal of pharmacology. 2020 Sep 15:883():173301. doi: 10.1016/j.ejphar.2020.173301. Epub 2020 Jun 25 [PubMed PMID: 32592768]
McNicholas TA, Woo HH, Chin PT, Bolton D, Fernández Arjona M, Sievert KD, Schoenthaler M, Wetterauer U, Vrijhof EJ, Gange S, Montorsi F. Minimally invasive prostatic urethral lift: surgical technique and multinational experience. European urology. 2013 Aug:64(2):292-9. doi: 10.1016/j.eururo.2013.01.008. Epub 2013 Jan 19 [PubMed PMID: 23357348]
Rukstalis D, Grier D, Stroup SP, Tutrone R, deSouza E, Freedman S, David R, Kamientsky J, Eure G. Prostatic Urethral Lift (PUL) for obstructive median lobes: 12 month results of the MedLift Study. Prostate cancer and prostatic diseases. 2019 Sep:22(3):411-419. doi: 10.1038/s41391-018-0118-x. Epub 2018 Dec 12 [PubMed PMID: 30542055]
Sievert KD, Schonthaler M, Berges R, Toomey P, Drager D, Herlemann A, Miller F, Wetterauer U, Volkmer B, Gratzke C, Amend B. Minimally invasive prostatic urethral lift (PUL) efficacious in TURP candidates: a multicenter German evaluation after 2 years. World journal of urology. 2019 Jul:37(7):1353-1360. doi: 10.1007/s00345-018-2494-1. Epub 2018 Oct 3 [PubMed PMID: 30283994]
Knight L, Dale M, Cleves A, Pelekanou C, Morris R. UroLift for Treating Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia: A NICE Medical Technology Guidance Update. Applied health economics and health policy. 2022 Sep:20(5):669-680. doi: 10.1007/s40258-022-00735-y. Epub 2022 Jul 18 [PubMed PMID: 35843995]
Al-Singary W, Patel R, Obi-Njoku O, Patel HRH. The UroLift(®) System for lower urinary tract obstruction: patient selection for optimum clinical outcome. Minimally invasive therapy & allied technologies : MITAT : official journal of the Society for Minimally Invasive Therapy. 2022 Mar:31(3):456-461. doi: 10.1080/13645706.2020.1816554. Epub 2020 Sep 11 [PubMed PMID: 32915085]
Level 2 (mid-level) evidenceJones P, Rai BP, Aboumarzouk O, Somani BK. UroLift: a new minimally-invasive treatment for benign prostatic hyperplasia. Therapeutic advances in urology. 2016 Dec:8(6):372-376 [PubMed PMID: 27904652]
Level 3 (low-level) evidenceRoehrborn CG, Chin PT, Woo HH. The UroLift implant: mechanism behind rapid and durable relief from prostatic obstruction. Prostate cancer and prostatic diseases. 2022 Mar:25(1):79-85. doi: 10.1038/s41391-021-00434-0. Epub 2021 Aug 6 [PubMed PMID: 34363010]
Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, Cantwell AL, McVary KT, Te AE, Gholami SS, Moseley WG, Chin PT, Dowling WT, Freedman SJ, Incze PF, Coffield KS, Herron S, Rashid P, Rukstalis DB. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. The Canadian journal of urology. 2017 Jun:24(3):8802-8813 [PubMed PMID: 28646935]
Level 1 (high-level) evidenceMiller LE, Chughtai B, Dornbier RA, McVary KT. Surgical Reintervention Rate after Prostatic Urethral Lift: Systematic Review and Meta-Analysis Involving over 2,000 Patients. The Journal of urology. 2020 Nov:204(5):1019-1026. doi: 10.1097/JU.0000000000001132. Epub 2020 May 12 [PubMed PMID: 32396049]
Level 1 (high-level) evidenceWong K, Kinsella N, Seth J, Nicol D, Cahill D, Kasivisvanathan R, Withington J, Moghul M, Moss CL, Van Hemelrijck M, Giorgakoudi K, Cottrell C, Yates E, Khoo V, James ND. COmparing Urolift and Standard Transurethral resection of prostate Ahead of Radiotherapy in men with urinary symptoms secondary to prostate enlargement in Southwest London and North Cumbria (CO-STAR): a study protocol for a randomised feasibility study. BMJ open. 2023 Oct 6:13(10):e076621. doi: 10.1136/bmjopen-2023-076621. Epub 2023 Oct 6 [PubMed PMID: 37802612]
Level 1 (high-level) evidenceBiswal NC, Swann B, McKenna MG, Singh R. UroLift as a surrogate for fiducial markers in IGRT planning of prostate cancer in BPH patients. Practical radiation oncology. 2018 Jul-Aug:8(4):e231-e233. doi: 10.1016/j.prro.2017.11.014. Epub 2017 Dec 24 [PubMed PMID: 29452872]
Keehn A, Fram E, Garg M, Maria P. UroLift in Place of Fiducial Markers for Patients With Benign Prostatic Hyperplasia Undergoing External Beam Radiation Therapy. Urology. 2017 Jun:104():230-234. doi: 10.1016/j.urology.2016.11.029. Epub 2016 Dec 14 [PubMed PMID: 27988266]
Munien K, Leslie SW, Desai D. Water Vapor Thermal Ablation of the Prostate. StatPearls. 2025 Jan:(): [PubMed PMID: 38261694]
Woo H, Levin R, Cantrill C, Zhou S, Neff D, Sutton M, Bailen J, Darson M, Horgan J, Zantek P, Marty-Roix R, Rezūm Clinical Trials Group. Prospective Trial of Water Vapor Thermal Therapy for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia in Subjects with a Large Prostate: 6- and 12-month Outcomes. European urology open science. 2023 Dec:58():64-72. doi: 10.1016/j.euros.2023.10.006. Epub 2023 Nov 8 [PubMed PMID: 38152482]
McVary KT, El-Arabi A, Roehrborn C. Preservation of Sexual Function 5 Years After Water Vapor Thermal Therapy for Benign Prostatic Hyperplasia. Sexual medicine. 2021 Dec:9(6):100454. doi: 10.1016/j.esxm.2021.100454. Epub 2021 Oct 30 [PubMed PMID: 34731779]
Babar M, Loloi J, Tang K, Syed U, Ciatto M. Emerging outcomes of water vapor thermal therapy (Rezum) in a broad range of patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review. Lower urinary tract symptoms. 2022 May:14(3):140-154. doi: 10.1111/luts.12435. Epub 2022 Mar 1 [PubMed PMID: 35233955]
Level 1 (high-level) evidenceElliott SP, Coutinho K, Robertson KJ, D'Anna R, Chevli K, Carrier S, Aube-Peterkin M, Cantrill CH, Ehlert MJ, Te AE, Dann J, DeLong JM, Brandes SB, Hagedorn JC, Levin R, Schlaifer A, DeSouza E, DiMarco D, Erickson BA, Natale R, Husmann DA, Morey A, Olsson C, Virasoro R. One-Year Results for the ROBUST III Randomized Controlled Trial Evaluating the Optilume(®) Drug-Coated Balloon for Anterior Urethral Strictures. The Journal of urology. 2022 Apr:207(4):866-875. doi: 10.1097/JU.0000000000002346. Epub 2021 Dec 2 [PubMed PMID: 34854748]
Level 1 (high-level) evidenceVanDyke ME, Morey AF, Coutinho K, Robertson KJ, D'Anna R, Chevli K, Cantrill CH, Ehlert MJ, Te AE, Dann J, DeLong JM, Virasoro R, Hagedorn JC, Levin R, DeSouza E, DiMarco D, Erickson BA, Olsson C, Elliott SP. Optilume drug-coated balloon for anterior urethral stricture: 2-year results of the ROBUST III trial. BJUI compass. 2024 Mar:5(3):366-373. doi: 10.1002/bco2.312. Epub 2023 Dec 18 [PubMed PMID: 38481667]
Kaplan SA, Moss J, Freedman S, Coutinho K, Wu N, Efros M, Elterman D, D'Anna R, Padron O, Robertson KJ, Lawindy S, Mistry S, Shore N, Spier J, Kaminetsky J, Mazzarella B, Cahn D, Jalkut M, Te A. The PINNACLE Study: A Double-blind, Randomized, Sham-controlled Study Evaluating the Optilume BPH Catheter System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. The Journal of urology. 2023 Sep:210(3):500-509. doi: 10.1097/JU.0000000000003568. Epub 2023 Aug 9 [PubMed PMID: 37555604]
Level 1 (high-level) evidenceBalakrishnan D, Jones P, Somani BK. iTIND: the second-generation temporary implantable nitinol device for minimally invasive treatment of benign prostatic hyperplasia. Therapeutic advances in urology. 2020 Jan-Dec:12():1756287220934355. doi: 10.1177/1756287220934355. Epub 2020 Jun 25 [PubMed PMID: 32655690]
Level 3 (low-level) evidenceKadner G, Valerio M, Giannakis I, Manit A, Lumen N, Ho BSH, Alonso S, Schulman C, Barber N, Amparore D, Porpiglia F. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World journal of urology. 2020 Dec:38(12):3235-3244. doi: 10.1007/s00345-020-03140-z. Epub 2020 Mar 2 [PubMed PMID: 32124019]
Tutrone RF, Schiff W. Early patient experience following treatment with the UroLift prostatic urethral lift and Rezum steam injection. The Canadian journal of urology. 2020 Jun:27(3):10213-10219 [PubMed PMID: 32544043]
Ulchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. ClinicoEconomics and outcomes research : CEOR. 2018:10():29-43. doi: 10.2147/CEOR.S148195. Epub 2017 Dec 29 [PubMed PMID: 29343977]
Rukstalis D, Rashid P, Bogache WK, Tutrone RF, Barkin J, Chin PT, Woo HH, Cantwell AL, Cowan BE, Bolton DM. 24-month durability after crossover to the prostatic urethral lift from randomised, blinded sham. BJU international. 2016 Oct:118 Suppl 3():14-22. doi: 10.1111/bju.13666. Epub [PubMed PMID: 27684483]
Level 1 (high-level) evidenceShore N, Freedman S, Gange S, Moseley W, Heron S, Tutrone R, Brown T, Barkin J. Prospective multi-center study elucidating patient experience after prostatic urethral lift. The Canadian journal of urology. 2014 Feb:21(1):7094-101 [PubMed PMID: 24529008]
Sønksen J, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D, Sievert KD, Chapple CR, Montorsi F, Patterson JM, Fahrenkrug L, Schoenthaler M, Gratzke C. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. European urology. 2015 Oct:68(4):643-52. doi: 10.1016/j.eururo.2015.04.024. Epub 2015 Apr 30 [PubMed PMID: 25937539]
Level 1 (high-level) evidenceDenisenko A, Somani B, Agrawal V. Recent advances in UroLift: A comprehensive overview. Turkish journal of urology. 2022 Jan:48(1):11-16. doi: 10.5152/tud.2022.21149. Epub [PubMed PMID: 35118985]
Level 3 (low-level) evidenceDutta R, Matz EL, Deebel NA, Terlecki RP. Persistent need for ongoing medical or surgical therapy despite UroLift: Data from an academic center. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2023 Aug 29:17(12):E408-11. doi: 10.5489/cuaj.8394. Epub 2023 Aug 29 [PubMed PMID: 37787590]
Rochester M, Doherty R, Page T, Barber N, Kavia R, Thiruchelvam N, Gange S, Mueller T, Eure G, Chin P, Kayes O. Prostatic urethral lift for subjects in urinary retention (PULSAR): 12-Month results of a prospective controlled trial compared with real-world outcomes. BJUI compass. 2024 Jan:5(1):60-69. doi: 10.1002/bco2.280. Epub 2023 Sep 8 [PubMed PMID: 38179018]
Hosseini M, Ebrahimi SM, SeyedAlinaghi S, Mahmoodi M. Sensitivity and specificity of international prostate symptom score (IPSS) for the screening of Iranian patients with prostate cancer. Acta medica Iranica. 2011:49(7):451-5 [PubMed PMID: 21960078]
Tsuru T, Tsujimura A, Mizushima K, Kurosawa M, Kure A, Uesaka Y, Nozaki T, Shirai M, Kobayashi K, Horie S. International Prostate Symptom Score and Quality of Life Index for Lower Urinary Tract Symptoms Are Associated with Aging Males Symptoms Rating Scale for Late-Onset Hypogonadism Symptoms. The world journal of men's health. 2023 Jan:41(1):101-109. doi: 10.5534/wjmh.210171. Epub 2022 Jan 6 [PubMed PMID: 35021314]
Level 2 (mid-level) evidenceBurnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh J, Khera M, McVary KT, Miner MM, Nelson CJ, Sadeghi-Nejad H, Seftel AD, Shindel AW. Erectile Dysfunction: AUA Guideline. The Journal of urology. 2018 Sep:200(3):633-641. doi: 10.1016/j.juro.2018.05.004. Epub 2018 May 7 [PubMed PMID: 29746858]
Rhoden EL, Telöken C, Sogari PR, Vargas Souto CA. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. International journal of impotence research. 2002 Aug:14(4):245-50 [PubMed PMID: 12152112]
Abdelmoteleb H, Jefferies ER, Drake MJ. Assessment and management of male lower urinary tract symptoms (LUTS). International journal of surgery (London, England). 2016 Jan:25():164-71. doi: 10.1016/j.ijsu.2015.11.043. Epub 2015 Nov 30 [PubMed PMID: 26654899]
Sasaki R, Habuchi T, Sato K, Akao T, Kakinuma H, Zhang LQ, Wang L, Matsuo S, Sasaki S, Ogawa O, Kato T. The clinical utility of measuring total PSA, PSA density, gamma-seminoprotein and gamma-seminoprotein/total PSA in prostate cancer prediction. Japanese journal of clinical oncology. 2000 Aug:30(8):337-42 [PubMed PMID: 11059338]
Jiang YH, Kuo HC. Recent research on the role of urodynamic study in the diagnosis and treatment of male lower urinary tract symptoms and urinary incontinence. Tzu chi medical journal. 2017 Apr-Jun:29(2):72-78. doi: 10.4103/tcmj.tcmj_19_17. Epub [PubMed PMID: 28757770]
Nunez-Nateras R, Andrews JR, Martin GL, Andrews PE, Humphreys MR, Ferrigni RG, Eversman WG, Castle EP. Accuracy of ultrasound in estimation of prostate weight: comparison of urologists and radiologists. The Canadian journal of urology. 2010 Feb:17(1):4985-8 [PubMed PMID: 20156377]
Aaronson DS, Walsh TJ, Smith JF, Davies BJ, Hsieh MH, Konety BR. Meta-analysis: does lidocaine gel before flexible cystoscopy provide pain relief? BJU international. 2009 Aug:104(4):506-9; discussion 509-10. doi: 10.1111/j.1464-410X.2009.08417.x. Epub 2009 Feb 23 [PubMed PMID: 19239453]
Level 1 (high-level) evidenceMukerji G, Munasinghe I, Raza A. A survey of the peri-operative management of urological patients on clopidogrel. Annals of the Royal College of Surgeons of England. 2009 May:91(4):313-20. doi: 10.1308/003588409X391820. Epub 2009 Apr 2 [PubMed PMID: 19344548]
Level 3 (low-level) evidenceIqbal M, Jones R, Hughes S, Shergill I. Low power HOLEP after failed urolift: A case report using 50 Watt laser. Urology case reports. 2018 Jan:16():114-115. doi: 10.1016/j.eucr.2017.11.029. Epub 2017 Dec 5 [PubMed PMID: 29255680]
Level 3 (low-level) evidenceXiang P, Wang M, Guan D, Liu D, Wang Y, Hao Y, Li S, Liu Y, Ping H. A Systematic Review and Meta-analysis of Prostatic Urethral Lift for Male Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. European urology open science. 2020 Jul:19():3-15. doi: 10.1016/j.euros.2020.05.001. Epub 2020 Jun 4 [PubMed PMID: 34337448]
Level 1 (high-level) evidencePorto JG, Arbelaez MCS, Blachman-Braun R, Bhatia A, Bhatia S, Satyanarayana R, Marcovich R, Shah HN. Complications associated with minimally invasive surgical therapies (MIST) for surgical management of benign prostatic hyperplasia: a Manufacturer and User Facility Device Experience (MAUDE) database review. World journal of urology. 2023 Jul:41(7):1975-1982. doi: 10.1007/s00345-023-04440-w. Epub 2023 May 24 [PubMed PMID: 37222779]
Juliebø-Jones P, Somani BK, Tzelves L, Haugland JN, Moen CA, Honoré A, Beisland C. Complications and device failures associated with urolift: Findings from the MAUDE database. Urologia. 2023 Nov:90(4):636-641. doi: 10.1177/03915603231180016. Epub 2023 Jun 8 [PubMed PMID: 37292024]
Benidir T, Austhof E, Ward RD, Ream J, Bullen J, Turkbey B, Pinto PA, Giganti F, Klein EA, Purysko AS. Impact of Prostate Urethral Lift Device on Prostate Magnetic Resonance Image Quality. The Journal of urology. 2023 Jan 11:():101097JU0000000000003156. doi: 10.1097/JU.0000000000003156. Epub 2023 Jan 11 [PubMed PMID: 36630568]
Level 2 (mid-level) evidenceZhang Y, Yuan P, Ma D, Gao X, Wei C, Liu Z, Li R, Wang S, Liu J, Liu X. Efficacy and safety of enucleation vs. resection of prostate for treatment of benign prostatic hyperplasia: a meta-analysis of randomized controlled trials. Prostate cancer and prostatic diseases. 2019 Dec:22(4):493-508. doi: 10.1038/s41391-019-0135-4. Epub 2019 Feb 28 [PubMed PMID: 30816336]
Level 1 (high-level) evidenceMagistro G, Stief CG, Gratzke C. Prostatic Urethral Lift Versus Transurethral Resection of the Prostate (TURP). Current urology reports. 2017 Aug 29:18(10):82. doi: 10.1007/s11934-017-0725-4. Epub 2017 Aug 29 [PubMed PMID: 28852996]
Fiori C, Checcucci E, Gilling P, Amparore D, Volpi G, De Cillis S, Aimar R, Sica M, Cattaneo G, Alleva G, Manfredi M, Porpiglia F, ESUT Lower Tract Group. All you need to know about "Aquablation" procedure for treatment of benign prostatic obstruction. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2020 Apr:72(2):152-161. doi: 10.23736/S0393-2249.20.03654-1. Epub 2020 Feb 19 [PubMed PMID: 32083415]
Pavone C, Abbadessa D, Scaduto G, Caruana G, Scalici Gesolfo C, Fontana D, Vaccarella L. Sexual dysfunctions after transurethral resection of the prostate (TURP): evidence from a retrospective study on 264 patients. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica. 2015 Mar 31:87(1):8-13. doi: 10.4081/aiua.2015.1.8. Epub 2015 Mar 31 [PubMed PMID: 25847889]
Level 2 (mid-level) evidenceBarry MJ, Fowler FJ Jr, O'leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT, Measurement Committee of the American Urological Association. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. The Journal of urology. 2017 Feb:197(2S):S189-S197. doi: 10.1016/j.juro.2016.10.071. Epub 2016 Dec 22 [PubMed PMID: 28012747]
Long Depaquit T, Reus R, Uleri A, Bastide C, Berchiche W, Peyrottes A, Eghazarian C, Baboudjian M, Fourmarier M. Comparative analysis of functional and economic outcomes of prostatic urethral lift and water vapor thermal therapy for male LUTS: A French perspective. The French journal of urology. 2025 Feb 19:35(5):102872. doi: 10.1016/j.fjurol.2025.102872. Epub 2025 Feb 19 [PubMed PMID: 39983907]
Level 2 (mid-level) evidence