Introduction
Chondroid syringoma, also known as a mixed tumor of the skin, is a rare, typically benign neoplasm that arises from the sweat glands in the skin. These tumors predominantly occur in the head and neck region, especially on the nose, cheeks, and upper lip, though they can also be found on the extremities.[1][2] In 1892, Nasse described the histopathologic components of both epithelial and mesenchymal elements, resembling the structure of mixed tumors in other tissues, such as pleomorphic adenoma of the salivary gland.[3] The term chondroid syringoma was suggested by Hirsch and Helwig in 1961.[4] Chondroid syringomas generally grow slowly and present as painless, firm nodules. While most are benign, there have been rare cases of malignancy. Treatment typically involves surgical excision, and the prognosis is generally good following complete removal of the tumor.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of chondroid syringoma remains unclear, but it is believed to originate from pluripotent cells within the sweat gland ductal epithelium.[4] Some studies suggest a potential association with trauma, chronic irritation, or hormonal influences, although no definitive causal factors have been identified. Histologically, chondroid syringoma exhibits a biphasic pattern with both epithelial and mesenchymal components, characterized by nests of basaloid epithelial cells and areas of chondroid or myxoid stroma.[6] While the tumor typically presents as a slow-growing, painless, solitary nodule, diagnosis primarily relies on histopathological examination. Although some recent developments have been made to identify genomic aberrations of various salivary gland tumors, further research is needed to elucidate the precise etiology and pathogenesis of chondroid syringoma.[7]
Epidemiology
Chondroid syringoma is a relatively uncommon benign tumor arising from sweat gland structures, most frequently observed in the head and neck area. While precise epidemiological data on chondroid syringoma are limited, this neoplasm predominantly affects adults, with a slight predominance in men.[4] The condition typically manifests between the third and sixth decades of life; pediatric cases have been reported.[8][9]
There is a consensus that chondroid syringoma is rare, comprising less than 0.01% of all primary skin tumors.[10][11] Although it can occur sporadically, some reports suggest an association with previous trauma or chronic irritation at the tumor site. However, further research is warranted to establish more comprehensive epidemiological characteristics and risk factors associated with this condition.
Pathophysiology
Mixed tumors of the skin originate from eccrine or apocrine sweat glands or their ductal structures.[3] The exact pathophysiological mechanisms underlying chondroid syringoma development remain unclear. The condition is believed to arise from pluripotent myoepithelial cells within the sweat gland ductal epithelium, undergoing differentiation towards both epithelial and mesenchymal components. The epithelial component usually consists of apocrine or eccrine differentiation, while the mesenchymal components are generally a chondroid or myxoid stroma.[12] Therefore, histologically, chondroid syringoma demonstrates a biphasic pattern characterized by nests of basaloid epithelial cells surrounded by a chondroid or myxoid stroma.
PLAG1 gene rearrangements have been identified in a significant number of chondroid syringomas.[13] More than 87% of apocrine-type cutaneous mixed tumors overexpress PLAG1. However, eccrine-type cutaneous mixed tumors lack PLAG1 expression.[12] Pleomorphic adenoma, chondroid syringoma's homologous neoplasm of the salivary gland, lung, and breast, also demonstrates PLAG1 fusions.[12]
Despite the limited understanding of its pathophysiology, chondroid syringoma is recognized as a benign tumor with low recurrence rates following surgical excision, underscoring its relatively indolent nature. Continued research efforts to unravel the molecular mechanisms driving chondroid syringoma development may provide insights into its pathophysiology and potentially identify novel therapeutic targets.
Histopathology
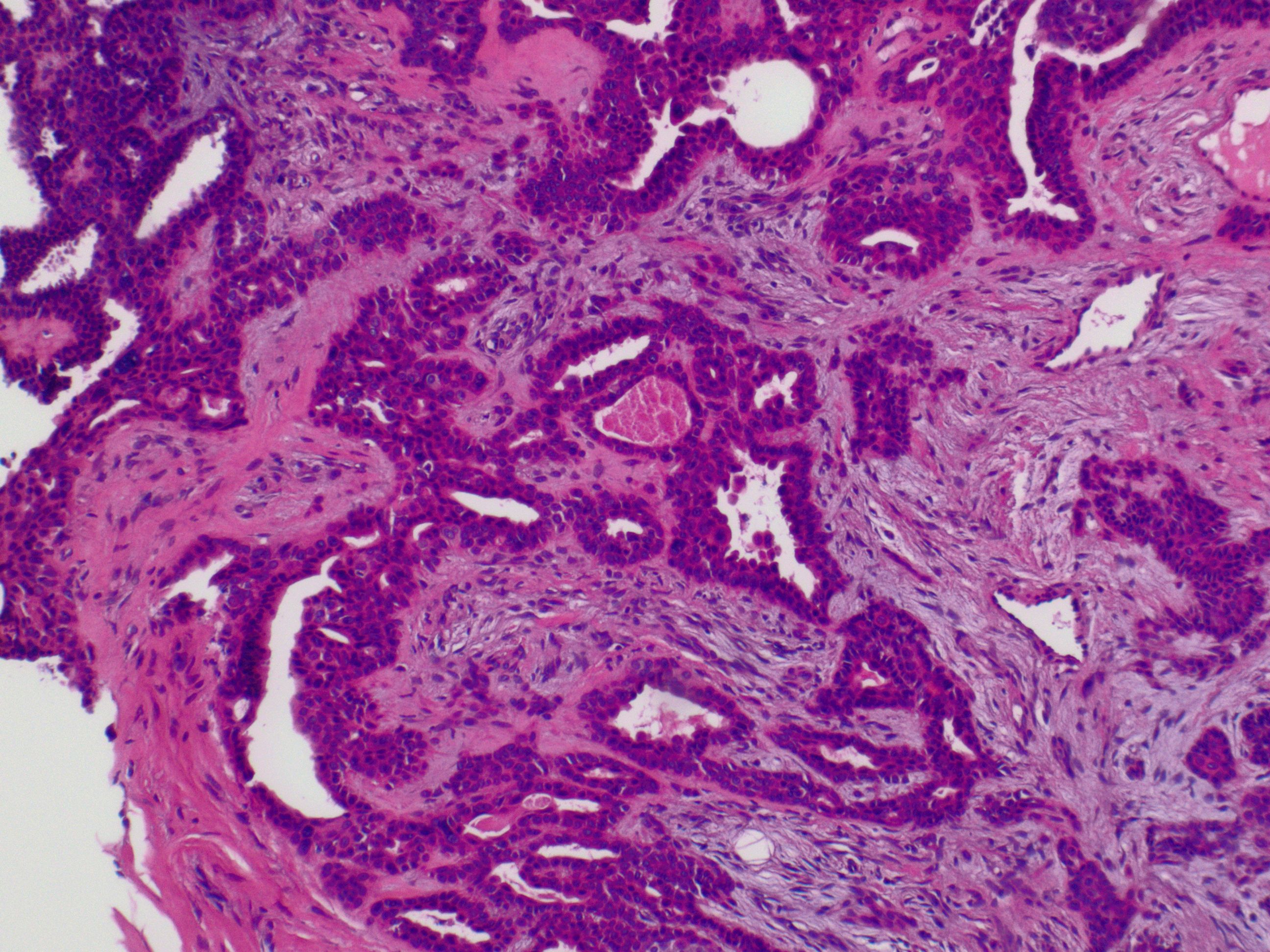
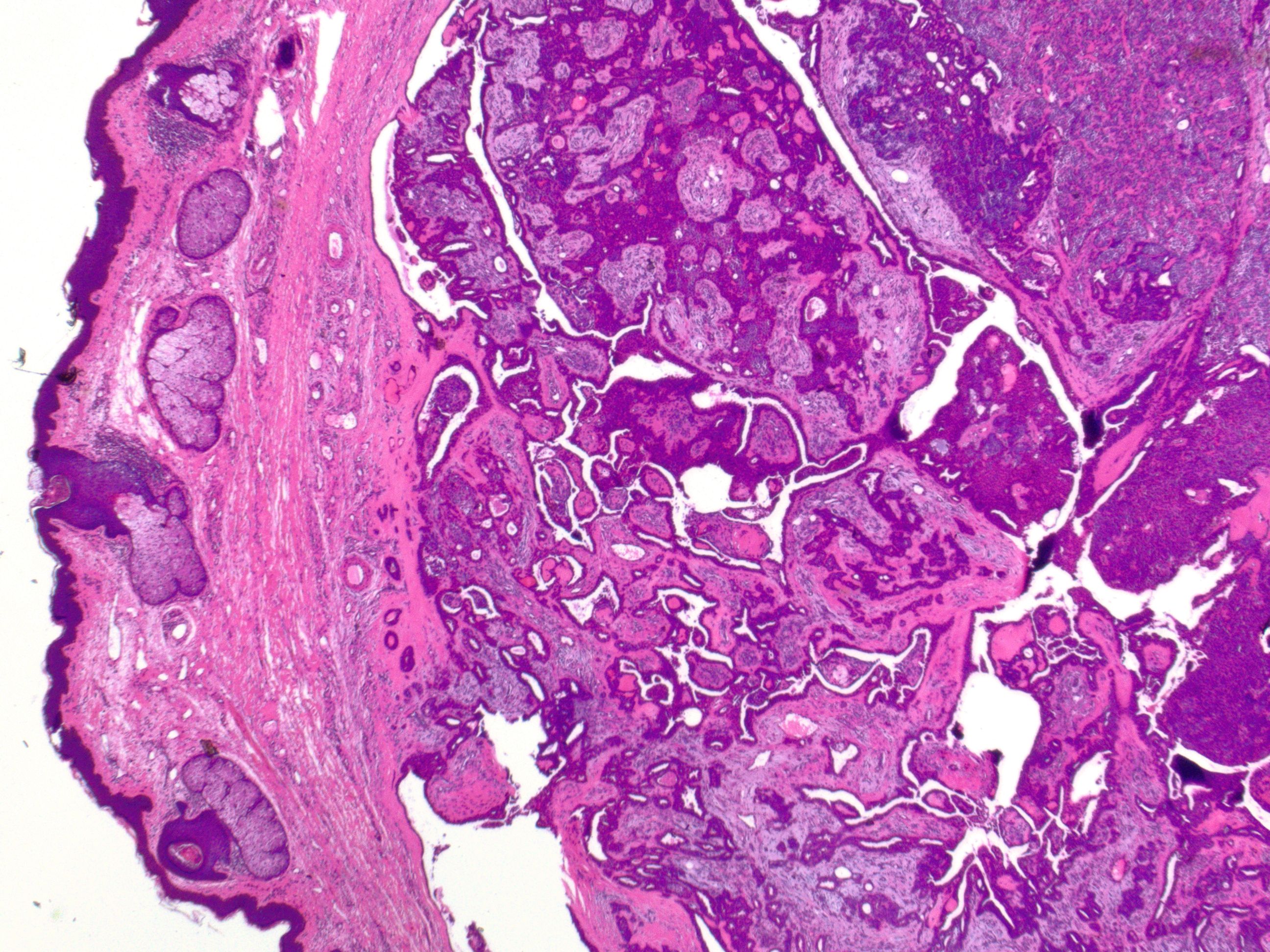
Chondroid syringoma exhibits distinctive histopathological features that aid in its diagnosis (see Image. Cutaneous Mixed Tumor at 2x Magnification). Histologically, chondroid syringoma demonstrates a circumscribed dermal or subcutaneous tumor with a biphasic pattern characterized by nests or cords of basaloid epithelial cells surrounded by islands or strands of myoepithelial cells within a chondromyxoid or myxoid stroma.[13][14] The basaloid cells typically form duct-like structures, while the myoepithelial cells appear spindled or stellate. A spectrum of differentiations exists for both the epithelial and stromal components. Epithelial alterations include apocrine, eccrine, or less commonly follicular, sebaceous, or plasmacytoid differentiation. The various stromal components that have been identified include myxoid, chondroid, or less commonly collagenous, osteoid, or lipoid metaplasia.[15]
Further, chondroid syringoma can be subclassified based on histologic characteristics into the most common apocrine type or the rarer eccrine type.[16] The apocrine variety typically involves differentiation toward hair follicle and sebaceous gland structures, with an epithelial part made up of branching tubules and cyst-like spaces set in a myxoid or chondromyxoid stroma (see Image. Cutaneous Mixed Tumor at 10x Magnification). The tubules consist of 2 layers: an outer layer of myoepithelial cells and an inner layer of epithelial cells, which sometimes exhibit secretion through decapitation.[17] In the eccrine type, the epithelial part consists of small tubes lined with 1 layer of uniform cells that are either cuboidal, oval, or round. These cells have a cytoplasm that is either eosinophilic or has a clear, glass-like appearance and vesicular nuclei. There is no decapitation secretion present.
Immunohistochemical staining plays a valuable role in confirming the diagnosis of chondroid syringoma and distinguishing it from other cutaneous neoplasms. Commonly used immunohistochemical markers include cytokeratin, epithelial membrane antigen (EMA), S-100 protein, and smooth muscle actin (SMA). Cytokeratin and EMA typically show positive staining in the epithelial component of chondroid syringoma, highlighting the ductal structures. S-100 protein and SMA, on the other hand, demonstrate positive staining in the myoepithelial cells, aiding in identifying this component within the tumor.[12] The characteristic histopathological features and immunohistochemical staining patterns help accurately diagnose chondroid syringoma and differentiate it from other benign and malignant cutaneous neoplasms.
History and Physical
The clinical presentation of mixed tumors is indistinctive. These neoplasms can present with varying morphology but are most commonly misdiagnosed as cysts. The history and physical examination of a patient with chondroid syringoma play a critical role in diagnosis and management. Clinical evaluation typically involves a comprehensive assessment of the patient's medical history and a thorough examination of the lesion.
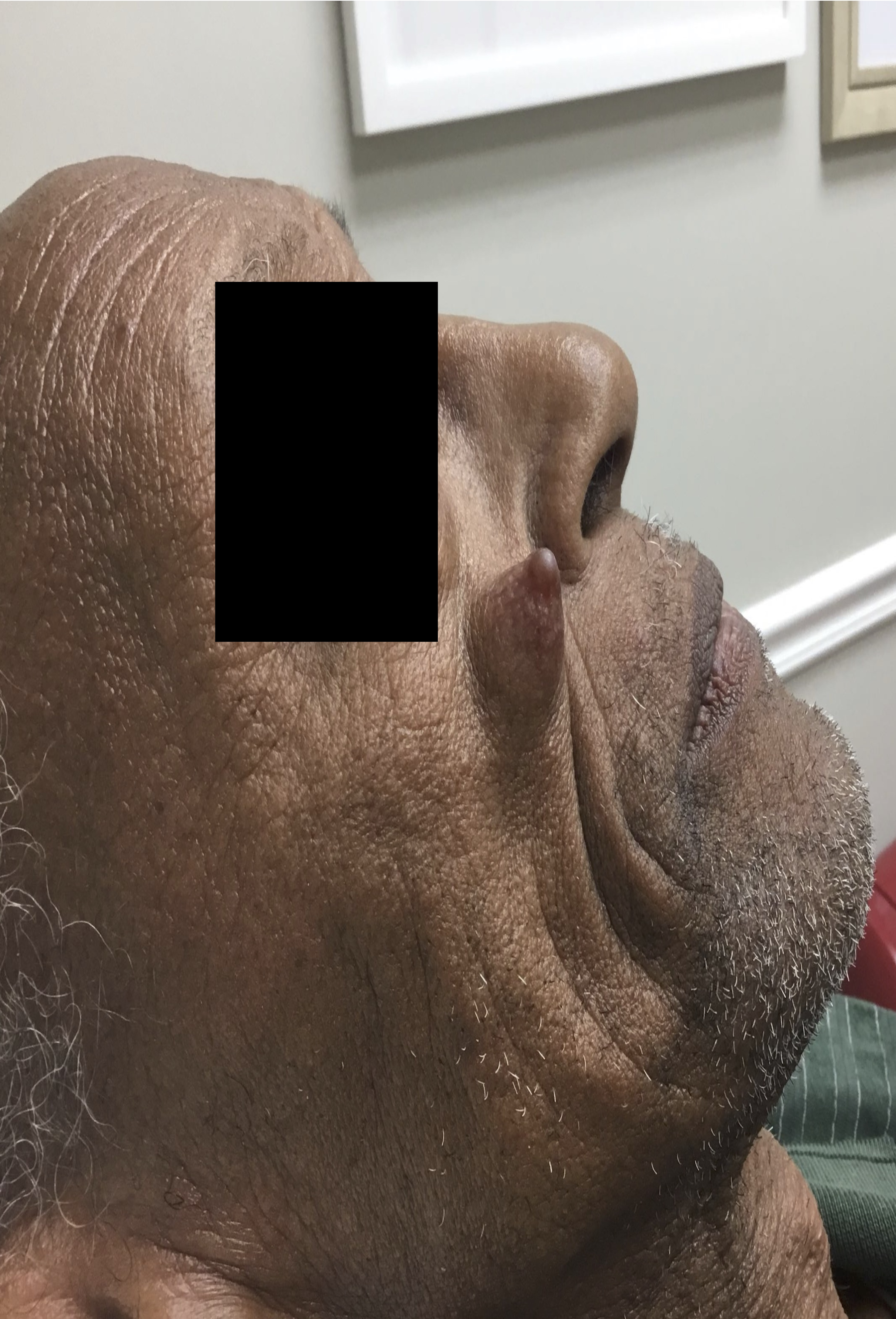
History-taking should include inquiries about the lesion's onset, duration, progression, and associated symptoms, such as pain, tenderness, or changes in size. Patients may report a slow-growing, painless nodule present for months to years, often in the head and neck region, particularly around the eyelids or nose (see Image. Cutaneous Mixed Tumor). Additionally, clinicians should inquire about any relevant past medical history, including previous skin lesions, trauma, or radiation exposure.
The physical examination of a chondroid syringoma typically reveals a well-defined, firm, mobile nodule within the dermis or subcutaneous tissue. The overlying skin may appear normal or show erythematous, ulcerative, or crusting features. Lesions are often solitary but can occasionally be multiple. Size and location may vary, with some lesions being small and superficial while others may be larger and deeper-seated. While the history and physical examination provide valuable clues, definitive diagnosis typically requires histopathological examination of a tissue biopsy or excisional specimen.
Evaluation
Evaluation typically involves a combination of clinical assessment, histopathological examination, and, in some cases, immunohistochemistry to confirm the diagnosis and assess the extent of the tumor. While there are no current guidelines and limited literature specifically addressing the evaluation of chondroid syringoma, recent studies have highlighted the role of various diagnostic modalities in its assessment. Histopathological examination remains the gold standard for diagnosing chondroid syringoma.[7] Tissue biopsy or excisional biopsy of the lesion allows for microscopic evaluation, revealing characteristic features such as nests of basaloid cells, tubular structures, and areas of chondroid or myxoid stroma. Fine-needle aspiration has also been reported as an initial biopsy method for deeper subcutaneous tumors.[18][19] Immunohistochemistry may be utilized to support the diagnosis, with markers such as cytokeratin, EMA, and S-100 protein often showing positive staining patterns consistent with chondroid syringoma.
Imaging studies such as ultrasonography, magnetic resonance imaging (MRI), or computed tomography (CT) may help characterize the lesion's depth, relationship to surrounding structures, and potential involvement of deeper tissues, particularly in larger, subcutaneous, or recurrent cases where more complex surgical planning is necessary.[20] Ultrasonography is often used as an initial imaging modality due to its accessibility and ability to assess superficial lesions; MRI or CT may provide additional information in cases of larger or deeper lesions.[21] While specific laboratory tests are not typically indicated for evaluating chondroid syringoma, a complete blood count and a comprehensive metabolic panel may be performed as part of a preoperative assessment or to evaluate any underlying systemic conditions.
Treatment / Management
Chondroid syringoma treatment typically involves surgical excision, which is considered curative in most cases.[10] The primary goal of surgical intervention is complete removal of the tumor with adequate margins to minimize the risk of recurrence and, although extremely rare, avoid malignant transformation. The extent of excision depends on factors such as tumor size, location, and depth of invasion. While chondroid syringoma is generally well-demarcated and encapsulated, careful dissection is essential to prevent damage to surrounding structures and ensure complete excision.
Moreover, although recurrence rates following surgical excision are generally low, the possibility of recurrence necessitates long-term surveillance and follow-up care to ensure optimal patient outcomes. Recurrent lesions may require additional surgeries, potentially increasing patient morbidity and healthcare costs. In cases where surgical excision is not feasible due to tumor size, location, recurrence, or patient comorbidities, alternative treatment modalities such as Mohs micrographic surgery or radiation therapy may be considered.[22] However, the efficacy of these approaches in chondroid syringoma management remains less established, and further research is needed to delineate their role.(B3)
Due to its rarity, guidelines for the management of patients with chondroid syringoma are limited. Therefore, treatment decisions are typically guided by expert consensus and clinical experience. Histopathological examination of the excised specimen is essential for confirming the diagnosis and assessing margins to ensure complete tumor removal. Genetic studies and immunohistochemical markers may provide insights into tumor behavior and aid in risk stratification.
Differential Diagnosis
When evaluating a patient with a suspected chondroid syringoma, it is essential to consider a range of differential diagnoses due to its nonspecific clinical presentation. Key conditions to include in the differential diagnosis to ensure accurate identification and management include:
- Epidermal inclusion cyst: A benign entity, which chondroid syringoma is often misdiagnosed as, based on clinical features
- Eccrine spiradenoma: Another benign adnexal tumor originating from eccrine sweat glands, which can resemble chondroid syringoma histologically
- Trichoepithelioma: A benign hair follicle tumor that may show overlapping histological features with chondroid syringoma, including basaloid cells and myxoid stroma
- Pilomatricoma: A benign skin tumor derived from hair matrix cells, which may exhibit calcification and chondroid differentiation histologically
- Dermatofibroma: A common benign skin lesion that can sometimes mimic chondroid syringoma clinically, especially when presenting as a firm, nodular lesion
- Dermatofibrosarcoma protuberans: A rare, locally aggressive soft tissue tumor that may share histological similarities with chondroid syringoma, such as a storiform pattern
- Adnexal carcinomas (eccrine carcinoma, apocrine carcinoma): Malignant adnexal tumors that may resemble chondroid syringoma histologically, particularly in cases of poorly differentiated or metastatic carcinoma
- Basal cell carcinoma: The most common malignant skin tumor, which may display diverse histological patterns, including those resembling chondroid syringoma, such as cribriform or trabecular patterns
- Cutaneous metastases: Cutaneous metastases from various internal malignancies can mimic chondroid syringoma clinically and histologically (rare)
- Neurofibroma: A benign peripheral nerve sheath tumor occasionally demonstrates histological overlap with chondroid syringoma, particularly in cases with myxoid stroma
- Syringoma: A benign tumor of eccrine sweat duct origin, which can mimic chondroid syringoma clinically, especially in cases presenting as multiple, small papules
Prognosis
Chondroid syringoma typically carries an excellent long-term prognosis due to its benign nature, particularly when the tumor is diagnosed early and fully excised. Complete surgical removal usually leads to resolution without significant long-term complications.[23] Although it may occasionally recur after surgical excision, recurrence rates are low, ranging from 2% to 9%, according to various study results.[22][24][25]
The risk of malignant transformation is exceedingly rare, with only a few documented cases reported in the literature.[25][26] Malignant chondroid syringoma most commonly occurs on the extremities.[20] Furthermore, metastasis has not been observed in chondroid syringoma.
Complications
Complications associated with chondroid syringoma are generally rare due to its benign nature; however, certain factors can lead to potential challenges in management. First, misdiagnosis or delayed diagnosis may occur due to the rarity of this condition and its varied clinical presentation, potentially resulting in inappropriate treatment or unnecessary patient anxiety. Moreover, incomplete excision of the tumor during surgery can lead to recurrence, albeit infrequent, requiring additional interventions and potentially impacting patient outcomes. Careful surgical planning and meticulous dissection are essential to minimize the risk of recurrence. Surgical complications may include wound infection, hematoma formation, nerve injury, or cosmetic deformity—particularly in cases involving lesions in cosmetically sensitive areas such as the face.
Consultations
In patients with chondroid syringoma, consultation with a dermatologist is crucial for accurate diagnosis and initial management. If the tumor is located in a cosmetically sensitive area, such as the face or neck, involving a plastic surgeon is advisable to ensure optimal cosmetic outcomes during surgical excision. Pathology consultation is also essential to distinguish chondroid syringoma from other benign and malignant neoplasms through histopathological examination. In rare cases of suspected malignancy or when the tumor exhibits atypical features, an oncologist may be consulted for further evaluation and management. Multidisciplinary collaboration enhances patient care and treatment outcomes.
Deterrence and Patient Education
Patient education is integral in managing chondroid syringoma, facilitating informed decision-making, reducing anxiety, and promoting adherence to treatment and follow-up care. By providing comprehensive education encompassing the nature of the condition, diagnostic process, treatment options, post-treatment care, and psychosocial support, healthcare professionals can empower patients to participate in their care journey and actively achieve optimal outcomes.
Patients should understand the benign nature of chondroid syringoma and that simple excision is essentially curative, with low recurrence rates after surgical excision. Histological characteristics and typical clinical presentation should be explained to facilitate patient education. Guidance on wound care, pain management, and expected postoperative recovery should be provided to ensure optimal healing outcomes. Patients should also be educated about signs of infection or recurrence warranting medical attention.
Pearls and Other Issues
Certain key insights can significantly improve diagnosis and treatment outcomes when managing care for patients with chondroid syringoma. Essential clinical pearls that healthcare professionals should keep in mind to optimize patient care include the following:
- This condition typically presents as a slow-growing, painless nodule, often on the head and neck.
- This is most commonly seen in middle-aged men.
- Early biopsy is essential for accurate diagnosis, as it can mimic other skin lesions.
- Differentiation from malignant chondroid syringoma and other neoplasms is critical.
- Surgical excision is the preferred treatment, aiming for complete removal to prevent recurrence.
- Consider a multidisciplinary approach to optimize functional and aesthetic outcomes, especially in cosmetically sensitive areas.
Enhancing Healthcare Team Outcomes
Diagnosing and managing chondroid syringoma involves an interprofessional approach that combines the expertise of dermatologists, pathologists, surgeons, primary care clinicians, advanced care clinicians, nurses, pharmacists, and other healthcare professionals. This team works together to establish an accurate diagnosis using clinical assessment, possible imaging studies, and biopsies. This diagnostic process requires expertise in recognizing the varied clinical presentations and skills to perform a biopsy. Treatment typically involves surgical excision, with the team ensuring patient safety and providing comprehensive perioperative care.
Care coordination includes managing pain, providing wound care, and monitoring the patient's recovery. Interprofessional communication is key to optimizing patient-centered care and outcomes. Team members share important information about the patient's condition and treatment plan, allowing for seamless collaboration.
Ethical standards guide all healthcare professionals caring for patients with chondroid syringoma, prioritizing patient safety and informed consent. The team collaborates to deliver evidence-based treatment options and emotionally support patients and their families. By maintaining clear, open lines of communication and working cohesively, the interprofessional team can enhance patient-centered care, safety, and clinical outcomes associated with chondroid syringoma.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sivamani R, Wadhera A, Craig E. Chondroid syringoma: case report and review of the literature. Dermatology online journal. 2006 Sep 8:12(5):8 [PubMed PMID: 16962023]
Level 3 (low-level) evidenceSulochana S, Manoharan M, Anitha. Chondroid syringoma-an unusual presentation. Journal of clinical and diagnostic research : JCDR. 2014 Jul:8(7):FD13-4. doi: 10.7860/JCDR/2014/7567.4627. Epub 2014 Jul 20 [PubMed PMID: 25177574]
HEADINGTON JT. Mixed tumors of skin: eccrine and apocrine types. Archives of dermatology. 1961 Dec:84():989-96 [PubMed PMID: 13905736]
HIRSCH P, HELWIG EB. Chondroid syringoma. Mixed tumor of skin, salivary gland type. Archives of dermatology. 1961 Nov:84():835-47 [PubMed PMID: 13907712]
Martora F, Mascolo M, Fabbrocini G, Annunziata A, Picone V, DE Fata Salvadores G, Russo D, Marasca C. Chondroid syringoma: an asymptomatic benign tumor. Italian journal of dermatology and venereology. 2022 Oct:157(5):450-452. doi: 10.23736/S2784-8671.22.07191-2. Epub 2022 Mar 11 [PubMed PMID: 35274880]
Sami A, Jain VK, Khan NP, Jeyaraman N, Jeyaraman M. Soft-tissue Chondroma Masquerading as Chondroid Syringoma - A Case Report. Journal of orthopaedic case reports. 2024 Sep:14(9):92-97. doi: 10.13107/jocr.2024.v14.i09.4742. Epub [PubMed PMID: 39253677]
Level 3 (low-level) evidenceToper MH, Sarioglu S. Molecular Pathology of Salivary Gland Neoplasms: Diagnostic, Prognostic, and Predictive Perspective. Advances in anatomic pathology. 2021 Mar 1:28(2):81-93. doi: 10.1097/PAP.0000000000000291. Epub [PubMed PMID: 33405400]
Level 3 (low-level) evidenceTurhan-Haktanir N, Sahin O, Bukulmez A, Demir Y. Chondroid syringoma in a child. Pediatric dermatology. 2007 Sep-Oct:24(5):505-7 [PubMed PMID: 17958797]
Purkayastha P, Thomson R, Wilson Jones N, Ng S. Chondroid syringoma: an unusual presentation in a 7-year-old boy. BMJ case reports. 2021 Jul 26:14(7):. doi: 10.1136/bcr-2019-232943. Epub 2021 Jul 26 [PubMed PMID: 34312123]
Level 3 (low-level) evidenceChen AH, Moreano EH, Houston B, Funk GF. Chondroid syringoma of the head and neck: clinical management and literature review. Ear, nose, & throat journal. 1996 Feb:75(2):104-8 [PubMed PMID: 8714424]
Yavuzer R, Başterzi Y, Sari A, Bir F, Sezer C. Chondroid syringoma: a diagnosis more frequent than expected. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2003 Feb:29(2):179-81 [PubMed PMID: 12562350]
Macagno N, Sohier P, Kervarrec T, Pissaloux D, Jullie ML, Cribier B, Battistella M. Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers. 2022 Jan 18:14(3):. doi: 10.3390/cancers14030476. Epub 2022 Jan 18 [PubMed PMID: 35158743]
Level 3 (low-level) evidenceAoun A, Dufrenot-Petitjean-Roget L, Amazan E, Derancourt C, Alexandre M, Quist D, Grossin M, Molinié V. [Cutaneous chondroid syringoma]. Annales de pathologie. 2015 Aug:35(4):275-80. doi: 10.1016/j.annpat.2015.04.002. Epub 2015 Jul 16 [PubMed PMID: 26188668]
Wan H, Xu M, Xia T. Clinical and pathological study on mixed tumors of the skin. Medicine. 2018 Sep:97(36):e12216. doi: 10.1097/MD.0000000000012216. Epub [PubMed PMID: 30200138]
Kazakov DV, Belousova IE, Bisceglia M, Calonje E, Emberger M, Grayson W, Hantschke M, Kempf W, Kutzner H, Michal M, Spagnolo DV, Virolainen S, Zelger B. Apocrine mixed tumor of the skin ("mixed tumor of the folliculosebaceous-apocrine complex"). Spectrum of differentiations and metaplastic changes in the epithelial, myoepithelial, and stromal components based on a histopathologic study of 244 cases. Journal of the American Academy of Dermatology. 2007 Sep:57(3):467-83 [PubMed PMID: 17707152]
Level 3 (low-level) evidenceKisova D, Dikov T, Ivanova V, Stoyanov H, Yordanova G. Mixed Eccrine Cutaneous Tumor with Folliculo-Sebaceous Differentiation: Case Report and Literature Review. Medicina (Kaunas, Lithuania). 2023 Aug 16:59(8):. doi: 10.3390/medicina59081465. Epub 2023 Aug 16 [PubMed PMID: 37629755]
Level 3 (low-level) evidenceKunikane H, Ishikura H, Yamaguchi J, Yoshiki T, Itoh T, Aizawa M. Chondroid syringoma (mixed tumor of the skin). A clinicopathological study of 13 cases. Acta pathologica japonica. 1987 Apr:37(4):615-25 [PubMed PMID: 2441570]
Level 3 (low-level) evidenceLamba S, Nanda A, Kumar U. Chondroid Syringoma: Fine-needle Aspiration Cytology of a Rare Entity at an Unusual Site. Journal of clinical and diagnostic research : JCDR. 2017 Jul:11(7):ED06-ED07. doi: 10.7860/JCDR/2017/28405.10135. Epub 2017 Jul 1 [PubMed PMID: 28892909]
Kim NI, Lim HS, Lee JS. Chondroid syringoma of the axilla: A potential for misdiagnosis as metastatic carcinoma on fine needle aspiration cytology. Diagnostic cytopathology. 2022 Nov:50(11):E315-E319. doi: 10.1002/dc.25007. Epub 2022 Jun 24 [PubMed PMID: 35748195]
Ge C, Gu J, Deng A, Joshi G, Most M, Tai R. Atypical Chondroid Syringoma of the Toe. Journal of the American Podiatric Medical Association. 2023 May-Jun:113(3):. pii: 22-094. doi: 10.7547/22-094. Epub [PubMed PMID: 37463189]
Min KH, Byun JH, Lim JS, Lee HK, Lee WM, Joo JE. Chondroid Syringoma on Face. Archives of craniofacial surgery. 2016 Sep:17(3):173-175. doi: 10.7181/acfs.2016.17.3.173. Epub 2016 Sep 23 [PubMed PMID: 28913278]
Prajapati CK, Mehta MJ, Kunikullaya US. Chondroid syringoma of an upper eyelid tumor: Unusual case report. Indian journal of cancer. 2023 Apr-Jun:60(2):245-247. doi: 10.4103/ijc.IJC_164_21. Epub [PubMed PMID: 36861706]
Level 3 (low-level) evidencePark SH, Kang SG, Choi HJ. Chondroid Syringoma of a Cheek. The Journal of craniofacial surgery. 2017 Jul:28(5):e480-e481. doi: 10.1097/SCS.0000000000003689. Epub [PubMed PMID: 28582289]
Mandeville JT, Roh JH, Woog JJ, Gonnering RS, Levin PS, Mazzoli RA, Ainbinder DJ, Older JJ, Moulin AP, Kiel R, Kim YD, Dryja TP. Cutaneous benign mixed tumor (chondroid syringoma) of the eyelid: clinical presentation and management. Ophthalmic plastic and reconstructive surgery. 2004 Mar:20(2):110-6 [PubMed PMID: 15083078]
Barnett MD, Wallack MK, Zuretti A, Mesia L, Emery RS, Berson AM. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. American journal of clinical oncology. 2000 Jun:23(3):227-32 [PubMed PMID: 10857882]
Level 3 (low-level) evidenceZufall AG, Mark EJ, Gru AA. Malignant chondroid syringoma: A systematic review. Skin health and disease. 2023 Apr:3(2):e144. doi: 10.1002/ski2.144. Epub 2022 Jul 10 [PubMed PMID: 37013126]
Level 1 (high-level) evidence