 Central Post-Stroke Pain Syndrome
Central Post-Stroke Pain Syndrome
Introduction
Central Poststroke Pain Syndrome Overview
Central poststroke pain syndrome (CPSPS) is a neuropathic pain syndrome, manifesting as continuous or paroxysmal pains or paresthesias secondary to a cerebrovascular accident (CVA). CPSPS mimics thalamic pain syndrome but distinguishes itself by its broader neural involvement. The condition encompasses damage beyond the thalamus, commonly involving sensory pathways such as the spinothalamic and trigeminothalamic tracts.[1][2] The disease presentation exhibits remarkable variability but commonly features paresthesias, allodynia, and hyperalgesia.[3][4] Onset is typically 3 to 6 months post-CVA, but early and delayed presentations have also been documented.[5][6]
Diagnosing CPSPS is challenging as it requires ruling out other poststroke conditions systematically, including pain attributed to spasticity, headaches, and musculoskeletal issues. Only 30% of patients get treatment in the acute and subacute phases poststroke. This undertreatment is associated with severe consequences like sleep disturbances, depression, and even suicide.[7][8] However, recent literature has compiled common clinical signs and treatment options to enhance outcomes.
Pain Pathways in CPSPS: A Brief Review
CPSPS arises from damage to diverse areas of the central nervous system (CNS) after a stroke. The specific anatomical involvement can vary, but several key structures and pathways are commonly implicated. The thalamus is a pivotal relay center for sensory information. Thalamic damage is often associated with CPSPS, given its role in transmitting sensory signals to the cortex. The spinothalamic tract conveys pain and temperature sensations to the thalamus. Injury to this tract often contributes to CPSPS development.
CPSPS may manifest as facial pain or trigeminal neuralgia when the stroke affects regions governing trigeminal nerve function. Damage to the somatosensory cortex's postcentral gyrus, responsible for processing tactile sensations, can result in abnormal pain perception. Disrupting the descending pain modulation pathways, which typically regulate pain transmission, may also contribute to CPSPS pathophysiology.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Poststroke pain arises from damage to key somatosensory structures like the spinothalamic tract, trigeminothalamic tract, and thalamus post-CVA. However, not all damage to these tracts leads to CPSPS. The stroke's nature plays a role, with ischemic strokes more likely to cause CPSPS than hemorrhagic strokes.[9] Reports substantiate this, with an 86.1% incidence among patients who had an ischemic stroke in contrast to 13.9% among individuals who experienced a hemorrhagic stroke.[10]
The stroke's anatomical location does not always predict CPSPS. Some reports suggest lesions near the thalamus and brainstem are more likely to trigger the condition than lower spinal cord damage. Other studies highlight a greater risk of damage to the spinothalamic tract than thalamic lesions, though the exact mechanisms increasing this risk remain elusive.[11] Identifying patients prone to CPSPS remains a significant challenge due to limited knowledge of the condition's precipitating factors.
Epidemiology
The prevalence of CPSPS post-CVA ranges from 1% to 12%, with some studies suggesting it could be up to 25% or more.[12][13][14] Accurately determining the condition's prevalence is challenging due to the delayed symptom onset and the subjectiveness of pain reporting poststroke. However, reported risk factors include older age, female sex, depression, alcohol use, peripheral vascular disease, and statin use.[15][16][17] Additionally, spasticity, reduced upper extremity movements, and sensory deficits poststroke, especially in ischemic strokes, are notable risk factors.
Pathophysiology
Multiple hypotheses have been proposed to elucidate the pathophysiology of CPSPS, which originated from its early association with thalamic syndrome in the early 20th century.[18] Contemporary understanding posits that poststroke pain may arise from lesions affecting the sensory pathways within the CNS. Pain and temperature signals from the limbs and periphery travel through the CNS via the spinothalamic tract, ascending through the spine and brainstem to synapse within the thalamus (see Image. Central Poststroke Pain Syndrome, Potential Sites of Involvement). The spinothalamic tract consists of 2 divisions: the lateral spinothalamic tract, which transmits noxious pain stimuli and temperature, and the anterior spinothalamic tract, which conveys crude touch.
The lateral spinothalamic tract plays a significant role in CPSPS. This tract begins with first-order neurons, which transmit sensory signals from the periphery to the spinal cord through dorsal roots. These neurons synapse within the spinal cord with second-order neurons in the posterior gray horn. The second-order neurons decussate within the cord, ascend through the brainstem to the cerebrum, and reach the ventral posterolateral (VPL) thalamic nucleus. Third-order neurons then convey signals to the primary sensory cortex (postcentral gyrus) via the posterior limb of the internal capsule and corona radiata. Lesions within (intrathalamic) or around (extrathalamic) this pathway may contribute to CPSPS.
Studies using positron emission tomography, functional magnetic resonance imaging (fMRI), and peripheral laser stimulation suggest that the thalamus modulates and inhibits ascending pain signals from the periphery. Increased activity in the insula and thalamus has been observed while perceiving noxious stimuli.[19] Deafferentation, or the destruction of one sensory tract, may lead to the overactivation of an opposing sensory pathway, intensifying sensations of pain, heat, or cold allodynia. Recent literature underscores the significance of lesions involving specific thalamic nuclei, particularly the VPL and, to some extent, the ventral posterior medial nucleus (VPM). VPL nuclear damage, especially in the posterolateral and inferior portions, is associated with symptoms such as allodynia or heightened pain sensitivity, as confirmed by radiographic imaging.
Recent human and animal models have identified molecular mechanisms underlying pain sensitization. One such mechanism involves a microglia-mediated inflammatory pathway, which upregulates excitatory glutamate and downregulates the inhibitory γ-aminobutyric acid (GABA) molecule, leading to disinhibition within the thalamus. Notably, the microglial P2X7 receptor has been implicated in inducing cytokine release following ischemic events.[20] This postischemic cytokine and interleukin release may increase glutamate levels, leading to neuronal hyperexcitability within the VPL.[21] Recent investigations suggest that P2X7 receptor antagonism reduces central neuropathic pain.
Postischemic microglial activation triggers the release of brain-derived neurotrophic factor (BDNF), which is implicated in potassium-chloride cotransporter disruption and subsequent interference with neuronal hyperpolarization via chloride ions. This blockade ultimately attenuates the neuronal response to GABA.[22] Poststroke inflammatory mediators like the NLRP3 inflammasome may also indirectly reduce GABA release from GABAergic neurons, leading to unopposed excitatory signals from specific thalamic regions, consequently affecting pain and sensory modulation.[23] New pharmacotherapeutic agents, such as epoxyeicosatrienoic acids, have effectively alleviated mechanical allodynia in rodent CPSPS models by antagonizing microglia-mediated inflammatory cytokines. However, such pharmacotherapy awaits testing in human trials.
Extrathalamic lesions contributing to CPSPS have been proposed, as seen in patients with Wallenberg syndrome (lateral medullary syndrome), despite the absence of direct thalamic involvement. This form of central pain resembles trigeminal neuralgia symptoms, with facial allodynia and intermittent pain sensitivity resulting from a lesion of the ventral trigeminothalamic tract within the spinothalamic tract. Biochemical changes within the cerebrum may also occur following a peripheral nerve injury. One suggested pathway involves reduced serotonin release in the contralateral ventrobasal thalamic complex, potentially decreasing inhibitory inputs to the spinal cord and facilitating pain perception.[24][25][26] Further research is needed to comprehend the complex pathophysiology of CPSPS fully. Hypotheses regarding intrathalamic and extrathalamic disinhibition offer a promising framework for elucidating the mechanisms behind the diverse symptomatology associated with this condition.
History and Physical
CPSPS presents variably, with symptoms including throbbing, stabbing, shooting, and burning pain with or without a trigger. Onset typically occurs 1 to 6 months poststroke, sometimes extending beyond a year, making timely recognition challenging. Symptoms may be categorized into broad groups: heat allodynia, cold allodynia, spontaneous dysaesthesia, evoked dysaesthesia, hyperalgesia, and paresthesias.
Accurate CPSPS identification requires a thorough clinical history and physical examination, as current practice is based on excluding other poststroke conditions. Common conditions like spasticity, headaches, shoulder pain, and musculoskeletal issues may be confounding factors. Musculoskeletal complaints, including shoulder pain found in up to 40% of patients with poststroke, must be carefully differentiated from CPSPS.[27] Understanding the temporal progression of CPSPS is crucial. Musculoskeletal and shoulder pain typically appear within 2 weeks to 3 months post-CVA.[28] Spasticity and related pain may arise within 2 to 12 months.[29][30] Clinicians must differentiate these timelines through focused musculoskeletal and neurological exams to formulate an accurate diagnosis.
A focused history highlighting sensory aspects in CPSPS against motor or range-of-motion deficits in shoulder- and joint-related pain or spasticity helps clarify the presenting problem. The Ashworth Scale may diagnose and characterize spasticity severity.[31] A comprehensive physical exam, particularly of cranial nerves and sensory function, helps localize lesions, differentiating CPSPS from other poststroke maladies. Evaluating the spinothalamic tract involves various modalities, including fine-needle pinprick tests, cold or hot stimuli application for pain sensation, and blunt objects for crude touch assessment. These methods facilitate lesion localization and identify responses to pain sensation, hyperalgesia, hypoalgesia, and evoked dysesthesias. Pain and paresthesias typically occur contralaterally to the CVA site in cases involving the thalamus, spinothalamic, or trigeminothalamic tract.
Evaluation
Objective findings should also be integrated within the clinical gestalt. No laboratory result or molecular inflammatory mediators have been identified for diagnosing CPSPS. Imaging techniques like MRI, fMRI, and computed tomography may be crucial in diagnosing CPSPS and localizing the inciting lesion. Combining these imaging modalities with a thorough history and physical exam enhances spatial lesion identification, enabling clinicians to arrive at an accurate diagnosis more quickly.
Treatment / Management
CPSPS management is controversial, prompting an exploration of diverse therapeutic approaches. Treatment strategies may be broadly categorized into nonpharmacologic and pharmacologic interventions. Nonpharmacologic approaches include physical therapy, stretching, acupuncture, and transcranial magnetic and deep brain stimulation. Pharmacologic treatment includes agents like anticonvulsants, antidepressants, corticosteroids, and opioids.[32][33](B3)
Pharmacotherapy
CPSPS pharmacotherapy remains contentious, with literature supporting different treatment models. A common first-line medication is amitriptyline. This tricyclic antidepressant inhibits serotonin and norepinephrine reuptake.[34] Other initial pharmacotherapeutic options include pregabalin, gabapentin, and lamotrigine. Gabapentinoids, including pregabalin and gabapentin, indirectly reduce neurotransmitter release by binding to α2δ subunits within calcium ion channels, effectively managing CPSPS symptoms.[35]
Lamotrigine, an anticonvulsant that blocks voltage-gated sodium channels, has also been studied for its pain-modulating effects on CPSPS.[36][37] Some literature supports combining amitriptyline and a gabapentinoid to enhance pain control if monotherapy fails to provide adequate pain relief.[38] The Canadian Best Stroke Practice and the Royal College of Physicians recommend amitriptyline, gabapentin, pregabalin, and lamotrigine as effective first- or second-line agents.[39][40](A1)
Common alternatives for failed first-line agents include serotonin and norepinephrine reuptake inhibitors, anticonvulsants like carbamazepine, and opioids such as tramadol.[41] However, opioids are generally avoided due to their abuse potential. Less common options include phenytoin, pamidronate, steroids, lidocaine, and ketamine. Phenytoin has shown some success, albeit with limited research.[42] A network meta-analysis of 13 randomized controlled trials demonstrated significant pain reduction with pamidronate and prednisone compared to placebo and conventional agents like pregabalin and carbamazepine. Ketamine's efficacy in CPSPS remains uncertain due to limited research.[43][44][45] Lidocaine has shown only modest pain control.[46] Although not specifically studied for CPSPS, cannabis-based derivatives, and topical agents have demonstrated some efficacy in modulating neuropathic pain.[47] The use of these agents in CPSP warrants further investigation to better understand their potential benefits.(A1)
Nonpharmacological Interventions
Repetitive transcranial magnetic stimulation (rTMS) has emerged as a promising nonpharmacological intervention for alleviating neuropathic pain and CPSPS symptoms. This technique involves applying transcranial electromagnets on the scalp to generate magnetic impulses, which potentiate or inhibit nerve signals. High-frequency rTMS (over 1 hertz) appears more effective than low-frequency stimulation.[48][49] The mechanism may involve modulating inhibitory signals between cerebral hemispheres and descending pain circuits.[50][51] Although recent literature supports this modality's effectiveness in pain control, long-term outcomes beyond 3 months remain underexplored.[52](A1)
Placement of electromagnets over the M1 region or territory of the brain has demonstrated pain relief in various trials.[53][54][55] Motor-evoked potentials in intrinsic hand muscles can guide accurate coil placement during treatment.[56] Deep brain stimulation, particularly targeting the brain's M1 section, has shown promise in treating refractory CPSPS, resulting in significant pain reduction.[57][58] Neuroplastic changes observed via fMRI indicate pain modulation through tracts beyond the spinothalamic tract, particularly the primary motor cortex and corticothalamic and thalamocortical loops.[59][60] However, due to its invasiveness, deep brain stimulation is typically reserved for more treatment-resistant CPSPS cases.(A1)
Acupuncture shows promise in reducing poststroke pain, though empirical support is limited. A systematic review indicated consistent pain reduction compared to pharmacotherapy, albeit with caution due to potential biases in some studies.[61][62] Further research is warranted to establish the efficacy of acupuncture and derivatives like electroacupuncture.[63] Physical therapy, including stretching and exercise, is potentially beneficial but lacks sufficient empirical validation for its role in relieving CPSPS. However, physical therapy may indirectly improve functional status and quality of life in affected individuals by impacting pain related to stroke progression and spasticity.(A1)
Differential Diagnosis
Neuropathic pain's prevalence is 3% to 17%. Thus, distinguishing it from similar disease states is vital.[64] A thorough evaluation requires collaboration among neurologists, pain specialists, general practitioners, and other relevant healthcare professionals. Conditions unrelated to stroke but involving central neuropathic pain include multiple sclerosis, spinal cord trauma, syringomyelia, Parkinson disease-related pain, and psychogenic pain disorders like conversion or somatic symptom disorder. A comprehensive assessment involving clinical history, neurological examination, and diagnostic imaging is crucial in differentiating CPSPS from these conditions.
CPSPS may not present until many months after the initial CVA event. As mentioned earlier, post-CVA pathology must be effectively ruled out before making a diagnosis of CPSPS. Besides other neuropathic pain sources, headache syndromes and musculoskeletal disorders must be distinguished from CPSPS.
Prognosis
Data on the chronicity or duration timeline of CPSPs is limited. Some may experience improvement or remission due to neuroplastic changes or treatment. Others face persistent challenges influenced by the lesion's severity or problematic location. A more thorough understanding of CPSP's timeline and recovery becomes feasible as awareness grows among clinicians and diagnostic precision improves.
Complications
PSPS is associated with a significant psychological burden. Patients with this condition are at increased risk for worsening anxiety and depression. Chronic pain may limit mobility and function, impacting daily activities and causing a decline in overall physical health. In severe cases, the chronicity of CPSPS and its sequelae can contribute to the development of behavioral issues, major depressive disorder, and suicidal ideation. CPSPS management's complexity also creates treatment adherence challenges, potentially leading to suboptimal pain control and a poorer prognosis. Early identification of these psychological and behavioral manifestations by healthcare providers is crucial to optimizing treatment adherence, therapeutic outcomes, and patient well-being.
Deterrence and Patient Education
CPSPS typically assumes a chronic course, with pain lasting months or even years after the stroke. Patients often exhibit heightened sensitivity to stimuli like touch or temperature changes, experiencing normal sensations as painful. This chronic pain significantly impacts daily life, decreasing mobility, sleep, and quality of life. Managing CPSPS involves an interprofessional approach using medications, physical therapy, and psychological support strategies. Treatment plans may require ongoing adjustments to find the most effective combination. Rehabilitation programs, such as physical and occupational therapy, can improve function, mobility, and pain severity. Management should be tailored to each individual's experience of CPSPS due to its varied presentation.
Enhancing Healthcare Team Outcomes
Addressing CPSPS's multifaceted nature necessitates a collaborative approach among healthcare professionals. Pharmacists are essential in managing CPSPS, ensuring optimal medication regimens, and addressing potential side effects in coordination with neurologists. Radiologists are vital in the diagnostic process, using advanced imaging to localize lesions for appropriate treatment planning precisely. Neurologists are the primary CPSPS clinicians who conduct evaluations, create treatment plans, and prescribe suitable medications. These professionals' understanding of CPSPS's neural pathways is vital for targeted interventions. Collaboration with radiologists integrates clinical and imaging data, improving diagnostic precision.
Primary care providers act as care coordinators for patients with CPSPS, conducting initial assessments, making specialist referrals, and managing ongoing care. These professionals' holistic approach should include general health monitoring and addressing patient concerns, ensuring a cohesive healthcare journey. Synergy among pharmacists, radiologists, neurologists, and primary care providers creates a comprehensive, patient-centered approach to CPSPS. This collaboration aims to alleviate symptoms and improve overall quality of life. Refining treatment strategies and enhancing collective expertise will advance care and outcomes for individuals with CPSPS as research progresses.
Media
(Click Image to Enlarge)
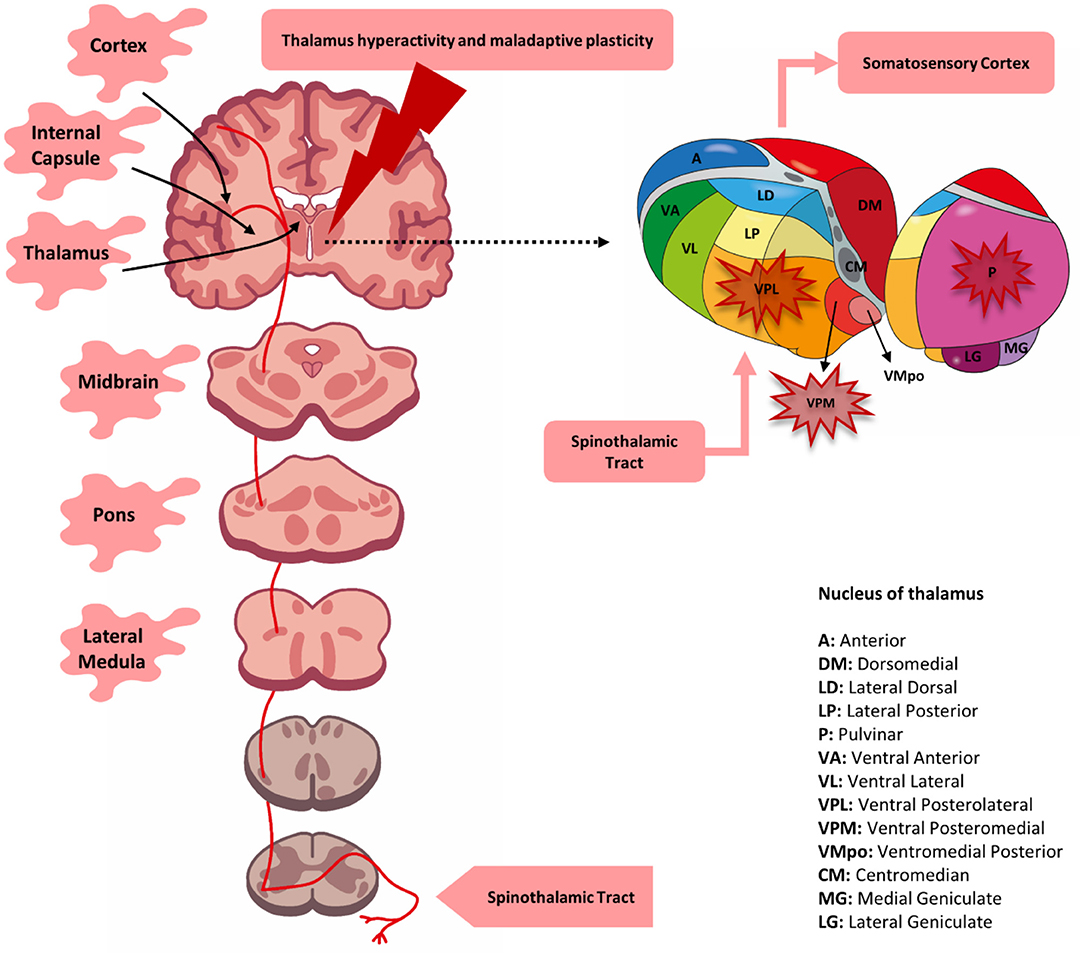
Central Poststroke Pain Syndrome, Potential Sites of Involvement. Lesions at various levels of the spinothalamic tract, including the thalamus, can contribute to central poststroke pain syndrome. Thalamic lesions were initially thought to be solely responsible, but later research identified the involvement of the lateral medulla, pons, lenticulocapsular area, and cortex. The condition may result from the loss of somatosensory integration and changes in cortical plasticity.
Betancur DFA, da Graça Lopes Tarragó M, da Silva Torres IL, Fregni F, Caumo W. Central post-stroke pain: an integrative review of somatotopic damage, clinical symptoms, and neurophysiological measures. Front Neurol. 2021;12:678198. doi: 10.3389/fneur.2021.678198.
References
Jang SH, Lee J, Yeo SS. Central post-stroke pain due to injury of the spinothalamic tract in patients with cerebral infarction: a diffusion tensor tractography imaging study. Neural regeneration research. 2017 Dec:12(12):2021-2024. doi: 10.4103/1673-5374.221159. Epub [PubMed PMID: 29323041]
Vartiainen N, Perchet C, Magnin M, Creac'h C, Convers P, Nighoghossian N, Mauguière F, Peyron R, Garcia-Larrea L. Thalamic pain: anatomical and physiological indices of prediction. Brain : a journal of neurology. 2016 Mar:139(Pt 3):708-22. doi: 10.1093/brain/awv389. Epub 2016 Feb 8 [PubMed PMID: 26912644]
Vestergaard K, Nielsen J, Andersen G, Ingeman-Nielsen M, Arendt-Nielsen L, Jensen TS. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. Pain. 1995 May:61(2):177-186. doi: 10.1016/0304-3959(94)00140-A. Epub [PubMed PMID: 7659427]
Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008 Aug 31:138(2):343-353. doi: 10.1016/j.pain.2008.01.006. Epub 2008 Mar 4 [PubMed PMID: 18289791]
Paolucci S, Iosa M, Toni D, Barbanti P, Bovi P, Cavallini A, Candeloro E, Mancini A, Mancuso M, Monaco S, Pieroni A, Recchia S, Sessa M, Strambo D, Tinazzi M, Cruccu G, Truini A, Neuropathic pain special interest group of the Italian Neurological Society. Prevalence and Time Course of Post-Stroke Pain: A Multicenter Prospective Hospital-Based Study. Pain medicine (Malden, Mass.). 2016 May:17(5):924-30. doi: 10.1093/pm/pnv019. Epub 2015 Dec 14 [PubMed PMID: 26814255]
Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. The Lancet. Neurology. 2009 Sep:8(9):857-68. doi: 10.1016/S1474-4422(09)70176-0. Epub [PubMed PMID: 19679277]
Misra UK, Kalita J, Kumar B. A study of clinical, magnetic resonance imaging, and somatosensory-evoked potential in central post-stroke pain. The journal of pain. 2008 Dec:9(12):1116-22. doi: 10.1016/j.jpain.2008.06.013. Epub 2008 Oct 10 [PubMed PMID: 18848810]
MacGowan DJ, Janal MN, Clark WC, Wharton RN, Lazar RM, Sacco RL, Mohr JP. Central poststroke pain and Wallenberg's lateral medullary infarction: frequency, character, and determinants in 63 patients. Neurology. 1997 Jul:49(1):120-5 [PubMed PMID: 9222179]
Harrison RA, Field TS. Post stroke pain: identification, assessment, and therapy. Cerebrovascular diseases (Basel, Switzerland). 2015:39(3-4):190-201. doi: 10.1159/000375397. Epub 2015 Mar 5 [PubMed PMID: 25766121]
Barbosa LM, da Silva VA, de Lima Rodrigues AL, Mendes Fernandes DTR, de Oliveira RAA, Galhardoni R, Yeng LT, Junior JR, Conforto AB, Lucato LT, Lemos MD, Peyron R, Garcia-Larrea L, Teixeira MJ, Ciampi de Andrade D. Dissecting central post-stroke pain: a controlled symptom-psychophysical characterization. Brain communications. 2022:4(3):fcac090. doi: 10.1093/braincomms/fcac090. Epub 2022 Apr 5 [PubMed PMID: 35528229]
Hong JH, Choi BY, Chang CH, Kim SH, Jung YJ, Lee DG, Kwon YH, Jang SH. The prevalence of central poststroke pain according to the integrity of the spino-thalamo-cortical pathway. European neurology. 2012:67(1):12-7. doi: 10.1159/000333012. Epub 2011 Dec 3 [PubMed PMID: 22142796]
Kuptniratsaikul V, Kovindha A, Suethanapornkul S, Manimmanakorn N, Archongka Y. Complications during the rehabilitation period in Thai patients with stroke: a multicenter prospective study. American journal of physical medicine & rehabilitation. 2009 Feb:88(2):92-9. doi: 10.1097/PHM.0b013e3181909d5f. Epub [PubMed PMID: 19077674]
Kong KH, Woon VC, Yang SY. Prevalence of chronic pain and its impact on health-related quality of life in stroke survivors. Archives of physical medicine and rehabilitation. 2004 Jan:85(1):35-40 [PubMed PMID: 14970965]
Level 2 (mid-level) evidenceBowsher D. Stroke and central poststroke pain in an elderly population. The journal of pain. 2001 Oct:2(5):258-61 [PubMed PMID: 14622804]
Sommerfeld DK, Welmer AK. Pain following stroke, initially and at 3 and 18 months after stroke, and its association with other disabilities. European journal of neurology. 2012 Oct:19(10):1325-30. doi: 10.1111/j.1468-1331.2012.03747.x. Epub 2012 May 8 [PubMed PMID: 22568638]
Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008 Feb:39(2):414-20 [PubMed PMID: 18096834]
Appelros P. Prevalence and predictors of pain and fatigue after stroke: a population-based study. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation. 2006 Dec:29(4):329-33 [PubMed PMID: 17106351]
Treister AK, Hatch MN, Cramer SC, Chang EY. Demystifying Poststroke Pain: From Etiology to Treatment. PM & R : the journal of injury, function, and rehabilitation. 2017 Jan:9(1):63-75. doi: 10.1016/j.pmrj.2016.05.015. Epub 2016 Jun 16 [PubMed PMID: 27317916]
Youell PD, Wise RG, Bentley DE, Dickinson MR, King TA, Tracey I, Jones AK. Lateralisation of nociceptive processing in the human brain: a functional magnetic resonance imaging study. NeuroImage. 2004 Nov:23(3):1068-77 [PubMed PMID: 15528107]
Wan L, Li Z, Liu T, Chen X, Xu Q, Yao W, Zhang C, Zhang Y. Epoxyeicosatrienoic acids: Emerging therapeutic agents for central post-stroke pain. Pharmacological research. 2020 Sep:159():104923. doi: 10.1016/j.phrs.2020.104923. Epub 2020 May 24 [PubMed PMID: 32461186]
Mohanan AT, Nithya S, Nomier Y, Hassan DA, Jali AM, Qadri M, Machanchery S. Stroke-Induced Central Pain: Overview of the Mechanisms, Management, and Emerging Targets of Central Post-Stroke Pain. Pharmaceuticals (Basel, Switzerland). 2023 Aug 4:16(8):. doi: 10.3390/ph16081103. Epub 2023 Aug 4 [PubMed PMID: 37631018]
Level 3 (low-level) evidenceKuan YH, Shih HC, Tang SC, Jeng JS, Shyu BC. Targeting P(2)X(7) receptor for the treatment of central post-stroke pain in a rodent model. Neurobiology of disease. 2015 Jun:78():134-45. doi: 10.1016/j.nbd.2015.02.028. Epub 2015 Mar 30 [PubMed PMID: 25836422]
Ri S. The Management of Poststroke Thalamic Pain: Update in Clinical Practice. Diagnostics (Basel, Switzerland). 2022 Jun 10:12(6):. doi: 10.3390/diagnostics12061439. Epub 2022 Jun 10 [PubMed PMID: 35741249]
Brüggemann J, Galhardo V, Apkarian AV. Immediate reorganization of the rat somatosensory thalamus after partial ligation of sciatic nerve. The journal of pain. 2001 Aug:2(4):220-8 [PubMed PMID: 14622820]
Salt TE, Eaton SA. Function of non-NMDA receptors and NMDA receptors in synaptic responses to natural somatosensory stimulation in the ventrobasal thalamus. Experimental brain research. 1989:77(3):646-52 [PubMed PMID: 2572448]
Goettl VM, Huang Y, Hackshaw KV, Stephens RL Jr. Reduced basal release of serotonin from the ventrobasal thalamus of the rat in a model of neuropathic pain. Pain. 2002 Sep:99(1-2):359-66 [PubMed PMID: 12237215]
Jönsson AC, Lindgren I, Hallström B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients' perspectives. Journal of neurology, neurosurgery, and psychiatry. 2006 May:77(5):590-5 [PubMed PMID: 16354737]
Zhang Q, Chen D, Shen Y, Bian M, Wang P, Li J. Incidence and Prevalence of Poststroke Shoulder Pain Among Different Regions of the World: A Systematic Review and Meta-Analysis. Frontiers in neurology. 2021:12():724281. doi: 10.3389/fneur.2021.724281. Epub 2021 Nov 4 [PubMed PMID: 34803873]
Level 1 (high-level) evidenceBavikatte G, Subramanian G, Ashford S, Allison R, Hicklin D. Early Identification, Intervention and Management of Post-stroke Spasticity: Expert Consensus Recommendations. Journal of central nervous system disease. 2021:13():11795735211036576. doi: 10.1177/11795735211036576. Epub 2021 Sep 20 [PubMed PMID: 34566442]
Level 3 (low-level) evidenceWatkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clinical rehabilitation. 2002 Aug:16(5):515-22 [PubMed PMID: 12194622]
Harb A, Kishner S. Modified Ashworth Scale. StatPearls. 2024 Jan:(): [PubMed PMID: 32119459]
Widar M, Ek AC, Ahlström G. Coping with long-term pain after a stroke. Journal of pain and symptom management. 2004 Mar:27(3):215-25 [PubMed PMID: 15010100]
Radiansyah RS, Hadi DW. Repetitive transcranial magnetic stimulation in central post-stroke pain: current status and future perspective. The Korean journal of pain. 2023 Oct 1:36(4):408-424. doi: 10.3344/kjp.23220. Epub [PubMed PMID: 37752663]
Level 3 (low-level) evidencePlecash AR, Chebini A, Ip A, Lai JJ, Mattar AA, Randhawa J, Field TS. Updates in the Treatment of Post-Stroke Pain. Current neurology and neuroscience reports. 2019 Nov 13:19(11):86. doi: 10.1007/s11910-019-1003-2. Epub 2019 Nov 13 [PubMed PMID: 31720885]
Frese A, Husstedt IW, Ringelstein EB, Evers S. Pharmacologic treatment of central post-stroke pain. The Clinical journal of pain. 2006 Mar-Apr:22(3):252-60 [PubMed PMID: 16514325]
Vestergaard K, Andersen G, Gottrup H, Kristensen BT, Jensen TS. Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology. 2001 Jan 23:56(2):184-90 [PubMed PMID: 11160953]
Level 1 (high-level) evidenceKalita J, Chandra S, Misra UK. Pregabalin and lamotrigine in central poststroke pain: A pilot study. Neurology India. 2017 May-Jun:65(3):506-511. doi: 10.4103/neuroindia.NI_45_16. Epub [PubMed PMID: 28488610]
Level 3 (low-level) evidenceHolbech JV, Jung A, Jonsson T, Wanning M, Bredahl C, Bach FW. Combination treatment of neuropathic pain: Danish expert recommendations based on a Delphi process. Journal of pain research. 2017:10():1467-1475. doi: 10.2147/JPR.S138099. Epub 2017 Jun 26 [PubMed PMID: 28721089]
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016 Jun:47(6):e98-e169. doi: 10.1161/STR.0000000000000098. Epub 2016 May 4 [PubMed PMID: 27145936]
Level 2 (mid-level) evidenceTeasell R, Salbach NM, Foley N, Mountain A, Cameron JI, Jong A, Acerra NE, Bastasi D, Carter SL, Fung J, Halabi ML, Iruthayarajah J, Harris J, Kim E, Noland A, Pooyania S, Rochette A, Stack BD, Symcox E, Timpson D, Varghese S, Verrilli S, Gubitz G, Casaubon LK, Dowlatshahi D, Lindsay MP. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. International journal of stroke : official journal of the International Stroke Society. 2020 Oct:15(7):763-788. doi: 10.1177/1747493019897843. Epub 2020 Jan 27 [PubMed PMID: 31983296]
Vranken JH, Hollmann MW, van der Vegt MH, Kruis MR, Heesen M, Vos K, Pijl AJ, Dijkgraaf MGW. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain. 2011 Feb:152(2):267-273. doi: 10.1016/j.pain.2010.09.005. Epub [PubMed PMID: 21078545]
Level 1 (high-level) evidenceAgnew DC, Goldberg VD. A brief trial of phenytoin therapy for thalamic pain. Bulletin of the Los Angeles neurological societies. 1976 Jan:41(1):9-12 [PubMed PMID: 1016820]
Angstadt R, Esperti S, Mangano A, Meyer S. Palliative ketamine: the use of ketamine in central post-stroke pain syndrome-a case report. Annals of palliative medicine. 2021 Jun:10(6):6974-6978. doi: 10.21037/apm-20-972. Epub 2020 Nov 10 [PubMed PMID: 33183017]
Level 3 (low-level) evidenceVick PG, Lamer TJ. Treatment of central post-stroke pain with oral ketamine. Pain. 2001 May:92(1-2):311-3 [PubMed PMID: 11323153]
Vranken JH, Dijkgraaf MG, Kruis MR, van Dasselaar NT, van der Vegt MH. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: a placebo-controlled trial. Pain. 2005 Nov:118(1-2):224-31 [PubMed PMID: 16202531]
Bo Z, Jian Y, Yan L, Gangfeng G, Xiaojing L, Xiaolan L, Zhao C, Ke H, Yang F, Maoxia L, Jian W. Pharmacotherapies for Central Post-Stroke Pain: A Systematic Review and Network Meta-Analysis. Oxidative medicine and cellular longevity. 2022:2022():3511385. doi: 10.1155/2022/3511385. Epub 2022 Aug 18 [PubMed PMID: 36035203]
Level 1 (high-level) evidenceMücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. The Cochrane database of systematic reviews. 2018 Mar 7:3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2. Epub 2018 Mar 7 [PubMed PMID: 29513392]
Level 1 (high-level) evidencePan LJ, Zhu HQ, Zhang XA, Wang XQ. The mechanism and effect of repetitive transcranial magnetic stimulation for post-stroke pain. Frontiers in molecular neuroscience. 2022:15():1091402. doi: 10.3389/fnmol.2022.1091402. Epub 2023 Jan 6 [PubMed PMID: 36683849]
Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S, Smith AR, Madan A, Patterson D, George MS. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. The Clinical journal of pain. 2011 Jul-Aug:27(6):486-94. doi: 10.1097/AJP.0b013e31820d2733. Epub [PubMed PMID: 21415720]
Level 1 (high-level) evidenceGuilbaud G, Benoist JM, Levante A, Gautron M, Willer JC. Primary somatosensory cortex in rats with pain-related behaviours due to a peripheral mononeuropathy after moderate ligation of one sciatic nerve: neuronal responsivity to somatic stimulation. Experimental brain research. 1992:92(2):227-45 [PubMed PMID: 1337325]
Morishita T, Inoue T. Brain Stimulation Therapy for Central Post-Stroke Pain from a Perspective of Interhemispheric Neural Network Remodeling. Frontiers in human neuroscience. 2016:10():166. doi: 10.3389/fnhum.2016.00166. Epub 2016 Apr 21 [PubMed PMID: 27148019]
Level 3 (low-level) evidenceYang S, Chang MC. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Frontiers in neurology. 2020:11():114. doi: 10.3389/fneur.2020.00114. Epub 2020 Feb 18 [PubMed PMID: 32132973]
Level 1 (high-level) evidenceZhao CG, Sun W, Ju F, Jiang S, Wang H, Sun XL, Mou X, Yuan H. Analgesic Effects of Navigated Repetitive Transcranial Magnetic Stimulation in Patients With Acute Central Poststroke Pain. Pain and therapy. 2021 Dec:10(2):1085-1100. doi: 10.1007/s40122-021-00261-0. Epub 2021 Apr 17 [PubMed PMID: 33866522]
Ojala J, Vanhanen J, Harno H, Lioumis P, Vaalto S, Kaunisto MA, Putaala J, Kangasniemi M, Kirveskari E, Mäkelä JP, Kalso E. A Randomized, Sham-Controlled Trial of Repetitive Transcranial Magnetic Stimulation Targeting M1 and S2 in Central Poststroke Pain: A Pilot Trial. Neuromodulation : journal of the International Neuromodulation Society. 2022 Jun:25(4):538-548. doi: 10.1111/ner.13496. Epub 2022 Feb 2 [PubMed PMID: 35670063]
Level 1 (high-level) evidenceKobayashi M, Fujimaki T, Mihara B, Ohira T. Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation : journal of the International Neuromodulation Society. 2015 Jun:18(4):249-54. doi: 10.1111/ner.12301. Epub 2015 Apr 23 [PubMed PMID: 25906811]
Betancur DFA, Tarragó MDGL, Torres ILDS, Fregni F, Caumo W. Central Post-Stroke Pain: An Integrative Review of Somatotopic Damage, Clinical Symptoms, and Neurophysiological Measures. Frontiers in neurology. 2021:12():678198. doi: 10.3389/fneur.2021.678198. Epub 2021 Aug 18 [PubMed PMID: 34484097]
Holland MT, Zanaty M, Li L, Thomsen T, Beeghly JH, Greenlee JDW, Reddy CG. Successful deep brain stimulation for central post-stroke pain and dystonia in a single operation. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2018 Apr:50():190-193. doi: 10.1016/j.jocn.2018.01.036. Epub 2018 Feb 1 [PubMed PMID: 29396066]
Akyuz G, Kuru P. Systematic Review of Central Post Stroke Pain: What Is Happening in the Central Nervous System? American journal of physical medicine & rehabilitation. 2016 Aug:95(8):618-27. doi: 10.1097/PHM.0000000000000542. Epub [PubMed PMID: 27175563]
Level 1 (high-level) evidenceXerri C, Zennou-Azogui Y, Sadlaoud K, Sauvajon D. Interplay between intra- and interhemispheric remodeling of neural networks as a substrate of functional recovery after stroke: adaptive versus maladaptive reorganization. Neuroscience. 2014 Dec 26:283():178-201. doi: 10.1016/j.neuroscience.2014.06.066. Epub 2014 Jul 8 [PubMed PMID: 25014877]
Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. Journal of neurosurgery. 1998 Oct:89(4):585-91 [PubMed PMID: 9761052]
Li W, Chen S. Acupuncture for thalamic pain after stroke: A systematic review and meta-analysis. Medicine. 2023 Mar 3:102(9):e33006. doi: 10.1097/MD.0000000000033006. Epub [PubMed PMID: 36862907]
Level 1 (high-level) evidenceCho SY, Park JY, Jung WS, Moon SK, Park JM, Ko CN, Park SU. Bee venom acupuncture point injection for central post stroke pain: a preliminary single-blind randomized controlled trial. Complementary therapies in medicine. 2013 Jun:21(3):155-7. doi: 10.1016/j.ctim.2013.02.001. Epub 2013 Mar 13 [PubMed PMID: 23642945]
Level 1 (high-level) evidenceYun SP, Sun BC. Apipuncture treatment for central post-stroke pain. Journal of alternative and complementary medicine (New York, N.Y.). 2010 Feb:16(2):223-4 [PubMed PMID: 20180697]
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. International journal of immunopathology and pharmacology. 2019 Jan-Dec:33():2058738419838383. doi: 10.1177/2058738419838383. Epub [PubMed PMID: 30900486]
Level 3 (low-level) evidence