Introduction
Medical imaging has significantly improved over the past 30 years, enhancing its clinical utility and enabling clinicians to refine therapeutic strategies. In wound care, imaging is pivotal in facilitating early and accurate diagnosis, which is crucial for optimizing patient outcomes. While imaging is often employed as an adjunct to traditional clinical evaluation, it significantly supports wound healing assessment and monitoring, mainly when clinical examination alone may fall short.
The most common initial assessment of acute or chronic wounds involves visual inspection and clinical examination.[1][2][3] However, these methods are inherently subjective, especially when evaluating key metrics such as wound size and changes in the wound bed over time. Traditional grading systems like the Bates-Jensen wound assessment tool, pressure ulcer grade recording charts, Wagner grading scale, the wound, ischemia, and foot infection, and the site, ischemia, bacterial infection, area, and depth assessments provide useful frameworks. Still, they do not offer insights into whether healing has stalled, the depth of the wound, or the presence of infection.[1] Clinical assessment relies on the clinician's experience, and even a well-trained eye may not accurately assess wound depth or the presence of underlying infection.[3][4] This limitation underscores the importance of advanced imaging tools in wound care.
Photography, alongside more traditional imaging modalities like plain radiographs, computed tomography scans, magnetic resonance imaging, and ultrasound, offers an invaluable complement to clinical assessments. These imaging techniques are particularly useful in detecting deeper or life-threatening infections, evaluating underlying bone trauma or abnormalities, and assessing distal perfusion. Digital imaging, often through digital cameras or smartphone-based systems, has emerged as the most cost-effective and minimally invasive method for documenting wound progress. These images can be uploaded to electronic medical records and integrated with artificial intelligence to measure the wound area and perimeter (see Image. Stage 2 Pressure Ulcer).[1] They can also be customized to provide data regarding the tissue type and used to build wound databases that support education and clinical decision-making.
Despite its advantages, most imaging techniques struggle to measure wound depth accurately, limiting their ability to assess wound volume. Specialized equipment like "time-of-flight" cameras can address this challenge by measuring the phase shift of light reflected from the wound bed to calculate depth and volume. However, these devices are expensive and logistically challenging to use. Nonetheless, digital photography remains the predominant imaging tool in wound care, providing a balance of accessibility, ease of use, and valuable clinical insights. This article explores various imaging techniques and their relevance to treating acute or chronic wounds.
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The anatomy associated with wounds encompasses the structures of the skin and underlying tissues that are affected when a wound occurs. Understanding the anatomy of these tissues is crucial in assessing the severity of wounds, their healing process, and appropriate treatment strategies. Depending on the depth and nature of the injury, wounds can involve various layers of the skin and may extend into deeper structures, including muscles, bones, and organs.
Skin Anatomy
The skin is the body's largest organ and acts as a barrier to protect underlying tissues from pathogens, physical damage, and dehydration. The skin comprises three primary layers: the epidermis, dermis, and subcutaneous tissue.
- Epidermis
- This outermost layer comprises stratified squamous epithelium and functions as a protective barrier. This avascular layer depends on diffusion from the dermis for nutrient supply. The epidermis contains keratinocytes, melanocytes, and Langerhans cells. In wound healing, keratinocytes are vital in reepithelialization, migrating to cover the wound bed.
- Dermis
- The dermis is a thicker, more supportive layer beneath the epidermis. Composed primarily of collagen, elastin, and fibroblasts, it provides structural support to the skin and houses blood vessels, nerves, hair follicles, and sweat glands. The dermis plays a crucial role in wound healing, particularly through the activity of fibroblasts, which produce collagen and extracellular matrix essential for tissue repair.
- Subcutaneous tissue (hypodermis)
- This deepest layer of skin consists of loose connective tissue, fat cells, blood vessels, and nerves. This layer acts as an insulator and shock absorber, anchoring the skin to underlying muscles and bones. Damage to the subcutaneous tissue in deeper wounds may impair perfusion and lead to complications like delayed healing or necrosis.
Cutaneous Wounds
Cutaneous wounds occur when the skin's protective barrier is broken, exposing underlying tissues such as fat, muscle, or bone. This exposure places these tissues at increased risk of infection, dehydration, and tissue death. Muscles, tendons, or bones may be exposed in more severe wounds, resulting in potential functional impairment or infection. In the case of diabetic ulcers, poor circulation can hinder the wound's healing and increase the risk of infection.
Musculoskeletal System
Wounds extending beyond the dermis and subcutaneous layers may involve muscles, tendons, and bones. The anatomy of these structures influences both the severity of the wound and its healing process.
- Muscles and tendons
- Muscle tissue is composed of long fibers capable of contraction. Wounds involving muscles can impair mobility and lead to functional deficits. Tendons, which connect muscles to bones, are less vascular than muscle tissue; thus, tendon injuries may heal more slowly. Wounds involving muscle or tendons may require surgical intervention for repair.
- Bone
- Bone is a dense, mineralized tissue that provides structural support. When a wound penetrates the skin to expose bone, the injury is more severe and may require interventions such as debridement or fixation. Bone infections (osteomyelitis) can also complicate wound healing, particularly in cases of open fractures. Imaging techniques like x-rays, computed tomography, and magnetic resonance scans are essential for identifying bone involvement and guiding treatment.
Vascular System
The vascular system, comprising blood vessels (arteries, veins, and capillaries), is critical to wound healing. Blood vessels provide oxygen and nutrients necessary for tissue repair and waste removal. The arteries supply oxygenated blood, while veins return deoxygenated blood to the heart. Capillaries, the smallest blood vessels, facilitate the exchange of gases, nutrients, and waste products at the cellular level.
Adequate blood flow to the wound site is vital for healing. Impaired perfusion due to vascular injury, peripheral vascular disease, or other conditions can lead to delayed healing and an increased risk of infection. The distal perfusion to extremities, in particular, is crucial for maintaining tissue viability and preventing complications like necrosis.
Nervous System
The nervous system, including sensory and motor nerves, plays a role in wound healing and recovery. Sensory nerves transmit pain, temperature, and touch sensations, which can be crucial for assessing the severity of a wound. Motor nerves control the movement of muscles, and damage to these nerves may result in functional deficits. When injured in a wound, nerves can regenerate, but this process is often slow and incomplete, especially with deeper wounds. Neuropathic wounds, resulting from conditions like diabetes, can lead to altered sensations, making it harder for patients to detect injuries and prompting delays in treatment.
Lymphatic System
The lymphatic system plays a role in the immune response and fluid balance. Lymphatic vessels drain excess interstitial fluid, which can accumulate in the wound bed, leading to edema and impaired healing. Additionally, the lymphatic system helps transport immune cells to the injury site to combat infection.
Infections and Pathogen Entry
The skin's surface is covered with a protective barrier that helps prevent pathogen entry. When a wound breaches this barrier, pathogens can enter, leading to infection. The type and depth of the wound, along with the patient's immune status, will determine the likelihood of infection. Common wound pathogens include bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa, as well as fungi and viruses.
In deeper or more complicated wounds, infection may spread to adjacent tissues or bones, causing systemic complications. Imaging techniques can help identify underlying infections, particularly when the wound is not responding to initial treatment. Gas-producing organisms, for example, are often associated with necrotizing soft tissue infections, such as necrotizing fasciitis, which can spread rapidly and lead to death if not treated promptly. Imaging can reveal the presence of air within deep tissues, a hallmark sign of these infections.
Wound Healing Phases
Wound healing occurs in several overlapping phases:
- Hemostasis
- The body immediately attempts to stop bleeding by constricting blood vessels and forming a clot. Platelets release growth factors that promote the next phase.
- Inflammation
- White blood cells, including neutrophils and macrophages, remove pathogens and debris while preparing the tissue for repair.
- Proliferation
- This phase involves the formation of new tissue, including epithelial cells, collagen, and new blood vessels (angiogenesis). Fibroblasts and keratinocytes are particularly active during this phase.
- Maturation (remodeling)
- Over time, the new tissue is strengthened, and collagen fibers are reorganized to improve the strength and functionality of the healed tissue.
Advanced Imaging in Wound Care
In some cases, wound imaging is essential for identifying underlying complications that may not be immediately apparent on visual inspection. For example, imaging can help assess poor tissue blood flow, underlying infections, or pressure injuries and is particularly important for rapidly worsening wounds, suspected subcutaneous fluid collections, or foreign bodies in traumatic wounds.
In diabetic foot ulcers, for instance, imaging can help identify osteomyelitis or significant bone loss, which is critical for determining whether surgical intervention, such as amputation, is required. Imaging techniques also play a key role in detecting necrotizing soft tissue infections (NSTIs), which progress rapidly and can be fatal without intervention. Imaging can reveal signs such as the presence of air within deep tissues, which is characteristic of gas-producing organisms responsible for NSTIs.
Plain Films
X-rays, also known as plain radiography, are a widely used imaging technique that uses ionizing radiation with wavelengths of less than 10 nm.[5] These rays are emitted from a generator and pass through the body, interacting with tissues differently. The x-ray beam is either scattered, absorbed, or transmitted through the patient’s tissues, and a detector collects this data to form an image.[5] The resulting image is a negative representation of what was detected by the detector: areas that absorb or scatter the x-rays (such as bones) appear bright white. In contrast, areas that transmit the x-rays (such as air) appear black. Radio-opaque objects, like bones, block x-rays and thus appear white, whereas radiolucent objects, like air, absorb or transmit the rays, appearing black on the film.
Plain films are often the first imaging modality to assess patients with suspected trauma or infections, particularly chronic wounds. This is primarily because of their low cost and wide availability. X-rays are beneficial in evaluating bone injuries, detecting fractures, and assessing inflammation, as inflamed tissues show increased radioopacity (see Image. Osteomyelitis on Foot Radiograph).[2] In wound care, x-rays help identify underlying bone involvement, foreign bodies, or deep infections, providing critical diagnostic information early in treatment.
Detection of Foreign Bodies
One of the most common applications of plain films in wound imaging is the identification of foreign bodies that may be embedded in the wound. This can be particularly important for nonhealing wounds, where a foreign object is suspected of obstructing healing. Radiopaque foreign bodies, such as metal or glass, are easily visualized on an x-ray, appearing as bright white objects in the tissue. This helps guide treatment, allowing for the removal of the foreign body, which can be a key factor in wound resolution.
Assessment of Bone Involvement
In traumatic injuries, x-rays, such as fractures or cracks, are essential for evaluating bone involvement. In wounds that expose bone, x-rays can assess the degree of injury, alignment of the bone fragments, and the presence of complications such as osteomyelitis (bone infection). For wounds associated with fractures, x-rays can help determine whether the fracture is displaced or if surgical intervention is required to realign the bone properly. The ability to assess bone integrity is crucial for managing complex wounds, particularly in extremities like the lower limbs, where fractures are common.
Evaluation of Soft Tissue and Deeper Structures
While x-rays are less sensitive in evaluating soft tissues than other imaging techniques like magnetic resonance imaging (MRI) or computed tomography (CT) scans, they can still provide valuable information about soft tissue damage. For example, gas in the soft tissues, a hallmark of certain infections like NSTI, can be visualized on x-ray as areas of radiolucency (black spaces) within the tissues. This indicates the presence of gas-producing organisms, such as Clostridium species, which cause gas gangrene and necrotizing fasciitis. Early identification of such infections is crucial, as these conditions can progress rapidly and be fatal if untreated. X-rays can also detect soft tissue swelling, effacement of fat planes (a sign of inflammation), and changes in the surrounding tissues that suggest infection or trauma.[6] These findings are nonspecific, but help guide further diagnostic and therapeutic interventions when combined with clinical evaluation.
Infection Detection
X-rays can reveal inflammatory changes that may indicate the presence of infection. For instance, soft tissue swelling or the blurring of fat planes (normally visible on plain films) can suggest the presence of an underlying infection. Additionally, air in the soft tissues, seen as radiolucent areas on the x-ray, can suggest a gas-producing infection, such as necrotizing fasciitis. Other signs that may raise suspicion of infection include foreign bodies, bony fractures, and changes in the bone structure, like osteomyelitis. In cases where a foreign body is suspected beneath a chronic wound that does not heal, x-rays can help identify the object, even when deeply embedded. This is critical for preventing continued infection or impaired healing associated with retained foreign material.
Limitations of Plain Films in Wound Imaging
- Limited soft tissue resolution
- X-rays are less effective in evaluating soft tissues than other imaging modalities like CT or MRI, limiting their ability to provide detailed information about soft tissue injury or wound healing.
- Two-dimensional (2D) imaging
- X-rays provide a 2D representation of a 3D object, which may not fully capture the depth or complexity of the wound or underlying structures.
- Radiation exposure
- Although the radiation dose from x-rays is generally low, repeated use may accumulate exposure, which can be of concern to certain patient populations.
Indications for Using X-rays in Wound Imaging
- Traumatic wounds
- When there is suspicion of fractures or bone involvement.
- Nonhealing wounds
- In cases where foreign bodies are suspected beneath the wound.
- Signs of infection
- When there are concerns about soft tissue infection, gas gangrene, or osteomyelitis.
- Monitoring bone healing
- X-rays can track the healing process of bone fractures or assess the development of osteomyelitis or other complications in chronic wounds.
Computed Tomography
Computed Tomography in Wound Imaging
CT is a specialized imaging modality that employs a series of x-rays, which are then reconstructed via computer to produce detailed cross-sectional images of the scanned region.[7] This modality is widely available, provides high-resolution images quickly, and allows for detailed visualization of a wound's soft tissues, bone, and other structures.[2][5] CT is invaluable in assessing complex wounds, particularly those involving deeper tissues or those complicated by infection or foreign bodies. The ability to view tissues in 3 dimensions makes CT an important diagnostic tool, allowing clinicians to quickly and accurately assess wound severity and potential complications.
How Computed Tomography Works
CT imaging uses a rotating x-ray beam to capture data from multiple angles. A computer then processes these images to create a series of thin cross-sectional slices stacked together to form a detailed 3D image of the affected area. Each slice is essentially a "picture" of a thin body section, which helps clinicians identify both superficial and deep injuries. Dense tissues, like bone, appear white, while softer tissues like fat and muscle appear in shades of gray. In certain cases, contrast agents can enhance the image, making it easier to assess blood flow, identify abscesses, or highlight areas of inflammation.[1]
Role of Computed Tomography in Wound Imaging
- Evaluation of soft tissue injuries
- CT scans provide high-resolution images of soft tissue injuries, allowing clinicians to assess the extent of damage to muscles, fascia, and skin. This is particularly useful in complex wounds where damage extends beyond the superficial layers of the skin. CT effectively identifies subcutaneous gas (a hallmark of infections like necrotizing fasciitis), abscesses, and necrotic tissue, which are often difficult to visualize on plain radiographs or through clinical examination alone.
- Foreign body detection
- CT is excellent for detecting foreign bodies embedded in wounds, particularly those made of nonradiopaque materials, such as wood, plastic, or glass. Unlike plain x-rays, which may miss foreign objects that do not absorb or scatter x-rays, CT can identify subtle differences in tissue density, making it a more sensitive tool for locating and characterizing foreign bodies.
- Infection detection and diagnosis
- CT plays a critical role in diagnosing deep infections, especially in complex wounds where the involvement of soft tissues, fascia, and bone is unclear due to ease of emergent availability, speed, resolution, and ability to view the image in 3D.[8] One of the primary applications of CT in wound imaging is distinguishing between NSTI and cellulitis.
- NSTIs are rapidly progressing and potentially life-threatening infections that affect deep tissues and fascia. CT can help identify subcutaneous gas, often seen in NSTIs, though it may not always be present in the early stages (see Image. Necrotizing Soft Tissue Infection on Computed Tomography). CT imaging has a 100% negative predictive value for NSTI and a 98% specificity, making it an invaluable diagnostic tool.[9]
- Combining CT findings with the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can improve diagnostic accuracy. The score uses C-reactive protein, sodium, glucose, creatinine, total white blood cell count, and hemoglobin concentration to score the patient's risk of NSTI.[10] LRINEC has a wide range of sensitivities from 43% to 80%. A high LRINEC score (≥7) in conjunction with CT findings of multicompartmental inflammation and thickening of the deep fascia greater than 3 mm significantly increases the sensitivity of NSTI diagnosis up to 96%.[9]
- Bone and joint pathology
- CT is particularly effective in evaluating bone and joint pathology, which is critical when wounds involve bone injury or complications like osteomyelitis. CT offers great anatomic detail, making assessing bone destruction, periosteal reactions, and other signs of infection or injury easier. This is crucial for patients with chronic wounds, such as diabetic foot ulcers, where underlying bone involvement can complicate wound healing.
- Evaluation of blood flow and vascular structures
- CT, particularly when combined with CT angiography (CTA), is useful in assessing blood flow to the wound area. This is especially important in wounds complicated by vascular insufficiency, such as those in diabetic patients or individuals with peripheral arterial disease (PAD). CTA provides a detailed view of the blood vessels, helping to identify any blockages or abnormalities that could impair wound healing. In cases where revascularization is needed, CT imaging can guide surgical planning.
- Preoperative planning
- In complex cases, CT scans provide detailed anatomical information that helps in preoperative planning. This includes assessing the extent of tissue damage, identifying necrosis or infection that requires surgical debridement, and evaluating the involvement of deep structures like bone or fascia. CT can also guide the surgical removal of foreign bodies or infected tissue and help in the decision to perform amputation if osteomyelitis or severe tissue necrosis is present.
Limitations of Computed Tomography in Wound Imaging
- Radiation exposure
- CT scans involve exposure to ionizing radiation, which may not be appropriate for repeated imaging, especially in vulnerable populations.
- Contrast-related risks
- The use of contrast agents can pose risks, such as nephrotoxicity, especially in patients with renal impairment or those taking medications that affect kidney function.
- Cost
- CT scans can be more expensive than other imaging modalities, which may limit their accessibility in some healthcare settings.
Indications for Using CT in Wound Imaging
CT is especially useful in the following clinical scenarios:
- Deep or complex wounds
- Wounds involving deep tissues, bone, or multiple anatomical structures require detailed imaging to assess the full extent of the injury.
- Infection diagnosis
- When suspected of an NSTI, osteomyelitis, or a deep abscess
- Foreign body detection
- In cases where nonradiopaque foreign bodies are suspected and need to be located
- Preoperative planning
- CT provides critical information to guide debridement, revascularization, or amputation when surgery is required.
- Vascular assessment
- CT angiogram can help assess blood flow and guide treatment decisions for wounds with suspected vascular insufficiency.
Magnetic Resonance
MRI in Wound Imaging
MRI is a radiation-free imaging modality that uses magnetic fields and radiofrequency waves to excite hydrogen atoms within tissues, generating detailed images of soft tissues, bones, and fluids. Unlike CT or x-rays, MRI produces superior resolution of soft tissues, making it a critical tool in assessing complex wounds, detecting infections, and diagnosing underlying conditions like osteomyelitis or NSTI.[1][5] When compared to CT or x-ray, MRI is more time-intensive and expensive. As a result, a smaller field of view is usually obtained with MRI, compared to whole-body scanning with CT.[5] This increased acquisition time is also clinically relevant, as it introduces more risk for motion artifact and is unsuitable for unstable or critically ill individuals, who cannot be sequestered in an imaging department or be off monitors for extended periods.[5]
MRI Technology
MRI works by aligning hydrogen protons in the body using a strong magnetic field. Radiofrequency pulses excite these protons, and as they return to their baseline state, they emit signals that are detected and processed into high-resolution images by a computer. The distinct signals generated by various tissue types allow MRI to produce unparalleled soft-tissue contrast compared to other imaging modalities. Contrast agents, such as gadolinium, can enhance visualization of inflamed tissues, abscesses, sinus tracts, or necrotic areas.[2][4][8] However, their use should be carefully considered in patients with chronic kidney disease or other contraindications.
Applications in Wound Imaging
- Soft tissue evaluation
- MRI provides exceptional soft tissue detail, making it invaluable for identifying subtle changes such as edema, necrosis, or inflammation. This is useful for assessing complex wounds or soft tissue infections like cellulitis and NSTI.
- Infection detection
- Cellulitis
- Phlegmon or abscess
- MRI can differentiate between phlegmon (intensely enhancing with poorly defined borders) and abscesses (fluid collections surrounded by a hypointense wall with a "double-layer" configuration).[4] Contrast-enhanced MRI is particularly effective in defining abscess boundaries and identifying sinus tracts.[4]
- NSTI
- NSTI can be distinguished from cellulitis on MRI by high signal intensity along the deep intermuscular fascia, which has a high negative predictive value and sensitivity of 86.4% for the disease.[8][9] However, such a finding is not specific, as it can be seen postradiation in inflammatory myositis, trauma, lymphedema, and vasculitis. Soft tissue gas is characteristic of the disease but can be absent early, as with CT imaging.[8] Contrast enhancement of the deep fascia can also be lost in the late stages of the disease, as necrosis progresses and blood flow to the tissues is lost.[4] Despite the superior soft tissue resolution, the speed of CT scanning makes MRI a less optimal choice when life-threatening NSTI is suspected.[9]
- Chronic wounds
- MRI is invaluable for detecting complications such as abscesses, sinus tracts, or osteomyelitis in diabetic foot ulcers or pressure sores (see Image. Magnetic Resonance Imaging of Osteomyelitis of the Foot).[1][4] Contrast-enhanced images can highlight devitalized tissue and help guide surgical intervention.
- Bone pathology
- MRI is the gold standard for diagnosing osteomyelitis, especially in chronic wounds.
- T1-weighted images
- Infected bone appears hypointense (dark) due to marrow changes.
- T2-weighted images
- These will show hyperintense (bright) signals indicating edema or inflammation.
- Postcontrast images
- These highlight cortical disruption and bone enhancement, which are highly specific for osteomyelitis.[4] The infected bone will exhibit an ill-defined, low-intensity signal with intense postcontrast enhancement (see Image. Knee Osteomyelitis on T1-Weighted Postcontrast Magnetic Resonance Imaging).
- Despite its accuracy, MRI may overestimate the extent of osteomyelitis due to the inability to distinguish infection from surrounding edema.
- T1-weighted images
- MRI is the gold standard for diagnosing osteomyelitis, especially in chronic wounds.
- Vascular assessment
- While less commonly used for vascular imaging than CTA, MRI can assess blood flow abnormalities contributing to wound chronicity, such as ischemia.
Advantages of MRI in Wound Imaging
- Superior soft tissue resolution
- MRI excels at distinguishing tissue types, making it ideal for evaluating infections, necrosis, and structural abnormalities.
- Radiation-free
- MRI avoids the risks associated with ionizing radiation.
- Detailed bone and soft tissue interaction
- This interaction is critical for diagnosing OM and NSTI.
- Multiplanar imaging
- MRI provides comprehensive visualization of wounds and surrounding anatomy.
Limitations of MRI in Wound Imaging
- Cost and time
- MRI is more expensive and time-consuming than CT or x-rays.
- Motion artifacts
- Prolonged acquisition times increase the risk of artifacts from patient movement.
- Field of view
- This is smaller with MRI as compared to whole-body CT scans, which limits its utility for extensive trauma.
- Contraindications
- Patients with metal implants, life-support devices, or severe claustrophobia may be unable to undergo MRI. Sedation or anxiolytics may be needed, increasing risk and resource use.[5]
- Less practical for emergencies
- MRI’s longer imaging times make it less suitable for critically ill patients or emergent conditions like NSTI, which require rapid diagnosis.
Ultrasonography
Ultrasonography in Wound Imaging
Ultrasonography (US) has emerged as a versatile, cost-effective, and safe imaging modality for wound care, offering radiation-free, real-time imaging.[1] Utilizing sound waves, US provides a dynamic view of soft tissue structures and can assess both superficial and deep tissues. The technology supports advanced imaging formats, including 2D, 3D, and even 4D imaging, enabling clinicians to monitor and evaluate wound healing precisely. 3D visualization of tissue structures up to a depth of several inches makes ultrasound imaging a strong tool in full-thickness wounds.
Mechanism and Technology
US imaging involves directing an acoustic pulse into tissues, with reflections detected and converted into visual images.[11] The resolution and depth of imaging depend on the frequency of the sound waves:
- High-frequency sound waves
- These offer increased spatial resolution for evaluating superficial structures, such as collagen deposition, microvascular growth, and epithelialization.[11] However, their penetration depth is limited. High-frequency ultrasound is used to evaluate wound structure and assess wound healing quantitatively.[1][11]
- Low-frequency sound waves
- These provide deeper imaging at the cost of resolution, making them suitable for evaluating full-thickness wounds.
Technological advances have allowed ultrasound devices to exist in smaller, more portable formats like hand-held devices. Despite their smaller size, these devices are still capable of high-resolution images, similar to those of their larger counterparts.[12][13] These devices are practical in diverse healthcare settings and support the creation of databases for long-term monitoring and research.
Applications in Wound Care
- Wound evaluation
- Depth and structure
- US accurately measures wound dimensions, tissue thickness, and healing progress. In chronic wounds, it aids in assessing granulation tissue formation and detecting complications like abscesses or fluid collections (see Image. Ultrasound of Abscess).[14]
- Pressure necrosis and injuries
- Depth and structure
- Infection assessment
- Abscesses and fluid collections
- US is a valuable initial modality for detecting abscesses, showing fluid collections with posterior acoustic enhancement and soft tissue stranding indicative of inflammation.
- Deeper infections
- While the US can detect perifascial fluid or early signs of infection, deeper involvement, like necrotizing infections, often requires complementary imaging, such as CT or MRI.
- Abscesses and fluid collections
- Vascular Analysis
- Doppler US
- Doppler US enables assessment of blood flow, helping differentiate cellulitis from venous thrombosis or inflammation.[8] Doppler is particularly useful in chronic wounds with suspected vascular insufficiency.
- Lower extremity arterial duplex
- This application assesses vessel composition, stenosis, or occlusion near the wound site. Parameters like ankle peak systolic velocity and pedal peak systolic velocity provide alternative metrics for peripheral ischemia, correlating with ankle-brachial and toe-brachial indexes.[16] These parameters are effective even in the presence of vessel calcification, gangrene, or amputation.[17]
- Doppler US
- Advanced technologies
- Laser Doppler imaging
- This offers a noncontact method for evaluating microvascular perfusion, which is particularly effective for assessing deep burns and tracking wound healing.[1] This method is effective for assessing deep burns in the early period of injury (see Image. Laser Doppler of Foot Burn) and can improve the length of stay and the cost of treatment of burns.[18][19] This modality has also been useful in wound imaging for measuring healing progress.[19] However, it is not as accurate in diabetics or patients with peripheral vascular disease.[1]
- Laser Doppler imaging
- Chronic wound monitoring
- Images can be uploaded into wound databases, enabling data analysis, research, and educational use.
Limitations of US
- Depth limitations
- US struggles to visualize deeper structures, such as bone or fascia, and is less effective in diagnosing osteomyelitis or necrotizing infections.
- Operator dependency
- Image quality and interpretation depend heavily on the sonographer's expertise, making it less accessible to front-line wound care personnel without specialized training.[2]
- Focus on anatomy
- While US excels in anatomical evaluation, it provides limited physiological data about the complex processes involved in wound healing.
Nuclear Medicine
Nuclear Medicine in Wound Imaging
Nuclear medicine imaging is a functional imaging technique crucial in diagnosing and monitoring treatment outcomes for infections and the inflammatory phases of wounds. Nuclear medicine imaging is a functional imaging that is pivotal in diagnosing and monitoring treatment outcomes of infections and the inflammatory phases of wounds.[20]
Positron Emission Tomography/CT Imaging and Limitations
Positron emission tomography (PET) evaluates functional changes and treatment responses, especially when combined with CT (PET/CT). The most common radiotracer, [18F]fluorodeoxyglucose (FDG), highlights areas of increased glucose metabolism. However, FDG-PET/CT lacks specificity, as both infection and inflammation demonstrate elevated uptake. Additionally, postsurgical or radiation-induced healing responses complicate interpretation due to tracer accumulation in granulation tissue. Another drawback of this approach is that the equipment and radioisotope tracers used to perform these scans are expensive.[2]
Emerging Molecular Imaging Targets
New radiotracers target specific cells and molecules involved in wound pathology. Radiolabeled lymphocytes, macrophages, and fibroblast activation protein inhibitors (FAPI) are under study to detect active fibroblasts and inflammatory responses. Tracers like [68Ga] and [18F]-FAPI show promise in identifying fibroblast activity. Pilot studies exploring bacterial-targeted tracers could soon allow infection detection and bacterial identification, guiding early antibiotic treatment.
Hybrid Imaging Techniques: PET/MRI and SPECT/CT
PET/MRI combines functional and anatomical imaging, offering superior soft tissue detail and reduced radiation, making it beneficial for pediatric cases. Although costly and complex, PET/MRI is especially useful in diabetic foot infections and osteomyelitis. Single-photon emission computed tomography (SPECT/CT) integrates functional 3D imaging with high-resolution anatomical detail. This modality is particularly effective for assessing lower extremity microvascular perfusion in peripheral artery disease and chronic wounds.
Radiotracers and Safety Considerations
Technetium-99m (99mTc)-labeled perfusion radiotracers are widely used for wound imaging due to their favorable imaging quality and lower radiation dose. Despite their value, nuclear imaging techniques are costly and expose patients to ionizing radiation. Additionally, PET and SPECT hold future potential for monitoring bioengineered scaffolds in regenerative wound therapies.
Risks of Radiopharmaceutical Extravasation
Radiopharmaceutical administration carries the risk of extravasation, where the tracer leaks into the surrounding tissue. This may lead to local radiation exposure and alter tracer biodistribution, compromising imaging accuracy. Careful technique and monitoring during intravenous injection are essential to minimize these risks and ensure diagnostic reliability.
Angiography
Angiography in Wound Imaging
Angiography is pivotal in wound imaging, especially when evaluating vascular health and perfusion in ischemic or nonhealing wounds. This technique assesses macrovasculature and microvasculature, providing critical insights into tissue perfusion—a key determinant of wound healing. Tissue oxygen saturation is an important marker of wound perfusion and can be measured using various techniques.[21] Angiographic studies are complemented by other diagnostic methods, such as the ABI, pulse volume recording (PVR), and transcutaneous oxygen (TcPO2), to assess vascular status comprehensively.[22]
Noninvasive Techniques in Angiographic Wound Imaging
- CT Angiography
- Mechanism
- Computed tomography angiography (CTA) uses intravenous iodinated contrast and CT to produce high-resolution, cross-sectional images of macrovasculature.
- Applications
- Preferred for assessing peripheral vascular disease, especially in nonhealing wounds or ulcers
- Identifies stenosis, occlusion, or aneurysms affecting blood flow to the wound bed (see Image. Computed Tomography Angiogram of Aorta with Bilateral Runoff)
- Maps vascular abnormalities for surgical or interventional planning (eg, angioplasty, stenting)
- Advantages
- Faster scanning times compared to magnetic resonance angiography (MRA)
- High spatial resolution and the ability to assess distal vasculature and perfusion
- Limitations
- Radiation exposure and potential nephrotoxicity or allergies associated with iodinated contrast agents
- Mechanism
- MRA
- Mechanism
- MRA uses magnetic fields and gadolinium-based contrast (or noncontrast techniques) to visualize vascular structures.
- Applications
- An alternative to CTA for patients with contraindications to iodinated contrast [1]
- Suitable for evaluating soft tissue perfusion and identifying vascular lesions
- Advantages
- Avoids ionizing radiation [1]
- Superior soft tissue resolution
- Limitations
- Longer acquisition times
- Makes it less suitable for critically ill individuals
- Higher cost and limited availability compared to CTA
- Longer acquisition times
- Mechanism
- SPECT
- Mechanism
- SPECT combines functional imaging with anatomical resolution to evaluate microvascular perfusion.
- Applications
- High-sensitivity assessment of tissue-level perfusion in complex wounds
- Limitations
- High cost and radiation exposure [1]
- Mechanism
- Venous Duplex Studies
- Mechanism
- These studies combine ultrasound with Doppler imaging to evaluate venous insufficiency and vascular flow.
- Applications
- Identifies venous reflux or obstruction contributing to delayed wound healing
- Guides venoocclusive therapies by locating incompetent perforators near the wound bed [23]
- Mechanism
Invasive Techniques in Angiographic Wound Imaging
- Catheter-Based Digital Subtraction Angiography
- Mechanism
- Digital subtraction angiography (DSA) is a direct injection of contrast into vessels with real-time x-ray imaging, subtracting soft tissue and bone from the image.
- Applications
- The gold standard for vascular assessment in ischemic wounds and peripheral artery disease (PAD)
- Allows simultaneous therapeutic interventions (eg, angioplasty, stenting)
- Advantages
- High accuracy for small vessels
- Provides real-time feedback for interventional procedures
- Limitations
- Invasive, with risks of bleeding, infection, or vascular injury
- Mechanism
- Immunofluorescent Angiography
- Mechanism
- This technique uses fluorescent dyes to assess perfusion at the microvascular level.
- Applications
- Evaluates perfusion directly in the wound bed
- Guides decisions on debridement or flap viability
- Limitations
- Requires specialized equipment and training
- Mechanism
Supplementary Perfusion Measurement Techniques
- Ankle brachial index
- This measures large-vessel perfusion and is a standard test for PAD. However, due to calcified, noncompressible vessels, it may be unreliable in diabetics.
- Pulse volume recording
- This analyzes Doppler waveforms to assess vascular disease. Subjective interpretation may impact accuracy and potentially lead to suboptimal care.[24]
- Transcutaneous oximetry, TcPO2
- This measures tissue oxygenation but is limited for plantar foot wounds due to the difficulty of assessing the entire surface area.
- Toe brachial index and toe pressures
- These are more reliable than ABI in diabetics for evaluating distal perfusion.
Clinical Applications of Angiography in Wound Care
- Ischemic wounds and PAD
- Diabetic foot ulcers
- CTA and DSA are critical in mapping macrovascular and microvascular perfusion deficits.
- Guides interventions for restoring blood flow and promoting healing
- Chronic nonhealing wounds
- Identifies perfusion deficits in wounds with delayed healing.
- Combines imaging with physiological markers like ABI and TcPO2 to assess overall vascular status
- Traumatic wounds
- Determines vascular integrity and guides reconstructive surgery
- Immunofluorescent angiography evaluates tissue perfusion for flap viability.
Advantages of Angiography
- High-resolution imaging of vascular structures
- Direct visualization of perfusion deficits, guiding targeted interventions
- Comprehensive assessment of both macrovasculature and microvasculature in complex wounds
Limitations of Angiography
- Radiation and contrast risks
- Associated with CTA and DSA, limiting use in patients with renal impairment or allergies
- Invasiveness
- Catheter-based studies carry procedural risks.
- Cost and accessibility
- Advanced modalities like SPECT and immunofluorescent angiography are costly and less available in resource-limited settings.
Anterior Segment Optical Coherence Tomography
Dynamic Optical Coherence Tomography in Wound Imaging
Optical coherence tomography (OCT) and its recent variant, dynamic OCT (D-OCT), permit rapid, noninvasive, depth-resolved imaging of the capillaries in the superficial dermis via a handheld probe, showing the morphology and density of vessels down to 20 µm in diameter.[27] D-OCT is an advanced imaging modality that builds upon conventional OCT by incorporating motion contrast analysis to evaluate the microcirculatory environment, tissue architecture, and wound healing processes.[1][11] This noninvasive technique provides high-resolution, real-time visualization of the microcirculatory environment in wounds, making it particularly valuable in assessing wound healing processes. By capturing blood flow patterns within the wound bed and surrounding tissues, D-OCT allows clinicians to monitor the regeneration of microvascular networks, epithelialization, and dermal remodeling.
One of the key advantages of D-OCT is its ability to visualize both structural and functional changes in the wound simultaneously. For instance, it can evaluate vascular density, perfusion dynamics, tissue oxygenation, and wound morphology. This dual capability is especially beneficial for detecting early signs of ischemia, impaired perfusion, or delayed healing in chronic wounds, diabetic ulcers, and especially burns.[28] Additionally, D-OCT has been used to assess the effects of therapeutic interventions, such as vascular therapies or debridement, by tracking real-time changes in microvascular activity. Although D-OCT offers promising applications in wound imaging, challenges such as high equipment costs and the need for specialized training in interpretation remain barriers to widespread adoption. However, ongoing advancements in technology and software integration may enhance its accessibility and clinical utility in improving patient outcomes.
Fluorescent and Thermal Imaging
Advanced imaging technologies, including fluorescent imaging, thermal imaging, and spectroscopic techniques, transform wound assessment and management. These noninvasive and noncontact modalities provide critical insights into tissue physiology, perfusion, oxygenation, and infection status, enabling earlier diagnosis, personalized treatment, and improved outcomes.[29]
Hyperspectral and Multispectral Imaging
Hyperspectral and multispectral imaging (HSI and MSI) are advanced optical techniques that expand upon human vision by dividing the primary color bands (red, green, and blue) into numerous spectral bands. These modalities provide detailed insights into tissue physiology by measuring key optical signals, such as oxyhemoglobin and deoxyhemoglobin, allowing for perfusion mapping and evaluation of microcirculatory status.[3][11] These signals provide a measure of local oxygenation and microcirculatory status.[30] HSI and MSI generate 3D representations and 2D images of wounds, offering valuable information for assessing burn depth, diabetic foot ulcer healing, and microvascular disease in PAD (see Image. Hyperspectral Imaging of Foot Ulcer).[1][3][11]
These modalities give insights into tissue health and the healing potential of wounds and convey a comprehensive picture of tissue health and the healing capacity of wounds.[31] Studies highlight hyperspectral imaging's (HSI) high sensitivity and predictive value in wound care, with 1 study demonstrating superior sensitivity compared to blood oxygen, SpO2, measurements.[3] HSI has shown the ability to predict pressure ulcers with 95% specificity up to 58 days before clinical manifestation and has demonstrated 100% sensitivity in detecting wound infections.[3] However, the technology has limitations, including the large datasets generated and the need for specialized equipment and expertise for interpretation.[32] Despite these challenges, HSI and MSI hold significant potential for proactive wound care and the early detection of tissue compromise.
Spatial Frequency Domain Imaging
Spatial frequency domain imaging (SFDI) is a noncontact optical imaging technique with significant potential in wound assessment.[1] Like spectral imaging, SFDI primarily quantifies oxygen saturation, oxyhemoglobin and deoxyhemoglobin concentrations, and tissue water content.[1][2] This modality provides valuable insights into the physiological state of tissues and is particularly promising in burn care, where it can measure wound depth and severity and detect inflammation or infection (see Image. Spatial Frequency Domain Imaging of Foot Burn). While still under extensive research, SFDI shows promise in advancing wound care, especially burn wounds, through precise and noninvasive assessments of tissue health.
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is an emerging, noninvasive, and noncontact imaging modality that leverages light absorption properties to provide critical insights into tissue physiology. NIRS measures parameters such as oxygen saturation, hemoglobin concentration, and water content, making it particularly useful for evaluating wound characteristics like burn depth, local tissue edema, and ischemic or diabetic ulcer healing (see Image. Near-Infrared Imaging of a Foot Ulcer).[1] This technology can detect subtle variations in tissue oxygenation and perfusion, aiding in identifying inflammation or ischemia. For example, an increased signal (red) may indicate inflammation, while surrounding mottling suggests ischemia, offering valuable diagnostic and prognostic information.
A notable advantage of NIRS is its capability for mobile, hand-held applications, which allows for consistent, repeat imaging across clinical settings.[33] This portability makes it a practical tool for monitoring wound healing over time, particularly in chronic wounds or ulcers. The technology's ability to provide real-time, quantitative data enhances its utility in guiding therapeutic decisions and evaluating treatment efficacy. Despite its promise, further advancements and integration into standard clinical workflows are needed to maximize its potential in improving patient outcomes.
Thermal Imaging
Thermal imaging provides critical diagnostic and prognostic value in wound care by utilizing infrared cameras to measure tissue temperature indicative of inflammation and infection.[1][2][34] Temperature changes serve as key indicators of wound pathology. Research has shown that a temperature difference between a chronically infected wound and normal tissue has a specific elevated thermal gradient range of 3 °C to 4 °C.[35] Thermal imaging can also detect ischemia, which manifests as reduced tissue temperature due to compromised blood flow, commonly observed in ischemic ulcers, pressure injuries, and trauma.[36] Furthermore, this modality is valuable in assessing burn depth and severity, identifying increased blood flow indicative of inflammation and decreased flow reflective of poor perfusion.[37][38]
A major advantage of thermal imaging is its ability to detect changes in skin temperature before clinical manifestations of ischemia, inflammation, or infection, allowing for early intervention. Long-wave infrared thermography has been particularly effective as an adjunct in detecting inflammation and infection. Emerging smartphone-based applications combine thermal imaging with visual wound monitoring to enhance traditional evaluation methods. However, challenges such as accuracy, specificity, and sensitivity remain, emphasizing the need for further refinement and integration into routine clinical practice.[39][40]
Fluorescent Imaging
Fluorescent imaging in wound care is categorized based on its fluorescence source: natural fluorescence from bacteria and tissue components or fluorescence from exogenous agents like indocyanine green used to visualize perfusion. While exogenous dye-based imaging has applications in assessing wound size, depth, healing progress, collagen/elastin content, and vascularization, its practicality in clinical settings is hindered by the need for dyes and prolonged imaging times.[41] Autofluorescence imaging is beneficial for identifying bacterial colonization. Portable and user-friendly autofluorescent cameras are increasingly utilized in clinical settings. However, these devices do not diagnose infections but highlight areas of clinically significant bacterial presence (see Image. Autofluorescence of Wound).[2] Study results demonstrate 78% sensitivity and specificity in detecting bacterial load, outperforming culture swabs alone.[42] By identifying bacterial hotspots, fluorescent imaging supports targeted wound debridement, aids in assessing antimicrobial needs, and ultimately enhances clinical outcomes in wound management.[43]
Patient Positioning
Proper patient positioning is critical in ensuring the accuracy and reproducibility of wound imaging results. Positioning must be tailored to the anatomical location of the wound, the imaging modality being used, and the goals of the assessment. For instance, wounds on the extremities often require elevation to reduce dependent edema, which may interfere with imaging results, especially when assessing perfusion or inflammation using thermal imaging or NIRS. For pressure ulcers on the sacrum or buttocks, patients may need to be positioned in lateral decubitus or prone positions to optimize wound exposure while minimizing further pressure on the affected area.
Maintaining a consistent positioning protocol is essential for serial wound assessments. This ensures that successive images are comparable, allowing for accurate healing or disease progression tracking. Certain imaging techniques, such as SFDI or HSI, require precise alignment between the imaging device and the wound to avoid distortion or variability in data. Patient comfort must also be prioritized, especially for individuals with limited mobility, pain, or comorbidities such as diabetes or vascular disease. Care teams should collaborate to secure stable positioning while preventing additional harm or discomfort, using supportive devices as needed.
Clinical Significance
Effective utilization of wound imaging requires a multidisciplinary approach that emphasizes skills, strategy, interprofessional communication, and care coordination. Advanced clinicians bring their diagnostic expertise and clinical decision-making to interpret imaging results and guide treatment plans. They must be skilled in selecting appropriate imaging modalities based on the wound type, patient history, and clinical indications. Nurses are critical in preparing patients for imaging procedures, monitoring for adverse reactions, and integrating imaging findings into wound care protocols. Pharmacists contribute by assessing medication interactions, mainly when contrast agents or medications for imaging-related anxiety are used. Additionally, technicians and imaging specialists ensure high-quality image acquisition and collaborate with clinicians to provide accurate results.
Interprofessional communication and care coordination enhance patient-centered care and outcomes by fostering a shared understanding of wound imaging's role in treatment planning. Regular team huddles, clear documentation, and standardized protocols facilitate seamless information exchange. Care coordination ensures imaging findings inform the broader treatment strategy, including surgical interventions, debridement, or antimicrobial therapy. This teamwork approach also promotes patient safety by minimizing errors, such as unnecessary imaging or adverse contrast reactions, and ensures timely follow-up on critical findings. Ultimately, a cohesive, multidisciplinary strategy improves team performance and optimizes the healing trajectory for patients with complex wounds.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Wound Infection. Post-contrast T1W image demonstrating abnormal marrow signal and enhancement within the middle finger distal phalanx with a 5.5 mm subperiosteal abscess within the anterior soft tissues of the distal phalanx. There is edema and enhancement throughout the anterior soft tissues to the level of the MCP joint. The imaging features are compatible with osteomyelitis of the middle finger distal phalanx with anterior subperiosteal abscess.
Contributed by A Thomas, MD
(Click Image to Enlarge)
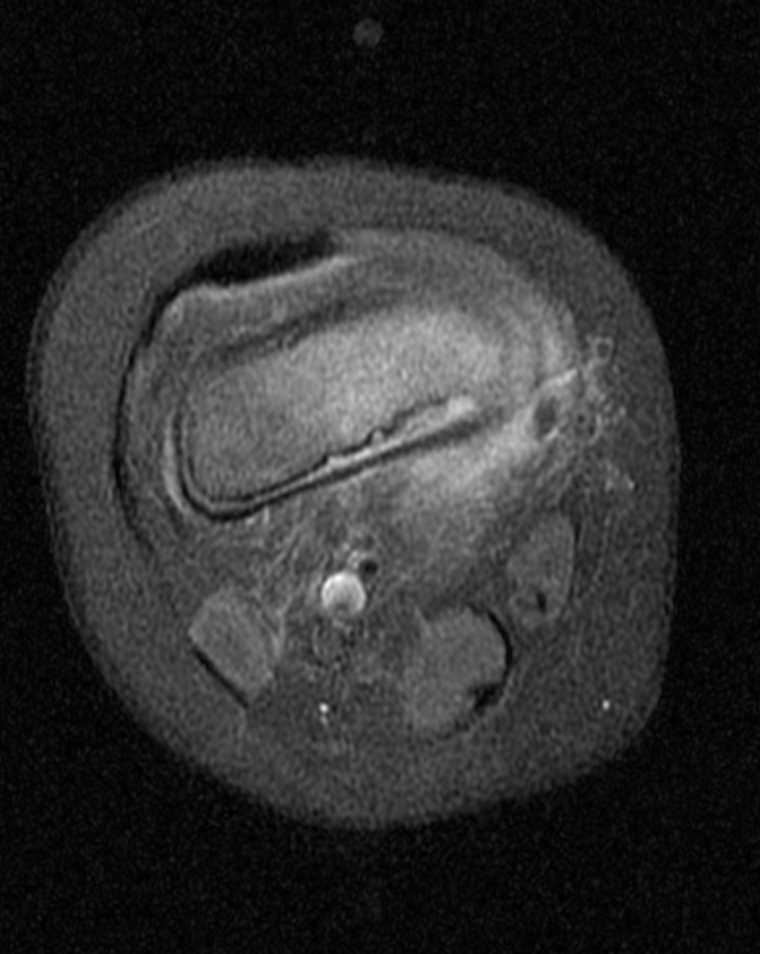
(Click Image to Enlarge)

Wound Imaging. Post-contrast T1W fat-saturated MRI in a patient with prior transmetatarsal amputation with soft tissue swelling compatible with cellulitis and plantar soft tissue abscess as annotated. Additionally, multifocal areas of bone infarction/osteonecrosis involving the distal tibia (annotated) and multiple tarsal bones.
Contributed by A Thomas, MD
(Click Image to Enlarge)
(Click Video to Play)
Wound Imaging. Post-contrast CT imaging in a patient status post right great toe amputation with soft tissue defect and scattered subcutaneous emphysema overlying the great toe metatarsal stump and overlying
subcutaneous tissues of the second and third toe. Additionally, osseous erosion of the proximal phalanx of the second toe is consistent with gangrenous osteomyelitis.
Contributed by A Thomas, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Video to Play)
Wound Imaging. CT Aortogram with distal vessel runoff.
Contributed by A Thomas, MD
(Click Image to Enlarge)
References
Li S, Mohamedi AH, Senkowsky J, Nair A, Tang L. Imaging in Chronic Wound Diagnostics. Advances in wound care. 2020 May 1:9(5):245-263. doi: 10.1089/wound.2019.0967. Epub 2020 Mar 19 [PubMed PMID: 32226649]
Level 3 (low-level) evidenceLi S, Renick P, Senkowsky J, Nair A, Tang L. Diagnostics for Wound Infections. Advances in wound care. 2021 Jun:10(6):317-327. doi: 10.1089/wound.2019.1103. Epub 2020 Jul 7 [PubMed PMID: 32496977]
Level 3 (low-level) evidenceSaiko G, Lombardi P, Au Y, Queen D, Armstrong D, Harding K. Hyperspectral imaging in wound care: A systematic review. International wound journal. 2020 Dec:17(6):1840-1856. doi: 10.1111/iwj.13474. Epub 2020 Aug 23 [PubMed PMID: 32830443]
Level 1 (high-level) evidenceSoldatos T, Durand DJ, Subhawong TK, Carrino JA, Chhabra A. Magnetic resonance imaging of musculoskeletal infections: systematic diagnostic assessment and key points. Academic radiology. 2012 Nov:19(11):1434-43. doi: 10.1016/j.acra.2012.05.022. Epub 2012 Aug 11 [PubMed PMID: 22884398]
Level 1 (high-level) evidenceFlorkow MC, Willemsen K, Mascarenhas VV, Oei EHG, van Stralen M, Seevinck PR. Magnetic Resonance Imaging Versus Computed Tomography for Three-Dimensional Bone Imaging of Musculoskeletal Pathologies: A Review. Journal of magnetic resonance imaging : JMRI. 2022 Jul:56(1):11-34. doi: 10.1002/jmri.28067. Epub 2022 Jan 19 [PubMed PMID: 35044717]
Lázaro Martínez JL, García Álvarez Y, Tardáguila-García A, García Morales E. Optimal management of diabetic foot osteomyelitis: challenges and solutions. Diabetes, metabolic syndrome and obesity : targets and therapy. 2019:12():947-959. doi: 10.2147/DMSO.S181198. Epub 2019 Jun 21 [PubMed PMID: 31417295]
Schulz RA, Stein JA, Pelc NJ. How CT happened: the early development of medical computed tomography. Journal of medical imaging (Bellingham, Wash.). 2021 Sep:8(5):052110. doi: 10.1117/1.JMI.8.5.052110. Epub 2021 Oct 29 [PubMed PMID: 34729383]
Hayeri MR, Ziai P, Shehata ML, Teytelboym OM, Huang BK. Soft-Tissue Infections and Their Imaging Mimics: From Cellulitis to Necrotizing Fasciitis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016 Oct:36(6):1888-1910 [PubMed PMID: 27726741]
Kwee RM, Kwee TC. Diagnostic performance of MRI and CT in diagnosing necrotizing soft tissue infection: a systematic review. Skeletal radiology. 2022 Apr:51(4):727-736. doi: 10.1007/s00256-021-03875-9. Epub 2021 Jul 24 [PubMed PMID: 34302500]
Level 1 (high-level) evidenceHoesl V, Kempa S, Prantl L, Ochsenbauer K, Hoesl J, Kehrer A, Bosselmann T. The LRINEC Score-An Indicator for the Course and Prognosis of Necrotizing Fasciitis? Journal of clinical medicine. 2022 Jun 22:11(13):. doi: 10.3390/jcm11133583. Epub 2022 Jun 22 [PubMed PMID: 35806870]
Weigelt MA, Lev-Tov HA, Tomic-Canic M, Lee WD, Williams R, Strasfeld D, Kirsner RS, Herman IM. Advanced Wound Diagnostics: Toward Transforming Wound Care into Precision Medicine. Advances in wound care. 2022 Jun:11(6):330-359. doi: 10.1089/wound.2020.1319. Epub 2021 Jul 21 [PubMed PMID: 34128387]
Level 3 (low-level) evidenceLee L, DeCara JM. Point-of-Care Ultrasound. Current cardiology reports. 2020 Sep 17:22(11):149. doi: 10.1007/s11886-020-01394-y. Epub 2020 Sep 17 [PubMed PMID: 32944835]
Le MT, Voigt L, Nathanson R, Maw AM, Johnson G, Dancel R, Mathews B, Moreira A, Sauthoff H, Gelabert C, Kurian LM, Dumovich J, Proud KC, Solis-McCarthy J, Candotti C, Dayton C, Arena A, Boesch B, Flores S, Foster MT, Villalobos N, Wong T, Ortiz-Jaimes G, Mader M, Sisson C, Soni NJ. Comparison of four handheld point-of-care ultrasound devices by expert users. The ultrasound journal. 2022 Jul 7:14(1):27. doi: 10.1186/s13089-022-00274-6. Epub 2022 Jul 7 [PubMed PMID: 35796842]
O'Rourke K, Kibbee N, Stubbs A. Ultrasound for the Evaluation of Skin and Soft Tissue Infections. Missouri medicine. 2015 May-Jun:112(3):202-5 [PubMed PMID: 26168591]
Scheiner J, Farid K, Raden M, Demisse S. Ultrasound to Detect Pressure-related Deep Tissue Injuries in Adults Admitted via the Emergency Department: A Prospective, Descriptive, Pilot Study. Ostomy/wound management. 2017 Mar:63(3):36-46 [PubMed PMID: 28355138]
Level 3 (low-level) evidenceBishara RA, Taha W, Akladious I, Allam MA. Ankle peak systolic velocity: new parameter to predict nonhealing in diabetic foot lesions. Vascular. 2009 Sep-Oct:17(5):264-8 [PubMed PMID: 19769805]
Junaidi F, Muradi A, Pratama D, Suhartono R, Kekalih A. Effectiveness of Doppler Ultrasonography as a Predictor of Wound Healing after Below-Knee Amputation for Peripheral Arterial Disease. Chirurgia (Bucharest, Romania : 1990). 2020 Sept-Oct:115(5):618-625. doi: 10.21614/chirurgia.115.5.618. Epub [PubMed PMID: 33138899]
Venclauskiene A, Basevicius A, Zacharevskij E, Vaicekauskas V, Rimdeika R, Lukosevicius S. Laser Doppler imaging as a tool in the burn wound treatment protocol. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques. 2014 Mar:9(1):24-30. doi: 10.5114/wiitm.2014.40273. Epub 2014 Feb 18 [PubMed PMID: 24729806]
Gill P. The critical evaluation of laser Doppler imaging in determining burn depth. International journal of burns and trauma. 2013:3(2):72-7 [PubMed PMID: 23638324]
Glaudemans AWJM, Gheysens O. Expert opinions in nuclear medicine: Finding the "holy grail" in infection imaging. Frontiers in medicine. 2023:10():1149925. doi: 10.3389/fmed.2023.1149925. Epub 2023 Feb 27 [PubMed PMID: 36923013]
Level 3 (low-level) evidenceArnold JF. Is There Adequate Perfusion for Healing? What Routine Noninvasive Vascular Studies are Missing? Wounds : a compendium of clinical research and practice. 2018 Sep:30(9):E89-E92 [PubMed PMID: 30256756]
Donohue CM, Adler JV, Bolton LL. Peripheral arterial disease screening and diagnostic practice: A scoping review. International wound journal. 2020 Feb:17(1):32-44. doi: 10.1111/iwj.13223. Epub 2019 Nov 3 [PubMed PMID: 31680419]
Level 2 (mid-level) evidenceLazarides AL, Saltzman EB, Visgauss JD, Mithani SK, Eward WC, Brigman BE. Intraoperative angiography imaging correlates with wound complications following soft tissue sarcoma resection. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2022 Oct:40(10):2382-2390. doi: 10.1002/jor.25270. Epub 2022 Jan 20 [PubMed PMID: 35005805]
Guilcher A, Lanéelle D, Hoffmann C, Guillaumat J, Constans J, Bressollette L, Le Hello C, Boissier C, Bura-Rivière A, Jaquinandi V, Omarjee L, Lacroix P, Pernod G, Abbadie F, Sevestre MA, Boulon C, Mahé G. Comparison of the Use of Arterial Doppler Waveform Classifications in Clinical Routine to Describe Lower Limb Flow. Journal of clinical medicine. 2021 Jan 26:10(3):. doi: 10.3390/jcm10030464. Epub 2021 Jan 26 [PubMed PMID: 33530374]
Alam W, Hasson J, Reed M. Clinical approach to chronic wound management in older adults. Journal of the American Geriatrics Society. 2021 Aug:69(8):2327-2334. doi: 10.1111/jgs.17177. Epub 2021 May 17 [PubMed PMID: 34002364]
Kim DU, Rao A, Kaplan S, Baksh F, Caprioli R, Haight J, Ferguson RG, Pliskin M, Oropallo A. The use of indocyanine green fluorescence angiography to assess perfusion of chronic wounds undergoing hyperbaric oxygen therapy. Undersea & hyperbaric medicine : journal of the Undersea and Hyperbaric Medical Society, Inc. 2018 Nov-Dec:45(6):663-671 [PubMed PMID: 31158933]
Holmes J, Schuh S, Bowling FL, Mani R, Welzel J. Dynamic Optical Coherence Tomography Is a New Technique for Imaging Skin Around Lower Extremity Wounds. The international journal of lower extremity wounds. 2019 Mar:18(1):65-74. doi: 10.1177/1534734618821015. Epub 2019 Jan 7 [PubMed PMID: 30612479]
Dalicho V, Straube T, Kelly K, Larsen B, Wünsch L, Lindert J. Depth of intact vascular plexus - visualized with optical coherence tomography - correlates to burn depth in thoracic thermic injuries in children. Innovative surgical sciences. 2024 Jun:9(2):83-91. doi: 10.1515/iss-2023-0066. Epub 2024 Jun 12 [PubMed PMID: 39100719]
Mukherjee R, Tewary S, Routray A. Diagnostic and Prognostic Utility of Non-Invasive Multimodal Imaging in Chronic Wound Monitoring: a Systematic Review. Journal of medical systems. 2017 Mar:41(3):46. doi: 10.1007/s10916-016-0679-y. Epub 2017 Feb 13 [PubMed PMID: 28194684]
Level 1 (high-level) evidencede Keijzer IN, Massari D, Sahinovic M, Flick M, Vos JJ, Scheeren TWL. What is new in microcirculation and tissue oxygenation monitoring? Journal of clinical monitoring and computing. 2022 Apr:36(2):291-299. doi: 10.1007/s10877-022-00837-x. Epub 2022 Mar 11 [PubMed PMID: 35275312]
Lucas Y, Niri R, Treuillet S, Douzi H, Castaneda B. Wound Size Imaging: Ready for Smart Assessment and Monitoring. Advances in wound care. 2021 Nov:10(11):641-661. doi: 10.1089/wound.2018.0937. Epub 2020 Sep 25 [PubMed PMID: 32320356]
Level 3 (low-level) evidenceCui R, Yu H, Xu T, Xing X, Cao X, Yan K, Chen J. Deep Learning in Medical Hyperspectral Images: A Review. Sensors (Basel, Switzerland). 2022 Dec 13:22(24):. doi: 10.3390/s22249790. Epub 2022 Dec 13 [PubMed PMID: 36560157]
Kaile K, Godavarty A. Development and Validation of a Smartphone-Based Near-Infrared Optical Imaging Device to Measure Physiological Changes In-Vivo. Micromachines. 2019 Mar 9:10(3):. doi: 10.3390/mi10030180. Epub 2019 Mar 9 [PubMed PMID: 30857323]
Level 1 (high-level) evidenceZhang S, Gnyawali S, Huang J, Ren W, Gordillo G, Sen CK, Xu R. Multimodal imaging of cutaneous wound tissue. Journal of biomedical optics. 2015 Jan:20(1):016016. doi: 10.1117/1.JBO.20.1.016016. Epub [PubMed PMID: 25604545]
Queen D, Harding KG. Importance of imaging to wound care practice. International wound journal. 2023 Feb:20(2):235-237. doi: 10.1111/iwj.14082. Epub [PubMed PMID: 36715140]
Burke-Smith A, Collier J, Jones I. A comparison of non-invasive imaging modalities: Infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns : journal of the International Society for Burn Injuries. 2015 Dec:41(8):1695-1707. doi: 10.1016/j.burns.2015.06.023. Epub 2015 Sep 28 [PubMed PMID: 26421694]
Schilrreff P, Alexiev U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. International journal of molecular sciences. 2022 Apr 28:23(9):. doi: 10.3390/ijms23094928. Epub 2022 Apr 28 [PubMed PMID: 35563319]
Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010 Mar:89(3):219-29. doi: 10.1177/0022034509359125. Epub 2010 Feb 5 [PubMed PMID: 20139336]
Chojnowski M. Infrared thermal imaging in connective tissue diseases. Reumatologia. 2017:55(1):38-43. doi: 10.5114/reum.2017.66686. Epub 2017 Mar 22 [PubMed PMID: 28386141]
Fraiwan L, AlKhodari M, Ninan J, Mustafa B, Saleh A, Ghazal M. Diabetic foot ulcer mobile detection system using smart phone thermal camera: a feasibility study. Biomedical engineering online. 2017 Oct 3:16(1):117. doi: 10.1186/s12938-017-0408-x. Epub 2017 Oct 3 [PubMed PMID: 28974212]
Level 2 (mid-level) evidenceFrykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Advances in wound care. 2015 Sep 1:4(9):560-582 [PubMed PMID: 26339534]
Level 3 (low-level) evidenceOttolino-Perry K, Chamma E, Blackmore KM, Lindvere-Teene L, Starr D, Tapang K, Rosen CF, Pitcher B, Panzarella T, Linden R, DaCosta RS. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. International wound journal. 2017 Oct:14(5):833-841. doi: 10.1111/iwj.12717. Epub 2017 Feb 28 [PubMed PMID: 28244218]
Yang H, Kim J, Nam W, Kim HJ, Cha IH, Kim D. Handheld Near-Infrared Fluorescence Imaging Device Using Modified Action Cameras for Peri-Operative Guidance of Microvascular Flap Surgery. Journal of clinical medicine. 2021 Jan 21:10(3):. doi: 10.3390/jcm10030410. Epub 2021 Jan 21 [PubMed PMID: 33494469]