Introduction
Dermatopathology is essential in diagnosing and managing skin tumors, providing detailed histologic insights that inform clinical decisions. Skin tumors comprise a diverse range of neoplasms, requiring a strong understanding of their histopathologic characteristics for accurate classification and treatment. The World Health Organization (WHO) categorizes skin tumors into 7 groups, highlighting their complexity and the necessity of specialized diagnostic methods.
Besides conventional hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC) and molecular testing are critical in evaluating cutaneous neoplasms. H&E staining reveals key morphological features, while IHC and molecular techniques enhance diagnostic precision. IHC uses specific antibodies to detect target antigens in tissue sections, aiding in tumor origin identification, distinguishing benign from malignant lesions, and classifying cutaneous neoplasms. For instance, IHC markers such as S-100, human melanoma black 45 (HMB-45), and melan-A (also known as melanoma antigen recognized by T cells 1 or MART-1) are essential for diagnosing melanocytic tumors, whereas cytokeratins (CKs) and p63 help identify epithelial tumors.
Molecular techniques, including polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS), detect genetic alterations and mutations in tumors. These methods are invaluable for identifying specific genetic markers linked to various skin cancers, such as BRAF mutations in melanoma and TP53 mutations in squamous cell carcinoma (SCC). Molecular testing also aids in prognosis assessment, therapeutic target identification, and minimal residual disease monitoring.
Together, IHC and molecular testing enhance conventional H&E staining by offering a more comprehensive diagnostic approach, improving the accuracy of cutaneous neoplasm diagnosis, and guiding personalized treatment strategies. Additionally, collaboration between clinicians and dermatopathologists, as highlighted by Liersch et al, is essential for optimizing diagnostic precision and therapeutic outcomes.[1]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Accurate diagnosis and management of cutaneous tumors require a multifaceted approach that includes selecting the appropriate biopsy technique and applying IHC staining for precise classification. Biopsy techniques must be carefully chosen based on tumor type, anatomical location, and suspected malignancy to ensure adequate tissue sampling while minimizing diagnostic errors. Improper biopsy methods, inadequate sampling, and processing artifacts can compromise histopathological interpretation, leading to misdiagnosis or delayed treatment. Additionally, IHC plays a crucial role in distinguishing between benign and malignant lesions, subclassifying tumors, and identifying metastatic involvement.
Biopsy Methods and Tumor Sampling
Effective dermatopathologic evaluation begins with obtaining a high-quality biopsy. The choice of biopsy method depends on factors such as tumor type, depth, and suspected malignancy, with proper technique minimizing diagnostic errors and ensuring adequate tissue sampling.
Biopsy
To obtain a representative biopsy that includes the relevant anatomical structures, clinicians must understand the tumor’s potential biological behavior and growth pattern. Maximizing diagnostic yield requires differentiating between nonmelanocytic and melanocytic tumors and selecting the appropriate biopsy method, such as punch or excisional biopsy. Histopathological evaluation can be significantly affected by crushing artifacts and electrocaustic changes.
Biopsies of potentially neoplastic lesions may aim to excise the entire lesion for both diagnosis and treatment or serve as partial biopsies solely for diagnostic purposes. The approach depends on whether the tumor is nonmelanocytic or melanocytic. Many melanocytic lesions exhibit heterogeneous histologic features, with a melanocytic nevus component present in 20% to 60% of melanomas.[2] Partial biopsies risk missing evolving melanoma areas not captured in the sample, while definitive treatment typically follows once the lesion’s nature is determined.[3]
The simplest incisional biopsy technique is the shave biopsy, where a scalpel or hand-held blade removes a superficial skin sample of the desired thickness. This method is best suited for superficial lesions or diagnosing nonmelanoma skin cancers (NMSCs). However, overly superficial biopsies may fail to capture deeper aggressive histologic changes. Curettage biopsy is commonly used for both diagnosis and treatment, particularly when determining whether a lesion is benign or malignant, such as distinguishing seborrheic keratosis from SCC.
Punch biopsy is another incisional technique that uses a cylindrical blade to obtain a full-thickness skin specimen. Offering greater depth, this approach is particularly useful for diagnosing inflammatory dermatoses and conditions affecting the deeper dermis and subcutaneous tissue. Punch biopsy can also sample larger pigmented lesions. The punch size, typically ranging from 2 to 6 mm, is selected to ensure adequate tissue for histologic evaluation while minimizing patient disfigurement. However, partial sampling, especially with punch biopsies, increases the risk of melanoma misdiagnosis.
Wedge biopsy, another incisional technique, involves taking a narrow elliptical section from a larger skin neoplasm, oriented radially to capture a cross-section from the lesion's center extending into normal-appearing skin. When performed with sufficient depth, this method is useful for diagnosing keratoacanthoma and suspected melanoma when excisional biopsy is impractical.[4]
For accurate sample identification, each vial should contain only 1 specimen. The histopathology request form must include precise patient information, the suspected clinical diagnosis, and the biopsy site.
Excision
Excisional biopsy typically involves using a scalpel to remove the full thickness of the skin, often including some subcutaneous tissue. This technique is preferred for evaluating small lesions or suspected melanoma, provided it is feasible. A biopsy of adequate depth is essential when melanoma is suspected, as tumor thickness determines staging and treatment. Inaccurate staging may occur if the base is transected.[4]
To minimize the need for reexcision, surgical margins should be at least 1 mm, as microscopic control requires a minimum of tumor-free tissue. Margins also depend on tumor characteristics. For example, Campoli et al. reported that a 4-mm margin is necessary to achieve a 95% tumor clearance rate in SCC.[5] However, wide local excision (WLE) is recommended for melanoma lesions larger than 1 mm or Merkel cell carcinoma (MCC), with margins of 2.5 to 3 cm.[6][7]
Micrographic control of excisional margins requires 1 or 2 suture markings. For elliptical excisions intended to remove a lesion entirely, margin assessment is expected. To ensure precise identification of excisional margins, 2 suture markings are recommended: 1 caudally to the biopsy (or at 12 o’clock) and 1 laterally or medially, following anatomical orientation.
If the ellipse is oriented by a suture or other means, different-colored inks should be applied to the 2 long margins for specific margin identification. Sections should be taken at 2- to 3-mm intervals to allow an accurate margin assessment.
Mohs surgery
Mohs micrographic surgery (MMS) involves the precise removal of a visible tumor along with a thin layer of surrounding tissue. The excised tissue is sectioned, mapped, and processed for immediate on-site microscopic examination. If cancer cells are identified in any area, an additional layer is removed precisely from that location. This process is repeated until no cancer cells remain, minimizing the loss of healthy tissue.
Indications for MMS depend on tumor characteristics, anatomic location, and patient-specific factors, categorizing tumors as low- or high-risk. This procedure should be performed in cases of high-risk basal cell carcinoma (BCC), high-risk SCC, and rare NMSCs, such as dermatofibrosarcoma protuberans (DFSP) and mucinous carcinoma. Melanoma in situ (MIS) in high-risk areas is also an indication. Invasive melanomas are increasingly treated with MMS, demonstrating cure rates comparable to or exceeding those of wide local excision.
MMS enables comprehensive histopathological analysis of both peripheral and deep surgical margins while minimizing tissue removal. Certain skin cancers have small extensions or “roots” that may be missed when tumors are serially cross-sectioned in a “bread-loaf” fashion, a common practice in traditional excision specimens.[8]
With its microscopic margin control, MMS has become the preferred treatment for extensively invasive, infiltrative, or recurrent tumors. This procedure maximizes the preservation of healthy tissue while ensuring clear margins, significantly reducing local recurrence rates.
Grossing
For small specimens, such as tissue obtained by shave or punch biopsy measuring less than 10 mm, the entire tissue must be submitted for examination. Optimal grossing procedures for skin excision samples are essential to ensure accurate diagnosis and adequate margin evaluation. The process begins with proper specimen orientation using anatomical landmarks, followed by inking the margins to differentiate them from the rest of the tissue. Different ink colors may be applied to specific margins, such as superior and inferior, to aid in microscopic examination.
After orientation and inking, the specimen should be immediately fixed in formalin to preserve tissue architecture and prevent autolysis. Adequate fixation, typically 24 to 48 hours for small-to-medium specimens, is crucial for optimal tissue preservation. The specimen should then be serially sectioned at 2- to 4-mm intervals in a “bread-loaf” pattern.
Critical areas, such as ulcerated regions and areas of maximum thickness, particularly in SCC or BCC cases, must be sampled meticulously. Special attention should be given to the specimen’s edges to ensure the surgical margins are included in the sections. Documentation of the orientation, inking, and sectioning process should be clear and thorough, including photographs taken before and after inking and sectioning for reference.
If the lesion is not grossly visible, the entire specimen should be submitted for histological examination. In cases of suspected malignancy or frequently recurrent benign tumors, thorough margin evaluation is essential to confirm complete excision. Detailed clinical information, including the lesion’s location, size, and clinical suspicion, should be provided to the pathologists. Additionally, any areas of concern requiring special attention during histological examination should be highlighted.[9]
Immunohistochemical Applications in Dermatopathology
IHC is essential in dermatopathology, providing critical diagnostic and prognostic information for various cutaneous neoplasms. By utilizing specific markers, IHC facilitates the differentiation and classification of skin tumors, including melanocytic, hematolymphoid, epithelial, adnexal, mesenchymal (soft tissue), and metastatic tumors.
Melanocytic tumors
S100 is the most sensitive melanocytic marker, though it lacks specificity. More specific markers include MART-1, microphthalmia-associated transcription factor (MITF), and HMB-45. MART-1 and MITF help identify melanoma cells, while HMB-45 aids in differentiating benign nevi from malignant melanoma. Benign lesions typically show decreased expression with lesion depth, whereas melanoma often exhibits consistent staining throughout the lesion.[10]
Sex-determining region Y-related high-mobility group box 10 (SOX10) is a highly sensitive marker for both benign and malignant melanocytic lesions, including nearly all primary melanomas. However, its staining may be less intense in metastatic melanomas. SOX10 is particularly useful for distinguishing desmoplastic melanoma from histologically similar lesions, such as fibrohistiocytic and mesenchymal neoplasms. However, this marker should not be used alone for diagnosing metastatic lesions due to its expression in some breast and salivary gland carcinomas.[11][12]
Hematolymphoid tumors
The basic panel for hematolymphoid tumors includes CD2, CD3, CD5, and CD7 as pan-T-cell markers, with CD4 and CD8 used to assess T-cell subset differentiation. CD20 and CD79a serve as pan-B-cell markers, while plasma cells typically express CD38, CD138, and multiple myeloma oncogene 1 (MUM1). Markers such as CD30 and anaplastic lymphoma kinase (ALK) aid in identifying specific lymphomas, such as anaplastic large cell lymphoma. Ki-67, a proliferation marker, provides insight into the aggressiveness of hematolymphoid malignancies. Additionally, CD117 and tryptase are used to detect neoplastic mast cells in cutaneous mastocytosis and other mast cell neoplasms.[13]
Epithelial tumors
Keratinocytic tumors, including BCC and SCC, are among the most common skin malignancies. BCC typically expresses markers such as Ber-EP4 and epithelial membrane antigen (EMA), whereas SCC stains positively for high-molecular-weight cytokeratins (HMWCK) like CK5/6. CK7 and Cam5.2 aid in differentiating extramammary Paget disease (EMPD) from SCC and in excluding pagetoid radial growth phase melanoma.
The biomarker p63 is strongly expressed in squamous cell layers and helps distinguish SCC and BCC from other epithelial neoplasms, including EMPD. Ber-EP4 is highly sensitive for EMPD but not for pagetoid or bowenoid SCC or melanoma. Therefore, a diagnostic panel including Ber-EP4, p63, and a melanoma-specific marker, such as MART-1, can effectively differentiate these neoplasms.
Adnexal tumors
Adnexal tumors, which arise from skin appendages such as sweat glands and hair follicles, may be identified using specific markers. Carcinoembryonic antigen (CEA) and epithelial membrane antigen highlight normal eccrine and apocrine sweat glands and are useful in diagnosing microcystic adnexal carcinoma (MAC). Follicular tumors, such as desmoplastic trichoepithelioma (DTE), share some markers with BCC. Ber-EP4 staining is positive in 100% of BCC cases and 57% to 75% of DTE cases. Eccrine poromas typically express CK5/6 and p63, while apocrine tumors may be positive for androgen receptors and gross cystic disease fluid protein 15 (GCDFP-15).
Mesenchymal soft tissue tumors
The IHC markers for mesenchymal tumors vary based on their origin, whether from fat, muscle, nerves, or blood vessels. Smooth muscle tumors, such as leiomyomas and leiomyosarcomas, are identified using SMA and desmin. S100 is essential for detecting nerve sheath tumors like schwannomas and neurofibromas, as it highlights neural axons.
For vascular tumors, including hemangiomas, endothelial markers such as CD31, CD34, ETS-related gene (ERG-1), and von Willebrand factor (vWF) are used. CD34 and factor XIII help differentiate DFSP from dermatofibroma. DFSP demonstrates diffuse CD34 positivity and factor XIII negativity, whereas dermatofibroma typically exhibits a patchy CD34 pattern and factor XIII positivity. Angiosarcomas often express vascular markers such as CD31, CD34, and ERG. Verhoeff–Van Gieson staining, which highlights elastic fibers, is useful for confirming elastofibroma.
Metastatic tumors
Metastatic tumors of the skin can originate from various primary sites, including the breast, lung, colon, and melanoma. Specific markers aid in identifying the primary tumor. For instance, breast cancer metastases commonly express estrogen receptor (ER), progesterone receptor (PR), and HER2. Lung adenocarcinoma metastases may stain positively for thyroid transcription factor 1 (TTF-1) and napsin A. Melanoma metastases typically express melanocytic markers such as S100, HMB-45, and MART-1.
Clinical Significance
Classifying cutaneous neoplasms is essential for guiding diagnosis, prognostication, and treatment strategies. Cutaneous neoplasms are broadly categorized into epidermal, melanocytic, adnexal, hematolymphoid, mesenchymal, and metastatic tumors.
Epidermal Neoplasms
Epidermal tumors encompass a broad spectrum of benign, premalignant, and malignant lesions arising from keratinocytes, making them among the most frequently encountered in dermatopathology. Although much less common, MCC is classified as an epidermal tumor in the WHO Classification of Tumors, 5th edition, due to its origin from skin-derived neuroendocrine cells.
Benign epidermal tumors
Benign epidermal tumors include verrucae, acanthomas, and seborrheic keratoses. Verrucae (warts) are caused by human papillomavirus (HPV) and histologically exhibit hyperkeratosis, papillomatosis, and acanthosis (see Image. Wart). Clear cell acanthomas are well-circumscribed lesions with psoriasiform epidermal hyperplasia, elongated and fused rete ridges, and thinning of the papillary plates while sparing adnexal structures (see Image. Clear Cell Acanthoma). These lesions consist of periodic acid-Schiff (PAS) stain-positive keratinocytes with pale-to-clear cytoplasm. Large cell acanthoma, a variant, features enlarged keratinocytes with nuclei twice the size of adjacent normal keratinocytes. These sharply demarcated lesions display mild acanthosis, epidermal flattening, and overlying basket-weave hyperkeratosis.
Seborrheic keratoses are among the most common benign epidermal tumors, characterized by intraepidermal proliferation of monomorphic basaloid cells with horn cysts and pseudohorn cysts. These well-circumscribed lesions have papillomatous outlines and a flat base (see Image. Seborrheic Keratosis). Differentiating an irritated or inflamed seborrheic keratosis from Bowen disease or well-differentiated SCC can be challenging, particularly in shave biopsy specimens.
Premalignant epidermal tumors
Premalignant lesions include actinic keratoses, arsenical keratoses, and psoralen and ultraviolet A (PUVA) keratoses. All of these conditions have the potential to progress to SCC.
Actinic keratoses, often regarded as SCC in situ, is characterized by dysplasia of the basal epidermal layer, loss of maturation, dyskeratosis, isolated mitotic figures, absence of granular cell layers, and alternating orthokeratosis and hyperkeratosis or parakeratosis. Actinic elastosis is typically present in the superficial dermis (see Image. Actinic Keratosis). Unlike full-thickness squamous dysplasia, actinic keratosis involves only the lower portion of the epidermis.
Arsenical keratoses appear as multiple hyperkeratotic lesions on the hands and feet following chronic arsenic exposure. Affected individuals may also exhibit diffuse or localized hyperpigmentation, raindrop-like pigmentation on the trunk, and yellowish hyperkeratotic papules or verrucous plaques on palmoplantar skin. Histopathologically, these lesions range from acanthosis and hyperkeratosis to squamous dysplasia, often featuring vacuolated dysplastic keratinocytes and atypical mitoses.[14]
PUVA keratoses develop on sun-protected skin after prolonged PUVA therapy. Clinically, the lesions present as multiple warty, hyperkeratotic, and scaly papules with a broad base, while histologically, they are characterized by sharply demarcated acanthosis with overlying hyperkeratosis.[15]
Malignant epidermal tumors
Keratinocytic malignancies, including BCC and SCC, are the most frequently encountered cancers in clinical and pathological practice. The incidence of these conditions surpasses that of all other cancers combined.
BCC is the most common malignancy, accounting for about 80% of all skin cancers.[16] This tumor arises from keratinocytes, particularly in fair-skinned populations, and typically grows slowly over months to years, with rare metastatic potential.[17]
Histopathologically, BCC is diverse, encompassing over 20 recognized patterns, including nodular, superficial, micronodular, infiltrating, and sclerosing or morphoeic types.[18] Despite this variation, all BCC types share key features, including basaloid tumor cells with hyperchromatic nuclei and scant cytoplasm. Apoptotic cells are frequently observed within the nests, while stromal changes often include fibromyxoid alteration, tumor nest retraction from the stroma, calcification, and amyloid deposition. Tumor nests may also be colonized by melanocytes and contain melanin pigment. Additionally, solar elastosis is typically present in the underlying dermis (see Image. Basal Cell Carcinoma).
SCC is more prevalent than BCC in immunosuppressed populations and carries a significant metastatic potential.[19] This neoplasm is the 2nd most common malignancy worldwide, primarily affecting the skin and mucosa of the head and neck.[20] Classic SCC in situ, known as Bowen disease, presents as erythematous, scaling plaques with full-thickness keratinocyte atypia, loss of maturation, mitoses at all levels, and dyskeratotic cells. Approximately 4% of SCC in situ lesions progress to invasive SCC.[21]
Invasive SCCs range from well to poorly differentiated. Well-differentiated forms, such as keratoacanthomas, feature polygonal cells, pleomorphism, and significant keratin production (see Image. Squamous Cell Carcinoma). Histological subtypes include acantholytic SCC, characterized by dyscohesive cells and pseudolumina; clear cell SCC, distinguished by abundant clear cytoplasm and PAS-positive glycogen; and spindle cell SCC, also known as sarcomatoid SCC, which exhibits spindled tumor cells with focal or absent keratinization. While clear cell SCC may show focal androgen receptor positivity, sebaceous carcinoma—typically diffusely positive for androgen receptors and adipophilin—can be excluded.[22]
Sarcomatoid SCC is a rare and aggressive variant with a poor prognosis. This tumor may be distinguished from other spindle cell malignancies by uniform positivity for p63 or CK5/6.[23][24] Conditions such as Paget disease and MIS can mimic SCC in situ. Strong CK7 or carcinoembryonic antigen (CEA) positivity in Paget disease and SOX10 expression in melanoma serve as diagnostic markers.
MCC is a rare but aggressive skin cancer, primarily affecting elderly individuals with sun-damaged skin. This pathology typically presents as a rapidly growing, flesh-colored or violaceous nodule, most often on the head or neck. MCC originates from Merkel cells in the basal layer of the epidermis, which function as part of the skin’s neuroendocrine system.
Histopathologically, MCC appears as a “blue” nodule with either circumscribed or infiltrative growth patterns in the dermis or subcutis. The tumor cells are densely packed in large or small aggregates, with nuclei exhibiting a finely stippled chromatin pattern. Mitotic figures and apoptotic bodies are common, and lymphatic invasion is frequently observed. MCC can be positive or negative for Merkel cell polyomavirus (MCPyV), with virus-negative cases often associated with or potentially originating from SCC.[25]
On IHC studies, MCC tumor cells express both epithelial and neuroendocrine markers. Perinuclear dot-like staining for CK20 or neurofilament is a reliable diagnostic feature, while TTF-1 staining is almost always negative (see Image. Merkel Cell Carcinoma).
Melanocytic Neoplasms
Melanocytic tumors are classified into nevi, melanocytomas, and melanomas. Nevi are benign lesions characterized by a single mutation without additional pathogenic alterations. Some lesions previously categorized as nevi are now referred to as "melanocytomas," indicating neoplasms with multiple mutations that suggest progression toward malignancy. These lesions often exhibit atypical histological features or clinical behavior, with mutations typically occurring in specific pathways, such as WNT and BAP1.
Primary cutaneous melanomas have traditionally been classified based on histopathological and clinical features. However, advances in genetic research have identified biologically distinct melanoma subtypes with unique characteristics. Integrating genetic data with clinical and histopathological findings has refined melanoma classification, revealing variations in genetic profiles, clinical presentation, age of onset, anatomic site, and cell of origin.
Benign melanocytic neoplasms
Benign melanocytic lesions include junctional, compound, and dermal nevi, which are localized clonal proliferations of nevoid melanocytes. These nevi are classified based on their location within the skin. Simple lentigo and lentiginous melanocytic nevi are distinct entities. Simple lentigo (lentigo simplex) is characterized by hyperpigmented keratinocytes with a variable number of intraepidermal melanocytes dispersed along the epidermal basal layer without forming nests. In contrast, a lentiginous melanocytic nevus exhibits a lentiginous proliferation of melanocytes along with nests of 3 or more melanocytes within the basal epidermis. Lentiginous compound nevi contain both intraepidermal lentiginous melanocytes and nests, as well as dermal melanocytic components within the papillary dermis.
A dysplastic nevus is a benign melanocytic lesion that appears clinically atypical and exhibits histological features of architectural disorder and cytologic atypia. A halo nevus is defined by circumferential depigmentation, often accompanied by a brisk lymphocytic infiltrate histologically. A combined nevus is a histopathologically benign melanocytic neoplasm composed of 2 or more phenotypically distinct tumor cell populations. However, this classification excludes molecularly defined representations of stepwise tumor progression, such as WNT-activated deep penetrating/plexiform melanocytoma (nevus), combined BAP1-inactivated melanocytoma, and pigmented epithelioid melanocytoma (PEM), as well as collision tumors.
Melanocytomas
WNT-activated deep penetrating/plexiform melanocytomas are rare melanocytic lesions that can be challenging to distinguish from malignant melanoma due to their atypical histological features. These lesions typically present as solitary, symmetrical, pigmented papules or nodules and are more common in young individuals. Histologically, the tumors appear as well-demarcated, wedge-shaped lesions composed of loosely arranged nests of slightly enlarged epithelioid to spindle-shaped pigmented melanocytes, interspersed with melanophages, extending into the reticular dermis and subcutis.[26]
Key distinguishing features of WNT-activated deep penetrating/plexiform melanocytomas include infrequent mitotic activity and minimal pagetoid spread. Additionally, these lesions frequently harbor CTNNB1 gene mutations, resulting in continuous activation of the β-catenin signaling pathway. This molecular characteristic aids in differentiating them from other nevi and melanomas, which typically lack these mutations.[27]
PEM is a rare melanocytic neoplasm composed of heavily pigmented epithelioid and spindle cells. Although occasional mitotic figures and necrosis may be present, these features are generally less pronounced than in more aggressive melanomas. Histologically, PEMs often display a biphasic pattern, with tumor cells predominantly confined to the dermis and frequently extending into the subcutis. Approximately 2/3 of PEMs exhibit PRKAR1A gene loss. IHC staining shows expression of routine melanocytic markers, while loss of PRKAR1A expression can confirm PRKAR1A inactivation in up to 80% of cases.[28]
BAP1-inactivated melanocytomas are characterized by epithelioid cell morphology and genetic inactivation of the BAP1 gene. Clinically, the lesions present as flesh-colored papules or nodules and are primarily intradermal melanocytic proliferations, sometimes with junctional involvement.
The hallmark cells of BAP1-inactivated melanocytomas are large epithelioid melanocytes with well-defined cytoplasmic borders, abundant eosinophilic cytoplasm, and vesicular nuclei with prominent nucleoli. These melanocytes, typically unpigmented, reside within the dermis and exhibit varying degrees of nuclear and nucleolar size variation, including nuclear pleomorphism with dispersed chromatin patterns.[29] Mitotic activity is low, and tumor-infiltrating lymphocytes are often observed. Loss of nuclear BAP1 expression, as detected by IHC, is diagnostically equivalent to biallelic BAP1 inactivation on molecular testing and can confirm the diagnosis.
Malignant melanocytic neoplasms
"Cumulative sun damage" (CSD) refers to the long-term effects of UV radiation exposure and serves as a basis for classifying UV-related melanomas. Lesions include low-CSD, high-CSD, and desmoplastic melanoma, whereas melanomas associated with congenital or blue nevi, along with Spitz melanoma, are not linked to UV exposure. Melanomas on sun-exposed skin are most prevalent in individuals with fair skin and poor tanning ability.
Low-CSD melanomas, including superficial spreading melanoma and low-CSD nodular melanoma, typically arise on intermittently sun-exposed areas such as the back and proximal extremities, often in individuals younger than 55. These melanomas are linked to UV radiation from sunlight or tanning beds and frequently develop from common or dysplastic nevi, progressing through a rapid transition or inverse progression cascade. Histopathologically, the lesions are characterized by nested proliferation and pagetoid scatter of large neoplastic melanocytes with dusty cytoplasmic melanin pigmentation. Genomic alterations predominantly include BRAF p.V600E mutations, along with CDKN2A inactivation and TERT promoter mutations.
High-CSD melanomas, such as lentigo maligna melanoma and high-CSD nodular melanoma, occur on chronically sun-exposed skin, including the face and distal extremities, and are more common in individuals older than 55. These melanomas often arise from MIS and are associated with extensive solar elastosis. Histologically, neoplastic melanocytes display a lentiginous growth pattern, appearing smaller, with minimal cytoplasm and reduced pigment (see Image. Lentigo Maligna with Invasive Melanoma). Patients frequently present with concurrent sun-induced lesions such as actinic keratoses and SCC. Genomic alterations include BRAF p.V600K and other non-p.V600E mutations, biallelic NF1 inactivation, as well as NRAS and KIT mutations.
Desmoplastic melanoma is a distinct variant that manifests as an intradermal, sarcomatoid spindle cell proliferation, commonly developing on severely sun-damaged skin. This neoplasm has a high mutation burden with a strong UV radiation signature and frequently harbors NF1 inactivation and other MAPK pathway-activating mutations. While it predominantly occurs in areas with severe elastosis, desmoplastic melanoma can also arise in acral or mucosal sites, sharing genomic characteristics with melanomas from those locations.[30] On IHC studies, the tumor is typically positive for S100 protein, SOX10, and nerve growth factor receptor (NGFR) but negative for HMB45 and melan-A. Additionally, PRAME expression has been observed in 35% of cases.[31]
Melanomas that arise at sun-shielded sites or without a known UV radiation association include biologically distinct subtypes with unique clinical, histopathological, and molecular features. These tumors often lack the classic UV-induced mutational signatures seen in cutaneous melanomas and may be influenced by alternative pathogenic mechanisms, such as genetic fusions or mechanical trauma.
Spitz melanoma, also known as malignant Spitz tumor, represents the malignant end of a spectrum that includes benign Spitz nevi and intermediate Spitz melanocytomas, also called "atypical Spitz tumors" (ASTs). Spitz melanoma often presents as a growing or changing amelanotic or pigmented plaque, papule, or nodule. These tumors are generally larger than Spitz nevi or ASTs, with most exceeding 6 mm and often surpassing 10 mm.[32]
Histologically, the lesions consist of nests of large spindle or epithelioid melanocytes with abundant eosinophilic cytoplasm extending from the epidermis into the reticular dermis in a wedge-shaped configuration. While this pattern resembles Spitz nevi, Spitz melanomas exhibit greater atypia and typically lack solar elastosis or UV-related mutational signatures. Common genetic alterations include HRAS mutations and kinase fusions such as MAP3K8.[33]
The term "spitzoid" describes tumors with Spitz-like morphology but without characteristic Spitz-associated genetic fusions. In cases with highly atypical features, identifying chromosomal copy number aberrations or mutations in BRAF, NRAS, NF1, or the TERT promoter can support a diagnosis of melanoma over Spitz melanocytoma.[34] On IHC tests, Spitz melanomas may display deep, rather than stratified, HMB-45 staining and patchy rather than diffuse melan-A expression, while p16 expression is often lost, and the Ki-67 proliferation index is frequently above 20%.[35]
Acral melanoma arises on glabrous skin, including the palms, soles, and nail apparatus, and is not associated with sun exposure. Chronic mechanical trauma has been suggested as a contributing factor. The most common subtype is acral lentiginous melanoma (ALM), followed by nodular melanoma and low-CSD superficial spreading melanoma (SSM).
Histologically, in situ ALM is characterized by an increased number of irregularly distributed basal melanocytes with hyperchromatic nuclei and prominent dendrites, progressing to confluence and eventual dermal invasion (see Image. In Situ Acral Lentiginous Melanoma). Acral melanomas generally have a low tumor mutation burden but frequently harbor oncogenic mutations in BRAF, NRAS, KIT, and NF1, along with recurrent amplifications of CCND1, TERT, CDK4, GAB2, and MDM2.[36] On IHC testing, SOX10 and MITF help assess intraepidermal melanocyte density and distribution in early lesions, while SOX10 and S100 are preferred for delineating melanocytes in desmoplastic acral melanoma.
Adnexal Neoplasms
Cutaneous adnexal tumors are a diverse and complex group of neoplasms that differentiate into 1 or more types of skin appendages, including sweat gland, follicular, sebaceous, or mixed differentiation. Sweat gland tumors make up the largest subgroup, followed by hair follicle and sebaceous tumors. These neoplasms may present as solitary lesions or multiple papules, nodules, and plaques, with syndromic cases typically exhibiting multiple lesions. Diagnosis can be challenging due to their tendency to form cysts and the existence of pigmented variants.
Sweat gland tumors
Sweat gland tumors are classified based on their apocrine or eccrine differentiation and exhibit a broad spectrum of behaviors, ranging from indolent to highly metastatic. These lesions typically present as solitary or multiple papules, nodules, or plaques and are most commonly found in the head and neck region due to the high density of sweat glands in these areas. Diagnosis relies primarily on microscopic examination, as clinical presentation can be highly variable. While irregular borders, color variation, induration, and ulceration often suggest malignancy, benign tumors can also exhibit these characteristics.
Classifying sweat gland tumors is challenging due to overlapping characteristics, requiring distinct histological findings for accurate differentiation. These tumors are broadly categorized into low-grade and high-grade types. Low-grade tumors exhibit locally destructive growth with a risk of recurrence but have low metastatic potential, whereas high-grade tumors pose a significant risk of metastasis and disease-related mortality.
Among benign sweat gland tumors, eccrine poromas are characterized histologically by small, round, basophilic poroid cells interspersed with eosinophilic squamoid cuticular cells. Syringomas typically present as multiple small, flesh-colored to yellowish papules on the lower eyelids and cheeks, featuring ducts embedded in a fibrous stroma (see Image. Syringoma). Hidradenomas, such as nodular hidradenoma, usually appear as solitary, firm nodules with both cystic and solid areas containing clear and dark cells (see Image. Hidradenoma). These benign tumors are generally asymptomatic but may be excised to improve cosmesis or reduce discomfort.
Porocarcinoma, the most common malignant sweat gland tumor, originates from the eccrine sweat glands. Histopathologically, this neoplasm is characterized by atypical poroid cells and ductal structures and often presents as a rapidly growing nodule or ulcer with potential for regional and distant metastasis (see Image. Porocarcinoma). Other notable malignant sweat gland tumors include adenoid cystic carcinoma, mucinous carcinoma, hidradenocarcinoma, and apocrine carcinoma.
Adenoid cystic carcinoma consists of basaloid cells forming cribriform and tubular structures, with a high risk of local recurrence and perineural invasion (see Image. Adenoid Cystic Carcinoma). Mucinous carcinoma is distinguished by clusters of tumor cells floating in pools of mucin and is most commonly found on the head and neck (see Image. Primary Cutaneous Mucinous Carcinoma). Hidradenocarcinoma is an aggressive tumor characterized by pleomorphic cells and high mitotic activity. Apocrine carcinoma arises in areas rich in apocrine glands, such as the axilla and anogenital region, and features large cells with abundant eosinophilic cytoplasm.
Follicular tumors
Follicular tumors arise from the hair follicle, which consists of a matrix, inner and outer root sheaths of clear cells, and connections to sebaceous and apocrine glands. The follicle’s distinct mesenchyme includes the follicular papilla and perifollicular hair sheath. A key challenge in dermatopathology is distinguishing benign follicular tumors from BCC, with ongoing debate regarding whether BCC should be classified as an epidermal or follicular tumor.
Trichoblastoma, a benign follicular tumor, presents as a well-circumscribed dermal lesion composed of epithelial cell aggregates and characteristic follicular stroma, lacking the peritumoral clefting seen in BCC (see Image. Trichoblastoma). BCC itself exhibits various histological subtypes, including nodular, superficial, infiltrative, and special variants such as the Pinkus tumor. Nodular basaloid proliferations with peripheral palisading and peritumoral clefting define the lesion.
Other benign follicular tumors, such as trichofolliculoma and sebaceous gland tumors, also require differentiation from BCC (see Image. Trichofolliculoma). IHC studies, such as anti-Ber-Ep4 staining, aid in this distinction by highlighting follicular structures present in both BCC and trichoblastoma. Differentiating sclerosing BCC from MAC can be particularly challenging, but anti-Ber-Ep4 staining helps, as MAC exhibits minimal to no staining.
Sebaceous gland tumors
Sebaceous gland tumors are diverse and can be classified into 3 main categories: sebaceoma, low-grade sebaceous carcinoma (LGSC) or borderline sebaceous neoplasm (BSN), and sebaceous carcinoma. These tumors commonly arise on the palpebral and extraocular skin and may be associated with Muir-Torre syndrome.[37]
Sebaceoma is a benign neoplasm with a well-defined architecture and no cellular atypia. Low-grade sebaceous carcinoma or borderline sebaceous neoplasm represents an intermediate malignancy characterized by a well-organized structure but with nuclear atypia. Sebaceous carcinoma, the malignant counterpart, exhibits invasive growth and marked nuclear atypia.
Histopathologically, sebaceoma presents as well-circumscribed lobules in the dermis with mature sebaceous differentiation, lacking pleomorphism or mitotic activity. In contrast, sebaceous carcinoma features pleomorphic cells, mitotic figures, and areas of necrosis (see Image. Sebaceous Carcinoma). IHC markers such as adipophilin, known for its high sensitivity and specificity, aid in diagnosing sebaceous carcinoma.[38]
Mixed-differentiation tumors
Mixed differentiation tumors are a subset of cutaneous adnexal tumors characterized by features of multiple skin appendages, including apocrine, eccrine, follicular, and sometimes sebaceous differentiation. The complex histological composition of these neoplasms makes them relatively rare and diagnostically challenging, as they exhibit overlapping morphological features that can complicate classification.
Neoplasms of the Nail Unit
Nail neoplasms include both benign and malignant growths arising from the nail or periungual tissues, often causing nail deformities and growth disturbances. Malignant tumors tend to infiltrate and disrupt surrounding structures, whereas benign tumors generally preserve adjacent tissue integrity. The unique anatomy of the nail unit, which comprises the nail plate, nail bed, and matrix, makes predicting and evaluating nail tumors particularly challenging.
These tumors are broadly classified as benign or malignant. Common benign tumors, such as onychomatricoma, glomus tumor, and pyogenic granuloma, typically maintain the structural integrity of surrounding tissues but may occasionally involve the underlying bone. Malignant tumors, including melanoma and SCC, can mimic benign conditions such as benign melanonychia or onychomycosis, creating significant diagnostic challenges. Early detection of melanoma is critical due to its aggressive nature, while SCC may also resemble benign lesions, increasing the risk of misdiagnosis.[39]
Hematolymphoid Neoplasms
The skin may be affected by hematological malignancies either as a secondary manifestation of systemic disease or as the primary site of involvement. When evaluating a skin lesion with hematopoietic cell infiltration, both architectural patterns and cytologic morphology must be assessed. An initial IHC panel is crucial for characterizing the infiltrating cells and refining the differential diagnosis.
Myeloid neoplasms
Cutaneous mastocytosis is a form of mastocytosis that primarily affects the skin without histologically apparent involvement of other organs and is most commonly seen in pediatric patients. In contrast, cutaneous lesions in systemic mastocytosis, often in the setting of indolent systemic mastocytosis, typically present in adulthood.[40] A bone marrow biopsy is generally recommended alongside a skin biopsy in suspected cases of mastocytosis to evaluate potential systemic involvement.
Cutaneous mastocytosis includes maculopapular cutaneous mastocytosis (MPCM), diffuse cutaneous mastocytosis (DCM), and mastocytoma. Skin biopsies reveal varying numbers of dermal mast cells, which may be detected using Giemsa, toluidine blue, mast cell tryptase, or KIT (CD117) staining (see Image. Mastocytosis). In maculopapular cutaneous mastocytosis, mast cells are loosely scattered, often aggregating around blood vessels and adnexal structures in the upper dermis. Diffuse cutaneous mastocytosis is characterized by a marked increase in mast cells, whereas mastocytomas display nodular mast cell infiltrates. On H&E staining, mast cells appear as unremarkable histiocytoid cells, while Giemsa staining may highlight spindle-shaped-to-round mast cells with hypergranulated cytoplasm, indicating a well-differentiated morphology.[41]
Myeloid sarcoma is a rare condition characterized by 1 or more tumor masses composed of immature myeloid cells at an extramedullary site. The skin is the most common site of presentation (28.2%), followed by the lymph nodes.[42]
Cutaneous myeloid sarcoma disrupts normal skin architecture, with immature myeloid cells diffusely infiltrating the dermis or subcutaneous tissue while sparing the epidermis (grenz zone). These neoplastic cells are typically medium to large with fine chromatin and prominent nucleoli.
The IHC profile of myeloid sarcoma varies depending on the degree of differentiation (monocytic versus granulocytic) and maturation (blasts versus maturing myeloid cells) within the myeloid lineage. Neoplastic cells generally express CD43, CD68, and myeloperoxidase (~90%). Myeloid neoplasms with monocytic differentiation often lack CD34 or CD117, whereas those with granulocytic differentiation typically express these markers. Additionally, approximately 40% of myeloid sarcomas exhibit abnormal cytoplasmic nucleophosmin 1 (NPM1) expression, which is associated with monocytic differentiation.[43]
Histiocytic neoplasms
Histiocytoses are rare disorders characterized by the accumulation of macrophage-, dendritic cell-, or monocyte-derived cells in various tissues. These conditions exhibit a broad spectrum of clinical presentations, ranging from localized skin lesions to multisystem involvement. C.M. Luder et al have classified the cutaneous manifestations of histiocytoses into 3 groups.
The Langerhans group includes Langerhans cell histiocytosis, which presents with eczematous, psoriasiform, or acneiform lesions. Histologically, Langerhans cell histiocytosis is characterized by S100- and CD1a-positive cells, often harboring BRAF p.V600E mutations, indicating a neoplastic origin from bone marrow-derived monocytes. This condition primarily affects children but can also occur in adults.
The cutaneous and mucocutaneous histiocytosis group includes juvenile xanthogranuloma, necrobiotic xanthogranuloma, and multicentric reticulohistiocytosis. These conditions present with diverse skin findings, from small nodular lesions in juvenile xanthogranuloma to indurated plaques in necrobiotic xanthogranuloma. Juvenile xanthogranuloma is typically benign and primarily affects children, whereas necrobiotic xanthogranuloma is associated with monoclonal gammopathies, and multicentric reticulohistiocytosis affects adults, often leading to destructive arthritis. These histiocytoses typically stain positively for CD68 and negatively for S100, CD1a, and Langerin.
The Rosai-Dorfman disease (RDD) and related histiocytoses (R group) include RDD, which presents with massive lymphadenopathy and cutaneous lesions. Histologically, the lesions are positive for S100 and CD68 but negative for CD1a. RDD primarily affects children and young adults, while the cutaneous form shows a predilection for older women.[44]
Cutaneous B-cell neoplasms
Primary cutaneous B-cell lymphomas (PCBCL) account for approximately 20% to 25% of all primary cutaneous lymphomas (PCLs).[45] According to the most recent WHO Classification of Tumors, 5th edition, the 3 most common types are primary cutaneous marginal zone lymphoma (PCMZL), primary cutaneous follicle center lymphoma (PCFCL), and primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL-LT). While PCMZL and PCFCL typically exhibit indolent behavior, PCDLBCL-LT is more aggressive.[46] In addition to these PCBCLs, cutaneous B-cell lymphoproliferative disorders may also arise from secondary involvement by other B-cell lymphomas, including mantle cell lymphoma (MCL), Burkitt lymphoma, and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
The initial approach to significant lymphatic infiltration in the skin generally involves immunophenotyping. A typical antibody panel includes CD20, CD3, and CD30, addressing the most prevalent cutaneous lymphoproliferations. Prominent dermal lymphatic infiltration rich in CD20-positive B cells is typically absent in normal skin except in reactive B-cell follicles associated with cutaneous pseudolymphomas. Evaluating intradermal B-cell infiltration requires careful assessment of cytology and growth patterns to refine the differential diagnosis, guiding the selection of an appropriate staining panel.[47]
Oschlies et al described 3 distinct patterns of PCBCL. The 1st pattern, follicular or nodular dermal infiltration, is the most common and features mixed cellularity, with varying numbers of plasma cells and large cells. This pattern is also the most challenging to assess. The primary differential diagnoses include PCFCL, PCMZL, pseudolymphomas, and B cell-rich primary cutaneous small/medium-sized CD4+ T-cell lymphomas.
A crucial diagnostic indicator is the presence of clonal B-cell receptor (BCR) gene rearrangement without clonal T-cell receptor (TCR) gene rearrangement. Secondary skin manifestations of systemic B-cell lymphomas, such as systemic follicular lymphoma (SFL) and marginal zone lymphoma/lymphoplasmacytic lymphoma (MZL/LPL), as well as nodular or perivascular minor infiltrations by CLL or rare cases of MCL, should also be considered in the differential diagnosis.
The 2nd pattern, diffuse dermal infiltration by small- to medium-sized B cells, is rare but easily identifiable as neoplastic. Reactive B-cell infiltrations with dense dermal diffuse B cells are uncommon, in contrast to reactive dense dermal T-cell infiltrations, such as in Jessner lymphocytic infiltration of the skin. Differential diagnoses include PCBCLs, specifically diffuse variants of PCFCL and PCMZL, as well as secondary manifestations of CLL. Rarer occurrences of systemic follicular lymphoma, MZL/LPL, and MCL should also be considered.
The 3rd pattern, diffuse sheet-like dermal infiltration by large or blastic B cells, is rare and generally straightforward to diagnose due to its clear neoplastic nature. The most common differential diagnosis in this scenario is PCDLBCL-LT. However, other differential diagnoses must still be excluded, including blastic/pleomorphic variants of MCL and large cell/spindle cell-rich variants of PCFCL. While PCDLBCL-LT and blastic MCL are rarely diagnosed in children or younger individuals, precursor B lymphoblastic lymphoma should be considered in these age groups.
Other exceptional differential diagnoses include CD20 coexpressing large-cell T-cell lymphomas and nonhematological neoplasms, such as MCC expressing B-cell markers. Furthermore, the potential association with Epstein-Barr virus (EBV) must be excluded in patients with an underlying immunodeficiency.
Cutaneous T-cell neoplasms
The majority (75%-80%) of all PCLs are cutaneous T-cell lymphomas (CTCLs). Among CTCLs, mycosis fungoides is the most common type, accounting for 60% of CTCLs and nearly 50% of all PCLs. Following mycosis fungoides, primary cutaneous CD30+ lymphoproliferative disorders (LPDs), including lymphomatoid papulosis (LyP) and primary cutaneous anaplastic large cell lymphoma (PC-ALCL), are also prevalent. While both LyP and PC-ALCL feature an infiltrate of medium-to-large atypical CD30+ neoplastic T cells, their clinical presentations and courses differ significantly, representing different ends of the same disease spectrum.[48]
Mycosis fungoides typically progresses through stages of patches, plaques, and tumors, each with distinct histological features. Key histological clues for diagnosing this condition include a range of epidermal changes, from hyperplastic to atrophic, often accompanied by parakeratosis. Epidermotropism of lymphocytes is a hallmark, characterized by lymphocytes appearing as single elements or in aggregates, without consensual spongiosis, and forming Pautrier microabscesses. Additionally, haloed intraepidermal lymphocytes, marked by clearings around the cells, often align along the dermal-epidermal junction. These intraepidermal lymphocytes are typically larger than intradermal lymphocytes and exhibit hyperchromatic, irregular nuclei (see Image. Mycosis Fungoides).[49]
Variants of mycosis fungoides include folliculotropic mycosis fungoides (FMF), pagetoid reticulosis, and granulomatous slack skin (GSS).[50] Classic mycosis fungoides generally demonstrates a CD2-positive, CD3-positive, TCR β-positive, CD5-positive, CD4-positive, CD8-negative, and CD30-positive/negative phenotype, with CD7 often dimly expressed or partially lost, particularly in advanced stages.[51] Most cases also express cutaneous lymphocyte antigen (CLA) and CCR4.[52]
LyP is a skin disorder that typically self-regresses but tends to relapse, characterized by papulonodular lesions that can sometimes become necrotic. Histological features vary significantly depending on the stage of the skin lesion biopsied.[53] Five histologic types of LyP have been identified based on the predominant cell type and tropism: A, B, C, D, and E. Subtypes A, B, and C predominantly express CD4, while subtypes D and E express CD8.[54] Additionally, LyP with DUSP22-IRF4 rearrangement often presents with a double-negative CD4/CD8 phenotype.[55] A characteristic perinuclear, dot-like Golgi staining pattern is observed in the large atypical lymphoid cells in 50% of LyP cases.[56]
PC-ALCL typically presents as a solitary, localized red-brown nodule or papule (>2 cm) or, in some cases, multifocal lesions with occasional ulceration and rare spontaneous regression, which contrasts with LyP. PC-ALCL is characterized by a diffuse dermal infiltrate of medium to large anaplastic, pleomorphic, or immunoblastic cells without epidermotropism, often displaying an angiocentric distribution. A key histological feature of PC-ALCL is the presence of large cells (15-50 µm) with eccentric kidney-shaped nuclei, multiple small basophilic nucleoli, and abundant amphophilic or basophilic cytoplasm with a prominent eosinophilic Golgi region. The neoplastic cells often express cytotoxic proteins, such as granzyme B, T-cell intracellular antigen 1 (TIA-1), and perforin, along with variable loss of CD2, CD3, CD5, and CD7.[57]
Mesenchymal (Soft Tissue) Neoplasms
Mesenchymal tumors typically develop in deep soft tissues and are rarely found in the skin or subcutaneous layers. Cutaneous sarcomas, which are an uncommon type of skin tumor, are much less frequent than carcinomas and benign mesenchymal skin neoplasms.[58] While various deep tissue mesenchymal neoplasms can present superficially, some tumors, such as cellular neurothekeoma, hobnail hemangioma, microvenular hemangioma, and DFSP, are exclusively superficial and do not have deep tissue counterparts.
Despite being the same entity, superficial mesenchymal neoplasms and their deep-seated counterparts can differ in both morphology and disease prognosis. For example, the rare leiomyoma of deep soft tissues typically presents as a well-defined nodular neoplasm, whereas pilar leiomyoma is a poorly defined, infiltrative lesion that may mimic more aggressive mesenchymal tumors. In comparison to deep tissue sarcomas, most cutaneous sarcomas generally have a more favorable prognosis, regardless of their morphological grade of malignancy.[59] Mesenchymal tumors are generally categorized based on their origin into several types. These categories include adipocytic, fibrohistiocytic, vascular, smooth muscle, neural, and tumors of uncertain differentiation.
Common benign adipocytic tumors include lipoma, angiolipoma, and spindle cell lipoma. An atypical lipomatous tumor (ALT) is considered a borderline tumor, while pleomorphic sarcoma represents a malignant tumor. Lipoma is characterized by lobular proliferation of mature adipocytes without atypia. Angiolipomas typically present as multiple small, tender subcutaneous nodules and consist of mature adipocytes interspersed with capillary vessels and fibrin microthrombi.[60]
Spindle cell lipoma presents as a painless nodule composed of varying proportions of mature adipocytes, bland spindle cells, and ropy collagen. ALT consists of mature adipocytes and atypical stromal cells with hyperchromasia. The amplification of MDM2 is a distinguishing feature of ALT, setting it apart from other benign adipose tumors.[61] In contrast, pleomorphic sarcoma is characterized by pleomorphic spindle cell sarcoma, pleomorphic lipoblasts, absence of MDM2 amplification, and a complex karyotype.[62]
Dermatofibroma, also known as fibrous histiocytoma, is a common benign lesion that typically appears as a solitary, dome-shaped nodule with hyperpigmentation. Microscopically, this pathology is found in the reticular dermis and often presents as a round, nonencapsulated, poorly defined lesion. Key histological features include a storiform pattern of spindle cells arranged in a whorled or cartwheel-like pattern, usually surrounded by collagen, with areas of sclerosis. The tumor cells typically have small, oval-to-spindle-shaped nuclei with inconspicuous nucleoli, and mitotic figures are sparse (see Image. Dermatofibroma).[63]
DFSP is an intermediate lesion with a typically indolent course. This condition usually presents as a painless, firm, slow-growing plaque or nodule that may exhibit various discolorations. In its early stage, known as preprotuberans, the lesion has nonspecific features and is often misdiagnosed.[64]
DFSP is characterized by poorly circumscribed tumors that infiltrate the entire dermis, destroying preexisting structures and spreading into the subcutaneous tissue. This infiltration often displays a "honeycomb" pattern in the subcutaneous fat. The primary histologic feature is a dense, uniform array of spindle-shaped cells embedded in collagen, usually arranged in a storiform pattern. Mitotic figures are typically infrequent (see Image. Dermatofibrosarcoma Protuberans).
DFSP is classified into various subtypes based on histological presentation. Most classic DFSP cases exhibit diffuse positive CD34 staining, which serves as a key diagnostic marker.[65][66] Additionally, identifying the COL1A1-PDGFB rearrangement through molecular testing strongly supports a diagnosis of DFSP.[67]
Common benign vascular tumors include angiokeratoma, lymphangioma, and hemangioma, which has various subtypes. Angiokeratoma is marked by the proliferation of dilated thin-walled vessels in the papillary dermis, accompanied by variable epidermal changes. Hemangiomas can usually be easily identified, but distinguishing them from malformations can be challenging. Angiosarcoma must be ruled out if atypia is present, especially in radiation-associated cases, including those following breast cancer treatment.[68]
Cutaneous smooth muscle tumors are rare and primarily benign, including smooth muscle hamartoma, cutaneous leiomyoma, and EBV-associated smooth muscle tumor (EBV-SMT). Atypical intradermal smooth muscle neoplasm is classified as an intermediate neoplasm. Smooth muscle hamartoma is characterized by haphazardly arranged dermal bundles of mature smooth muscle near the pilosebaceous units.[69]
Cutaneous leiomyoma, also known as pilar leiomyoma, originates from the arrector pili smooth muscle in the dermis and presents as a poorly defined nodule composed of well-differentiated smooth muscle bundles. These tumors are typically highlighted by smooth muscle actin (SMA), desmin, and caldesmon staining. EBV-associated smooth muscle tumors occur mainly in immunocompromised individuals and present as well-circumscribed tumors with interlacing fascicles of spindle-shaped myoid cells.[70] Given their similarity to other smooth muscle tumors, EBV-encoded RNA in situ hybridization (EBER-ISH) testing is recommended for immunocompromised patients. Atypical intradermal smooth muscle neoplasms share histological features with cutaneous leiomyoma but show more atypical and mitotic figures.
Benign cutaneous neural tumors include neuroma, Schwannoma, neurofibroma, and granular cell tumor, while malignant peripheral nerve sheath tumor (MPNST) is a malignant neoplasm. Solitary circumscribed neuroma presents as a small dermal nodule, with fascicles of bland spindle cells encapsulated by a fine capsule. This arrangement creates artifactual clefts at the tumor-stromal interface.[71] Neurofibromas are characterized by an infiltrative, hypocellular spindle cell lesion with a myxoid to collagenous stroma. These lesions are positive for S100 in neoplastic cells and display a characteristic stromal "fingerprint" pattern highlighted by CD34 (see Image. Neurofibroma).[72]
Schwannomas typically exhibit classic Antoni A or Antoni B areas. These tumors are composed of spindle-shaped cells arranged in distinctive patterns. Granular cell tumors consist of epithelioid cells with abundant granular eosinophilic cytoplasm. These cells stain diffusely positive for S100 and SOX10, aiding in their identification.
MPNSTs typically arise from a benign nerve sheath tumor and are distinguished by spindle cells with marked cellular atypia. These cells are arranged in fascicles or whorls. Unlike benign neurofibromas, MPNSTs lack a myxoid background and Schwannian differentiation. The lesions exhibit high mitotic activity and show patchy or focal expression of S100 and SOX10.
Tumors of uncertain differentiation
A cutaneous tumor of uncertain differentiation refers to a type of skin tumor whose cellular origin and lineage cannot be definitively determined based on histologic and IHC findings. These tumors are difficult to classify because they do not exhibit clear characteristics typical of specific cell types or tissue origins within the skin.
Benign examples of these tumors include cellular neurothekeoma, epithelioid fibrous histiocytoma, and perivascular epithelioid cell tumor (PEComa). Intermediate tumors include angiomatoid fibrous histiocytoma, NTRK-rearranged spindle cell neoplasm, atypical fibroxanthoma, and superficial CD34+ fibroblastic tumor. Malignant neoplasms within this category include pleomorphic dermal sarcoma, epithelioid sarcoma, dermal clear cell sarcoma, and Ewing sarcoma.
Metastases to the Skin
Cutaneous metastases typically result from hematogenous spread of an internal cancer. These lesions occur in 0.5% to 10.4% of patients with cancer and, in some cases, may be the first indication of the primary tumor before it is identified.[73] The most common sources of cutaneous metastases are breast cancer in women and melanoma in men, followed by lung, colon, head and neck, ovarian, and renal cancers.[74]
Microscopically, cutaneous metastases are generally located in the dermis, sparing the epidermis, and tend to share morphological features with the primary tumor.[75] For example, breast carcinoma metastases often present with solid or nested neoplastic cells that form ductal patterns and test positive for estrogen receptor, progesterone receptor, and mammaglobin.[76]
Distinguishing cutaneous metastatic melanoma from primary dermal melanoma can be challenging. Metastatic melanoma cells are confined to the dermis, lack an overlying precursor lesion, and extend into the deep dermis. These cells often show little pigmentation and test positive for S100 or SOX10, though staining for melan-A and HMB-45 can vary.[77]
Lung adenocarcinoma metastases typically present as moderately differentiated tumors, forming solid nests, sheets, and cords of cells without an epidermal connection. These metastases can be confirmed with positivity for TTF-1 and CK7. On the other hand, small cell carcinoma metastases retain neuroendocrine features, showing positivity for TTF-1 and CAM5.2 but negativity for CK7 and CK20.[78]
Inherited Disorders Associated with Skin Tumors
Inherited disorders that predispose individuals to skin tumors are often caused by genetic mutations impacting tumor suppressor genes, DNA repair mechanisms, and other essential regulatory pathways. These disorders typically result in a higher incidence of various skin tumors, along with systemic manifestations.
Familial melanoma
Familial melanoma is often linked to mutations in the CDKN2A gene, which encodes proteins that regulate the cell cycle and prevent uncontrolled cell proliferation. As a result, patients frequently develop multiple primary melanomas at a younger age compared to sporadic cases, with an increased risk of pancreatic cancer.[79] Histopathologically, melanomas in familial cases exhibit typical features, including asymmetry, irregular borders, multiple colors, and a high mitotic rate.
BAP1 tumor predisposition syndrome
This syndrome results from mutations in the BAP1 gene, which encodes a deubiquitinating enzyme involved in chromatin remodeling and DNA repair. Affected individuals often present with multiple atypical Spitz tumors, uveal melanoma, and increased risks of mesothelioma and renal cell carcinoma.[80] Histopathologically, BAP1-mutated melanocytic tumors demonstrate characteristic spitzoid morphology, with epithelioid and spindle cells, as well as nuclear pleomorphism.
Xeroderma pigmentosum
Xeroderma pigmentosum is caused by mutations in genes responsible for nucleotide excision repair (NER), leading to an inability to repair UV-induced DNA damage. Xeroderma pigmentosum is characterized by extreme sensitivity to UV light, early-onset skin cancers (such as BCC, SCC, and melanoma), freckling, and atrophic skin changes.[81] Histopathologically, skin biopsies from xeroderma pigmentosum patients reveal solar elastosis, actinic keratoses, and a range of skin cancers, often presenting in multiples and with atypical features.
Nevoid basal cell carcinoma syndrome (Gorlin Syndrome)
Gorlin syndrome is caused by mutations in the PTCH1 gene, which is part of the Hedgehog signaling pathway and plays a role in regulating cell growth.[82] Patients with Gorlin syndrome develop multiple BCCs at a young age, as well as jaw cysts and skeletal abnormalities.[83] Histopathologically, BCCs in this syndrome exhibit typical features such as nests of basaloid cells with peripheral palisading and stromal retraction.
Carney complex
Carney complex is caused by mutations in the PRKAR1A gene, which affects protein kinase A signaling and regulates cell proliferation.[84] Patients often present with lentigines, blue nevi, myxomas, and endocrine overactivity, with an increased risk of cardiac myxomas and extracutaneous tumors.[85] Histopathologically, skin lesions like blue nevi display nests of pigmented dendritic melanocytes, while myxomas show myxoid stroma interspersed with spindle cells.
Muir-Torre syndrome
Muir-Torre syndrome is associated with mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6), leading to microsatellite instability.[86] Patients typically develop sebaceous gland tumors, including adenomas, epitheliomas, and carcinomas, as well as keratoacanthomas, and have an increased risk of internal malignancies such as colorectal cancer. Histopathologically, sebaceous tumors exhibit lobules of sebocytes with varying degrees of differentiation, while keratoacanthomas feature well-differentiated squamous epithelium and a central keratin plug.
Brooke-Spiegler and related syndromes
These syndromes involve mutations in the CYLD gene, which encodes a deubiquitinating enzyme that regulates the NF-κB pathway.[87] Affected individuals often develop multiple cylindromas, trichoepitheliomas, and spiradenomas, usually from a young age.[88] Histopathologically, cylindromas are composed of basaloid cells arranged in a jigsaw puzzle pattern, trichoepitheliomas show nests of basaloid cells resembling hair follicles, and spiradenomas contain basaloid and epithelial cells with ductal differentiation.
Other Issues
Molecular testing plays a crucial role in diagnosing ambiguous cutaneous lesions by offering precise and objective genetic information that enhances the accuracy of traditional histopathologic evaluations. For example, noninvasive genomic testing of clinically ambiguous pigmented lesions can help identify melanomas that might otherwise be overlooked in visual assessments. In one study, 1,254 ambiguous lesions were evaluated, leading to the detection of 35 melanomas.[89] The introduction of a 35-gene expression profile (35-GEP) test has significantly advanced the differentiation between benign and malignant pigmented lesions, providing high sensitivity and specificity. This innovation helps refine diagnoses and reduce unnecessary procedures.[90]
Additionally, techniques such as FISH and comparative genomic hybridization (CGH) are used to detect chromosomal aberrations and mutations. These methods contribute to the accurate classification and treatment of melanocytic neoplasms. PCR and DNA sequencing are also essential in diagnosing infectious diseases, genetic disorders, and oncology, as they identify biomarkers and genetic variants crucial for prognosis and treatment decisions.[91] Furthermore, tape stripping offers a noninvasive method to profile genetic mutations in skin lesions, providing an alternative to biopsies for some cases.[92]
Enhancing Healthcare Team Outcomes
Enhancing healthcare team outcomes in the evaluation and management of cutaneous neoplasms requires a coordinated, interprofessional approach. Dermatologists, dermatopathologists, oncologists, and other healthcare professionals must demonstrate advanced clinical skills in biopsy techniques, histopathological evaluation, and the application of immunohistochemical and molecular testing.
Continuous professional development and keeping up with the latest diagnostic technologies are critical for accurate diagnoses and optimal patient care. A strategic, evidence-based approach is essential for developing treatment plans tailored to each patient’s needs, which involves selecting appropriate biopsy methods, accurately interpreting histopathological findings, and using advanced diagnostic tools effectively. These strategies ensure that care is precise and individualized, reducing unnecessary interventions and ultimately improving patient outcomes.
Ethical decision-making is crucial in dermatopathology, especially in complex cases. Providers must ensure informed consent and respect patient autonomy while guiding decisions with the principles of beneficence and nonmaleficence. Clear roles and responsibilities within the interprofessional team are essential for effective collaboration, with each member contributing their expertise to patient care.
Effective communication is a cornerstone of successful patient-centered care. Regular discussions, interprofessional meetings, and comprehensive documentation ensure that all team members stay informed, reducing errors and facilitating timely interventions. Coordinating care across specialties and settings guarantees a seamless patient journey, from diagnosis through treatment and follow-up, which ultimately improves safety and patient satisfaction.
By focusing on skills, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can enhance patient outcomes, ensure safety, and improve team performance in managing cutaneous neoplasms. This collaborative approach leads to better patient care and more effective management of skin tumors.
Media
(Click Image to Enlarge)

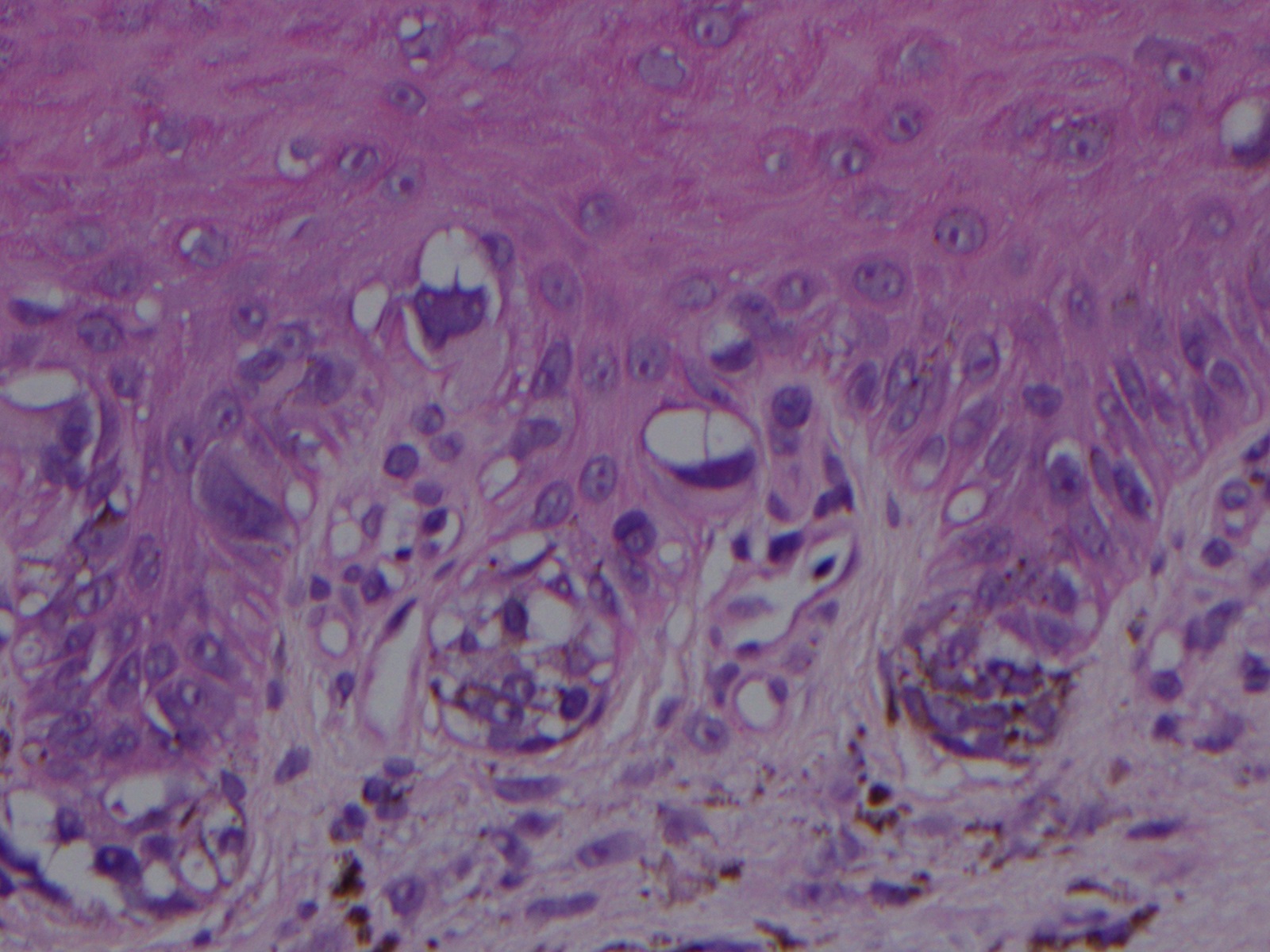
Wart. The main image (magnified 40x) shows hyperkeratosis, papillomatosis, and acanthosis in skin tissue stained with hematoxylin and eosin. Hyperkeratosis is stratum corneum thickening due to excess keratin. Papillomatosis means the appearance of surface undulations from elongated dermal papillae. Acanthosis arises from epidermal thickening due to an expanded spinous layer. Koilocytes are cells that exhibit perinuclear clearing (halo appearance), nuclear enlargement, hyperchromasia, and irregular nuclear contours (inset, magnified 400x).
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)
(Click Image to Enlarge)

Seborrheic Keratosis. This image (hematoxylin and eosin stain, original magnification ×100) of skin tissue shows marked epidermal thickening (acanthosis), excessive keratin buildup (hyperkeratosis), and multiple keratin-filled invaginations (pseudohorn cysts).
Contributed by M Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)

Actinic Keratosis. This image shows an intact epidermal surface with marked acanthosis. Cytological atypia, characterized by enlarged, elongated, and hyperchromatic nuclei, is present in the basal and suprabasal layers, along with disorganized squamous epithelium. The dermis contains minimal inflammatory infiltrate and scattered melanophages. Findings are consistent with bowenoid actinic keratosis (hematoxylin and eosin stain, original magnifications ×40 center and ×100 right).
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)

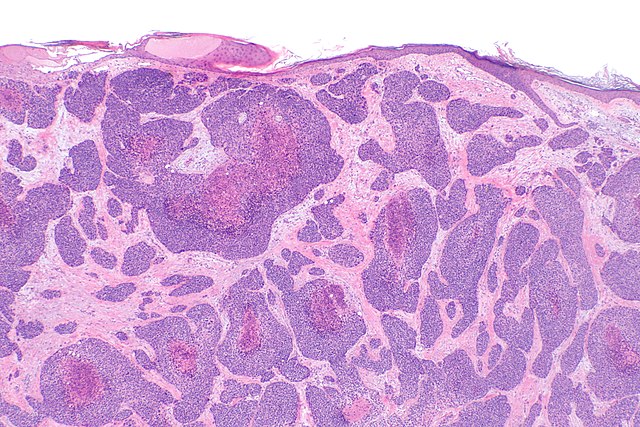
Basal Cell Carcinoma. This image shows 3 variants of basal cell carcinoma. The epidermis remains intact with irregular thickening and mild hyperkeratosis. In the papillary and upper reticular dermis, variable-sized basaloid islands are embedded in a fibromyxoid stroma and surrounded by peritumoral lacunae, characteristic of the nodular type. These islands are lined by basaloid cells exhibiting peripheral palisading. Some basaloid islands remain attached to the overlying epidermis, consistent with the superficial type. Additionally, multiple small basaloid nodules infiltrate the upper reticular dermis, representing the micronodular type (hematoxylin and eosin stain, original magnification ×100).
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)
(Click Image to Enlarge)

Merkel Cell Carcinoma. This image shows Merkel cell carcinoma with epidermal involvement. Hematoxylin-and-eosin staining (left) reveals densely packed tumor cells in the dermis and lymphatic invasion. Cytokeratin 20 staining (right) highlights the characteristic perinuclear dot-like staining pattern, a reliable diagnostic feature of this neoplasm.
Contributed by Muammar Arida, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Syringoma. This image shows a circumscribed proliferation of multiple cords and ductular structures lined by 2 layers of epithelial cells embedded in a densely fibrotic stroma. Some ducts exhibit elongated tails of epithelial cells, creating a characteristic comma-shaped or tadpole appearance (hematoxylin and eosin, original magnification ×100).
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)
(Click Image to Enlarge)

Adenoid Cystic Carcinoma. This frozen section shows multiple glandular and ductal structures (top) and cystic spaces surrounded by basaloid cells (bottom), findings consistent with adenoid cystic carcinoma.
Used with permission via open-access article (Source: Xu YG, Hinshaw M, Longley BJ, Ilyas H, Snow SN. Cutaneous adenoid cystic carcinoma with perineural invasion treated by mohs micrographic surgery-a case report with literature review. J Oncol. 2010;2010:469049.)
(Click Image to Enlarge)
(Click Image to Enlarge)
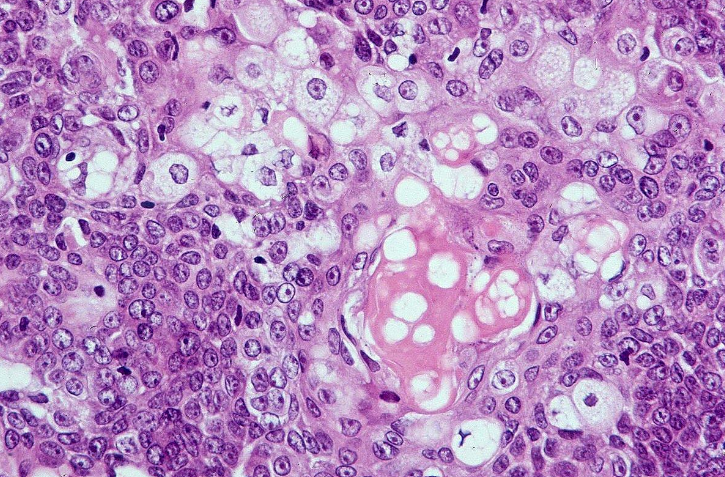
Porocarcinoma. This image shows atypical poroid cells and ductal structures, characteristic histopathologic features of eccrine-derived porocarcinoma.
Nephron, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
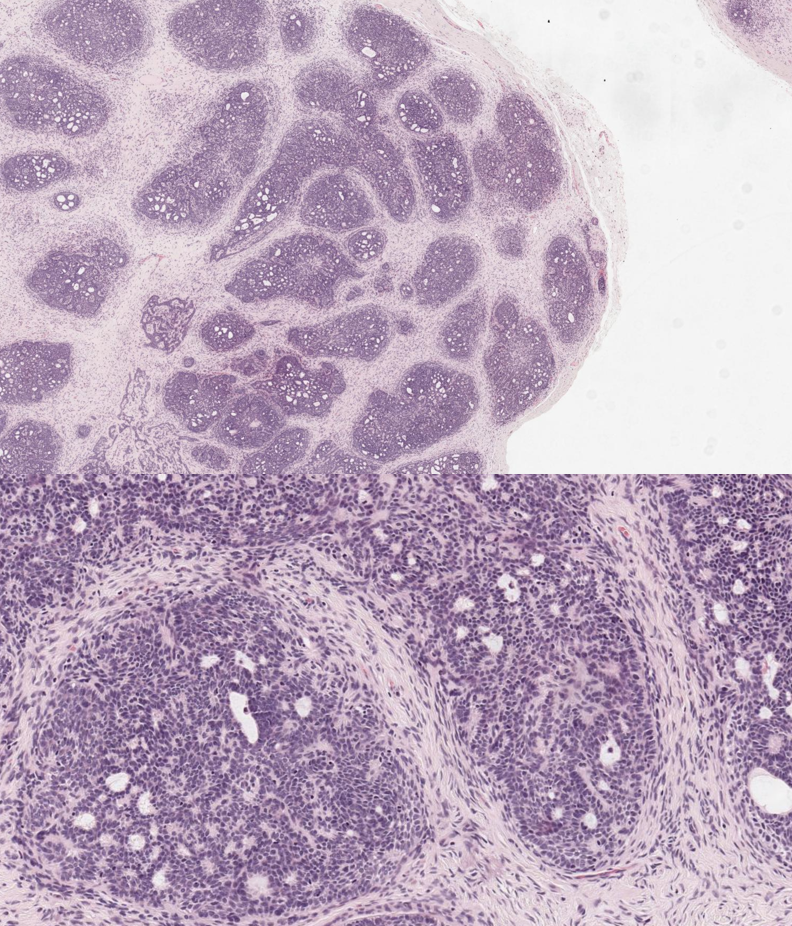
Trichoblastoma. This image shows a well-circumscribed dermal lesion with epithelial cell aggregates and characteristic follicular stroma, lacking the peritumoral clefting seen in basal cell carcinoma (hematoxylin and eosin stain, original magnifications ×40 top and ×100 bottom).
Contributed by N Sathe, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
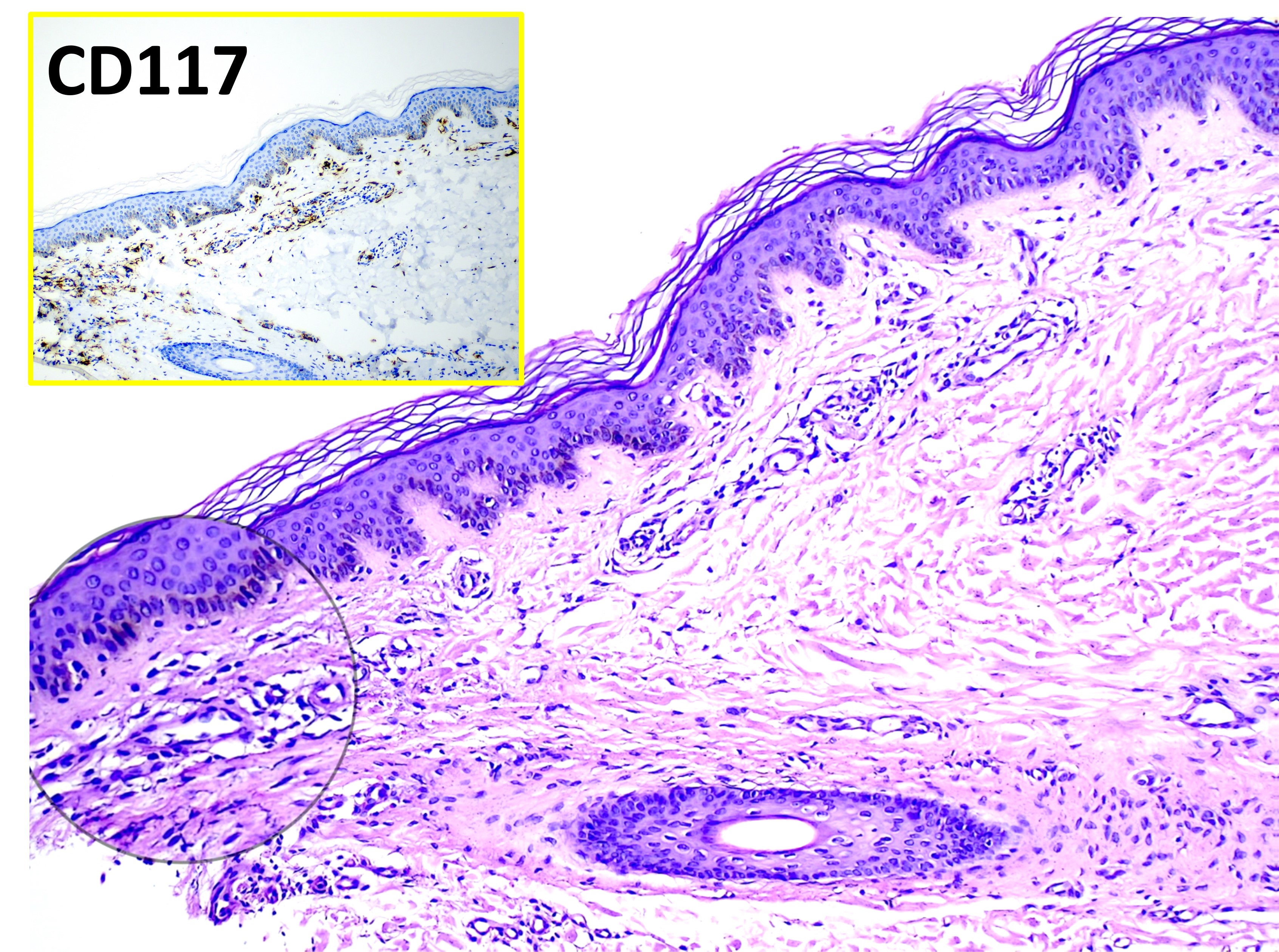
Mastocytosis. The main image (hematoxylin and eosin, original magnification ×100) shows varying numbers of dermal mast cells appearing as unremarkable histiocytoid cells. The inset highlights CD117-positive staining with immunohistochemistry, confirming mast cell proliferation.
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)

Mycosis Fungoides. The main image (hematoxylin and eosin, original magnification ×40) shows an intact epidermis with prominent epidermotropism of atypical lymphocytes and Pautrier microabscesses. The inset (hematoxylin and eosin, original magnification ×100) highlights medium-sized hyperchromatic lymphocytes with pericellular halos, some demonstrating basilar tagging. The upper and middle dermis exhibit dilated blood vessels, moderate perivascular lymphocytic infiltrates, melanophages, and mild dermal lymphocytic atypia, along with moderate fibroplasia in the papillary dermis. No large cell transformation is observed, supporting a diagnosis of mycosis fungoides.
Contributed by Mona Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
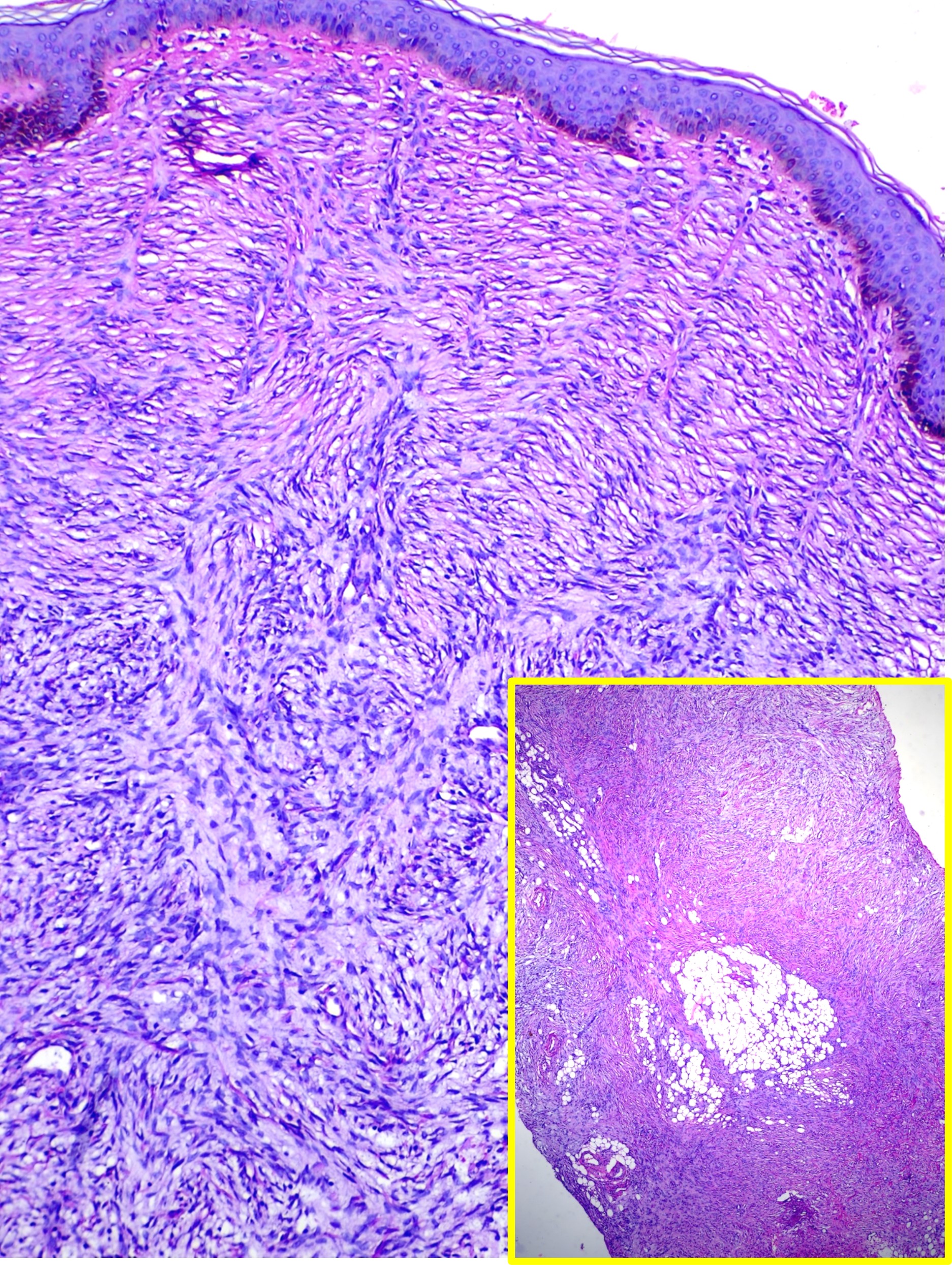
Dermatofibrosarcoma Protuberans. This image shows a spindle cell neoplasm with a storiform pattern involving the entire thickness of the dermis, findings consistent with dermatofibrosarcoma protuberans (hematoxylin and eosin stain, main high-power magnification, inset low-power magnification).
Contributed by M Abdel-Halim Ibrahim, MD
(Click Image to Enlarge)
References
Liersch J, von Köckritz A, Schaller J. Dermatopathology 101. Part 2 - Skin tumors. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2017 Sep:15(9):906-929. doi: 10.1111/ddg.13320. Epub 2017 Aug 25 [PubMed PMID: 28841778]
Harvey NT, Chan J, Wood BA. Skin biopsy in the diagnosis of neoplastic skin disease. Australian family physician. 2017:46(5):289-294 [PubMed PMID: 28472574]
McKee PH. Clues to the diagnosis of atypical melanocytic lesions. Histopathology. 2010 Jan:56(1):100-11. doi: 10.1111/j.1365-2559.2009.03451.x. Epub [PubMed PMID: 20055908]
Ramsey ML, Rostami S. Skin Biopsy. StatPearls. 2025 Jan:(): [PubMed PMID: 29262109]
Campoli M, Brodland DG, Zitelli J. A prospective evaluation of the clinical, histologic, and therapeutic variables associated with incidental perineural invasion in cutaneous squamous cell carcinoma. Journal of the American Academy of Dermatology. 2014 Apr:70(4):630-636. doi: 10.1016/j.jaad.2013.11.034. Epub 2014 Jan 13 [PubMed PMID: 24433872]
Puckett Y, Wilson AM, Farci F, Thevenin C. Melanoma Pathology. StatPearls. 2025 Jan:(): [PubMed PMID: 29083592]
Hitchcock CL, Bland KI, Laney RG 3rd, Franzini D, Harris B, Copeland EM 3rd. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Annals of surgery. 1988 Feb:207(2):201-7 [PubMed PMID: 3277546]
Bittner GC, Cerci FB, Kubo EM, Tolkachjov SN. Mohs micrographic surgery: a review of indications, technique, outcomes, and considerations. Anais brasileiros de dermatologia. 2021 May-Jun:96(3):263-277. doi: 10.1016/j.abd.2020.10.004. Epub 2021 Mar 24 [PubMed PMID: 33849752]
Ranjan R, Singh L, Arava SK, Singh MK. Margins in skin excision biopsies: principles and guidelines. Indian journal of dermatology. 2014 Nov:59(6):567-70. doi: 10.4103/0019-5154.143514. Epub [PubMed PMID: 25484385]
Compton LA, Murphy GF, Lian CG. Diagnostic Immunohistochemistry in Cutaneous Neoplasia: An Update. Dermatopathology (Basel, Switzerland). 2015 Jan-Mar:2(1):15-42. doi: 10.1159/000377698. Epub 2015 Apr 8 [PubMed PMID: 27047932]
Shin J, Vincent JG, Cuda JD, Xu H, Kang S, Kim J, Taube JM. Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. Journal of the American Academy of Dermatology. 2012 Oct:67(4):717-26. doi: 10.1016/j.jaad.2011.12.035. Epub 2012 Feb 9 [PubMed PMID: 22325460]
Ikenberg K, Pfaltz M, Rakozy C, Kempf W. Immunohistochemical dual staining as an adjunct in assessment of mitotic activity in melanoma. Journal of cutaneous pathology. 2012 Mar:39(3):324-30. doi: 10.1111/j.1600-0560.2011.01858.x. Epub [PubMed PMID: 22335591]
Chatterjee D, Bhattacharjee R. Immunohistochemistry in Dermatopathology and its Relevance in Clinical Practice. Indian dermatology online journal. 2018 Jul-Aug:9(4):234-244. doi: 10.4103/idoj.IDOJ_8_18. Epub [PubMed PMID: 30050812]
Hinojosa JA, Williams CL, Vandergriff T, Le LQ. Arsenical keratosis secondary to Fowler solution. JAAD case reports. 2018 Jan:4(1):72-74. doi: 10.1016/j.jdcr.2017.11.008. Epub 2017 Dec 19 [PubMed PMID: 29387754]
Level 3 (low-level) evidencevan Praag MC, Bavinck JN, Bergman W, Rosendaal FR, Mommaas AM, Bruynzeel I, Scheffer E, Vermeer BJ, Bruijn JA. PUVA keratosis. A clinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 1993 Mar:28(3):412-7 [PubMed PMID: 8445056]
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA dermatology. 2015 Oct:151(10):1081-6. doi: 10.1001/jamadermatol.2015.1187. Epub [PubMed PMID: 25928283]
Wadhera A, Fazio M, Bricca G, Stanton O. Metastatic basal cell carcinoma: a case report and literature review. How accurate is our incidence data? Dermatology online journal. 2006 Sep 8:12(5):7 [PubMed PMID: 16962022]
Level 3 (low-level) evidenceSaldanha G, Fletcher A, Slater DN. Basal cell carcinoma: a dermatopathological and molecular biological update. The British journal of dermatology. 2003 Feb:148(2):195-202 [PubMed PMID: 12588368]
Ponticelli C, Cucchiari D, Bencini P. Skin cancer in kidney transplant recipients. Journal of nephrology. 2014 Aug:27(4):385-94. doi: 10.1007/s40620-014-0098-4. Epub 2014 May 9 [PubMed PMID: 24809813]
Caruntu A, Moraru L, Lupu M, Ciubotaru DA, Dumitrescu M, Eftimie L, Hertzog R, Zurac S, Caruntu C, Voinea OC. Assessment of Histological Features in Squamous Cell Carcinoma Involving Head and Neck Skin and Mucosa. Journal of clinical medicine. 2021 May 27:10(11):. doi: 10.3390/jcm10112343. Epub 2021 May 27 [PubMed PMID: 34071843]
Cox NH, Eedy DJ, Morton CA, Therapy Guidelines and Audit Subcommittee, British Association of Dermatologists. Guidelines for management of Bowen's disease: 2006 update. The British journal of dermatology. 2007 Jan:156(1):11-21 [PubMed PMID: 17199561]
Bourlond F, Velter C, Cribier B. Androgen receptor expression in epidermal and adnexal tumours. Annales de dermatologie et de venereologie. 2021 Jun:148(2):116-121. doi: 10.1016/j.annder.2020.08.054. Epub 2021 Jan 19 [PubMed PMID: 33478823]
Ha Lan TT, Chen SJ, Arps DP, Fullen DR, Patel RM, Siddiqui J, Carskadon S, Palanisamy N, Harms PW. Expression of the p40 isoform of p63 has high specificity for cutaneous sarcomatoid squamous cell carcinoma. Journal of cutaneous pathology. 2014 Nov:41(11):831-8. doi: 10.1111/cup.12387. Epub 2014 Nov 3 [PubMed PMID: 25263756]
Lee Y, Lee D, Yeo H, Park H, Park H. Extraordinarily aggressive cutaneous sarcomatoid squamous cell carcinoma of the face: a case report. Archives of craniofacial surgery. 2022 Apr:23(2):77-82. doi: 10.7181/acfs.2022.00059. Epub 2022 Apr 20 [PubMed PMID: 35526843]
Level 3 (low-level) evidenceHarms PW, Verhaegen ME, Hu K, Hrycaj SM, Chan MP, Liu CJ, Grachtchouk M, Patel RM, Udager AM, Dlugosz AA. Genomic evidence suggests that cutaneous neuroendocrine carcinomas can arise from squamous dysplastic precursors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2022 Apr:35(4):506-514. doi: 10.1038/s41379-021-00928-1. Epub 2021 Sep 30 [PubMed PMID: 34593967]
Hung T, Yang A, Mihm MC, Barnhill RL. The plexiform spindle cell nevus nevi and atypical variants: report of 128 cases. Human pathology. 2014 Dec:45(12):2369-78. doi: 10.1016/j.humpath.2014.08.009. Epub 2014 Sep 2 [PubMed PMID: 25300464]
Level 3 (low-level) evidenceEbbelaar CF, Schrader AMR, van Dijk M, Meijers RWJ, de Leng WWJ, Bloem LT, Jansen AML, Blokx WAM, MOLecular Evaluation of Melanocytic Ambiguous Tumors (MOLEMAT) investigators. Towards diagnostic criteria for malignant deep penetrating melanocytic tumors using single nucleotide polymorphism array and next-generation sequencing. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2022 Aug:35(8):1110-1120. doi: 10.1038/s41379-022-01026-6. Epub 2022 Feb 19 [PubMed PMID: 35184152]
Zembowicz A, Knoepp SM, Bei T, Stergiopoulos S, Eng C, Mihm MC, Stratakis CA. Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. The American journal of surgical pathology. 2007 Nov:31(11):1764-75 [PubMed PMID: 18059235]
Donati M, Martinek P, Steiner P, Grossmann P, Vanecek T, Kastnerova L, Kolm I, Baneckova M, Donati P, Kletskaya I, Kalmykova A, Feit J, Blasch P, Szilagyi D, Baldi A, Persichetti P, Crescenzi A, Michal M, Kazakov DV. Novel insights into the BAP1-inactivated melanocytic tumor. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2022 May:35(5):664-675. doi: 10.1038/s41379-021-00976-7. Epub 2021 Dec 2 [PubMed PMID: 34857909]
Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Italian journal of dermatology and venereology. 2021 Jun:156(3):300-321. doi: 10.23736/S2784-8671.21.06958-3. Epub 2021 May 13 [PubMed PMID: 33982546]
Lezcano C, Jungbluth AA, Nehal KS, Hollmann TJ, Busam KJ. PRAME Expression in Melanocytic Tumors. The American journal of surgical pathology. 2018 Nov:42(11):1456-1465. doi: 10.1097/PAS.0000000000001134. Epub [PubMed PMID: 30045064]
Lott JP, Wititsuwannakul J, Lee JJ, Ariyan S, Narayan D, Kluger HH, Lazova R. Clinical characteristics associated with Spitz nevi and Spitzoid malignant melanomas: the Yale University Spitzoid Neoplasm Repository experience, 1991 to 2008. Journal of the American Academy of Dermatology. 2014 Dec:71(6):1077-82. doi: 10.1016/j.jaad.2014.08.026. Epub 2014 Oct 11 [PubMed PMID: 25308882]
Raghavan SS, Peternel S, Mully TW, North JP, Pincus LB, LeBoit PE, McCalmont TH, Bastian BC, Yeh I. Spitz melanoma is a distinct subset of spitzoid melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2020 Jun:33(6):1122-1134. doi: 10.1038/s41379-019-0445-z. Epub 2020 Jan 3 [PubMed PMID: 31900433]
Quan VL, Zhang B, Zhang Y, Mohan LS, Shi K, Wagner A, Kruse L, Taxter T, Beaubier N, White K, Zou L, Gerami P. Integrating Next-Generation Sequencing with Morphology Improves Prognostic and Biologic Classification of Spitz Neoplasms. The Journal of investigative dermatology. 2020 Aug:140(8):1599-1608. doi: 10.1016/j.jid.2019.12.031. Epub 2020 Jan 29 [PubMed PMID: 32004563]
Uguen A, Talagas M, Costa S, Duigou S, Bouvier S, De Braekeleer M, Marcorelles P. A p16-Ki-67-HMB45 immunohistochemistry scoring system as an ancillary diagnostic tool in the diagnosis of melanoma. Diagnostic pathology. 2015 Oct 26:10():195. doi: 10.1186/s13000-015-0431-9. Epub 2015 Oct 26 [PubMed PMID: 26503349]
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, Jakrot V, Kazakoff S, Holmes O, Leonard C, Sabarinathan R, Mularoni L, Wood S, Xu Q, Waddell N, Tembe V, Pupo GM, De Paoli-Iseppi R, Vilain RE, Shang P, Lau LMS, Dagg RA, Schramm SJ, Pritchard A, Dutton-Regester K, Newell F, Fitzgerald A, Shang CA, Grimmond SM, Pickett HA, Yang JY, Stretch JR, Behren A, Kefford RF, Hersey P, Long GV, Cebon J, Shackleton M, Spillane AJ, Saw RPM, López-Bigas N, Pearson JV, Thompson JF, Scolyer RA, Mann GJ. Whole-genome landscapes of major melanoma subtypes. Nature. 2017 May 11:545(7653):175-180. doi: 10.1038/nature22071. Epub 2017 May 3 [PubMed PMID: 28467829]
Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. The American journal of medicine. 1991 May:90(5):606-13 [PubMed PMID: 2029018]
Ansai SI. Topics in histopathology of sweat gland and sebaceous neoplasms. The Journal of dermatology. 2017 Mar:44(3):315-326. doi: 10.1111/1346-8138.13555. Epub [PubMed PMID: 28256768]
Park JH, Lee DY, Kim N. Nail neoplasms. The Journal of dermatology. 2017 Mar:44(3):279-287. doi: 10.1111/1346-8138.13702. Epub [PubMed PMID: 28256766]
Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, Matito A, Broesby-Olsen S, Siebenhaar F, Lange M, Niedoszytko M, Castells M, Oude Elberink JNG, Bonadonna P, Zanotti R, Hornick JL, Torrelo A, Grabbe J, Rabenhorst A, Nedoszytko B, Butterfield JH, Gotlib J, Reiter A, Radia D, Hermine O, Sotlar K, George TI, Kristensen TK, Kluin-Nelemans HC, Yavuz S, Hägglund H, Sperr WR, Schwartz LB, Triggiani M, Maurer M, Nilsson G, Horny HP, Arock M, Orfao A, Metcalfe DD, Akin C, Valent P. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. The Journal of allergy and clinical immunology. 2016 Jan:137(1):35-45. doi: 10.1016/j.jaci.2015.08.034. Epub 2015 Oct 21 [PubMed PMID: 26476479]
Level 3 (low-level) evidenceWolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leukemia research. 2001 Jul:25(7):519-28 [PubMed PMID: 11377676]
Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, Gentile A, Rinaldi P, Franco V, Vincelli D, Pileri A Jr, Gasbarra R, Falini B, Zinzani PL, Baccarani M. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007 Feb:21(2):340-50 [PubMed PMID: 17170724]
Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, Liso A, Martelli MP, Bolli N, Bacci F, Pettirossi V, Santucci A, Martelli MF, Pileri S, Stein H. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007 Jul:21(7):1566-70 [PubMed PMID: 17443224]
Luder CM, Nordmann TM, Ramelyte E, Mühleisen B, Kerl K, Guenova E, Dummer R. Histiocytosis - cutaneous manifestations of hematopoietic neoplasm and non-neoplastic histiocytic proliferations. Journal of the European Academy of Dermatology and Venereology : JEADV. 2018 Jun:32(6):926-934. doi: 10.1111/jdv.14794. Epub 2018 Jan 31 [PubMed PMID: 29341328]
Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019 Apr 18:133(16):1703-1714. doi: 10.1182/blood-2018-11-881268. Epub 2019 Jan 11 [PubMed PMID: 30635287]
Dumont M, Battistella M, Ram-Wolff C, Bagot M, de Masson A. Diagnosis and Treatment of Primary Cutaneous B-Cell Lymphomas: State of the Art and Perspectives. Cancers. 2020 Jun 8:12(6):. doi: 10.3390/cancers12061497. Epub 2020 Jun 8 [PubMed PMID: 32521744]
Level 3 (low-level) evidenceOschlies I, Wehkamp U. Cutaneous B cell lymphomas: standards in diagnostic and clinical work-up. Hints, pitfalls and recent advances. Histopathology. 2022 Jan:80(1):184-195. doi: 10.1111/his.14556. Epub [PubMed PMID: 34958501]
Level 3 (low-level) evidenceOrtiz-Hidalgo C, Pina-Oviedo S. Primary Cutaneous Anaplastic Large Cell Lymphoma-A Review of Clinical, Morphological, Immunohistochemical, and Molecular Features. Cancers. 2023 Aug 14:15(16):. doi: 10.3390/cancers15164098. Epub 2023 Aug 14 [PubMed PMID: 37627126]
Vitiello P, Sagnelli C, Ronchi A, Franco R, Caccavale S, Mottola M, Pastore F, Argenziano G, Creta M, Calogero A, Fiorelli A, Casale B, Sica A. Multidisciplinary Approach to the Diagnosis and Therapy of Mycosis Fungoides. Healthcare (Basel, Switzerland). 2023 Feb 18:11(4):. doi: 10.3390/healthcare11040614. Epub 2023 Feb 18 [PubMed PMID: 36833148]
Schukow C, Ahmed A. Dermatopathology, Cutaneous Lymphomas. StatPearls. 2025 Jan:(): [PubMed PMID: 36944007]
Miyagaki T. Diagnosis and prognostic stratification of cutaneous lymphoma. The Journal of dermatology. 2022 Feb:49(2):210-222. doi: 10.1111/1346-8138.16099. Epub 2021 Aug 4 [PubMed PMID: 34346516]
Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. The Journal of investigative dermatology. 2003 Nov:121(5):1045-52 [PubMed PMID: 14708605]
Level 2 (mid-level) evidenceWillemze R, Meijer CJ. Primary cutaneous CD30-positive lymphoproliferative disorders. Hematology/oncology clinics of North America. 2003 Dec:17(6):1319-32, vii-viii [PubMed PMID: 14710887]
Martinez-Cabriales SA, Walsh S, Sade S, Shear NH. Lymphomatoid papulosis: an update and review. Journal of the European Academy of Dermatology and Venereology : JEADV. 2020 Jan:34(1):59-73. doi: 10.1111/jdv.15931. Epub 2019 Oct 14 [PubMed PMID: 31494989]
Bergqvist C, Beylot-Barry M, Ram-Wolff C, Vergier B, Bagot M, Battistella M, Dalle S, Balme B, Merlio JP, Durupt F, Le Corre Y, Bonnet N, Le Bozec P, Skowron F, Vivard-Wallee I, Dereure O, Brunet-Possenti F, Ingen-Housz-Oro S, Ortonne N. Lymphomatoid papulosis types D and E: a multicentre series of the French Cutaneous Lymphomas Study Group. Clinical and experimental dermatology. 2021 Dec:46(8):1441-1451. doi: 10.1111/ced.14730. Epub 2021 Jul 28 [PubMed PMID: 33987864]
Olsen SH, Ma L, Schnitzer B, Fullen DR. Clusterin expression in cutaneous CD30-positive lymphoproliferative disorders and their histologic simulants. Journal of cutaneous pathology. 2009 Mar:36(3):302-7. doi: 10.1111/j.1600-0560.2008.01036.x. Epub [PubMed PMID: 19220628]
Bekkenk MW, Geelen FA, van Voorst Vader PC, Heule F, Geerts ML, van Vloten WA, Meijer CJ, Willemze R. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000 Jun 15:95(12):3653-61 [PubMed PMID: 10845893]
Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Annals of oncology : official journal of the European Society for Medical Oncology. 2010 May:21(5):1106-11. doi: 10.1093/annonc/mdp415. Epub 2009 Oct 25 [PubMed PMID: 19858086]
Mentzel T. Cutaneous mesenchymal tumours: an update. Pathology. 2014 Feb:46(2):149-59. doi: 10.1097/PAT.0000000000000046. Epub [PubMed PMID: 24378387]
Sheng W, Lu L, Wang J. Cellular angiolipoma: a clinicopathological and immunohistochemical study of 12 cases. The American Journal of dermatopathology. 2013 Apr:35(2):220-5. doi: 10.1097/DAD.0b013e3182685731. Epub [PubMed PMID: 22935891]
Level 3 (low-level) evidenceSirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L, Ranchere-Vince D, Terrier P, Pedeutour F. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. The American journal of surgical pathology. 2007 Oct:31(10):1476-89 [PubMed PMID: 17895748]
Ghadimi MP, Liu P, Peng T, Bolshakov S, Young ED, Torres KE, Colombo C, Hoffman A, Broccoli D, Hornick JL, Lazar AJ, Pisters P, Pollock RE, Lev D. Pleomorphic liposarcoma: clinical observations and molecular variables. Cancer. 2011 Dec 1:117(23):5359-69. doi: 10.1002/cncr.26195. Epub 2011 May 19 [PubMed PMID: 21598240]
Canelas MM, Cardoso JC, Andrade PF, Reis JP, Tellechea O. Fibrous histiocytomas: histopathologic review of 95 cases. Anais brasileiros de dermatologia. 2010 Mar-Apr:85(2):211-5 [PubMed PMID: 20520936]
Level 3 (low-level) evidenceMartin L, Piette F, Blanc P, Mortier L, Avril MF, Delaunay MM, Dréno B, Granel F, Mantoux F, Aubin F, Sassolas B, Adamski H, Dalac S, Pauwels C, Dompmartin A, Lok C, Estève E, Guillot B, French Group for Cutaneous Oncology. Clinical variants of the preprotuberant stage of dermatofibrosarcoma protuberans. The British journal of dermatology. 2005 Nov:153(5):932-6 [PubMed PMID: 16225602]
Abenoza P, Lillemoe T. CD34 and factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. The American Journal of dermatopathology. 1993 Oct:15(5):429-34 [PubMed PMID: 7694515]
Llombart B, Serra-Guillén C, Monteagudo C, López Guerrero JA, Sanmartín O. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Seminars in diagnostic pathology. 2013 Feb:30(1):13-28. doi: 10.1053/j.semdp.2012.01.002. Epub [PubMed PMID: 23327727]
Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJ, López-Terrada D. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Human pathology. 2008 Feb:39(2):184-93 [PubMed PMID: 17950782]
Level 3 (low-level) evidenceCozzi S, Najafi M, Bardoscia L, Ruggieri MP, Giaccherini L, Blandino G, Botti A, Ciammella P, Iotti C. Radiation-induced breast angiosarcoma: report of two patients after accelerated partial breast irradiation (APBI) and review of the literature. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2021:26(5):827-832. doi: 10.5603/RPOR.a2021.0080. Epub 2021 Sep 30 [PubMed PMID: 34760317]
Johnson MD, Jacobs AH. Congenital smooth muscle hamartoma. A report of six cases and a review of the literature. Archives of dermatology. 1989 Jun:125(6):820-2 [PubMed PMID: 2658846]
Level 3 (low-level) evidencePitjadi TM, Grayson W. Epstein-Barr Virus-Associated Smooth Muscle Tumour: A Case Series with a Significant Proportion of Tumours Showing Proclivity for Cutaneous Soft Tissues. Dermatopathology (Basel, Switzerland). 2019 Apr-Jun:6(2):133-146. doi: 10.1159/000497075. Epub 2019 Jun 26 [PubMed PMID: 31700854]
Level 2 (mid-level) evidenceReed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Archives of dermatology. 1972 Dec:106(6):865-70 [PubMed PMID: 4639250]
Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. Journal of cutaneous pathology. 2011 Aug:38(8):625-30. doi: 10.1111/j.1600-0560.2011.01700.x. Epub 2011 Apr 4 [PubMed PMID: 21457155]
Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. Journal of the American Academy of Dermatology. 1993 Aug:29(2 Pt 1):228-36 [PubMed PMID: 8335743]
Level 2 (mid-level) evidenceStrickley JD, Jenson AB, Jung JY. Cutaneous Metastasis. Hematology/oncology clinics of North America. 2019 Feb:33(1):173-197. doi: 10.1016/j.hoc.2018.08.008. Epub [PubMed PMID: 30497674]
Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. The American Journal of dermatopathology. 2012 Jun:34(4):347-93. doi: 10.1097/DAD.0b013e31823069cf. Epub [PubMed PMID: 22617133]
Habermehl G, Ko J. Cutaneous Metastases: A Review and Diagnostic Approach to Tumors of Unknown Origin. Archives of pathology & laboratory medicine. 2019 Aug:143(8):943-957. doi: 10.5858/arpa.2018-0051-RA. Epub 2018 Dec 28 [PubMed PMID: 30605024]
Weissinger SE, Keil P, Silvers DN, Klaus BM, Möller P, Horst BA, Lennerz JK. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 Apr:27(4):524-34. doi: 10.1038/modpathol.2013.162. Epub 2013 Sep 20 [PubMed PMID: 24051699]
Yang DT, Holden JA, Florell SR. CD117, CK20, TTF-1, and DNA topoisomerase II-alpha antigen expression in small cell tumors. Journal of cutaneous pathology. 2004 Mar:31(3):254-61 [PubMed PMID: 14984578]
Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, MacKie RM, Magnusson V, Mann GJ, Bishop JN, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E, Lund Melanoma Study Group, Melanoma Genetics Consortium (GenoMEL). Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. Journal of medical genetics. 2007 Feb:44(2):99-106 [PubMed PMID: 16905682]
Walpole S, Pritchard AL, Cebulla CM, Pilarski R, Stautberg M, Davidorf FH, de la Fouchardière A, Cabaret O, Golmard L, Stoppa-Lyonnet D, Garfield E, Njauw CN, Cheung M, Turunen JA, Repo P, Järvinen RS, van Doorn R, Jager MJ, Luyten GPM, Marinkovic M, Chau C, Potrony M, Höiom V, Helgadottir H, Pastorino L, Bruno W, Andreotti V, Dalmasso B, Ciccarese G, Queirolo P, Mastracci L, Wadt K, Kiilgaard JF, Speicher MR, van Poppelen N, Kilic E, Al-Jamal RT, Dianzani I, Betti M, Bergmann C, Santagata S, Dahiya S, Taibjee S, Burke J, Poplawski N, O'Shea SJ, Newton-Bishop J, Adlard J, Adams DJ, Lane AM, Kim I, Klebe S, Racher H, Harbour JW, Nickerson ML, Murali R, Palmer JM, Howlie M, Symmons J, Hamilton H, Warrier S, Glasson W, Johansson P, Robles-Espinoza CD, Ossio R, de Klein A, Puig S, Ghiorzo P, Nielsen M, Kivelä TT, Tsao H, Testa JR, Gerami P, Stern MH, Paillerets BB, Abdel-Rahman MH, Hayward NK. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. Journal of the National Cancer Institute. 2018 Dec 1:110(12):1328-1341. doi: 10.1093/jnci/djy171. Epub [PubMed PMID: 30517737]
Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, Emmert S, Pike KM, Raziuddin A, Plona TM, DiGiovanna JJ, Tucker MA, Kraemer KH. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. Journal of medical genetics. 2011 Mar:48(3):168-76. doi: 10.1136/jmg.2010.083022. Epub 2010 Nov 19 [PubMed PMID: 21097776]
Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science (New York, N.Y.). 1996 Jun 14:272(5268):1668-71 [PubMed PMID: 8658145]
Kimonis VE, Goldstein AM, Pastakia B, Yang ML, Kase R, DiGiovanna JJ, Bale AE, Bale SJ. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. American journal of medical genetics. 1997 Mar 31:69(3):299-308 [PubMed PMID: 9096761]
Hafeez F, Krakowski AC, Lian CG, Nazarian RM, Maleszewski JJ. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression. Histopathology. 2022 May:80(6):1001-1003. doi: 10.1111/his.14568. Epub 2022 Mar 17 [PubMed PMID: 34532875]
Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine. 1985 Jul:64(4):270-83 [PubMed PMID: 4010501]
Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, Bapat B, Bernstein I, Capellá G, den Dunnen JT, du Sart D, Fabre A, Farrell MP, Farrington SM, Frayling IM, Frebourg T, Goldgar DE, Heinen CD, Holinski-Feder E, Kohonen-Corish M, Robinson KL, Leung SY, Martins A, Moller P, Morak M, Nystrom M, Peltomaki P, Pineda M, Qi M, Ramesar R, Rasmussen LJ, Royer-Pokora B, Scott RJ, Sijmons R, Tavtigian SV, Tops CM, Weber T, Wijnen J, Woods MO, Macrae F, Genuardi M. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nature genetics. 2014 Feb:46(2):107-115. doi: 10.1038/ng.2854. Epub 2013 Dec 22 [PubMed PMID: 24362816]
Level 2 (mid-level) evidenceYoung AL, Kellermayer R, Szigeti R, Tészás A, Azmi S, Celebi JT. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clinical genetics. 2006 Sep:70(3):246-9 [PubMed PMID: 16922728]
Kazakov DV, Zelger B, Rütten A, Vazmitel M, Spagnolo DV, Kacerovska D, Vanecek T, Grossmann P, Sima R, Grayson W, Calonje E, Koren J, Mukensnabl P, Danis D, Michal M. Morphologic diversity of malignant neoplasms arising in preexisting spiradenoma, cylindroma, and spiradenocylindroma based on the study of 24 cases, sporadic or occurring in the setting of Brooke-Spiegler syndrome. The American journal of surgical pathology. 2009 May:33(5):705-19. doi: 10.1097/PAS.0b013e3181966762. Epub [PubMed PMID: 19194280]
Level 3 (low-level) evidenceRuiz J, Hyde M, Perry A, Brouha B. Genomic Evaluation of Clinically Ambiguous Pigmented Lesions. The Journal of clinical and aesthetic dermatology. 2023 Jun:16(6):44-45 [PubMed PMID: 37361365]
Marks E, Jarell A, Ludzik J, Farberg AS, Rabinovitz HS, Phelps RG, Cockerell CJ, Witkowski A. A Physician's Guide to the Use of Gene Expression Profile Ancillary Diagnostic Testing for Cutaneous Melanocytic Neoplasms. The Journal of clinical and aesthetic dermatology. 2023 Apr:16(4):12-20 [PubMed PMID: 37077930]
Has C. Molecular diagnostics in dermatology. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2022 Mar:20(3):251-252. doi: 10.1111/ddg.14760. Epub [PubMed PMID: 35304963]
Kränke T, Loibner M, Arzberger E, Wieczorek G, Krenz T, Attig H, Hofmann-Wellenhof E, Fischer B, Eberhard A, Oberauner-Wappis L, Ulz CM, Fried I, Cerroni L, Oelmueller U, Zatloukal K, Hofmann-Wellenhof R, Heitzer E. Non-invasive molecular profiling of skin lesions using tape-stripping. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI). 2023 May:29(5):e13322. doi: 10.1111/srt.13322. Epub [PubMed PMID: 37231928]