Introduction
The interpretation of dermatopathology specimens can be confounded by artifacts unrelated to the primary pathology. Before making a histopathological diagnosis, tissue specimens must undergo a series of steps in preparation for microscopic examination. When errors occur in any stage of the tissue preparation process, artifact introduction to tissue specimens may result.
Tissue artifact refers to an artificial structure on a microscopic slide resulting from an extraneous factor.[1][2] Many artifacts in dermatopathology specimens are present before tissue removal from the patient. Artifacts in tissue specimens can make interpretation difficult, especially if the pathologist is unfamiliar with the underlying etiology. Clinically, artifacts can be the primary reason for a biopsy, as in the case of a graphite tattoo.[3] Both clinicians and pathologists should be aware of potential artifacts in tissue specimens, the etiology of the artifacts, and if the artifact is related to the primary pathology.
Tissue Preparation
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
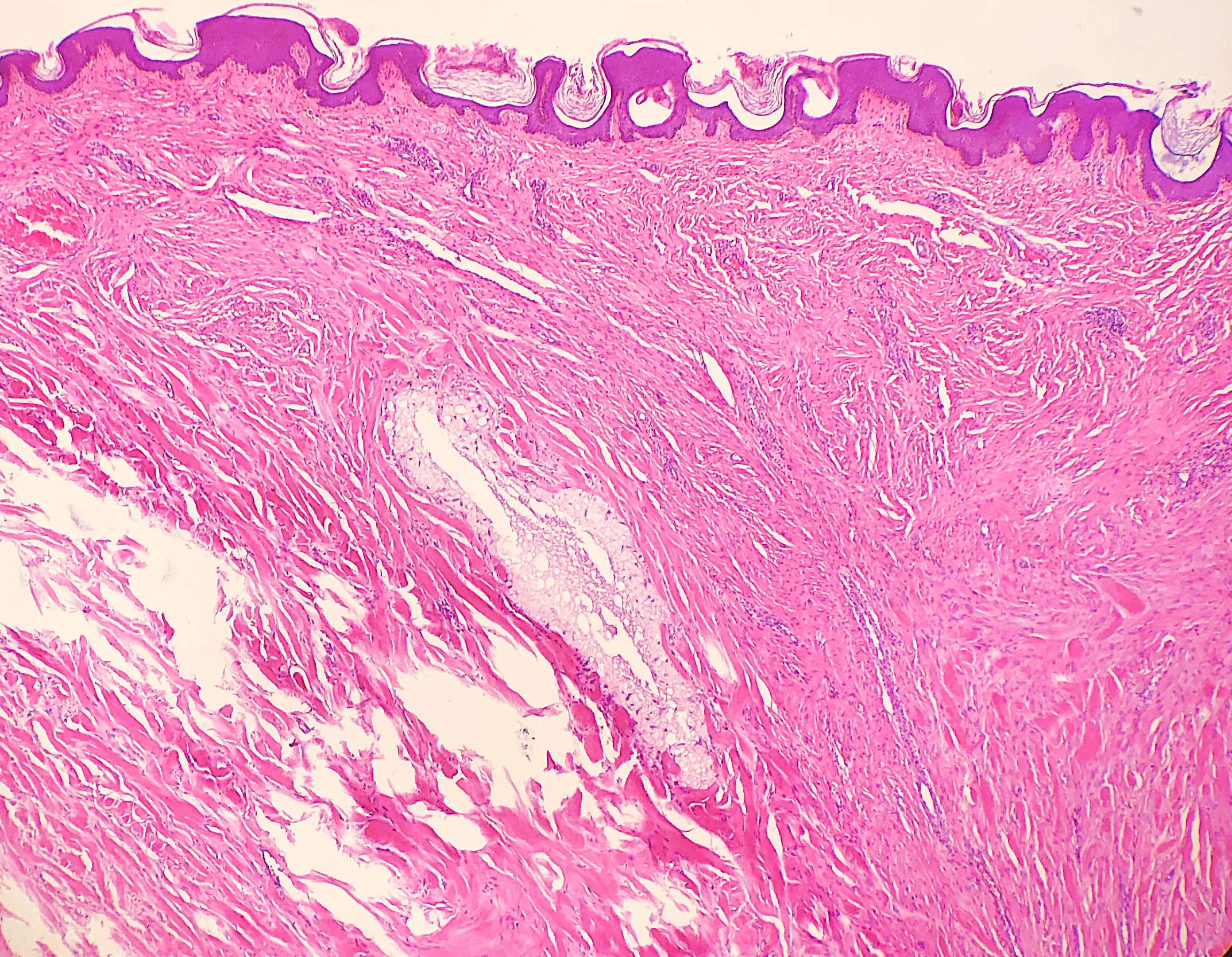
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Tissue Preparation
Tissue artifacts may occur at any step of specimen preparation. To understand the etiology of different artifacts, it is essential to be familiar with the steps in tissue processing. There are several methods and variations in tissue processing. The details of tissue processing are beyond the scope of this paper. The tissue processing steps are described in a condensed and simplistic view below.
For dermatopathology specimens, tissue is typically acquired by shave biopsy, punch biopsy, or an excision. The tissue is placed in a fixative solution, commonly 10% formalin (3% to 4% formaldehyde). The goal of fixation is to preserve the tissue in its original state, stopping further metabolic reactions and preventing alterations in morphologic quality.[4] For fixation to occur, formaldehyde must react with the tissue through a series of chemical bonds. This process takes time, and fixation is typically allowed to occur over 24 to 48 hours.[5] The estimated timeline for completion of the chemical bonding process is 1 hour per millimeter (mm) of tissue thickness.[6] Due to the demand for quick turnaround times, tissue fixation is sometimes accelerated. When formalin fixation is inadequately complete, the specimen quality may suffer, resulting in distorted nuclear features such as artifactual nuclear bubbling or the appearance of washed-out nuclei.[5]
Following fixation in formalin, the tissue is processed grossly. Typically, tissue specimens are sectioned into pieces less than 2 centimeters (cm) in length and less than 0.5 cm thick.[7] Thicker sections prevent proper penetration of substances in subsequent steps. After gross preparation, the specimen will go through a sequence of steps termed dehydration, clearing, and infiltration, typically in an automated processor.[7]
The dehydration step takes the tissue through increasing concentrations of alcohol, commonly ethanol or isopropanol, to remove water from the specimen through osmosis. Following dehydration, tissue is often bathed in xylene to remove the dehydrating agents, as they are not miscible with paraffin and will dissolve lipids. The tissue specimen will then undergo infiltration as it is placed in a supportive medium to preserve the tissue's form during microtomy. The most commonly used medium for infiltration is paraffin.[7]
The melting point of the paraffin may be affected by additives. The melting points have implications for the introduction of tissue artifacts. Thinner sections can be obtained using higher melting points, but mediums with higher melting points may be more susceptible to thermal effects. Thinner sections are more challenging when using supportive mediums with lower melting points but are also less likely to succumb to thermal effects.[7]
In most settings, these three steps are accomplished with automated tissue processing machines. Microwave-assisted tissue processing has allowed for an accelerated process in which progressively graded alcohol concentrations are not required for dehydration.[7]
Tissue specimens can be overprocessed if excessively dehydrated or cleared. Overprocessing may result in hardened or brittle tissue that is difficult to cut.[7] In contrast, underprocessed tissue is underexposed to the dehydration or clearing step, resulting in inadequate penetration of paraffin. Many factors can lead to the underprocessing of tissue specimens, such as specimens with more extensive adipose layers, thick sections, or water contamination. Underprocessing can lead to tissue that is difficult to cut, tissue that is not transparent, and staining artifacts.[7]
After infiltration, the specimen is embedded in paraffin wax to create a tissue block before microtomy. The histotechnician sections the tissue from the block using a microtome into smaller pieces that may vary in size (often 4 to 10 micrometers in thickness) depending on the type of specimen or the pathologist's preference. The sectioned tissue is subsequently placed in a warm water bath to aid in proper positioning onto a glass slide.
After placement of tissue onto a glass slide, the specimen will undergo routine hematoxylin and eosin staining or other special stains as needed. The tissue is first deparaffinized with substances such as xylene.[5] The tissue specimen will undergo a series of steps, which may vary in the protocol, resulting in the specimen's final hematoxylin and eosin staining. The glass slide, with stained tissue, will have a coverslip mounted with formulations and methods designed to prevent specimen degradation and preserve quality over time.[5] After the coverslip is mounted to the glass slide, the specimen is ready to be examined by the pathologist.
Histochemistry and Cytochemistry
Minor deficiencies in the tedious tissue preparation process allow for the introduction of artifacts. Artifacts in tissue specimens can lead to distraction and make an accurate diagnosis difficult. An aberrant diagnosis may result when first encountering specific artifacts unfamiliar to the pathologist. The types of artifacts can be subclassified based on the step in which they occur in tissue processing.[2]
- Prefixation artifact
- Fixation artifact
- Processing artifact
- Mounting artifact
Microscopy, Light
Most dermatopathology specimens are examined via light microscopy with hematoxylin and eosin staining.
Pathophysiology
Prefixation Artifacts
Hemorrhage
Hemorrhage of erythrocytes into surrounding tissue is a finding in some tissue specimens. Bleeding can be related to the underlying pathology, as seen in solar purpura. Hemorrhage artifact refers to extravasated erythrocytes that occur during the procedure and have no relation to the underlying pathology. (see Image. Histology Slide Hemorrhage)
Thermal/Heat
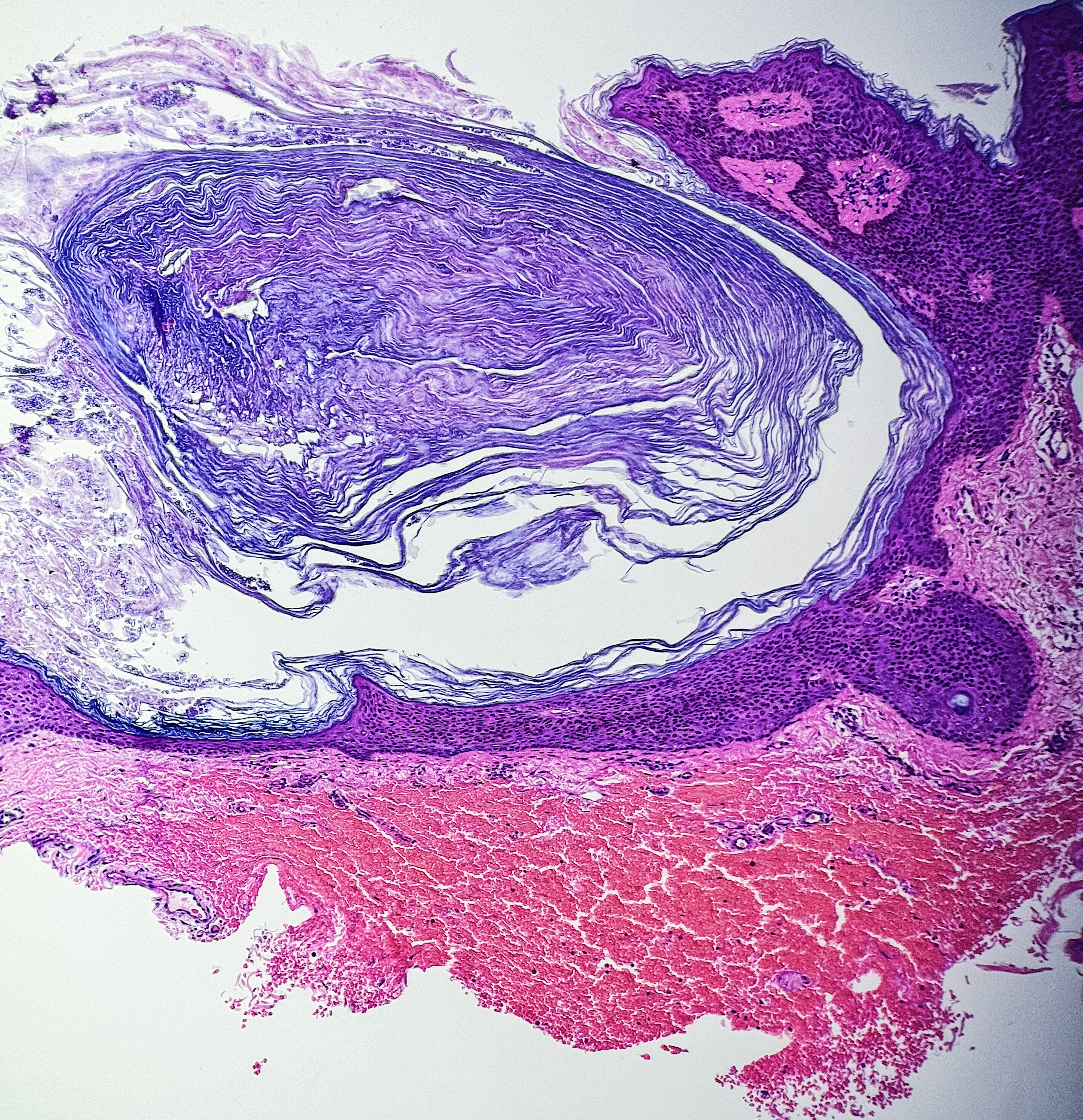
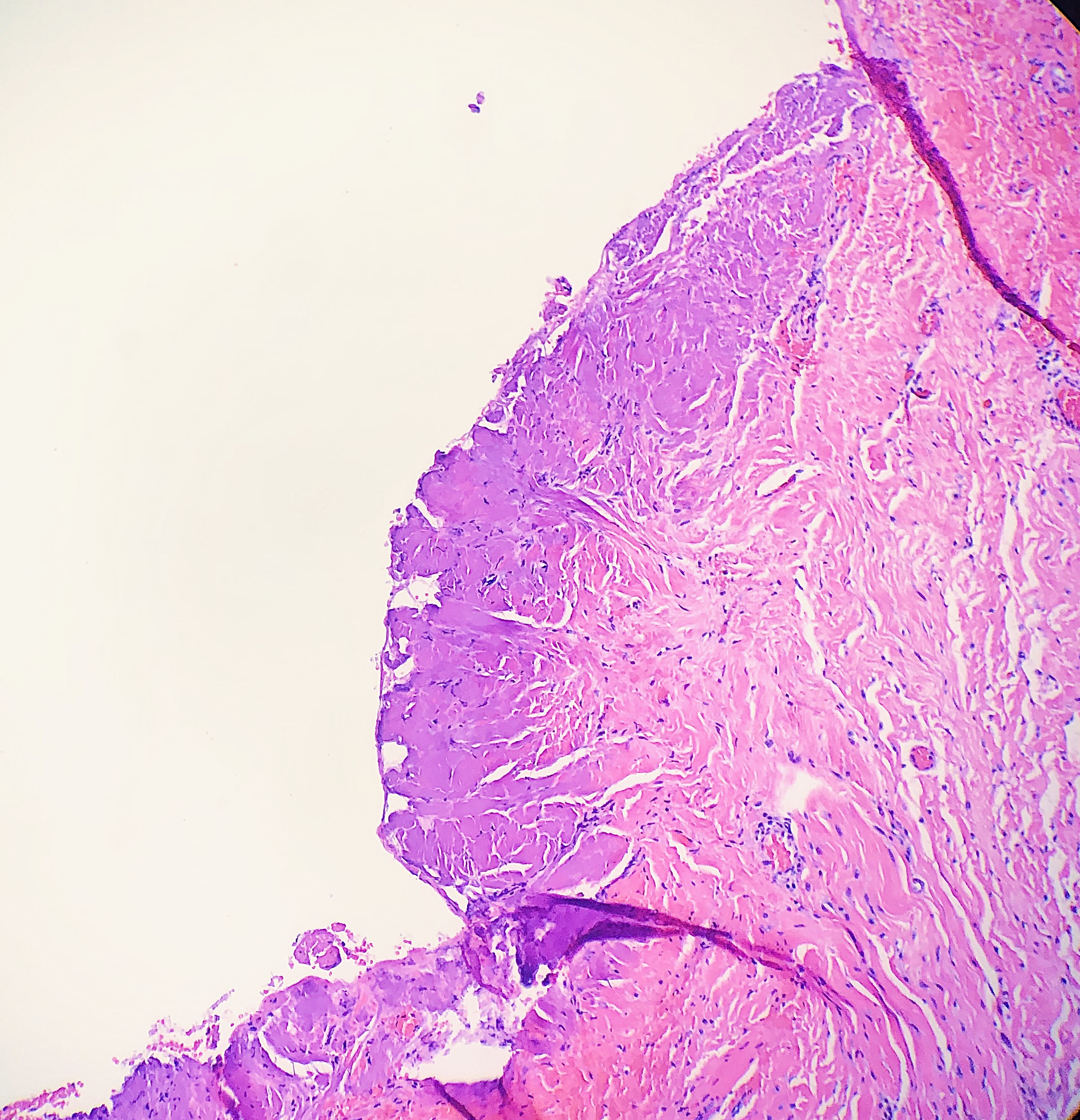
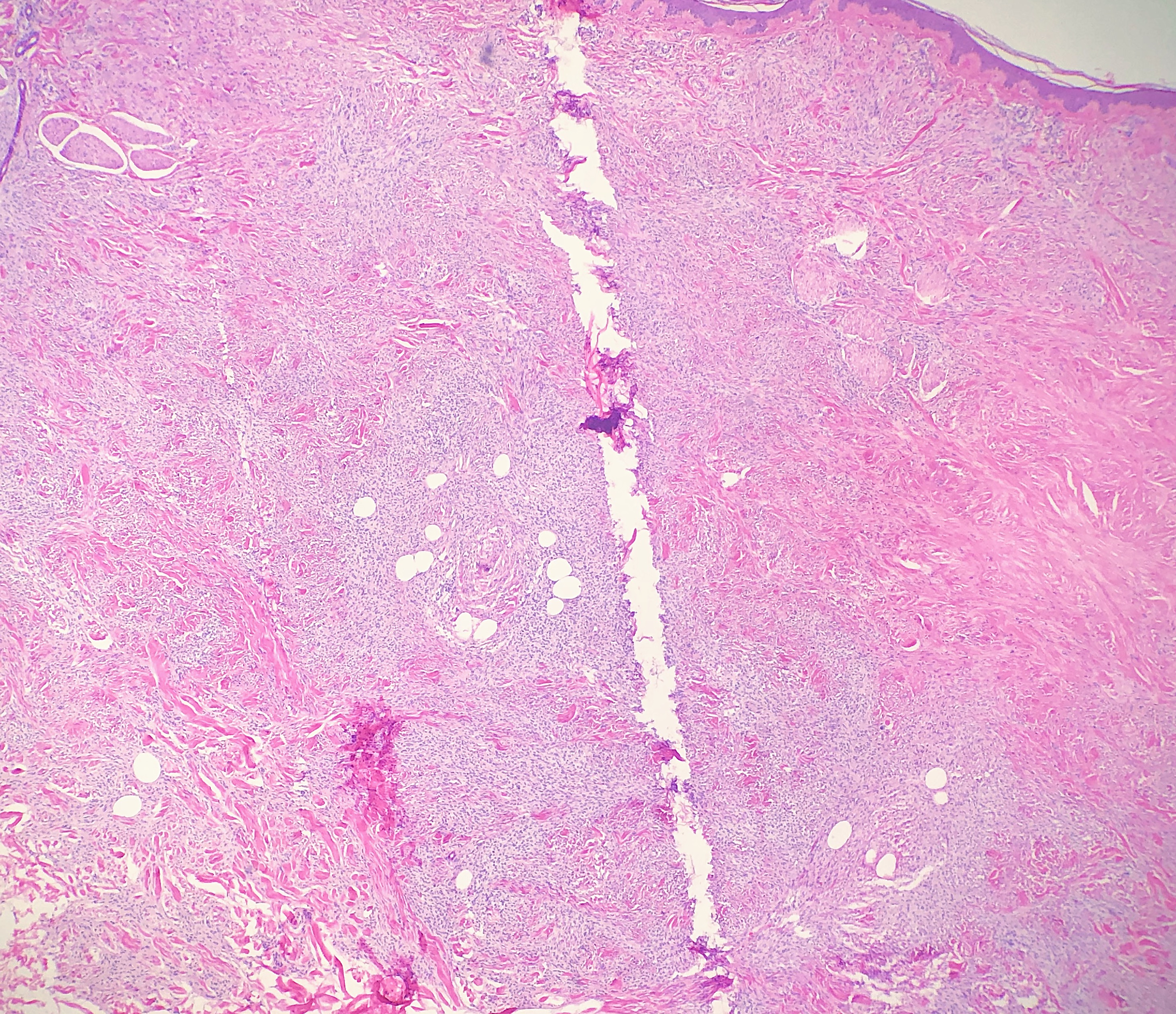
Thermal or heat artifacts are common in specimens where the provider utilizes excessive heat via laser or electrosurgery. The characteristic epidermal changes include loss of polarity of the epidermis, spindled keratinocytes, and, in extreme cases, epidermal necrosis with separation from the underlying basement membrane.[8] When deeper tissues are exposed to excessive heat, similar findings can occur, but the tissue may also take on more of an opaque or amorphous appearance.[9] (see Images. Histology Collagen and Histology Collagen Folds)
Freeze Artifact
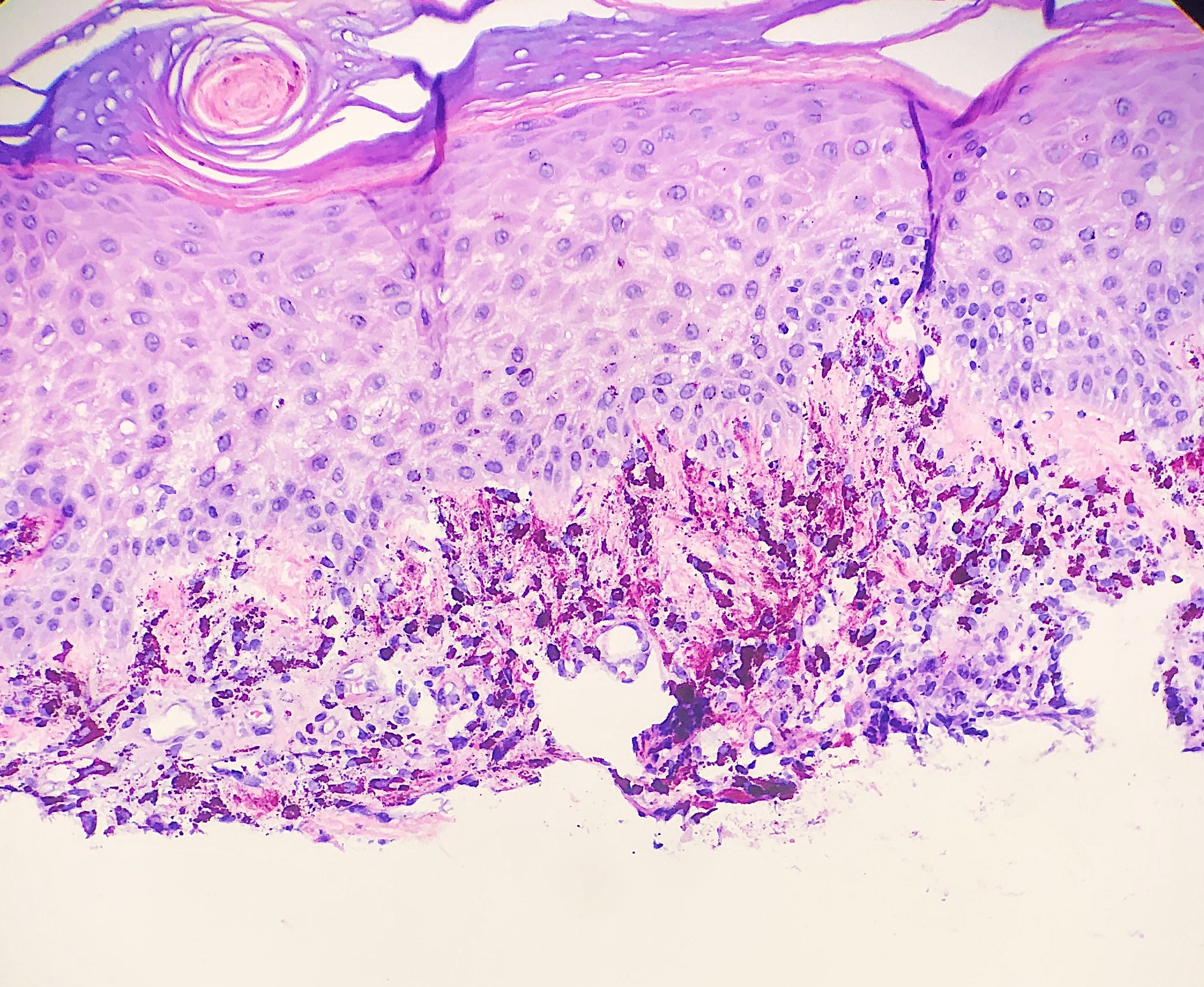
In dermatopathology, freeze or ice artifacts are commonly encountered in the setting of frozen specimens utilized in Mohs micrographic surgery. Freeze artifacts can also be found in tissue frozen with liquid nitrogen before removal. The characteristic finding is vacuolated keratinocytes in the epidermis, but other findings include splaying of dermal collagen and loss of cell architecture.[10] (see Image. Vacuolar Change)
Suture
Surgeons may place sutures as a marker for orientation during the grossing process of the tissue specimen. Sutures can also be encountered at previous biopsy sites that were subsequently excised. The appearance of the suture will depend on the composition of the suture placed. Polyfilament sutures often appear as numerous round structures filling a lumen and are often birefringent when polarized.[10] (see Images. Polyfilament Suture and Suture Birefringence.)
Injected Material
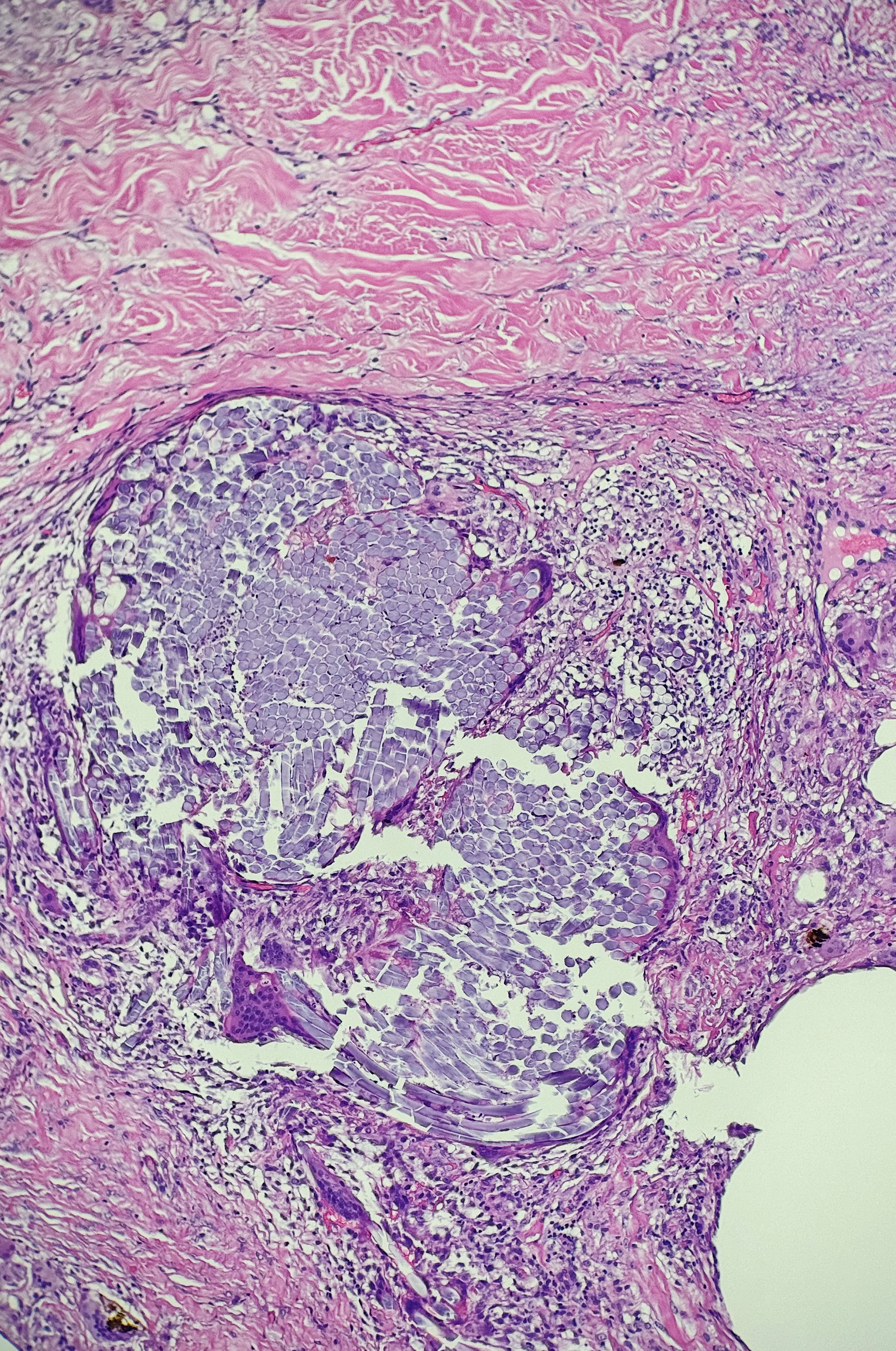
Tissue samples may have injectable substances, such as intralesional corticosteroids. Intralesional corticosteroid is a common finding in keloids. The steroid may resemble light blue pools of mucin with occasional granulomatous inflammation.[11] Histologic findings are similar regardless of the type of corticosteroid injected and the steroid dosage.[12] (see Image. Corticosteroid Keloid)
Aluminum Chloride
Aluminum chloride is used as an antiperspirant and hemostatic agent. Aluminum chloride is commonly encountered in dermatopathology excision specimens when used as a hemostatic agent at a prior biopsy site. The aluminum particles are seen within histiocytes with a granular cytoplasm. Aluminum chloride can also result in basophilic collagen alteration.[13][14] Granular deposition of aluminum chloride is a potential pitfall for considering other causes of parasitized histiocytes.[13]
Ferrous Subsulfate (Monsel's solution)
Ferrous subsulfate is sometimes used as a hemostatic agent and is also commonly encountered at previous biopsy sites in excision specimens. Ferrous subsulfate will appear as prominent pigmented deposits that are granular or more diffuse and "smudgy." The pigment may be found in macrophages, fibroblasts, or around collagen bundles.[15] If unfamiliar with this solution, distinguishing the pigmented ferrous subsulfate deposits from hemosiderin or melanin can be difficult.[14][15] (see Image. Ferrous Subsulfate)
Gelfoam
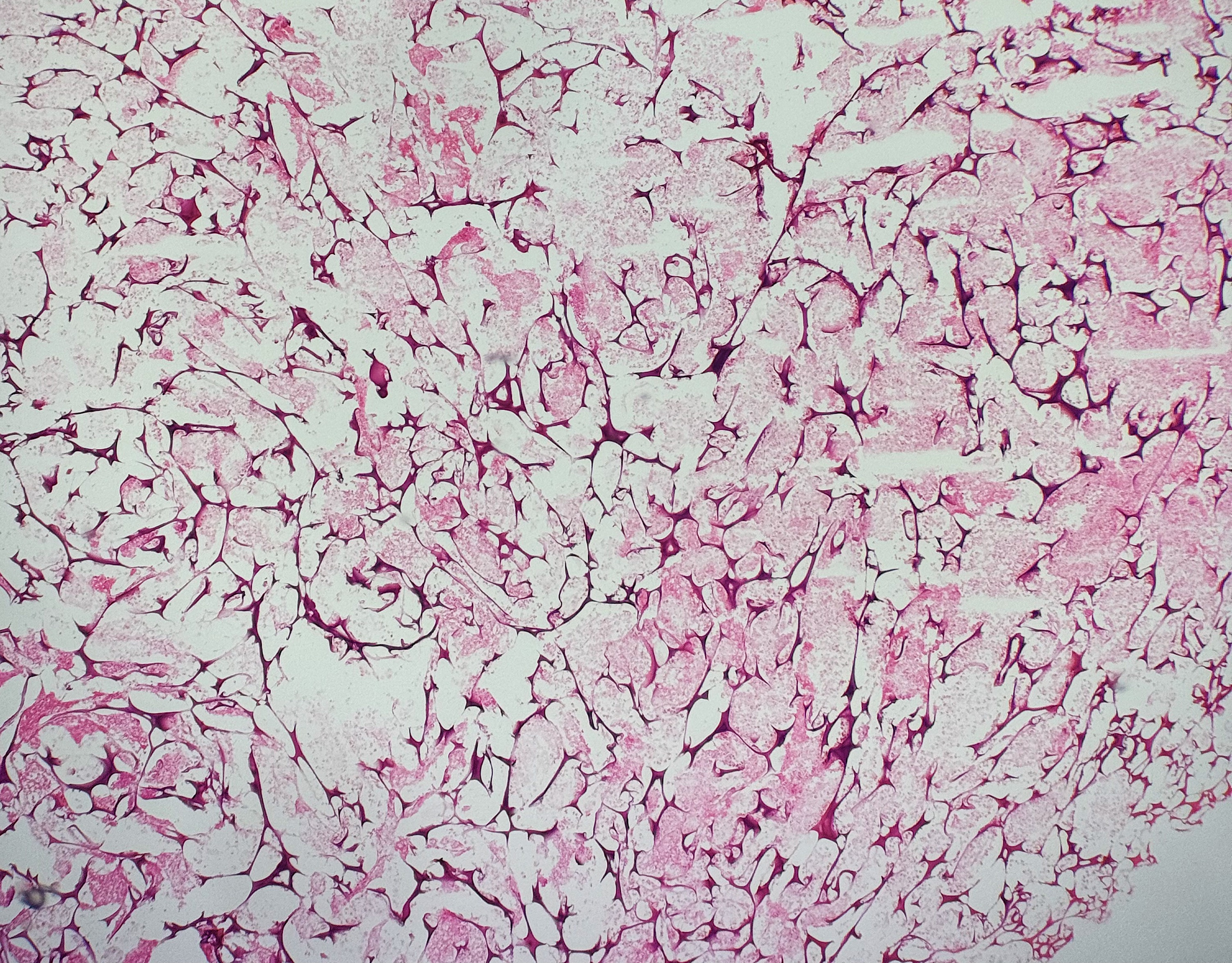
Gelfoam is sometimes used as a hemostatic agent and placed after a punch biopsy. Excision of the prior biopsy site will reveal the striking nature of the gelfoam. Gelfoam appears as distorted spaces surrounded by basophilic gelatin walls.[16] (see Image. Gelfoam)
Tattoo Pigment
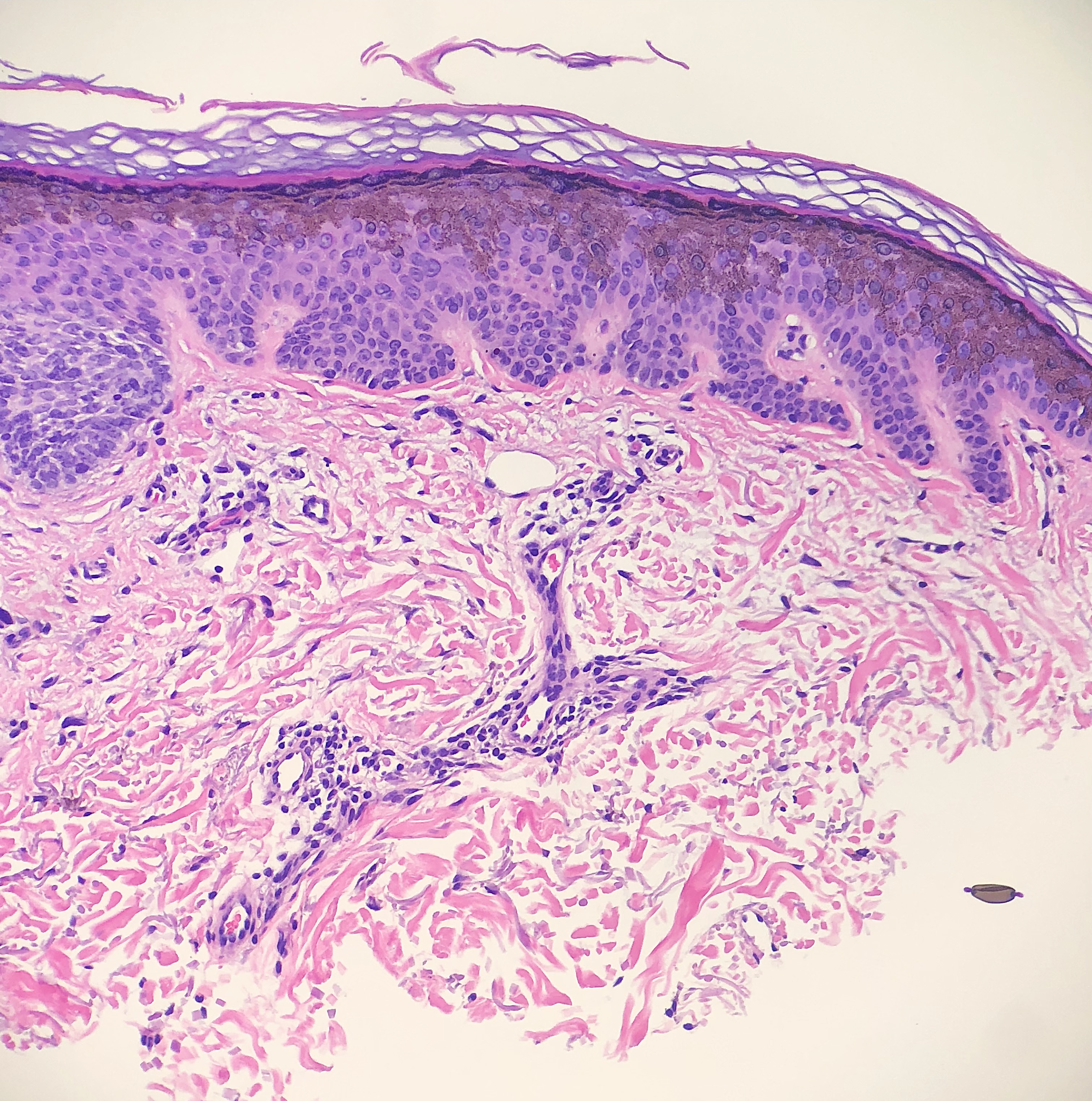
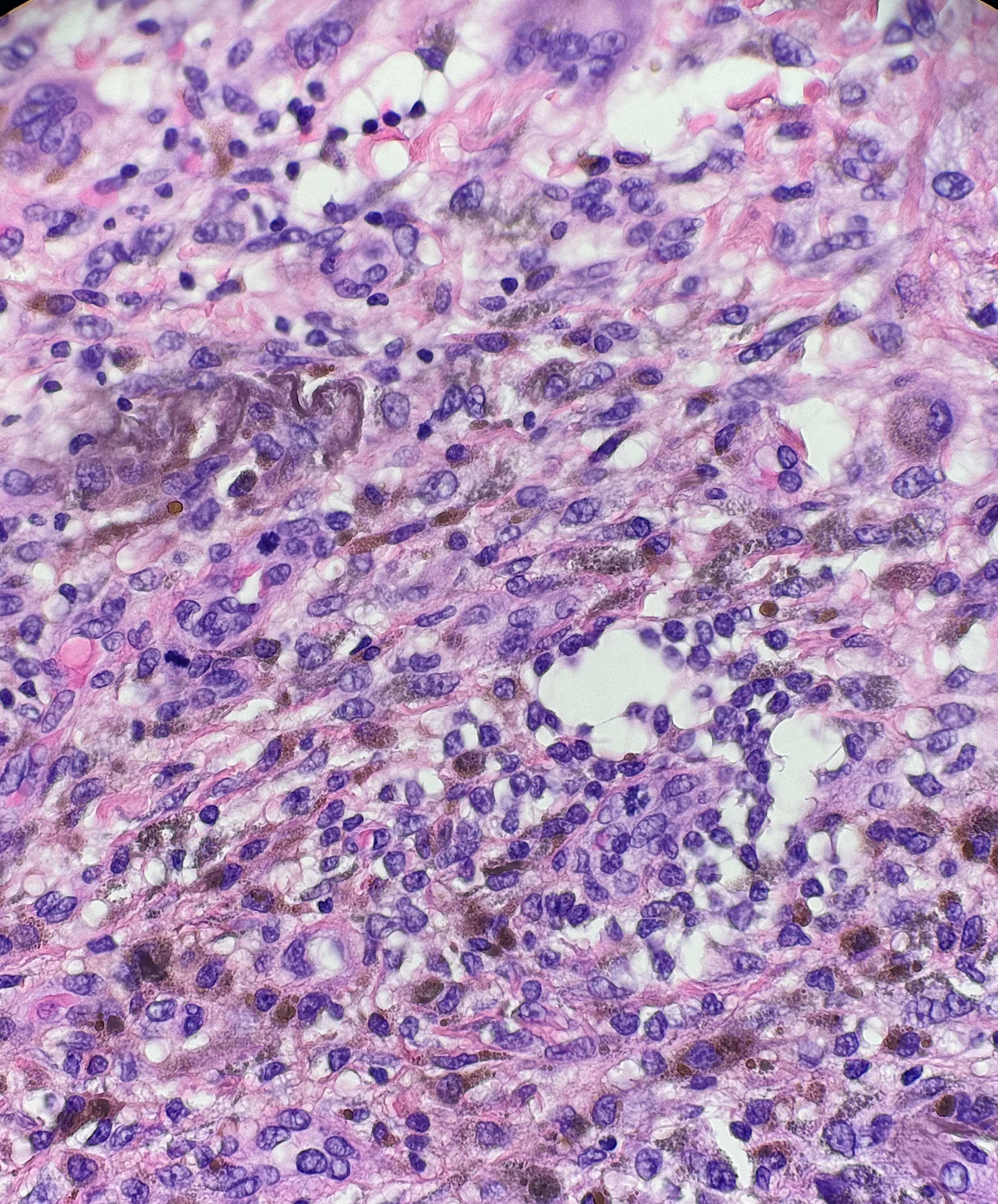
Tattoo pigment may be found in dermatopathology specimens as a part of the primary pathology, as in granulomatous tattoo reactions. Tattoo pigment can also be an artifact or incidental finding unrelated to the primary pathology, such as in the background of skin cancer. Tattoo pigment is often found around the superficial dermal plexus but can sometimes be found with macrophages or between collagen bundles.[17] (see Image. Tattoo Pigment)
Other Prefixation Artifacts
Many other potential prefixation artifacts can be found in tissue related or unrelated to the primary pathology. The list is not exhaustive and includes injectable fillers, glass, metals, and many other substances.
Fixation Artifacts
Incomplete Fixation
Ideally, tissue is fixed in an appropriate solution immediately upon removal from the patient to prevent any significant tissue alterations or protein degradation. Delay in fixation is not typically an issue in dermatopathology, but increased demand for turnaround time could lead to a decrease in total fixation time. If the tissue specimen is placed in an inadequate volume of formalin or not given enough time in formalin, tissue microtomy is compromised.[18] (see Image. Poorly-Fixed Specimen)
Microwave Fixation Artifact
Microwave fixation aids in the speed of tissue fixation, but the process can result in tissue texture alteration, tissue shrinkage, and breakdown of erythrocytes.[6] Microwave-induced tissue artifacts appear as increased vacuolization of cells, overstaining of cytoplasm, or pyknotic nuclei.[2]
Improper Fixation (Saline)
The storage of a tissue specimen in an improper fixative, such as saline for transport or as a temporary medium, can result in vacuolization of the basal layer epithelium, widespread vacuolization of cells, and eventually lysis of cells. The dermis displays the separation of collagen and eventual lysis of cell architecture.[19]
Processing Artifacts
Embedding
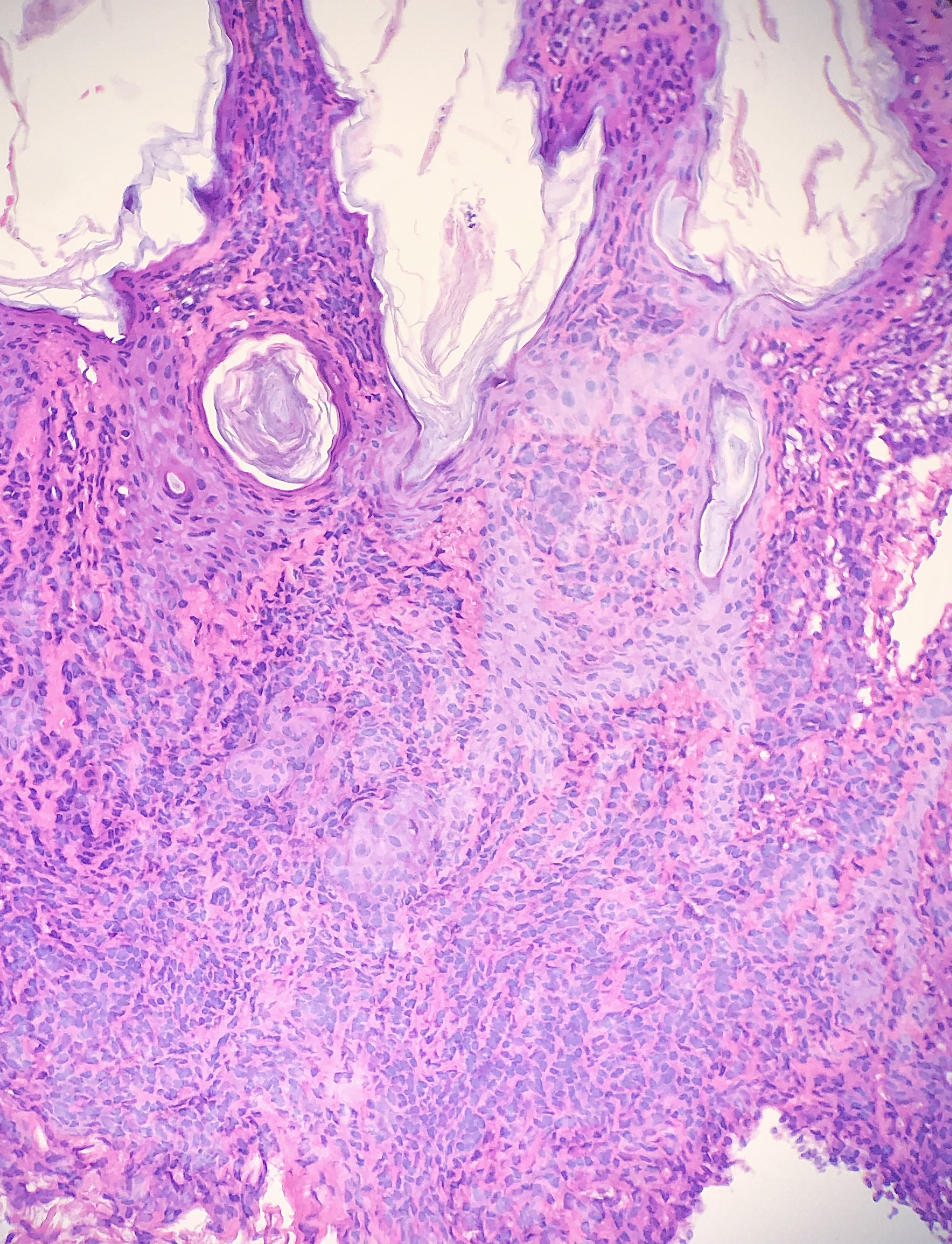
During the embedding and microtomy process, if the tissue specimen is not aligned correctly, the specimen can be cut at an unfavorable angle. Improper embedding can lead to deeper connective tissue of the dermis appearing in the same plane as the epidermis. The result can give an appearance similar to an invasive squamous cell carcinoma.[2]
Chatter/Venetian Blind Artifact
Chatter, also commonly referred to as Venetian blind artifact, is multiple parallel bands of tissue separated by narrow clear spaces that are said to resemble Venetian blinds.[20] The introduction of chatter artifact is often the result of the vibration of the specimen in the paraffin block during the operation of the microtome knife, especially if the blade is not tightly screwed down into its mount. Chatter artifact is also more common in certain conditions where nodular aggregates of inflammatory or neoplastic cells replace the stromal support within the dermis typically provided by collagen.[20]
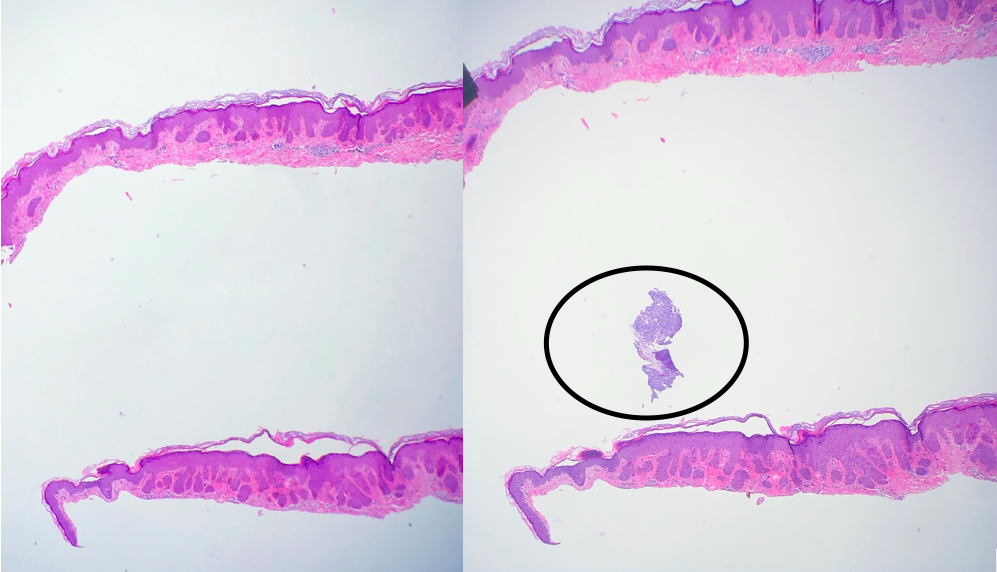
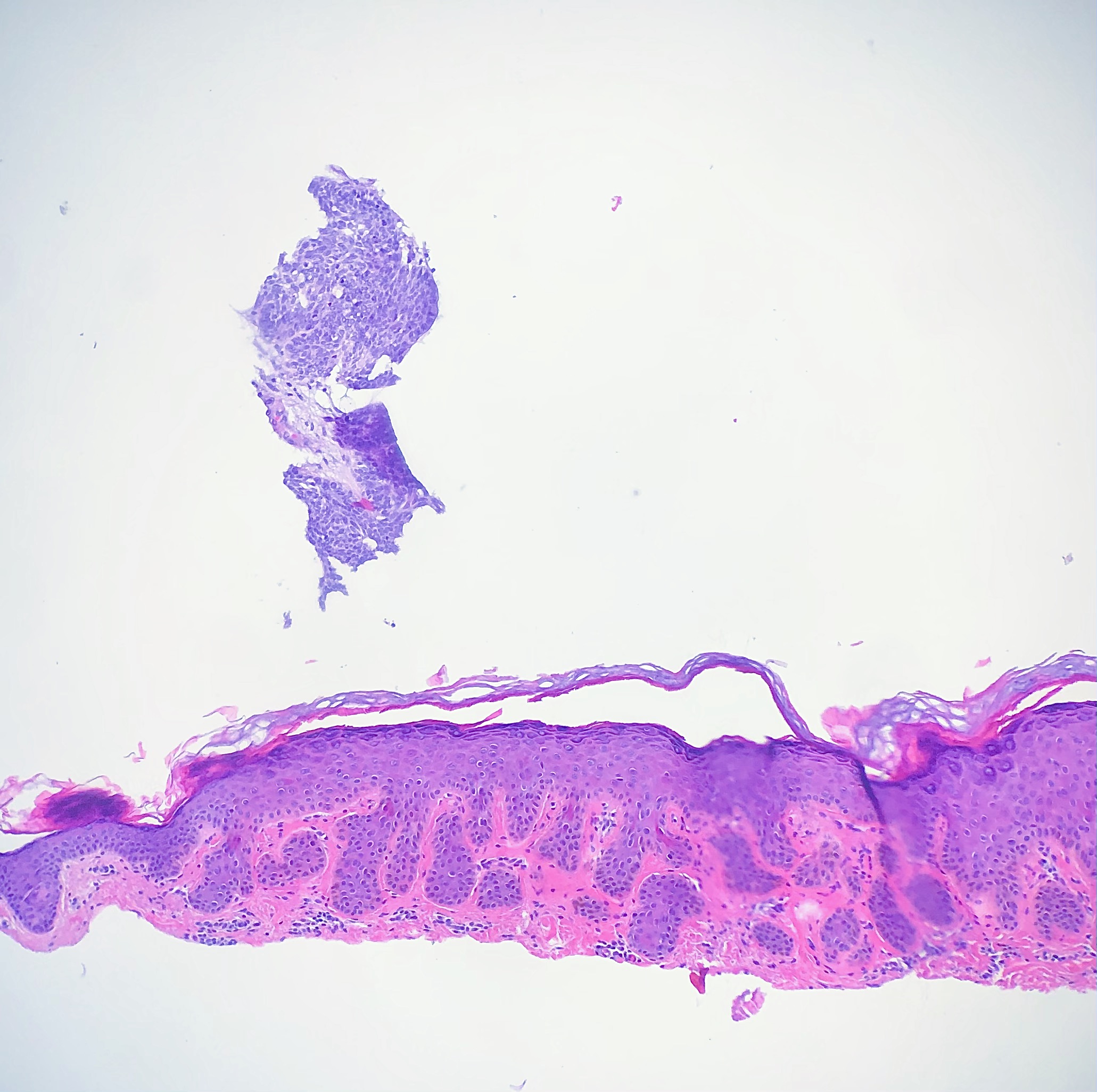
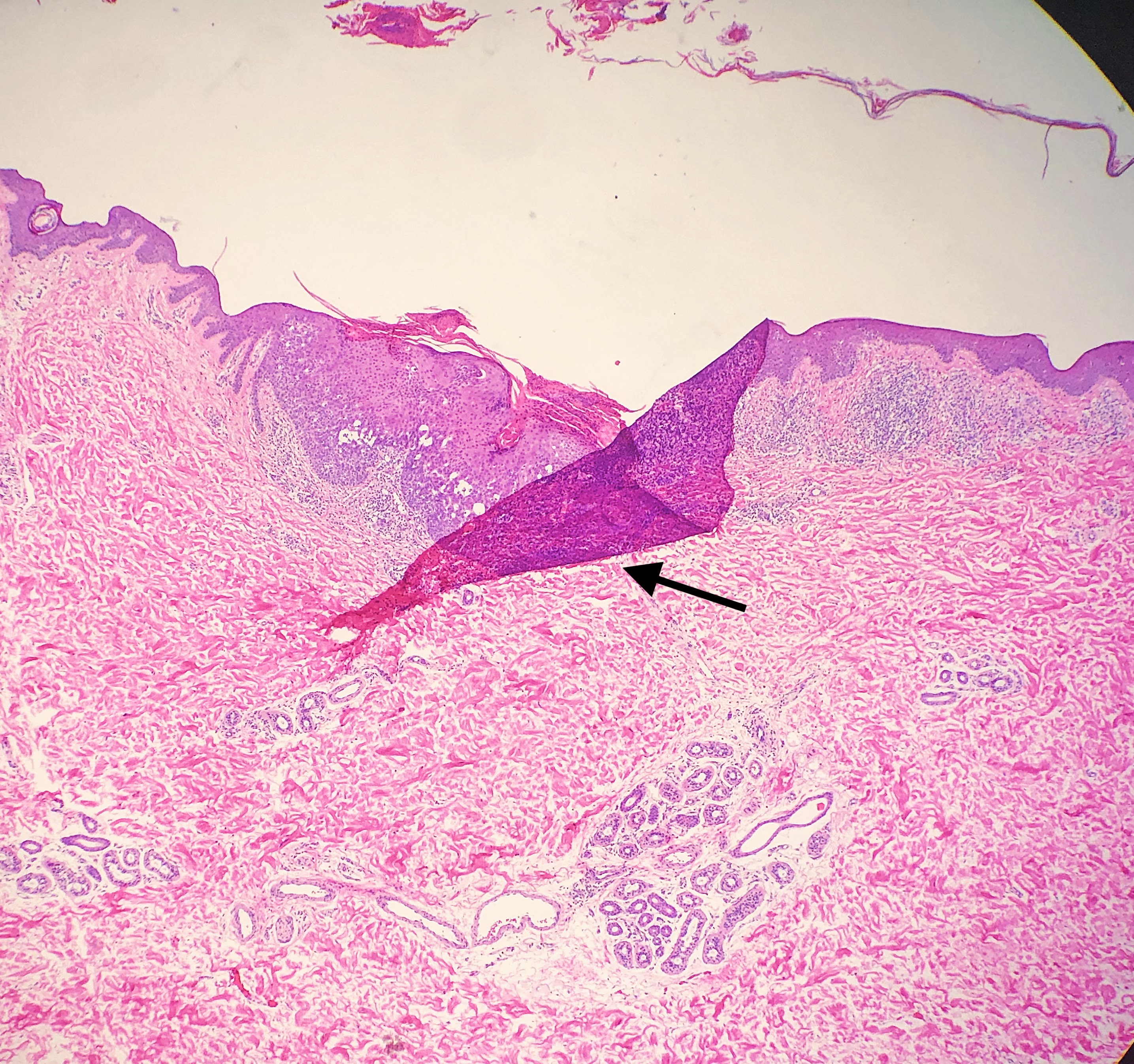
Floater
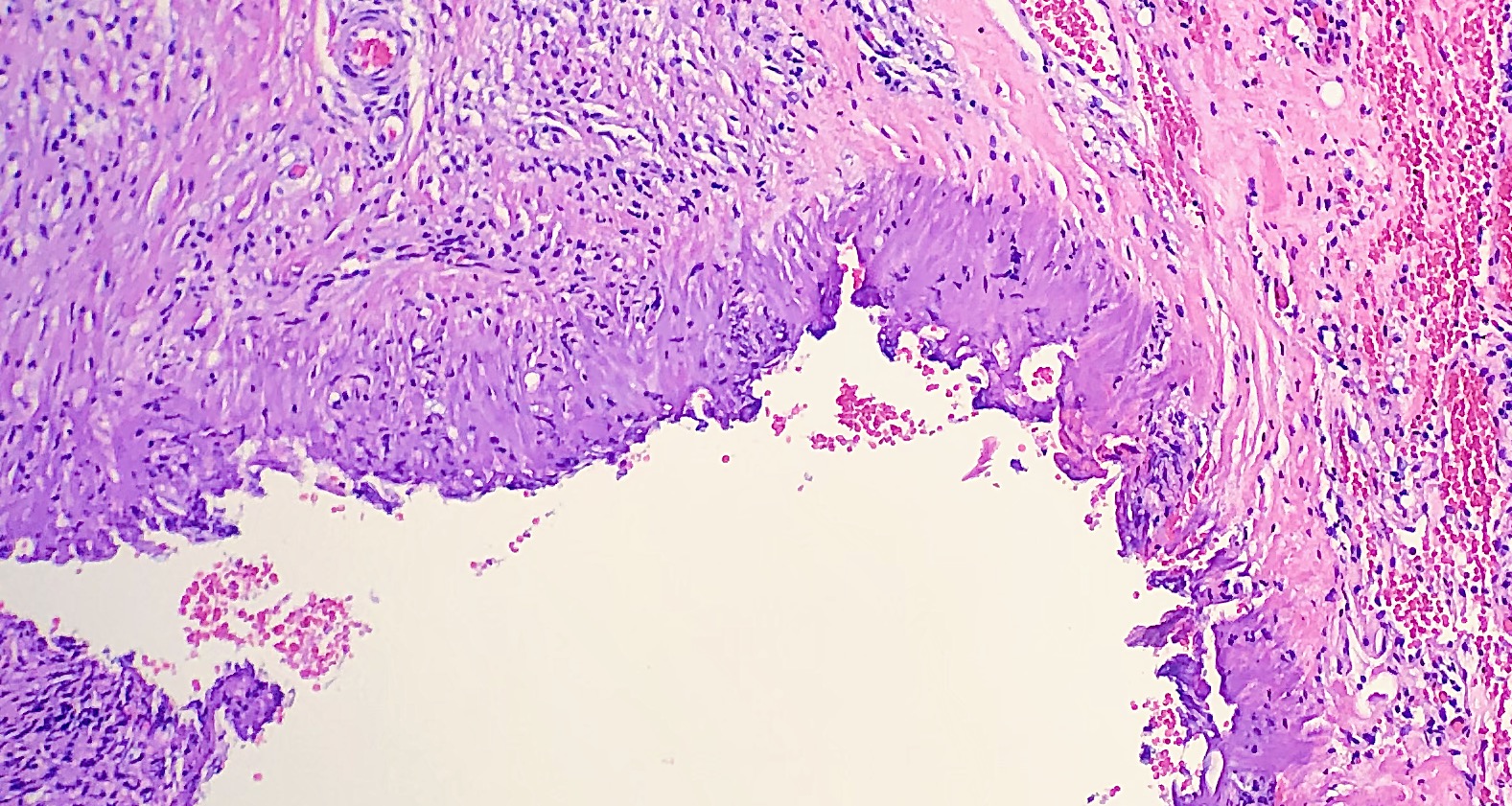
Tissue floater artifact refers to tissue specimens contaminated by pathology from extraneous tissue. The classic example of a floater artifact in dermatopathology is a piece of basal cell carcinoma contaminating an unrelated tissue specimen, leading to a diagnostic dilemma. Although the grossing process can induce tissue floaters, the most common cause of tissue floater introduction is contamination of the water bath.[21] (see Image. Floater Artifact)
Tissue Folds
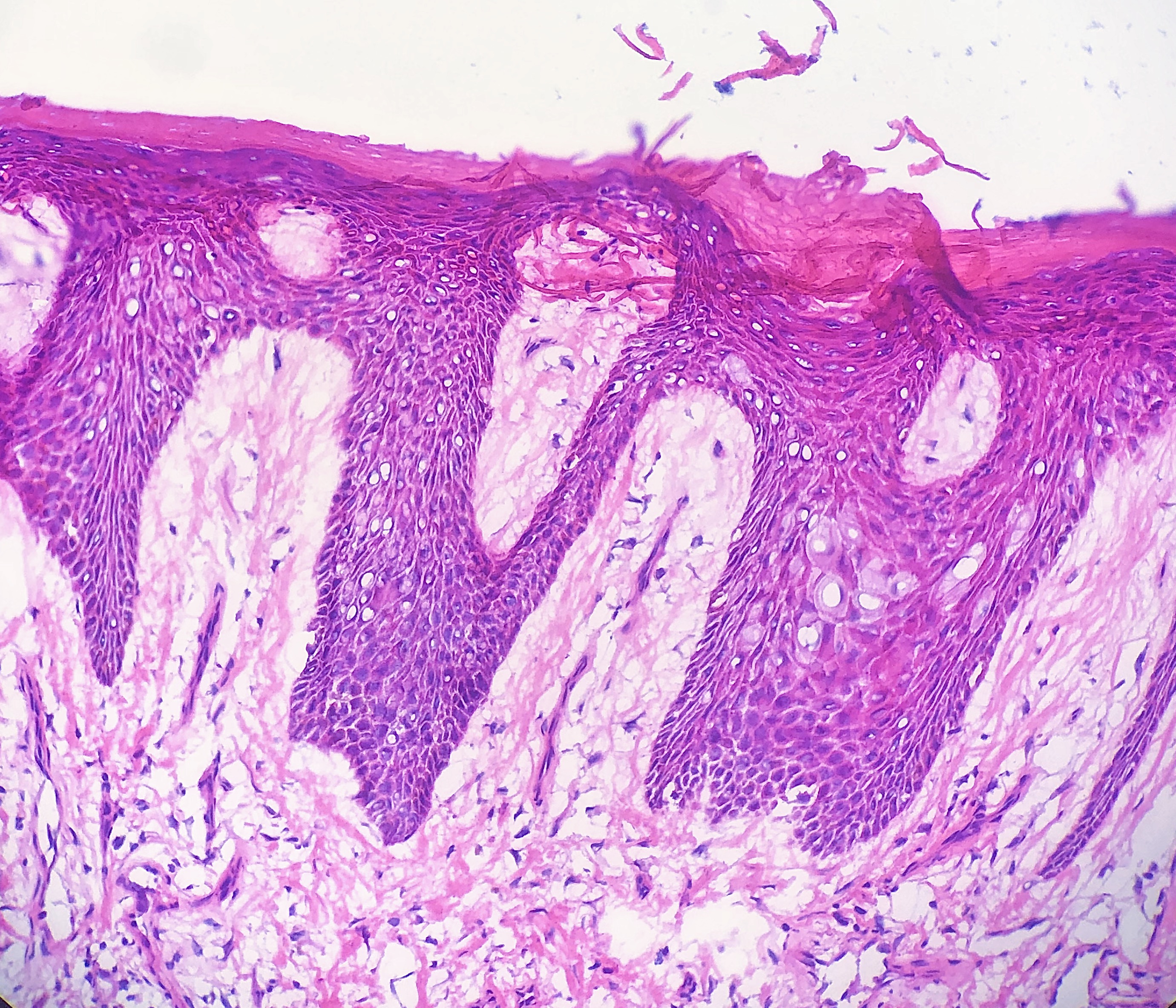
Tissue folds are among the most common artifacts encountered in daily practice. Tissue folds, or wrinkles, occur when thin sections fold over on themselves when placed onto the slide.[22] Tissue folds appear as darker stained sections due to the folded tissue being thicker and retaining more of the stain.[2] (see Image. Tissue Fold)
Tissue Scores/Tears
Tissue scoring artifact refers to a tissue tear extending linearly across the specimen.[2] The artifact results from imperfections in the microtome knife edge or "nicks" in the microtome blade. This artifact can result when sectioning hard material such as calcium.[2] (see Image. Tissue Tear)
Crush Artifact
Crush artifact refers to clusters of cells, often "small blue cells," with cellular details blending to become unrecognizable. Nuclei are less sensitive to crush artifacts and often appear stacked with distorted chromatin.[23]
Mounting Artifacts
Even while mounting the coverslip to the slide, there is potential for the insertion of artifacts. Air bubble entrapment can occur between the slide and coverslip.[9] Artifacts can also occur if a coverslip is mounted to a dry specimen or the mounting media peels away from the coverslip. Improper mounting can give a brown appearance over the areas where the coverslip is peeling away from the slide, making interpretation difficult. Distinguishing features of the specimen can be impossible to discern depending on the degree of artifact. (see Image. Improper Mounting)
Clinical Significance
The artifacts can be introduced before or during the procedure, in tissue processing, or during coverslip mounting. Dermatopathologists encounter artifacts daily. Some artifacts may be trivial at times and do not contribute to the primary process or hinder the interpretation of the specimen. However, understanding how and when these artifacts occur can help the pathologist decipher why the artifact is present and if it is of importance in the diagnosis.
The clinician is often aware of prefixation artifacts that are present. Clinicians should be familiar with potential pitfalls pathologists may encounter to understand when to communicate information about prefixation artifacts to the pathologist. Informing the pathologist that the site was previously biopsied could prevent confusion about a granulomatous reaction to ferrous subsulfate or suture material.
Artifacts related to fixation may result from inadequate fixation time, the volume of fixative, or the use of an improper fixative solution.[5][18] Understanding the potential for introducing artifacts with improper fixation techniques can ensure clinicians and pathologists follow conventional fixation protocols.
Tissue processing artifacts can lead to many different artifacts based on the stage in which they occur. Microtomy deficiencies account for Venetian blind artifacts or chatter and tissue tears. The water bath often accounts for tissue floaters. Despite these artifacts seeming benign and negligible, they may be of diagnostic importance. Tissue tears may denote a firm substance in the tissue, such as a foreign body or calcium.[2]
Mounting artifacts can make discernible features necessary for diagnosis impossible to detect. Troubleshooting the cause of the mounting artifact, whether it is a nonadherent coverslip or a missing coverslip, can aid the pathologist in correcting the issue to make the diagnosis.
Artifacts in dermatopathology specimens are common. An unlimited number of potential artifacts may appear on a slide. Understanding the possible etiologies and recognizing the specific artifacts can help pathologists avoid pitfalls during slide interpretation.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Seoane J, Varela-Centelles PI, Ramírez JR, Cameselle-Teijeiro J, Romero MA. Artefacts in oral incisional biopsies in general dental practice: a pathology audit. Oral diseases. 2004 Mar:10(2):113-7 [PubMed PMID: 14996282]
Taqi SA, Sami SA, Sami LB, Zaki SA. A review of artifacts in histopathology. Journal of oral and maxillofacial pathology : JOMFP. 2018 May-Aug:22(2):279. doi: 10.4103/jomfp.JOMFP_125_15. Epub [PubMed PMID: 30158787]
Level 2 (mid-level) evidenceMoraes RM, Gouvêa Lima Gde M, Guilhermino M, Vieira MS, Carvalho YR, Anbinder AL. Graphite oral tattoo: case report. Dermatology online journal. 2015 Oct 16:21(10):. pii: 13030/qt0z57p9xr. Epub 2015 Oct 16 [PubMed PMID: 26632800]
Level 3 (low-level) evidenceSampedro-Carrillo EA. Sample Preparation and Fixation for Histology and Pathology. Methods in molecular biology (Clifton, N.J.). 2022:2422():33-45. doi: 10.1007/978-1-0716-1948-3_3. Epub [PubMed PMID: 34859397]
Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods in molecular biology (Clifton, N.J.). 2014:1180():31-43. doi: 10.1007/978-1-4939-1050-2_3. Epub [PubMed PMID: 25015141]
Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. The American journal of pathology. 2002 Dec:161(6):1961-71 [PubMed PMID: 12466110]
Level 3 (low-level) evidenceAziz SJ, Zeman-Pocrnich CE. Tissue Processing. Methods in molecular biology (Clifton, N.J.). 2022:2422():47-63. doi: 10.1007/978-1-0716-1948-3_4. Epub [PubMed PMID: 34859398]
Carqueville JC, Chesnut C. Histologic Comparison of Upper Blepharoplasty Skin Excision Using Scalpel Incision Versus Microdissection Electrocautery Needle Tip Versus Continuous Wave CO2 Laser. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2021 Oct 1:47(10):1376-1378. doi: 10.1097/DSS.0000000000003178. Epub [PubMed PMID: 34352836]
Chatterjee S. Artefacts in histopathology. Journal of oral and maxillofacial pathology : JOMFP. 2014 Sep:18(Suppl 1):S111-6. doi: 10.4103/0973-029X.141346. Epub [PubMed PMID: 25364159]
Erickson QL, Clark T, Larson K, Minsue Chen T. Flash freezing of Mohs micrographic surgery tissue can minimize freeze artifact and speed slide preparation. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2011 Apr:37(4):503-9. doi: 10.1111/j.1524-4725.2011.01926.x. Epub [PubMed PMID: 21481069]
Level 3 (low-level) evidenceSanta Cruz DJ, Ulbright TM. Mucin-like changes in keloids. American journal of clinical pathology. 1981 Jan:75(1):18-22 [PubMed PMID: 7457425]
Balogh K. The histologic appearance of corticosteroid injection sites. Archives of pathology & laboratory medicine. 1986 Dec:110(12):1168-72 [PubMed PMID: 3778146]
Elston DM, Bergfeld WF, McMahon JT. Aluminum tattoo: a phenomenon that can resemble parasitized histiocytes. Journal of cutaneous pathology. 1993 Aug:20(4):326-9 [PubMed PMID: 8227609]
Gottesman SP, Junkins-Hopkins JM. Histochemical features of aluminum chloride histiocytic reaction and the use of PAS stain to provide a clue to prior subtle biopsy sites. Journal of cutaneous pathology. 2017 Nov:44(11):993-994. doi: 10.1111/cup.13023. Epub 2017 Sep 5 [PubMed PMID: 28875554]
Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. Journal of the American Academy of Dermatology. 1980 Nov:3(5):492-8 [PubMed PMID: 7217377]
Level 3 (low-level) evidenceBindhu P, Krishnapillai R, Thomas P, Jayanthi P. Facts in artifacts. Journal of oral and maxillofacial pathology : JOMFP. 2013 Sep:17(3):397-401. doi: 10.4103/0973-029X.125206. Epub [PubMed PMID: 24574659]
Torre-Castro J, Nájera L, Suárez D, García-Fresnadillo D, Freites-Martínez A, Briz AS, Rodríguez Peralto JL, Requena L. Histopathology of Dermatologic Complications of Tattoos. The American Journal of dermatopathology. 2022 Sep 1:44(9):632-649. doi: 10.1097/DAD.0000000000002183. Epub 2022 May 4 [PubMed PMID: 35503881]
Buesa RJ, Peshkov MV. How much formalin is enough to fix tissues? Annals of diagnostic pathology. 2012 Jun:16(3):202-9. doi: 10.1016/j.anndiagpath.2011.12.003. Epub 2012 Apr 5 [PubMed PMID: 22483550]
Sengupta S, Prabhat K, Gupta V, Vij H, Vij R, Sharma V. Artefacts produced by normal saline when used as a holding solution for biopsy tissues in transit. Journal of maxillofacial and oral surgery. 2014 Jun:13(2):148-51. doi: 10.1007/s12663-013-0473-z. Epub 2013 Jan 26 [PubMed PMID: 24822006]
Joshi R. 'Venetian blinds' artifact in dermatohistopathology. Indian dermatology online journal. 2012 Jan:3(1):59-61. doi: 10.4103/2229-5178.93497. Epub [PubMed PMID: 23130268]
Layfield LJ, Witt BL, Metzger KG, Anderson GM. Extraneous tissue: a potential source for diagnostic error in surgical pathology. American journal of clinical pathology. 2011 Nov:136(5):767-72. doi: 10.1309/AJCP4FFSBPHAU8IU. Epub [PubMed PMID: 22031316]
Kothari S, Phan JH, Wang MD. Eliminating tissue-fold artifacts in histopathological whole-slide images for improved image-based prediction of cancer grade. Journal of pathology informatics. 2013:4():22. doi: 10.4103/2153-3539.117448. Epub 2013 Aug 31 [PubMed PMID: 24083057]
Flamminio F, Tosi AL, Fellegara G. Crushing artifacts resulting in small blue cellular clusters that simulate small cell carcinoma. International journal of surgical pathology. 2011 Aug:19(4):487-91. doi: 10.1177/1066896911411187. Epub 2011 Jun 1 [PubMed PMID: 21632632]