Mohs Micrographic Surgery Evaluation and Treatment of Dermatofibrosarcoma Protuberans
Mohs Micrographic Surgery Evaluation and Treatment of Dermatofibrosarcoma Protuberans
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive soft tissue skin tumor.[1] DFSP originates from the dermal layer of the skin, typically manifesting as a slow-growing, indurated plaque or nodule.[2] The tumor's predilection for the trunk and proximal extremities reflects its origin in the reticular dermis.[3] DFSP rarely metastasizes. However, some tumors show high-grade sarcomatous change, potentially impacting evaluation and treatment.[4] DFSP spreads locally along tissue planes, infiltrating adjacent structures while respecting tissue boundaries. Metastasis to regional lymph nodes or distant sites is rare but usually involves high-grade or fibrosarcomatous transformation. Micrographic dermatologic surgery (MDS) is vital in achieving tumor-free margins while preserving function and cosmesis.[5] MDS, including Mohs micrographic surgery (MMS) and other peripheral and deep en-face margin assessment techniques, has become a cornerstone in managing DFSP, offering precise tumor removal while sparing healthy tissue.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of DFSP remains incompletely understood, with no definitive causative factors identified. However, several genetic and environmental factors have been implicated in its pathogenesis.[7] Genetic aberrations play a central role in DFSP development, with over 90% of cases characterized by a specific chromosomal translocation involving chromosomes 17 and 22. This translocation, t(17;22)(q22;q13), results in the fusion of the type I α1 collagen (COL1A1) and platelet-derived growth factor-β (PDGF-β) genes, leading to constitutive activation of the PDGF-β receptor.[8] Activation of this receptor promotes cell proliferation and angiogenesis, contributing to DFSP tumorigenesis.
Besides genetic predisposition, environmental factors such as ultraviolet (UV) radiation exposure and trauma have been proposed as potential contributors to DFSP development.[9] UV radiation-induced deoxyribonucleic acid (DNA) damage may exacerbate genomic instability, facilitating the accumulation of genetic mutations conducive to DFSP progression.[10] Trauma, particularly repetitive injury or scarring, has been suggested to trigger DFSP growth in predisposed individuals, although the exact mechanisms remain unclear.
Epidemiology
DFSP accounts for approximately 1% of all soft tissue sarcomas in the United States, translating to an estimated annual incidence of 4.1 cases per million individuals. The condition typically affects adults in their 3rd to 5th decades of life, with a median age at diagnosis ranging from 30 to 40 years. However, cases have been reported across all age groups, including pediatric and older adult populations. Studies have not demonstrated a clear sex predominance. Racial and ethnic disparities in DFSP incidence have been noted, with almost double the incidence in Black patients compared to White patients.[11] Internationally, DFSP incidence rates vary across different regions and populations. Limited population-based studies and registries hinder the comprehensive assessment of global DFSP epidemiology.
Histopathology
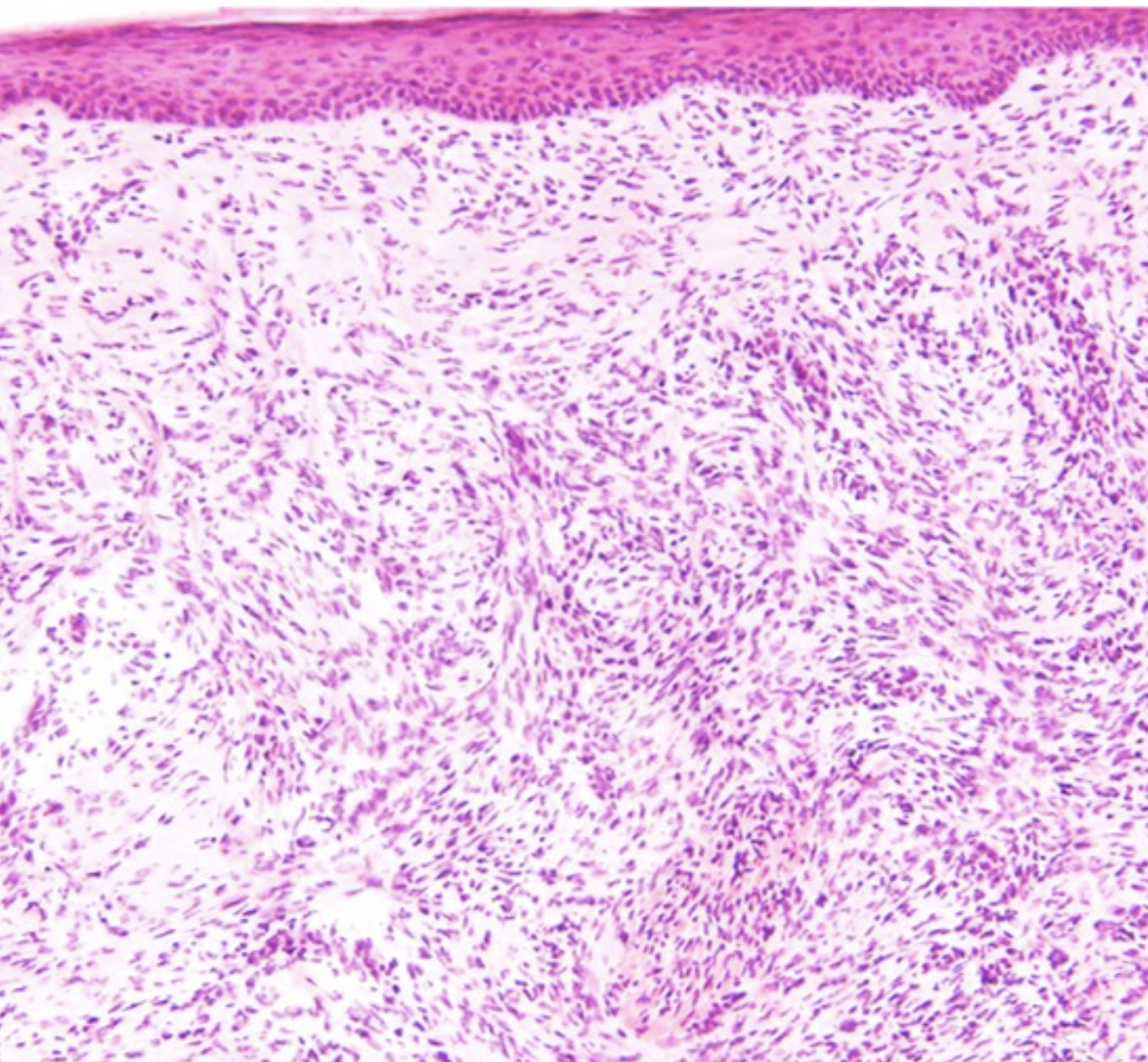
Histopathologically, DFSP is characterized by spindle-shaped fibroblast-like cells arranged in a storiform or cartwheel pattern within the dermis.[12] These cells typically exhibit low-to-moderate nuclear atypia and scant cytoplasm (see Image. Dermatofibrosarcoma Protuberans Histology).[13] Collagen deposition and myxoid changes may also be observed within the tumor stroma. Immunohistochemical staining often shows positivity for CD34, a marker commonly associated with DFSP.[13]
Despite its benign histological appearance, DFSP is locally aggressive, tends to infiltrate surrounding tissues, and recurs if not adequately excised with clear margins. Rarely, DFSP undergoes fibrosarcomatous transformation, characterized by increased cellularity, nuclear pleomorphism, and mitotic activity, indicating a more aggressive phenotype (see Image. Fibrosarcomatous Change).[14]
History and Physical
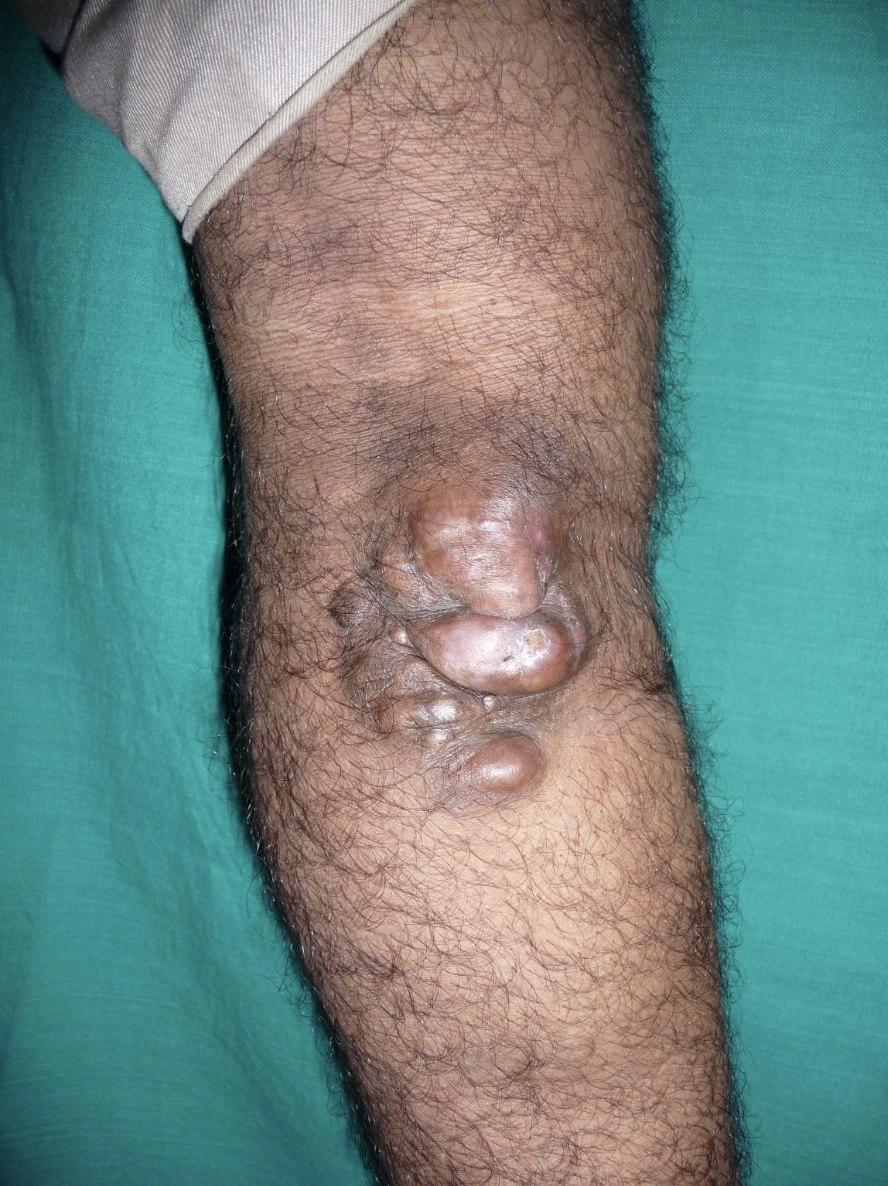
DFSP presents variably, often posing diagnostic challenges due to its insidious growth pattern and nonspecific symptoms. Patients commonly report a slow-growing, painless, firm, indurated skin plaque or nodule, which may mimic benign lesions such as dermatofibroma or keloid scars (see Image. Dermatofibrosarcoma Protuberans, Right Lower Extremity).[15]
DFSP most commonly occurs on the trunk, proximal extremities, head, and neck.[16][17][18] The lesion typically arises in the skin's dermal layer and may exhibit a reddish-brown or violaceous hue, although coloration can vary. The typical DFSP lesion is characterized by its firm consistency and tendency to protrude above the skin surface, thus its protuberant appearance. The lesion may be well-demarcated or ill-defined, with a rubbery or fibrotic texture upon palpation. Advanced DFSP may also exhibit overlying telangiectasias or ulceration.[19] Tumor diameter can range from 0.5 cm to greater than 10 cm, with an average of 2 to 3.5 cm.[20][21]
Evaluation
The evaluation of DFSP typically involves a combination of clinical assessment, histopathological examination, and imaging studies to establish a definitive diagnosis and guide treatment planning. Much of the evaluation is guided by National Comprehensive Cancer Network guidelines for DFSP. Interprofessional consultation in treatment centers experienced in DFSP should be considered, especially for patients with large or recurrent DFSP.
Clinical Evaluation
Clinical examination remains the cornerstone of DFSP evaluation, with thorough inspection and palpation of the lesion to assess its size, location, consistency, and associated symptoms. Other relevant history and physical examination, including family history, social history, personal medical history, and a complete skin examination, are pertinent in evaluating a patient with a lesion suspicious of DFSP. Dermoscopy may aid the clinical diagnosis by revealing characteristic features, such as a pigment network, linear vessels, and a central, white, scar-like area.[22] However, definitive diagnosis requires histopathological confirmation through biopsy or surgical excision, specifically a punch, incisional, or core biopsy, to ensure adequate tissue sampling through the deeper subcutaneous layer.
Histopathologic Evaluation
Histopathological examination of a biopsy specimen is essential for diagnosing DFSP and differentiating it from other cutaneous neoplasms. Qualified and experienced pathologists should evaluate lesions suspicious for DFSP. The characteristic histological features of DFSP include spindle-shaped fibroblast-like cells arranged in a storiform or cartwheel pattern within the dermis, often accompanied by collagen deposition and myxoid changes. Immunohistochemical staining for CD34 is typically positive in DFSP, supporting the diagnosis. Staining negative for factor XIIIa would differentiate DFSP from its benign counterpart.[23]
A nondiagnostic specimen should be rebiopsied without undermining to prevent difficulty interpreting reexcisions. Fluorescent in situ hybridization for platelet-derived growth factor receptor (PDGFR) translocation should be performed for equivocal lesions. Polymerase chain reaction and conventional cytogenetics may also be used to confirm characteristic genetic changes.[24]
Debulking specimens from the biopsy should be evaluated thoroughly to rule out fibrosarcomatous transformation. If observed, the lesion will need further evaluation as soft tissue sarcoma for multimodal therapy. Lesions with fibrosarcomatous changes are considered soft tissue sarcomas of the extremity, body wall, head, or neck. Besides adequate imaging, a core needle (preferred) or incisional biopsy should be performed. Ancillary diagnostic methods may be needed. Additional imaging may be indicated in select patients with genetic tumor syndromes, including patients with neurofibromatosis, Li-Fraumeni syndrome, and hereditary nonpolyposis colorectal cancer syndrome (ie, Lynch syndrome).
Radiographic Evaluation
Radiographic imaging, such as ultrasonography, magnetic resonance imaging (MRI), and computed tomography (CT), may be warranted to assess tumor depth, size, and extent of local invasion, particularly for further evaluation of inconclusive cases or planning surgical intervention. MRI is the preferred imaging modality for evaluating DFSP, offering superior soft tissue contrast resolution and multiplanar imaging capabilities.[25] MRI defines the tumor's size, extent, and association with neighboring neuromuscular structures and bone. MRI is thus a useful modality for preoperative and postoperative evaluation, especially if extensive subcutaneous extension of the DFSP is evident.[26] Staging evaluations, including chest radiography or CT scans, may also be recommended to assess for metastatic disease in high-risk cases or in the presence of suspicious clinical findings. However, routine staging imaging is not typically indicated for localized DFSP without evidence of advanced disease or concerning symptoms.
Treatment / Management
DFSP management requires a comprehensive approach aimed at achieving complete tumor excision while minimizing morbidity and reducing the risk of recurrence. Treatment modalities encompass surgical, medical, and, in select cases, adjuvant therapies tailored to the individual patient's disease characteristics and clinical presentation.
Surgical excision should be performed with MDS with peripheral and deep en-face margin assessment. If the tumor is borderline or unresectable, tumor mutation analysis and neoadjuvant imatinib should be considered.[27] Regular monitoring should be initiated for tumors with negative margins, while those with positive margins should be reexcised where possible. When positive margins remain, or surgery becomes infeasible, patients should undergo interprofessional evaluation and management, which may include radiation therapy or other medical interventions.[28](A1)
Surgical Interventions
Surgical excision remains the treatment mainstay for localized DFSP, with the primary goal of achieving tumor-free margins while preserving function and cosmesis. MDS, including MMS or other forms of peripheral and deep en-face margin assessment, has emerged as a preferred surgical technique for managing DFSP, offering precise tumor removal with high cure rates while sparing healthy tissue.[29] MMS involves serial excision and examination of tissue layers under microscopy until tumor-free margins are achieved, minimizing the risk of recurrence and maximizing tissue conservation, particularly in cosmetically sensitive areas or tumors with ill-defined borders.
Notably, DFSP can occasionally exhibit "skip growth" with neoplastic cells extending past the clinically apparent tumor margins. This phenomenon may lead to incomplete excision of the tumor despite the meticulous layer-by-layer strategy characteristic of MMS. Extensive undermining postsurgical excision is also not recommended, as it could potentially seed malignant cells if the tumor is not completely resected. This measure may also complicate the interpretation of subsequent reexcisions. Tumors initially excised with positive margins or those that have relapsed warrant further excision to attain clear margins whenever feasible.
For surgical treatment of DFSP, tumor bed excision should be based on tumor infiltration depth. Superficial (stages I and IIA) tumors may be excised without including the underlying fascia. In contrast, excision of the muscle or periosteum's investing fascia is recommended for deeper (stage IIB) tumors. Due to limited space and complex structures, the digits pose a challenge for wide local excision (WLE). MMS appears to be the more appropriate treatment modality for DFSP of the digits, keeping in mind that periosteal involvement may necessitate partial or total amputation of the digit to achieve tumor-free margins.[30](B3)
Reconstruction should be postponed until a thorough examination confirms that all peripheral and deep margins are negative. Presently, no consensus exists on the ideal width of margins. Results from a 1997 study of 58 patients with primary and recurrent DFSP treated with MMS found that 70% of patients had positive margins with a 1 cm margin, 39.7% with a 2 cm margin, 15.5% with a 3 cm margin, and 5.2% with a 5 cm margin.[31]
Further, results from a 2022 single-center study of 222 DFSP cases treated with MMS found that an average of 1.47 stages and a minimum margin of 1.23 cm were required for tumor clearance. Tumors located in the head and neck region necessitated more stages and a significantly wider margin. Tumor size also showed a positive correlation with time to diagnosis, the patient’s age, and the number of stages taken.[32](B3)
The literature has shown significant variation in local recurrence rates with different surgical margins. Postponing reconstruction after MMS is advised for cases necessitating extensive resection or tissue manipulation. This approach enables confirmation of negative margins before proceeding with reconstruction.[33] Reconstruction options include rotational flaps or full- or split-thickness skin grafts. The choice of reconstruction depends on multiple factors, including the defect's size, depth, and location.
Slow Mohs, also known as paraffin MMS, is preferred for evaluating tumors with high-risk histological characteristics like DFSP. This modified MMS method utilizes paraffin-embedded permanent sections and requires at least 24 to 48 hours of processing time.[34] In contrast to conventional MMS, paraffin MMS enables tissue morphology preservation, complete tumor staging, residual tumor tissue identification, and the ability to perform immunohistochemistry studies.[35][36] However, given the apparent practical advantages of frozen MMS, both techniques may be considered effective.(B3)
Appropriate Use Criteria
According to the MMS appropriate use criteria, MMS is appropriate for DFSP in all locations and patient types.[37] In cases where MMS is not feasible or is contraindicated, WLE with 2- to 3-cm margins extending to the fascia may be performed, followed by a histopathological examination to confirm clear margins.[38] MMS has demonstrated a lower local recurrence rate than WLE when utilized for DFSP.[39][40][41][42] Conclusions from a 2024 meta-analysis of 136 cases revealed that the disease-specific mortality rate was not significantly different between patients treated with MMS vs WLE for DFSP. However, for recurrent tumors, the MMS treatment cohort exhibited a statistically significant reduction in disease-specific mortality compared to patients treated with WLE.[43] MMS is also less costly than WLE for all surgical sites.[44](A1)
Potential Drawbacks
Drawbacks of MMS include the potential for tumor cells to be mistaken for normal dermal spindle cells in frozen sections and the high CD34-staining variability of frozen sections. Some authors question the reliability of this technique altogether.[45][46] This concern may be addressed by excising an extra layer and sending it for permanent paraffin-embedded assessment once negative frozen margins are achieved. However, clearing deep margins can be challenging if the tumor has invaded the underlying fascia or muscle.(B3)
A study of 40 cases demonstrated that 43% of patients needed resection of fascia, muscle, or periosteum to achieve tumor clearance.[47] MMS procedures can also be intricate, time-consuming, and necessitate extensive procedures for patients and subsequent reconstruction.[48] For tumors invading deep structures, eg, bone, nerves, major blood vessels, MMS may be used for marginal surfaces with an interprofessional discussion of nonvisualized areas.
Recurrence and Metastasis
Recurrence warrants tumor re-resection. Radiation therapy should be considered if it was not previously used and resection is infeasible. Imatinib therapy may be used if the disease is unresectable or resection cannot be performed due to anatomic restrictions. Patients with metastatic lesions should have interprofessional consultations to determine the best individualized treatment approach, possibly including surgery. Radiation therapy is not recommended if MDS is used. Otherwise, radiation therapy may be considered for surgical cases rendering positive margins if not previously used and re-resection is infeasible.
DFSP with fibrosarcomatous differentiation (DFSP-FS) is a highly aggressive DFSP subtype with 50% local recurrence and 10% to 15% metastasis rates.[49][50] DFSP-FS treatment includes WLE with clear margins or MMS for stages IA, IB, II, and III disease. Other adjuvant therapies (eg, systemic or radiation therapy) may also be needed to achieve tumor clearance. Resectable stage-II, -III, and -IV disease with unacceptable functional outcomes or unresectable primary disease may require radiation therapy, chemoradiation, systemic therapy, isolated limb perfusion/infusion, or amputation/radical resection.[51](B3)
Synchronous stage IV disease with oligometastases with limited tissue bulk amenable to local therapy should undergo similar treatments for earlier-stage disease. However, patients with diffuse metastases may also consider metastasectomy, stereotactic body radiation therapy, ablation, embolization (for nonlung metastases), observation, or palliative care. These same treatments are considered for metastatic disease and recurrences.[52](B2)
Adjuvant Therapy
Adjuvant therapies, such as radiotherapy or systemic therapy, may be considered in select DFSP cases with high-risk features, including large tumor size, positive margins, and unresectability. Radiotherapy may be employed as adjuvant therapy following surgical excision to reduce the risk of local recurrence, particularly in cases with close or positive margins. Systemic therapies, including imatinib, a tyrosine kinase inhibitor targeting the platelet-derived growth factor-β receptor, may be considered in unresectable, metastatic, or recurrent DFSP. However, further research is needed to define optimal treatment strategies and long-term outcomes.[53](A1)
Differential Diagnosis
Key clinical and histopathologic differential diagnoses that must be considered when evaluating for DFSP include the following:
- Dermatofibroma
- Schwannoma
- Cutaneous neurofibroma
- Solitary fibrous tumor
- Intradermal spindle cell lipoma
- Spindle cell/desmoplastic melanoma
While these diagnoses should be considered in the differential diagnosis, each entity has features distinguishing it from DFSP.[13] For example, dermatofibroma stains negative for CD34, spindle cell lipomas are S100-positive, and spindle cell melanoma expresses S100, SOX-10, and glial fibrillary acidic protein. Accurate diagnosis requires careful clinical evaluation and histopathological examination facilitated by collaboration among dermatologists, dermatopathologists, and surgical oncologists to ensure appropriate management and optimize patient outcomes.
Radiation Oncology
Radiation therapy plays a significant role in DFSP management, particularly as adjuvant therapy following surgical excision in cases with high-risk features or positive margins. Adjuvant radiation therapy should be considered for resected tumors with positive margins or gross disease. Doses typically range from 50 to 60 Gy for indeterminate or positive margins and up to 66 Gy for positive margins or gross tumor, given in 2-Gy fractions daily. The treatment can extend 3 to 5 cm beyond surgical margins when feasible.[28] Radiation therapy is recommended for negative margins only if MDS is not used. The same radiation therapy is recommended for positive margins if relapse or metastasis occurs previous radiation therapy has not been administered, or surgery is infeasible.[54][55]
Studies show that adjuvant radiation therapy following surgical excision significantly reduces the risk of local recurrence and may improve long-term outcomes in patients with DFSP. However, the optimal timing, dose, and fractionation of radiation therapy remain areas of ongoing research and debate. Some authors recommend that 60 Gy for indeterminate/microscopic positive margins and 70 Gy for macroscopic positive margins should be utilized. Individual doses may be administered 5 times a week at 2 Gy daily. Additionally, ongoing clinical trials are investigating novel radiation techniques, such as intensity-modulated radiation therapy (IMRT) and proton beam therapy, to improve treatment efficacy and minimize toxicity in patients with DFSP.
For DFSP with fibrosarcomatous change, multiple radiation therapy strategies are suggested based on the type of treatment. Neoadjuvant radiation therapy should include a preoperative dose of 50 to 50.4 Gy (1.8-2 Gy per fraction) using electron beam therapy, 3-dimensional conformal radiation therapy, IMRT, or proton beam therapy with imaging. An additional 14 to 20 Gy may be used with fractionated external beam radiation therapy (EBRT) or brachytherapy if appropriate.[56][57]
Adjuvant radiation therapy should be performed on postoperative disease, with EBRT doses of 50 to 50.4 Gy at 1.8 to 2 Gy per fraction and a 10- to 16-Gy boost as tolerated. If brachytherapy is combined with EBRT, 16- to 20-Gy or 14- to 16-Gy brachytherapy with 50-Gy EBRT should be used for positive margins, while 45-Gy or 36-Gy brachytherapy (3.6 Gy twice daily for 10 fractions in 5 days) should be used for tumors with negative margins.[58][59] For definitive radiation therapy in unresectable disease, initial doses of 50 Gy with a boost of 63 Gy may be used, though higher doses up to 70 to 80 Gy may be considered based on tolerance.[60]
Medical Oncology
Medical oncology plays a crucial role in treating DFSP, particularly when surgical intervention is infeasible, or the tumor recurrence risk is high. A significant advancement in the medical management of DFSP is targeted therapy, particularly with imatinib mesylate. Imatinib, a tyrosine kinase inhibitor, has shown remarkable efficacy in managing DFSP, especially when complete surgical resection is challenging due to tumor location, size, or involvement of critical structures. The drug inhibits the activity of specific tyrosine kinases, including platelet-derived growth factor receptors, which are often overexpressed in DFSP.
Clinical studies have demonstrated the effectiveness of imatinib in controlling DFSP growth, inducing tumor regression, and improving progression-free survival rates.[61] The decision to initiate imatinib therapy is based on various factors, including disease extent, tumor size, location, and metastasis. Treatment regimens typically involve oral administration at a recommended dose of 400 to 800 mg daily, adjusted to individual response and tolerance. Other tyrosine kinase inhibitors, such as sunitinib and sorafenib, have been used as additional treatment options for DFSP.[62]
For DFSP with fibrosarcomatous change, medical therapy depends on whether a regimen is used as neoadjuvant/adjuvant, first-line, or subsequent-line therapy. The preferred regimen for neoadjuvant/adjuvant therapy includes ifosfamide and mesna with either doxorubicin or epirubicin. Gemcitabine, docetaxel, or trabectedin may be useful in certain circumstances.[63][64] For first-line therapy of advanced or metastatic disease, the preferred regimen may include combinations of doxorubicin, epirubicin, dacarbazine, ifosfamide, larotrectinib, or entrectinib. Gemcitabine, docetaxel, pazopanib, trabectedin, and selpercatinib (RET fusion-positive tumors) may be useful in select circumstances.[65][66] For subsequent-line therapy of advanced or metastatic disease, the preferred regimen may include combinations of pazopanib, eribulin, trabectedin, gemcitabine, docetaxel, dacarbazine, ifosfamide, temozolomide, vinorelbine, and regorafenib. Vinorelbine with gemcitabine, pazopanib with gemcitabine, pembrolizumab, nivolumab with ipilimumab, or cabozantinib may be useful in certain circumstances.[67][68]
Staging
DFSP staging currently lacks a standard system. However, a modified staging system has been proposed by Hao et al, outlined below.
- Stage I tumors are nonprotuberant lesions that may include atrophic/sclerotic plaques, maculae, or small nodules.
- Stage II includes protuberant primary tumors, further divided into IIA and IIB.
- Stage IIA tumors are superficial and do not invade the underlying fascia.
- Stage IIB tumors are deeper, either located superficial to the fascia with deeper infiltration or occurring entirely beneath the superficial fascia.
- Stage III tumors include those with lymph node metastasis.
- Stage IV tumors include those with metastasis to distant organs.
Prognosis
The prognosis of DFSP is generally favorable, with a low risk of metastasis and high rates of long-term survival, mainly when diagnosed and treated early. The overall prognosis is primarily influenced by factors such as tumor size, depth of invasion, margin status, and presence of metastasis. After surgical excision with margin evaluation, DFSP has 5- and 10-year recurrence-free survival rates of 86% and 76%, respectively. Male sex, Black race, head/limb tumor location, increased tumor size, fibrosarcomatous transformation, positive margins, and advanced age are all associated with a decreased overall survival rate. Recurrence rates after excision range from 20% to 50%, with an average time to recurrence of 32 to 38 months. Long-term surveillance every 6 to 12 months is thus encouraged.
Complications
MMS is highly effective in treating DFSP. However, MMS carries certain risks, as with other surgical procedures. Possible complications include seeding tumor cells, delayed wound healing, wound dehiscence, infection, and cosmetic concerns such as noticeable scars or skin texture changes. Depending on tumor size and location, MMS may also lead to functional impairment or limitations in range of motion, particularly in areas involving critical anatomical structures.
Despite its high cure rates, the risk of local recurrence following MMS remains, especially in cases with close or positive surgical margins. DFSP can occasionally exhibit skip growth, with neoplastic cells extending beyond the clinically apparent tumor margins. This occurrence produces microscopic disease foci that can evade initial detection. This characteristic poses a challenge for MMS, as the focal clusters of skipped tumor cells may remain undetected and lead to incomplete excision, increasing the risk of local recurrence. Rare complications include nerve damage, bleeding, hematoma formation, and scar contracture. Mitigating these risks requires careful preoperative evaluation, meticulous surgical technique, and diligent postoperative care, emphasizing the importance of close communication between the surgical team and the patient to optimize outcomes and address concerns following MMS for DFSP.
Postoperative and Rehabilitation Care
Postoperative care following MMS for DFSP is essential to promote wound healing, prevent complications, and optimize functional and cosmetic outcomes. Patients should be instructed to keep the surgical site clean and dry, avoiding excessive manipulation or trauma to the area. Wound care instructions, including dressing changes and wound monitoring, should be provided, and close follow-up should be scheduled to assess healing progress and monitor for signs of infection or other complications.
Depending on the size and location of the tumor, patients may benefit from physical therapy or rehabilitation exercises to restore range of motion, strength, and function in affected areas. Early mobilization and gentle stretching exercises can help prevent stiffness and promote tissue healing, particularly when DFSP involves critical anatomical structures or requires extensive tissue excision.
Cosmetic concerns following MMS for DFSP should be addressed through appropriate wound management and scar care techniques. Patients should be educated on scar massage, silicone gel application, and sun protection measures to minimize scar hypertrophy, pigmentation changes, and other aesthetic issues. Long-term follow-up care is essential to monitor for signs of local recurrence, assess functional and cosmetic outcomes, and address any concerns or complications that may arise. Patients should be encouraged to maintain regular dermatologic surveillance every 6 to 12 months to detect any potential recurrence or new skin lesions early.
Deterrence and Patient Education
Patient education plays a crucial role in the deterrence and management of DFSP. Raising awareness among patients about the signs and symptoms of DFSP is essential, emphasizing the importance of early detection and prompt medical evaluation for any suspicious skin lesions. Patients should be educated about DFSP risk factors, including previous trauma or surgical scars, and encouraged to perform regular skin self-examinations to monitor for changes in existing lesions or the development of new ones.
Furthermore, patients should be informed about the significance of seeking care from dermatologists or healthcare providers with expertise in skin cancer diagnosis and management. Patients should understand the diagnostic and treatment options available for DFSP, including MMS and WLE, and be actively involved in shared decision-making regarding their care. Sun protection measures, including sunscreen, protective clothing, and seeking shade during peak UV hours, should also be emphasized to reduce the risk of developing DFSP and other malignancies. Additionally, patients should be educated about the importance of regular dermatologic surveillance and long-term follow-up care post-DFSP treatment to monitor for recurrence and ensure optimal outcomes.
Enhancing Healthcare Team Outcomes
Successfully managing DFSP with MMS necessitates a highly coordinated, interprofessional healthcare team approach. Physicians, including dermatologic surgeons and surgical oncologists, lead the team and apply their expertise in skin cancer diagnosis and MMS techniques. Clinicians must possess advanced procedural skills, meticulous attention to detail, and sound clinical judgment to ensure complete tumor removal while preserving surrounding healthy tissue.
Referral to an interdisciplinary oncology team (tumor board) with broad knowledge and experience with DFSP is important, especially in fibrosarcomatous DFSP cases. Collaborative multidisciplinary management proves beneficial for managing infiltrative DFSP of the head and neck and large tumors on the trunk. In this approach, the Mohs surgeon conducts tumor mapping and histologic examination of margins in coordination with another ablative surgeon.[69]
Results from a 2023 study regarding socioeconomic factors associated with MMS for DFSP demonstrated that MMS was more commonly performed at academic centers, likely due to increased resources and access to interprofessional experts. Higher incomes were also found to be predictive of MMS, revealing potential healthcare disparities. Advanced practitioners, such as physician assistants and nurse practitioners, collaborate closely with physicians to provide comprehensive patient care throughout the MMS process. These providers play key roles in patient assessment, intraoperative assistance, wound care, and postoperative follow-up, serving as liaisons between patients and the healthcare team.
Nurses are integral members of the MMS team, responsible for preoperative preparation, intraoperative assistance, and postoperative care. Nursing staff members ensure patient safety, comfort, and adherence to infection control protocols during MMS procedures. Nurses also provide patient education, wound assessment, and symptom management, facilitating optimal recovery and outcomes.
Pharmacists contribute expertise in medication management, ensuring appropriate perioperative pharmacotherapy and minimizing potential drug interactions or adverse effects. These providers collaborate with the healthcare team to optimize pain management, prophylactic antibiotic use, and wound healing—promoting patient safety and treatment efficacy.
Ethical considerations, including informed consent, patient autonomy, and beneficence, guide healthcare team members in providing patient-centered care during MMS for DFSP. Respect for patient preferences, cultural sensitivity, and confidentiality fosters trust and rapport between patients and the healthcare team, enhancing patient satisfaction and treatment adherence. By leveraging their collective skills, expertise, and resources, the healthcare team can optimize patient-centered care, minimize complications, and achieve favorable outcomes. Collaborative efforts among healthcare professionals facilitate efficient procedural workflows, streamline patient care pathways, and promote a culture of excellence in DFSP management.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
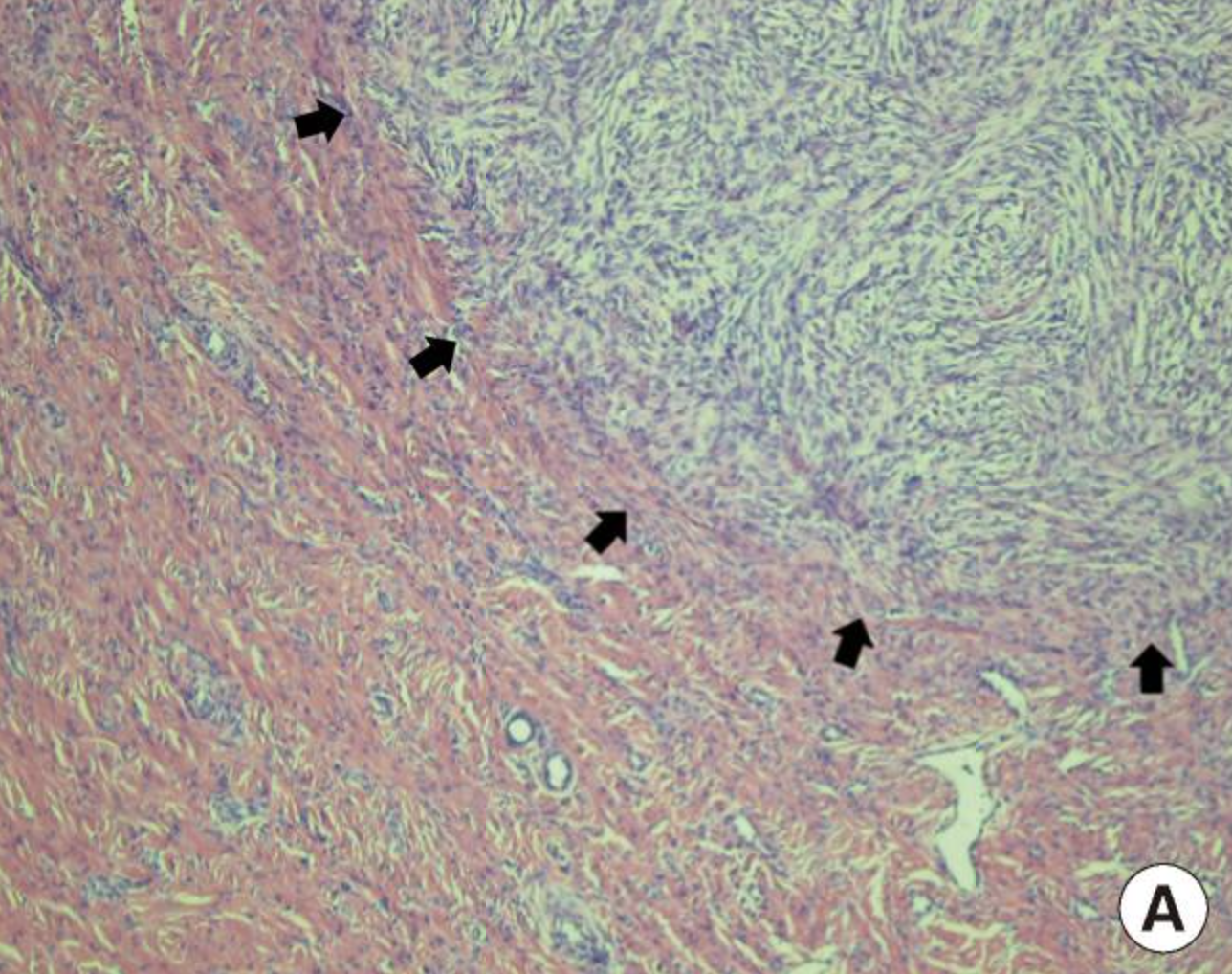
Fibrosarcomatous Change. Dermatofibrosarcoma protuberans (DFSP) with fibrosarcomatous change, thigh. Hematoxylin-and-eosin-stained DFSP with fibrosarcomatous component indicated by black arrows at 100 times magnification.
Used with permission via Creative Commons license (open-access article) from: Kim SM, Rha EY, Jung SN, Lim JS, Yoo G, Byeon JH. Dermatofibrosarcoma protuberans with pulmonary metastasis in the absence of local recurrence. Arch Plast Surg. 2012;39(3):265-267.
(Click Image to Enlarge)
References
Yoshida KI, Fujii J, Honma K, Nakai S. An unusual presentation of dermatofibrosarcoma protuberans: A case of fibrosarcomatous dermatofibrosarcoma protuberans with pleomorphic angiectatic tumor-like changes. Journal of cutaneous pathology. 2024 Aug:51(8):609-613. doi: 10.1111/cup.14641. Epub 2024 May 6 [PubMed PMID: 38711216]
Level 3 (low-level) evidenceVitiello GA, Lee AY, Berman RS. Dermatofibrosarcoma Protuberans: What Is This? The Surgical clinics of North America. 2022 Aug:102(4):657-665. doi: 10.1016/j.suc.2022.05.004. Epub [PubMed PMID: 35952694]
King L, López-Terrada D, Jakacky J, McCarville MB, Spunt SL, Bridge JA, Bahrami A. Primary intrathoracic dermatofibrosarcoma protuberans. The American journal of surgical pathology. 2012 Dec:36(12):1897-902. doi: 10.1097/PAS.0b013e31826b7919. Epub [PubMed PMID: 23108023]
Ramirez-Fort MK, Meier-Schiesser B, Niaz MJ, Niaz MO, Feily A, Fort M, Lange CS, Caba D. Dermatofibrosarcoma Protuberans: The Current State of Multidisciplinary Management. Skinmed. 2020:18(5):288-293 [PubMed PMID: 33160438]
Mansilla-Polo M, Morgado-Carrasco D, Toll A. Review on the Role of Paraffin-embedded Margin-controlled Mohs Micrographic Surgery to Treat Skin Tumors. Actas dermo-sifiliograficas. 2024 Jun:115(6):T555-T571. doi: 10.1016/j.ad.2024.04.019. Epub 2024 Apr 21 [PubMed PMID: 38648936]
Sanabria A, Pinillos P, Chiesa-Estomba C, Guntinas-Lichius O, Kowalski LP, Mäkitie AA, Rao KN, Ferlito A. Comparing Mohs micrographic surgery and wide local excision in the management of head and neck dermatofibrosarcoma protuberans: a scoping review. The Journal of dermatological treatment. 2024 Dec:35(1):2295816. doi: 10.1080/09546634.2023.2295816. Epub 2023 Dec 26 [PubMed PMID: 38146660]
Level 2 (mid-level) evidenceJouary T, Beltran C, Coindre JM, Plagnol P, Taieb A, Ebran N, Pédeutour F, Delaunay M. Dermatofibrosarcoma protuberans occurring in two brothers: Role of environmental or genetic factors? Journal of the American Academy of Dermatology. 2007 Aug:57(2 Suppl):S58-60 [PubMed PMID: 17637382]
Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2016 Jan:42 Suppl 1():S24-31. doi: 10.1097/DSS.0000000000000300. Epub [PubMed PMID: 26730971]
Laikova KV, Oberemok VV, Krasnodubets AM, Gal'chinsky NV, Useinov RZ, Novikov IA, Temirova ZZ, Gorlov MV, Shved NA, Kumeiko VV, Makalish TP, Bessalova EY, Fomochkina II, Esin AS, Volkov ME, Kubyshkin AV. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules (Basel, Switzerland). 2019 Apr 17:24(8):. doi: 10.3390/molecules24081516. Epub 2019 Apr 17 [PubMed PMID: 30999681]
Level 3 (low-level) evidenceBehroozan DS, Glaich A, Goldberg LH. Dermatofibrosarcoma protuberans following tanning bed use. Journal of drugs in dermatology : JDD. 2005 Nov-Dec:4(6):751-4 [PubMed PMID: 16302562]
Desai AD, Behbahani S, Soliman Y, Samie FH. Factors associated with Mohs micrographic surgery in dermatofibrosarcoma protuberans of the head and neck: A cohort study. Indian journal of dermatology, venereology and leprology. 2023 Jun 14:():1-3. doi: 10.25259/IJDVL_991_2022. Epub 2023 Jun 14 [PubMed PMID: 37436022]
Kang SY, Choi EJ, Jang KY. Fibrosarcomatous Transformation in Dermatofibrosarcoma Protuberans of the Male Breast and its Association with Magnetic Resonance Imaging and Immunohistopathologic Features. Current medical imaging. 2024 May 16:():. doi: 10.2174/0115734056309290240513101648. Epub 2024 May 16 [PubMed PMID: 38757329]
Hao X, Billings SD, Wu F, Stultz TW, Procop GW, Mirkin G, Vidimos AT. Dermatofibrosarcoma Protuberans: Update on the Diagnosis and Treatment. Journal of clinical medicine. 2020 Jun 5:9(6):. doi: 10.3390/jcm9061752. Epub 2020 Jun 5 [PubMed PMID: 32516921]
Saikia BK, Das I, Mandal GK. Fibrosarcomatous change in the background of dermatofibrosarcoma protuberans in male breast: Study of a rare case and review of the entity. Journal of mid-life health. 2016 Jan-Mar:7(1):45-8. doi: 10.4103/0976-7800.179171. Epub [PubMed PMID: 27134483]
Level 3 (low-level) evidenceBogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma protuberans: a review of the literature. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2012 Apr:38(4):537-51. doi: 10.1111/j.1524-4725.2011.02292.x. Epub 2012 Jan 30 [PubMed PMID: 22288484]
Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, Brennan MF, Lewis JJ. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000 Jun 15:88(12):2711-20 [PubMed PMID: 10870053]
Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2004 Apr:30(3):341-5 [PubMed PMID: 15028319]
Gloster HM Jr. Dermatofibrosarcoma protuberans. Journal of the American Academy of Dermatology. 1996 Sep:35(3 Pt 1):355-74; quiz 375-6 [PubMed PMID: 8784271]
Acosta AE, Vélez CS. Dermatofibrosarcoma Protuberans. Current treatment options in oncology. 2017 Aug 10:18(9):56. doi: 10.1007/s11864-017-0498-5. Epub 2017 Aug 10 [PubMed PMID: 28795284]
Li Y, Wang C, Xiang B, Chen S, Li L, Ji Y. Clinical Features, Pathological Findings and Treatment of Recurrent Dermatofibrosarcoma Protuberans. Journal of Cancer. 2017:8(7):1319-1323. doi: 10.7150/jca.17988. Epub 2017 May 12 [PubMed PMID: 28607608]
Fiore M, Miceli R, Mussi C, Lo Vullo S, Mariani L, Lozza L, Collini P, Olmi P, Casali PG, Gronchi A. Dermatofibrosarcoma protuberans treated at a single institution: a surgical disease with a high cure rate. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Oct 20:23(30):7669-75 [PubMed PMID: 16234529]
Escobar GF, Ribeiro CK, Leite LL, Barone CR, Cartell A. Dermoscopy of Dermatofibrosarcoma Protuberans: What Do We Know? Dermatology practical & conceptual. 2019 Apr:9(2):139-145. doi: 10.5826/dpc.0902a10. Epub 2019 Apr 30 [PubMed PMID: 31106017]
Khamdan F, Brailsford C, Dirr MA, Sagut P, Nietert PJ, Elston D. Dermatofibroma Versus Dermatofibrosarcoma Protuberans: A Nuclear Morphology Study. The American Journal of dermatopathology. 2023 Sep 1:45(9):631-634. doi: 10.1097/DAD.0000000000002526. Epub [PubMed PMID: 37625803]
Navarrete-Dechent C, Mori S, Barker CA, Dickson MA, Nehal KS. Imatinib Treatment for Locally Advanced or Metastatic Dermatofibrosarcoma Protuberans: A Systematic Review. JAMA dermatology. 2019 Mar 1:155(3):361-369. doi: 10.1001/jamadermatol.2018.4940. Epub [PubMed PMID: 30601909]
Level 1 (high-level) evidenceRen Q, Li J, Shangguan J, Feng X, Ma X. Imaging features of dermatofibrosarcoma protuberans. Journal of cancer research and therapeutics. 2022 Apr:18(2):476-481. doi: 10.4103/jcrt.jcrt_1619_21. Epub [PubMed PMID: 35645117]
Riggs K, McGuigan KL, Morrison WB, Samie FH, Humphreys T. Role of magnetic resonance imaging in perioperative assessment of dermatofibrosarcoma protuberans. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2009 Dec:35(12):2036-41. doi: 10.1111/j.1524-4725.2009.01330.x. Epub [PubMed PMID: 19732100]
Rota S, Franza A, Fabbroni C, Paolini B, Greco FG, Alessi A, Padovano B, Casali P, Sanfilippo R. COL1A1::PDGFB fusion-associated uterine fibrosarcoma: A case report and review of the literature. Cancer reports (Hoboken, N.J.). 2024 Feb:7(2):e1969. doi: 10.1002/cnr2.1969. Epub 2024 Jan 26 [PubMed PMID: 38279510]
Level 3 (low-level) evidenceFionda B, Loperfido A, Di Stefani A, Lancellotta V, Paradisi A, De Angeli M, Cappilli S, Rossi E, Caretto AA, Zinicola T, Schinzari G, Gentileschi S, Morganti AG, Rembielak A, Peris K, Tagliaferri L. The Role of Postoperative Radiotherapy in the Management of Dermatofibrosarcoma Protuberans: A Multidisciplinary Systematic Review. Journal of clinical medicine. 2024 Mar 21:13(6):. doi: 10.3390/jcm13061798. Epub 2024 Mar 21 [PubMed PMID: 38542022]
Level 1 (high-level) evidenceMalan M, Xuejingzi W, Quan SJ. The efficacy of Mohs micrographic surgery over the traditional wide local excision surgery in the cure of dermatofibrosarcoma protuberans. The Pan African medical journal. 2019:33():297. doi: 10.11604/pamj.2019.33.297.17692. Epub 2019 Aug 13 [PubMed PMID: 31692830]
Shah KK, McHugh JB, Folpe AL, Patel RM. Dermatofibrosarcoma Protuberans of Distal Extremities and Acral Sites: A Clinicopathologic Analysis of 27 Cases. The American journal of surgical pathology. 2018 Mar:42(3):413-419. doi: 10.1097/PAS.0000000000000998. Epub [PubMed PMID: 29240584]
Level 3 (low-level) evidenceRatner D, Thomas CO, Johnson TM, Sondak VK, Hamilton TA, Nelson BR, Swanson NA, Garcia C, Clark RE, Grande DJ. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. Journal of the American Academy of Dermatology. 1997 Oct:37(4):600-13 [PubMed PMID: 9344201]
Serra-Guillén C, Llombart B, Nagore E, Guillén C, Sanmartín O. Determination of Margins for Tumor Clearance in Dermatofibrosarcoma Protuberans: A Single-Center Study of 222 Cases Treated With Modified Mohs Surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2022 Jan 1:48(1):51-56. doi: 10.1097/DSS.0000000000003269. Epub [PubMed PMID: 34743125]
Level 3 (low-level) evidenceWilder F, D'Angelo S, Crago AM. Soft tissue tumors of the trunk: management of local disease in the breast and chest and abdominal walls. Journal of surgical oncology. 2015 Apr:111(5):546-52. doi: 10.1002/jso.23843. Epub 2014 Nov 23 [PubMed PMID: 25418423]
Nieto-Benito LM, Ciudad-Blanco C, Sanmartin-Jimenez O, Garces JR, Rodríguez-Prieto MA, Vilarrasa E, de Eusebio-Murillo E, Miñano-Medrano R, Escutia-Muñoz B, Gonzalez-Sixto B, Artola-Igarza JL, Alfaro Rubio A, Redondo P, Delgado-Jiménez Y, Sánchez-Schmidt JM, Allende-Markixana I, Alonso-Pacheco ML, García-Bracamonte B, de la Cueva Dobao P, Navarro-Tejedor R, Suarez-Fernández R, Carnero-González L, Vázquez-Veiga H, Barchino-Ortiz L, Ruiz-Salas V, Sánchez-Sambucety P, López-Estebaranz JL, Botella-Estrada R, Feal-Cortizas C, Martorell Calatayud A, Gil P, Morales-Gordillo V, Toll-Abelló A, Ocerin-Guerra I, Mayor-Arenal M, Garcia-Donoso C, Cano-Martinez N, Sainz-Gaspar L, Descalzo MA, Garcia-Doval I, REGESMOHS (Registro Español de Cirugía de Mohs). Mohs micrographic surgery in dermatofibrosarcoma protuberans: Rate and risk factors for recurrence in a prospective cohort study from the Spanish Registry of Mohs Surgery (REGESMOHS) and review of the literature. Experimental dermatology. 2021 May:30(5):717-722. doi: 10.1111/exd.14291. Epub 2021 Feb 13 [PubMed PMID: 33523531]
Saiag P, Grob JJ, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, Peris K, Stratigos A, Middelton M, Basholt L, Testori A, Garbe C. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. European journal of cancer (Oxford, England : 1990). 2015 Nov:51(17):2604-8. doi: 10.1016/j.ejca.2015.06.108. Epub 2015 Jul 16 [PubMed PMID: 26189684]
Level 3 (low-level) evidenceEbede TL, Lee EH, Dusza SW, Busam KJ, Nehal KS. Clinical value of paraffin sections in association with Mohs micrographic surgery for nonmelanoma skin cancers. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2012 Oct:38(10):1631-8. doi: 10.1111/j.1524-4725.2012.02570.x. Epub 2012 Sep 7 [PubMed PMID: 22958072]
Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Smith Begolka W, Ratings Panel, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, McBurney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct:67(4):531-50. doi: 10.1016/j.jaad.2012.06.009. Epub 2012 Sep 5 [PubMed PMID: 22959232]
Mullen JT. Dermatofibrosarcoma Protuberans: Wide Local Excision Versus Mohs Micrographic Surgery. Surgical oncology clinics of North America. 2016 Oct:25(4):827-39. doi: 10.1016/j.soc.2016.05.011. Epub 2016 Aug 3 [PubMed PMID: 27591501]
Martin ECS, Vyas KS, Batbold S, Erwin PJ, Brewer JD. Dermatofibrosarcoma Protuberans Recurrence After Wide Local Excision Versus Mohs Micrographic Surgery: A Systematic Review and Meta-Analysis. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2022 May 1:48(5):479-485. doi: 10.1097/DSS.0000000000003411. Epub 2022 Mar 30 [PubMed PMID: 35353755]
Level 1 (high-level) evidenceParadisi A, Abeni D, Rusciani A, Cigna E, Wolter M, Scuderi N, Rusciani L, Kaufmann R, Podda M. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer treatment reviews. 2008 Dec:34(8):728-36. doi: 10.1016/j.ctrv.2008.06.002. Epub 2008 Aug 5 [PubMed PMID: 18684568]
Lowe GC, Onajin O, Baum CL, Otley CC, Arpey CJ, Roenigk RK, Brewer JD. A Comparison of Mohs Micrographic Surgery and Wide Local Excision for Treatment of Dermatofibrosarcoma Protuberans With Long-Term Follow-up: The Mayo Clinic Experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2017 Jan:43(1):98-106. doi: 10.1097/DSS.0000000000000910. Epub [PubMed PMID: 27749444]
Loghdey MS, Varma S, Rajpara SM, Al-Rawi H, Perks G, Perkins W. Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2014 Oct:67(10):1315-21. doi: 10.1016/j.bjps.2014.05.021. Epub 2014 May 23 [PubMed PMID: 25012249]
Crum OM, O'Hern K, Demer AM, Brewer JD. Disease-Specific Mortality of Dermatofibrosarcoma Protuberans After Mohs Surgery Versus Wide Local Excision: A Systematic Review and Meta-Analysis. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2024 Apr 1:50(4):317-321. doi: 10.1097/DSS.0000000000004088. Epub 2024 Feb 8 [PubMed PMID: 38335454]
Udkoff J, Russell E, Beal BT, Holzer AM, Brodland DG, Knackstedt T. Cost effectiveness of dermatofibrosarcoma protuberans treated with Mohs micrographic surgery compared with wide local excision. Journal of the American Academy of Dermatology. 2022 Nov:87(5):1156-1157. doi: 10.1016/j.jaad.2022.02.028. Epub 2022 Feb 22 [PubMed PMID: 35202774]
Massey RA, Tok J, Strippoli BA, Szabolcs MJ, Silvers DN, Eliezri YD. A comparison of frozen and paraffin sections in dermatofibrosarcoma protuberans. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1998 Sep:24(9):995-8 [PubMed PMID: 9754088]
Garcia C, Viehman G, Hitchcock M, Clark RE. Dermatofibrosarcoma protuberans treated with Mohs surgery. A case with CD34 immunostaining variability. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1996 Feb:22(2):177-9 [PubMed PMID: 8608381]
Level 3 (low-level) evidenceSnow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB. Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer. 2004 Jul 1:101(1):28-38 [PubMed PMID: 15221986]
Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma Protuberans. Dermatologic clinics. 2019 Oct:37(4):483-488. doi: 10.1016/j.det.2019.05.006. Epub [PubMed PMID: 31466588]
Abbott JJ, Oliveira AM, Nascimento AG. The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans. The American journal of surgical pathology. 2006 Apr:30(4):436-43 [PubMed PMID: 16625088]
Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. The American journal of surgical pathology. 1998 May:22(5):576-87 [PubMed PMID: 9591728]
Level 3 (low-level) evidenceAbdouh S, Boujguenna I, Soleh A, Abkari I, Rais H. Navigating diagnostic challenges-distinguishing malignant melanoma and clear cell sarcoma of soft tissues: a case report and review of the literature. Journal of medical case reports. 2024 May 17:18(1):249. doi: 10.1186/s13256-024-04542-y. Epub 2024 May 17 [PubMed PMID: 38755643]
Level 3 (low-level) evidenceMuto Y, Fujimura T, Takahashi A, Namikawa K, Ogata D, Nakano E, Jinnai S, Hashimoto A, Kambayashi Y, Asano Y, Yamazaki N. Analysis of surgical margins and prognostic factors in dermatofibrosarcoma protuberans after wide local excision: A multicenter study of 116 Japanese patients. The Journal of dermatology. 2024 May 22:():. doi: 10.1111/1346-8138.17280. Epub 2024 May 22 [PubMed PMID: 38775205]
Level 2 (mid-level) evidenceHenry OS, Platoff R, Cerniglia KS, Batchu S, Goodwin BJ, Sandilos G, Adams A, Hong YK. Tyrosine kinase inhibitors versus radiation therapy in unresectable dermatofibrosarcoma protuberans (DFSP): A narrative systematic review. American journal of surgery. 2023 Feb:225(2):268-274. doi: 10.1016/j.amjsurg.2022.09.040. Epub 2022 Sep 26 [PubMed PMID: 36184329]
Level 1 (high-level) evidenceCastle KO, Guadagnolo BA, Tsai CJ, Feig BW, Zagars GK. Dermatofibrosarcoma protuberans: long-term outcomes of 53 patients treated with conservative surgery and radiation therapy. International journal of radiation oncology, biology, physics. 2013 Jul 1:86(3):585-90. doi: 10.1016/j.ijrobp.2013.02.024. Epub 2013 Apr 26 [PubMed PMID: 23628134]
Williams N, Morris CG, Kirwan JM, Dagan R, Mendenhall WM. Radiotherapy for dermatofibrosarcoma protuberans. American journal of clinical oncology. 2014 Oct:37(5):430-2. doi: 10.1097/COC.0b013e31827dee86. Epub [PubMed PMID: 23388563]
Haas RL, Delaney TF, O'Sullivan B, Keus RB, Le Pechoux C, Olmi P, Poulsen JP, Seddon B, Wang D. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? International journal of radiation oncology, biology, physics. 2012 Nov 1:84(3):572-80. doi: 10.1016/j.ijrobp.2012.01.062. Epub 2012 Apr 18 [PubMed PMID: 22520481]
Salerno KE, Alektiar KM, Baldini EH, Bedi M, Bishop AJ, Bradfield L, Chung P, DeLaney TF, Folpe A, Kane JM, Li XA, Petersen I, Powell J, Stolten M, Thorpe S, Trent JC, Voermans M, Guadagnolo BA. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Practical radiation oncology. 2021 Sep-Oct:11(5):339-351. doi: 10.1016/j.prro.2021.04.005. Epub 2021 Jul 26 [PubMed PMID: 34326023]
Level 1 (high-level) evidenceO'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet (London, England). 2002 Jun 29:359(9325):2235-41 [PubMed PMID: 12103287]
Level 1 (high-level) evidenceDavis AM, O'Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, Goddard K, Freeman C, Sadura A, Zee B, Day A, Tu D, Pater J, Canadian Sarcoma Group, NCI Canada Clinical Trial Group Randomized Trial. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2005 Apr:75(1):48-53 [PubMed PMID: 15948265]
Level 1 (high-level) evidenceNielsen OS, Cummings B, O'Sullivan B, Catton C, Bell RS, Fornasier VL. Preoperative and postoperative irradiation of soft tissue sarcomas: effect of radiation field size. International journal of radiation oncology, biology, physics. 1991 Nov:21(6):1595-9 [PubMed PMID: 1938569]
Rutkowski P, Dębiec-Rychter M, Nowecki Z, Michej W, Symonides M, Ptaszynski K, Ruka W. Treatment of advanced dermatofibrosarcoma protuberans with imatinib mesylate with or without surgical resection. Journal of the European Academy of Dermatology and Venereology : JEADV. 2011 Mar:25(3):264-70. doi: 10.1111/j.1468-3083.2010.03774.x. Epub [PubMed PMID: 20569296]
Shah A, Tassavor M, Sharma S, Torbeck R. The various treatment modalities of dermatofibrosarcoma protuberans. Dermatology online journal. 2021 Jun 15:27(6):. doi: 10.5070/D327654070. Epub 2021 Jun 15 [PubMed PMID: 34387070]
. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet (London, England). 1997 Dec 6:350(9092):1647-54 [PubMed PMID: 9400508]
Level 1 (high-level) evidencePervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008 Aug 1:113(3):573-81. doi: 10.1002/cncr.23592. Epub [PubMed PMID: 18521899]
Level 1 (high-level) evidenceGrobmyer SR,Maki RG,Demetri GD,Mazumdar M,Riedel E,Brennan MF,Singer S, Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Annals of oncology : official journal of the European Society for Medical Oncology. 2004 Nov; [PubMed PMID: 15520069]
Level 2 (mid-level) evidenceEdmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, Parkinson DR. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993 Jul:11(7):1269-75 [PubMed PMID: 8315424]
Level 1 (high-level) evidenceFrustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti G, Barbieri E, Apice G, Zmerly H, Serraino D, Picci P. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 Mar 1:19(5):1238-47 [PubMed PMID: 11230464]
Level 1 (high-level) evidenceMack LA, Crowe PJ, Yang JL, Schachar NS, Morris DG, Kurien EC, Temple CL, Lindsay RL, Magi E, DeHaas WG, Temple WJ. Preoperative chemoradiotherapy (modified Eilber protocol) provides maximum local control and minimal morbidity in patients with soft tissue sarcoma. Annals of surgical oncology. 2005 Aug:12(8):646-53 [PubMed PMID: 15965732]
Foshee JP, Trofymenko O, Zeitouni NC. Surgical and Functional Considerations of Dermatofibrosarcoma Protuberans Involving Facial Nerve Danger Zones. The Journal of clinical and aesthetic dermatology. 2019 Dec:12(12):39-43 [PubMed PMID: 32038764]