Mohs Micrographic Surgery Interpretation of Technical Problems Impacting Slide Quality
Mohs Micrographic Surgery Interpretation of Technical Problems Impacting Slide Quality
Introduction
Mohs micrographic surgery (MMS) has a 5-year cure rate of 99%, 94.4%, 92% to 99%, and 90% for primary basal cell carcinoma (BCC), recurrent BCC, primary squamous cell carcinoma (SCC), and recurrent SCC, respectively.[1][2][3] The utility of MMS extends beyond nonmelanoma skin cancer, including treating rare tumors.[4] The high cure rate consistently reported in MMS relies on the integrity and reproducibility of the prepared histology slides to be accurately interpreted by the Mohs surgeon. Aspects of MMS slide preparation depend on the surgeon and histology technicians. Regardless of practice preferences, the entire care team needs to recognize the technical problems that may impact slide quality. In addition to being competent in obtaining, processing, and staining tissue, clinicians should be aware of common pitfalls encountered at each step and the strategies that should be implemented to reduce potential errors.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Debulking
Whether a specimen is debulked in vivo (ie, before removal from the patient), ex vivo (ie, after removal from the patient), or not at all is surgeon-dependent. Debulking allows for easier tissue relaxation. Blunt debulking with a curette is simple and fast and can provide a more accurate understanding of the size and depth of the tumor via tactile sensation. Sharp debulking with a bendable blade or 15-blade can allow for examining vertical sections and any high-risk features of the tumor, which will contribute to appropriate staging in, for example, SCC.[5][6]
A randomized study evaluating the treatment of head and neck BCCs revealed that tumor debulking resulted in significantly larger wounds and more first layers taken, but no statistical difference in the number of layers needed or reconstruction method. This study included well-demarcated BCCs measuring less than 2 cm in diameter.[7] No significant evidence comparing the presence of false positives or negatives or floaters (ie, fragments of tumor or tissue which inadvertently attach to slide) between sharp and blunt debulking has been documented; arguments have been made for and against both methods. The approach to debulking is ultimately dictated by the Mohs surgeon's preference and the histology technician's needs. However, it is essential that the debulk is not considered an additional layer or a biopsy.
Obtaining Tissue
Beveling or taking an "oblique" section relates to the degree to which the blade is angled from the surface of the epidermis. This technique is also dependent on the surgeon and histology technician. A retrospective review compared beveled and nonbeveled incisions and found similar slide quality and margin adequacy.[8] Some experts have argued that a 90-degree incision perpendicular to the skin surface with relaxing incisions is adequate for complete circumferential and deep margin assessment. In contrast, others prefer a bevel between 80 degrees and the traditionally described 45-degree angle.[9] The angle of the bevel can also be greater or lesser depending on the type and thickness of tissue or the location from which the specimen is being obtained. For example, when operating over cartilage, which is inelastic and does not retract like the dermis or subcutis when frozen, a larger margin and more acute bevel between 45 and 20 degrees have been suggested to account for the retraction of the overlying dermis and yield a more complete margin after processing.[10]
Grossing
Grossing involves taking a 3-dimensional specimen and converting it into a 2-dimensional representation of the entire margin that is transferred to a slide for analysis. This process should be efficient and ease the further slide preparation steps of embedding and cutting. Many techniques and devices have been described in the literature to improve this step, the discussion of which is beyond the scope of this topic.[7] However, the principle of grossing specimens is relatively simple: maintaining tissue orientation while laying out the peripheral and deep margins into a single plane.
Tissue Orientation
Orienting tissue is generally performed in vivo and is paramount in creating an accurate and easy-to-follow Mohs map. Furthermore, tissue orientation is essential for attaining the high cure rates associated with Mohs surgery. Methods for orienting tissue vary widely and by surgeon preference, including nicks in the tissue or using various dyes in vivo.[7] However, consistency and accuracy are more critical than the specific orientation method.
Tissue Transfer
Transferring tissue while maintaining orientation is important. Again, several methods have been described in the literature, including placing the specimen on pre-oriented filter paper, a transfer card, or even a photograph of the surgical site.[7]
Tissue Flattening
Relaxing incisions, firm pressure, and dividing thick or large tissue specimens are helpful in tissue flattening and fitting the tissue on the slide.[7]
Inking and Mapping
Many techniques are employed in inking and mapping. However, the most crucial aspect is that the Mohs surgeon is consistent and accurate in their inking, reflecting the tissue orientation on the map.[7]
Mounting
Mounting involves securing the 2-dimensional marginal tissue to be frozen for sectioning. Different techniques exist, but some popular methods include the heat sink method, the glass slide method, and the Miami special.[11][12][13] Some advantages and limitations of each method include:
- The heat sink method generally involves placing the margin over the top of a chilled metal cylinder. This method is fast and relatively simple but is time-sensitive, as the warmer tissue immediately adheres to the cold heat sink. More air bubbles may be present with this method.[11]
- The glass slide method is also simple and relatively fast, allowing the tissue to be manipulated into a single plane better than the heat sink method. The glass slide method produces fewer air bubbles, allowing visualization of the marginal surface. The tissue, however, will take slightly longer to freeze.[12] A single, large slide for large specimens has been proposed, saving the time needed to divide the tissue into smaller pieces.[14]
- The Miami special involves sponge forceps that have been modified by adding a freezing plate and chuck holder. The Miami special is an efficient method for mounting and preparing a specimen.[13]
Embedding
Embedding involves preparing the tissue into a block and subsequently freezing the specimen. Various media are available to facilitate this step. Some of the most common include Tissue Freezing Medium™, Optimum Cutting Temperature Medium™ (OTC™), and Frozen Section Medium™. A small study by Reserva et al from 2017 compared these 3 common embedding media products and found no real difference in slide readability but did find easier cutting and more efficient slide preparation times with Frozen Section Medium™. Although the contents of the products are proprietary information, the authors speculated that a higher concentration of polyethylene glycol, a plasticizer, or a higher molecular weight polyvinyl alcohol may have been present in this product compared to the others, as these can contribute to more ease with producing sections.[15]
Freezing
Generally, a cryostat is kept between -25 and -30 °C. This temperature range allows for faster tissue freezing and less ice crystal formation.[16] For even more rapid tissue freezing, a heat sink, additional liquid nitrogen spraying, or a cooled isopentane bath are several methods employed.[12] Clinicians should consider a few special issues, however.
Specimens with increased adipose generally require colder temperatures or longer freeze times to produce quality sections. Spraying the embedded specimen with liquid nitrogen, using an isopentane bath, or cutting thicker sections are methods to produce quality sections of tissue rich in adipose.[12][17] Cartilage is also somewhat temperature-dependent as it may shatter at very cold temperatures; warmer temperatures of approximately -16 to -22 °C may reduce shattering. Keeping the specimen moist while processing, using positively charged slides (as cartilage is high in negatively charged sulfates), as well as including perichondrium when cartilage is a deep margin as perichondrium adheres to the slide and sections more easily than cartilage, all may be helpful when processing a specimen with cartilage.[12]
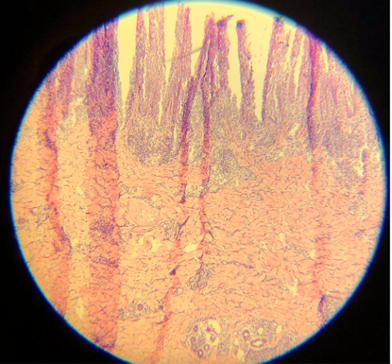
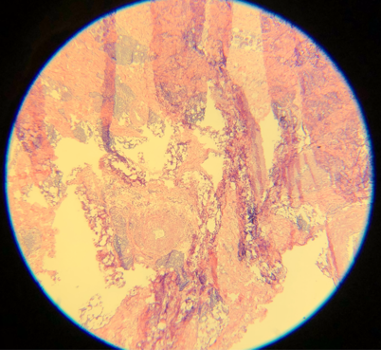
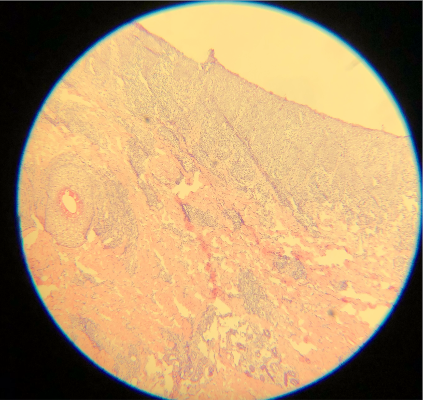
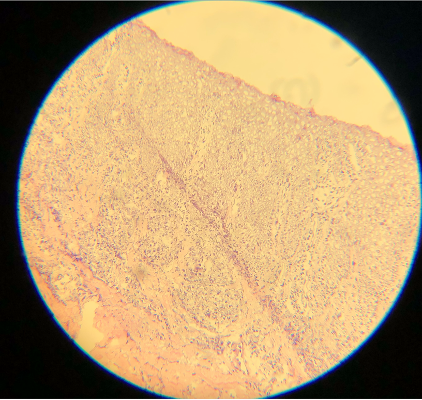
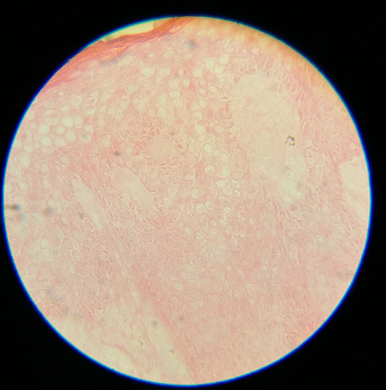
Artifacts may occur due to sectioning tissue specimens that are too cold (see Image. Cold Tissue Artifact). This may result in nicks and tears in the epidermis and dermis or even "chunking out of tissue," where entire specimen sections are lost. Cold tissue can also create a prominent "freeze artifact" in tissue frozen slowly to a very low temperature (see Image. Freeze Artifact). Conversely, tissue that is too warm when sectioned may result in "accordion folding" (see Image. Accordion Folding of Warm Tissue) as well as poor sectioning of fat (see Image. Poor Sectioning of Fat of Warm Tissue).
Cutting
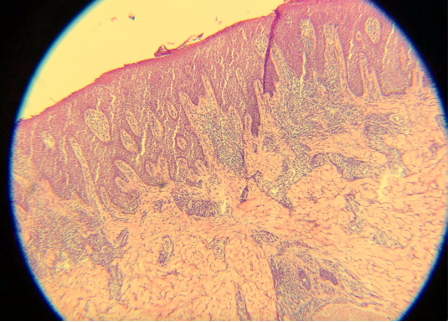
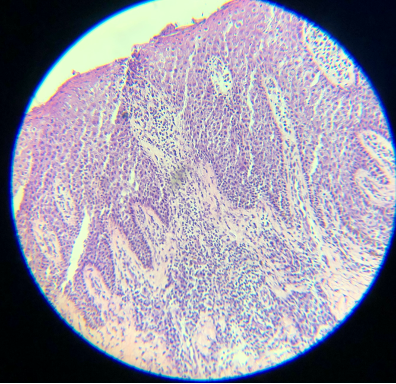
Epithelial cells are 8 µm thick on average, so optimal cutting typically ranges from 4 to 6 µm. All tissue, especially cartilage, may adhere better to dry, positively charged slides. An adhesive can also help keep cartilage on the slide. Cutting in a constant motion and using either a delicate brush or the antiroll plate may help facilitate the cutting of a thin, flat section that can be placed on a slide.[5] Despite adequate 12-µm thick sections, these cuts are darker than the typical thinner sections (see Images. Thick Sections A and B). Though not represented well in the images, fine nuclear and cytoplasmic details on very high power are more obscured with thicker sections. Specimens measuring 3 µm are ultra-thin, very faint, and difficult to interpret (see Images. Thin Section A and B). In addition, cutting sections too thin can be challenging for a histology technician to prepare and may result in the specimen "chunking" out of the block.[12] Cutting with a damaged microtome blade may result in significant tears in the tissue (see Images. Damaged Microtome Blade A and B).
Staining
Staining involves several steps, which can also vary. The following steps are a general outline of the staining process:
- Fixation preserves the specimen's integrity, including cellular and stromal details, throughout the staining process. Fixatives include formalin, alcohol, or acetone. Acetone can be particularly helpful in fixing fat.
- Tap water can rinse the fixative and provide some hydration to the tissue for staining. A recent communication recommended this step as an amorphous, eosinophilic artifact was repeatedly affecting histological interpretation. Adding a 15-second water rinse eliminated this problem.[18]
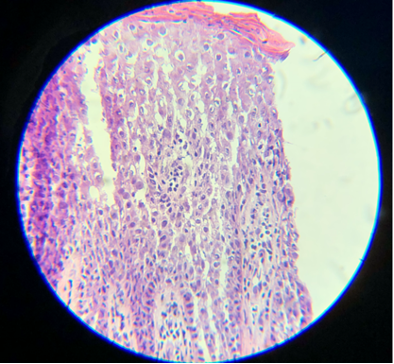
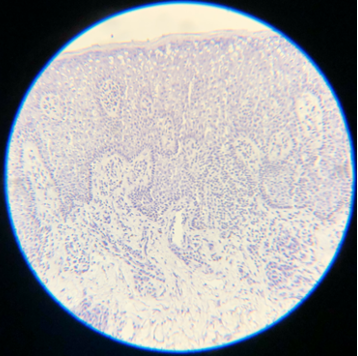
- Hematoxylin is a basic dye that stains the acidic components of tissue, which include nucleic acids, a dark purple or blue color, and is very important for the differentiation of various cellular details, particularly the nucleus. Slides are virtually uninterpretable when hematoxylin staining is skipped (see Images. Skipped Hematoxylin A and B).
- Tap water, again, can be used to rinse excess hematoxylin from the slide.
- An additional weakly acidic alcohol bath can help in differentiation, which involves further rinsing off the basic hematoxylin dye. If skipped, the entire slide can be overly purple or blue, the nucleus can be a very dark purple, collagen will not be as vibrant pink, and other structures can be more difficult to differentiate.
- Additional tap water can rinse the alcohol and prevent excessive destaining.
- A bluing agent can darken structures like the nucleus. One example is Scott tap water, magnesium sulfate buffered with sodium bicarbonate. Leaving tissue in an ammonia solution for too long can cause sections to fall off the slide.
- A water rinse then clears the excess bluing agent and neutralizes the tissue for additional staining with eosin.
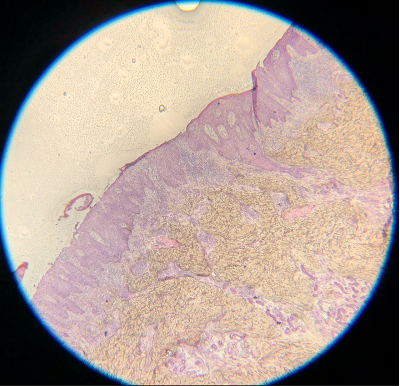
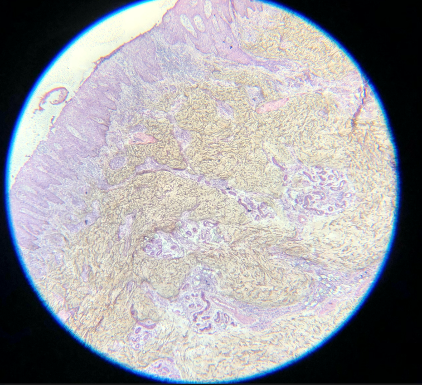
- Eosin is an acidic, negatively charged dye that highlights cytoplasm, mitochondria, secretory granules, collagen, keratin, and erythrocytes, providing an excellent counterstain to hematoxylin. If eosin is skipped, slides will also be challenging to interpret. Although some structural and cellular details may be apparent, the background pink color when eosin is used provides much better differentiation for making appropriate diagnoses (see Images. Skipped Eosin A and B).
- Alcohol concentrated at 95% can again be used to help rinse the eosin and improve differentiation.
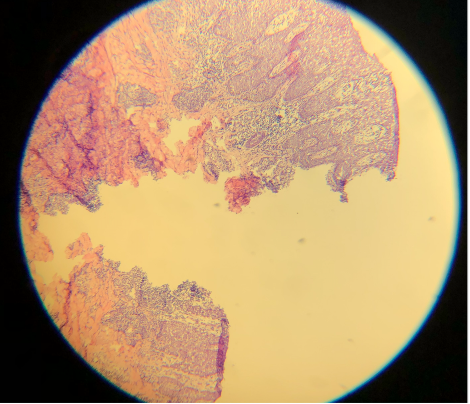
- Alcohol concentrated at 100% is used for dehydration and additional differentiation. Without additional alcohol baths, the slide may be left with water beads or a haze (see Images. Lacking Additional Alcohol Baths A and B). This can occur when water mixes with the final clearing agent.[19]
- A clearing agent, generally xylene, limonene, or naphthenic or aliphatic hydrocarbon, improves the formation of a homogenous solution with the glue used for coverslipping. The clearing agent also helps prevent excess bleeding of eosin due to alcohol remaining on the tissue. Some figures in the literature show an insufficient clearing agent or incompatibility of the clearing agent with the mounting media.[12] This hinders the formation of a homogenous solution over the slide, leading to bubbles and lifting of the coverslip off the slide. Lifting of the coverslip may also occur with insufficient mounting medium. Of note, xylene is neurotoxic.[12]
Hematoxylin is a stain that comes from the Haematoxylon campechianum (ie, logwood tree) and is a basic dye that stains acidic portions of tissue, such as the nucleic acids of the nucleus, glycosaminoglycans, and glycoproteins. Eosin is an acidic, negatively charged dye that highlights various tissue portions, eg, cytoplasm, collagen, and keratin. These stains are reliable, simple to perform, inexpensive, and are the standard for histology.[19]
Toluidine blue is a rapid metachromatic stain; it stains different substances in various colors depending on the charge density of the tissue. It stains basal and squamous cells dark blue.[20] Importantly, toluidine blue stains the mucopolysaccharides that make up the mucinous stroma of some tumors, which is a vibrant magenta color. This staining helps identify the stroma surrounding BCC (as opposed to that of a follicle). Toluidine blue can also draw attention to areas of inflammation and subtle foci of SCC surrounding or involving nerves. This effect has been described as the perineural corona sign.[21] Some literature also exists on the use of toluidine blue in identifying the stromal elements of microcystic adnexal tumors and dermatofibrosarcoma protuberans.[22][23] Toluidine blue is inhibited by aluminum chloride and Monsel's solution.[24]
Enhancing Healthcare Team Outcomes
In Mohs micrographic surgery, high-quality slide preparation is essential to achieving optimal patient outcomes, maintaining high cure rates, and minimizing risks. This process requires interprofessional skills and coordination, as each step—obtaining, processing, and staining tissue—presents technical challenges that can affect interpretability. Subsequently, a better understanding of the technical problems impacting slide quality by the entire care team will improve patient-centered care, outcomes, patient safety, and team performance.
Physicians, advanced practitioners, and histology technicians must work closely to ensure precise tissue mapping, accurate orientation, and controlled freezing to avoid common pitfalls such as improper tissue temperature, section thickness issues, or air bubbles caused by inadequate clearing agents. Nurses and medical assistants play a crucial role by transporting specimens efficiently and carefully, ensuring proper handling from surgery to the laboratory. Effective communication across this team fosters rapid recognition and correction of slide quality issues, while pharmacists can provide guidance on suitable clearing agents. This collaborative approach supports accurate diagnoses, enhances patient-centered care, ensures safety, and elevates overall team performance in delivering successful Mohs surgery outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
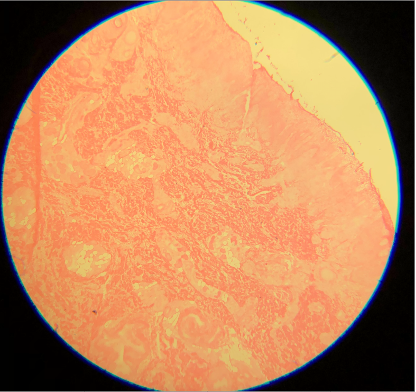
Skipped Hematoxylin A. Hematoxylin is a basic dye that stains the acidic components of tissue, which include nucleic acids, a dark purple or blue color, and is very important for the differentiation of various cellular details, which can not be identified well if this staining process is not performed.
Contributed by D Knabel, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
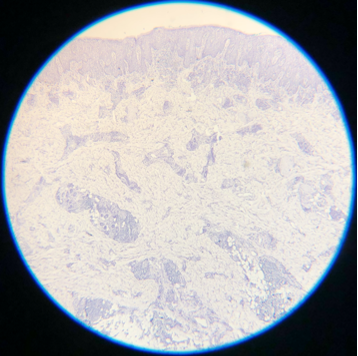
Skipped Eosin A. Eosin is an acidic, negatively charged dye that highlights cytoplasm, mitochondria, secretory granules, collagen, keratin, and erythrocytes, providing an excellent counterstain to hematoxylin. If eosin is skipped, slides will also be challenging to interpret.
Contributed by D Knabel, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. The Journal of dermatologic surgery and oncology. 1989 Mar:15(3):315-28 [PubMed PMID: 2646336]
Roenigk RK, Roenigk HH Jr. Current surgical management of skin cancer in dermatology. The Journal of dermatologic surgery and oncology. 1990 Feb:16(2):136-51 [PubMed PMID: 2406310]
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. Journal of the American Academy of Dermatology. 1992 Jun:26(6):976-90 [PubMed PMID: 1607418]
Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2007 Mar:33(3):333-9 [PubMed PMID: 17338692]
Davis DA, Pellowski DM, William Hanke C. Preparation of frozen sections. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Dec:30(12 Pt 1):1479-85 [PubMed PMID: 15606735]
Silapunt S, Peterson SR, Alcalay J, Goldberg LH. Mohs tissue mapping and processing: a survey study. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2003 Nov:29(11):1109-12; discussion 1112 [PubMed PMID: 14641335]
Level 3 (low-level) evidenceLi JY, Silapunt S, Migden MR, McGinness JL, Nguyen TH. Mohs Mapping Fidelity: Optimizing Orientation, Accuracy, and Tissue Identification in Mohs Surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2018 Jan:44(1):1-9. doi: 10.1097/DSS.0000000000001215. Epub [PubMed PMID: 28654580]
Yu SH, Olbricht SM, Tiger JB. A single-institution retrospective evaluation of Mohs incision angles and histopathologic specimen quality. International journal of dermatology. 2019 Oct:58(10):1210-1211. doi: 10.1111/ijd.14591. Epub 2019 Jul 17 [PubMed PMID: 31317543]
Level 2 (mid-level) evidenceTilleman TR, Tilleman MM, Neumann MH. Minimal bevelling angle in Mohs micrographic surgery cut: a 10 degrees angle is sufficient. The British journal of dermatology. 2005 May:152(5):1081-3 [PubMed PMID: 15888185]
Krishnan RS, Donnelly HB. A better technique for taking Mohs sections involving cartilage. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2007 Jun:33(6):716-21 [PubMed PMID: 17550450]
Bakhtar O, Close A, Davidson TM, Baird SM. Tissue preparation for MOHS' frozen sections: a comparison of three techniques. Virchows Archiv : an international journal of pathology. 2007 May:450(5):513-8 [PubMed PMID: 17406894]
Aslam A, Aasi SZ. Frozen-Section Tissue Processing in Mohs Surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2019 Dec:45 Suppl 2():S57-S69. doi: 10.1097/DSS.0000000000002260. Epub [PubMed PMID: 31764292]
Nouri K, O'Connell C, Alonso J, Rivas MP, Alonso Y. The Miami Special: a simple tool for quality section mounting in Mohs surgery. Journal of drugs in dermatology : JDD. 2004 Mar-Apr:3(2):175-7 [PubMed PMID: 15098973]
Arutyunyan S, Basehore BM, Farhang S, Saleeby E. Processing Sections in Mohs Micrographic Surgery Too Big to Fit on a Single Conventional Microscope Slide. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2022 Jul 1:48(7):784-786. doi: 10.1097/DSS.0000000000003484. Epub [PubMed PMID: 35778251]
Reserva J, Krol C, Speiser J, Adams W, Tung-Hahn E, Alam M, Tung R. Comparison of three embedding media for preparation of frozen sections for Mohs micrographic surgery. International journal of dermatology. 2017 Dec:56(12):1499-1501. doi: 10.1111/ijd.13704. Epub 2017 Aug 1 [PubMed PMID: 28762468]
Erickson QL, Clark T, Larson K, Minsue Chen T. Flash freezing of Mohs micrographic surgery tissue can minimize freeze artifact and speed slide preparation. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2011 Apr:37(4):503-9. doi: 10.1111/j.1524-4725.2011.01926.x. Epub [PubMed PMID: 21481069]
Reserva J, Kozel Z, Krol C, Speiser J, Adams W, Tung R. Processing Adipose-Rich Mohs Samples: A Comparative Study of Effectiveness of Pretreatment With Liquid Nitrogen Versus Flash Freezing Spray. The American Journal of dermatopathology. 2017 Nov:39(11):838-841. doi: 10.1097/DAD.0000000000000826. Epub [PubMed PMID: 28178008]
Level 2 (mid-level) evidenceFlint N, Friedman P, Frigerio A, Tinklepaugh A. Incomplete Staining Artifact: A Confounding Frozen Section Pathology Artifact Encountered During Mohs Micrographic Surgery. Journal of drugs in dermatology : JDD. 2022 May 1:21(5):544. doi: 10.36849/JDD.542. Epub [PubMed PMID: 35533040]
Larson K, Ho HH, Anumolu PL, Chen TM. Hematoxylin and eosin tissue stain in Mohs micrographic surgery: a review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2011 Aug:37(8):1089-99. doi: 10.1111/j.1524-4725.2011.02051.x. Epub 2011 Jun 2 [PubMed PMID: 21635628]
Styperek AR, Goldberg LH, Goldschmidt LE, Kimyai-Asadi A. Toluidine Blue and Hematoxylin and Eosin Stains are Comparable in Evaluating Squamous Cell Carcinoma During Mohs. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2016 Nov:42(11):1279-1284 [PubMed PMID: 27662051]
Drosou A, Trieu D, Goldberg LH, Kimyai-Asadi A. The perineural corona sign: enhancing detection of perineural squamous cell carcinoma during Mohs micrographic surgery with toluidine blue stain. Journal of the American Academy of Dermatology. 2014 Oct:71(4):826-7. doi: 10.1016/j.jaad.2014.04.076. Epub [PubMed PMID: 25219703]
Wang SQ, Goldberg LH, Nemeth A. The merits of adding toluidine blue-stained slides in Mohs surgery in the treatment of a microcystic adnexal carcinoma. Journal of the American Academy of Dermatology. 2007 Jun:56(6):1067-9 [PubMed PMID: 17504725]
Ahadiat O, Higgins S, Ly A, Wysong A. Toluidine Blue Stain of Dermatofibrosarcoma Protuberans: Highlighting Its Use in Mohs. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2017 Dec:43(12):1496-1498. doi: 10.1097/DSS.0000000000001078. Epub [PubMed PMID: 28346254]
Chen CL, Wilson S, Afzalneia R, Chen CJ. Topical Aluminum Chloride and Monsel's Solution Block Toluidine Blue Staining in Mohs Frozen Sections: Mechanism and Solution. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2019 Aug:45(8):1019-1025. doi: 10.1097/DSS.0000000000001761. Epub [PubMed PMID: 30640770]