Introduction
Optical coherence tomography (OCT) is a noninvasive imaging technique that uses visible and infrared electromagnetic waves to provide detailed, cross-sectional images of body tissues. OCT has widespread application in ocular imaging to diagnose and monitor various ophthalmic pathologies in both the anterior and posterior segments. OCT is commonly employed in evaluating and managing vitreoretinal and macular diseases in addition to processes affecting the optic nerve head, including glaucoma.[1]
OCT has evolved considerably since its invention in the early 1990s and the introduction of the first commercial ophthalmological device in 1996.[2] The 3 main types of OCT are time-domain, spectral-domain, and swept-source. These types differ in image acquisition, scanning speed, axial and transverse resolution, and range of imaging.
Time-Domain Optical Coherence Tomography
Time-domain OCT (TD-OCT) is a first-generation technology that uses a low-coherence interferometer to measure the time delay and magnitude of backscattered light from different tissue depths, subsequently constructing two-dimensional images in a manner similar to ultrasound technology.[3] However, TD-OCT measures only one point at a time, and the coherence length of the light source limits depth resolution.[3] TD-OCT typically uses a superluminescent diode with a relatively broad spectrum as its light source, resulting in decreased image resolution compared to newer technologies. TD-OCT has largely been replaced by techniques that offer faster acquisition speeds, higher image resolution, and a better range of imaging.
Spectral-Domain Optical Coherence Tomography
Spectral-domain OCT (SD-OCT) is a second-generation technology with significantly faster acquisition speeds, deeper tissue penetration, and higher image resolution.[4] SD-OCT uses a spectrometer to detect the spectrum of backscattered light, allowing simultaneous measurement of multiple tissue points.[4] Higher-speed data acquisition enables three-dimensional tissue imaging and improved resolution.
SD-OCT uses Fourier transformations to generate high-resolution, cross-sectional images of biological tissues.[5] In SD-OCT, a broadband superluminescent diode source is used to illuminate the tissue. Light reflected from the tissue is detected using a spectrometer that separates the reflected light into its constituent wavelengths. Interference patterns between the reflected light and a reference beam are measured for each wavelength and processed using Fourier transformations to generate high-resolution images.[6]
Due to its improved imaging capabilities, SD-OCT has become the standard for clinical use in ophthalmology, enabling earlier diagnosis and management of conditions such as age-related macular degeneration, diabetic retinopathy, and glaucoma. SD-OCT also has clinical applications in dermatology, cardiology, and gastroenterology.[7][8]
Enhanced Depth Imaging
Enhanced depth imaging (EDI) in SD-OCT is an imaging modality that places the objective lens in a closer scanning position to the eye, permitting better depth sensitivity and improved visualization of deeper ocular structures, particularly the choroid.[9][10] EDI-OCT is particularly useful when diagnosing and managing diseases that affect the choroid, such as age-related macular degeneration, choroidal neovascularization, polypoidal choroidal vasculopathy, and central serous chorioretinopathy.[10] EDI-OCT can also provide crucial information about the thickness of the retina and the presence of fluid or swelling in the retina or choroid.[11] Modern SD-OCT and swept-source OCT (SS-OCT) machines are capable of acquiring enhanced depth images.[12]
Swept-Source Optical Coherence Tomography
SS-OCT is an advanced noninvasive medical imaging technique that uses a wavelength-sweeping laser and a single dual-balanced photodetector to capture high-resolution images of the anterior segment, retina, optic nerve, and choroid.[13] The longer wavelength of the light source permits deeper tissue penetration and faster scanning speeds, resulting in excellent widefield visualization of posterior segment eye structures superior to SD-OCT with EDI.[14]
Optical Coherence Tomography Angiography
OCT angiography (OCTA) is a noninvasive imaging method that gives a three-dimensional visualization of blood vessels at different tissue levels within the retina and choroid. The images captured by OCTA provide details far superior to those obtained using conventional fundus fluorescein angiography or indocyanine green angiography; OCTA does not carry the time requirement or risks associated with systemic contrast administration.[15] OCTA has multiple applications in neuro-ophthalmology, including multiple sclerosis, anterior ischemic neuropathy, hereditary optic neuropathy, and glaucoma.[15]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
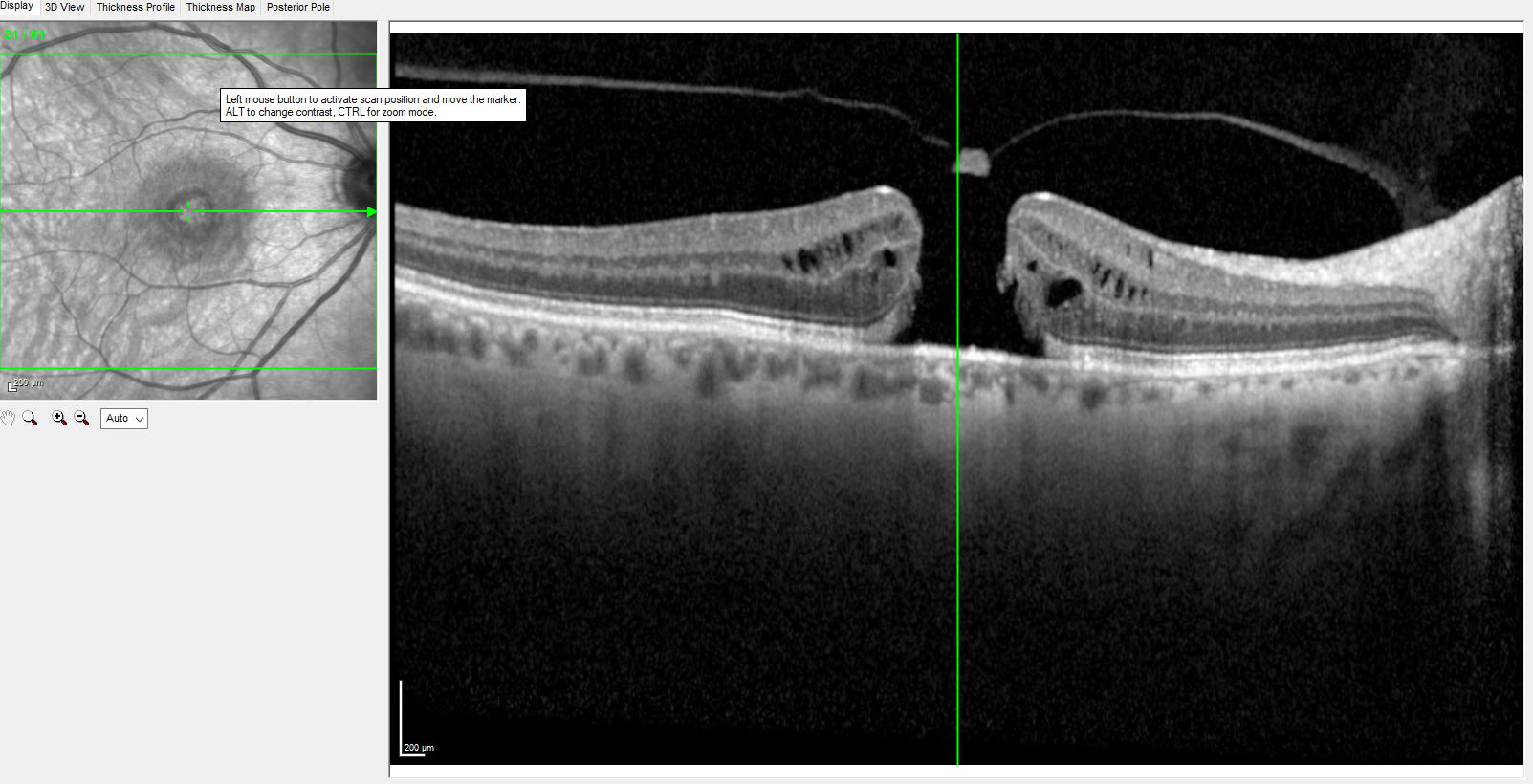
Effective use of OCT when diagnosing and managing ophthalmic disorders requires a solid understanding of the retinal anatomy provided by OCT images (see Image. Optical Coherence Tomography). OCT provides a two-dimensional cross-sectional image of all retinal layers from the internal limiting membrane to the retinal pigment epithelium. SS-OCT can deliver high-resolution scans of all retinal layers through the sclera-choroid junction.
Adjacent to the choroid is the hyperreflective retinal pigment epithelium/Bruch membrane complex. The interdigitation zone is also hyperreflective and represents the retinal pigment epithelium-photoreceptor junction. The hyperreflective ellipsoid zone is the junction of the inner and outer photoreceptor segments (IS-OS junction); these segments are hyporeflective. The IS-OS junction abuts the hyperreflective external limiting membrane. The hyporeflective outer nuclear layer is adjacent to the hyperreflective outer plexiform layer. Other retinal layers include the inner nuclear layer, the hyperreflective inner plexiform layer, the hyporeflective ganglion cell layer, and the retinal nerve fiber layer.[16] The normal macula is slightly depressed at the fovea due to the migration of the inner retinal layers towards the periphery, exposing the densely packed photoreceptors in the outer retina.[17]
Changes in the normal reflectance of OCT images can indicate underlying ocular pathology. Fluid collections, such as intraretinal, subretinal, or sub-retinal pigment epithelium collections, appear hyporeflective and dark. Hyperreflective substances include but are not limited to exudates, blood, fibrosis, choroidal neovascular membranes, and epiretinal membranes.
Various parameters can be assessed with OCT. Macular thickness is the distance in microns between the internal limiting membrane and retinal pigment epithelium.[18] The central retinal thickness, which includes the central 1-mm diameter retinal thickness, is widely used in clinical practice for diagnosis, treatment protocols, and follow-up.
The normal ranges of common anatomic measurements vary between OCT devices based on the manufacturer, model, and reference population used for calibration. However, typical ranges of normal values are generally reported as follows:
- Central macular thickness: 200 to 250 μm
- Retinal nerve fiber layer: 90 to 110 μm
- Ganglion cell layer: 70 to 90 μm
- Inner plexiform layer: 50 to 70 μm
- Choroidal thickness: 200 to 400 μm; varies significantly with the technique and location of measurement.
Indications
OCT may be indicated for any patient with otherwise unexplained vision loss. Although OCT is a useful clinical tool, it does not replace the need to obtain a comprehensive medical history or perform a thorough ophthalmoscopic examination.
Retinal Disease
OCT has become essential in diagnosing and managing many macular diseases. The macula is the central part of the retina responsible for sharp, detailed vision. Patients with macular disease commonly present with central scotoma, metamorphopsia, or blurred central vision. OCT is recommended even if ophthalmoscopy reveals macular or foveal abnormalities; OCT typically yields results that better correlate with clinical findings. Examples of macular diseases that benefit from OCT include but are not limited to:
- Macular edema, which may be secondary to diabetic retinopathy, intraocular infections, systemic conditions with ocular manifestations, retinal vein occlusion, medications, or postoperative complications including Irvine-Gass syndrome [19]
- Maculopathies, such as wet age-related macular degeneration or choroidal neovascular membrane
- Macular holes [20]
- Vitreomacular traction
- Cellophane maculopathy and macular pucker from epiretinal membranes
- Genetic maculopathies and macular dystrophies such as Stargardt or Best disease.
When monitoring the progression of macular diseases and responses to therapeutic intervention, OCT is indicated for evaluating changes in retinal anatomy and retinal thickness to facilitate the timing of surgical intervention, visualizing and monitoring changes in fluid accumulation over time, and assessing the integrity of the photoreceptor layer.[21]
EDI-OCT, SS-OCT, and OCTA are frequently indicated in the evaluation and management of various choroidopathies, particularly those involving the vasculature, including central serous chorioretinopathy, choroidal neovascularization, and polypoidal choroidal vasculopathy. OCTA is frequently used for imaging patients with diabetic retinopathy, age-related macular degeneration, retinal vein occlusion, and macular telangiectasia.
OCT can also evaluate other retinal abnormalities causing vision loss, such as retinal detachment, retinal pigment epithelial detachments, central serous retinopathy, or pathological myopia.
Optic Nerve
OCT is indicated in the evaluation of optic neuritis, an optic nerve inflammation that can cause vision loss and eye pain. OCT is also crucial for evaluating and managing glaucoma, providing valuable details about the retinal nerve fiber layer and ganglion cell layer thickness, both of which are reduced in the glaucomatous eye.[22] OCT is also used as a screening tool in patients at increased risk of developing glaucoma.[23]
Anterior Segment
Anterior segment OCT (AS-OCT) enables visualization of the entire anterior segment structures in a single image and precise quantitative measurements of these structures, including angle opening distance, angle recess area, and trabecular iris space area.[24][25] The trabecular meshwork, canal of Schlemm, angle configuration, and iris configuration can all be visualized using AS-OCT. AS-OCT is particularly useful in identifying patients with shallow angles at risk of acute angle-closure glaucoma and could benefit from preventative iridotomy. AS-OCT can also be used to evaluate and map the cornea, including in cases of corneal opacities, scars, or dystrophies.[24] Intraoperative AS-OCT has been used widely in keratoplasties, refractive surgeries, implantation of intrastromal rings for keratoconus, and trabeculectomy procedures.[26][27] Please see the StatPearls' companion resource, "Acute Angle-Closure Glaucoma," for more information.
Contraindications
There are no absolute contraindications to OCT. However, as OCT uses light waves passing through the pupil and reflecting off the retina, medial opacities can compromise image quality. Therefore, OCT may be of limited utility in patients with congenital or acquired corneal opacities, severe cataracts, or intravitreous hemorrhage.
Equipment
An OCT image is obtained by directing a beam of light, typically in the near-infrared spectrum, onto a tissue of interest; in ophthalmology, this tissue is typically the retina. The light is simultaneously directed onto a reference mirror. The 2 reflected light beams form an interference pattern, generating A-scans. Numerous adjacent A-scans produce a live cross-sectional image of the tissue called a B-scan.[28] TD-OCT has a typical scanning speed of 400 A-scans per second. In contrast, SD-OCT can produce as many as 100,000 A-scans per second, resulting in faster acquisition of higher-resolution images.[28][29][30] SS-OCT uses a tunable laser with a longer wavelength, typically 1050 nm, with a scanning speed of up to 4 million A-scans per second.[31] AS-OCT also uses a longer wavelength to achieve the width and depth required to scan the entire anterior segment.[32]
Equipment
Historically, OCT machines were large, immobile equipment requiring patients to visit a medical facility for testing. However, this is no longer the case with the development of portable OCT devices. Mobile OCT devices are small and lightweight, with a frequently simplified user interface. Many portable OCT devices are handheld and battery-powered, enabling their use in various settings, such as rural or medically underserved areas, emergency departments, or operating rooms.
Intraoperative OCT is an innovative medical imaging technology that provides high-resolution images of the surgical field, offering superior detail compared to traditional one- and two-dimensional images. Technological advancements have integrated the OCT device into the surgical microscope. The benefits of intraoperative OCT include increased precision and safety during surgical procedures, a reduced risk of complications, and improved surgical outcomes. This technology has widespread applications for procedures of both the anterior and posterior segments.
Personnel
OCT can be performed by any eye care professional who has received appropriate training and demonstrated competency.
Preparation
Patient Preparation
OCT is a noninvasive, noncontact, transpupillary imaging technique. Routine preprocedural pharmacologic pupillary dilatation is not typically performed in clinical practice but may be considered in patients with small pupils to improve image capture and quality. Patients should be counseled on the possibility of pupillary dilatation and advised of the risk of temporary light sensitivity and blurred vision; some patients may experience difficulty driving after the procedure due to these transient effects.
Artifacts can be introduced in OCT images due to either the patient or the machine. Patient-related artifacts are primarily due to excessive eye movements and can be controlled by eye-tracking devices. Patients should be advised to try to remain still during the short scanning period.
Technique or Treatment
Scanning Protocols
Numerous OCT scanning protocols exist. The commonly utilized macular scanning protocols for the widely used SD-OCT are the three-dimensional cube, raster, and radial scans.[28] The cube scan provides a three-dimensional comprehensive macular image and comprises horizontal adjacent line scans over an area measuring 6 × 6, 7 × 7, or 12 × 9 mm. A raster scan is a series of parallel line scans oriented at any angle; raster scans are of higher resolution.[28] A radial scan comprises multiple line scans positioned at equal angles with a common axis; if the axis coincides with the fovea, the position of pathologic lesions can be estimated with respect to that structure.[28]
Measuring Choroidal Thickness Using Spectral-Domain Optical Coherence Tomography
Choroidal thickness measurement with SD-OCT requires specialized training and should only be performed by a qualified eye care professional. Pupillary dilatation can temporarily increase choroidal thickness and negatively affect the accuracy of choroidal thickness measurements; choroidal thickness should be measured before any dilatation is performed. Accurate choroidal thickness measurements require the zero-delay line to be set as close as possible to the choroid without touching it.
Optical Coherence Tomography Angiography
OCTA is a widely used imaging modality for visualizing the vasculature of the retina and the choroid. This technique evaluates signal changes over time caused by moving objects such as intravascular erythrocytes compared to the surrounding static retinal tissue.[33] OCTA allows for visualization of the retinal and choroidal vasculature in a single scan, particularly the superficial vascular complex, deep vascular complex, avascular complex, choriocapillaris, and choroid. OCTA cannot delineate low-flow vascular abnormalities such as choroidal polyps.[34][35] OCTA can assist in distinguishing between type 1 and 2 choroidal neovascular membranes and monitor responses to treatment.[36][37][38] Please see StatPearls' companion resource, "Optical Coherence Tomography Angiography," for more information.
Complications
The main complications of OCT are related to artifacts. The most common software-related artifact is a segmentation error. This error causes the software to incorrectly identify certain layers in the OCT image and most commonly occurs in patients with vitreomacular traction or wet age-related macular degeneration.[39][40]
Artifacts can also affect the accuracy of OCT measurements in patients with glaucoma. Minimizing artifacts requires proper patient positioning and fixation during image capture. The clinician should systematically exclude the presence of artifacts before interpreting OCT results. Typically encountered artifacts include motion or shadow artifacts and segmentation errors, which can induce erroneous measurements of the retinal nerve fiber layer, ganglion cell layer, and inner plexiform layer. The most common etiology of a shadow artifact is a cataract, which blocks or scatters the light beam and produces a shadow or signal dropout in the resulting image. SS-OCT and EDI-OCT are less susceptible to artifacts when evaluating the glaucomatous eye. To minimize artifacts, it is important to ensure proper patient positioning and fixation during the OCT scan and to carefully review the OCT images for any artifacts before interpreting the results.
Clinical Significance
Macular Edema
OCT has revolutionized the diagnosis of macular edema. Macular edema can arise from various conditions, with diabetes mellitus being the most prevalent cause.[41][42] Several OCT-based grading systems for diabetic macular edema have been proposed. OCT is also useful in evaluating uveitic macula edema due to any etiology, including Irvine-Gass syndrome.[43] Please see StatPearls' companion resources, "Diabetic Macular Edema," "Macular Edema," and "Uveitic Macular Edema," for more information.
Age-Related Macular Degeneration
OCT can be used to assess the area and volume of drusen observed in age-related macular degeneration, which can serve as a prognostic marker.[36] OCT can also demonstrate Type 1 and 2 choroidal neovascular membranes.[44][28] OCT and OCTA are also used to evaluate, classify, and monitor disease progression and response to treatment in wet age-related macular degeneration.[45] Please see StatPearls' companion resources, "Macular Degeneration" and "Wet Age-Related Macular Degeneration," for more information.[44]
Vitreoretinal Interface Disorders
The International Vitreomacular Traction Study Group has developed an OCT classification system for vitreomacular interface disorders, including vitreomacular adhesion, vitreomacular traction, and macular holes.[46] OCT has greatly improved our understanding of the pathophysiology of these disorders and allows for objective assessment of the relationship between the posterior vitreous and retina. A posterior vitreous detachment can be readily observed as a hyperreflective band that has separated from the retina (see Image. Optical Coherence Tomography, Full-Thickness Macular Hole). A posterior cortical attachment that is within 3 mm of the fovea and does not cause any foveal distortion or loss of foveal contour is called a vitreomacular adhesion.[47]
Vitreomacular traction occurs when the posterior cortical vitreous is tightly adherent to the fovea and remains so even when the posterior vitreous detaches. This traction on the fovea causes distortion, macular edema, or foveal detachment.[46][48] Idiopathic macular holes are believed to be caused by the same vitreomacular tractional forces, and OCT scanning has revolutionized the understanding of idiopathic macular holes.
There are various stages in forming a macular hole, from a pre-macular hole to a full-thickness macular hole with a complete posterior vitreous detachment.[28] Please see StatPearls' companion resources, "Posterior Vitreous Detachment" and "Macular Hole," for more information.
Pachychoroid Spectrum of Diseases
The pachychoroid spectrum of disease includes central serous chorioretinopathy, pachychoroid neovasculopathy, pachychoroid pigment epitheliopathy, and polypoidal choroidal vasculopathy. These pachychoroid disorders share the common feature of thickened choroid and large caliber choroidal vessels visible on OCT and are best evaluated using SS-OCT.[49] The OCT findings typical of central serous chorioretinopathy include a neurosensory detachment, most commonly at the fovea. Chronic central serous chorioretinopathy may demonstrate hyperreflective foci underneath the detachment or cystoid changes within the detached retina.[50] OCT may reveal retinal pigment epithelial detachment in acute or chronic serous chorioretinopathy.[51][52] Doppler OCT permits analysis of choroidal perfusion, optimizing patient compliance by minimizing motion artifacts to improve the accuracy of results.[53]
Glaucoma
OCT is the dominant imaging modality in patients with glaucoma. SD-OCT can detect glaucomatous changes before they become apparent on visual field testing, often many years before the clinical signs of glaucoma are present. OCT also permits the objective assessment of vision parameters and monitors temporal changes over time.[54] OCT can be used to measure the cup-to-disc ratio, rim area, and other parameters of the optic nerve head. OCT effectively evaluates retinal nerve fiber layer thickness; the glaucomatous eye frequently demonstrates thinning of this layer, particularly in the superior and inferior regions. OCT is also used to assess the thickness of the ganglion cell and inner plexiform layers, which typically show thinning in the inferior and temporal regions. Please see the StatPearls' companion resources, "Glaucoma," "Chronic Closed Angle Glaucoma," "Open-Angle Glaucoma," and "Acute Angle-Closure Glaucoma," for more information.
Other Disease Processes
Paracentral acute middle maculopathy: This retinal finding is often observed in individuals with risk factors for vascular or ischemic disease. Fundus visualization reveals a focal paracentral intraretinal pallid sign, and cross-sectional OCT images show hyperreflective band lesions within the inner nuclear layer, which cast hyporeflective shadows onto underlying retinal layers. These findings are believed to represent deep inner retinal capillary plexus vascular insufficiency. Acute superficial retinal vascular pathology due to diabetes mellitus, sickle cell disease, hypertension, or congenital vascular anomalies is common.[55]
Ethambutol toxicity: Ethambutol toxicity is characterized by perimacular thinning of the retinal nerve fiber layer, visible with OCT. Later stages of toxicity may be associated with generalized or diffuse ganglion cell layer loss.[56]
Plaquenil retinopathy: Advanced plaquenil toxicity is characterized by the loss of the subfoveal retinal pigment epithelial layer with diffuse central retinal attenuation of the outer photoreceptor-pigment epithelium complex.[57]
Crystalline retinopathy: In this condition, OCT reveals discrete focal crystalloid deposits in the outer plexiform layer.[58]
Stargardt disease: OCT reveals characteristic crystalline deposits at the retinal pigment epithelium and possibly Bruch membrane. Mild-to-moderate loss of the characteristic macular contour may be observed.[59]
Best disease: OCT demonstrates hyperreflective diffuse subfoveal material around the interdigitation zone or anterior to the retinal pigment epithelium and Bruch membrane.[60]
Retinitis pigmentosa: Cross-sectional OCT images typically demonstrate cystic foveal degeneration, extensive hyporeflective spaces within neuroretinal layers, outer retinal rosettes, and thinning of the ellipsoid layer. These findings correlate with the retinal bone spicules and diffuse retinal vascular attenuation characteristic of the disease.[61]
Subretinal perfluorocarbon liquid: OCT findings of retained subretinal perfluorocarbon liquid typically include a subretinal hyporeflective dome-shaped cyst, compression of overlying adjacent retinal layers, a hyperreflective band around the retinal pigment epithelium layer, and associated hyperreflective shadows over the underlying choroidal section. Stepping of the retinal pigment epithelium has been described as a key differential finding among patients with residual perfluorocarbon liquid versus residual silicon oil; this finding is attributed to the refractive index of the retained subretinal transparent medium.[62]
Solar retinopathy: OCT shows generalized foveal thinning with a hyporeflective area of varying depth that depends on the extent of visual reduction. This OCT finding often accounts for the loss of photoreceptor inner and outer segments and may extend to the retinal pigment epithelial layer.[63]
Foveoschisis: Foveoschisis is frequently associated with vitreomacular traction, and OCT reveals progressive separation within neuroretinal layers around the foveal pit. Please see StatPearls' companion resource, "Myopic Foveoschisis," for more information.
Optic disc pit maculopathy: OCT shows detachment of the pigment epithelial layer extending from the temporal optic cup margin and affecting the macula; dome-shaped macular distortion is a frequent finding.[64]
Acute idiopathic maculopathy: Outer neuroretinal submacular exudative detachment is a common OCT finding during the acute phase. Following the resolution of exudative detachments, typical OCT findings include hyperreflectivity of the photoreceptor outer segments layer with retinal pigment epithelial thickening.[65]
Acute macular neuroretinopathy: OCTA typically shows mild hypoautofluorescent petaloid patterns around the paramacular area.[66]
Enhancing Healthcare Team Outcomes
OCT imaging is a widely used tool for diagnosing and monitoring various retinal diseases, including glaucoma.[67] OCT provides an excellent two-dimensional cross-sectional image of the tissue and, as previously described, has significantly improved our understanding of many conditions, including vitreoretinal interface disorders. OCT is frequently used as a sole investigative tool to support clinical diagnosis.
In the acute setting, modern OCT, with multimodal imaging and OCTA, can be key for differentiating several ocular ischemic emergencies without systemic injection of dyes. OCTA is gaining popularity and can image several disorders of the anterior segment and retina.[68] OCT mapping of the peripapillary retinal nerve fiber layer also forms a valuable adjunct to standard automated perimetry and neuroimaging for detecting or predicting the localization of defects within the visual pathway.[69][70][71] SS-OCT machines also take advantage of their high resolution and penetration to facilitate preoperative biometry in the presence of dense cataracts.[72]
Amid these advanced practice applications of OCT technology in ophthalmic imaging, available OCT systems still pose some limitations.[73] Although there are pitfalls with OCT imaging, it forms the cornerstone of retinal imaging, and several ongoing advancements are being made to develop this technology further.[74] Further research and practice advancements, with or without the application of machine learning, are bound to promote the significance, precision, and hopefully accessibility of OCT in everyday practice.[75]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
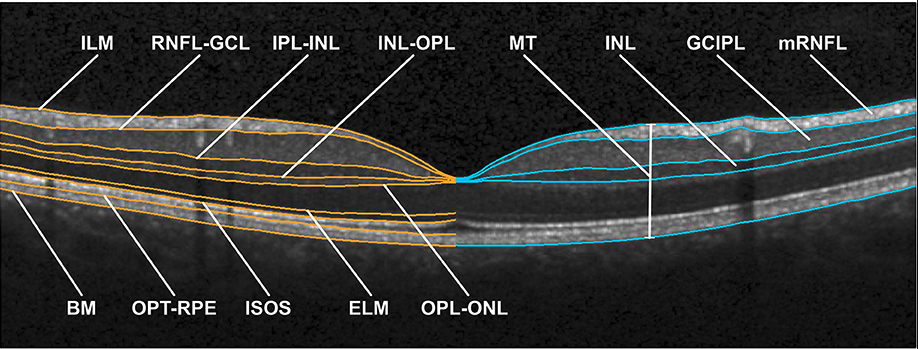
Optical Coherence Tomography. The image shows an optical coherence tomography scan of the macula in a normal eye. ILM, inner limiting membrane; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; ELM, external limiting membrane; ISOS, inner and outer segment; OPT, outer photoreceptor tip; RPE, retinal pigment epithelium; BM, Bruch membrane; MT, macular thickness; mRNFL, macular retinal nerve fiber layer; GCIPL, combined ganglion cell and plexiform layer; INL, inner nuclear layer.
Motamedi S, Gawlik K, Ayadi N, et al. Normative data and minimally detectable change for inner retinal layer thicknesses using a semi-automated OCT image segmentation pipeline. Front Neurol. 2019;10:1117. doi: 10.3389/fneur.2019.01117.
References
Michelessi M, Li T, Miele A, Azuara-Blanco A, Qureshi R, Virgili G. Accuracy of optical coherence tomography for diagnosing glaucoma: an overview of systematic reviews. The British journal of ophthalmology. 2021 Apr:105(4):490-495. doi: 10.1136/bjophthalmol-2020-316152. Epub 2020 Jun 3 [PubMed PMID: 32493760]
Level 3 (low-level) evidenceFujimoto J, Swanson E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investigative ophthalmology & visual science. 2016 Jul 1:57(9):OCT1-OCT13. doi: 10.1167/iovs.16-19963. Epub [PubMed PMID: 27409459]
Bengtsson B, Andersson S, Heijl A. Performance of time-domain and spectral-domain Optical Coherence Tomography for glaucoma screening. Acta ophthalmologica. 2012 Jun:90(4):310-5. doi: 10.1111/j.1755-3768.2010.01977.x. Epub 2010 Oct 14 [PubMed PMID: 20946342]
Böhringer HJ, Boller D, Leppert J, Knopp U, Lankenau E, Reusche E, Hüttmann G, Giese A. Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers in surgery and medicine. 2006 Jul:38(6):588-97 [PubMed PMID: 16736504]
Level 3 (low-level) evidenceAsghari H. Visible wavelength time-stretch optical coherence tomography. Optics express. 2023 Jul 17:31(15):24085-24096. doi: 10.1364/OE.492753. Epub [PubMed PMID: 37475244]
Chauhan P, Kho AM, Srinivasan VJ. From Soma to Synapse: Imaging Age-Related Rod Photoreceptor Changes in the Mouse with Visible Light OCT. Ophthalmology science. 2023 Dec:3(4):100321. doi: 10.1016/j.xops.2023.100321. Epub 2023 Apr 29 [PubMed PMID: 37388138]
Szczepanik M, Balicki I, Śmiech A, Szadkowski M, Gołyński M, Osęka M, Zwolska J. The use of optical coherence tomography for skin evaluation in healthy rats. Veterinary dermatology. 2022 Aug:33(4):296-e69. doi: 10.1111/vde.13071. Epub 2022 May 30 [PubMed PMID: 35635296]
Ming Y, Hao S, Wang F, Lewis-Israeli YR, Volmert BD, Xu Z, Goestenkors A, Aguirre A, Zhou C. Longitudinal morphological and functional characterization of human heart organoids using optical coherence tomography. Biosensors & bioelectronics. 2022 Jul 1:207():114136. doi: 10.1016/j.bios.2022.114136. Epub 2022 Mar 9 [PubMed PMID: 35325716]
Meng QY, Miao ZQ, Liang ST, Wu X, Wang LJ, Zhao MW, Guo LL. Choroidal thickness, myopia, and myopia control interventions in children: a Meta-analysis and systemic review. International journal of ophthalmology. 2023:16(3):453-464. doi: 10.18240/ijo.2023.03.17. Epub 2023 Mar 18 [PubMed PMID: 36935799]
Level 1 (high-level) evidenceWang J, Yin LR. The Application of Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography in Macular Diseases. Journal of ophthalmology. 2020:2020():9503795. doi: 10.1155/2020/9503795. Epub 2020 Aug 24 [PubMed PMID: 32908688]
Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D'Antonio F, De Vico U, Imbriano L, Campanelli A, De Lena C. Choroidal thinning as a new finding in Alzheimer's disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. Journal of Alzheimer's disease : JAD. 2014:40(4):907-17. doi: 10.3233/JAD-132039. Epub [PubMed PMID: 24577467]
Erbahçeci Timur İE, Açıkgöz V, Uğurlu N, Yalçın B, Şendur MAN, Hızal M, Kara H. Tamoxifen related chorioretinal structural changes. Cutaneous and ocular toxicology. 2023 Sep:42(3):109-117. doi: 10.1080/15569527.2023.2220388. Epub 2023 Jun 15 [PubMed PMID: 37272809]
Sayanagi K, Hara C, Fukushima Y, Sato S, Kawasaki R, Nishida K. Three cases of macular retinal detachment exacerbated during follow-up with myopic foveoschisis around myopic choroidal neovascularization. American journal of ophthalmology case reports. 2023 Dec:32():101899. doi: 10.1016/j.ajoc.2023.101899. Epub 2023 Jul 20 [PubMed PMID: 37564973]
Level 3 (low-level) evidencede Boer JF, Leitgeb R, Wojtkowski M. Twenty-five years of optical coherence tomography: the paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomedical optics express. 2017 Jul 1:8(7):3248-3280. doi: 10.1364/BOE.8.003248. Epub 2017 Jun 15 [PubMed PMID: 28717565]
Lupidi M, Cerquaglia A, Chhablani J, Fiore T, Singh SR, Cardillo Piccolino F, Corbucci R, Coscas F, Coscas G, Cagini C. Optical coherence tomography angiography in age-related macular degeneration: The game changer. European journal of ophthalmology. 2018 Jul:28(4):349-357. doi: 10.1177/1120672118766807. Epub 2018 Apr 6 [PubMed PMID: 29623720]
Drenser KA, Pieramici DJ, Gunn JM, Rosberger DF, Kozma P, Fineman MS, Duchateau L, Khanani AM. Retrospective Study of Ellipsoid Zone Integrity Following Treatment with Intravitreal Ocriplasmin (OZONE Study). Clinical ophthalmology (Auckland, N.Z.). 2021:15():3109-3120. doi: 10.2147/OPTH.S285464. Epub 2021 Jul 16 [PubMed PMID: 34295149]
Level 2 (mid-level) evidenceKondo H. Foveal hypoplasia and optical coherence tomographic imaging. Taiwan journal of ophthalmology. 2018 Oct-Dec:8(4):181-188. doi: 10.4103/tjo.tjo_101_18. Epub [PubMed PMID: 30637189]
Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, Enzmann V, Wolf S. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Investigative ophthalmology & visual science. 2009 Jul:50(7):3432-7. doi: 10.1167/iovs.08-2970. Epub 2009 Feb 21 [PubMed PMID: 19234346]
Level 2 (mid-level) evidenceEdgar A, Ward J, Woods C. Optical coherence tomography in the assessment of simultaneous macula oedema and papilloedema. Clinical & experimental optometry. 2020 Nov:103(6):905-907. doi: 10.1111/cxo.13061. Epub 2020 Apr 1 [PubMed PMID: 32239530]
Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS, Sebag J, Gaudric A, Staurenghi G, Haritoglou C, Kadonosono K, Thompson JT, Chang S, Bottoni F, Tadayoni R. Optical coherence tomography-based consensus definition for lamellar macular hole. The British journal of ophthalmology. 2020 Dec:104(12):1741-1747. doi: 10.1136/bjophthalmol-2019-315432. Epub 2020 Feb 27 [PubMed PMID: 32107208]
Level 3 (low-level) evidenceKhalid S, Akram MU, Hassan T, Jameel A, Khalil T. Automated Segmentation and Quantification of Drusen in Fundus and Optical Coherence Tomography Images for Detection of ARMD. Journal of digital imaging. 2018 Aug:31(4):464-476. doi: 10.1007/s10278-017-0038-7. Epub [PubMed PMID: 29204763]
Ran AR, Tham CC, Chan PP, Cheng CY, Tham YC, Rim TH, Cheung CY. Correction: Deep learning in glaucoma with optical coherence tomography: a review. Eye (London, England). 2021 Jan:35(1):357. doi: 10.1038/s41433-020-01244-9. Epub [PubMed PMID: 33097920]
Klein BE, Johnson CA, Meuer SM, Lee K, Wahle A, Lee KE, Kulkarni A, Sonka M, Abràmoff MD, Klein R. Nerve Fiber Layer Thickness and Characteristics Associated with Glaucoma in Community Living Older Adults: Prelude to a Screening Trial? Ophthalmic epidemiology. 2017 Apr:24(2):104-110. doi: 10.1080/09286586.2016.1258082. Epub 2016 Dec 29 [PubMed PMID: 28032805]
Angmo D, Nongpiur ME, Sharma R, Sidhu T, Sihota R, Dada T. Clinical utility of anterior segment swept-source optical coherence tomography in glaucoma. Oman journal of ophthalmology. 2016 Jan-Apr:9(1):3-10. doi: 10.4103/0974-620X.176093. Epub [PubMed PMID: 27013821]
Campbell P, Redmond T, Agarwal R, Marshall LR, Evans BJ. Repeatability and comparison of clinical techniques for anterior chamber angle assessment. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2015 Mar:35(2):170-8. doi: 10.1111/opo.12200. Epub [PubMed PMID: 25761580]
Han SB, Liu YC, Noriega KM, Mehta JS. Applications of Anterior Segment Optical Coherence Tomography in Cornea and Ocular Surface Diseases. Journal of ophthalmology. 2016:2016():4971572 [PubMed PMID: 27721988]
Venkateswaran N, Galor A, Wang J, Karp CL. Optical coherence tomography for ocular surface and corneal diseases: a review. Eye and vision (London, England). 2018:5():13. doi: 10.1186/s40662-018-0107-0. Epub 2018 Jun 12 [PubMed PMID: 29942817]
Bhende M, Shetty S, Parthasarathy MK, Ramya S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian journal of ophthalmology. 2018 Jan:66(1):20-35. doi: 10.4103/ijo.IJO_902_17. Epub [PubMed PMID: 29283118]
Badaró E, Novais E, Prodocimo LM, Sallum JM. Spectral-domain optical coherence tomography for macular edema. TheScientificWorldJournal. 2014:2014():191847. doi: 10.1155/2014/191847. Epub 2014 May 14 [PubMed PMID: 24963500]
Wolf S, Wolf-Schnurrbusch U. Spectral-domain optical coherence tomography use in macular diseases: a review. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2010:224(6):333-40. doi: 10.1159/000313814. Epub 2010 May 4 [PubMed PMID: 20453539]
Level 3 (low-level) evidenceBarteselli G, Bartsch DU, Weinreb RN, Camacho N, Nezgoda JT, Marvasti AH, Freeman WR. REAL-TIME FULL-DEPTH VISUALIZATION OF POSTERIOR OCULAR STRUCTURES: Comparison Between Full-Depth Imaging Spectral Domain Optical Coherence Tomography and Swept-Source Optical Coherence Tomography. Retina (Philadelphia, Pa.). 2016 Jun:36(6):1153-61. doi: 10.1097/IAE.0000000000000842. Epub [PubMed PMID: 26562563]
Shan J, DeBoer C, Xu BY. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2019 MarchApril 01:8(2):146-157. doi: 10.22608/APO.201910. Epub [PubMed PMID: 31020820]
Hagag AM, Gao SS, Jia Y, Huang D. Optical coherence tomography angiography: Technical principles and clinical applications in ophthalmology. Taiwan journal of ophthalmology. 2017 Jul-Sep:7(3):115-129. doi: 10.4103/tjo.tjo_31_17. Epub 2017 Sep 19 [PubMed PMID: 28966909]
Gass JD. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. V. Retinal telangiectasis. Archives of ophthalmology (Chicago, Ill. : 1960). 1968 Nov:80(5):592-605 [PubMed PMID: 5684308]
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina (Philadelphia, Pa.). 2012 Feb:32 Suppl 1():1-8 [PubMed PMID: 22451948]
Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014 Jul:121(7):1435-44. doi: 10.1016/j.ophtha.2014.01.034. Epub 2014 Mar 27 [PubMed PMID: 24679442]
Level 3 (low-level) evidenceKuehlewein L, Bansal M, Lenis TL, Iafe NA, Sadda SR, Bonini Filho MA, De Carlo TE, Waheed NK, Duker JS, Sarraf D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. American journal of ophthalmology. 2015 Oct:160(4):739-48.e2. doi: 10.1016/j.ajo.2015.06.030. Epub 2015 Jul 9 [PubMed PMID: 26164826]
El Ameen A, Cohen SY, Semoun O, Miere A, Srour M, Quaranta-El Maftouhi M, Oubraham H, Blanco-Garavito R, Querques G, Souied EH. TYPE 2 NEOVASCULARIZATION SECONDARY TO AGE-RELATED MACULAR DEGENERATION IMAGED BY OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retina (Philadelphia, Pa.). 2015 Nov:35(11):2212-8. doi: 10.1097/IAE.0000000000000773. Epub [PubMed PMID: 26441269]
Domalpally A, Danis RP, Zhang B, Myers D, Kruse CN. Quality issues in interpretation of optical coherence tomograms in macular diseases. Retina (Philadelphia, Pa.). 2009 Jun:29(6):775-81. doi: 10.1097/IAE.0b013e3181a0848b. Epub [PubMed PMID: 19373128]
Level 1 (high-level) evidenceGiani A, Cigada M, Esmaili DD, Salvetti P, Luccarelli S, Marziani E, Luiselli C, Sabella P, Cereda M, Eandi C, Staurenghi G. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina (Philadelphia, Pa.). 2010 Apr:30(4):607-16. doi: 10.1097/IAE.0b013e3181c2e09d. Epub [PubMed PMID: 20094011]
Romero-Aroca P. Managing diabetic macular edema: The leading cause of diabetes blindness. World journal of diabetes. 2011 Jun 15:2(6):98-104. doi: 10.4239/wjd.v2.i6.98. Epub [PubMed PMID: 21860693]
Chung YR, Kim YH, Ha SJ, Byeon HE, Cho CH, Kim JH, Lee K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. Journal of diabetes research. 2019:2019():8164250. doi: 10.1155/2019/8164250. Epub 2019 Dec 20 [PubMed PMID: 31930145]
Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye (London, England). 2016 Oct:30(10):1277-1292. doi: 10.1038/eye.2016.115. Epub 2016 Jun 3 [PubMed PMID: 27256304]
Mrejen S, Sarraf D, Mukkamala SK, Freund KB. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina (Philadelphia, Pa.). 2013 Oct:33(9):1735-62. doi: 10.1097/IAE.0b013e3182993f66. Epub [PubMed PMID: 23873168]
Anantharaman G, Sheth J, Bhende M, Narayanan R, Natarajan S, Rajendran A, Manayath G, Sen P, Biswas R, Banker A, Gupta C. Polypoidal choroidal vasculopathy: Pearls in diagnosis and management. Indian journal of ophthalmology. 2018 Jul:66(7):896-908. doi: 10.4103/ijo.IJO_1136_17. Epub [PubMed PMID: 29941728]
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013 Dec:120(12):2611-2619. doi: 10.1016/j.ophtha.2013.07.042. Epub 2013 Sep 17 [PubMed PMID: 24053995]
Tian J, Lin C, Fang Y, Cao K, Duan A, Qi Y, Wang N. Multimodal Analysis on Clinical Characteristics of the Advanced Stage in Myopic Traction Maculopathy. Ophthalmology and therapy. 2023 Oct:12(5):2569-2581. doi: 10.1007/s40123-023-00745-6. Epub 2023 Jul 8 [PubMed PMID: 37420080]
Level 2 (mid-level) evidenceMirza RG, Johnson MW, Jampol LM. Optical coherence tomography use in evaluation of the vitreoretinal interface: a review. Survey of ophthalmology. 2007 Jul-Aug:52(4):397-421 [PubMed PMID: 17574065]
Level 3 (low-level) evidenceLehmann M, Bousquet E, Beydoun T, Behar-Cohen F. PACHYCHOROID: an inherited condition? Retina (Philadelphia, Pa.). 2015 Jan:35(1):10-6. doi: 10.1097/IAE.0000000000000287. Epub [PubMed PMID: 25046398]
Level 2 (mid-level) evidenceIida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina (Philadelphia, Pa.). 2003 Feb:23(1):1-7; quiz 137-8 [PubMed PMID: 12652224]
Yang L, Jonas JB, Wei W. Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Investigative ophthalmology & visual science. 2013 Jul 12:54(7):4659-65. doi: 10.1167/iovs.12-10991. Epub 2013 Jul 12 [PubMed PMID: 23737472]
Berger L, Bühler V, Yzer S. Central Serous Chorioretinopathy - an Overview. Klinische Monatsblatter fur Augenheilkunde. 2021 Sep:238(9):971-979. doi: 10.1055/a-1531-5605. Epub 2021 Aug 20 [PubMed PMID: 34416788]
Level 3 (low-level) evidenceBek T, Jeppesen SK. Doppler OCT as a tool for studying localized disturbances in blood flow in larger retinal vessels. Acta ophthalmologica. 2022 Nov:100(7):834-835. doi: 10.1111/aos.15106. Epub 2022 Feb 3 [PubMed PMID: 35113483]
Tatham AJ, Medeiros FA. Detecting Structural Progression in Glaucoma with Optical Coherence Tomography. Ophthalmology. 2017 Dec:124(12S):S57-S65. doi: 10.1016/j.ophtha.2017.07.015. Epub [PubMed PMID: 29157363]
Moura-Coelho N, Gaspar T, Ferreira JT, Dutra-Medeiros M, Cunha JP. Paracentral acute middle maculopathy-review of the literature. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020 Dec:258(12):2583-2596. doi: 10.1007/s00417-020-04826-1. Epub 2020 Jul 13 [PubMed PMID: 32661700]
Pavan Taffner BM, Mattos FB, Cunha MCD, Saraiva FP. The use of optical coherence tomography for the detection of ocular toxicity by ethambutol. PloS one. 2018:13(11):e0204655. doi: 10.1371/journal.pone.0204655. Epub 2018 Nov 8 [PubMed PMID: 30408036]
Yusuf IH, Charbel Issa P, Ahn SJ. Novel imaging techniques for hydroxychloroquine retinopathy. Frontiers in medicine. 2022:9():1026934. doi: 10.3389/fmed.2022.1026934. Epub 2022 Oct 13 [PubMed PMID: 36314000]
Gaucher D, Saleh M, Sauer A, Bourcier T, Speeg-Schatz C. Spectral OCT analysis in Bietti crystalline dystrophy. European journal of ophthalmology. 2010 May-Jun:20(3):612-4 [PubMed PMID: 20099228]
Level 3 (low-level) evidenceMishra Z, Wang Z, Sadda SR, Hu Z. Automatic Segmentation in Multiple OCT Layers For Stargardt Disease Characterization Via Deep Learning. Translational vision science & technology. 2021 Apr 1:10(4):24. doi: 10.1167/tvst.10.4.24. Epub [PubMed PMID: 34004000]
Makati R, Shechtman D, Besada E, Pizzimenti JJ. Electrooculography and optical coherence tomography reveal late-onset Best disease. Optometry and vision science : official publication of the American Academy of Optometry. 2014 Nov:91(11):e274-7. doi: 10.1097/OPX.0000000000000403. Epub [PubMed PMID: 25259760]
Level 3 (low-level) evidenceOh JK, Nuzbrokh Y, Lima de Carvalho JR Jr, Ryu J, Tsang SH. Optical coherence tomography in the evaluation of retinitis pigmentosa. Ophthalmic genetics. 2020 Oct:41(5):413-419. doi: 10.1080/13816810.2020.1780619. Epub 2020 Jun 19 [PubMed PMID: 32552399]
Ma CC, Chang YH. Multimodal Imaging of Retained Subretinal Perfluorocarbon Liquid. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2022 Sep 1:11(5):492. doi: 10.1097/APO.0000000000000456. Epub 2022 Sep 1 [PubMed PMID: 35342175]
Jain A, Desai RU, Charalel RA, Quiram P, Yannuzzi L, Sarraf D. Solar retinopathy: comparison of optical coherence tomography (OCT) and fluorescein angiography (FA). Retina (Philadelphia, Pa.). 2009 Oct:29(9):1340-5. doi: 10.1097/IAE.0b013e3181b0da88. Epub [PubMed PMID: 19934824]
Level 2 (mid-level) evidenceChiu YT, Chen HY, Tsai YY, Lin JM, Chiang CC. Stratus optical coherence tomography for evaluating optic disc pits associated with maculopathy before and after vitrectomy: two case reports. The Kaohsiung journal of medical sciences. 2006 May:22(5):229-34 [PubMed PMID: 16793558]
Level 3 (low-level) evidenceFernández-Avellaneda P, Breazzano MP, Fragiotta S, Xu X, Zhang Q, Wang RK, Freund KB. BACILLARY LAYER DETACHMENT OVERLYING REDUCED CHORIOCAPILLARIS FLOW IN ACUTE IDIOPATHIC MACULOPATHY. Retinal cases & brief reports. 2022 Jan 1:16(1):59-66. doi: 10.1097/ICB.0000000000000943. Epub [PubMed PMID: 31764886]
Level 3 (low-level) evidenceBhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET Jr. Acute macular neuroretinopathy: A comprehensive review of the literature. Survey of ophthalmology. 2016 Sep-Oct:61(5):538-65. doi: 10.1016/j.survophthal.2016.03.003. Epub 2016 Mar 10 [PubMed PMID: 26973287]
Level 3 (low-level) evidenceShaimova VA, Kuchkildina SK, Islamova GR, Arslanov GM, Kravchenko TG, Askaeva AA. [Age-related changes in human vitreous]. Vestnik oftalmologii. 2023:139(3):106-111. doi: 10.17116/oftalma2023139031106. Epub [PubMed PMID: 37379116]
Hassan M, Agarwal A, Afridi R, daSilva MJ, Karaca I, Sadiq MA, Nguyen QD, Do DV. The Role of Optical Coherence Tomography Angiography in the Management of Uveitis. International ophthalmology clinics. 2016 Fall:56(4):1-24. doi: 10.1097/IIO.0000000000000130. Epub [PubMed PMID: 27575755]
Banc A, Stan C, Florian IS. Optical coherence tomography impacts the evaluation of visual pathway tumors. Neurosurgical review. 2018 Apr:41(2):415-426. doi: 10.1007/s10143-016-0772-1. Epub 2016 Jul 28 [PubMed PMID: 27465394]
Coric D, Nij Bijvank JA, van Rijn LJ, Petzold A, Balk LJ. The role of optical coherence tomography and infrared oculography in assessing the visual pathway and CNS in multiple sclerosis. Neurodegenerative disease management. 2018 Oct:8(5):323-335. doi: 10.2217/nmt-2018-0011. Epub 2018 Sep 18 [PubMed PMID: 30226111]
van der Sijde JN, Karanasos A, van Ditzhuijzen NS, Okamura T, van Geuns RJ, Valgimigli M, Ligthart JM, Witberg KT, Wemelsfelder S, Fam JM, Zhang B, Diletti R, de Jaegere PP, van Mieghem NM, van Soest G, Zijlstra F, van Domburg RT, Regar E. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. European heart journal. Cardiovascular Imaging. 2017 Apr 1:18(4):467-474. doi: 10.1093/ehjci/jew037. Epub [PubMed PMID: 26992420]
Kane JX, Chang DF. Intraocular Lens Power Formulas, Biometry, and Intraoperative Aberrometry: A Review. Ophthalmology. 2021 Nov:128(11):e94-e114. doi: 10.1016/j.ophtha.2020.08.010. Epub 2020 Aug 13 [PubMed PMID: 32798526]
Shahlaee A, Samara WA, Sridhar J, Kasi SK, Hsu J, Ho AC. Accentuation of optical coherence tomography angiography projection artefacts on hyper-reflective retinal layers. Acta ophthalmologica. 2018 Nov:96(7):e883-e884. doi: 10.1111/aos.13164. Epub 2016 Jul 1 [PubMed PMID: 27369697]
Khan A, Pin K, Aziz A, Han JW, Nam Y. Optical Coherence Tomography Image Classification Using Hybrid Deep Learning and Ant Colony Optimization. Sensors (Basel, Switzerland). 2023 Jul 26:23(15):. doi: 10.3390/s23156706. Epub 2023 Jul 26 [PubMed PMID: 37571490]
Balyen L, Peto T. Promising Artificial Intelligence-Machine Learning-Deep Learning Algorithms in Ophthalmology. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2019 May-Jun:8(3):264-272. doi: 10.22608/APO.2018479. Epub 2019 May 31 [PubMed PMID: 31149787]