Introduction
More than 100 distinct types of cancer have been identified. Cancer frequently affects multiple organs due to uncontrolled cell division, migration, and invasion. Understanding its pathogenesis is crucial, given the rising number of cases and the limitations of standard treatment options, which contribute to poor overall and disease-free survival rates for many patients.[1][2]
Despite improved survival rates in developed countries, the incidence of cancer continues to rise sharply. More than 90% of cancers are directly attributable to environmental and lifestyle factors, making them largely preventable. Over the past decade, significant progress has been made in understanding cancer, including the processes of carcinogenesis (the initiation of cancer), oncogenesis, and the progression of cells to malignant metastasis. Gaining insights into the molecular and cellular mechanisms driving carcinogenesis is crucial for developing effective strategies for cancer prevention, diagnosis, and treatment.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
The epidemiology and history of cancer reveal a complex and evolving disease that has impacted humanity for millennia. Our understanding of cancer has grown significantly from ancient descriptions in historical texts to modern epidemiological studies. Today, insights into its prevalence, causes, and progression shape public health strategies and medical advancements, highlighting the importance of historical context in the ongoing fight against cancer.
Epidemiology
Lung cancer is projected to be the leading cause of death in both males and females by 2040.[3] Current statistics from the Global Cancer Observatory (GLOBOCAN) estimate that cancer cases will reach 28.4 million by 2040, an increase of nearly 50% compared to 2020.[4] Cancer severity is primarily age-related, with middle-aged individuals at the highest risk of developing malignant tumors. Hereditary factors contribute 5% to 10% of cancers arising from pathogenic variations in cancer-predisposing genes.[5]
Cancers can also occur somatically, where genomic variations arise de novo and are not passed down to offspring.[6] Therefore, it is essential to understand the disease's biology, its pathogenesis, and the mechanisms by which normal cells transform into malignant cells.
Institutional History
References to cancer can be found in historical records as far back as 1700 BC, including the Babylonian Code of Hammurabi, the ancient Egyptian Ebers and Smith Papyrus, and the Chinese Rites of the Zhou Dynasty. Early mentions of cancer are also documented in medieval Islamic texts and the ancient Indian Ramayana.[7][8] In the United States, a definitive effort to combat cancer and advance scientific research began with the establishment of the National Cancer Institute in 1937. This initiative gained further momentum with the passage of the National Cancer Act in 1971.[9]
Scientific Advancement History
Humankind has studied benign and malignant tumors since ancient times, speculating on their causes. Recent findings by Randolph-Quinney et al support the ancient origins of cancer, with the discovery of osteosarcoma in a hominin foot bone over one million years old in South Africa—suggesting that cancer has been present as long as humanity itself.[10]
Until the 18th century, hypotheses about the origins of cancer ranged from superstitions to the belief that lymph was responsible for the disease. Percival Pott was the first to identify cancerous growths in the scrotums of individuals exposed to chimney sweeps, providing early evidence that environmental factors could cause cancer.[11]
Rudolf Virchow's cell theory established the cellular origin of cancer in 1858. Following this, several hypotheses emerged regarding the initiating agents of cancer, including Warburg's theory of enhanced aerobic glycolysis in 1956, Knudson's 2-hit hypothesis in 1971, theories of chromosomal aberrations, and the somatic mutation theory.
In 1914, Boveri proposed that cancer originates from a single cell that spreads to neighboring cells, resulting in abnormal proliferation. In 1917, Yamagiwa built upon Pott's hypotheses by conducting the first study to demonstrate a direct link between chemicals and cancer using coal tar—a mixture of various substances. This breakthrough paved the way for identifying specific cancer-causing chemicals. Later, Cook et al discovered that polycyclic hydrocarbons act as activators of these carcinogens.[10]
The somatic mutation theory became widely accepted as the primary cause of cancer. However, certain exceptions highlight the need to consider all entities of molecular interactions (EoMIs) in understanding carcinogenesis. EoMI includes various processes such as synthetic, mechanical, electrical, concentration, and heat generation-related interactions, all of which should be factored into cancer research.[12]
Currently, the most widely accepted theory is the multistep theory, which explains the genetic and epigenetic events involved in transforming a single cell into numerous malignant cells capable of metastasis. Moreover, identifying cancer hallmarks has redefined the understanding of carcinogenesis, providing a more comprehensive view of cancer as a process driven by the accumulation of these hallmarks.[13][14]
Causes
Cancer usually develops through the transformation of normal cells into malignant tumor cells, often progressing from a precancerous condition to a fully developed tumor. This transition is influenced by an individual’s genetics and exposure to 3 main categories, as mentioned below.
Physical Carcinogens
- Ultraviolet and ionizing radiation
Chemical Carcinogens
- Polycyclic hydrocarbons
- Asbestos
- Components of tobacco smoke
- Alcohol
- Aflatoxin (a grain contaminant)
- Arsenic (a drinking water contaminant)
Biological Carcinogens
- Viral infections such as hepatitis B and human papillomavirus (HPV)
- Bacterial infections, such as Helicobacter pylori
- Parasitic infections, such as Schistosoma haematobium
Anatomical Pathology
The origin of cancer cells can be traced back to 3 potential sources—the cells where modifications occur to induce cancer growth and the cells that lead to the development of the cancer cell clone. The sources include:
- Stem cells, such as mesenchymal stem cells for solid tumors or hematopoietic stem cells for leukemia
- Intermediate progenitor cells
- Dedifferentiated tissue cells
Biochemical and Genetic Pathology
Cancer is a group of diseases characterized by diverse molecular signatures, yet all share common cellular phenotypic traits and functional capabilities. Hanahan and Weinberg identified these traits as the hallmarks of cancer, which include increased cell proliferation, achieved through sustained proliferative signaling or evasion of growth suppressors. Additionally, cancer cells promote tumor progression and spread by inducing or accessing vasculature and activating invasion and metastasis.
Two enabling characteristics facilitate the acquisition of these traits: genomic instability and tumor-promoting inflammation. Additionally, deregulated tumor metabolism has emerged as a defining feature of cancer cells. Carcinogenesis can be viewed as a series of genetic and epigenetic changes that gradually allow cells to acquire these hallmarks, ultimately leading to full cancer development.[15]
The carcinogenesis process involves multiple events of genetic DNA damage (mutations) and epigenetic modifications in gene expression that accumulate sequentially. These changes contribute to the gradual acquisition of the hallmarks of cancer, ultimately resulting in fully developed, metastatic cancer (see Image. The Carcinogenesis Pathway).
DNA Damage
DNA damage plays a crucial role in carcinogenesis. Proper cell function depends on the precise regulation of various cellular processes, including DNA replication and repair. When DNA is damaged, it typically activates repair mechanisms. As DNA serves as the genetic blueprint for all living organisms, damage can significantly impact the cell and its future progeny. To counteract this, cells have highly efficient DNA repair systems designed to prevent harmful mutations.
The 2015 Nobel Prize in Chemistry was awarded to Tomas Lindahl and Aziz Sancar for their groundbreaking research on fundamental DNA repair mechanisms, including mismatch repair, base excision repair, and nucleotide excision repair. If DNA damage is irreparable, the affected cells undergo programmed cell death, also known as apoptosis. When apoptosis fails, a third line of defense—the immune system—is activated to recognize and attack cells with mutated genes. However, if the immune system is compromised or overwhelmed by the mutated cells, these cells can persist and potentially progress to full-blown cancer under favorable conditions.
Recent studies estimate the rate of mutation accumulation to range from 13 to 44 mutations per genome per year. This rate increases with exposure to environmental factors that induce DNA abnormalities, such as radiation, smoking, pollutants, and alcohol. Endogenous factors, including abnormalities in DNA repair mechanisms, hormonal imbalances, and oxidative stress, also contribute to mutation accumulation. Research has shown that even normal tissues can harbor clonal expansions of cells carrying carcinogenic driver mutations. These clonal expansions increase with age and exposure to both exogenous and endogenous mutagenic factors. Certain mutated cell clones are more likely to progress to cancer, depending on specific cancer driver mutations within the clone. Beyond cancer, these mutated cell clones can also contribute to other diseases, including inflammatory and cardiovascular conditions.
DNA damage can result from endogenous factors, extrinsic factors, or a combination of both. Endogenous factors include normal metabolic processes, such as oxidative stress, while extrinsic factors encompass environmental exposures, such as radiation and toxins.
Endogenous DNA damage: Endogenous factors usually result from spontaneous disruptions in normal metabolic processes, such as defects in DNA replication and disruptions in cellular signaling, leading to errors. These factors often originate from hereditary predispositions. Common forms of endogenous DNA damage include oxidative and methylation reactions occurring naturally within cells. In some cases, endogenous factors may interact with external environmental influences, as seen in conditions such as xeroderma pigmentosum, which can further increase the risk of cancer.
Normal cellular processes, such as the Fenton reaction, combine hydrogen peroxide (H2O2) with iron to generate highly reactive hydroxyl (•OH) free radicals, which can cause severe DNA damage and lead to significant mutations. Additionally, DNA damage can occur internally through methylation, typically mediated by physiological methylation reactions facilitated by methyltransferase enzymes that utilize S-adenosylmethionine as a methyl donor. This process results in the formation of over 4600 methylated guanine and adenine residues, which can hinder DNA repair and polymerization, as well as disrupt the normal regulation of transcription.[16]
Exogenous DNA damage: External factors such as chemicals, toxins, radiation, and environmental hazards are common sources of DNA damage and are largely preventable causes of carcinogenesis. Chemical combinations found in tobacco smoke, industrial processes, and chemotherapy drugs induce DNA aberrations through alkylation. Alkylating agents attach an alkyl group to the guanine base of DNA, disrupting normal protein synthesis and causing mutations. Tobacco smoke, fossil fuels, industrial chemicals such as pesticides, and high-temperature cooking can also produce aromatic amines. These amines can be converted into alkylating agents, esters, and sulfates, which target guanine bases in DNA, resulting in damage that may initiate cancer.
Recent studies indicate that environmental pollution is a significant exogenous source of DNA damage. High levels of air pollution, particularly particulate matter, have been strongly linked to an increased incidence of lung cancer. Additionally, the long-term effects of extreme temperatures resulting from global warming contribute to DNA damage. Environmental pollution now ranks as the second most common cause of cancer, surpassed only by tobacco smoke.
Since 2007, lung cancer deaths have increased by approximately 30%, a trend likely attributed to rising environmental pollution, particularly given the substantial decline in tobacco smoking rates. Particulate matter with a diameter of 2.5 µm, consisting of solid and liquid mixtures of gases, is particularly concerning. When inhaled, these particles penetrate deep into the terminal bronchioles, where they are absorbed, triggering both local and systemic inflammation. Chronic inflammation can induce significant DNA damage through the production of reactive oxygen species and free radicals.[17]
Epigenetics
Epigenetics is defined as changes in DNA expression that are not controlled by inherited factors. This process is characterized by strict regulation and a degree of plasticity, allowing cells to differentiate. Epigenetic regulation involves the following 6 key processes:
- Posttranslational histone modifications and histone variants
- DNA methylation and demethylation
- ATP-dependent chromatin remodeling complexes
- Polycomb (PcG) and Trithorax (TrxG) complexes
- Noncoding RNAs and nuclear dynamics
- 3-Dimensional genome organization
Hyperplasticity of epigenetic regulation refers to a deviation from strict genomic control, which is characterized by a baseline plasticity that preserves cellular identity. This shift can result in the upregulation of oncogenes or the downregulation of tumor suppressor genes. Epigenetic modifications are crucial in carcinogenesis, with several key alterations frequently observed in cancer cells. These changes can arise from both genetic and nongenetic sources, including environmental, metabolic, and developmental factors such as aging.
Genetic causes include mutations in enzymes that regulate epigenetic processes, such as DNA methylases. These mutations can affect the expression of multiple genes, potentially leading to cancer initiation and progression. Similar to genetic mutations, epimutations can influence genes involved in establishing cancer hallmarks. Key epigenetic changes include global DNA hypomethylation, which results in genomic instability and oncogene activation, and promoter hypermethylation of tumor suppressor genes, effectively silencing their protective roles.
Cancer cells also exhibit altered histone modifications, including changes in acetylation and methylation, which affect chromatin structure and gene expression. Additionally, the dysregulation of microRNAs—essential for posttranscriptional gene regulation—is common in many cancers. Mutations or changes in the expression of enzymes that regulate DNA methylation and histone modifications further contribute to these epigenetic alterations. Collectively, these changes can interact with genetic mutations to initiate and drive cancer progression, making them attractive therapeutic targets due to their potentially reversible nature.
Morphology
Morphologically, a cell undergoing initiation in the carcinogenesis process displays distinct microscopic changes. The initiated cell often appears irregular, with a significant increase in the nuclear-to-cytoplasmic ratio (N/C ratio), indicating that the nucleus occupies a larger volume than the cytoplasm. This N/C ratio, also referred to as the karyoplasmic ratio, has become a crucial diagnostic parameter for cancer, as it is closely associated with cancer progression and the potential for metastasis.[18]
Mechanisms
Carcinogenesis is a multistep process involving molecular and cellular events at both the genetic and epigenetic levels, driven by the interplay of environmental, genetic, and metabolic factors. Regardless of the specific sequence of genetic or epigenetic alterations involved, this process is typically categorized into 3 main stages—initiation, promotion, and progression.
Initiation
Cancer initiation occurs when mutations or epigenetic alterations accumulate, giving a cell a growth or survival advantage over surrounding tissues. These changes allow the initiated cell to proliferate more rapidly or evade normal regulatory mechanisms, thereby setting the stage the groundwork for tumor development.
This mutation can be hereditary or acquired. If repair mechanisms or apoptosis fail to correct the mutation after a driver gene mutation, an initiated clone of cells emerges, signaling the onset of the initiation phase with irreparable DNA damage. Initiation confers proliferative capabilities to the cell, which may or may not progress to the promotion stage, depending on factors such as the dose and duration of exposure to an initiator. Notably, initiation is a reversible stage that can be corrected by removing the initiator. Understanding initiation is, therefore, crucial for both prophylactic and therapeutic interventions.
Upon exposure to chemical, physical, or biological factors, a cell can undergo irreversible genetic changes, potentially evolving into a neoplastic cell clone. This transformation occurs rapidly, within minutes to hours. The initiated cell can remain in this state indefinitely without causing harm or being detected by the organism’s defense systems, as it does not exhibit any noticeable phenotypic changes. Sporadic mutations, which account for approximately 75% of colorectal tumors, are particularly prevalent in this stage.
The initiation of cancer involves critical factors such as DNA damage, genetic mutations, and inflammation. Notably, there may be considerable overlap in the mechanisms through which genetic alteration, DNA damage, and inflammation contribute to the initiation process. The mutated cell, known as an initiated cell or initiated clone, subsequently undergoes clonal expansion. During this phase, the mutated cell and its progeny replicate, forming a population of cells that share identical genetic alterations.
After initiation, carcinogenesis advances through the promotion and progression stages. Understanding the mechanisms involved in the initiation phase is crucial for developing strategies to prevent, detect, and treat cancer. Identifying specific carcinogens and recognizing the genetic alterations associated with initiation have led to significant progress in cancer research, paving the way for more targeted therapies.
Recent research suggests that cell initiation may result from epigenetic changes rather than genetic mutations.[19] Environmental factors, such as exposure to non-mutagenic carcinogens, can trigger these epigenetic events. These modifications may serve as the initial alteration in cell properties, potentially leading to the development of the first hallmark of cancer. Alternatively, these epigenetic changes may prime cells to respond more readily to a subsequent genetic mutation. Conversely, an epigenetic mutation may arise from a mutation in genes that regulate epigenetic processes, such as DNA methyltransferase genes DNMT1 and DNMT3A.[20]
Promotion and Progression
Promotion involves the expansion of initiated cells into a detectable tumor, whereas progression entails the acquisition of additional genetic alterations that increase tumor aggressiveness. Progression can also include the development of metastatic capabilities, enabling the tumor to spread to distant sites in the body.
Promotion refers to the stage of cancer development where altered or damaged cells, having evaded apoptosis, become immortal and acquire mutations that support their proliferation and survival from a single progenitor cell. Although still considered premalignant and reversible, the growth and sustained survival of these transformed cells are stimulated, eventually leading to the formation of a discernible tumor.
This intricate process involves various intrinsic and extrinsic factors, including hormones, growth factors such as transforming growth factor-β (TGF-β), and chronic exposure to certain chemicals or irritants, all of which shape the tumor microenvironment. These agents facilitate carcinogenesis by promoting the growth of initiated cells. The promotion stage depends on the duration and intensity of exposure to these promoting agents, which can accelerate the progression of initiated cells toward distant tissue invasion.
Some events may overlap between the promotion and progression stages. Hormones, such as estrogen, can act as stimulatory agents in certain cancers. For instance, estrogen promotes breast tissue growth and is associated with both the promotion and progression of specific breast cancers. During the promotion stage, cellular proliferation is stimulated, particularly in cells with acquired genetic alterations that drive uncontrolled growth and increased responsiveness to these signals.
Epigenetic modifications, such as alterations in DNA methylation patterns and histone modifications, can promote cancer development by influencing the expression of genes that regulate the cell cycle and apoptosis, ultimately affecting cell behavior. Furthermore, chronic inflammation can facilitate cancer progression by creating a microenvironment rich in growth-promoting signals and factors that enhance cell survival.
Inflammatory cells release cytokines and growth factors that promote the survival and proliferation of cancer cells. Angiogenesis, the formation of new blood vessels, is often associated with tumor growth during the promotion stage. Tumors depend on an adequate blood supply for their growth and development, and angiogenic factors secreted by tumor cells or adjacent stromal cells stimulate blood vessel formation within the tumor. The tumor microenvironment—including immune cells, fibroblasts, and extracellular matrix components—supports tumor growth. The interactions and cooperation between tumor cells and their surrounding environment are essential for the survival and proliferation of cancer cells.
Understanding the progression stage is crucial for developing interventions to halt the carcinogenic process. By targeting factors that promote cancer cell growth and disrupting the pathways that support them, it may be possible to impede or slow the transformation of precancerous cells into malignancies. Additionally, identifying and addressing modifiable risk factors that contribute to cancer progression is essential for effective cancer prevention and control. In the final stage of carcinogenesis, cells that have undergone initiation and promotion acquire the necessary mutations to invade surrounding tissues, marking the onset of invasive cancer.
Clinicopathologic Correlations
Inflammation and mutation are critical factors in the development and progression of cancer. Chronic inflammation, often resulting from persistent infections, autoimmune disorders, or prolonged exposure to irritants, creates an environment conducive to DNA damage and cellular changes. Mutations, whether inherited or acquired, disrupt normal cellular functions and can lead to uncontrolled cell proliferation. Together, these processes contribute to the initiation and progression of cancer by altering the genetic and epigenetic landscape of affected cells, promoting their survival and aggressive behavior. Understanding the interplay between inflammation and mutation is essential for developing targeted interventions and preventive strategies against cancer.
Inflammation
Rudolf Virchow first recognized the connection between inflammation and cancer in the 19th century. The debate continues: Is inflammation the root cause of cancer, or is cancer a result of chronic inflammation? Chronic inflammation typically involves cellular damage, which leads to increased cellular proliferation and the activation of repair pathways.
In the early stages of chronic inflammatory conditions, individuals face an increased risk of various cancers, particularly in rapidly dividing cells found in the skin, gut, and blood. Chronic inflammation is estimated to contribute to approximately 15% of all cancers globally.
Various factors, including microorganisms such as H pylori, can trigger inflammation. Chronic infection with H pylori significantly increases nitric oxide production, which damages DNA in the gastric mucosa by influencing transcriptional regulation through DNA methyltransferase activity. Additionally, H pylori promotes the proliferation of epithelial cells by stimulating the release of various inflammatory cytokines.[21]
Mutation
Mutations in the gene suppressor of mothers against decapentaplegic 4 (SMAD4), which is crucial in the TGF-β signaling pathway, are implicated in over 50% of pancreatic adenocarcinomas. Several studies highlight its role in both initiation and progression, depending on the tumor type.[16] SMAD4 is involved in TGF-β signaling, regulating cellular migration, proliferation, and apoptosis. In cholangiocarcinoma, SMAD4 acts as a tumor initiator and is associated with the clinical severity of the disease in over 40% of intrahepatic cholangiocarcinoma cases. Research using murine models indicates that SMAD4 facilitates the progression of pancreatic cancer initiated by KRAS mutations. Similarly, substantial evidence suggests that SMAD4 aids in the progression of colorectal cancer initiated by adenomatous polyposis coli mutations.
The TP53 gene, located on chromosome 17p13, is often referred to as the "guardian of the cell" due to its vital role in preventing abnormal cell proliferation. TP53 encodes the P53 protein—one of the most extensively studied proteins in carcinogenesis. The role of P53 in cancer development varies depending on the context, influencing initiation, promotion, or progression. P53 is essential for the expression of genes involved in DNA repair, apoptosis, and cell cycle arrest. In breast and ovarian cancers, especially those classified as basal cancers due to the expression of cytokeratins, the TP53 gene is crucial for initiation. Germline mutations in TP53 are significantly associated with tumor initiation. Li-Fraumeni syndrome, inherited in an autosomal dominant manner, is characterized by a high risk of various cancers, including early-onset breast cancers and sarcomas.[22]
Clinical Significance
Carcinogenesis refers to the process of transforming normal cells into cancerous ones and has significant clinical implications. The significance of carcinogenesis lies in its profound impact on human health, influencing the diagnosis, treatment, and prevention of cancer. Understanding the mechanisms and stages of carcinogenesis is crucial for healthcare professionals across various fields, including oncology, pathology, pharmacy, family medicine, and public health. Below are some essential clinical significances of carcinogenesis.
Early Detection and Diagnosis
Understanding carcinogenesis allows for the identification of risk factors and early warning signs for cancer. This knowledge facilitates the development of screening tests and diagnostic biomarkers for early detection. In clinical practice, imaging techniques such as mammography, colonoscopy, and Papanicolaou (Pap) smears are used to identify early-stage tumors before they progress to more advanced stages.
Treatment Selection and Personalized Medicine
Molecular profiling enables clinicians to identify specific genetic abnormalities involved in cancer development. This knowledge is essential for selecting targeted therapies that disrupt critical tumor growth and survival pathways. Personalized medicine strategies aim to tailor treatments based on the unique genetic characteristics of individual tumors, enhancing treatment efficacy and minimizing potential adverse effects.
Prognostic Stratification
Clinicians can classify patients into distinct risk categories by assessing the molecular traits of tumors influenced by carcinogenesis. Detecting biomarkers associated with various stages of carcinogenesis offers critical prognostic insights. These insights inform therapeutic decisions and guide the development of tailored posttreatment care plans.
Prevention and Risk Reduction
Understanding carcinogenesis is essential for developing effective public health strategies to minimize cancer risk. By targeting modifiable risk factors—such as smoking cessation, adopting a balanced diet, and maintaining regular physical activity—the likelihood of carcinogenesis and subsequent cancer development can be significantly reduced. Vaccination against cancer-causing viruses, such as HPV and hepatitis B, is also critical in cancer prevention.
Development of Therapeutic Agents
Understanding the critical steps of carcinogenesis is vital for innovating new therapeutic agents that target fundamental pathways involved in tumor initiation, promotion, and progression. For instance, agents, such as selective estrogen receptor modulators, are designed to disrupt specific phases of the carcinogenic process, thereby reducing the likelihood of cancer formation.
A promising avenue in cancer research involves the development of cancer vaccines. Researchers are investigating the E75 peptide vaccine, also known as nelipepimut-S, as a potential treatment for breast cancer. This vaccine utilizes a 9-amino acid peptide derived from the extracellular domain of the human epidermal growth factor receptor 2 (HER2) protein, aiming to halt tumor development at the promotion stage.[23]
Monitoring Disease Progression and Recurrence
Monitoring molecular changes during carcinogenesis allows clinicians to track disease progression and evaluate treatment effectiveness. Evaluating biomarkers for residual or minimal residual disease post-treatment offers valuable insights for monitoring recurrence and guiding posttreatment surveillance plans. Understanding the clinical implications of carcinogenesis is essential for advancing cancer diagnosis, treatment, and prevention. By unraveling the complex molecular mechanisms involved, healthcare providers can develop tailored interventions to effectively minimize the impact of cancer on individuals and communities.
Media
(Click Image to Enlarge)
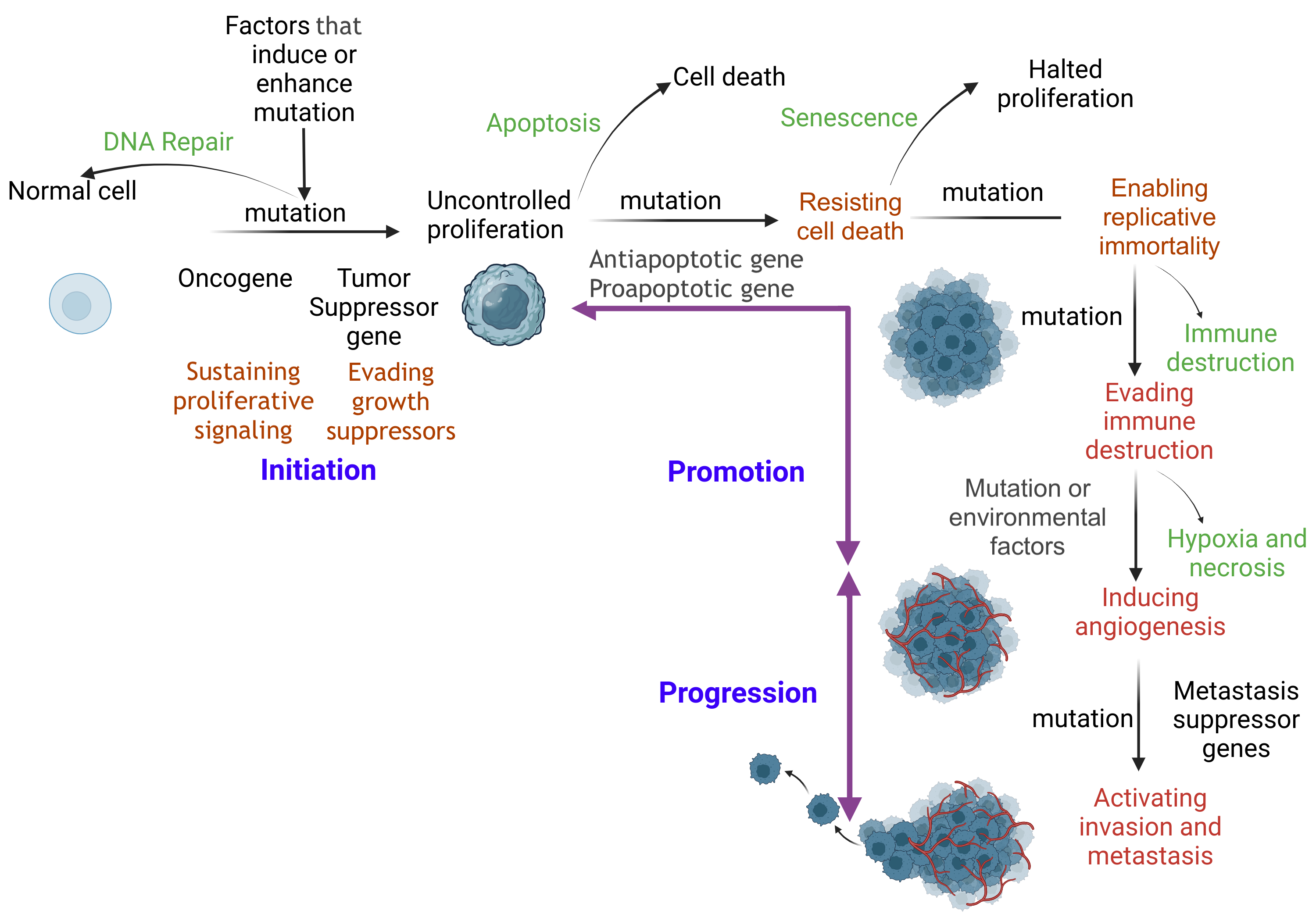
The Carcinogenesis Pathway. Prolonged disruption of cellular function can cause DNA damage. Damaged cells initiate repair mechanisms such as base excision repair, nucleotide excision repair, mismatch repair, homologous recombination, and nonhomologous end joining. If repair fails, apoptosis eliminates the damaged cell, preventing further carcinogenesis. Defective DNA repair and apoptosis characterize the initiation stage. The initiated cell evades tumor suppressor genes and undergoes uncontrolled proliferation. With additional mutations, the cell becomes immortal and bypasses immune checkpoints. As mutations accumulate, the cell establishes a self-sustaining nutritional supply, promoting growth and leading to distant tissue invasion (metastasis).
Contributed by SA Ibrahim, MBBCh, MSc, PhD
References
Huang Y, Lu H, Li H. DNA methyltransferase 3a-induced hypermethylation of the fructose-1,6-bisphosphatase-2 promoter contributes to gastric carcinogenesis. Molecular biology reports. 2024 Jan 6:51(1):78. doi: 10.1007/s11033-023-08966-5. Epub 2024 Jan 6 [PubMed PMID: 38183507]
Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. American journal of cancer research. 2021:11(4):1121-1131 [PubMed PMID: 33948349]
Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA network open. 2021 Apr 1:4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708. Epub 2021 Apr 1 [PubMed PMID: 33825840]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May:71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4 [PubMed PMID: 33538338]
Hart SN, Polley EC, Yussuf A, Yadav S, Goldgar DE, Hu C, LaDuca H, Smith LP, Fujimoto J, Li S, Couch FJ, Dolinsky JS. Mutation prevalence tables for hereditary cancer derived from multigene panel testing. Human mutation. 2020 Aug:41(8):e1-e6. doi: 10.1002/humu.24053. Epub 2020 Jul 9 [PubMed PMID: 32442341]
Qing T, Mohsen H, Marczyk M, Ye Y, O'Meara T, Zhao H, Townsend JP, Gerstein M, Hatzis C, Kluger Y, Pusztai L. Germline variant burden in cancer genes correlates with age at diagnosis and somatic mutation burden. Nature communications. 2020 May 15:11(1):2438. doi: 10.1038/s41467-020-16293-7. Epub 2020 May 15 [PubMed PMID: 32415133]
Level 2 (mid-level) evidenceJavier RT, Butel JS. The history of tumor virology. Cancer research. 2008 Oct 1:68(19):7693-706. doi: 10.1158/0008-5472.CAN-08-3301. Epub [PubMed PMID: 18829521]
Hajdu SI. A note from history: landmarks in history of cancer, part 1. Cancer. 2011 Mar 1:117(5):1097-102. doi: 10.1002/cncr.25553. Epub 2010 Oct 19 [PubMed PMID: 20960499]
Parascandola M, Pearlman PC, Eldridge L, Gopal S. The Development of Global Cancer Research at the United States National Cancer Institute. Journal of the National Cancer Institute. 2022 Sep 9:114(9):1228-1237. doi: 10.1093/jnci/djac104. Epub [PubMed PMID: 35640108]
Peters JM, Gonzalez FJ. The Evolution of Carcinogenesis. Toxicological sciences : an official journal of the Society of Toxicology. 2018 Oct 1:165(2):272-276. doi: 10.1093/toxsci/kfy184. Epub [PubMed PMID: 30629266]
Lipsick J. A History of Cancer Research: Carcinogens and Mutagens. Cold Spring Harbor perspectives in medicine. 2021 Mar 1:11(3):. doi: 10.1101/cshperspect.a035857. Epub 2021 Mar 1 [PubMed PMID: 33649023]
Level 3 (low-level) evidenceHanselmann RG, Welter C. Origin of Cancer: Cell work is the Key to Understanding Cancer Initiation and Progression. Frontiers in cell and developmental biology. 2022:10():787995. doi: 10.3389/fcell.2022.787995. Epub 2022 Mar 1 [PubMed PMID: 35300431]
Level 3 (low-level) evidenceHanahan D. Hallmarks of Cancer: New Dimensions. Cancer discovery. 2022 Jan:12(1):31-46. doi: 10.1158/2159-8290.CD-21-1059. Epub [PubMed PMID: 35022204]
Loh JJ, Ma S. Hallmarks of cancer stemness. Cell stem cell. 2024 May 2:31(5):617-639. doi: 10.1016/j.stem.2024.04.004. Epub [PubMed PMID: 38701757]
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4:144(5):646-74. doi: 10.1016/j.cell.2011.02.013. Epub [PubMed PMID: 21376230]
Level 3 (low-level) evidenceChatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environmental and molecular mutagenesis. 2017 Jun:58(5):235-263. doi: 10.1002/em.22087. Epub 2017 May 9 [PubMed PMID: 28485537]
Berg CD, Schiller JH, Boffetta P, Cai J, Connolly C, Kerpel-Fronius A, Kitts AB, Lam DCL, Mohan A, Myers R, Suri T, Tammemagi MC, Yang D, Lam S, International Association for the Study of Lung Cancer (IASLC) Early Detection and Screening Committee. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2023 Oct:18(10):1277-1289. doi: 10.1016/j.jtho.2023.05.024. Epub 2023 Jun 3 [PubMed PMID: 37277094]
Schirmer EC, Latonen L, Tollis S. Nuclear size rectification: A potential new therapeutic approach to reduce metastasis in cancer. Frontiers in cell and developmental biology. 2022:10():1022723. doi: 10.3389/fcell.2022.1022723. Epub 2022 Oct 10 [PubMed PMID: 36299481]
Bhartiya D, Raouf S, Pansare K, Tripathi A, Tripathi A. Initiation of Cancer: The Journey From Mutations in Somatic Cells to Epigenetic Changes in Tissue-resident VSELs. Stem cell reviews and reports. 2024 May:20(4):857-880. doi: 10.1007/s12015-024-10694-7. Epub 2024 Mar 8 [PubMed PMID: 38457060]
You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer cell. 2012 Jul 10:22(1):9-20. doi: 10.1016/j.ccr.2012.06.008. Epub [PubMed PMID: 22789535]
Khandia R, Munjal A. Interplay between inflammation and cancer. Advances in protein chemistry and structural biology. 2020:119():199-245. doi: 10.1016/bs.apcsb.2019.09.004. Epub 2019 Nov 26 [PubMed PMID: 31997769]
Level 3 (low-level) evidenceMalkin D. The role of p53 in human cancer. Journal of neuro-oncology. 2001 Feb:51(3):231-43 [PubMed PMID: 11407595]
Lau KH, Tan AM, Shi Y. New and Emerging Targeted Therapies for Advanced Breast Cancer. International journal of molecular sciences. 2022 Feb 18:23(4):. doi: 10.3390/ijms23042288. Epub 2022 Feb 18 [PubMed PMID: 35216405]