Introduction
The overlap syndrome, introduced by David C. Flenely in 1985, characterizes the combination of obstructive sleep apnea (OSA) with respiratory disorders such as chronic obstructive pulmonary disease (COPD).[1] COPD involves persistent respiratory symptoms and irreversible distal airflow limitation.[2] Individuals with COPD frequently experience challenges in initiating sleep and frequent nighttime arousals, leading to poor sleep quality and excessive daytime fatigue.
Nocturnal hypoxia and hypoventilation associated with COPD generally occur during rapid eye movement (REM) sleep, stemming from reduced chest wall motility and the relaxation of intercostal muscles. Conversely, patients with OSA experience frequent nighttime arousals and excessive daytime sleepiness due to upper airway collapse, reduced intrathoracic pressures, and activation of the sympathetic nervous system.[2]
Patients affected by overlap syndrome exhibit higher levels of nocturnal oxygen desaturations than those with COPD or OSA alone, heightening their risk of cardiovascular events, including pulmonary hypertension, right heart failure, and atrial fibrillation. The overlapping presentation of COPD and OSA complicates diagnosis and treatment.[1] Given the increased morbidity and mortality associated with overlap syndrome, healthcare professionals must be aware of this condition for timely recognition and treatment.
With the increasing prevalence of COPD and OSA, studies suggest that 1 out of 10 individuals with 1 condition will also have the other disorder, by chance alone.[1] However, whether the prevalence of OSA is higher in patients with COPD than in the general population is debatable, as some studies show no increase.[3] Conversely, others have identified a high prevalence of OSA in patients with moderate to severe COPD referred to pulmonary rehabilitation.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Evidence exists of a bidirectional relationship between COPD and OSA.[5] Overlap syndrome can emerge in patients with COPD who later develop OSA or in those with OSA who are later diagnosed with COPD. Older age, male gender, exposure to cigarette smoke and alcohol, and reduced reported physical activity are all associated with the development of overlap syndrome.[1][6]
Cigarette smoking is a common and independent risk factor for developing COPD and OSA. Smoking accelerates the underlying disease process by inducing oxidative stress and inflammatory mediator release, thereby triggering upper airway inflammation and increasing the susceptibility to OSA.[7] Moreover, obesity, notably increased neck circumference, poses a significant risk for developing OSA. These anthropomorphic changes result in upper airway narrowing, predisposing patients to nocturnal oxygen desaturations.[8] Additional risk factors for OSA include:
- Shortening of the mandible or maxilla
- Hypothyroidism
- Acromegaly
- Male gender
- Age 40 to 65
- Myotonic dystrophy
- Ehlers-Danlos syndrome
COPD encompasses a spectrum of airway diseases, including emphysema, chronic bronchitis, and chronic obstructive asthma. The likelihood of developing OSA varies depending on the patient's underlying pathophysiology. Patients with emphysema classically demonstrate hyperinflated lungs and a lower BMI due to prominent dyspnea and increased work of breathing encountered in the advanced stages of COPD. Conversely, individuals with chronic bronchitis often have an elevated BMI, increasing their risk of developing OSA, particularly when adipose tissue accumulates around the neck.[7] In the developing world, occupational and environmental exposures significantly contribute to the onset and development of COPD.
Epidemiology
Despite being among the most widespread pulmonary diseases globally, there's variability in the prevalence of concurrent COPD and OSA.[9] Nevertheless, studies suggest approximately 1 in 10 individuals diagnosed with either COPD or OSA will, by chance alone, have the other disorder as well.[1] A systematic review demonstrates a 1.0% to 3.6% rate of coexistence in the general and hospital populations.[5]
As of 2019, COPD stands as the third leading cause of death globally, accounting for 3.23 million deaths. In 2017, 55% of the estimated 544.9 million individuals living with chronic respiratory disease could attribute their diagnosis to COPD.[10] The global prevalence of COPD is estimated at 10%.[11][12][13] Cigarette smoking contributes to 70% of cases in high-income countries and 30% in low- and middle-income countries, while indoor air pollution is a significant risk factor in low- and middle-income countries.
The prevalence of isolated OSA in the US is approximately 30%, affecting 1 billion people worldwide.[14] According to the Wisconsin Sleep Cohort, OSA prevalence in men and women aged 30 to 60 is 24% and 9%, respectively.[15] Rising rates of obesity contribute to the increased prevalence of OSA.[16][17]
Epidemiological data regarding the prevalence of overlap syndrome is continually evolving. A review of available literature regarding the prevalence of overlap syndrome reveals significant variations in disease rates, making it challenging to calculate the true incidence accurately. For patients with a primary diagnosis of COPD, the reported incidence of overlap syndrome varies between 2.9% and 65.9%. Among those primarily diagnosed with OSA, the incidence falls between 7.6% and 55.7%.[5] In studies focusing on patients with COPD older than 70 and those on long-term oxygen therapy, overlap syndrome incidence rates were 21.4% and 15.7%, respectively.[5]
Limitations in determining the true prevalence of overlap syndrome are:
- Differing diagnostic criteria for COPD and OSA across studies
- Lack of a standardized definition and specific diagnostic code for overlap syndrome
- Updated scoring criteria to categorize nocturnal respiratory events by the American Academy of Sleep Medicine [18][19]
- Inability to define and include additional disordered breathing-related physiologic disturbances like hypoxic burden, respiratory-related arousal, and autonomic disturbances [20][21]
- Overlooking COPD or OSA once establishing the other diagnosis
- Study selection bias evidenced by small convenient samples or heterogeneous groups
- Results of home versus traditional overnight polysomnography
- Varying cut-offs for apnea-hypopnea index (AHI) [22]
- Effects of long-term oxygen therapy on overnight polysomnography results
- Cost of overnight polysomnography
Pathophysiology
The underlying pathophysiology of overlap syndrome encompasses a combination of the individual effects of COPD and OSA. To comprehend the physiologic alterations involved, it is essential to recognize the typical changes during sleep. These include:
- Reduced upper airway muscle activity
- Decreased pharyngeal caliber
- Increased upper airway resistance
- Increased upper airway compliance
- Increased internal load on the respiratory system
- Changes in the ratio of the rib cage and abdominal muscle contribution to tidal volume
- During non-REM sleep, rib cage muscles contribute more than abdominal muscles to tidal volume
- Increased partial PaCO2
Obstructive Sleep Apnea
OSA involves recurrent pharyngeal airway obstruction, causing hypoxia and sleep disruption.[23][24][25] During normal sleep, humans experience a diminished drive to breathe compared to wakefulness, resulting in decreased ventilatory motor output to the respiratory muscles. This decreased activity and immediate decrease in inspiratory flow are particularly evident in muscles like the tensor palatini and genioglossus that maintain upper airway patency.[26][27][28][29] Respiration becomes dependent on the level of chemoreceptor and mechanoreceptor stimuli.[5] Consequently, patients are more vulnerable to central apnea and upper airway obstruction.[2][30][31] While reduced upper airway muscle activity typically has little impact on healthy patients, it can result in upper airway narrowing for those at risk.
Upper airway narrowing, causing turbulent airflow, is a routine finding during sleep. As the resistance in the upper airway rises, turbulent flow increases, along with a restriction in inspiratory flow. This phenomenon causes the soft palate and upper airway soft tissue to flutter.
The narrowed upper airway and increased resistance to flow create an increased internal load on the lungs. These loads trigger an automatic respiratory effort during wakefulness to counteract their effects. However, during sleep, ventilation decreases, leading to an elevation of PaCO2. This rise in PaCO2 prompts ventilation to return to normal levels in healthy individuals. However, patients with COPD or other abnormal respiratory mechanics may experience worsening ventilation issues due to the increased internal load.
Repetitive respiratory events are associated with increased airway resistance and can lead to increased patient end-tidal CO2 and alternating periods of hypoventilation and hyperventilation (see Image. Airway Resistance and End-Tidal CO2). The oxidative stress caused by breathing interruptions during sleep results in sporadic reductions in airflow to the lungs and significant variations in the partial pressure of oxygen between the proximal and distal airways.[32]
In a healthy individual with ideal anatomy, maintaining normal oxygen saturation and sleep patterns is achievable even with a slightly elevated PaCO2. Patients with a less ideal anatomy may experience inspiratory flow limitation and snore. Breathing during wakefulness and in patients who do not snore typically does not encounter flow limitation. Snoring suggests flow limitation and the risk of upper airway collapse. Patients with significant upper airway compromise may develop OSA.
For individuals with obesity, the fat deposition surrounding the pharyngeal airway can increase its susceptibility to collapse.[26] This contributes to increased neck circumference, a standard measurement to assess the risk of developing OSA. A neck circumference exceeding 16 inches in a female or 17 inches in a male is associated with an increased risk of developing OSA.[30]
Chronic Obstructive Pulmonary Disease
COPD is characterized by airflow obstruction that is minimally reversible and aberrant inflammatory responses in the lungs. It encompasses a group of overlapping diseases consisting of emphysema and chronic bronchitis, primarily involving the airways but potentially involving the lung parenchyma and vasculature, depending on the underlying condition.[31][32] The pathophysiologic changes of COPD typically have a more pronounced impact during physiological challenges such as exercise or sleep. The overall effects of the pathogenic processes include:
- Mucous hypersecretion
- Ciliary dysfunction
- Airflow obstruction
- Hyperinflation
- Gas exchange abnormalities
- Pulmonary hypertension
- Systemic effects like cachexia and polycythemia
The mechanism of respiratory function impairment can be broadly categorized into 2 main groups. Each category, including mechanical and ventilatory, represents distinct pathways contributing to respiratory dysfunction. These classifications help elucidate the diverse factors underlying respiratory impairment, aiding in diagnostic and therapeutic approaches.
Mechanical factors
Peripheral airway obstruction, destruction of pulmonary connective tissue, or a combination of both leads to alterations in the mechanical properties of the respiratory system. These structural changes set off a cascade of events that can contribute to the morbidity and chronicity of the disease.
Inflammation of the small airways due to cigarette smoke or air pollutants triggers an exaggerated response to inhaled toxins. The amplified natural protective mechanism causes tissue destruction, impairs the defense mechanisms that typically limit such destruction, and disrupts natural healing processes. Patients with COPD also have increased protease production by neutrophils, macrophages, and matrix metalloproteinases, while antiproteases like Alpha1-antitrypsin become inactivated. This imbalance can lead to structural damage to the alveolar air sacs, resulting in emphysematous changes. Additionally, cigarette smoke and inflammatory cells release oxidants, the production of which further increases during an exacerbation of COPD. This heightened oxidant production exacerbates protease activity, mucous production, and the release of inflammatory mediators. The overall results are as follows:
- Reduction in forced expiratory volume (FEV) due to the inflammatory reaction and blockage of the airways
- Airflow obstruction and reduced gas exchange due to tissue damage [31]
- Air trapping from airway collapse on exhalation [31]
- Hyperinflation leading to an increase in the anterior-posterior diameter of the lower rib cage, causing depression and shortening of the diaphragm, making diaphragmatic contraction less mechanically advantageous [33]
- Increased airway resistance with chronic bronchitis due to mucous gland enlargement and mucociliary dysfunction resulting in excessive mucus production and reduced airway caliber
- Fibrosis and smooth muscle hypertrophy due to increased mucus production [34]
- Loss of elastic recoil in patients with emphysema causing diminished expiratory flow rates, air trapping, and airway collapsing [34]
- Skeletal muscle myopathy from long-term corticosteroid usage, systemic inflammatory response with oxidative damage, and persistent hypoxemia [35]
- Increased airway resistance during inspiration and expiration
Ventilatory factors
Ventilatory control remains relatively unaffected in individuals with mild COPD. However, as COPD progresses, alterations in ventilatory motor output become more apparent, leading to significant changes in gas exchange and respiratory function during wakefulness and sleep.[36] Expected ventilatory changes include:
- Prolonged inspiratory time and reduced expiratory time exacerbate hyperinflation due to a reduced ventilatory drive, resulting in a significant decrease in tidal volume.[36]
- Transient episodes of nocturnal hypoxemia, particularly during REM sleep, are common in patients with chronic bronchitis. These patients often experience hypoxemia and CO2 retention while awake and breathing.[37]
- Increased respiratory frequency or work of breathing due to increased airway resistance during inspiration and expiration.
- Increased work of breathing causing increased oxygen extraction from the blood and increased carbon dioxide production.
- A reduction in hypercapnic ventilatory response due to increased mechanical load.[38]
- Increased oxygen levels contributing to hypercarbia due to several mechanisms like reduced ventilatory responses, ventilation/perfusion (V/Q) mismatch, and the Haldane effect.[39]
Overlap Syndrome
The link between OSA and COPD is reciprocal, where exacerbation of 1 condition can exacerbate the other. Severity scales exist to categorize patients with COPD and OSA. However, the point at which these combined disorders have additive or synergistic clinical significance is uncertain.[9]
Overlap syndrome results in more profound nocturnal oxygen desaturations than in COPD or OSA alone. Previous studies defined nocturnal oxygen desaturations as SaO2 below 88% or 90% during at least 30% of the recording time.[9][40]
Alveolar hypoventilation, decreased ventilation-perfusion matching, and reduced end-expiratory volume (ERV) are the 3 primary mechanisms contributing to nocturnal oxygen desaturations in patients with COPD.[8] This hypoxia causes the release of systemic inflammatory mediators such as C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), nuclear factor-kappa beta (NF-κB), tumor necrosis factor-alpha (TNF-α), and hypoxia-inducible factor (HIF).[8][32] Reactive oxygen species are also released, contributing to endothelial dysfunction and accelerated atherosclerosis, thereby increasing mortality and the risk of pulmonary hypertension.[8]
Patients with overlap syndrome exhibit higher sympathetic activity and reduced parasympathetic activity measured by sympathetic and parasympathetic modulation of heart rate, compared to those with OSA or COPD alone. Moreover, research suggests that individuals with overlap syndrome have increased arterial stiffness, surpassing even patients with OSA alone, indicating another underlying mechanism for cardiac disease.[7]
The precise mechanism for developing OSA in patients with COPD remains unclear. Studies propose protective mechanisms that may contribute to the development of OSA in individuals with COPD. These mechanisms include larger end-expiratory lung volumes (EELV), which promote tracheal traction, thereby reducing airway resistance and stabilizing the upper airway.[41]
Chronic oral steroid use and decreased exercise tolerance leading to obesity may increase the risk of OSA. The elevated pulmonary artery pressure associated with severe COPD can lead to right ventricular dysfunction and right heart failure, causing edema in the pharyngeal soft tissues and increasing the risk of OSA. Additionally, COPD is associated with generalized muscle weakness, which may contribute to higher upper airway collapse.
Obstructive sleep apnea can induce local and systemic inflammation, causing a reduction in airway lumen and potentially contributing to alveolar wall destruction. Patients with gastrointestinal reflux disease can have worsening COPD and OSA symptoms. Furthermore, allergic rhinitis is associated with sleep-disordered breathing and COPD.
History and Physical
The clinical characteristics specific to overlap syndrome often coexist with the common presentations of COPD and OSA. Typical findings include obesity, increased neck circumference, excessive daytime sleepiness, sleep disruptions, and hypertension.
Patients with COPD commonly present with sputum production, shortness of breath, cough, wheezing, forgetfulness, confusion, and disrupted sleep, likely related to sleep-disordered breathing. Conversely, patients with OSA commonly present with daytime sleepiness, loud snoring, gasping for air, nocturia, morning headache, and choking. Additionally, the patient's sleeping partner often observes snoring, which may progress to apneic episodes.
In addition to the common symptoms of the individual diseases, COPD and OSA, patients with overlap syndrome exhibit increased hypoxemia, hypercapnia, and pulmonary hypertension. Consequently, they may experience morning headaches resulting from hypercapnia, cyanosis resulting from hypoxemia, and peripheral edema resulting from cor pulmonale, which are additional possible findings associated with overlap syndrome.[7]
Evaluation
Clear guidelines do not currently exist for screening patients with COPD for OSA. However, utilizing the STOP-Bang questionnaire (SBQ), which assesses snoring, tiredness, observed apnea, blood pressure, body mass index, age, neck size, and gender in all patients with COPD, is recommended. Diagnostic testing should be considered in all patients with an intermediate-to-high risk for OSA.
Patients with an SBQ score of 0 to 2 are at low risk for moderate to severe OSA, while those scoring 5 to 8 are considered at high risk for moderate to severe OSA. Patients scoring 3 or 4 require further clinical assessment for classification. For example, a patient with a score of 3 or 4 and a BMI of 35 is at high risk for moderate to severe OSA.
Patients with COPD, pulmonary hypertension, and borderline or nocturnal hypoxemia warrant a sleep study. Current recommendations from the American Thoracic Society and European Respiratory Society suggest that individuals with mild COPD and clinical evidence of pulmonary hypertension should be evaluated with overnight testing to assess for possible OSA.[9][8]
Additional screening tools include the recently created NoSAS (neck, obesity, snoring, age, sex) questionnaire, utilized alongside objective clinical data, including anthropometrics and medical history.[7] A NoSAS score of 8 or higher indicates probable OSA. The Berlin Questionnaire also identifies patients at increased risk of suffering from sleep apnea.[42]
Patients with overlap syndrome demonstrate higher COPD Assessment Test (CAT), Epworth Sleepiness Scale (ESS), Charlson Comorbidity Index (CCI), and SBQ scores than patients with COPD alone.[43] Notably, excessive daytime sleepiness is not a universal finding. Some patients may complain of excessive daytime fatigue, leading to inaccuracies when using questionnaires to measure excessive sleepiness.
Diagnosis
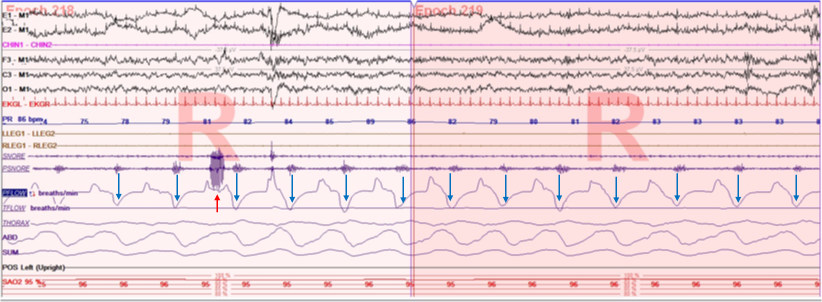
Polysomnography is the gold standard for detecting sleep disturbances. However, overnight oximetry is a valuable screening tool for identifying nocturnal hypoxia during sleep. A cyclical or sawtooth pattern on oximetry suggests OSA in patients with COPD, although confirmation is necessary with overnight polysomnography (see Image. Flow Limitation with Snoring).[8] Polysomnography establishes the diagnosis of OSA when:
- ≥5 predominantly obstructive respiratory events per hour of sleep occur accompanied by ≥1 of the following:
- Sleepiness, fatigue, insomnia, or other symptoms leading to impaired sleep-related quality of life
- Waking up with breath holding, gasping, or choking
- Habitual snoring or breathing interruptions noted by a bed partner or other observer
- ≥15 predominantly obstructive respiratory events per hour of sleep
The AHI and the respiratory disturbance index (RDI) determine the severity of OSA. The AHI is calculated as the total number of apneas and hypopneas divided by the total sleep time in hours, while the RDI includes additional respiratory effort-related arousals (RERAs) ([apneas + hypopneas + RERAs] / total sleep time in hours).
- Mild: 5 to 14 respiratory events per hour of sleep
- Moderate: 15 to 30 respiratory events per hour of sleep
- Severe: >30 respiratory events per hour of sleep
The severity of nocturnal hypoxemia is determined using the oxygen desaturation index (ODI), which quantifies the number of episodes when blood oxygen levels decrease by 3% or 4% below baseline. Elevated ODI levels are associated with the production of inflammatory markers. While the AHI determines the severity of OSA, the ODI is superior to the AHI in predicting cardiovascular risk.[44][7]
Current recommendations advise against the use of home sleep apnea testing (HSAT) in patients with severe chronic respiratory illnesses due to several limitations:
-
The inability of HSAT to detect hypoventilation as a cause of hypoxemia potentially leads to inadequate oxygen treatment or inappropriate positive airway pressure (PAP) prescription.
-
HSAT is often used with auto-PAP treatment, causing an unwarranted rise in expiratory positive airway pressure (EPAP). This elevation in EPAP may exacerbate hyperinflation and worsen lung function in patients with COPD.[45]
Pulmonary function testing
Spirometry conducted before and after bronchodilator administration is necessary to establish the diagnosis of COPD. Airflow limitation that is irreversible or only partially reversible with bronchodilator treatment is considered pathognomonic for COPD. Diagnosis depends on the FEV in 1 second (FEV1) and forced vital capacity (FVC) values. A postbronchodilator FEV1/FVC ratio less than 0.7 is the threshold for COPD. Using the FEV in 6 seconds (FEV6) is an acceptable alternative. The GOLD COPD classification classifies COPD severity as:
- GOLD 1 or mild disease: FEV1 ≥80% predicted
- GOLD 2 or moderate disease: FEV1 between 50% and 80% predicted
- GOLD 3 or severe disease: FEV1 between 30% and 50% predicted
- GOLD 4 or very severe disease: FEV1 <30% predicted
Patients with overlap syndrome may have a propensity for more severe lung hyperinflation, quantified by a reduced inspiratory capacity/total lung capacity (IC/TLC) ratio. Some researchers suggest that the IC/TLC ratio may be a more accurate measure of lung disease severity than FEV1. This hyperinflation could potentially contribute to deteriorating sleep quality in overlap syndrome, possibly stemming from increased work of breathing while in the recumbent position. However, this mechanism is poorly understood and requires further evaluation.[46]
The severity of obstruction measured by the FEV1/FVC ratio corresponds with the likelihood of persistent hypoxemia.[9] Limited documentation exists regarding the differences in pulmonary function testing related to overlap syndrome and COPD alone.
Laboratory evaluation
While unnecessary for establishing a diagnosis, laboratory abnormalities associated with overlap syndrome typically arise from the pathophysiology of overlap syndrome or a consequence of comorbidities. The risk of insulin resistance, regardless of obesity, diabetes, abnormal liver enzymes, and liver fibrosis, increases with OSA. Other potential laboratory abnormalities are normochromic normocytic anemia, polycythemia, and increased serum bicarbonate levels due to chronic hypercapnia.
Consideration of a complete blood count to evaluate for anemia, thyroid stimulating hormone levels, plasma brain natriuretic peptide (BNP) or N-terminal pro-BNP concentration for heart failure, serum electrolytes, and renal function is advisable for patients presenting with new onset dyspnea or excessive fatigue. In a single-center study that evaluated the association between microalbuminuria in patients with overlap syndrome versus OSA alone, microalbuminuria was more prevalent in patients with overlap syndrome. However, after adjusting for confounding variables such as diabetes and increased body mass index, both independent risk factors for microalbuminuria, researchers concluded that overlap syndrome is not an independent risk factor.[47]
Radiographic
Typical radiographic findings are:
- Lung hyperinflation
- Diaphragmatic flattening
- Increase in anterior-posterior diameter
- Bronchial wall thickening is associated with chronic bronchitis [31]
Treatment / Management
Overlap syndrome has a higher rate of morbidity and mortality than either condition alone. Therefore, concurrent management of both disorders is imperative to achieve optimal nocturnal oxygen saturation, prevent multiple arousals, and improve quality of life.
Lifestyle Modifications and Exercise
- Structured exercise programs and pulmonary rehabilitation improve AHI, daytime sleepiness, and overall sleep quality. Particularly in COPD patients, structured exercise regimens improve skeletal muscle atrophy.[8]
- Pulmonary rehabilitation improves mood index, dyspnea scores, and quality of life while reducing the frequency of hospitalization.
- Weight loss has well-documented benefits for patients with OSA and obesity. Conversely, cachexia increases mortality in patients with advanced COPD. A clear distinction exists between therapeutic goal-directed weight loss and cachexia as part of the pathologic process in end-stage COPD.[8][9]
- Healthcare professionals must counsel all patients on smoking cessation. Smoking cessation reduces morbidity and mortality associated with overlap syndrome.
Supplemental Oxygen
Supplemental oxygen is a cornerstone in managing COPD by improving daytime and nocturnal hypoxemia and reducing mortality risk in affected patients. However, while oxygen supplementation mitigates nocturnal oxygen desaturations, it is ineffective in diminishing obstructive events in managing OSA.
- Supplemental nocturnal oxygen is necessary when considerable nocturnal oxygen desaturations continue after the patient's medication regimen is optimized. Supplemental oxygen does not appear to be related to an increased risk of hypercapnia in these patients.[7]
- Earlier studies on nocturnal supplemental oxygen in apnea-prone patients with COPD reveal increased premature ventricular contractions. Patients with known coronary artery disease or left ventricular dysfunction should receive oxygen cautiously. Thus, oxygen therapy alone is not recommended to treat overlap syndrome.[9][48]
- In the recent International Nocturnal Oxygen (INOX) trial, nocturnal supplemental oxygen did not reduce mortality or delay the progression of long-term oxygen therapy in patients with COPD with nocturnal hypoxemia.[45][49] (A1)
- Studies suggest that the effects of nasal high-flow air on a nocturnal gas exchange may be superior to oxygen supplementation.[7]
- Researchers in a relatively small study containing 40 patients older than 65 compared nasal high-flow oxygen treatment (NHF-OT) to conventional O2 treatment. Nasal high-flow oxygen therapy improved the severity of OSA, with nearly half of the patients having an AHI of <5 events/hour.[50]
Pharmacologic Therapies
Treatment regimens for patients with COPD are determined by the severity of symptoms and the risk of future exacerbations. Maintenance therapy typically involves the use of inhaled bronchodilators, usually long-acting beta-agonists and muscarinic antagonists, either alone or in combination, with the potential addition of inhaled glucocorticoids. All patients with COPD should have access to a short-acting bronchodilator. Generally, the preferred choice is a short-acting beta-agonist (SABA) to avoid combining a short-acting muscarinic agent (SAMA) with a long-acting muscarinic agent. Patients treated with long-acting beta-agonists alone or who use only short-acting therapy should use a combination of SABA-SAMA for rescue therapy due to the superior bronchodilator response compared to either agent alone. Patients with COPD should also receive pneumococcal, influenza, pertussis, COVID-19, and respiratory syncytial virus vaccinations.
- Mean nocturnal oxygen saturation improves approximately 2% to 3% after treatment with inhaled long-acting beta-agonist and anticholinergic medications compared to a placebo alone. Neither medication affects sleep quality.[7]
- Theophylline reduces nocturnal hypoxemia and benefits patients with OSA by reducing the AHI. Stimulation of the central respiratory drive is the likely mechanism of action and may be more beneficial in central sleep apnea. Side effects, however, limit the use of this medication.[51] (A1)
- Patients with a history of COPD exacerbations benefit from inhaled corticosteroids. The efficacy of inhaled corticosteroids for overlap syndrome is under debate. A study shows improved AHI, nocturnal hypoxemia, daytime PaCO2, and lung function by decreasing inflammation. Unfortunately, other studies demonstrate that inhaled corticosteroids may increase myopathy risk, worsening upper airway collapsibility.[45] (B3)
- Respiratory stimulants such as acetazolamide improve oxygenation in patients with predominantly central sleep apnea, but the clinical benefit is not evident.[52] Additionally, worsening respiratory acidosis limits the use of these medications.[45] (B3)
Positive Airway Pressure and Noninvasive Ventilation
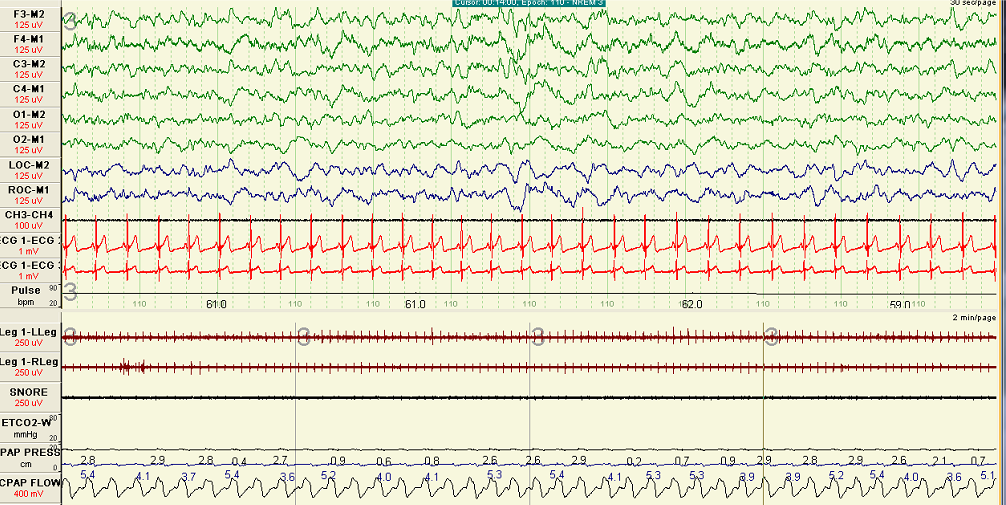
The most appropriate and well-established therapeutic option for COPD-OSA overlap is PAP therapy, which has demonstrated efficacy in eliminating respiratory events, flow limitation, and snoring, (see Image. Polygraph Study of Patient on CPAP). Studies show that patients with hypoxemia, COPD, and concomitant OSA who undergo PAP treatment exhibit an increased survival rate than those who decline PAP therapy.[7]
Likewise, patients undergoing PAP therapy experience fewer hospitalizations and emergency room visits from any cause, a lower rate of severe acute COPD exacerbations, and lower healthcare costs.[53] PAP therapy also decreases proinflammatory markers such as CRP and TNF-α, which are involved in developing and exacerbating cardiovascular disease.[45] (B3)
When selecting the type of PAP therapy for patients with COPD, it is essential to consider the underlying etiology of their condition. Patients with emphysema may experience increased sleep-related hypoventilation due to the downward displacement of the diaphragm and accessory muscle paralysis during REM sleep. These patients benefit from continuous positive airway pressure (CPAP) or EPAP to nullify patients' auto-positive end-expiratory pressure (PEEP) or the positive pressure that will remain in the airways at the end of the respiratory cycle. This therapy also supports ventilation and reduces muscle fatigue. Titrating CPAP is crucial, as studies indicate that if the applied PEEP exceeds auto-PEEP, it may increase expiratory load, dampen ventilation, and worsen lung function.
Some authors advocate using bilevel positive airway pressure (BPAP) in chronic bronchitis as it supports ventilation.[45] However, the superiority of BPAP over other forms of PAP therapy has not been conclusively demonstrated to date.[54](A1)
Recent studies evaluated the role of high-intensity noninvasive ventilation (NIV), particularly in patients with COPD and chronic hypercapnic respiratory failure.[55] This strategy utilizes high inspiratory positive airway pressure (IPAP) settings and a backup respiratory rate to elevate minute ventilation and normalize the PaCO2 or achieve a 20% decrease in CO2 levels.[56]. When titrated appropriately, this technique can potentially enhance these patients' survival outcomes.[57] (A1)
The titration of high-intensively NIV in stable patients with COPD-OSA and chronic hypercapnic respiratory failure requires an IPAP of 15 to 20 cm H2O. The EPAP level is determined based on the opening pressure identified during the PAP titration portion of the sleep study. If this information is unavailable, the EPAP setting can be empirically determined using the 10% rule based on the patient's body weight. For example, for a patient weighing 70 kg, the EPAP setting would be 7 cm H2O.[55]
Differential Diagnosis
COPD-OSA overlap syndrome may present similarly to other overlapping respiratory diseases, such as the coexistence of asthma and COPD.[55] To distinguish between these pathologies, pulmonary function testing and evaluation of symptomatology consistent with asthma are essential. Additionally, the absence of symptoms suggestive of OSA can aid in differentiation.
The differential diagnoses for COPD include:
- Chronic obstructive asthma without risk factors for COPD
- Central airway obstruction
- Chronic bronchitis with normal spirometry
- Bronchiectasis
- Heart failure
- Tuberculosis
- Constrictive bronchiolitis
- Diffuse panbronchiolitis
- Lymphangioleiomyomatosis
The differential diagnoses for obstructive sleep apnea alone include:
Pertinent Studies and Ongoing Trials
Multiple trials are ongoing to evaluate treatment strategies for overlap syndrome, focusing on the distinct phenotypic variations of COPD. One randomized controlled trial is investigating whether early diagnosis and initiation of PAP therapy in hospitalized patients can reduce 30-day readmission rates. Another clinical trial is comparing CPAP versus BPAP therapy in managing overlap syndrome. These trials hold promise in elucidating the optimal treatment approach for patients with overlap syndrome and potentially enhancing clinical outcomes.[45]
Prognosis
Overlap syndrome presents an increased risk of morbidity and mortality compared to either COPD or OSA alone. Patients affected by this syndrome face an elevated likelihood of cardiovascular morbidity, hospitalization due to COPD exacerbations, and increased all-cause mortality.[61]
Treatment of OSA with CPAP has been shown to significantly reduce the risk of death and cardiovascular events independent of age, gender, BMI, and preexisting cardiovascular comorbidities. Additionally, strategies such as smoking cessation, long-term oxygen therapy, noninvasive ventilation, and pharmacologic interventions play crucial roles in mitigating the morbidity and mortality associated with COPD.
Complications
As assessed by the St. George's Respiratory Questionnaire, patients with overlap syndrome report a worse quality of life than those with COPD alone.[9]
Complications associated with overlap syndrome include the following:
- Hypertension
- Coronary artery disease
- Myocardial infarction
- Peripheral arterial disease
- Heart failure
- Hyperlipidemia
- New-onset atrial fibrillation
- Pulmonary hypertension
- COPD exacerbations
- Bronchiectasis
- Motor vehicle collisions
- Impaired executive function
- Increased medical costs [41][61][5]
Improper treatment strategies in overlap syndrome may lead to reduced ventilation, deterioration of lung function, and poor adherence to therapy, ultimately impacting clinical outcomes. Research indicates that patients with emphysema exhibit lower adherence rates to CPAP therapy compared to other phenotypic variants, resulting in inadequate treatment and worsened clinical outcomes.[45] Moreover, bronchiectasis, associated with more severe nocturnal oxygen desaturations and increased sleep time spent with SpO2 below 90%, occurs more frequently in individuals with overlap syndrome.[5] This association underscores the significance of disease severity, particularly COPD severity, in shaping the clinical outcomes of overlap syndrome.[62]
Complications associated with COPD include the following:
- Cachexia
- Skeletal muscle wasting and disuse atrophy
- Normochromic normocytic anemic
- Secondary polycythemia
- Osteoporosis
- Depression and anxiety
Complications noted with OSA include:
Consultations
Patients exhibiting symptoms suggestive of overlap syndrome should be promptly referred to a pulmonary and sleep medicine specialist for comprehensive evaluation. Consultation with these specialists enables thorough assessment through polysomnography and pulmonary function tests, facilitating accurate diagnosis and management. These evaluations aid in determining the need for interventions such as PAP titration, pharmacologic therapy, and supplemental oxygen as necessary.
Deterrence and Patient Education
Overlap syndrome describes the coexistence of COPD with OSA. COPD is a chronic lung disease primarily associated with cigarette smoke exposure in high-income countries and indoor air pollution in others. COPD is the third leading cause of death worldwide, and individuals with COPD typically experience symptoms such as shortness of breath and a persistent cough accompanied by sputum production.
OSA occurs when a patient's airway obstructs or narrows, leading to brief pauses in breathing during sleep. While individuals with OSA may not be aware of these breathing interruptions, they may experience waking episodes characterized by choking or gasping for air. Often, their sleep partners notice loud snore, breating pauses, choking, or gasping sounds.
Common symptoms of OSA are restless sleep, morning headaches, dry mouth, sore throat, waking up often to urinate, waking up feeling unrested or groggy, and difficulty thinking clearly or remembering things. OSA increases a patient's risk of insulin resistance, diabetes, heart disease, stroke, hypertension, heart attack, nonalcoholic fatty liver disease, and death.
Diagnosing overlap syndrome can be challenging due to the overlapping symptoms of COPD and OSA. This condition significantly impacts patients' quality of life and raises their risk of various complications, including nighttime hypoxia, heart disease, exacerbations of COPD, atrial fibrillation, and mortality. Healthcare providers must maintain a high suspicion level to identify overlap syndrome accurately. While pulmonary function tests are essential for diagnosing COPD, a polysomnogram or sleep study is necessary to confirm obstructive sleep apnea. Combining these diagnostic tools enables clinicians to identify and manage overlap syndrome effectively.
Effective COPD and sleep apnea management is essential to mitigate cardiovascular and overall mortality risks. Addressing obesity, a significant risk factor for OSA, necessitates targeted interventions like structured weight loss programs. Treatment options encompass pulmonary rehabilitation, PAP therapy, smoking cessation efforts, supplemental oxygen therapy, and pharmacological interventions. Furthermore, clinicians prioritize administering recommended vaccinations to safeguard against respiratory illnesses, which pose elevated risks for individuals with COPD.
Pearls and Other Issues
Key facts to keep in mind about overlap syndrome are as follows:
- Overlap syndrome refers to the coexistence of COPD and OSA.
- COPD is characterized by airflow limitation and is often associated with smoking, while OSA involves recurrent airway obstruction during sleep.
- Risk factors for overlap syndrome include older age, male gender, smoking, obesity, and chronic bronchitis.
- Patients with overlap syndrome may experience worsened symptoms, increased hypoxemia, and higher rates of morbidity and mortality compared to those with COPD or OSA alone.
- Diagnosis typically involves polysomnography for OSA and pulmonary function tests for COPD.
- Treatment strategies may include lifestyle modifications, positive airway pressure therapy, pulmonary rehabilitation, and pharmacotherapy.
- Effective management of overlap syndrome requires a multidisciplinary approach involving pulmonologists, sleep specialists, and other healthcare professionals.
- Regular monitoring and follow-up are crucial to optimize treatment outcomes and improve patients' quality of life.
Enhancing Healthcare Team Outcomes
Overlap syndrome is associated with a higher incidence of morbidity and mortality than OSA or COPD alone. The overlap in excessive daytime sleepiness and fatigue, sleep disruption, and executive dysfunction makes diagnosing overlap syndrome challenging. Healthcare professionals need a high index of suspicion to diagnose overlap syndrome. Providing a timely diagnosis and treatment for patients with overlap syndrome will improve the overall quality of life, reduce sleep disruption and nocturnal oxygen desaturation, as well as decrease overall morbidity and mortality.
Caring for patients with overlap syndrome requires an interprofessional team approach to provide the best possible patient care. Clinicians must understand the clinical presentations and associated morbidity and mortality of COPD and OSA. Of equal importance is predicting the risk of OSA in patients with COPD. An interprofessional team involving primary care, pulmonology, cardiology, pulmonary rehabilitation, nutrition, sleep medicine, pharmacy, nursing, and sleep lab personnel will help increase awareness of overlap syndrome and provide early diagnosis and treatment.
All medical staff play a crucial role in the comprehensive management of OSA by assisting in assessing, diagnosing, and treating patients. Standardized questionnaires and scoring systems help clinicians identify comorbid OSA and refer patients to appropriate specialists. Sleep lab technicians run and maintain equipment during sleep studies, while nursing staff provide patient care and coordinate appointments. Titrations of PAP therapy in the lab are essential for tailoring treatment to individual needs based on disease severity and anatomical variations. Home nursing care may also be necessary for patients who struggle with daily activities, as skilled nurses can identify and address care deficiencies during home visits. Pharmacists are integral interdisciplinary team members, providing respiratory medications and patient education.
Effective collaboration among healthcare team members facilitates timely diagnosis, risk factor reduction, treatment, and, ultimately, improved quality of life for patients. Seamless interprofessional communication ensures cohesive information exchange and collaborative decision-making.
Media
(Click Image to Enlarge)
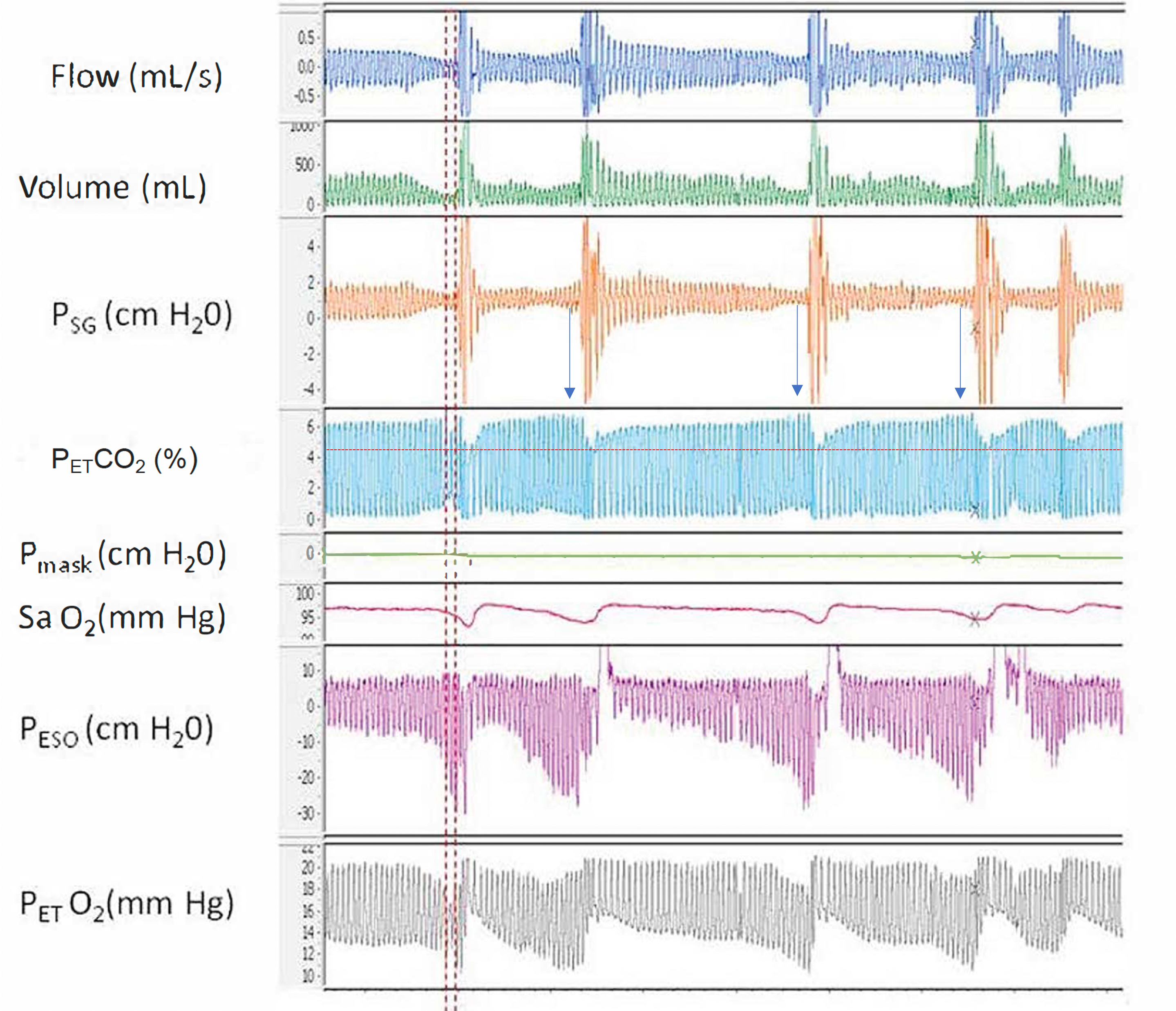
Airway Resistance and End-Tidal O2. A polygraph illustrating the relationship between increased airway resistance during respiratory events and increased end-tidal CO2, along with alternating periods of hypoventilation and hyperventilation corresponding to hypopnea and hyperpnea, respectively, throughout sleep. Abbreviations: Psg, supraglottic pharyngeal pressure; Pmask, mask pressure; PETCO2, end-tidal CO2; SaO2, Oxygen saturation using pulse oximetry; Peso, esophageal pressure; PETO2, end-tidal O2.
Contributed by Abdulghani Sankari, MD, PhD (courtesy to M. Safwan Badr, MD)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Adler D, Bailly S, Benmerad M, Joyeux-Faure M, Jullian-Desayes I, Soccal PM, Janssens JP, Sapène M, Grillet Y, Stach B, Tamisier R, Pépin JL. Clinical presentation and comorbidities of obstructive sleep apnea-COPD overlap syndrome. PloS one. 2020:15(7):e0235331. doi: 10.1371/journal.pone.0235331. Epub 2020 Jul 9 [PubMed PMID: 32645005]
Slowik JM, Sankari A, Collen JF. Obstructive Sleep Apnea. StatPearls. 2025 Jan:(): [PubMed PMID: 29083619]
Zhao YY, Blackwell T, Ensrud KE, Stone KL, Omachi TA, Redline S, Osteoporotic Fractures in Men (MrOS) Study Group. Sleep Apnea and Obstructive Airway Disease in Older Men: Outcomes of Sleep Disorders in Older Men Study. Sleep. 2016 Jul 1:39(7):1343-51. doi: 10.5665/sleep.5960. Epub 2016 Jul 1 [PubMed PMID: 27091524]
Soler X, Gaio E, Powell FL, Ramsdell JW, Loredo JS, Malhotra A, Ries AL. High Prevalence of Obstructive Sleep Apnea in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2015 Aug:12(8):1219-25. doi: 10.1513/AnnalsATS.201407-336OC. Epub [PubMed PMID: 25871443]
Shawon MS, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep medicine reviews. 2017 Apr:32():58-68. doi: 10.1016/j.smrv.2016.02.007. Epub 2016 Mar 2 [PubMed PMID: 28169105]
Level 2 (mid-level) evidenceNair S, Paul T, Mehta AA, Haridas N, Kunoor A, Sudhakar N. Prevalence of overlap syndrome in patients with obstructive sleep apnea in a quaternary care center of Kerala. Indian journal of public health. 2022 Nov:66(Supplement):S12-S16. doi: 10.4103/ijph.ijph_1085_22. Epub [PubMed PMID: 36412466]
McNicholas WT. COPD-OSA Overlap Syndrome: Evolving Evidence Regarding Epidemiology, Clinical Consequences, and Management. Chest. 2017 Dec:152(6):1318-1326. doi: 10.1016/j.chest.2017.04.160. Epub 2017 Apr 23 [PubMed PMID: 28442310]
Singh S, Kaur H, Singh S, Khawaja I. The Overlap Syndrome. Cureus. 2018 Oct 15:10(10):e3453. doi: 10.7759/cureus.3453. Epub 2018 Oct 15 [PubMed PMID: 30564532]
Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respiratory care. 2010 Oct:55(10):1333-44; discussion 1344-6 [PubMed PMID: 20875160]
Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet (London, England). 2022 Jun 11:399(10342):2227-2242. doi: 10.1016/S0140-6736(22)00470-6. Epub 2022 May 6 [PubMed PMID: 35533707]
Raherison C, Girodet PO. Epidemiology of COPD. European respiratory review : an official journal of the European Respiratory Society. 2009 Dec:18(114):213-21. doi: 10.1183/09059180.00003609. Epub [PubMed PMID: 20956146]
Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC { 0.70 in diagnosing COPD: an evidence-based review. Respiratory medicine. 2011 Jun:105(6):907-15. doi: 10.1016/j.rmed.2011.01.008. Epub 2011 Feb 5 [PubMed PMID: 21295958]
Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. The Lancet. Respiratory medicine. 2022 May:10(5):447-458. doi: 10.1016/S2213-2600(21)00511-7. Epub 2022 Mar 10 [PubMed PMID: 35279265]
Level 1 (high-level) evidenceBenjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet. Respiratory medicine. 2019 Aug:7(8):687-698. doi: 10.1016/S2213-2600(19)30198-5. Epub 2019 Jul 9 [PubMed PMID: 31300334]
Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. Journal of thoracic disease. 2015 May:7(5):920-9. doi: 10.3978/j.issn.2072-1439.2015.04.52. Epub [PubMed PMID: 26101650]
Level 2 (mid-level) evidenceYoung T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29:328(17):1230-5 [PubMed PMID: 8464434]
Salman LA, Shulman R, Cohen JB. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Current cardiology reports. 2020 Jan 18:22(2):6. doi: 10.1007/s11886-020-1257-y. Epub 2020 Jan 18 [PubMed PMID: 31955254]
Duce B, Kulkas A, Langton C, Töyräs J, Hukins C. The AASM 2012 recommended hypopnea criteria increase the incidence of obstructive sleep apnea but not the proportion of positional obstructive sleep apnea. Sleep medicine. 2016 Oct:26():23-29. doi: 10.1016/j.sleep.2016.07.013. Epub 2016 Oct 18 [PubMed PMID: 28007356]
He B, Al-Sherif M, Wu Y, Higgins S, Schwarz EI, Luo Y, Said AF, Refat N, Abdel Wahab NH, Steier J. Apnoea-hypopnoea-index comparing the 2007 and 2012 American Academy of Sleep Medicine criteria in chronic obstructive pulmonary disease/obstructive sleep apnoea overlap syndrome. Journal of thoracic disease. 2020 Oct:12(Suppl 2):S112-S119. doi: 10.21037/jtd-cus-2020-008. Epub [PubMed PMID: 33214916]
Sankari A, Ravelo LA, Maresh S, Aljundi N, Alsabri B, Fawaz S, Hamdon M, Al-Kubaisi G, Hagen E, Badr MS, Peppard P. Longitudinal effect of nocturnal R-R intervals changes on cardiovascular outcome in a community-based cohort. BMJ open. 2019 Jul 17:9(7):e030559. doi: 10.1136/bmjopen-2019-030559. Epub 2019 Jul 17 [PubMed PMID: 31315880]
Sankari A, Pranathiageswaran S, Maresh S, Hosni AM, Badr MS. Characteristics and Consequences of Non-apneic Respiratory Events During Sleep. Sleep. 2017 Jan 1:40(1):. doi: 10.1093/sleep/zsw024. Epub [PubMed PMID: 28364453]
Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS. Sleep-Disordered Breathing and Spinal Cord Injury: A State-of-the-Art Review. Chest. 2019 Feb:155(2):438-445. doi: 10.1016/j.chest.2018.10.002. Epub 2018 Oct 12 [PubMed PMID: 30321507]
Dempsey JA, Skatrud JB, Jacques AJ, Ewanowski SJ, Woodson BT, Hanson PR, Goodman B. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002 Sep:122(3):840-51 [PubMed PMID: 12226022]
Level 2 (mid-level) evidenceBadr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. Journal of applied physiology (Bethesda, Md. : 1985). 1995 May:78(5):1806-15 [PubMed PMID: 7649916]
Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. American journal of respiratory and critical care medicine. 2009 Feb 15:179(4):313-9. doi: 10.1164/rccm.200805-741OC. Epub 2008 Nov 21 [PubMed PMID: 19201929]
Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008 Feb 15:5(2):144-53. doi: 10.1513/pats.200707-114MG. Epub [PubMed PMID: 18250206]
Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. Journal of applied physiology (Bethesda, Md. : 1985). 2010 Aug:109(2):469-75. doi: 10.1152/japplphysiol.00373.2010. Epub 2010 May 27 [PubMed PMID: 20507968]
Level 1 (high-level) evidenceEckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. The Journal of physiology. 2007 Jun 15:581(Pt 3):1193-205 [PubMed PMID: 17395627]
Badr MS. Effect of ventilatory drive on upper airway patency in humans during NREM sleep. Respiration physiology. 1996 Jan:103(1):1-10 [PubMed PMID: 8822218]
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine. 2000 May 11:342(19):1378-84 [PubMed PMID: 10805822]
Agarwal AK, Raja A, Brown BD. Chronic Obstructive Pulmonary Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32644707]
Locke BW, Lee JJ, Sundar KM. OSA and Chronic Respiratory Disease: Mechanisms and Epidemiology. International journal of environmental research and public health. 2022 Apr 30:19(9):. doi: 10.3390/ijerph19095473. Epub 2022 Apr 30 [PubMed PMID: 35564882]
Walsh JM, Webber CL Jr, Fahey PJ, Sharp JT. Structural change of the thorax in chronic obstructive pulmonary disease. Journal of applied physiology (Bethesda, Md. : 1985). 1992 Apr:72(4):1270-8 [PubMed PMID: 1592714]
Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. American journal of respiratory cell and molecular biology. 2005 May:32(5):367-72 [PubMed PMID: 15837726]
Level 3 (low-level) evidenceCouillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. The European respiratory journal. 2005 Oct:26(4):703-19 [PubMed PMID: 16204604]
Jacono FJ. Control of ventilation in COPD and lung injury. Respiratory physiology & neurobiology. 2013 Nov 1:189(2):371-6. doi: 10.1016/j.resp.2013.07.010. Epub 2013 Jul 12 [PubMed PMID: 23856486]
Flenley DC. Sleep in chronic obstructive lung disease. Clinics in chest medicine. 1985 Dec:6(4):651-61 [PubMed PMID: 2935359]
CHERNIACK RM, SNIDAL DP. The effect of obstruction to breathing on the ventilatory response to CO2. The Journal of clinical investigation. 1956 Nov:35(11):1286-90 [PubMed PMID: 13376721]
Drechsler M, Morris J. Carbon Dioxide Narcosis. StatPearls. 2025 Jan:(): [PubMed PMID: 31869084]
Lacasse Y, Sériès F, Vujovic-Zotovic N, Goldstein R, Bourbeau J, Lecours R, Aaron SD, Maltais F. Evaluating nocturnal oxygen desaturation in COPD--revised. Respiratory medicine. 2011 Sep:105(9):1331-7. doi: 10.1016/j.rmed.2011.04.003. Epub 2011 May 10 [PubMed PMID: 21561753]
Level 3 (low-level) evidenceMessineo L, Lonni S, Magri R, Pedroni L, Taranto-Montemurro L, Corda L, Tantucci C. Lung air trapping lowers respiratory arousal threshold and contributes to sleep apnea pathogenesis in COPD patients with overlap syndrome. Respiratory physiology & neurobiology. 2020 Jan:271():103315. doi: 10.1016/j.resp.2019.103315. Epub 2019 Oct 3 [PubMed PMID: 31586648]
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of internal medicine. 1999 Oct 5:131(7):485-91 [PubMed PMID: 10507956]
Zhang P, Chen B, Lou H, Zhu Y, Chen P, Dong Z, Zhu X, Li T, Lou P. Predictors and outcomes of obstructive sleep apnea in patients with chronic obstructive pulmonary disease in China. BMC pulmonary medicine. 2022 Jan 4:22(1):16. doi: 10.1186/s12890-021-01780-4. Epub 2022 Jan 4 [PubMed PMID: 34983482]
Jung da W, Hwang SH, Lee YJ, Jeong DU, Park KS. Oxygen Desaturation Index Estimation through Unconstrained Cardiac Sympathetic Activity Assessment Using Three Ballistocardiographic Systems. Respiration; international review of thoracic diseases. 2016:92(2):90-7. doi: 10.1159/000448120. Epub 2016 Aug 23 [PubMed PMID: 27548650]
Suri TM, Suri JC. A review of therapies for the overlap syndrome of obstructive sleep apnea and chronic obstructive pulmonary disease. FASEB bioAdvances. 2021 Sep:3(9):683-693. doi: 10.1096/fba.2021-00024. Epub 2021 Jun 11 [PubMed PMID: 34485837]
Level 3 (low-level) evidenceKwon JS, Wolfe LF, Lu BS, Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009 Dec:6(6):441-5. doi: 10.3109/15412550903433000. Epub [PubMed PMID: 19938967]
Level 2 (mid-level) evidenceMatsumoto T, Murase K, Tachikawa R, Minami T, Hamada S, Tanizawa K, Inouchi M, Handa T, Oga T, Yanagita M, Mishima M, Chin K. Microalbuminuria in Patients with Obstructive Sleep Apnea-Chronic Obstructive Pulmonary Disease Overlap Syndrome. Annals of the American Thoracic Society. 2016 Jun:13(6):917-25. doi: 10.1513/AnnalsATS.201510-655OC. Epub [PubMed PMID: 26966922]
Alford NJ, Fletcher EC, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest. 1986 Jan:89(1):30-8 [PubMed PMID: 3079693]
Lacasse Y, Sériès F, Corbeil F, Baltzan M, Paradis B, Simão P, Abad Fernández A, Esteban C, Guimarães M, Bourbeau J, Aaron SD, Bernard S, Maltais F, INOX Trial Group. Randomized Trial of Nocturnal Oxygen in Chronic Obstructive Pulmonary Disease. The New England journal of medicine. 2020 Sep 17:383(12):1129-1138. doi: 10.1056/NEJMoa2013219. Epub [PubMed PMID: 32937046]
Level 1 (high-level) evidenceSpicuzza L, Sambataro G, Schisano M, Ielo G, Mancuso S, Vancheri C. Nocturnal nasal high-flow oxygen therapy in elderly patients with concomitant chronic obstructive pulmonary disease and obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2023 Jun:27(3):1049-1055. doi: 10.1007/s11325-022-02702-2. Epub 2022 Sep 3 [PubMed PMID: 36057738]
Mulloy E, McNicholas WT. Theophylline in obstructive sleep apnea. A double-blind evaluation. Chest. 1992 Mar:101(3):753-7 [PubMed PMID: 1541143]
Level 1 (high-level) evidenceGinter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, Salloum A, Badr MS. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. Journal of applied physiology (Bethesda, Md. : 1985). 2020 Apr 1:128(4):960-966. doi: 10.1152/japplphysiol.00532.2019. Epub 2020 Feb 20 [PubMed PMID: 32078469]
Sterling KL, Pépin JL, Linde-Zwirble W, Chen J, Benjafield AV, Cistulli PA, Cole KV, Emami H, Woodford C, Armitstead JP, Nunez CM, Wedzicha JA, Malhotra A. Impact of Positive Airway Pressure Therapy Adherence on Outcomes in Patients with Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease. American journal of respiratory and critical care medicine. 2022 Jul 15:206(2):197-205. doi: 10.1164/rccm.202109-2035OC. Epub [PubMed PMID: 35436176]
Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM 3rd, Hess DR, Knight SL, Ohar JA, Orr JE, Piper AJ, Punjabi NM, Rahangdale S, Wijkstra PJ, Yim-Yeh S, Drummond MB, Owens RL. Long-Term Noninvasive Ventilation in Chronic Stable Hypercapnic Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2020 Aug 15:202(4):e74-e87. doi: 10.1164/rccm.202006-2382ST. Epub [PubMed PMID: 32795139]
Level 1 (high-level) evidenceGong Y, Sankari A. Noninvasive Ventilation. StatPearls. 2025 Jan:(): [PubMed PMID: 35201716]
Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010 Apr:65(4):303-8. doi: 10.1136/thx.2009.124263. Epub [PubMed PMID: 20388753]
Level 1 (high-level) evidenceColeman JM 3rd, Wolfe LF, Kalhan R. Noninvasive Ventilation in Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2019 Sep:16(9):1091-1098. doi: 10.1513/AnnalsATS.201810-657CME. Epub [PubMed PMID: 31185181]
Dhillon K, Sankari A. Idiopathic Hypersomnia. StatPearls. 2025 Jan:(): [PubMed PMID: 36251850]
Ghimire P, Sankari A, Antoine MH, Bollu PC, Kaul P. Obesity-Hypoventilation Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 31194373]
Maggard MD, Sankari A, Cascella M. Upper Airway Resistance Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 33232072]
Fitzgibbons CM, Goldstein RL, Gottlieb DJ, Moy ML. Physical Activity in Overlap Syndrome of COPD and Obstructive Sleep Apnea: Relationship With Markers of Systemic Inflammation. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2019 Jul 15:15(7):973-978. doi: 10.5664/jcsm.7874. Epub 2019 Jul 15 [PubMed PMID: 31383234]
Yang X, Tang X, Cao Y, Dong L, Wang Y, Zhang J, Cao J. The Bronchiectasis in COPD-OSA Overlap Syndrome Patients. International journal of chronic obstructive pulmonary disease. 2020:15():605-611. doi: 10.2147/COPD.S243429. Epub 2020 Mar 18 [PubMed PMID: 32256061]
Türkay C, Ozol D, Kasapoğlu B, Kirbas I, Yıldırım Z, Yiğitoğlu R. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respiratory care. 2012 Feb:57(2):244-9. doi: 10.4187/respcare.01184. Epub 2011 Jul 12 [PubMed PMID: 21762556]
Level 2 (mid-level) evidenceLiu L, Kang R, Zhao S, Zhang T, Zhu W, Li E, Li F, Wan S, Zhao Z. Sexual Dysfunction in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. The journal of sexual medicine. 2015 Oct:12(10):1992-2003. doi: 10.1111/jsm.12983. Epub 2015 Sep 22 [PubMed PMID: 26395783]
Level 1 (high-level) evidence