Introduction
Bispecific antibodies (bsAbs) have gained significant attention in recent years as a promising class of therapeutic agents designed to target multiple antigens simultaneously (see Image. Blinatumomab Mechanism of Action). These unique antibodies are engineered to recognize and bind to 2 distinct epitopes, allowing for a broader range of applications in treating various diseases, including cancer, autoimmune disorders, and infectious diseases. The FDA has already approved the following bsAbs for marketing in the US:
- Blinatumomab (2014): For CD19-positive B-cell precursor acute lymphoblastic leukemia therapy
- Emicizumab (2017): For reducing bleeding frequency in Hemophilia A with factor VIII inhibitors
- Amivantamab (2021): For treating locally advanced or metastatic non–small cell lung cancer with epidermal growth factor receptor exon-20 insertion mutation
- Faricimab (2022): For therapy of neovascular age-related macular degeneration and diabetic macular edema
- Teclistamab (2022): For relapsed or refractory multiple myeloma treatment
- Mosunetuzumab (2022): For relapsed or refractory follicular lymphoma therapy
- Epcoritamab and glofitamab (2023): For treating relapsed or refractory diffuse large B-cell lymphoma
- Talquetamab and elranatamab (2023): For therapy of relapsed or refractory multiple myeloma
While bsAbs offer exciting therapeutic possibilities, their use raises concerns about potential toxicity. These agents' adverse effects include cytopenias, diarrhea, transaminitis, and tumor lysis syndrome (TLS), which are also seen with conventional chemotherapy. Side effects unique to bsAbs include cytokine release syndrome (CRS), neurotoxicity (including immune effector cell-associated neurotoxicity syndrome or ICANS), and tumor flare.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most naturally occurring human antibodies are bivalent monospecific, which implies that they have identical antigen-binding sites targeting the same antigen. Biologically engineered bsAbs can bind to 2 different antigen epitopes, allowing them to interact with both immune and tumor cells simultaneously. This interaction activates the immune system, destroying tumor cells.[2] Tumor killing occurs via antibody-dependent cellular cytotoxicity mediated by perforins and granzymes. Proinflammatory cytokines like interleukin-2 (IL-2), IL-6, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) increase in concentration within the tumor microenvironment. However, this cytokine response can also lead to systemic inflammation, producing bsAb side effects. The 5 main toxicities associated with bsAbs include infections, on-target off-tumor toxicities, CRS, ICANS, and TLS.[3]
Epidemiology
Information on bsAb toxicity's prevalence comes mainly from clinical trials. Real-world data is limited. The incidence of infections in various trials was between 38% and 76%, and grade 3 infection's incidence ranged from 14% to 44%.[4] CRS' incidence was 27% to 59% in various clinical trials, but only 1% to 3.5% were grade 3 or higher.[5] Neurological toxicity and ICANS were seen in 10% to 65% of patients treated with bsABs, with 10% to 15% of patients experiencing grade 3 or higher toxicity.[6] TLS' incidence depends mainly on the cancer type being treated but is much lower than conventional chemotherapy.
Pathophysiology
The increased risk of bacterial, viral, and opportunistic infections in bsAb therapy is attributed to bsAb-induced T-cell exhaustion, neutropenia, and hypogammaglobulinemia. This heightened risk arises from both direct T-cell activation and on-target off-tumor toxicities, where antibody targets are present not only on tumor cells but also on some healthy tissues. Examples include B-cell depletion from CD-19 and CD-20 bsAb binding, normal plasma cell and antibody elimination from B-cell maturation antigen targeting by bsAbs, and cytopenias from bsAbs attaching to CD123/CD33. Patient-specific factors, such as cancer-related immunodeficiency and previous anticancer treatments, can also influence these outcomes variably.[7]
CRS arises from proinflammatory cytokine production by activated T lymphocytes, macrophages, and endothelial cells. Specifically, IFN-γ is released by activated T cells to stimulate macrophages. The macrophages, in turn, produce IL-6, TNF-α, and IL-10. IL-6 produces vascular leakage, cardiomyopathy, complement activation, and coagulation cascades. IFN-γ is associated with fever, chills, headache, dizziness, and fatigue. TNF-α is responsible for flu-like symptoms, diarrhea, and acute lung injury.[8]
ICANS is thought to be due to endothelial cell activation, disrupting the blood-brain barrier. This inflammatory cascade within the central nervous system causes altered cortical and subcortical function, leading to diffuse cerebral edema. TNF-α, IL-6, and IL-1 are the implicated cytokines.[9] TLS is another infrequent but grave side effect seen with bsAB use. This condition occurs more commonly when bsAbs are used in non-Hodgkin lymphoma treatment. Rapid bsAb-induced lymphoma cell death ensues, resulting in massive potassium, phosphate, and nucleic acid release into the circulation that can produce serious metabolic derangements.[10]
History and Physical
Toxicity with these biologics can occur in the prehospital or in-hospital setting. Individuals on bsAbs may present unconscious, apneic, and pulseless. Airway, breathing, and circulation must be immediately assessed, and resuscitation should be performed promptly for individuals on cardiorespiratory arrest. A more detailed evaluation may be pursued once the patient is hemodynamically stable.
Patients treated with bsAbs present with a wide variety of toxicity symptoms. Infusion reactions may occur during or soon after administration of the drug. Monitoring should be done for fevers, chills, and allergy symptoms. Patients developing fevers while on these drugs should be evaluated thoroughly to rule out all infectious sources. Patients may acquire opportunistic infections like Epstein-Barr virus, cytomegalovirus, adenovirus, herpes simplex virus, hepatitis B, hepatitis C, pneumocystis jirovecii, toxoplasma, and aspergillosis. On-target off-tumor toxicities may present with symptoms like diarrhea, skin rash, nail disorders, loss of taste, and transaminitis.
CRS can manifest with mild flu-like symptoms or severe and potentially life-threatening disease. Mild CRS may present with fever, rash, fatigue, headache, diarrhea, or arthralgia. In contrast, severe CRS can cause hypotension, circulatory collapse, peripheral edema, pulmonary edema, renal and cardiac failure, and ultimately, multiorgan failure. Symptoms can develop within a few minutes to hours after bsAb infusion. CRS is classified into the following severity-based categories as per the common terminology criteria for adverse events (CTCAE) v5:
- Grade 1: Fever with or without constitutional symptoms
- Grade 2: Hypotension responding to fluids; hypoxia responding to <40% FiO2
- Grade 3: Hypotension managed with one vasopressor; hypoxia requiring ≥40% FiO2
- Grade 4: Life-threatening consequences; urgent intervention indicated
- Grade 5: Death [11]
ICANS may present concurrently with or after CRS. However, CRS does not always precede the onset of ICANS, which may occur independently. Symptoms include headache, confusion, hallucinations, tremors, ataxia, aphasia, behavioral changes, visual or auditory hallucinations, motor impairment, seizures, cerebral edema, and even death. As per CTCAE v5, individual ICANS symptoms are graded from grade 1 to 5, similar to CRS.[12] TLS produces hyperuricemia, hyperkalemia, hyperphosphatemia, renal failure, and cardiac arrhythmias.
Any patient receiving bsAB treatment presenting with new signs or symptoms should be evaluated for all the above potentially serious side effects. History should focus on the disease's nature, cancer stage, bsAB type being infused, and time since starting bsAb administration. Patients with fever should also be asked about fever duration, recent travel, exposure to sick contacts, infection-localizing symptoms like cough and urinary frequency or urgency, and insertion of medical devices like a port-a-cath. A thorough physical examination should be performed, including vital signs assessment, oxygen saturation measurement, chest auscultation, abdominal evaluation, and neurological assessment. Serial neurological exams focusing on mental status, language, attention, and motor function changes compared to baseline help identify subtle changes.
Evaluation
The evaluation process for bsAb toxicity encompasses several key components. Diagnostic results can provide valuable insights into the severity of adverse reactions, supporting clinical diagnosis and management decisions.
Inpatient Monitoring
Close inpatient monitoring during the initial days of bsAb therapy is routinely advised. Inpatient stay duration is usually based on the bsAb type, product-specific guidelines, and clinician discretion. Some of these antibodies follow a specific protocol with slow dosage escalation. Most clinicians prefer to monitor patients closely for 24 to 48 hours after the completion of the ramp-up phase.
Laboratory Testing
Laboratory tests include a complete blood count with differential, comprehensive metabolic panel, uric acid, lactate dehydrogenase, coagulation tests, blood cultures, urinalysis, and respiratory viral panels. Blood counts may reveal neutropenia (absolute neutrophil count <1500 cells/μL), thrombocytopenia, and anemia. Creatinine, uric acid, and lactate dehydrogenase elevation and electrolyte abnormalities may suggest TLS. Coagulation tests like prothrombin and partial thromboplastin time, D-dimer, and fibrinogen can help evaluate for disseminated intravascular coagulation. Blood cultures, urinalysis, and respiratory viral panels can help identify or rule out infectious sources.
Imaging
Chest x-rays can identify pneumonia and assess for other potential bsAb-related complications, such as pulmonary edema, pleural effusion, and cardiac enlargement. Ultrasound can help rule out venous thromboembolism in hospitalized patients with sudden-onset dyspnea. An echocardiogram can help assess for cardiomyopathy.
Computed tomography (CT) and magnetic resonance imaging (MRI) may be obtained to evaluate neurologic symptoms that may arise from ICANS. CT scans can help promptly rule out infarcts and hemorrhages. However, this modality is less sensitive than MRI in detecting subtle brain tissue changes. In contrast, MRI is more sensitive than CT in identifying soft tissue abnormalities, including brain edema and inflammation. MRI can also detect white matter changes associated with ICANS. However, this diagnostic procedure often takes longer to perform and may not be suitable for patients who cannot tolerate the test or have contraindications to MRI scans. CT and MRI may also be used to evaluate other forms of organ dysfunction, eg, gastrointestinal and renal, depending on clinical presentation.
Physiologic Tests
An electrocardiogram should be obtained in patients with suspected TLS or presenting with chest pain and dyspnea. An electroencephalogram may be warranted in some individuals with neurologic symptoms, particularly seizures.
Invasive Procedures
Lumbar puncture is not routinely advised. Cerebral edema should be ruled out by neuroimaging before performing a lumbar tap, which can lead to brain herniation.
Inflammatory Markers
Although CRS and ICANS are mainly clinical diagnoses, testing for inflammatory markers like erythrocyte sedimentation rate, C-reactive protein, ferritin, IL-6, soluble IL-2Rα, and IFN-γ may support the diagnosis. These markers' degree of elevation generally correlates with toxicity severity.
Treatment / Management
Prompt diagnosis and management of bsAb toxicity is vital to prevent irreversible end-organ damage and death. The management depends on the specific toxicity profile and clinical presentation. General strategies include the ones below.
Supportive Care
Supportive treatment includes antipyretics for fever, fluids with or without vasopressors for hypotension, and respiratory support with mechanical ventilation if needed. Early initiation of broad-spectrum antibiotics with an ongoing infectious workup is essential.
Immunosuppression
Immunosuppressive therapies should be considered to dampen the immune response in severe immune-related toxicities like CRS or ICANS. Proper grading of these side effects can help clinicians initiate the appropriate treatment. Mild-to-moderate CRS may be managed with intravenous fluids, antihistamines, and antipyretics. However, patients with severe CRS should be monitored and treated in the intensive care unit.
In grades 3 to 4 CRS, dexamethasone 8 mg every 8 hours intravenously or orally should be administered for 3 days. Steroids should be tapered over 4 days afterward. An inadequate response to these measures warrants intravenous tocilizumab at a dose of 8 mg/kg. Tocilizumab is an IL-6 antagonist that has produced good responses in severe CRS.
Dexamethasone 10 mg every 6 to 12 hours is recommended for grade 2 or higher ICANS, tapered over the next 2 to 5 days. Antiseizure prophylaxis with levetiracetam 500 mg twice a day is recommended in patients with ICANS who are at high risk of seizures. Antiseizure medications should be adjusted to therapeutic doses if clinical seizures develop. While early glucocorticoids are the standard of care, ICANS does not warrant tocilizumab unless associated with CRS per the Mayo Stratification for Myeloma And Risk-Adapted Therapy guidelines.[13][14](B3)
BsAb Discontinuation or Dose Adjustment
Depending on the severity of toxicity, temporary or permanent bsAb withdrawal or dose adjustments may be necessary. Treatment interruption is suggested for grade 3 toxicities, while permanent discontinuation is suggested for grade 4 toxicities.
Consultation with Specialists
Consultation with specialists in relevant fields, such as neurology, pulmonology, and infectious disease, may be required to tailor management in complex cases. Interprofessional care helps ensure comprehensive treatment.
TLS Management
TLS arising from bsAB toxicity is managed similarly as in other causes of this condition. Patients with a high tumor burden or uric acid levels before treatment should preemptively be started on intravenous fluids and allopurinol prophylaxis. Allopurinol should be initiated at doses of 100 mg/m2 every 8 hours if TLS develops after initiation of bsAb infusion. Rasburicase may be added in the presence of renal dysfunction. Close renal function and uric acid monitoring are recommended with a low threshold for involving nephrologists.
Differential Diagnosis
The symptoms of bsAb toxicity can mimic other clinical entities. The differential diagnosis includes the following:
Tumor Progression
Some presentations of bsAb toxicity can mimic tumor progression. For example, ICANS symptoms are similar to those of brain metastasis. Similarly, respiratory distress or cough may be mistaken for worsening pulmonary involvement rather than bsAb-related pulmonary toxicity. These scenarios underscore the importance of careful clinical evaluation and consideration of bsAb toxicity in the differential diagnosis to ensure appropriate management.
Infections and Sepsis
Symptoms of CRS can overlap with severe infections and sepsis. Infections to consider are viral and bacterial pneumonia, urinary tract infection, and hepatitis.
Cardiovascular Complications
Most bsAbs are used as 3rd- or 4th-line therapy. Thus, patients might have already been exposed to cardiotoxic chemotherapeutic agents. Heart failure, arrhythmias, and myocardial infarction may be confused with bsAb toxicity. Electrocardiography, echocardiography, brain natriuretic peptide, and troponin can help differentiate these conditions.
Venous Thromboembolism
Cancer patients have an increased risk of venous thrombosis, being in a procoagulant state. Deep venous thrombosis or pulmonary embolism can develop, with symptoms of leg swelling, shortness of breath, chest pain, and hemodynamic compromise. The care team should have a high suspicion index for this condition and obtain imaging tests like leg Doppler ultrasonography and chest CT angiography.
Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) is a grave syndrome of immune activation presenting with fever, lymphadenopathy, hepatosplenomegaly, cytopenias, hemophagocytosis, hypertriglyceridemia, hyperferritinemia, transaminitis, elevated soluble IL-2R, and decreased natural killer cell function. HLH can resemble CRS, as both involve immune activation. Hemophagocytosis confirmation and specialized inflammatory marker tests may be used to distinguish HLH from CRS.
Cerebrovascular Conditions
Ischemic stroke, hemorrhagic stroke, and metabolic encephalopathy may all be confused with ICANS. Brain MRI is the preferred modality for determining the etiology of neurologic symptoms.
Prognosis
The prognosis of bsAb toxicity varies depending on several factors, including the severity of toxicity, promptness of intervention, and patient-specific characteristics. With timely recognition and appropriate management, many bsAb-related toxicities can be effectively controlled, allowing patients to continue benefiting from these innovative therapies.[15] However, severe or untreated toxicities can lead to adverse outcomes and, in some cases, may necessitate the discontinuation of bsAb treatment.
Complications
Complications associated with bsAb toxicity may be diverse and include the following:
- Secondary infections: Immunosuppressive therapies used to manage toxicity can increase the risk of secondary infections.
- Organ damage: Prolonged or severe bsAb toxicity can injure the liver, kidney, or other vital organs.
- Treatment discontinuation: In some cases, toxicity may necessitate the discontinuation of bsAb therapy, limiting treatment options for the underlying disease.
These conditions underscore the importance of vigilant monitoring, prompt intervention, and balanced risk assessment in optimizing the safety and efficacy of bsAb therapies.
Deterrence and Patient Education
Patient education is vital in preventing and managing bsAb toxicity. The following measures are recommended:
- Educate patients: Patients should receive comprehensive education about the bsAb therapy type they are receiving, the potential side effects, and the importance of promptly reporting any unusual symptoms.
- Treatment adherence: To optimize results, patients must adhere to the prescribed treatment plan, including dosing schedules and monitoring appointments.
- Symptom recognition: Patients should be educated on recognizing early toxicity signs and instructed on when to seek immediate medical attention.
- Medication storage: Patients should be informed about proper medication storage to maintain treatment efficacy when applicable.
Together, these measures underscore the pivotal role of patient education in enhancing bsAb therapy outcomes and promoting patient well-being.
Pearls and Other Issues
BsAbs offer a promising avenue for targeted cancer therapy. Understanding these agents' potential toxicities is crucial for safe and effective use. One key consideration is the risk of CRS, a systemic inflammatory response triggered by bsAb-induced immune cell activation. Monitoring for CRS signs, such as fever, hypotension, and organ dysfunction, is essential for early detection and prompt management. Additionally, bsAbs may lead to on-target off-tumor toxicities, where the antibody binds to both tumor cells and healthy tissues expressing the target antigen. Close observation for adverse effects related to specific target antigens, such as B-cell depletion and cytopenias, is necessary to mitigate these risks.
Other bsAb side effects include infections, ICANS, and TLS. Regular monitoring for signs of infection, such as fever or localized symptoms, coupled with appropriate cultures and antimicrobial therapy, is essential for early infection detection and treatment. Evaluation of ICANS involves serial neurological assessments, with management strategies including supportive care, corticosteroids, and tocilizumab in severe cases. Additionally, TLS risk stratification and prophylaxis, alongside close monitoring of electrolytes and renal function, aid in the timely detection and management of this complication. TLS potentially requires interventions such as hydration and urate-lowering therapy.
Patient-specific factors, including underlying comorbidities and previous treatments, can influence the risk and severity of bsAb-related toxicities. Tailoring treatment strategies and closely monitoring patients for signs of toxicity are essential for maximizing the therapeutic benefits of bsAbs while minimizing the risk of adverse events.
Enhancing Healthcare Team Outcomes
Managing bsAb toxicity requires an interprofessional team approach. The team should include clinicians, advanced practitioners, nurses, pharmacists, and specialists in relevant fields. All healthcare team members should receive proper education and training regarding bsAb toxicity management. Relevant guidelines are constantly evolving, and the healthcare team should have access to appropriate educational resources.
The emergency physician is pivotal in promptly recognizing and stabilizing patients with bsAb toxicity. These professionals provide emergent interventions and coordinate further management. The oncologist collaborates closely with the emergency physician to guide treatment decisions, monitor bsAb therapy response, and adjust treatment plans as needed. The intensivist oversees the critical care management of patients experiencing severe bsAb toxicity, ensuring timely interventions and optimization of organ support to stabilize and improve patient condition.
Nurses should educate patients on detecting side effects and contacting the healthcare team for new symptoms. Pharmacists examine the indications for bsAb treatment before approving the titrations of these agents. Advanced practitioners and physicians of all specialties should respond promptly to any new concerns of patients undergoing bsAb treatment. The healthcare team should have a system enabling patients to communicate anytime. Patients should be informed that bsAb treatment is a prolonged course, and toxicity can arise at any time. Special consideration should be given to patients residing far from the closest treatment center, ensuring they have access to necessary care and support despite geographical distance.
Care coordination and communication between all healthcare team members are crucial to prevent morbidity and mortality from this condition. Concerns about new symptoms should be addressed promptly by nurses and communicated immediately to the treating oncologists. Periodic communication between oncologists, emergency medicine physicians, and nurses can help initiate mitigative measures promptly. This coordination reduces delays, minimizes errors, and enhances patient safety, ultimately producing improved outcomes and patient-centered care.
Media
(Click Image to Enlarge)
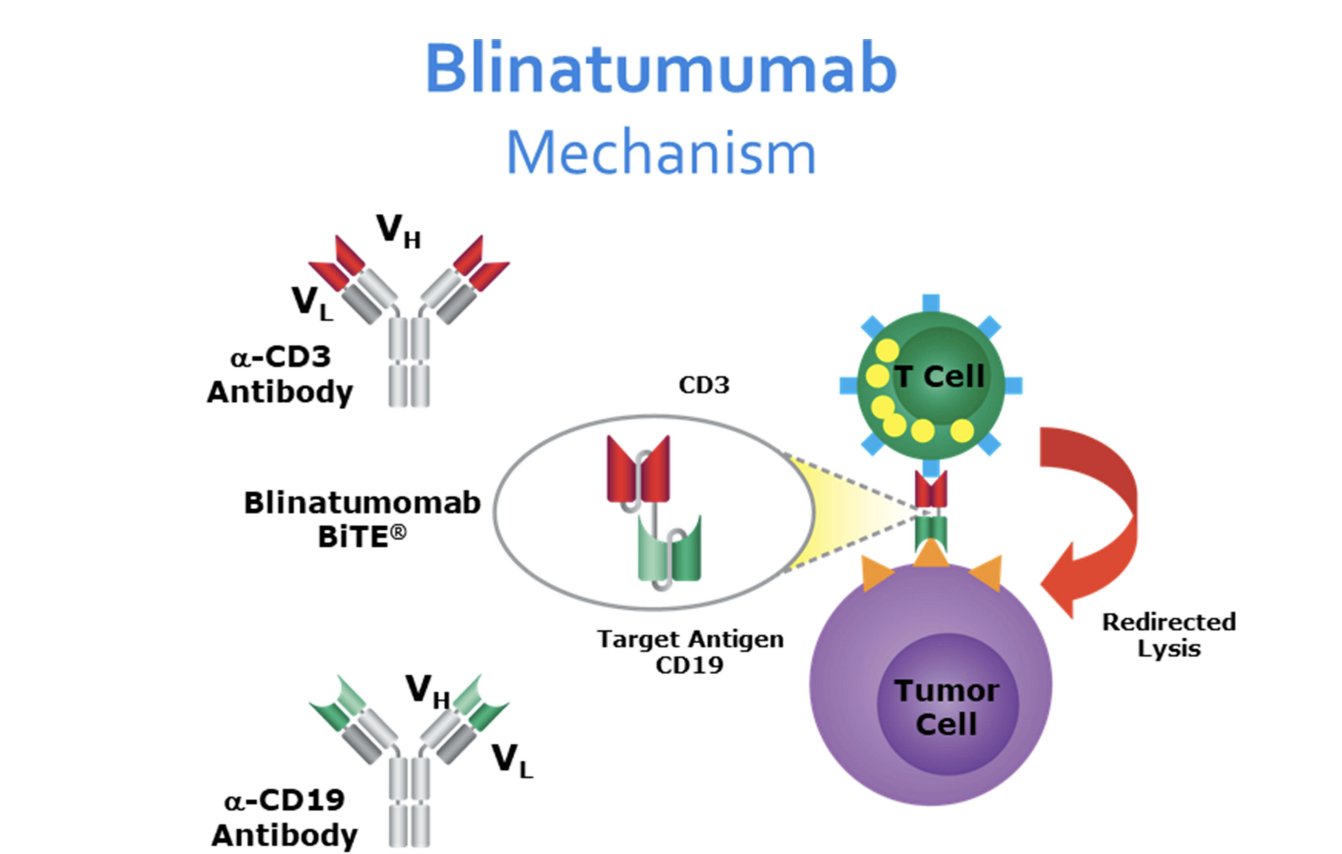
Blinatumomab Mechanism of Action. The diagram illustrates how blinatumomab, a bispecific single-chain antibody construct targeting CD19 and CD3, facilitates the interaction between B cell acute lymphoblastic leukemia blast cells and cytotoxic T cells, leading to T cell activation and subsequent cytotoxicity against CD19-positive cells.
References
Salvaris R, Ong J, Gregory GP. Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas. Journal of personalized medicine. 2021 Apr 29:11(5):. doi: 10.3390/jpm11050355. Epub 2021 Apr 29 [PubMed PMID: 33946635]
Moon D, Tae N, Park Y, Lee SW, Kim DH. Development of Bispecific Antibody for Cancer Immunotherapy: Focus on T Cell Engaging Antibody. Immune network. 2022 Feb:22(1):e4. doi: 10.4110/in.2022.22.e4. Epub 2022 Feb 14 [PubMed PMID: 35291652]
Cosenza M, Sacchi S, Pozzi S. Cytokine Release Syndrome Associated with T-Cell-Based Therapies for Hematological Malignancies: Pathophysiology, Clinical Presentation, and Treatment. International journal of molecular sciences. 2021 Jul 17:22(14):. doi: 10.3390/ijms22147652. Epub 2021 Jul 17 [PubMed PMID: 34299273]
Mazahreh F, Mazahreh L, Schinke C, Thanendrarajan S, Zangari M, Shaughnessy JD, Zhan F, van Rhee F, Al Hadidi S. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: a pooled analysis. Blood advances. 2023 Jul 11:7(13):3069-3074. doi: 10.1182/bloodadvances.2022009435. Epub [PubMed PMID: 36857755]
Level 3 (low-level) evidenceMartin TG, Mateos MV, Nooka A, Banerjee A, Kobos R, Pei L, Qi M, Verona R, Doyle M, Smit J, Sun W, Trancucci D, Uhlar C, van de Donk NWCJ, Rodriguez C. Detailed overview of incidence and management of cytokine release syndrome observed with teclistamab in the MajesTEC-1 study of patients with relapsed/refractory multiple myeloma. Cancer. 2023 Jul 1:129(13):2035-2046. doi: 10.1002/cncr.34756. Epub 2023 Mar 29 [PubMed PMID: 36991547]
Level 2 (mid-level) evidenceMoreau P, Garfall AL, van de Donk NWCJ, Nahi H, San-Miguel JF, Oriol A, Nooka AK, Martin T, Rosinol L, Chari A, Karlin L, Benboubker L, Mateos MV, Bahlis N, Popat R, Besemer B, Martínez-López J, Sidana S, Delforge M, Pei L, Trancucci D, Verona R, Girgis S, Lin SXW, Olyslager Y, Jaffe M, Uhlar C, Stephenson T, Van Rampelbergh R, Banerjee A, Goldberg JD, Kobos R, Krishnan A, Usmani SZ. Teclistamab in Relapsed or Refractory Multiple Myeloma. The New England journal of medicine. 2022 Aug 11:387(6):495-505. doi: 10.1056/NEJMoa2203478. Epub 2022 Jun 5 [PubMed PMID: 35661166]
van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet (London, England). 2023 Jul 8:402(10396):142-158. doi: 10.1016/S0140-6736(23)00521-4. Epub 2023 Jun 1 [PubMed PMID: 37271153]
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nature reviews. Immunology. 2022 Feb:22(2):85-96. doi: 10.1038/s41577-021-00547-6. Epub 2021 May 17 [PubMed PMID: 34002066]
Danish H, Santomasso BD. Neurotoxicity Biology and Management. Cancer journal (Sudbury, Mass.). 2021 Mar-Apr 01:27(2):126-133. doi: 10.1097/PPO.0000000000000507. Epub [PubMed PMID: 33750072]
Omer MH, Shafqat A, Ahmad O, Alkattan K, Yaqinuddin A, Damlaj M. Bispecific Antibodies in Hematological Malignancies: A Scoping Review. Cancers. 2023 Sep 14:15(18):. doi: 10.3390/cancers15184550. Epub 2023 Sep 14 [PubMed PMID: 37760519]
Level 2 (mid-level) evidenceLee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10:124(2):188-95. doi: 10.1182/blood-2014-05-552729. Epub 2014 May 29 [PubMed PMID: 24876563]
Level 3 (low-level) evidenceLee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019 Apr:25(4):625-638. doi: 10.1016/j.bbmt.2018.12.758. Epub 2018 Dec 25 [PubMed PMID: 30592986]
Level 3 (low-level) evidenceCobb DA, Lee DW. Cytokine Release Syndrome Biology and Management. Cancer journal (Sudbury, Mass.). 2021 Mar-Apr 01:27(2):119-125. doi: 10.1097/PPO.0000000000000515. Epub [PubMed PMID: 33750071]
Ludwig H, Terpos E, van de Donk N, Mateos MV, Moreau P, Dimopoulos MA, Delforge M, Rodriguez-Otero P, San-Miguel J, Yong K, Gay F, Einsele H, Mina R, Caers J, Driessen C, Musto P, Zweegman S, Engelhardt M, Cook G, Weisel K, Broijl A, Beksac M, Bila J, Schjesvold F, Cavo M, Hajek R, Touzeau C, Boccadoro M, Sonneveld P. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. The Lancet. Oncology. 2023 Jun:24(6):e255-e269. doi: 10.1016/S1470-2045(23)00159-6. Epub [PubMed PMID: 37269857]
Level 3 (low-level) evidenceNoori M, Yazdanpanah N, Rezaei N. Safety and efficacy of T-cell-redirecting bispecific antibodies for patients with multiple myeloma: a systematic review and meta-analysis. Cancer cell international. 2023 Sep 5:23(1):193. doi: 10.1186/s12935-023-03045-y. Epub 2023 Sep 5 [PubMed PMID: 37670301]
Level 1 (high-level) evidence