Introduction
Diborane (B2H6) is a colorless gas at room temperature with a repulsively sweet odor. Other names for diborane include boroethane and boron hydride. The odor threshold at 25 °C (77 °F) and 760 mm Hg is 2 to 4 ppm. Rapid hydrolysis occurs when diborane mixes with water, resulting in a highly exothermic reaction and boric acid and hydrogen gas production. This substance is highly soluble in ether.[1] Diborane explodes when exposed to a spark and mixed with air in concentrations ranging from 2% to 25%, with the most violent explosion occurring at 10% concentration or higher. Diborane is extremely combustible and burns in air to form boron trioxide and water.[2]
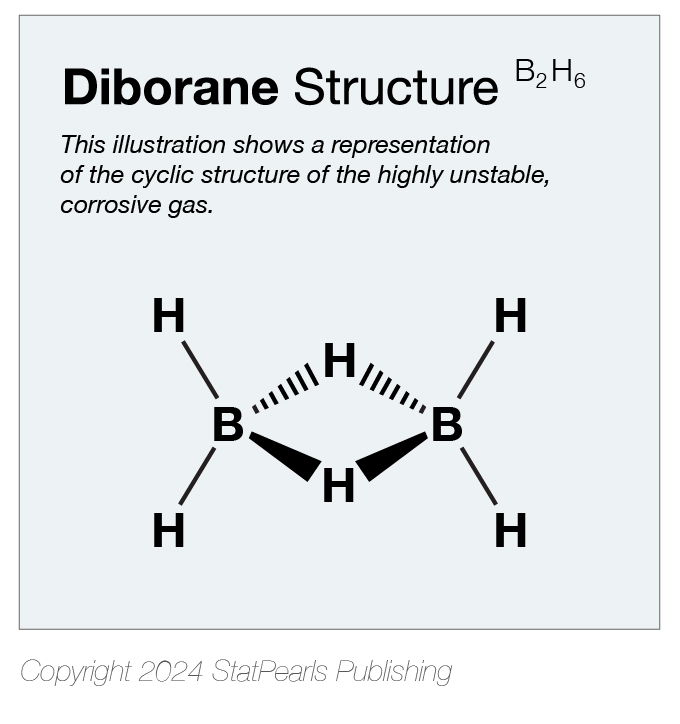
Diborane's reactivity arises from its structural instability, having 2 hydrogen atoms forming 2 covalent bonds in a highly sterically strained ring with 2 sp3-hybridized boron atoms (see Image. Diborane Structure).[3] Diborane is used as a rocket propellant, reducing agent, rubber vulcanizer, electronic semiconductor dopant, hydrogen polymerization catalyst, and flame-speed accelerator.[4] Cases of toxic diborane exposure are very rare due to the chemical's limited uses and handling difficulties.
Diborane is considered an occupational and environmental toxin and is on the Right-to-Know Hazardous Substance List. The Occupational Safety and Health Administration's legal airborne permissible exposure limit is 0.1 ppm, averaged over an 8-hour work shift. By comparison, the National Institute for Occupational Safety and Health's recommended airborne exposure limit is 0.1 ppm, averaged over a 10-hour work shift, while the American Conference of Governmental Industrial Hygienists' threshold limit value is 0.1 ppm, averaged over an 8-hour work shift.
Diborane's chemical identifiers include the following:
- Chemical Abstracts Service number (CAS number): 19287-45-7
- Right-to-Know substance number (RTK substance number): 0629
- Department of Transportation number (DOT number): UN 1911
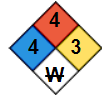
These numbers are identifiers used for regulatory and safety purposes. Meanwhile, the NFPA 704 Diamond, also known as the NFPA Diamond or Fire Diamond, is a standard system used to quickly and easily convey hazardous chemical information. This symbol consists of a diamond divided into 4 colored sections, each representing a different hazard category: health (blue), flammability (red), instability or reactivity (yellow), and special hazards (white). For diborane, the NFPA 704 Diamond indicates severe hazards in all categories, highlighting its extreme toxicity, flammability, and reactivity. This information helps emergency responders and individuals working with diborane to assess the potential risks and take appropriate safety precautions quickly (see Image. NFPA 704 Diamond for Diborane Gas).
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Diborane gas is used in electronics manufacturing, rocket fuels, and chemical laboratories. This explosive and irritating substance must be stored and shipped in stainless steel and commercial steel cylinders at -20 ºC or -4 ºF. Materials such as stainless steel, lead, Monel, copper, brass, paraffin, Glyptol, and Kel-F are unaffected by diborane contact. On the other hand, natural rubber is quickly destroyed on diborane exposure.
Personal protective equipment should be readily available for personnel handling diborane gas due to its hazards. A supplied-air respirator with a full facepiece approved by the National Institute for Occupational Safety and Health should be used. Working with diborane requires good ventilation. Diborane manufacturers recommend handling the chemical wearing plastic, butyl, or rubber gloves and Tychem® BR, LV, Responder, or TK as protective clothing for exposures of less than one hour.
Epidemiology
The incidence of diborane gas exposure has not been established due to its rarity. Individuals at risk for exposure to diborane gas are laboratory workers who handle the chemical, microelectronics and rocket fuel manufacturers, and environmental cleanup crews. Exposures, while rare, may occur during cleanups mandated by the Environmental Protection Agency. Diborane has been found in at least 3 of the 1,585 National Priorities List sites identified by the Environmental Protection Agency.
Pathophysiology
Diborane gas exerts its toxic effects primarily through lung and mucous membrane irritation. Signs and symptoms include cough, chest tightness, and dyspnea. Thermal burns of the eyes, skin, and upper respiratory tract can occur from the highly exothermic hydrolytic reaction with water, which abounds in mucosal surfaces. The reaction has the following chemical equation:
- B2H6 + 6 H2O → 2 H3BO3 + 6 H2 + heat
Boric acid, hydrogen gas, and heat are the products of this reaction. Boric acid is absorbed by the lung mucosa and excreted by the kidneys.[5] Ingestion of large boric acid amounts has been found to cause testicular atrophy and spermiation (release of mature spermatids) inhibition. However, this effect is not observed in inadvertent diborane exposures due to the hydrolytic reaction's little boric acid production.[6][7] Nevertheless, this diborane hydrolysis product is a potential tissue irritant, even in small amounts. The heat produced by the exothermic reaction can likewise injure exposed tissues. Hydrogen gas can fill the alveoli and cause asphyxiation.
Liquid diborane exposure may result in frostbite injuries from rapid cooling when evaporating. Direct irritant effects resulting in burns and irritation have also been reported. Diborane's carcinogenic potential and reproductive effects are currently unknown.
Histopathology
Acute diborane exposure in rats causes a diffuse panbronchiolitis-like lesion characterized as inflammatory cell infiltration of the terminal bronchioles and surrounding alveoli. Pulmonary congestion and bleeding, with or without edema, are notable.
Subacute exposure demonstrates perivascular and peribronchial lymphoid hyperplasia. Alveolar plasma cell and macrophage infiltration are also present.[8] Bronchoalveolar lavage analysis for subacute exposures shows a dose-dependent neutrophilic increase, followed by a gradual decline for 3 days after initial exposure. However, macrophages were reduced on initial exposure.
α1-antitrypsin levels and superoxide dismutase activity rise rapidly on the day of exposure. A delayed phospholipid increase occurs on day 3. The levels of superoxide dismutase, α1-antitrypsin, and phospholipids return to baseline after 14 days.[9]
Toxicokinetics
Diborane exists in the gaseous phase at room temperature and is heavier than air, resulting in its accumulation in low-lying areas. Diborane exposure primarily affects the lungs and causes congestion, pulmonary edema, hemorrhage, and anoxic cell death. A 31.5-ppm concentration of diborane results in a 50% kill after 4 hours of exposure in mice. Toxic effects are concentration-dependent, and higher concentrations kill faster—a concentration of 1000 ppm can result in death within 30 minutes. Some studies show exposure to as low as 1 ppm for 4 hours demonstrates alveolar cell and pulmonary capillary damage, while other studies report a no-observed effect at a level of 1 ppm.[10] The Committee on Threshold Limits of the American Conference of Governmental Industrial Hygienists has established a tentative threshold limit value of 0.1 ppm for humans.[11]
Exposure to high diborane concentrations results in anoxic death. Pulmonary fluid accumulation can be so great that it flows from the tracheal canal to neighboring tissues. Initial diborane exposure leads to increased respirations, decreased blood pressure, greater intestinal smooth muscle activity, and reduced cortical activity.[12] Electrocardiogram (ECG) readings may show sinus bradycardia with an increased T wave voltage.
History and Physical
Patients with preexisting conditions may develop severe reactions to diborane and quickly deteriorate. The healthcare team must be prepared to initiate resuscitation and stabilization measures after a quick primary survey when caring for these patients. A more thorough investigation may be pursued once the patient's airway, breathing, and circulation are stabilized.
History often reveals known diborane exposure or inhalation of an unknown gas with a "sickly sweet odor." Symptoms and severity depend on the chemical's concentration and the patient's exposure duration. Acute exposure can produce cutaneous and mucous membrane burns, chest tightness, diaphragmatic pain, dyspnea, cough, wheezing, dizziness, headache, weakness, central nervous system (CNS) depression, and incoordination. Chronic low-level exposures can cause pulmonary irritation, seizures, convulsions, fatigue, drowsiness, confusion, altered electroencephalogram responses, and involuntary muscle spasms. Less-reported effects include headache, vertigo, chills, and fever.
Physical exam may reveal signs of respiratory distress. Stridor, wheezing, tachypnea, decreased breath sounds, and crackles may be evident on pulmonary auscultation. Nonrespiratory findings include hypotension, cardiac arrhythmias, and signs of CNS depression such as seizures, somnolence, or coma. Clinicians should thoroughly inspect the skin and mucous membranes for signs of burns or frostbite injury.
Evaluation
The diagnosis of diborane toxicity is clinical, made when acute diborane exposure precedes the development of manifestations consistent with the condition. Individuals showing signs of instability, especially those with preexisting pulmonary disease or high susceptibility to pulmonary infections, should be monitored closely. These patients must be hooked to a cardiac and vital signs monitor with a pulse oximeter while receiving supplemental oxygen.
In cases with multiple casualties, patients known to have significant diborane exposure or showing severe poisoning signs should be transported to the nearest medical center for immediate evaluation. Individuals who develop a severe or persistent cough, dyspnea, chemical burns, or inability to protect the airway should be triaged first. Patients with minor symptoms, such as transitory eye or throat irritation, may be discharged after evaluation and observation. Hospital staff is at minimal risk of secondary contamination when caring for patients exposed to diborane gas.
Laboratory, imaging, and physiologic studies are not required to make the diagnosis. However, these tests help assess organ damage severity and rule out other serious conditions. Laboratory studies may include a complete blood count, glucose, a comprehensive metabolic panel, arterial blood gases, and urinalysis. A complete blood count can help rule out the presence of infections and other causes of respiratory distress, such as anemia. Glucose tests, eg, via fingerstick, can help rule out hypoglycemia as the cause of altered sensorium. A comprehensive metabolic panel can help assess electrolyte balance and hepatic status. Arterial blood gases with pH can help guide treatment, as heavy diborane exposures can cause hyperchloremic metabolic acidosis. Urinalysis and metabolic panel results combined can help assess kidney function.
Chest radiography and ECG can help evaluate cardiopulmonary status. Chest radiography may show bilateral infiltrates. ECG helps determine the need for further cardiac tests, eg, cardiac enzymes.
Treatment / Management
Diborane-exposed patients should be immediately removed from the source and decontaminated with saline irrigation. Temporary protection from diborane may be initiated using a chemical cartridge respirator charged with active Hopcalite.[13]
Diborane toxicity has no known antidote, and treatment is primarily supportive. Supplemental oxygen can be administered to patients with respiratory symptoms. Endotracheal intubation and subsequent ventilation must be performed for patients with frank respiratory failure or CNS depression.
Thermal airway injury can result in airway obstruction from soft tissue edema.[14] Children who develop stridor may receive aerosolized racemic epinephrine (0.25 to 0.75 mL of 2.25%) every 20 minutes as needed. Inhaled and intravenous corticosteroid administration have been found to help with lung function recovery in toxic gas exposures with a similar mechanism to diborane. Adults with bronchospasm should receive an aerosolized bronchodilator such as albuterol.[15] (B3)
Patients who become comatose, hypotensive, or actively seizing should be treated according to advanced life support guidelines. Adults who develop shock or hypoperfusion should receive fluid resuscitation with normal saline or lactated Ringer solution at a rate of 1,000 mL/h. Children with shock or hypoperfusion should receive a 20 mL/kg normal saline bolus over 10 to 20 minutes, followed by an infusion of 2 to 3 mL/kg/hour. Patients with evidence of severe burns or pulmonary damage, such as persistent shortness of breath, severe cough, or chest tightness, should be admitted to the hospital for treatment until symptom-free.
Patients with minor exposures should experience symptom resolution in an hour or less. These individuals may be released from observation several hours after symptoms disappear to ensure no delayed effects are missed. Clinically relevant exposures often produce extensive lower respiratory tract damage. However, symptoms may be delayed up to 24 hours owing to diborane's high lipid solubility. Outpatient follow-up is advised due to the likelihood of long-term respiratory problems after diborane inhalation. Patients with skin and corneal injury must be reexamined within 24 hours.
Differential Diagnosis
The differential diagnosis of diborane toxicity includes conditions presenting with sudden dyspnea, such as the following:
- Acute asthma exacerbation
- Anaphylaxis
- Acute coronary syndrome
- Acute pneumonia
- Pneumothorax
- Toxicity from other irritant gases, such as chlorine, phosgene, sulfur dioxide, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, ozone, and ammonia
However, diborane has a characteristic odor that able patients may describe during history-taking. Additionally, diborane is highly regulated and available only in a few places and thus easy to pinpoint as the cause of sudden breathlessness. The presence of mucosal and skin damage in other parts of the body also helps clinch the diagnosis.
Prognosis
The prognosis of diborane exposure depends on the concentration and length of exposure, but most patients are expected to survive and recover with minimal complications. Patients with preexisting pulmonary disease and heavy exposure have a poorer prognosis. Smokers also have a higher morbidity.[16]
Complications
Complications can range from minor pulmonary conditions to severe and debilitating sequelae. Minor complications may include prolonged cough, respiratory muscle weakness, acute upper airway inflammation, delayed pulmonary edema, residual psychogenic dyspnea, and reactive airway dysfunction. Severe sequelae may include bronchiectasis, chronic airflow obstruction, bronchial hyperreactivity, bronchiolitis obliterans, and hemorrhagic pneumonitis. Pulmonology follow-up and management are recommended for patients with evidence of pulmonary complications.
Deterrence and Patient Education
Extreme caution should be used when handling diborane. The substance should only be kept and used in dry and well-ventilated areas. Storage and transportation require appropriate containers and labels. The proper personal protective equipment should be worn at all times.[2]
Pearls and Other Issues
Diborane poisoning is a clinical diagnosis. This unstable substance—available only in a few facilities—is highly regulated and thus should be considered a likely etiology of sudden dyspnea and mucosal and skin irritation in identified storage sites. This irritant gas, like others, primarily affects the pulmonary system and can cause life-threatening respiratory injury at high concentrations and long exposures.
No specific antidote for diborane toxicity exists. Treatment consists of removal of the victim from the gaseous source, decontamination, airway maintenance, and bronchodilator administration. Patients with severe pulmonary injuries may need to be intubated and admitted to the hospital for airway management and ventilation. Toxic effects may be delayed for up to 24 hours in patients with significant exposures.
Diborane toxicity prevention involves safe and secure storage, adherence to proper handling protocols, and use in well-ventilated areas. Healthcare providers, emergency responders, and individuals working with diborane should be familiar with the signs and symptoms of toxic exposure and be prepared to take action to mitigate its effects.
Enhancing Healthcare Team Outcomes
Managing toxic diborane exposure requires an interprofessional team of healthcare workers, including nursing staff, poison control center employees, laboratory technologists, respiratory therapists, and emergency medicine physicians. Depending on symptom severity, an internal medicine or critical care physician may also care for patients with diborane poisoning. The emergency medicine physician and assigned nurse are responsible for assessing and determining the severity of the exposure and coordinating the patient's care, including:
- Monitoring vital signs for hypotension, cardiac arrhythmias, and respiratory collapse
- Securing the airway in the case of respiratory failure with endotracheal intubation and mechanical ventilation
- Assessing the skin for burns and frostbite injury
- Checking laboratory values, including electrolytes, blood pH, complete blood count, and blood glucose
- Consulting the poison control center for treatment recommendations
- Consulting internal medicine or the intensivist if hospitalization is necessary
Once the patient has recovered, close follow-up with an outpatient provider is important to monitor for complications. If long-term pulmonary complications occur, follow-up with a pulmonologist can significantly improve outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
STUMPE AR. Toxicity of diborane in high concentrations. A.M.A. archives of industrial health. 1960 Jun:21():519-24 [PubMed PMID: 13835445]
. DIBORANE. American Industrial Hygiene Association journal. 1958 Oct:19(5):438-9 [PubMed PMID: 13582874]
Chou SL, Lo JI, Peng YC, Lin MY, Lu HC, Cheng BM, Ogilvie JF. Identification of diborane(4) with bridging B-H-B bonds. Chemical science. 2015 Dec 1:6(12):6872-6877. doi: 10.1039/c5sc02586a. Epub 2015 Aug 14 [PubMed PMID: 29861928]
LaDou J. Potential occupational health hazards in the microelectronics industry. Scandinavian journal of work, environment & health. 1983 Feb:9(1):42-6 [PubMed PMID: 6857187]
Hadrup N, Frederiksen M, Sharma AK. Toxicity of boric acid, borax and other boron containing compounds: A review. Regulatory toxicology and pharmacology : RTP. 2021 Apr:121():104873. doi: 10.1016/j.yrtph.2021.104873. Epub 2021 Jan 22 [PubMed PMID: 33485927]
Chapin RE, Ku WW. The reproductive toxicity of boric acid. Environmental health perspectives. 1994 Nov:102 Suppl 7(Suppl 7):87-91 [PubMed PMID: 7889888]
Level 3 (low-level) evidenceNomiyama T, Omae K, Ishizuka C, Hosoda K, Yamano Y, Nakashima H, Uemura T, Sakurai H. Evaluation of the subacute pulmonary and testicular inhalation toxicity of diborane in rats. Toxicology and applied pharmacology. 1996 May:138(1):77-83 [PubMed PMID: 8658516]
Level 3 (low-level) evidenceUemura T, Omae K, Nakashima H, Sakurai H, Yamazaki K, Shibata T, Mori K, Kudo M, Kanoh H, Tati M. Acute and subacute inhalation toxicity of diborane in male ICR mice. Archives of toxicology. 1995:69(6):397-404 [PubMed PMID: 7495378]
Level 3 (low-level) evidenceNomiyama T. Inhalation toxicity of diborane in rats assessed by bronchoalveolar lavage examination. Archives of toxicology. 1995:70(1):43-50 [PubMed PMID: 8750904]
Level 3 (low-level) evidenceNomiyama T, Omae K, Uemura T, Nakashima H, Takebayashi T, Ishizuka C, Yamazaki K, Sakurai H. No-observed-effect level of diborane on the respiratory organs of male mice in acute and subacute inhalation experiments. Sangyo eiseigaku zasshi = Journal of occupational health. 1995 May:37(3):157-60 [PubMed PMID: 7796306]
Level 3 (low-level) evidenceKUNKEL AM, MURTHA EF, OIKEMUS AH, STABILE DE, SAUNDERS JP, WILLS JH. Some pharmacologic effects of diborane. A.M.A. archives of industrial health. 1956 Apr:13(4):346-51 [PubMed PMID: 13301066]
JACOBSON KH, LAWSON LH. The effect of age of weight on the toxicity of diborane. Toxicology and applied pharmacology. 1962 Mar:4():215-9 [PubMed PMID: 14450734]
LONG JE, LEVINSKAS GJ, HILL WH, SVIRBELY JL. Gas-mask protection against diborane, pentaborane, and mixtures of boranes. A.M.A. archives of industrial health. 1957 Nov:16(5):393-402 [PubMed PMID: 13468805]
Chen TM, Malli H, Maslove DM, Wang H, Kuschner WG. Toxic inhalational exposures. Journal of intensive care medicine. 2013 Nov-Dec:28(6):323-33. doi: 10.1177/0885066611432541. Epub 2012 Jan 9 [PubMed PMID: 22232204]
Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta anaesthesiologica Scandinavica. 2005 Feb:49(2):183-90 [PubMed PMID: 15715619]
Level 3 (low-level) evidencedo Pico GA. Toxic gas inhalation. Current opinion in pulmonary medicine. 1995 Mar:1(2):102-8 [PubMed PMID: 15786599]
Level 3 (low-level) evidence