Introduction
Glycol ethers have been a versatile and essential solvent group widely utilized in industrial and commercial settings since their initial development in the 1930s. Glycol ethers possess a unique combination of physical properties, including high solvency, low evaporation rates, and miscibility with both water and organic substances.[1][2] These characteristics make glycol ethers essential in manufacturing various products, including paint and ink formulations, cleaners, cosmetics, pharmaceuticals, circuit boards, and hydraulic brake fluids.[3]
Traditionally, glycol ethers are classified based on their precursor molecules. Ethylene oxide-derived glycol ethers belong to the E-series, while molecules derived from propylene oxide are classified as P-series glycol ethers. The utility of E-series glycol ethers (EGEs), such as methyl-, ethyl-, butyl-, and hexyl-GEs, has diminished due to increasing reports of their toxic effects.[4][5][6][7] EGEs are metabolized into their respective alkoxyacetic acids, producing neurologic, hematologic, renal, hepatic, endocrine, teratogenic, and reproductive effects. Consequently, the relatively safer P-series glycol ethers (PGEs) have mostly replaced EGEs.[8][9][10][11][12][13]
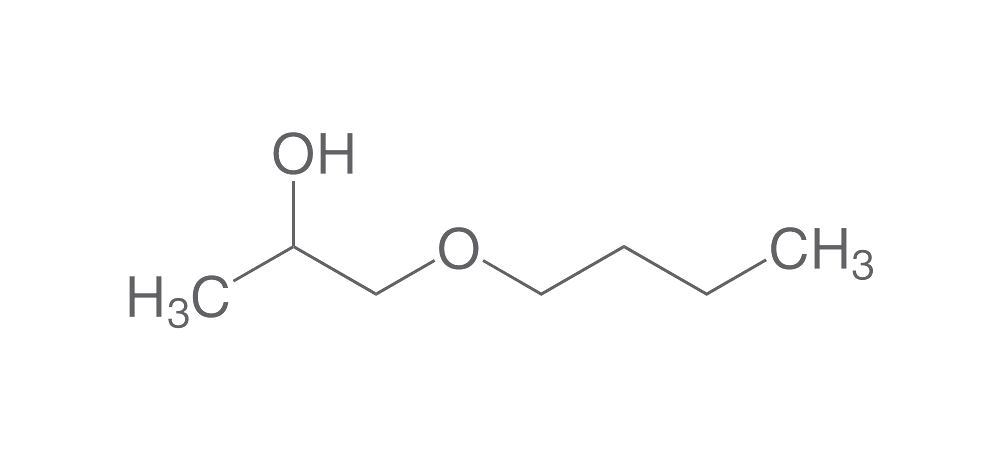
PGEs include methyl-, ethyl-, n-propyl-, butyl, and phenyl propylene glycol ethers (see Image. Propylene Glycol n-Butyl Ether Structure). These chemicals are often preferred due to their comparatively lower toxicity profiles. PGEs are commonly found in paints, coatings, semiconductor processes, resins, cleaners, sunscreen, and pharmaceuticals.[14] Propylene glycol has many applications due to its solvent properties. A key example is its use in lorazepam and diazepam infusions.[15]
Glycol ether exposure may occur in both household and occupational settings through inhalation, dermal contact, or ingestion. Occupations most vulnerable to glycol ether exposure include cleaners, printing machine operators, semiconductor manufacturing workers, chemical industry workers, automobile mechanics, cosmeticians, secretaries, and printers.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Glycol ethers are rapidly absorbed via dermal, inhalational, and gastrointestinal routes. Ingestion is the least likely mode of exposure but has the greatest potential to cause toxicity. Intentional ingestion of glycol ethers has been documented.[16][17] EGEs possess a primary alcohol and are metabolized by alcohol dehydrogenase, producing toxic alkoxyacetic acids. Organic acid accumulation is generally responsible for causing EGEs' renal, neurologic, and reproductive side effects. In contrast, PGEs lack a primary alcohol group and thus cannot produce toxic metabolites.[18][19]
Epidemiology
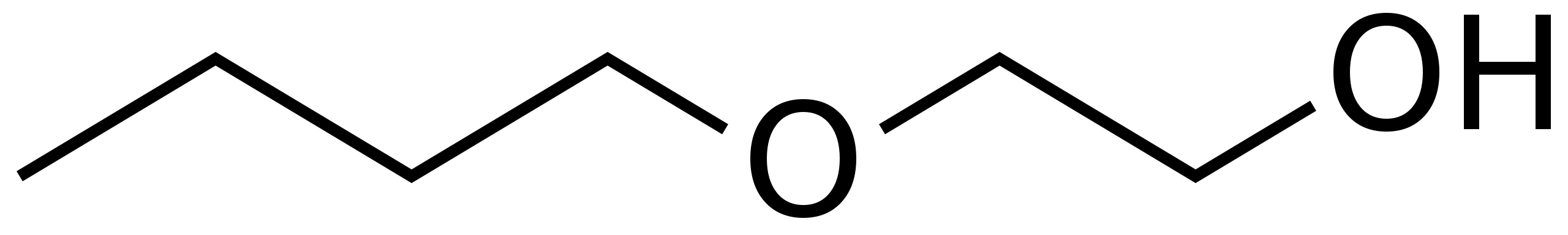
Limited human epidemiological data regarding the association between reproductive outcomes and glycol ethers is available. Epidemiological studies focus mostly on EGEs, particularly 2-methoxyethanol, 2-ethoxyethanol, and 2-butoxyethanol (see Image. Structure of 2-Butoxyethanol). These studies include case reports, animal research, and limited prospective cohorts.[20][21][22] Furthermore, these investigations are often confounded by exposures to other occupational chemicals.
Animal models first displayed EGEs' toxic reproductive effects in the 1970s, prompting investigations in the United States a decade later. France partially regulated the compound in 1992, but the first complete ban on using glycol ethers in drugs and cosmetics occurred in 1999.
Occupational exposures comprise most cases since glycol ethers have a ubiquitous industrial role. Semiconductor factory workers are the most vulnerable to reproductive toxicity. A 1996 American retrospective cohort study reported an increased relative risk of spontaneous abortions among female workers in semiconductor manufacturing facilities.[23] Similarly, a 1997 European case-controlled study reported that women exposed to glycol ethers had increased odds of having children with congenital disabilities, especially neural tube defects and cleft lips.[24]
Epidemiological studies likewise show that shipyard workers exposed to glycol ethers have a high prevalence of oligospermia and azoospermia.[25][26] Notably, a 2020 French study demonstrated the widespread detection of glycol ether metabolites in 6-year-old children undergoing neurodevelopmental evaluation[27]
The United States Poison Control Centers reported 6,411 ethylene glycol exposures in 2018, with most cases being unintentional (78%). Among these exposures, 469 occurred in children aged 5 years or younger.[88]
Pathophysiology
EGEs are metabolized by the liver enzyme alcohol dehydrogenase into toxic alkoxyacetic acids. These organic acids are primarily responsible for EGEs' renal, neurologic, and reproductive side effects.[11][12]. The most frequently studied EGE compounds include 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol, 2-propoxyethanol, 2-isopropoxyethanol, and 2-phenoxyethanol.
Neurological Injury
Acute encephalopathy characterized by confusion, agitation, and depressed mental status has been described in serious EGE poisonings.[28][29][30]. The onset of personality changes, memory loss, headaches, dizziness, ataxia, tremors, and lethargy characterizes chronic toxicity by occupational exposure to high EGE levels.[31] Animal models demonstrate EGEs' capacity to cross the blood-brain barrier. In vivo studies show that administering EGE mixtures to rats' frontal cortex and hippocampus leads to reduced antioxidant capacity, heightened lipid peroxidation, and elevated caspase-3 activity, indicating oxidative stress as the mechanism of EGE toxicity.
Renal Damage
Acute kidney injury and proteinuria have been reported following acute EGE ingestion.[32] Alkoxyacetic acid-induced renal tubular degeneration and necrosis have been found in both animal models and autopsies after fatal EGE poisoning.[33][34]
Hepatic Effects
Glycol ethers are predominantly metabolized in the liver. Hepatic injury arises from various mechanisms. The first is through mitochondrial disruption by EGE metabolism-derived reactive oxygen species, impairing cellular metabolism. The second is primarily mediated by hemolysis and iron deposition in the liver. Iron deposition activates Kupffer cells through the Fenton/Haber-Weiss reactions, releasing more reactive oxygen species and inducing TNF-α production.[35]
Studies also show that diethylene glycol monomethyl ether (DiEGME) and ethylene glycol monomethyl (EGME) increase microsomal protein concentrations and induce hepatic enzymes. Specifically, DiEGME increases cytochrome P-450 but not cytochrome b5 or NADPH-cytochrome c reductase.[36] EGME, on the other hand, has the opposite effect. The reason for the specificity of certain EGEs toward particular hepatic enzymes remains unclear, as does the correlation between the hepatotoxic effects of glycol ethers and the concentration of glycolic acid. These biochemical changes may manifest as hepatic steatosis and aminotransferase derangements due to toxic metabolite accumulation in the liver. Some studies conclude that patients with underlying liver disease would have less functional alcohol dehydrogenase to produce toxic metabolites, creating a less severe clinical presentation.[37]
Reproductive Toxicity
Certain EGEs are associated with reproductive toxicity and neurodevelopmental disorders.[38][39] Numerous animal studies demonstrate testicular weight changes, edema, and tubular atrophy. Routine EGE exposure has been shown to reduce sperm maturation steps.
Daily 2-methoxyethanol administration to pregnant mice delays neurogenesis and morphogenesis, specifically neural tube closure and limb and digit formation.[40] Researchers have determined that glycol ether metabolism to acetic acid is the primary driver of teratogenicity. The compound 2-methoxyacetic acid gets incorporated into the metabolic pathways during embryogenesis. Coenzyme A converts 2-methoxyacetic acid to a thioester, similar to acetic acid's transformation into the Krebs cycle intermediate acetyl CoA.
Researchers found that carbon-labeled CO2 was excreted in embryonic cultures exposed to carbon-labeled 2-methoxyacetic acid. However, competitive inhibitors such as acetate and serine could inhibit 2-methoxyacetic acid incorporation into the metabolic pathways. Serine was likewise found to counteract the decline in sperm production and micromorphological changes caused by toxicity related to 2-methoxyethanol.
Hematologic Manifestations
Butoxyethanol has been specifically identified as potentially toxic to the bone marrow. Studies show that animal exposure leads to hemolysis, hemoglobinuria, leukopenia, and decreased bone marrow cellularity. Further investigation reveals that β-aminolevulinic acid production is a critical factor in hemolysis induction.[41][42]
In vitro animal studies likewise demonstrate EGEs' time- and concentration-dependent hemolytic effect, preceded by a decrease in adenosine triphosphate levels and erythrocyte swelling. The underlying mechanisms behind this phenomenon remain poorly understood, but the erythrocyte membrane appears to be the damage site. Another possibility is that reactive oxygen species generated by the parent glycol ethers cause lipid peroxidation.
Lymphatic Damage
Early animal studies investigated male rat immune reactions after a 4-day EGME dermal exposure at doses of 150 to 1200 mg/kg/day. Thymus and spleen weights were found to decline across all amounts at higher doses. Additionally, lymphoproliferative responses were enhanced at 1200 mg/kg/day. However, 4 days of oral EGME administration impaired antibody response at 300 and 600 mg/kg/day. This immunosuppressive response was blocked with the administration of fomepizole, suggesting that 2-methoxyacetic acid is primarily responsible for mediating the process.[43] A follow-up study showed that different rodent species exhibit sensitivity differences toward EGE.[44] Research on the lymphogenic response to glycol ethers is limited and requires further investigation to elucidate its effects fully.
Dermatologic Injury
Glycol ethers are easily absorbed through the skin. Molecular weight is a significant determinant of these compounds' absorption rates. Smaller glycol ethers more easily penetrate the skin than larger molecules, with a lag time of 1.5 to 2 hours observed in human experiments.[45]
Other compounds can impact glycol ethers' dermal absorption. A study showed that an aqueous mixture of butoxyethanol was absorbed 6 times more efficiently than a pure glycol ether solvent, with the apparent permeability coefficient increasing as the glycol ether concentration in water decreased.[46] This absorption mechanism is thought to arise from glycol ethers' ability to induce stratum corneum lipid bilayer changes. Glycol ethers undergo cellular metabolism by cytosolic aldehyde dehydrogenase and alcohol dehydrogenase after absorption. These enzymes' concentrations vary in different body sites. Additional research is required to understand the dermatological toxicity of glycol ethers.
Toxicokinetics
Glycol ethers enter the body by ingestion, inhalation, or dermal exposure.[47][48][49] Ingestion is the most toxic route, while inhalation is the least harmful. Glycol ethers are swiftly absorbed following ingestion and have a large distribution volume. Glycol ethers are metabolized via alcohol dehydrogenase, producing toxic metabolites.
Similarly, glycol ethers are eliminated through urinary excretion, contrasting with methanol, which undergoes hepatic elimination.[50][51] Human studies indicate that EGME inhalation results in the excretion of unchanged alkoxyacetic acid in urine shortly after exposure. Animal research suggests that elimination occurs via the organic acid transport mechanism. The half-life of glycol ethers typically ranges from 1 to 24 hours, and variations occur between glycol ethers and their respective organic acids. For instance, ethylene glycol monobutyl ether has a half-life of approximately 1 hour, while its resulting organic acid, butoxyacetic acid, has a half-life of about 4 hours.[52][53]
History and Physical
History
Patients may present to the emergency room reporting accidental or nonaccidental exposure to a glycol ether-containing product. For example, some may disclose having ingested brake fluid or antifreeze. Symptoms vary in severity. Many exposures do not lead to significant illness, leaving most patients asymptomatic. Fatal cases are rare and typically result from significant ingestion. However, chronically exposed individuals may consult in the outpatient setting for insidiously developing symptoms or an incidental abnormal laboratory finding.
Acute glycol ether toxicity may present with gastrointestinal and acute kidney injury symptoms, such as nausea, emesis, decreased urine output, shortness of breath, and abdominal pain. Seizures, headaches, altered sensorium, agitation, weakness, chest pain, and upper gastrointestinal bleeding may also be reported. A history of ingesting large amounts of glycol ether is often elicited.[89] Acute hemolysis may also arise, manifesting as weakness, pallor, or syncope.
Patients initially with oliguria due to kidney damage and dehydration may later develop osmotic diuresis. This paradoxical manifestation may arise due to the accumulation of osmotically active substances in the renal tubules. Consequently, water reabsorption is inhibited and urine output increases despite ongoing renal injury.
A detailed past medical and social history is essential in elucidating glycol ether toxicity. Patients with acute exposures may have recent emotional trauma or belong to a home with safety deficiencies. Inquiring about using commonplace household items such as surface cleaner, all-purpose cleaner, window cleaner, paint, laundry detergents, and cosmetics is also essential. Remember that glycol ether poisoning may resemble toxicity from alcohols such as methanol and exacerbated medical conditions such as diabetic ketoacidosis. Obtaining a psychiatric history is also important because reports of intentional ingestion exist with suicide attempts. Patients with severe presentations may not provide history, highlighting the importance of collateral information from emergency medical services.
Occupational history is crucial in patients with chronic toxicity. Workers in various industries, such as electronics, printing, paint and coating, textiles, adhesives, cleaning, and personal care products may have been inadvertently exposed.[53][54] Additionally, workers in industries that use glycol ether solvents or as product components, such as laboratory technicians, janitorials, and construction workers, may also be at risk.[55][56]
Physical Examination
The physical examination of a patient who has ingested glycol ethers can vary based on several factors, including the amount consumed, the time since ingestion, and individual patient characteristics. As a result, examination findings can be classified into 3 categories based on disease onset and duration: acute, subacute, or chronic. Some features of these conditions may overlap.
Findings consistent with acute toxicity
Patients who ingested a large amount may be visibly agitated, confused, or unconscious, with symptoms developing within hours.[57] Metabolic acidosis from acute kidney injury may manifest as tachypnea or hyperventilation.[58] Heart blocks, refractory shock, and various metabolic derangements have been reported in some individuals.[59] Patients may have signs of severe dehydration from vomiting, such as dry oral mucosa, sunken eyeballs, tachycardia, and poor capillary refill.
Findings consistent with subacute toxicity
Neurological signs predominate after 5 to 10 days, including progressive lethargy, bilateral facial paralysis, dysphonia, and nonreactive pupils. Peripheral neuropathies impact specific nerve groups, including the 3rd, 5th, 6th, and 9th through 12th cranial nerves. Focal central nervous system involvement has been described bilaterally in the left partial lobe and occipital and cerebellar regions.[61] Localized skin erythema, dryness, and, in some cases, allergic reactions are possible, depending on the duration and frequency of exposure.[62]
Findings consistent with chronic toxicity
Chronic toxicity, occurring at least 2 weeks after initial exposure, may present with central and peripheral nervous system impairment and can progress to coma. Impaired respiratory function is possible, although the spectrum of long-term neurological injury is variable. Repetitive chronic exposure can also cause weakness, drowsiness, anisocoria (irregular pupils), and hypersomnolence. The resulting pancytopenia can present as a myriad of symptoms related to anemia and immunodeficiency. However, little research exists regarding the long-term sequelae of bone marrow suppression from glycol ether toxicity. Patients may also report a longstanding history of infertility, decreased testicular size, spontaneous abortions, and congenital malformations in the offspring.
Evaluation
Laboratory Tests
Basic laboratory tests, such as a complete metabolic panel and blood count, are recommended to assess for various electrolyte derangements and anemia. Hypocalcemia may be noted in the case of ethylene glycol toxicity. Elevated blood urea nitrogen (BUN), potassium, and creatinine are consistent with renal failure.
Respiratory distress signs like tachypnea warrant an arterial blood gas (ABG) analysis. This test provides information about acid-base balance, oxygenation status, and respiratory function. In cases of glycol ether toxicity, ABG can help assess the severity of metabolic acidosis and respiratory compensation. ABG results can guide treatment decisions, such as the need for respiratory support acid-base imbalance correction.
Metabolic acidosis in the setting of lactic acidosis is often accompanied by a high anion gap, calculated according to the following equation:
Anion Gap = ([Sodium concentration] + [Potassium concentration]) - ([Chloride concentration] + [Bicarbonate concentration])
In glycol ether toxicity cases, an elevated anion gap may be observed due to the accumulation of toxic metabolites, such as glycolic acid or oxalic acid, which are not routinely measured in standard laboratory tests but contribute to the blood's overall anion load.
The osmolality gap is the difference between the measured and calculated serum osmolality. This parameter provides an estimate of unmeasured osmotically active substances in the blood. The calculated serum osmolality is based on the concentrations of sodium, glucose, and BUN. An elevated osmolality gap may indicate the presence of substances not typically measured in standard laboratory tests, such as glycol ethers or their metabolites. Osmotic diuresis is likely accompanied by a high osmolality gap and a low anion gap.[54]
However, recognizing the limitations of utilizing the osmolality gap is important when evaluating glycol ether poisoning. Clinicians should be vigilant of overtly elevated osmolality gaps (ie, >30 mOsl/L) in the presence of altered mental status or other signs suggesting toxic ingestion.
Patients who present early may have a normal anion gap and elevated osmolality gap, as the parent compound has not transformed into its respective organic acid. On the other hand, patients who present late have had time to metabolize the glycol ether, which may produce a high anion gap. Metabolic conversion of the parent compound lowers its quantity, as seen in late-presenting patients. A normal osmolarity gap may be observed in such individuals.
Lactate levels are measured to assess tissue perfusion and determine lactate's contribution to metabolic acidosis. Elevated lactate levels may indicate tissue hypoperfusion and severe toxicity.
Coingestions of other toxic substances may occur with glycol ether exposure. Screening for common coingestants such as acetaminophen, ethanol, methanol, and salicylates helps identify additional toxicities that may require specific interventions or treatments.
Urinalysis is performed to assess renal function and detect any abnormalities such as hematuria, proteinuria, or crystalluria that may indicate kidney injury or dysfunction secondary to glycol ether toxicity. Urinalysis findings can also help monitor the progression of renal impairment and guide treatment decisions.
Time-consuming metabolite testing of organic acids is of little utility, especially in the acute setting. Many hospitals do not routinely test for metabolites.[55][56]
Electrocardiography
Electrocardiography is also essential for detecting conduction abnormalities, which can be a byproduct of various electrolyte derangements and acidosis. ECG findings should be interpreted in conjunction with the patient's clinical presentation, laboratory results, and overall clinical context to guide diagnosis and management.
Imaging
A computed tomography (CT) scan of the head may help rule out other causes of altered mental status. However, this modality may be of low value in assessing neurological damage due to the systemic nature of the primary etiology. Meanwhile, the utility of obtaining magnetic resonance imaging (MRI) is unclear, as evidence of neurologic injury may not be evident in this study.
A case series by Yahi et al reported that initial head CT scans in patients with glycol ether toxicity were without abnormality. Two patients initially had unrevealing MRIs, but a third demonstrated edema and herniation. These initial MRI findings may hold a poor prognosis. Other reports showed regular MRIs in patients with fatal outcomes. Yahi et al also mention brain atrophy in 2 of their surviving cases but with typical MRIs, questioning the true etiology of these findings. Brain imaging to evaluate glycol ether toxicity requires further investigation.
Chest imaging may also be considered to evaluate for pneumonitis from exposure to glycol ether vapor or aspiration in the setting of altered mental status. An abdominal ultrasound may help identify changes in size, texture, and even necrosis in the kidney and liver.
Electroencephalogram and Electromyography
Nerve conduction studies may also be beneficial in evaluating neuropathy. A case study reported that most patients with glycol ether exposure and elevated CSF protein, without pleocytosis, developed a neurological injury. An electroencephalogram may be helpful in patients with repeated seizures, although limited reports of this initial presentation exist.[57]
Treatment / Management
The initial treatment for glycol ether toxicity involves addressing the patient's airway, breathing, circulation, disability, and exposure. After the primary survey, additional therapies depend on the presentation's severity, often determined by which glycol ether was ingested, the route, and the time elapsed since ingestion. Glycol ether exerts toxicity primarily through its alkoxyacetic acid metabolite produced by alcohol dehydrogenase. Treatment aims to inhibit the production of the toxic metabolite and subsequent removal of the parent compound.[58] Fomepizole, an alcohol dehydrogenase inhibitor, is administered, and the parent compound is urgently eliminated via extracorporeal enhanced elimination by hemodialysis.[59] (B3)
Fomepizole monotherapy may be considered if the toxic compound has a short elimination half-life (ie, less than the half-life of fomepizole) in a patient with normal renal function. More importantly, these treatment strategies would benefit most during the early toxicity stages. As such, early suspicion of glycol ether toxicity is paramount. Note that fomepizole carries a half-life of about 12 hours and is therefore started at 15 mg/kg every 12 hours.[60][61] Additional adjustments may be required per hemodialysis and other patient-specific factors, such as a history of chronic kidney disease. The dialysability of a toxin primarily depends on its free plasma concentration, size, weight, and volume of distribution.[62] Small, unbound toxins with a low distribution volume are readily removed by dialysis.[63][64] Therefore, patients with toxicity to glycol ether exposure would benefit from hemodialysis. (A1)
Propylene glycol, a secondary alcohol, is structurally similar to glycol ethers but is generally less toxic. Both alcohols are metabolized by alcohol dehydrogenase and produce toxic metabolites. However, alcohol dehydrogenase does not readily metabolize propylene glycol because this glycol's hydroxyl group is bound to a secondary carbon. Nonetheless, propylene glycol toxicity management follows the same principle for glycol ether toxicity: inhibition of alcohol dehydrogenase with fomepizole and toxic alcohol removal with hemodialysis.[65][66]
Electrolyte management may also be beneficial, particularly in hyperkalemia and severe metabolic acidosis. Intubation and mechanical ventilation may become necessary if the patient has severe respiratory distress or no longer protecting their airway due to a depressed sensorium. Blood transfusions may also be needed if hemolysis results in severe anemia.
The use of gastric decontamination has not been closely studied. Some authors suggest gastric lavage or nasogastric decontamination, but the benefits of these interventions within the first 1 to 2 hours of ingestion have not been studied. Obtaining an accurate history is another limitation of using gastric lavage. Moreover, as neurotoxicity is a common finding, gastric decontamination may increase the risk of pulmonary aspiration.
Activated charcoal is also not recommended, as the substance has a poor affinity for alcohol.[67] No precise dedicated study suggests using N-acetylcysteine (NAC) to treat glycol ether-induced hepatic injury. However, given that the hepatic injury is mediated by oxidative stress, NAC may provide some utility in theory.[68] Animal studies suggest that NAC prevents genetic damage in testicular germ cells induced by 2-methylacetic acid, an organic acid that also mediates the disease process in glycol ether toxicity.[68](B3)
Differential Diagnosis
The differential diagnosis of glycol ether toxicity includes ingesting similar toxic alcohols such as methanol and isopropyl alcohol. Toxic ingestions of these compounds produce metabolic acidosis similar to glycol ether toxicity. However, the lethal dose and toxicokinetics differ. Drug overdoses may also present with neurological and metabolic derangements.
Toxicity and Adverse Effect Management
Managing glycol ether toxicity depends on factors such as ingested amount, time since exposure, and coingestions. Additionally, underlying comorbid conditions, such as chronic renal failure, should be considered when evaluating treatment options.
Prognosis
A favorable prognosis is associated with early hospitalization. Respiratory compensation offsetting metabolic acidosis also correlates with a better prognosis. Key findings associated with a poor prognosis include severe neurological damage (eg, coma), respiratory failure, profound metabolic arrangements requiring dialysis, acidosis with a pH of less than 7, and delayed hospital evaluation.[69][70] Ethylene glycol toxicity fatalities have been reported to follow acute renal failure requiring hemodialysis.[71]
Complications
Glycol ether toxicity complications primarily affect the neurologic and renal systems. Seizures and evidence of cranial palsies and structural brain changes have been documented.[72] Clinical case reports and epidemic studies, such as the Panama mass poisoning incident, indicate that patients may experience axonal sensorimotor neuropathy, peripheral neuropathy, cranial nerve neuropathies, and demyelination. Some complications may occur in the acute to subacute stages, while others may develop months after the initial presentation in patients who survive the acute poisoning. However, limited long-term longitudinal studies are available.
Severe hypocalcemia and acidosis may result in cardiac dysrhythmias and acute renal failure, which may require dialysis. Electrolyte imbalances, pancreatitis, and acidemia are expected acute complications likely related to renal failure, encephalopathy, and peripheral neuropathies. Additionally, a few isolated case reports suggest that significant acute glycol ether ingestion can cause pulmonary edema.[73] However, further research is needed to determine whether the glycol ether or the overall critical presentation causes the respiratory insult.
In patients who survive glycol ether toxicity, the long-term complications can vary widely. For example, mild kidney injury may resolve, but those who require hemodialysis generally remain dialysis-dependent.[74][75][76] Patients may also experience neurological complications, such as neurocognitive changes or quadriplegia, due to oxidative stress and demyelinating injury. A higher risk of developing neurological sequelae has been observed in renal failure patients. The variability of these complications is likely related to the patient's initial presentation.
Oxidative stress caused by glycol ether metabolites can damage the liver, although the long-term hepatic effects have not been studied. Evidence is insufficient to support that glycol toxicity increases malignancy risk. Studies on glycol ether exposure and the risk of developing acute myeloid leukemia do not show statistically significant results. Furthermore, no concrete evidence proves that glycol ether toxicity causes genotoxic changes.[77] Some research indicates that in-utero exposure to glycol ether may reduce telomere length and increase chromatid exchange, but further research is needed to investigate genotoxicity.[78]
Consultations
Consulting with the nearest poison control center and, if available, the institutional medical toxicologist is highly recommended. Specialists such as nephrologists, neurologists, and intensivists may be needed to manage complications such as renal and respiratory failure, seizures, and hyperkalemia.
Deterrence and Patient Education
Household supplies such as cleaning, paint, and automotive liquids should be kept safe and secure, especially from young children, to prevent accidental pediatric ingestions.[58][66] The reduction of occupational exposure to glycol ethers requires the implementation of both respiratory and dermal protection strategies. Engineering controls, such as using exhaust ventilation in small closed spaces, are key examples. Personal protective equipment, such as gloves, respiratory masks, facial shields, and protective clothing, may be necessary to prevent dermal exposure. The choice of personal protective equipment is determined by the specific glycol ether used and the potential exposure.
Pearls and Other Issues
The following are the most important aspects to remember about glycol ether poisoning evaluation and management:
- Upon exposure, glycol ethers are metabolized to their respective alkoxyacetic acids, which orchestrate systemic toxicity.
- Alcohol dehydrogenase is the primary means of metabolism, and excretion is primarily by the kidneys.
- Although the half-life of glycol ethers is relatively short, evaluating for coingestions that can prolong clearance through competitive enzyme inhibition is paramount.
- Acute toxicity is generally mild to moderate and can result in skin irritation, metabolic derangements, central nervous system depression, hemolytic anemia, and spermatogenesis defects. Fatal cases are rare and are likely the result of significant ingestion.
- Chronic toxicity can result in hematological side effects (eg, anemia, bone marrow suppression), hepatic histologic changes, and reproductive and developmental damage.
- Managing glycol ether toxicity is similar to toxic alcohol ingestions and begins with assessing the airway, breathing, and circulation, followed by supportive measures.
- The 2 primary avenues for treatment are fomepizole and hemodialysis for managing acute toxicity, which may provide the most benefit if given early.
Patients with glycol ether toxicity may require long-term follow-up for monitoring of renal function, neurological sequelae, and potential development of chronic complications. Close collaboration with toxicology specialists and nephrologists can optimize patient care and outcomes.
Enhancing Healthcare Team Outcomes
Early diagnosis of glycol ether ingestion to prevent further toxic metabolite production is critical. Assessing and managing toxic ingestions requires an interprofessional approach that includes clinicians, specialists, nursing staff, and pharmacists. An institutional medical toxicologist and a local poison control center must be consulted, if available. Recommendations by a medical toxicologist and nephrologist may be necessary for managing metabolic acidosis and renal failure through hemodialysis. An intensivist may be involved in the care of patients requiring intubation, mechanical ventilation, and hemodynamic support.
Interprofessional care coordination between clinicians and specialists, nursing staff, and pharmacists is necessary. The nursing staff administers medication and monitors patient responses. Pharmacists can perform medication reconciliation, verify dosing, and assist in patient drug therapy counseling. Management of long-term side effects such as permanent disability or reproductive defects requires consultations with primary care physicians, neurologists, urologists, and gynecologists. The interprofessional care paradigm is crucial to optimal patient outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kelsey JR, Seidel S. Propylene oxide derived glycol ethers: A review of the alkyl glycol ethers potential to cause endocrine disruption. Regulatory toxicology and pharmacology : RTP. 2023 Jun 30:():105442. doi: 10.1016/j.yrtph.2023.105442. Epub 2023 Jun 30 [PubMed PMID: 37394030]
Kelsey JR. Ethylene oxide derived glycol ethers: A review of the alkyl glycol ethers potential to cause endocrine disruption. Regulatory toxicology and pharmacology : RTP. 2022 Mar:129():105113. doi: 10.1016/j.yrtph.2021.105113. Epub 2021 Dec 30 [PubMed PMID: 34974128]
Browning RG, Curry SC. Clinical toxicology of ethylene glycol monoalkyl ethers. Human & experimental toxicology. 1994 May:13(5):325-35 [PubMed PMID: 8043314]
Level 3 (low-level) evidenceKirtana A, Seetharaman B. Comprehending the Role of Endocrine Disruptors in Inducing Epigenetic Toxicity. Endocrine, metabolic & immune disorders drug targets. 2022:22(11):1059-1072. doi: 10.2174/1871530322666220411082656. Epub [PubMed PMID: 35410624]
Beattie PJ, Brabec MJ. Methoxyacetic acid and ethoxyacetic acid inhibit mitochondrial function in vitro. Journal of biochemical toxicology. 1986 Sep:1(3):61-70 [PubMed PMID: 3271880]
Chapin RE, Lamb JC 4th. Effects of ethylene glycol monomethyl ether on various parameters of testicular function in the F344 rat. Environmental health perspectives. 1984 Aug:57():219-24 [PubMed PMID: 6541998]
Level 3 (low-level) evidenceNagano K, Nakayama E, Koyano M, Oobayashi H, Adachi H, Yamada T. [Testicular atrophy of mice induced by ethylene glycol mono alkyl ethers (author's transl)]. Sangyo igaku. Japanese journal of industrial health. 1979 Jan:21(1):29-35 [PubMed PMID: 470211]
Grant D, Sulsh S, Jones HB, Gangolli SD, Butler WH. Acute toxicity and recovery in the hemopoietic system of rats after treatment with ethylene glycol monomethyl and monobutyl ethers. Toxicology and applied pharmacology. 1985 Feb:77(2):187-200 [PubMed PMID: 3975897]
Kalf GF, Post GB, Snyder R. Solvent toxicology: recent advances in the toxicology of benzene, the glycol ethers, and carbon tetrachloride. Annual review of pharmacology and toxicology. 1987:27():399-427 [PubMed PMID: 3555320]
Level 3 (low-level) evidenceRambourg-Schepens MO, Buffet M, Bertault R, Jaussaud M, Journe B, Fay R, Lamiable D. Severe ethylene glycol butyl ether poisoning. Kinetics and metabolic pattern. Human toxicology. 1988 Mar:7(2):187-9 [PubMed PMID: 3378807]
Level 3 (low-level) evidenceWelsch F. The mechanism of ethylene glycol ether reproductive and developmental toxicity and evidence for adverse effects in humans. Toxicology letters. 2005 Mar 28:156(1):13-28 [PubMed PMID: 15705484]
Level 3 (low-level) evidenceJohanson G. Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Critical reviews in toxicology. 2000 May:30(3):307-45 [PubMed PMID: 10852499]
Level 3 (low-level) evidenceWess JA. Reproductive toxicity of ethylene glycol monomethyl ether, ethylene glycol monoethyl ether and their acetates. Scandinavian journal of work, environment & health. 1992:18 Suppl 2():43-5 [PubMed PMID: 1514082]
Level 3 (low-level) evidenceSpencer PJ. New toxicity data for the propylene glycol ethers - a commitment to public health and safety. Toxicology letters. 2005 Mar 28:156(1):181-8 [PubMed PMID: 15705495]
Level 3 (low-level) evidenceZar T, Yusufzai I, Sullivan A, Graeber C. Acute kidney injury, hyperosmolality and metabolic acidosis associated with lorazepam. Nature clinical practice. Nephrology. 2007 Sep:3(9):515-20 [PubMed PMID: 17717564]
Level 3 (low-level) evidenceSchep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clinical toxicology (Philadelphia, Pa.). 2009 Jul:47(6):525-35. doi: 10.1080/15563650903086444. Epub [PubMed PMID: 19586352]
Level 3 (low-level) evidenceCicolella A. Glycol ethers: a ubiquitous family of toxic chemicals: a plea for REACH regulation. Annals of the New York Academy of Sciences. 2006 Sep:1076():784-9 [PubMed PMID: 17119255]
Level 3 (low-level) evidenceLockley DJ, Howes D, Williams FM. Cutaneous metabolism of glycol ethers. Archives of toxicology. 2005 Mar:79(3):160-8 [PubMed PMID: 15551062]
Level 3 (low-level) evidenceVecer J, Kubátová H, Charvát J, Soucek M, Kvapil M. [Ethylene glycol poisoning]. Casopis lekaru ceskych. 2001 Jun 21:140(12):381-2 [PubMed PMID: 11503188]
Level 3 (low-level) evidenceHanley TR Jr,Young JT,John JA,Rao KS, Ethylene glycol monomethyl ether (EGME) and propylene glycol monomethyl ether (PGME): inhalation fertility and teratogenicity studies in rats, mice and rabbits. Environmental health perspectives. 1984 Aug; [PubMed PMID: 6499821]
Level 3 (low-level) evidenceCordier S, Garlantézec R, Labat L, Rouget F, Monfort C, Bonvallot N, Roig B, Pulkkinen J, Chevrier C, Multigner L. Exposure during pregnancy to glycol ethers and chlorinated solvents and the risk of congenital malformations. Epidemiology (Cambridge, Mass.). 2012 Nov:23(6):806-12. doi: 10.1097/EDE.0b013e31826c2bd8. Epub [PubMed PMID: 23007043]
Chevrier C, Dananché B, Bahuau M, Nelva A, Herman C, Francannet C, Robert-Gnansia E, Cordier S. Occupational exposure to organic solvent mixtures during pregnancy and the risk of non-syndromic oral clefts. Occupational and environmental medicine. 2006 Sep:63(9):617-23 [PubMed PMID: 16644895]
Correa A, Gray RH, Cohen R, Rothman N, Shah F, Seacat H, Corn M. Ethylene glycol ethers and risks of spontaneous abortion and subfertility. American journal of epidemiology. 1996 Apr 1:143(7):707-17 [PubMed PMID: 8651233]
Level 2 (mid-level) evidenceCordier S, Bergeret A, Goujard J, Ha MC, Aymé S, Bianchi F, Calzolari E, De Walle HE, Knill-Jones R, Candela S, Dale I, Dananché B, de Vigan C, Fevotte J, Kiel G, Mandereau L. Congenital malformation and maternal occupational exposure to glycol ethers. Occupational Exposure and Congenital Malformations Working Group. Epidemiology (Cambridge, Mass.). 1997 Jul:8(4):355-63 [PubMed PMID: 9209847]
Level 2 (mid-level) evidenceBagchi G, Waxman DJ. Toxicity of ethylene glycol monomethyl ether: impact on testicular gene expression. International journal of andrology. 2008 Apr:31(2):269-74. doi: 10.1111/j.1365-2605.2007.00846.x. Epub 2007 Dec 30 [PubMed PMID: 18179559]
Level 3 (low-level) evidenceSnow JE. Occupational exposure to glycol ethers: implications for occupational health nurses. AAOHN journal : official journal of the American Association of Occupational Health Nurses. 1994 Sep:42(9):413-9 [PubMed PMID: 7945591]
Garlantézec R, Warembourg C, Le Gléau F, Montfort C, Rouget F, Multigner L, Cordier S, Chevrier C. Exposure to glycol ethers among 6-year-old children in France. International journal of hygiene and environmental health. 2020 Jun:227():113510. doi: 10.1016/j.ijheh.2020.113510. Epub 2020 Mar 12 [PubMed PMID: 32172156]
Gijsenbergh FP,Jenco M,Veulemans H,Groeseneken D,Verberckmoes R,Delooz HH, Acute butylglycol intoxication: a case report. Human toxicology. 1989 May; [PubMed PMID: 2744782]
Level 3 (low-level) evidenceDean BS, Krenzelok EP. Clinical evaluation of pediatric ethylene glycol monobutyl ether poisonings. Journal of toxicology. Clinical toxicology. 1992:30(4):557-63 [PubMed PMID: 1359160]
Pomierny B, Starek A, Krzyżanowska W, Starek-Swiechowicz B, Smaga I, Pomierny-Chamioło L, Regulska M, Budziszewska B. Potential neurotoxic effect of ethylene glycol ethers mixtures. Pharmacological reports : PR. 2013:65(5):1415-21 [PubMed PMID: 24399739]
ZAVON MR. Methyl Cellosolve intoxication. American Industrial Hygiene Association journal. 1963 Jan-Feb:24():36-41 [PubMed PMID: 14003323]
Nitter-Hauge S. Poisoning with ethylene glycol monomethyl ether. Report of two cases. Acta medica Scandinavica. 1970 Oct:188(4):277-80 [PubMed PMID: 5479659]
Level 3 (low-level) evidenceOhi G, Wegman DH. Transcutaneous ethylene glycol monomethyl ether poisoning in the work setting. Journal of occupational medicine. : official publication of the Industrial Medical Association. 1978 Oct:20(10):675-6 [PubMed PMID: 722353]
Laitinen J, Liesivuori J, Savolainen H. Urinary alkoxyacetic acids and renal effects of exposure to ethylene glycol ethers. Occupational and environmental medicine. 1996 Sep:53(9):595-600 [PubMed PMID: 8882116]
Starek-Świechowicz B, Starek A. [Ethylene glycol and propylene glycol ethers - Reproductive and developmental toxicity]. Medycyna pracy. 2015:66(5):725-37. doi: 10.13075/mp.5893.00219. Epub [PubMed PMID: 26647990]
Kawamoto T, Matsuno K, Kayama F, Hirai M, Arashidani K, Yoshikawa M, Kodama Y. Effect of ethylene glycol monomethyl ether and diethylene glycol monomethyl ether on hepatic metabolizing enzymes. Toxicology. 1990 Jun:62(3):265-74 [PubMed PMID: 2389243]
Level 3 (low-level) evidenceMing Xing Huang, Xiao Mou Peng, Lin Gu, Gui Hua Chen. Pre-existing liver cirrhosis reduced the toxic effect of diethylene glycol in a rat model due to the impaired hepatic alcohol dehydrogenase. Toxicology and industrial health. 2011 Sep:27(8):742-53. doi: 10.1177/0748233710397417. Epub 2011 May 4 [PubMed PMID: 21543465]
Level 3 (low-level) evidencePaustenbach DJ. Assessment of the developmental risks resulting from occupational exposure to select glycol ethers within the semiconductor industry. Journal of toxicology and environmental health. 1988:23(1):29-75 [PubMed PMID: 3275786]
Cordier S, Garlantézec R, Bonvallot N, Multigner L. Glycol ethers and congenital malformations. Epidemiology (Cambridge, Mass.). 2013 Nov:24(6):940. doi: 10.1097/EDE.0b013e3182a79ac7. Epub [PubMed PMID: 24077003]
Terry KK, Stedman DB, Bolon B, Welsch F. Effects of 2-methoxyethanol on mouse neurulation. Teratology. 1996 Nov:54(5):219-29 [PubMed PMID: 9035343]
Starek A, Szymczak W, Zapor L. Hematological effects of four ethylene glycol monoalkyl ethers in short-term repeated exposure in rats. Archives of toxicology. 2008 Feb:82(2):125-36 [PubMed PMID: 17874071]
Level 3 (low-level) evidenceStarek-Świechowicz B, Miranowicz-Dzierżawska K, Szymczak W, Budziszewska B, Starek A. Hematological effects of exposure to mixtures of selected ethylene glycol alkyl ethers in rats. Pharmacological reports : PR. 2012:64(1):166-78 [PubMed PMID: 22580533]
Level 3 (low-level) evidenceSmialowicz RJ, Riddle MM, Luebke RW, Copeland CB, Andrews D, Rogers RR, Gray LE, Laskey JW. Immunotoxicity of 2-methoxyethanol following oral administration in Fischer 344 rats. Toxicology and applied pharmacology. 1991 Jul:109(3):494-506 [PubMed PMID: 1853347]
Level 3 (low-level) evidenceRiddle MM, Williams WC, Smialowicz RJ. Repeated high dose oral exposure or continuous subcutaneous infusion of 2-methoxyacetic acid does not suppress humoral immunity in the mouse. Toxicology. 1996 May 3:109(1):67-74 [PubMed PMID: 8619254]
Level 3 (low-level) evidenceVenier M, Adami G, Larese F, Maina G, Renzi N. Percutaneous absorption of 5 glycol ethers through human skin in vitro. Toxicology in vitro : an international journal published in association with BIBRA. 2004 Oct:18(5):665-71 [PubMed PMID: 15251185]
Traynor MJ, Wilkinson SC, Williams FM. The influence of water mixtures on the dermal absorption of glycol ethers. Toxicology and applied pharmacology. 2007 Jan 15:218(2):128-34 [PubMed PMID: 17173944]
Level 3 (low-level) evidenceLEAF G, ZATMAN LJ. A study of the conditions under which methanol may exert a toxic hazard in industry. British journal of industrial medicine. 1952 Jan:9(1):19-31 [PubMed PMID: 14895799]
Frantz SW, Beskitt JL, Grosse CM, Tallant MJ, Dietz FK, Ballantyne B. Pharmacokinetics of ethylene glycol. I. Plasma disposition after single intravenous, peroral, or percutaneous doses in female Sprague-Dawley rats and CD-1 mice. Drug metabolism and disposition: the biological fate of chemicals. 1996 Aug:24(8):911-21 [PubMed PMID: 8869828]
Level 3 (low-level) evidenceDriver J, Tardiff RG, Sedik L, Wester RC, Maibach HI. In vitro percutaneous absorption of [14C] ethylene glycol. Journal of exposure analysis and environmental epidemiology. 1993 Jul-Sep:3(3):277-84 [PubMed PMID: 8260837]
Cheng JT, Beysolow TD, Kaul B, Weisman R, Feinfeld DA. Clearance of ethylene glycol by kidneys and hemodialysis. Journal of toxicology. Clinical toxicology. 1987:25(1-2):95-108 [PubMed PMID: 3035205]
Level 3 (low-level) evidenceHovda KE, Andersson KS, Urdal P, Jacobsen D. Methanol and formate kinetics during treatment with fomepizole. Clinical toxicology (Philadelphia, Pa.). 2005:43(4):221-7 [PubMed PMID: 16035197]
Groeseneken D, Veulemans H, Masschelein R, Van Vlem E. Experimental human exposure to ethylene glycol monomethyl ether. International archives of occupational and environmental health. 1989:61(4):243-7 [PubMed PMID: 2722247]
Deisinger PJ, Guest D. Metabolic studies with diethylene glycol monobutyl ether acetate (DGBA) in the rat. Xenobiotica; the fate of foreign compounds in biological systems. 1989 Sep:19(9):981-9 [PubMed PMID: 2815838]
Level 3 (low-level) evidenceBesenhofer LM, Adegboyega PA, Bartels M, Filary MJ, Perala AW, McLaren MC, McMartin KE. Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2010 Sep:117(1):25-35. doi: 10.1093/toxsci/kfq167. Epub 2010 Jun 7 [PubMed PMID: 20530232]
Level 3 (low-level) evidenceBlomme B, Lheureux P, Gerlo E, Maes V. Cobas Mira S endpoint enzymatic assay for plasma formate. Journal of analytical toxicology. 2001 Mar:25(2):77-80 [PubMed PMID: 11300510]
Hanton SL, Watson ID. An enzymatic assay for the detection of glycolic acid in serum as a marker of ethylene glycol poisoning. Therapeutic drug monitoring. 2013 Dec:35(6):836-43. doi: 10.1097/FTD.0b013e31828f019c. Epub [PubMed PMID: 24263643]
Imam YZB, Kamran S, Karim H, Elalamy O, Sokrab T, Osman Y, Deleu D. Neurological manifestation of recreational fatal and near-fatal diethylene glycol poisonings: case series and review of literature. Medicine. 2014 Aug:93(10):e62. doi: 10.1097/MD.0000000000000062. Epub [PubMed PMID: 25170933]
Level 3 (low-level) evidenceWang GS, Yin S, Shear B, Heard K. Severe poisoning after accidental pediatric ingestion of glycol ethers. Pediatrics. 2012 Oct:130(4):e1026-9. doi: 10.1542/peds.2011-3849. Epub 2012 Sep 24 [PubMed PMID: 23008459]
Level 3 (low-level) evidenceBurkhart KK, Donovan JW. Hemodialysis following butoxyethanol ingestion. Journal of toxicology. Clinical toxicology. 1998:36(7):723-5 [PubMed PMID: 9865242]
Level 3 (low-level) evidenceDrick N, Schmidt JJ, Wiesner O, Kielstein JT. [Fomepizole, ethanol or dialysis in the case of life-threatening ethylenglycol intoxication?]. Medizinische Klinik, Intensivmedizin und Notfallmedizin. 2021 Nov:116(8):698-701. doi: 10.1007/s00063-020-00718-8. Epub 2020 Aug 20 [PubMed PMID: 32820350]
Level 3 (low-level) evidenceSivilotti ML, Burns MJ, McMartin KE, Brent J. Toxicokinetics of ethylene glycol during fomepizole therapy: implications for management. For the Methylpyrazole for Toxic Alcohols Study Group. Annals of emergency medicine. 2000 Aug:36(2):114-25 [PubMed PMID: 10918102]
Ashby D, Borman N, Burton J, Corbett R, Davenport A, Farrington K, Flowers K, Fotheringham J, Andrea Fox RN, Franklin G, Gardiner C, Martin Gerrish RN, Greenwood S, Hothi D, Khares A, Koufaki P, Levy J, Lindley E, Macdonald J, Mafrici B, Mooney A, Tattersall J, Tyerman K, Villar E, Wilkie M. Renal Association Clinical Practice Guideline on Haemodialysis. BMC nephrology. 2019 Oct 17:20(1):379. doi: 10.1186/s12882-019-1527-3. Epub 2019 Oct 17 [PubMed PMID: 31623578]
Level 1 (high-level) evidencePatzer J. Principles of bound solute dialysis. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2006 Apr:10(2):118-24 [PubMed PMID: 16684212]
Sternby J. Significance of distribution volume in dialysis quantification. Seminars in dialysis. 2001 Jul-Aug:14(4):278-83 [PubMed PMID: 11489203]
Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clinical journal of the American Society of Nephrology : CJASN. 2008 Jan:3(1):208-25 [PubMed PMID: 18045860]
Brent J. Fomepizole for the treatment of pediatric ethylene and diethylene glycol, butoxyethanol, and methanol poisonings. Clinical toxicology (Philadelphia, Pa.). 2010 Jun:48(5):401-6. doi: 10.3109/15563650.2010.495347. Epub [PubMed PMID: 20586570]
Neuvonen PJ, Olkkola KT. Oral activated charcoal in the treatment of intoxications. Role of single and repeated doses. Medical toxicology and adverse drug experience. 1988 Jan-Dec:3(1):33-58 [PubMed PMID: 3285126]
Rao AV, Shaha C. N-acetylcysteine prevents MAA induced male germ cell apoptosis: role of glutathione and cytochrome c. FEBS letters. 2002 Sep 11:527(1-3):133-7 [PubMed PMID: 12220648]
Level 3 (low-level) evidenceHassanian-Moghaddam H, Pajoumand A, Dadgar SM, Shadnia Sh. Prognostic factors in methanol poisoning. Human & experimental toxicology. 2007 Jul:26(7):583-6 [PubMed PMID: 17884962]
Level 2 (mid-level) evidenceBENNETT IL Jr, CARY FH, MITCHELL GL Jr, COOPER MN. Acute methyl alcohol poisoning: a review based on experiences in an outbreak of 323 cases. Medicine. 1953 Dec:32(4):431-63 [PubMed PMID: 13110604]
Level 3 (low-level) evidenceKarlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. Journal of toxicology. Clinical toxicology. 1992:30(4):565-74 [PubMed PMID: 1433427]
Hasbani MJ, Sansing LH, Perrone J, Asbury AK, Bird SJ. Encephalopathy and peripheral neuropathy following diethylene glycol ingestion. Neurology. 2005 Apr 12:64(7):1273-5 [PubMed PMID: 15824363]
Level 3 (low-level) evidenceBauer P, Weber M, Mur JM, Protois JC, Bollaert PE, Condi A, Larcan A, Lambert H. Transient non-cardiogenic pulmonary edema following massive ingestion of ethylene glycol butyl ether. Intensive care medicine. 1992:18(4):250-1 [PubMed PMID: 1430593]
Level 3 (low-level) evidenceSingh J, Dutta AK, Khare S, Dubey NK, Harit AK, Jain NK, Wadhwa TC, Gupta SR, Dhariwal AC, Jain DC, Bhatia R, Sokhey J. Diethylene glycol poisoning in Gurgaon, India, 1998. Bulletin of the World Health Organization. 2001:79(2):88-95 [PubMed PMID: 11242827]
Conklin L, Sejvar JJ, Kieszak S, Sabogal R, Sanchez C, Flanders D, Tulloch F, Victoria G, Rodriguez G, Sosa N, McGeehin MA, Schier JG. Long-term renal and neurologic outcomes among survivors of diethylene glycol poisoning. JAMA internal medicine. 2014 Jun:174(6):912-7. doi: 10.1001/jamainternmed.2014.344. Epub [PubMed PMID: 24819553]
van Leusen R, Uges DR. [A patient with acute tubular necrosis as a consequence of drinking diethylene glycol-treated wine]. Nederlands tijdschrift voor geneeskunde. 1987 May 2:131(18):768-71 [PubMed PMID: 3587402]
Level 3 (low-level) evidenceMcGregor DB. Genotoxicity of glycol ethers. Environmental health perspectives. 1984 Aug:57():97-103 [PubMed PMID: 6541999]
Level 3 (low-level) evidenceBallantyne B, Vergnes JS. In vitro and in vivo genetic toxicology studies with diethylene glycol monohexyl ether. Journal of applied toxicology : JAT. 2001 Nov-Dec:21(6):449-60 [PubMed PMID: 11746191]
Level 3 (low-level) evidence