Preventing Cross Infection in the Dental Office
Preventing Cross Infection in the Dental Office
Introduction
In the dental setting, infectious agents can be transmitted by inhalation, injection, ingestion, or contact with mucosa or skin. Infection prevention and control measures aim to prevent or minimize the transmission of pathogenic agents between patients, patients, and dental healthcare workers, and vice-versa. Infection control also limits the spread of infection outside the dental practice. Standard precautions must be in place for all patients, and transmission-based precautions must be applied when patients are at risk of spreading infectious diseases, mainly airborne infections.
Dentists handle several sharp instruments during their daily practice and are, therefore, always at risk of percutaneous injuries. A percutaneous injury exposes the dental professional to the patient's blood and possibly infectious agents. Bloodborne pathogens of concern for dental personnel include hepatitis B (HBV), hepatitis C viruses (HCV), and human immunodeficiency virus (HIV). However, the occupational risk of bloodborne viruses depends on the prevalence of the virus in the population and working conditions.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Potential Routes of Transmission of Infectious Agents in Dental Practice
The spread of pathogens may occur through direct contact (person to person) via blood, saliva, secretions, or indirect contact through contaminated instruments, equipment, or surfaces. Inhalation of pathogens suspended in the air (airborne transmission) is also a possible route of infection in the dental setting.[1]
An important transmission route in the dental office is direct exposure to blood and body fluids.[2] Blood from gingival bleeding is usually found in saliva; hence, all saliva must be treated as potentially infectious material.[2]
In principle, any disease could be transmitted in the dental office, but some conditions are more relevant to dental professionals than others. Bloodborne pathogens of concern include hepatitis B and C viruses and human immunodeficiency virus (HIV). The most common mode of transmission of HBV in dentistry is by percutaneous injury, [2] which carries an average risk of transmission of HBV of 30%. The Hepatitis B virus can stay infectious in dried blood at room temperature for a week.[2] It is estimated that dentists are at a ten times greater risk of developing chronic hepatitis B (HBV) than the average population, but the risk of acquiring HIV is relatively low.[3]
The highest risk of HIV transmission is with percutaneous injuries; however, after a needlestick exposure to HIV-contaminate blood, the approximate risk of transmission is 0.3% per exposure.[4] By contrast, the risk of transmission of HBV is as high as 30% in the dental office. When exposed to hepatitis C virus-positive blood, the risk of transmission is 1.8%.
Other more common viruses can also be acquired in the dental office, including rubella, mumps, and measles; herpes viruses, human papillomaviruses; adenovirus; coxsackie viruses; and upper respiratory tract viruses like influenza A and B viruses and coronavirus (COVID-19).[5] Many of these viruses could be particularly problematic for immunocompromised patients and nonimmune pregnant women.[5] Bacterial infections like tuberculosis (TB), infections with methicillin-resistant Staphylococcus aureus, and streptococci may also be transmitted in the dental setting.[6]
For any of these pathogens to cause disease, the following factors must be present independently from the route.[1]
- A sufficient number of disease-producing microorganisms.
- A reservoir, most likely a body fluid (blood, saliva, oral and respiratory secretions).
- A route of transmission from the source to the host.
- An entrance to the host.
- A nonimmune host (susceptible host).
Infection control prevents infection transmission by acting on one or more of these factors through implementing standard and transmission-based precautions. Standard precautions - the basic strategies to prevent and minimize the risk of infection transmission - are taken with all patients. Extra protection, "transmission-based precautions," are used when patients are suspected or known to be infected with pathogens that can be transmitted by contact, droplets, or air. See Image. Yellow Rigid Container for Sharps Disposal.
Standard Precautions
- Regular hand washing.
- Personal protective equipment, including mask, eye protection, gloves, and gown.
- Appropriate protective equipment for the specific task, for example, using heavy-duty gloves for cleaning and processing instruments.
- Adequate management of sharps.
- Appropriate cleaning, disinfection, and sterilization of patient care equipment.
- Cleaning and disinfection of environmental surfaces.
- Preventing injuries through safe work practices.[1]
Standard precautions should be applied with all patients whenever there is contact with mucous membranes or non-intact skin, but also when:
- Cleaning the dental surgery.
- Handling objects that have been in contact with saliva, like an x-ray film.
- Cleaning and processing instruments.
- Handling body fluids, like blood, saliva, and other secretions.
Transmission-based Precautions
Transmission-based precautions are applied when standard precautions alone are insufficient to prevent the spread of certain infectious microbes. The measures used depend on the mode of transmission of the specific agent. Transmission-based precautions are mainly used in the dental setting to prevent airborne infections.
To prevent the spread of airborne infectious agents, evidence recommends using negatively pressurized rooms and a P2 (N95) surgical respirator, which achieves a better seal with the face. Non-emergency dental treatment must be postponed in patients with viral influenza, active tuberculosis, chickenpox, or measles. If patients with viral influenza require emergency care, transmission-based precautions must be applied:
- The patient must be scheduled for the last appointment of the day, minimizing contact with other patients, or at least 30 minutes must be left before the next appointment.
- A mouth rinse must be offered before initiating treatment.
- Dental dam isolation must be used for restorative treatments.
- The use of aerosol-generating procedures must be avoided or minimized.
- Environmental surfaces must be cleaned twice.
- Providers delivering treatment must be immunized against the current influenza strain.
After the appearance of coronavirus disease 2019 (COVID-19), extra infection control measures have been implemented in the dental office.[7] New literature suggests giving patients a hydrogen peroxide rinse with 1% distilled water before treatment to decrease the salivary viral load.[7] Furthermore, rubber dam isolation must be done when the procedure allows it to reduce the spread of aerosols.[7]
Most dental clinics are not designed to execute all transmission-based precautions, e.g., negatively pressurized rooms; effective systems must be in place to identify and manage potentially infected patients.[1]
Respiratory Hygiene
Respiratory hygiene is designed to prevent the transmission of pathogens spread by air or droplets. Recommendations include the following:[8]
- Implement measures to contain respiratory secretions, like having signs at entrances with recommendations to cover their mouths and noses when coughing or sneezing.
- Provide masks to patients with respiratory symptoms entering the dental setting.
- Ask patients and companions not to come to the dental office if they have respiratory infection symptoms unless it is an emergency.
- Wash hands after being in contact with respiratory secretions.
Management of Patients with Bloodborne Infections
Patients with confirmed bloodborne infections are treated using standard precautions. No extra cleaning and sterilization measures are taken. Dental health care personnel must be confident that the infection control and prevention protocols work well with every patient regardless of their infectious status.
Issues of Concern
Hand Hygiene
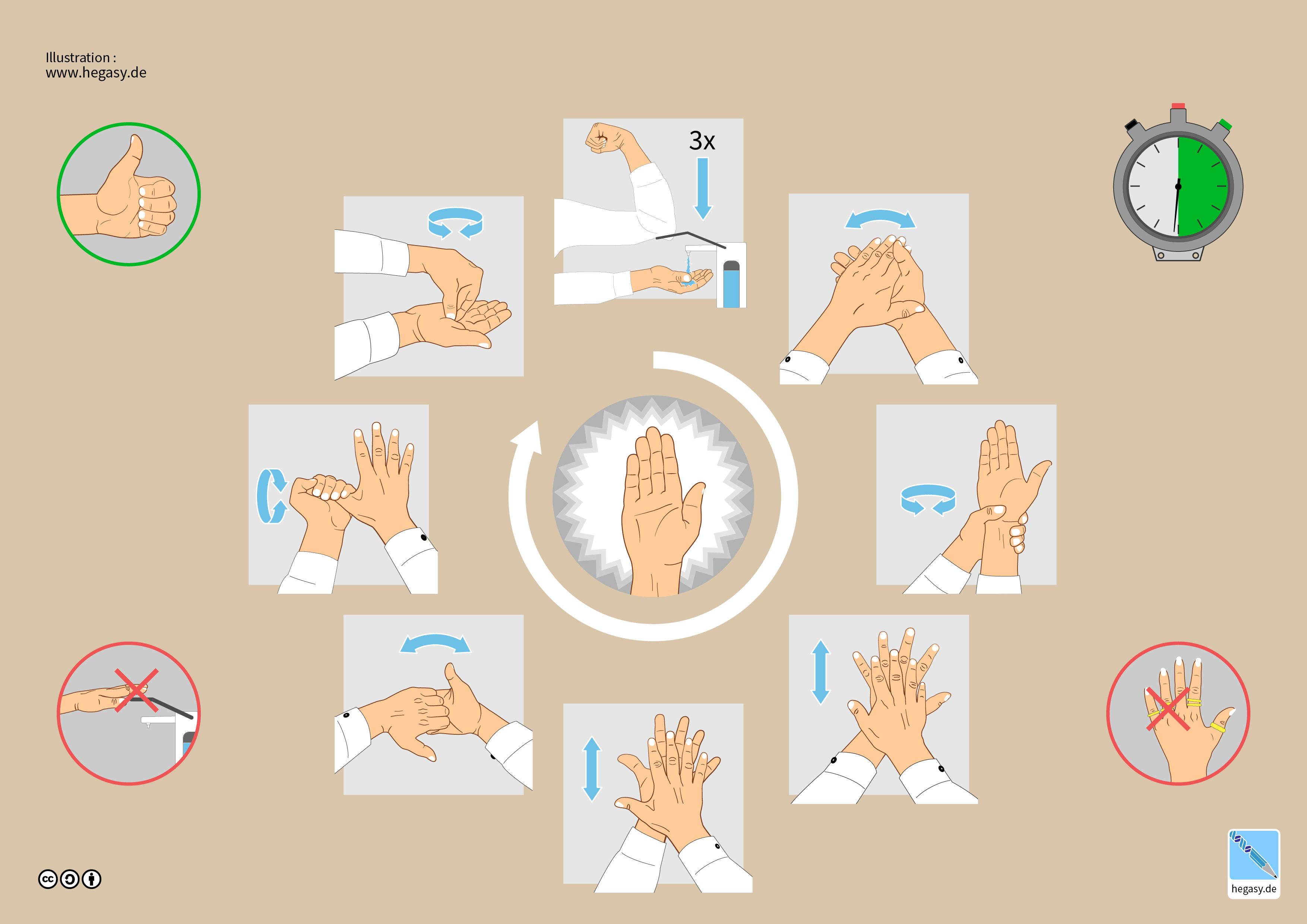
The most common path of pathogen transmission is the hands.[1] The spread of antibiotic resistance in healthcare environments can be reduced by hand washing.[1] Hand hygiene is an umbrella term that includes 1) routine hand washing, 2) antiseptic hand washing, 3) antiseptic hand rub, and 4) surgical hand antisepsis.[1] See Figure. Hand Hygiene Step by Step.
- Hand washing is washing hands with regular soap and water.
- Antiseptic hand washing is washing hands with an antiseptic-containing soap, like triclosan or chlorhexidine.
- Alcohol hand rub refers to using a waterless agent containing 60% to 95% ethanol or isopropanol alcohol-containing preparation. This should not be a substitute for regular hand wash.
- Surgical antisepsis is an antiseptic hand wash performed preoperatively by surgical personnel.[1]
When Should Hand Hygiene be Performed?
- Before and after treating each patient (before putting on personal protective equipment and after removing it).
- After touching objects contaminated with body fluids with bare hands (blood, saliva, oral and respiratory secretions).
- Before touching wrapped or unwrapped sterile instruments.
- Before placing gloves for a surgical procedure (surgical antisepsis).[1]
- When cleaning: after washing instruments, cleaning dental devices, and finishing decontamination tasks.
- Before leaving the dental office.
Personal Protection
Immunization
Dental healthcare workers are at risk of common vaccine-preventable infections. The goal of immunization is to reduce the risk of acquiring a disease. All clinical personnel must receive immunization against hepatitis B within ten days of patient contact.[2] They are allowed to see patients when needed to complete the vaccination scheme, usually between two to six months.[2] Immunization is achieved when the hepatitis B surface antibody (HbsAb) is more than 100 mIU/ml. A poor responder is when antibody levels are between 10 to 100 mIU/ml, and a non-responder is less than 10 mIU/ml.
Furthermore, immunization for common vaccine-preventable conditions is strongly recommended, including:[9]
- Varicella (if seronegative).
- Measles, mumps, and rubella (MMR) (if non-immune).
- Pertussis (whooping cough).
- Viral influenza (required every year to cover new).[8]
Personal Protective Equipment
Personal protective equipment (PPE) or protective barriers are essential components of standard precautions. PPE protects from sprays or spatters potentially containing infectious agents.[1] They include 1) masks, 2) gloves, 3) protective clothing, and 4) protective eyewear.[1]
Masks
Masks protect the operator's nasal and buccal mucosa and the skin of the face and neck from splatters. But they do not protect from aerosols. Masks must be tightly fitted to the face to improve their function.[2] Masks are single-use; a new mask must be used with each patient.
The filtration capacity of masks must be considered. Filtration effectiveness starts to decrease after 20 minutes of wearing due to moisture; therefore, masks must be changed during lengthy procedures if it is noticeably moistened. Wearing masks at all times also prevents the contamination of environmental surfaces with the operator's respiratory secretions.
Gloves
Wearing gloves is not a replacement for washing hands; hands must be washed before gloving and after de-gloving. Gloves must be replaced if they experience any breakage, cut, or puncture.[1] Keeping long fingernails and wearing rings are likely to puncture gloves; therefore, they are discouraged.[1]
Wedding rings may be kept if the skin underneath is washed and dried. Sweat underneath the glove containing skin microbes accumulates after wearing them for an extended period.[1] Hence, the importance of washing hands after de-gloving and removing them as far as possible from clean instruments.[1] Cuts or abrasions in the skin of the hands are a point of entry for microbes and must be covered before gloving.
Protective Clothing
Scrubs are usually used as the everyday uniform in dental practice. An appropriate layer (usually a disposable or reusable gown) must be used on scrubs when providing dental treatment. This gown should have short sleeves for non-surgical treatment and long sleeves for surgical procedures. Short sleeves allow the washing of the forearms simultaneously with hand hygiene. Long sleeves are used for surgical procedures because the gown is sterile. The protective layer used in the clinical area must be removed before moving to non-clinical areas, e.g., when taking a lunch break or leaving the clinic. Another option is wearing street clothes underneath the scrubs and removing the scrubs before moving to non-clinical zones.
Protective gowns should be worn when the procedure carries a risk of contamination with significant body fluids or blood - aerosol-generating procedures.[2] These gowns have long sleeves, which become contaminated during treatment and complicate hand hygiene, and therefore must be changed after every patient.[2]
Footwear must be closed to protect from sharp injuries when objects are accidentally dropped; non-slip and easy to clean.
Protective Eyewear
Eyewear protects the eyes from penetrating injuries, projectiles, splattering, or spraying with body fluids. Eye protection is essential during scaling, when using rotary devices, cleaning instruments, and cutting or working with wire. Protective eyewear must have side shields. Reading glasses provide insufficient coverage to the orbit to prevent injury and contamination.
Face shields are recommended when the procedure is expected to generate significant aerosols or splatters.[1] But, because they do not protect from airborne pathogens must always be used with a surgical mask. Dental safety glasses are advised for patients to decrease the risk of physical or chemical injury from materials during treatment. Tinted glasses have the advantage of protection from the glare of the operating light.
How to Remove PPE
It is essential to remove the PPE appropriately to avoid contact and contamination with blood and body secretions. PPE must be removed in the following order:
- Gloves must be removed first, ensuring the hands do not become contaminated. The gloves are left inside out. Hands must be washed if they become contaminated before moving to the next step.
- The disposable apron or gown is removed next by breaking the strap that goes in the neck. The apron must be touched only from the inside, gathered together, and disposed of.
- The mask is removed without touching its external surface by lifting it over the ears or pulling and breaking the straps.
- Protective eyewear must be removed without touching the outer surfaces.
- Hand hygiene must be performed after removing all PPE.[10]
Management of Sharps
A medical sharp is any object with the possibility of cutting, picking, or causing injury. The most common examples of sharps are needles and scalpel blades; however, other objects, like matrix bands, endodontic files, and wires, could also cause percutaneous injuries and must be managed appropriately.
Disposable sharps must be placed in a puncture-resistant (rigid) container located as close as possible to the area where they are being used.[7] They should never be overfilled; they are filled to up to two-thirds of their capacity.
Methods to prevent a sharp injury (percutaneous injury) include applying work practice and engineering controls. Engineering controls include:[11]
- Needle recapping devices.
- Self-sheathing needles and scalpels.
- Rigid containers for sharps disposal.
Work practice controls refer to good clinical practices, including:
- Avoid passing unsheathed needles between personnel.
- Avoid recapping needles with a one-hand technique.
- Restrict the use of fingers during anesthesia administration.[11]
Cleaning and Disinfection
Mechanical cleaning is preferred over manual cleaning of instruments before sterilization. This is because mechanical cleaning is more efficient and decreases the risk of percutaneous injuries and exposure to blood.[12] Mechanical cleaning can be performed using thermal disinfectors, also known as instrument washers or ultrasonic cleaners.
Manual cleaning is, therefore, discouraged. But if done, it must be carried out in a designated sink prefilled with lukewarm water and specific detergent for cleaning instruments. Hot water cannot be used since it promotes the coagulation of proteins, and cold water solidifies lipids, complicating the removal of debris from the instruments. The instruments must be cleaned with a long-handled brush while kept low and under the water in the sink. After cleaning, instruments must be rinsed with warm or hot water - hot water accelerates the drying of instruments - and then inspected under a magnifier device and appropriate light.
All equipment that can be removed from the dental unit should be sterilized, including the handpieces. X-ray equipment, lights, and the dental chair must be disinfected with chemical agents.[7] Digital radiography sensors should be protected with a cleared barrier to reduce contamination during use, followed by cleaning and heat sterilization or high-level disinfection between patients.[1]
Handpieces are considered semi-critical devices. They should be heat sterilized between patients. It is difficult for chemical germicides to reach the internal parts of the handpieces, which is why they should be heat sterilized.[1] Ultrasonic scalers should be soaked in 70% isopropyl alcohol to remove organic debris.[1]
Disinfectants like 0.1-0.5% sodium hypochlorite, 62 to 71% ethanol, or 2% glutaraldehyde can be used to disinfect surfaces like door handles, chairs, desks, elevators, and bathrooms.[7]
Clinical contact surfaces must be covered with protective barriers and changed after each patient. The immersion of instruments in sodium hypochlorite can also be used for disinfection reasons before sterilization.[7]
Methods of Sterilization
The purpose of sterilization is to destroy all active microorganisms, including bacterial spores, from instruments. The most common sterilization methods in dentistry are steam under pressure and dry heat sterilization. But other less common methods are available, including unsaturated chemical vapor pressure sterilization and ethylene oxide sterilization.[1]
Steam Pressure Sterilization (Autoclave)
Sterilization using steam under pressure (autoclave) is the most popular method in dentistry. It is practical and rapid, and the sterilization efficacy can be verified. Autoclave sterilization uses high temperatures of 121 C at a pressure of 15 pounds for 20 minutes.[2] Another cycle is 134 C for 3 to 4 minutes.[9]
The downsides include that instruments that can not be submitted to high temperatures cannot be sterilized by autoclave; autoclave sterilization tends to rust carbon steel instruments and burs; and the instruments must be air-dried at the end of the cycle.[1]
Dry Heat Sterilization
This method uses conventional dry heat ovens with a short cycle and high temperatures for items that cannot be exposed to moist heat, static air, or forced air. The high temperatures may damage heat-sensitive items; cycles are longer at lower temperatures, and ovens must be calibrated. Dry heat sterilization does not corrode instruments, and the effectiveness of sterilization can be verified. Industrial hot air ovens can sterilize more instruments at the same time.[1]
Unsaturated Chemical Vapor Pressure Sterilization
This method uses an unsaturated chemical vapor system of alcohol and formaldehyde. The cycles are short, and the load comes out dry; the effectiveness of the sterilization can be verified, and corrosion-sensitive instruments do not rust.
The disadvantage of this method is that instruments must be completely dry beforehand, and heavy cloth wrappings of surgical instruments may not be penetrated.[1]
Ethylene Oxide Sterilization
This method uses a fumigator. It works at low temperatures. The gas is penetrative and can be used for different equipment like handpieces. The efficacy of sterilization can be verified. It requires an aeration chamber and can be mutagenic and carcinogenic.[1]
Clinical Significance
Accidental Percutaneous Injuries
Dentists are always at risk of accidental sharps injuries in their daily practice. A sharp injury is an accidental skin-penetrating stab wound from a needle (needlestick injury) or a sharp object containing blood or body secretion.[13] This type of injury directly exposes the operator to the patient's blood, possibly contaminated with blood-borne pathogens.
Factors to Consider When Exposed to Blood-borne Viruses (BBVs)
- Type of exposure (penetrating injury or mucosal splash)
- Type of body fluid (blood, saliva)
- The volume of body fluids or blood
- Time of contact with the body fluid
- Time since the exposure
- Type of procedure and device involved
- If the injury was through a glove or clothing
- If the source patient is viraemic [8]
If a percutaneous injury occurs, like a needlestick, the operator must stop the procedure immediately and proceed with the post-exposure protocol.[2]
- The wound must be washed with soap and rinsed under running water, allowing it to bleed.[1]
- A supervisor must be notified, and the date and patient details must be recorded.
- Serological tests may be recommended for the operator and the source patient. These tests include HIV antibodies, HCV antibodies, and antibodies to hepatitis B surface antigens (anti-HBs).[8] Patients cannot be forced to be tested, but they must be encouraged to do so.[2]
- A medical consultation is required as the physician will determine the need for post-exposure prophylaxis according to the patient's medical history and risk for blood-borne infections.[2]
Source Positive for HIV
The risk of seroconversion after a sharps injury with HIV-infected blood is 0.3%, and after a mucous membrane exposure to HIV-infected blood is 0.09%.[8] Even when PEP is not received, postexposure testing and medical evaluation are required.
If the source is possibly infected with HIV, post-exposure prophylaxis (PEP) should be considered. The goal of post-exposure prophylaxis is to prevent viral replication. The basic HIV PEP regimen combines zidovudine (ZDV) and lamivudine (3TC). Alternatively, 3TC and stavudine or didanosine and stavudine can also be indiccated. The expanded regimen includes the basic regimen and indinavir, nelfinavir, efavirenz, or abacavir.[13]
Toxicity and side effects of HIV PEP should be considered. PEP is only indicated if there has been significant exposure and a proper risk assessment has been undertaken. Drugs should be administered between 24 to 36 hours post-exposure. HIV PEP should not be used in the case of exposure to nonblood-stained saliva.[8]
Postexposure testing for HIV antibodies is recommended at baseline, six weeks, 12 weeks, and six months postexposure.[13] Follow-up includes reporting any sudden or severe flu-like symptoms, any adverse events associated with PEP if this is administered, as well as signs and symptoms of possible retroviral illness.
If the status of the source is unknown, decisions should be based on the exposure risk and the chance that the source may be HIV positive, e.g., an IV drug user. If the source consents to HIV testing, post-exposure prophylaxis can be given as soon as possible after exposure and discontinued if the result is negative.[13]
Source Positive for Hepatitis C (HCV)
After exposure to the hepatitis C virus, the exposed professional must be closely followed up and referred for appropriate therapy if the infection does occur.[14] Currently, there is no effective post-exposure prophylaxis (PEP) for hepatitis C.
The risk of transmission after an injury from a positive source varies whether active viral replication is occurring or not. If the source is HCV RNA negative in polymerase chain reaction (PCR) assay, the risk is between 1.8 and 3.1%. If the source is PCR positive, the risk increases to 10%. It is recommended to be tested at baseline, three months, and six months. Liver function tests like aminotransferase (ALT) and aspartate aminotransferase (AST) at two, three, and six months are recommended. An infectious disease clinician or gastroenterologist should monitor clinical signs and symptoms.
Source Positive for Hepatitis B (HBV)
Postexposure management for hepatitis B virus (HBV) involves providing multiple doses of HBIG (hepatitis B immune globulin) and the hepatitis B vaccine.[14] HBIG is a human immunoglobulin used to prevent the development of hepatitis B after acute exposure to the surface antigen of the hepatitis B virus (HBsAg).[15]
The level of antibodies of the exposed individual should be tested if the source is positive for HBsAg. If the exposed person is not immune (has never been immunized, did not seroconvert to the vaccine, or has antibody levels to HBsAg less than 10 mIU/mL), the treatment is to give a single dose of hepatitis B immunoglobulin (HBIG) between 48 to 72 hours of the exposure and to start a course of HBV vaccination.[14]
The HBV vaccine should be given within seven days of exposure, one or two months after, and then six months after the first dose. The level of immunity should be tested two to four weeks later. The risk of transmission of HBV is more than 30% if the source is positive and post-exposure prophylaxis has not been used.[14]
Enhancing Healthcare Team Outcomes
Due to the nature of their work, dental personnel are exposed to possibly infectious agents daily that present an occupational risk. Vaccination, proper use of PPE, and disinfection and sterilization recommendations should be thoroughly followed to minimize the spread of infections.[16]
Protocols for minimizing percutaneous injuries must be in place in the dental office, explained to new personnel, and revised and checked regularly. An exposure protocol and risk assessment should be followed in case of an accident.[14] Infection control practices, continuing education, and vaccination should be reinforced as dental healthcare workers and their patients are at risk of infections and disease transmission. Proper sterilization techniques should be done to prevent cross-contamination between patients.[14][16]
The importance of infection control must always be in our minds: when working in the clinical environment, when treating each patient, when moving from clinical to non-clinical areas, when processing dental instruments, and when leaving the office to go home at the end of a working day. This will minimize the risk of pathogens transmission and ensure patient and personnel safety.
Media
(Click Image to Enlarge)
References
Sebastiani FR, Dym H, Kirpalani T. Infection Control in the Dental Office. Dental clinics of North America. 2017 Apr:61(2):435-457. doi: 10.1016/j.cden.2016.12.008. Epub [PubMed PMID: 28317575]
Thomas MV, Jarboe G, Frazer RQ. Infection control in the dental office. Dental clinics of North America. 2008 Jul:52(3):609-28, x. doi: 10.1016/j.cden.2008.02.002. Epub [PubMed PMID: 18501738]
Araujo MW, Andreana S. Risk and prevention of transmission of infectious diseases in dentistry. Quintessence international (Berlin, Germany : 1985). 2002 May:33(5):376-82 [PubMed PMID: 12014168]
Denault D, Gardner H. OSHA Bloodborne Pathogen Standards. StatPearls. 2024 Jan:(): [PubMed PMID: 34033323]
McCarthy GM. Risk of transmission of viruses in the dental office. Journal (Canadian Dental Association). 2000 Nov:66(10):554-5, 557 [PubMed PMID: 12584777]
Amato A, Caggiano M, Amato M, Moccia G, Capunzo M, De Caro F. Infection Control in Dental Practice During the COVID-19 Pandemic. International journal of environmental research and public health. 2020 Jul 2:17(13):. doi: 10.3390/ijerph17134769. Epub 2020 Jul 2 [PubMed PMID: 32630735]
Cabrera-Tasayco FDP, Rivera-Carhuavilca JM, Atoche-Socola KJ, Peña-Soto C, Arriola-Guillén LE. Biosafety Measures at the Dental Office After the Appearance of COVID-19: A Systematic Review. Disaster medicine and public health preparedness. 2021 Dec:15(6):e34-e38. doi: 10.1017/dmp.2020.269. Epub 2020 Jul 27 [PubMed PMID: 32713385]
Level 1 (high-level) evidenceSotomayor-Castillo C, Li C, Kaufman-Francis K, Nahidi S, Walsh LJ, Liberali SA, Irving E, Holden AC, Shaban RZ. Australian dentists' knowledge, preparedness, and experiences during the COVID-19 pandemic. Infection, disease & health. 2022 Feb:27(1):49-57. doi: 10.1016/j.idh.2021.10.001. Epub 2021 Oct 19 [PubMed PMID: 34750088]
Mahdi SS, Ahmed Z, Allana R, Amenta F, Agha D, Latif MW, Daood U, Mehanna C. Knowledge, Attitudes, and Perceptions of Dental Assistants regarding Dental Asepsis and Sterilization in the Dental Workplace. International journal of dentistry. 2021:2021():5574536. doi: 10.1155/2021/5574536. Epub 2021 Jun 16 [PubMed PMID: 34221016]
Lo Giudice R. The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2) in Dentistry. Management of Biological Risk in Dental Practice. International journal of environmental research and public health. 2020 Apr 28:17(9):. doi: 10.3390/ijerph17093067. Epub 2020 Apr 28 [PubMed PMID: 32354081]
Cleveland JL, Barker LK, Cuny EJ, Panlilio AL, National Surveillance System for Health Care Workers Group. Preventing percutaneous injuries among dental health care personnel. Journal of the American Dental Association (1939). 2007 Feb:138(2):169-78; quiz 247-8 [PubMed PMID: 17272371]
Miller CH, Tan CM, Beiswanger MA, Gaines DJ, Setcos JC, Palenik CJ. Cleaning dental instruments: measuring the effectiveness of an instrument washer/disinfector. American journal of dentistry. 2000 Feb:13(1):39-43 [PubMed PMID: 11763901]
McCarthy GM, Ssali CS, Bednarsh H, Jorge J, Wangrangsimakul K, Page-Shafer K. Transmission of HIV in the dental clinic and elsewhere. Oral diseases. 2002:8 Suppl 2():126-35 [PubMed PMID: 12164646]
Ayatollahi J, Ayatollahi F, Ardekani AM, Bahrololoomi R, Ayatollahi J, Ayatollahi A, Owlia MB. Occupational hazards to dental staff. Dental research journal. 2012 Jan:9(1):2-7. doi: 10.4103/1735-3327.92919. Epub [PubMed PMID: 22363355]
Habib S, Shaikh OS. Hepatitis B immune globulin. Drugs of today (Barcelona, Spain : 1998). 2007 Jun:43(6):379-94 [PubMed PMID: 17612709]
Villani FA, Aiuto R, Paglia L, Re D. COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. International journal of environmental research and public health. 2020 Jun 26:17(12):. doi: 10.3390/ijerph17124609. Epub 2020 Jun 26 [PubMed PMID: 32604906]