Introduction
Flow cytometry is a technique used to measure the physical and chemical properties of cells by analyzing their light scattering and fluorescence emission characteristics.[1] The term flow cytometry refers to the measurement of a cell in a flowing sample. This technology allows for the simultaneous assessment of multiple parameters on individual cells, making it valuable for immunophenotyping cells from various sources, including blood, bone marrow, body fluids, fine-needle aspirates, and cell suspensions from freshly biopsied specimens. Flow cytometry can analyze approximately 30,000 cells per second. Each cell is analyzed for visible light scatter and one or multiple fluorescence parameters.[2] This rapid, cost-effective, and widely available tool is used in diverse medical applications.
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Immunophenotyping is the most common application of flow cytometry. Using multiple immunomarkers, flow cytometry can be used for multiple purposes.
Diagnosis and Classification of Acute Leukemia
In peripheral blood or bone marrow aspirates, the blast cell population can be identified by flow cytometry through the dim-to-moderate expression of CD45 and the expression of immaturity markers such as CD34, CD117, or TdT. The lineage of these cells can be determined as B, T, myeloid, or ambiguous.[3] According to the WHO Classification of Tumours, often referred to as WHO Blue Books (5th edition), the lineage markers are as follows:
- B lineage: CD19 (strong) and one or more of CD10/CD22/CD79a. If the expression of CD19 is weak, then two or more of the additional markers should be strongly expressed.
- T lineage: Cytoplasmic or surface CD3 expression.
- Myeloid lineage: Myeloperoxidase expression or two or more of nonspecific esterase, CD11c, CD14, CD64, or lysozyme. The latter set of markers is for monocytic lineage. (International Agency for Research on Cancer. Haematolymphoid Tumours)
Acute promyelocytic leukemia is characterized by atypical promyelocytes in peripheral blood or bone marrow and has a peculiar flow cytometric pattern. These abnormal promyelocytes have high side and forward scatters. They are classically negative for CD34 and HLA-DR, and positive for CD13, CD33, and CD117. CD64 can be variably positive. The underlying cytogenetic abnormalities and prognosis can be predicted in some instances; CD10-negative and CD15-positive B-cell acute lymphoblastic leukemia (B-ALL) are associated with the t(9;11) translocation and have a poorer prognosis.[4]
Diagnosis of Chronic Leukemia
In peripheral blood or bone marrow aspirates, monoclonality and aberrant antigen expression can identify the neoplastic cells. For example, in chronic lymphocytic leukemia, peripheral blood lymphocytosis is observed, and the leukemic lymphocytes typically exhibit the following markers—CD5+, CD19+, CD20 dim+, CD23+, CD10-, CD200+, sIG dim+, and CD43+. These cells also express lymphoid enhancer-binding factor 1 (LEF1), which is a sensitive and specific marker for chronic lymphocytic leukemia and small lymphocytic lymphoma. These cells are generally negative for CD10, cyclin-D1, FMC7, and CD103. The number of blast cells is not increased.[5][6]
Plasma Cell Neoplasms
In these cases, bone marrow aspirates reveal the presence of neoplastic plasma cells confirmed by negative or partial-positive expression of CD45 and negative CD19 with a positive expression of CD38, CD138, and kappa or lambda restriction.[7]
Detection of Minimal Residual Disease
Flow cytometry allows for the identification of 0.1 to 0.001% of leukemic cells in posttherapeutic bone marrow samples from patients with leukemia.[8]
Myelodysplastic Disorders
Myelodysplastic syndromes are characterized by peripheral cytopenia, myelodysplasia, ineffective erythropoiesis, and an increased risk of progressing to acute leukemia.[9] Aberrant antigen expression patterns by the cells of different hematopoietic lineages can help detect myelodysplasia.[10]
Absolute CD4 T-Lymphocyte Counts in AIDS
The peripheral blood CD4 T-lymphocyte count is used for follow-up in HIV-positive patients to evaluate disease progression and response to antiretroviral therapy.[11] This enumeration is performed using flow cytometry.
Atypical Cells in Fine-Needle Aspirates and Body Fluids
Flow cytometric analysis of the body fluids with atypical cells, especially those of hematolymphoid origin, can be rewarding.[12] The approach may remain the same as in peripheral blood samples and bone marrow.
HLA-B27 Assay
HLA-B27 expression is studied on the CD3 T cells in suspected cases of ankylosing spondylitis using flow cytometry.[13] This investigation is routinely performed to diagnose ankylosing spondylitis.
DNA Ploidy and S-Phase Fraction
Flow cytometry measurements of DNA ploidy and S-phase fraction can serve as prognostic markers in carcinoma cases, such as cervical carcinoma, breast carcinoma, and hematolymphoid neoplasms.[14][15][14][16] Fine-needle aspirates, peripheral blood samples, and even paraffin-embedded tissue have been used for flow cytometry in these neoplasms.
Diagnosis of Paroxysmal Nocturnal Hemoglobinuria
Paroxysmal nocturnal hemoglobinuria is characterized by chronic recurrent intravascular hemolysis, thrombosis in unusual sites, and cytopenia due to bone marrow failure. Flow cytometry is used widely to diagnose this condition by analyzing the expression of markers such as FLAER (fluorescent aerolysin), CD55, CD59, and CD24 on neutrophils. Recently, CD157 was also introduced in the analysis. The negative expression of these markers is diagnostic for the condition.[17]
Detection of Fetal Hemoglobin
In cases of suspected fetomaternal hemorrhage in Rh-negative pregnant patients, fetal red blood cells can be identified and quantified using antibodies to fetal hemoglobin (anti-HbF) and flow cytometry. This accurate quantification is important to determine the appropriate dose of anti-D immunoglobulin for pregnant/postpartum patients.[18]
Role in Transfusion and Stem Cell Transplant
In transfusion medicine, flow cytometry has been utilized in evaluating ABO discrepancies, D variants/D(weak), and suspected chimerism, as well as for quality control of blood components.[19] The stored blood is evaluated for residual leukocytes and 90% of blood components should be less than 1x106 leukocytes/unit.
Role in Common Variable Immunodeficiency Syndrome
The peripheral blood lymphocytes can be analyzed for CD21, CD27, CD19, CD38, IgM, and IgD expression.[20]
Equipment
Types of Flow Cytometers
Flow cytometers can be classified into several types based on their functionality and complexity.
Traditional flow cytometer: These cytometers are commonly used instruments that utilize sheath fluid to focus the sample stream, enabling accurate analysis of cells and particles based on their optical properties.[2] A flow cytometer is most frequently used for the immunophenotyping of cells in suspension. Traditional flow cytometers can analyze multiple parameters at a time. Some of the recent flow cytometers can analyze up to 50 parameters.[21]
Cell sorters: This type of flow cytometer can segregate a specific cell population based on their specific immunoexpression. This cell population is collected in separate tubes or slides. Most cell sorters use electrostatic methods, also known as jet-in-air, whereby the stream exiting the flow cell is vibrated to generate droplets. The objective is to isolate single cells inside individual droplets. When a target cell is identified, a positive or negative electrostatic charge is applied to the droplet. The target droplet is deflected by charged plates and deposited in a collection tube. Uncharged droplets are directed to a waste receptacle.[22] Other cell sorters use different cell isolation methods, such as air diversion for large particles and microfluidic actuators to divert cells of interest inside a contained apparatus.[23]
Imaging flow cytometers: These flow cytometers allow rapid sample analysis for morphology and multiparameter fluorescence, combining fluorescence microscopy with traditional flow cytometry.[2][24] Imaging flow cytometry uses objective lenses to capture images of cells at the interrogation point, using charge-coupled device cameras to detect fluorescence across multiple wavelengths. These composite images enable researchers to visualize the spatial distribution of fluorescent probes within the cell, making them valuable for applications such as determining probe localization within specific cell regions, assessing cell-cell interactions, conducting morphological analyses, and performing spot counting.[25]
Mass cytometers: The cells are labeled with heavy metal ion-tagged antibodies, and the cell population is analyzed using time-of-flight spectrometry. Fluorescence-based antibodies are not used.[26] The strength of this technology is its ability to use a broad array of stable isotopes, thereby eliminating the limitations caused by spectral overlap typically encountered with fluorochromes.[21]
Spectral analyzers: These analyzers are designed to overcome the problems of compensation and spectral overlap in traditional flow cytometers. The entire fluorescent emission spectrum is captured for each fluorochrome. Because spectral cytometry removes the need for specific optical filters for each fluorochrome, it offers greater flexibility in selecting and combining fluorescent probes that can be utilized.[2]
Acoustic focusing cytometers: These cytometers use ultrasonic waves to enhance cell focusing for laser interrogation, significantly improving the efficiency and accuracy of flow cytometry. Acoustic focusing technology accommodates a wider variety of cell sizes, from small platelets to larger cells such as cardiomyocytes.[27]
Cytometers for bead array analysis: Cytometric bead arrays use different bead populations, each with distinct sizes and fluorescence intensities, to simultaneously measure multiple analytes. The assay typically follows a sandwich immunoassay format where the target protein binds to a capture antibody coated on the bead. A fluorescently labeled detection antibody is then added to visualize the captured protein, allowing for quantification based on the fluorescence intensity detected by the cytometer.[28][29]
Instrument and Principle
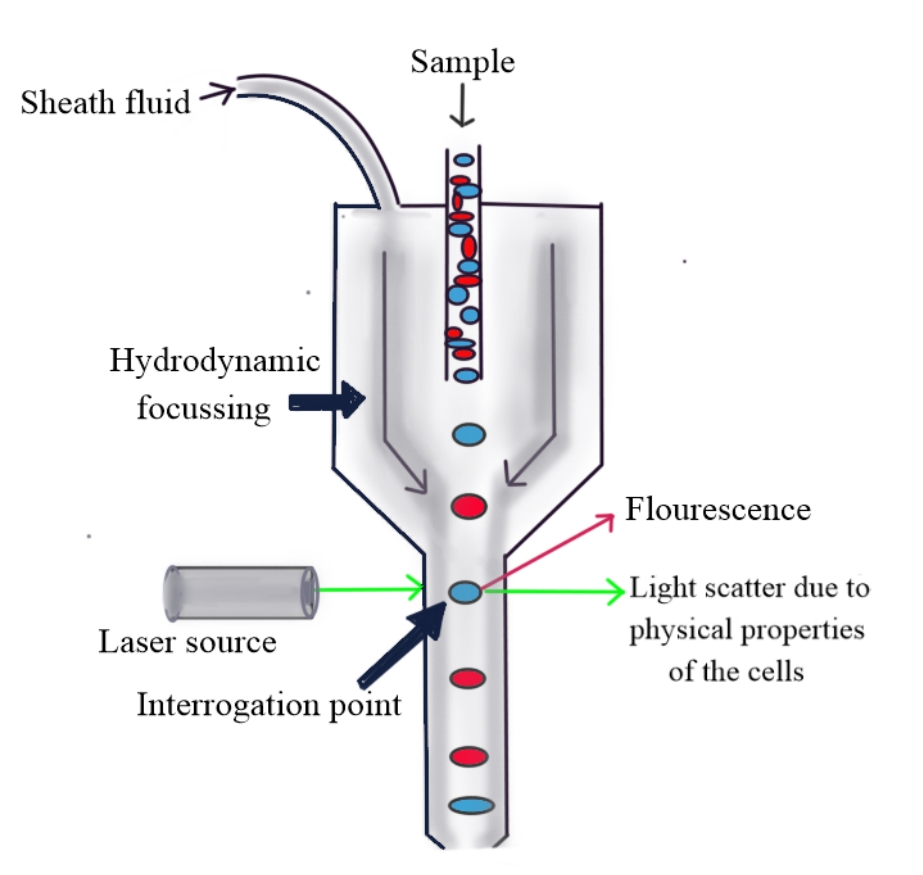
A multiparameter traditional flow cytometer consists of 3 main parts—fluidics, optics, and electronics. The fluidics system includes sheath fluid under pressure, which aids in focusing the central sample stream such that only a single cell can pass through the aperture at a time—a process known as hydrodynamic focusing.[2]
The laser is used to interrogate the stream of cells. A flow cytometer instrument can have 1 to 5 or more lasers. The laser beam is scattered by the cells and their contents, and the light scattered along the axis of the projected laser is called forward scatter, which depends on the size and surface area of the cell. The light scattered at approximately 90°, which depends on the cell granularity, nucleus, and internal complexity of the cell, is called side scatter or orthogonal light scatter. In addition to the physical properties of cells, fluorescence is also used to distinguish cell types by flow cytometry. Fluorochrome-labeled primary antibodies are used to target specific cell antigens. The fluorochromes are excited by the laser, and the emitted light of a longer wavelength is allowed to pass through the lens, mirrors, and filters, thus separating and directing lights of different wavelengths to appropriate detectors. This system of laser, lens, mirrors, and filters comprises optics (see Image. Principle of Flow Cytometry). The light signals are collected by the detectors, are changed into voltage pulses, and then converted into digital data, which is studied in the form of different plots and graphs using dedicated software.
Flow cytometry data analysis software traditionally uses worksheets containing a series of plots to visualize the data. Single-parameter (histograms) or two-parameter plots (dot plots) display each marker's expression pattern. Cell populations of interest are identified and segregated by drawing gates to form data boundaries and to isolate cells with common phenotypic patterns. Additional plots and gates narrow down the populations of interest using gating hierarchy or boolean logic. Statistics, such as frequency, are calculated from the data points within these gates.[30]
Flow cytometers generate data in the FCS (flow cytometry standard) file format. FCS files contain many keywords that can be used during analysis. Some of these are required by the file standard and autopopulated by the manufacturer's software. Other keywords require input from the user.[31]
In summary, flow cytometry involves focusing a cell suspension using hydrodynamic focusing, allowing single cells to pass through an aperture. Each cell is then analyzed by a laser, and the resulting light scatter is processed by software for quantification and further analysis.
Quality Management and Controls in Flow Cytometry
In clinical flow cytometry, an effective quality management system is crucial for maintaining consistent assay specifications, including precision, accuracy, sensitivity, specificity, reference ranges, and stability. Variations in flow cytometry results can arise due to factors related to the biological sample, such as sample variability and handling, and operational factors, such as equipment calibration, method validation, personnel competency, document control, and environmental conditions. By addressing biological and operational variables, clinical flow cytometry laboratories can enhance the reliability and accuracy of their assays, ultimately improving patient outcomes and trust in laboratory results.[32]
Quality control (QC) is a critical aspect of laboratory operations, especially in flow cytometry, where it plays a key role in monitoring all operational techniques and activities during the analytic phase to ensure valid and reproducible results.[33] QC in flow cytometry involves several key assessments to maintain assay integrity and reliability, including regular checks and calibrations of flow cytometry instruments, such as lasers, detectors, and fluidics systems, to ensure proper functioning. Reagent and antibody quality are also monitored to verify effectiveness and stability, with attention to expiration dates, storage conditions, and batch-to-batch consistency. In addition, process QC oversees the entire assay process from sample preparation to data acquisition and analysis, using control samples to confirm that the assay performs as expected. Maintaining specimen integrity through proper sample collection, handling, timely processing, and appropriate storage conditions is equally important.[2][34]
In flow cytometry, selecting the appropriate controls is essential for obtaining reliable and interpretable results, as the choice of controls can significantly impact the outcome of an experiment. Controls are vital for establishing baselines, validating assay performance, and troubleshooting potential issues. Key types of controls include fluorescent compensation controls, which correct for spectral overlap in multi-color assays, and isotype controls, which assess nonspecific binding and background fluorescence. Negative controls, which lack the target antigen, help define the threshold for positive signal detection. In contrast, positive controls containing known quantities of the target antigen validate the assay's sensitivity and specificity. In addition, biological controls, such as samples from known positive and negative patients or cell lines, provide real-world context for assay performance.[35][36]
Strategies for selecting controls include assessing sources of background fluorescence, optimizing control combinations, documenting control performance, and using an iterative approach to refine control selection. Ultimately, carefully chosen controls enhance the accuracy and reliability of flow cytometry results, contributing to successful experimental design and producing high-quality data for clinical and research applications.[37]
A written QC policy is essential, outlining clear objectives, detailed procedures, documentation guidelines, and a process for regular review and updates. Implementing a comprehensive QC system in flow cytometry ensures reliable and accurate assay results, enhancing diagnostic capabilities and improving patient care.[38]
Preparation
Specimen Requirements and Procedure
Flow cytometry is most commonly used to study peripheral blood and bone marrow aspirate specimens. Both types of specimens can be anticoagulated using EDTA or heparin. A well-preserved fresh specimen of 2 to 5 mL is ideal for flow cytometric analysis. For optimal results, it is recommended to process a fresh sample, preferably within 18 hours. The delay between the collection of the sample and its processing should not be more than 36 hours. The sample should be transported at room temperature and not refrigerated. Cell counts should be verified before proceeding with further processing.[39]
The sample is then processed using a stain-lyse-wash protocol, which involves incubating the cells with fluorochrome-labeled primary antibodies, followed by lysis of red blood cells using a lysis buffer. After lysis, the solution is washed and centrifuged. This procedure is used to stain cell surface antigens. To stain intracellular antigens, an additional step of cell membrane permeabilization is required, allowing the primary antibodies to enter the cells and bind to the intracellular targets.
Once the experiment is set up in the flow cytometer, all pre-analytical checks are conducted, and the sample is loaded into the flow cytometer. For optimal analysis, acquiring a minimum of 105 to 107 cells at a rate of 30,000 events/second is recommended.
Technique or Treatment
Identifying Cell Populations on Flow Cytometry
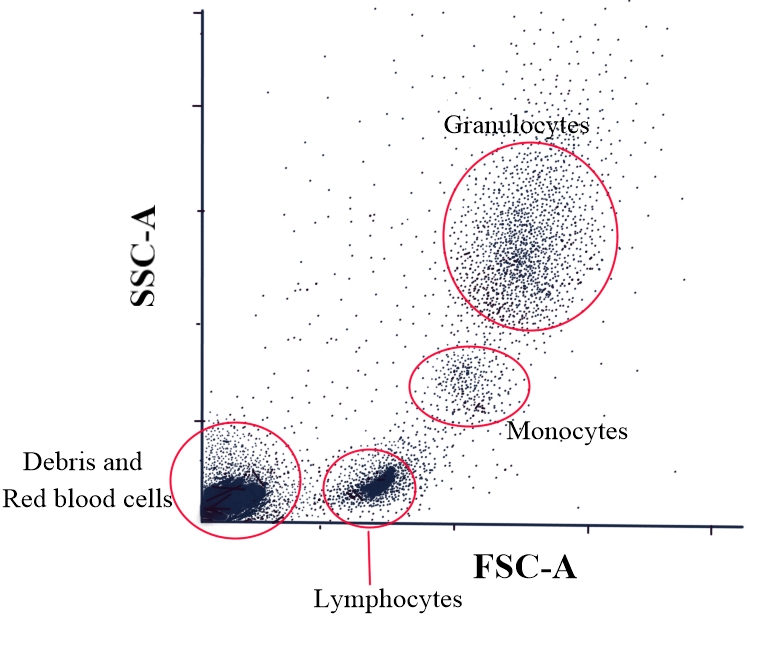
The forward scatter versus side scatter plot can help broadly identify cell types in peripheral blood. Forward scatter corresponds to the cell size, with smaller cells, such as lymphocytes, having a lower forward scatter and larger cells, such as granulocytes, having a higher forward scatter. The cells with agranular cytoplasm, such as lymphocytes, have a low side scatter, whereas those with granular cytoplasm, such as granulocytes, have a higher side scatter. Thus, it can be deduced that lymphocytes have low forward and side scatter. On the other hand, granulocytes, such as neutrophils, eosinophils, and basophils, have high forward and side scatter. The monocytes have intermediate forward and side scatters (see Image. Flow Cytometry: Side Scatter Versus Forward Scatter Dot Plot). This plot may not be sensitive enough to categorize cell types accurately using flow cytometry. Hence, this may not be an ideal or accurate approach.[40]
Different fluorochromes labeled with different immunophenotypic markers can help identify the cell populations more specifically. The expression of an immunophenotypic marker by a cell population can be categorized as negative or positive. The positive expression can be further categorized as dim, moderate, bright, or heterogenous, depending on the degree of positivity.
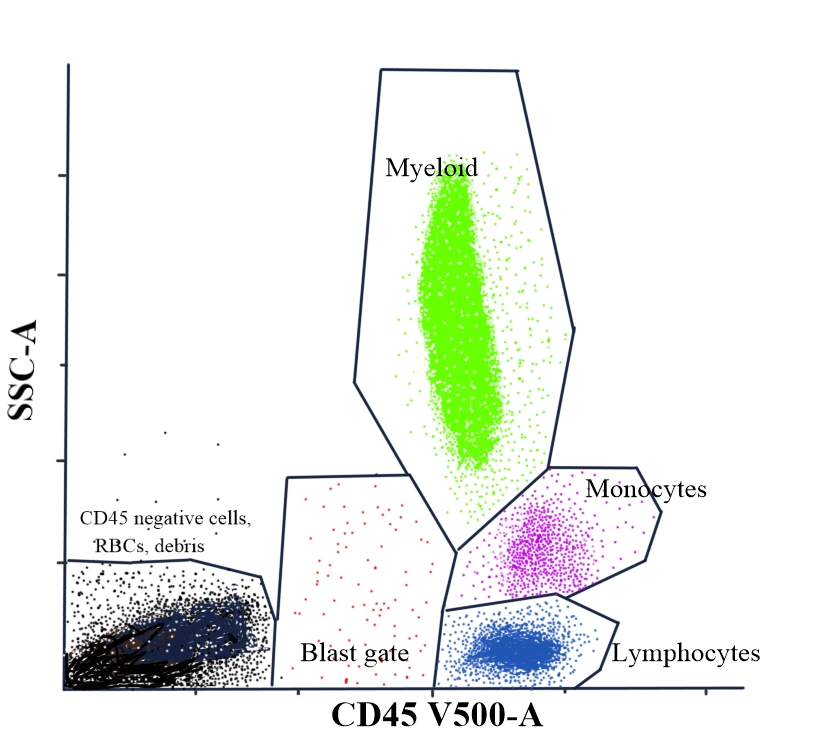
To identify different leukocytes in peripheral blood, a backbone marker, such as leukocyte common antigen or CD45, is used to stain different leukocytes. A plot between CD45 and side scatter distinguishes most of the leukocytes (see Image. Flow Cytometry: CD45 Versus Side Scatter Dot Plot for Differentiating Leukocytes).
- CD45 bright cells with a low side scatter are lymphocytes. T lymphocytes have a relatively brighter CD45 expression compared to B lymphocytes. The CD45 bright expression cells with a higher side scatter compared to lymphocytes are monocytes.[40]
- CD45 moderate expression cells with a high side scatter are neutrophils. CD45-negative, low-side scatter cells are red blood cells and cell debris. If the sample is degenerated, most of the cells may fall in the CD45-negative zone and have a variable side scatter. Propidium iodide can be used as a DNA stain in flow cytometry to evaluate cell viability.[41]
- Blast cells, including myeloblasts or lymphoblasts, have a dim-to-moderate CD45 expression and low side scatter. The latter is because of the higher nuclear-cytoplasmic ratio and scant cytoplasm. These cells constitute ≥20% of peripheral blood or bone marrow. The differential counts in flow cytometric analysis may not be accurate due to the loss of cells during the sample processing.[42]
Further identification of each cell population can be achieved by studying specific marker expressions, as detailed in the following table:
| Cell Type | Immunophenotypic Marker |
| T lymphocytes | CD3, CD2, CD5, CD7, CD4 or CD8 |
| B lymphocytes | CD19, CD20, CD79a, CD22 |
| Monocytes | CD14, CD64, CD11b, CD11c |
| Neutrophils | CD13, CD15, CD33 |
| Myeloblasts | CD13, CD33, myeloperoxidase, CD34(+/−), CD117(+/−), HLA-DR(+/−) |
| B lymphoblasts | CD34, CD19, CD20, CD22, CD79a, TdT(+/−), CD10 (+/−) |
| T lymphoblasts | Cytoplasmic CD3, CD34(+/−), TdT(+/−), CD2, CD7(+/−) |
| Atypical promyelocytes (in APML) | CD117, CD13, CD33, CD64(+/−), HLA-DR(−), CD34(−) |
APML, acute promyelocytic leukemia.
Clinical Significance
The immuno-expression of a cell population should be interpreted in comparison with the normal internal negative and positive controls. For example, CD19-positive cells, such as B cells, can be used as a positive control for determining the expression of CD19 in any abnormal cell population. On the other hand, T cells can be used as a negative control for CD19.
In cases of acute lymphoblastic leukemia on treatment, bone marrow aspirate smears can show atypical hematogones, which must be differentiated from residual lymphoblast cells on flow cytometry.[43][44]
Flow cytometry and morphology are complementary investigations in most cases. Therefore, it is advisable to perform a preliminary morphological evaluation of the sample before conducting flow cytometry, particularly when a hematolymphoid malignancy is suspected. This preliminary examination aids in selecting the appropriate immunophenotyping panel.[40]
At the same time, it is important to note that some cells may be lost during sample processing steps such as washing, which can affect the accuracy of differential cell counts, especially in leukemia cases. Thus, a morphological evaluation is necessary in borderline cases where blasts are less than 20% in flow cytometry analysis.
Enhancing Healthcare Team Outcomes
To ensure accurate and effective flow cytometry analysis, submitting a fully completed request form along with the specimens is crucial. The form should include essential patient identifiers, the name of the submitting facility, and the clinical diagnosis. Additionally, relevant clinical features and any hematological or supportive investigations that may provide context to the sample should be detailed. The time of specimen collection must be specified, as this can impact the quality and interpretation of the results. If a specific flow cytometry panel is required, it should be clearly indicated on the form. Providing all this information helps the laboratory facility prepare for and conduct the analysis appropriately.
In cases where previous flow cytometry reports are available, these should also be submitted with the current request. Historical data can provide valuable insights into the patient's condition, facilitate comparisons with previous results, and aid in the overall interpretation of the new data. Comprehensive documentation ensures that the flow cytometry laboratory can deliver precise and meaningful results, ultimately contributing to accurate diagnosis and effective patient management.
Nursing, Allied Health, and Interprofessional Team Interventions
The laboratory technical staff should receive comprehensive training to ensure successful flow cytometry results. Staff training should cover accurate sample processing, reagent handling, instrument maintenance, daily QC checks, preliminary result interpretation, and troubleshooting. New laboratory personnel should undergo thorough training and assessment to meet these standards.
All samples collected or received for flow cytometry must be handled with strict adherence to universal precautions. Safety measures include wearing gloves and laboratory coats during sample processing, avoiding aerosol formation and mouth pipetting, and following proper biomedical waste disposal protocols. Regular disinfection of the work area is essential.
Conducting QC with each experiment is advisable. External quality assurance programs are available from a few agencies (EuroFlow. EuroFlow External Quality Assessment Program). The laboratory facility can opt for them for better and quality results.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
El-Hajjar L, Ali Ahmad F, Nasr R. A Guide to Flow Cytometry: Components, Basic Principles, Experimental Design, and Cancer Research Applications. Current protocols. 2023 Mar:3(3):e721. doi: 10.1002/cpz1.721. Epub [PubMed PMID: 36946745]
McKinnon KM. Flow Cytometry: An Overview. Current protocols in immunology. 2018 Feb 21:120():5.1.1-5.1.11. doi: 10.1002/cpim.40. Epub 2018 Feb 21 [PubMed PMID: 29512141]
Level 3 (low-level) evidenceKhoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul:36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22 [PubMed PMID: 35732831]
Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul:36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22 [PubMed PMID: 35732829]
Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015 Jul 23:126(4):454-62. doi: 10.1182/blood-2015-02-585059. Epub 2015 Jun 11 [PubMed PMID: 26065657]
Mukkamalla SKR, Taneja A, Malipeddi D, Master SR. Chronic Lymphocytic Leukemia. StatPearls. 2024 Jan:(): [PubMed PMID: 29261864]
Flores-Montero J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, Sanoja L, Puig N, Lecrevisse Q, Vidriales MB, van Dongen JJ, Orfao A. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry. Part B, Clinical cytometry. 2016 Jan:90(1):61-72. doi: 10.1002/cyto.b.21265. Epub 2015 Jul 31 [PubMed PMID: 26100534]
Buldini B, Maurer-Granofszky M, Varotto E, Dworzak MN. Flow-Cytometric Monitoring of Minimal Residual Disease in Pediatric Patients With Acute Myeloid Leukemia: Recent Advances and Future Strategies. Frontiers in pediatrics. 2019:7():412. doi: 10.3389/fped.2019.00412. Epub 2019 Oct 11 [PubMed PMID: 31681710]
Level 3 (low-level) evidenceCazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood. 2013 Dec 12:122(25):4021-34. doi: 10.1182/blood-2013-09-381665. Epub 2013 Oct 17 [PubMed PMID: 24136165]
Della Porta MG, Picone C. Diagnostic Utility of Flow Cytometry in Myelodysplastic Syndromes. Mediterranean journal of hematology and infectious diseases. 2017:9(1):e2017017. doi: 10.4084/MJHID.2017.017. Epub 2017 Feb 15 [PubMed PMID: 28293405]
Westerman LE, Kohatsu L, Ortiz A, McClain B, Kaplan J, Spira T, Marston B, Jani IV, Nkengasong J, Parsons LM. A quality management systems approach for CD4 testing in resource-poor settings. American journal of clinical pathology. 2010 Oct:134(4):556-67. doi: 10.1309/AJCPP7MCHFYLX2FM. Epub [PubMed PMID: 20855636]
Level 2 (mid-level) evidenceGabali A. Serous fluids and hematolymphoid disorders. CytoJournal. 2022:19():17. doi: 10.25259/CMAS_02_12_2021. Epub 2022 Mar 19 [PubMed PMID: 35510123]
N P, Shanmugam SG, Devi SS, Chinambedu Dandapani MP, S R, D'Cruze L. Detection of Human Leukocyte Antigen B27 by Flowcytometry in Patients With Suspected Ankylosing Spondylitis in a Tertiary Care Centre. Cureus. 2021 Feb 25:13(2):e13560. doi: 10.7759/cureus.13560. Epub 2021 Feb 25 [PubMed PMID: 33791178]
Wilailak S, Rochanawutanon M, Srisupundit S, Aumkhyan A, Pattanapanyasat K. Flow cytometric analysis of DNA ploidy and S-phase fraction of Stage IIIB cervical carcinoma. European journal of gynaecological oncology. 2004:25(4):428-30 [PubMed PMID: 15285296]
Level 2 (mid-level) evidenceSpyratos F, Briffod M. DNA ploidy and S-phase fraction by image and flow cytometry in breast cancer fine-needle cytopunctures. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1997 Jun:10(6):556-63 [PubMed PMID: 9195572]
Gupta N, Mittal A, Dadu T, Choudhary D, Handoo A. Flow Cytometric DNA Ploidy Analysis in Haemato-Lymphoid Neoplasms: An Analysis of 132 Cases. International journal of hematology-oncology and stem cell research. 2022 Jan 1:16(1):34-46. doi: 10.18502/ijhoscr.v16i1.8440. Epub [PubMed PMID: 35975117]
Level 3 (low-level) evidenceBrando B, Gatti A, Preijers F. Flow Cytometric Diagnosis of Paroxysmal Nocturnal Hemoglobinuria: Pearls and Pitfalls - A Critical Review Article. EJIFCC. 2019 Nov:30(4):355-370 [PubMed PMID: 31814811]
Farias MG, Dal Bó S, Castro SM, da Silva AR, Bonazzoni J, Scotti L, Costa SH. Flow Cytometry in Detection of Fetal Red Blood Cells and Maternal F Cells to Identify Fetomaternal Hemorrhage. Fetal and pediatric pathology. 2016:35(6):385-391 [PubMed PMID: 27494244]
Chaudhary R, Das SS. Application of flow cytometry in transfusion medicine: The Sanjay Gandhi Post Graduate Institute of Medical Sciences, India experience. Asian journal of transfusion science. 2022 Jul-Dec:16(2):159-166. doi: 10.4103/ajts.ajts_61_22. Epub 2022 Sep 28 [PubMed PMID: 36687536]
Wienholt L, Lane M, Grey A, Hughes T. Recommendations for the reporting of B cell populations in the context of common variable immunodeficiency disorder (CVID). Pathology. 2019 Oct:51(6):640-641. doi: 10.1016/j.pathol.2019.04.013. Epub 2019 Aug 26 [PubMed PMID: 31466862]
Robinson JP, Ostafe R, Iyengar SN, Rajwa B, Fischer R. Flow Cytometry: The Next Revolution. Cells. 2023 Jul 17:12(14):. doi: 10.3390/cells12141875. Epub 2023 Jul 17 [PubMed PMID: 37508539]
Telford WG. Flow cytometry and cell sorting. Frontiers in medicine. 2023:10():1287884. doi: 10.3389/fmed.2023.1287884. Epub 2023 Nov 22 [PubMed PMID: 38076273]
Piyasena ME, Graves SW. The intersection of flow cytometry with microfluidics and microfabrication. Lab on a chip. 2014 Mar 21:14(6):1044-59. doi: 10.1039/c3lc51152a. Epub [PubMed PMID: 24488050]
Barteneva NS, Fasler-Kan E, Vorobjev IA. Imaging flow cytometry: coping with heterogeneity in biological systems. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012 Oct:60(10):723-33 [PubMed PMID: 22740345]
Rees P, Summers HD, Filby A, Carpenter AE, Doan M. Imaging flow cytometry: a primer. Nature reviews. Methods primers. 2022:2():. pii: 86. doi: 10.1038/s43586-022-00167-x. Epub 2022 Nov 3 [PubMed PMID: 37655209]
Leipold MD, Newell EW, Maecker HT. Multiparameter Phenotyping of Human PBMCs Using Mass Cytometry. Methods in molecular biology (Clifton, N.J.). 2015:1343():81-95. doi: 10.1007/978-1-4939-2963-4_7. Epub [PubMed PMID: 26420710]
Goddard GR, Sanders CK, Martin JC, Kaduchak G, Graves SW. Analytical performance of an ultrasonic particle focusing flow cytometer. Analytical chemistry. 2007 Nov 15:79(22):8740-6 [PubMed PMID: 17924647]
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clinical immunology (Orlando, Fla.). 2004 Mar:110(3):252-66 [PubMed PMID: 15047203]
Medeiros NI, Gomes JAS. Cytometric Bead Array (CBA) for Measuring Cytokine Levels in Chagas Disease Patients. Methods in molecular biology (Clifton, N.J.). 2019:1955():309-314. doi: 10.1007/978-1-4939-9148-8_23. Epub [PubMed PMID: 30868537]
Lugli E, Roederer M, Cossarizza A. Data analysis in flow cytometry: the future just started. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2010 Jul:77(7):705-13. doi: 10.1002/cyto.a.20901. Epub [PubMed PMID: 20583274]
Spidlen J, Moore W, Parks D, Goldberg M, Bray C, Bierre P, Gorombey P, Hyun B, Hubbard M, Lange S, Lefebvre R, Leif R, Novo D, Ostruszka L, Treister A, Wood J, Murphy RF, Roederer M, Sudar D, Zigon R, Brinkman RR. Data File Standard for Flow Cytometry, version FCS 3.1. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2010 Jan:77(1):97-100. doi: 10.1002/cyto.a.20825. Epub [PubMed PMID: 19937951]
Cossarizza A, Chang HD, Radbruch A, Abrignani S, Addo R, Akdis M, Andrä I, Andreata F, Annunziato F, Arranz E, Bacher P, Bari S, Barnaba V, Barros-Martins J, Baumjohann D, Beccaria CG, Bernardo D, Boardman DA, Borger J, Böttcher C, Brockmann L, Burns M, Busch DH, Cameron G, Cammarata I, Cassotta A, Chang Y, Chirdo FG, Christakou E, Čičin-Šain L, Cook L, Corbett AJ, Cornelis R, Cosmi L, Davey MS, De Biasi S, De Simone G, Del Zotto G, Delacher M, Di Rosa F, Di Santo J, Diefenbach A, Dong J, Dörner T, Dress RJ, Dutertre CA, Eckle SBG, Eede P, Evrard M, Falk CS, Feuerer M, Fillatreau S, Fiz-Lopez A, Follo M, Foulds GA, Fröbel J, Gagliani N, Galletti G, Gangaev A, Garbi N, Garrote JA, Geginat J, Gherardin NA, Gibellini L, Ginhoux F, Godfrey DI, Gruarin P, Haftmann C, Hansmann L, Harpur CM, Hayday AC, Heine G, Hernández DC, Herrmann M, Hoelsken O, Huang Q, Huber S, Huber JE, Huehn J, Hundemer M, Hwang WYK, Iannacone M, Ivison SM, Jäck HM, Jani PK, Keller B, Kessler N, Ketelaars S, Knop L, Knopf J, Koay HF, Kobow K, Kriegsmann K, Kristyanto H, Krueger A, Kuehne JF, Kunze-Schumacher H, Kvistborg P, Kwok I, Latorre D, Lenz D, Levings MK, Lino AC, Liotta F, Long HM, Lugli E, MacDonald KN, Maggi L, Maini MK, Mair F, Manta C, Manz RA, Mashreghi MF, Mazzoni A, McCluskey J, Mei HE, Melchers F, Melzer S, Mielenz D, Monin L, Moretta L, Multhoff G, Muñoz LE, Muñoz-Ruiz M, Muscate F, Natalini A, Neumann K, Ng LG, Niedobitek A, Niemz J, Almeida LN, Notarbartolo S, Ostendorf L, Pallett LJ, Patel AA, Percin GI, Peruzzi G, Pinti M, Pockley AG, Pracht K, Prinz I, Pujol-Autonell I, Pulvirenti N, Quatrini L, Quinn KM, Radbruch H, Rhys H, Rodrigo MB, Romagnani C, Saggau C, Sakaguchi S, Sallusto F, Sanderink L, Sandrock I, Schauer C, Scheffold A, Scherer HU, Schiemann M, Schildberg FA, Schober K, Schoen J, Schuh W, Schüler T, Schulz AR, Schulz S, Schulze J, Simonetti S, Singh J, Sitnik KM, Stark R, Starossom S, Stehle C, Szelinski F, Tan L, Tarnok A, Tornack J, Tree TIM, van Beek JJP, van de Veen W, van Gisbergen K, Vasco C, Verheyden NA, von Borstel A, Ward-Hartstonge KA, Warnatz K, Waskow C, Wiedemann A, Wilharm A, Wing J, Wirz O, Wittner J, Yang JHM, Yang J. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). European journal of immunology. 2021 Dec:51(12):2708-3145. doi: 10.1002/eji.202170126. Epub 2021 Dec 7 [PubMed PMID: 34910301]
McCoy JP Jr, Carey JL, Krause JR. Quality control in flow cytometry for diagnostic pathology. I. Cell surface phenotyping and general laboratory procedures. American journal of clinical pathology. 1990 Apr:93(4 Suppl 1):S27-37 [PubMed PMID: 1690504]
Level 2 (mid-level) evidenceOldaker TA, Wallace PK, Barnett D. Flow cytometry quality requirements for monitoring of minimal disease in plasma cell myeloma. Cytometry. Part B, Clinical cytometry. 2016 Jan:90(1):40-6. doi: 10.1002/cyto.b.21276. Epub 2015 Sep 4 [PubMed PMID: 26201282]
Level 2 (mid-level) evidenceMahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clinics in laboratory medicine. 2007 Sep:27(3):469-85, v [PubMed PMID: 17658403]
Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, Aghaeepour N, Akdis M, Allez M, Almeida LN, Alvisi G, Anderson G, Andrä I, Annunziato F, Anselmo A, Bacher P, Baldari CT, Bari S, Barnaba V, Barros-Martins J, Battistini L, Bauer W, Baumgart S, Baumgarth N, Baumjohann D, Baying B, Bebawy M, Becher B, Beisker W, Benes V, Beyaert R, Blanco A, Boardman DA, Bogdan C, Borger JG, Borsellino G, Boulais PE, Bradford JA, Brenner D, Brinkman RR, Brooks AES, Busch DH, Büscher M, Bushnell TP, Calzetti F, Cameron G, Cammarata I, Cao X, Cardell SL, Casola S, Cassatella MA, Cavani A, Celada A, Chatenoud L, Chattopadhyay PK, Chow S, Christakou E, Čičin-Šain L, Clerici M, Colombo FS, Cook L, Cooke A, Cooper AM, Corbett AJ, Cosma A, Cosmi L, Coulie PG, Cumano A, Cvetkovic L, Dang VD, Dang-Heine C, Davey MS, Davies D, De Biasi S, Del Zotto G, Dela Cruz GV, Delacher M, Della Bella S, Dellabona P, Deniz G, Dessing M, Di Santo JP, Diefenbach A, Dieli F, Dolf A, Dörner T, Dress RJ, Dudziak D, Dustin M, Dutertre CA, Ebner F, Eckle SBG, Edinger M, Eede P, Ehrhardt GRA, Eich M, Engel P, Engelhardt B, Erdei A, Esser C, Everts B, Evrard M, Falk CS, Fehniger TA, Felipo-Benavent M, Ferry H, Feuerer M, Filby A, Filkor K, Fillatreau S, Follo M, Förster I, Foster J, Foulds GA, Frehse B, Frenette PS, Frischbutter S, Fritzsche W, Galbraith DW, Gangaev A, Garbi N, Gaudilliere B, Gazzinelli RT, Geginat J, Gerner W, Gherardin NA, Ghoreschi K, Gibellini L, Ginhoux F, Goda K, Godfrey DI, Goettlinger C, González-Navajas JM, Goodyear CS, Gori A, Grogan JL, Grummitt D, Grützkau A, Haftmann C, Hahn J, Hammad H, Hämmerling G, Hansmann L, Hansson G, Harpur CM, Hartmann S, Hauser A, Hauser AE, Haviland DL, Hedley D, Hernández DC, Herrera G, Herrmann M, Hess C, Höfer T, Hoffmann P, Hogquist K, Holland T, Höllt T, Holmdahl R, Hombrink P, Houston JP, Hoyer BF, Huang B, Huang FP, Huber JE, Huehn J, Hundemer M, Hunter CA, Hwang WYK, Iannone A, Ingelfinger F, Ivison SM, Jäck HM, Jani PK, Jávega B, Jonjic S, Kaiser T, Kalina T, Kamradt T, Kaufmann SHE, Keller B, Ketelaars SLC, Khalilnezhad A, Khan S, Kisielow J, Klenerman P, Knopf J, Koay HF, Kobow K, Kolls JK, Kong WT, Kopf M, Korn T, Kriegsmann K, Kristyanto H, Kroneis T, Krueger A, Kühne J, Kukat C, Kunkel D, Kunze-Schumacher H, Kurosaki T, Kurts C, Kvistborg P, Kwok I, Landry J, Lantz O, Lanuti P, LaRosa F, Lehuen A, LeibundGut-Landmann S, Leipold MD, Leung LYT, Levings MK, Lino AC, Liotta F, Litwin V, Liu Y, Ljunggren HG, Lohoff M, Lombardi G, Lopez L, López-Botet M, Lovett-Racke AE, Lubberts E, Luche H, Ludewig B, Lugli E, Lunemann S, Maecker HT, Maggi L, Maguire O, Mair F, Mair KH, Mantovani A, Manz RA, Marshall AJ, Martínez-Romero A, Martrus G, Marventano I, Maslinski W, Matarese G, Mattioli AV, Maueröder C, Mazzoni A, McCluskey J, McGrath M, McGuire HM, McInnes IB, Mei HE, Melchers F, Melzer S, Mielenz D, Miller SD, Mills KHG, Minderman H, Mjösberg J, Moore J, Moran B, Moretta L, Mosmann TR, Müller S, Multhoff G, Muñoz LE, Münz C, Nakayama T, Nasi M, Neumann K, Ng LG, Niedobitek A, Nourshargh S, Núñez G, O'Connor JE, Ochel A, Oja A, Ordonez D, Orfao A, Orlowski-Oliver E, Ouyang W, Oxenius A, Palankar R, Panse I, Pattanapanyasat K, Paulsen M, Pavlinic D, Penter L, Peterson P, Peth C, Petriz J, Piancone F, Pickl WF, Piconese S, Pinti M, Pockley AG, Podolska MJ, Poon Z, Pracht K, Prinz I, Pucillo CEM, Quataert SA, Quatrini L, Quinn KM, Radbruch H, Radstake TRDJ, Rahmig S, Rahn HP, Rajwa B, Ravichandran G, Raz Y, Rebhahn JA, Recktenwald D, Reimer D, Reis e Sousa C, Remmerswaal EBM, Richter L, Rico LG, Riddell A, Rieger AM, Robinson JP, Romagnani C, Rubartelli A, Ruland J, Saalmüller A, Saeys Y, Saito T, Sakaguchi S, Sala-de-Oyanguren F, Samstag Y, Sanderson S, Sandrock I, Santoni A, Sanz RB, Saresella M, Sautes-Fridman C, Sawitzki B, Schadt L, Scheffold A, Scherer HU, Schiemann M, Schildberg FA, Schimisky E, Schlitzer A, Schlosser J, Schmid S, Schmitt S, Schober K, Schraivogel D, Schuh W, Schüler T, Schulte R, Schulz AR, Schulz SR, Scottá C, Scott-Algara D, Sester DP, Shankey TV, Silva-Santos B, Simon AK, Sitnik KM, Sozzani S, Speiser DE, Spidlen J, Stahlberg A, Stall AM, Stanley N, Stark R, Stehle C, Steinmetz T, Stockinger H, Takahama Y, Takeda K, Tan L, Tárnok A, Tiegs G, Toldi G, Tornack J, Traggiai E, Trebak M, Tree TIM, Trotter J, Trowsdale J, Tsoumakidou M, Ulrich H, Urbanczyk S, van de Veen W, van den Broek M, van der Pol E, Van Gassen S, Van Isterdael G, van Lier RAW, Veldhoen M, Vento-Asturias S, Vieira P, Voehringer D, Volk HD, von Borstel A, von Volkmann K, Waisman A, Walker RV, Wallace PK, Wang SA, Wang XM, Ward MD, Ward-Hartstonge KA, Warnatz K, Warnes G, Warth S, Waskow C, Watson JV, Watzl C, Wegener L, Weisenburger T, Wiedemann A, Wienands J, Wilharm A, Wilkinson RJ, Willimsky G, Wing JB, Winkelmann R, Winkler TH, Wirz OF, Wong A, Wurst P, Yang JHM, Yang J, Yazdanbakhsh M, Yu L, Yue A, Zhang H, Zhao Y, Ziegler SM, Zielinski C, Zimmermann J, Zychlinsky A. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). European journal of immunology. 2019 Oct:49(10):1457-1973. doi: 10.1002/eji.201970107. Epub [PubMed PMID: 31633216]
Hulspas R, O'Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry. Part B, Clinical cytometry. 2009 Nov:76(6):355-64. doi: 10.1002/cyto.b.20485. Epub [PubMed PMID: 19575390]
Wang D, Fang H, Ok CY, Jorgensen JL, Medeiros LJ, Wang W, Wang SA. Advancing Diagnostic Accuracy and Quality of Patient Care Through the Implementation of a Flow Cytometry Quality Assurance Program. Archives of pathology & laboratory medicine. 2024 Jun 14:():. doi: 10.5858/arpa.2024-0020-OA. Epub 2024 Jun 14 [PubMed PMID: 38871355]
Level 2 (mid-level) evidenceKárai B, Miltényi Z, Gergely L, Száraz-Széles M, Kappelmayer J, Hevessy Z. The impact of delayed sample handling and type of anticoagulant on the interpretation of dysplastic signs detected by flow cytometry. Biochemia medica. 2018 Jun 15:28(2):020704. doi: 10.11613/BM.2018.020704. Epub 2018 Apr 15 [PubMed PMID: 29666557]
Wood BL, Arroz M, Barnett D, DiGiuseppe J, Greig B, Kussick SJ, Oldaker T, Shenkin M, Stone E, Wallace P. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry. Part B, Clinical cytometry. 2007:72 Suppl 1():S14-22 [PubMed PMID: 17803189]
Level 3 (low-level) evidenceSaksena A, Gautam P, Desai P, Gupta N, Dubey AP, Singh T. Side scatter versus CD45 flow cytometric plot can distinguish acute leukaemia subtypes. The Indian journal of medical research. 2016 May:143(Supplement):S17-S22. doi: 10.4103/0971-5916.191743. Epub [PubMed PMID: 27748273]
Gupta T, Gupta R, Mittal N, Rahman K, Nityanand S. B-acute Lymphoblastic Leukemia with Bright CD45 and Moderate Side Scatter Simulating Monocytoid Population: An Unusual Phenotype. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2018 Apr:34(2):358-359. doi: 10.1007/s12288-017-0896-7. Epub 2017 Oct 28 [PubMed PMID: 29622886]
Das N, Gajendra S, Gupta R. Analytical Appraisal of Hematogones in B-ALL MRD Assessment Using Multidimensional Dot-Plots by Multiparametric Flow Cytometry: A Critical Review and Update. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2024 Jan:40(1):12-24. doi: 10.1007/s12288-023-01696-5. Epub 2023 Sep 16 [PubMed PMID: 38312180]
Li J, Wertheim G, Paessler M, Pillai V. Flow Cytometry in Pediatric Hematopoietic Malignancies. Clinics in laboratory medicine. 2017 Dec:37(4):879-893. doi: 10.1016/j.cll.2017.07.009. Epub 2017 Sep 28 [PubMed PMID: 29128074]