Introduction
The laboratory investigation of hematological disorders usually begins with a complete blood count performed using automated hematology analyzers that provide information on the number, size, and structure of the blood cells, including erythrocytes or red blood cells (RBCs). RBCs are ordinarily round and biconcave. RBCs have a thin cellular membrane and a cytoplasm that contains hemoglobin, a heme-containing protein for oxygen transport. Disorders that affect RBCs can lead to reduced production, abnormalities of shape or size, or qualitative and quantitative abnormalities of hemoglobin. Erythrocyte inclusions are seen in many RBC disorders. Erythrocyte inclusions may be pathological evidence of specific diseases or disorders when interpreted within the context of presenting clinical symptoms and signs.[1] Characterizing and identifying RBC inclusions can provide or support the diagnosis of various clinical disorders.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
The routine use of highly accurate and reliable automated hematology analyzers in hematology and clinical pathology laboratories has reduced the use of peripheral blood smears as a diagnostic tool. RBC inclusions can be identified on a peripheral smear, providing concise and valuable clues to the diagnosis or indicating further investigative avenues to arrive at a final diagnosis. The structure, compositional characterization, and pathophysiological link of RBC inclusions may provide valuable clues to underlying disease or disorder.[2] Many erythrocyte inclusions, if present in significant amounts, can interfere with the data obtained from an automated hematology analyzer data.[3] In particular, Heinz bodies and Hemoglobin H can cause erroneous results in automated reticulocyte counts.[4][3]
Causes
Various disorders may induce the development of RBC inclusions, including:
- Post-splenectomy asplenia and hyposplenism[5][6]
- Hemoglobinopathies, including thalassemias, unstable hemoglobinopathies, and sickle cell disease[7]
- Heavy metal poisoning due to lead, mercury, and arsenic[8]
- Enzymopathies such as glucose-6-phosphate dehydrogenase (G6PD) deficiency[9]
- Anemias, including but not limited to megaloblastic anemia and sideroblastic anemia[10]
- Autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and celiac disease[11][12]
- Neoplastic hematological disorders, including myelodysplastic syndrome or myelofibrosis[13]
- Various systemic diseases, such as amyloidosis, sarcoidosis, and cirrhosis[14]
Various infectious agents may live part or all of their life cycle within an erythrocyte. These infectious agents are not considered erythrocyte inclusions per se. Examples of this phenomenon include the agents of malaria and babesiosis. Both parasitic infections are seen as intracellular inclusions in erythrocytes. (Image. Ring stage of Malaria parasite Plasmodium falciparum) and (Image. Intracellular and extracellular Babesiosis)
Additionally, late normoblasts containing a pyknotic nucleus and reticulocytes containing RNA remnants are considered normal findings despite appearing morphologically similar to erythrocytes with inclusions.
Clinical Pathology
Most RBC inclusions can be identified and characterized on a routine peripheral blood smear using standard hematological Romanowsky stains, such as Wright or Giemsa. On the basis of their appearance, structural composition, and associated pathophysiology, the most common erythrocyte inclusions are usually categorized as Howell-Jolly, Heinz, or Pappenheimer bodies, basophilic stippling, Cabot rings, or precipitated hemoglobin inclusions, such as HbH.[15][9][8][16][10][17][7]
Morphology
Howell-Jolly Bodies
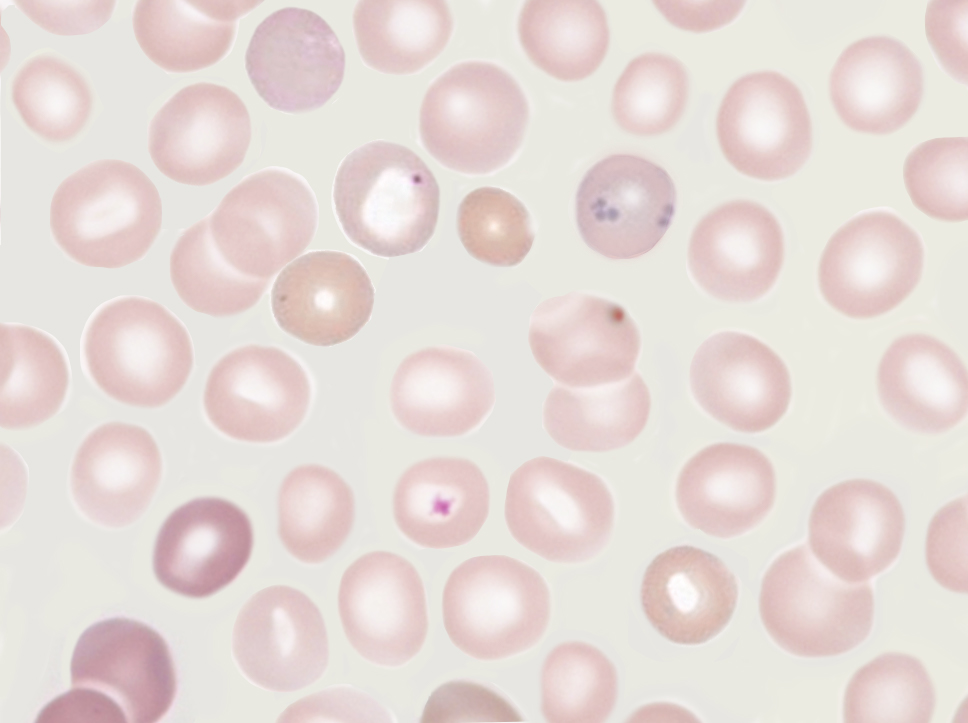
Howell-Jolly bodies represent residual nuclear DNA within a mature erythrocyte. These inclusions are small, dark, basophilic, smooth, round, punctate cytoplasmic bodies. Howell-Jolly bodies stain purple with Romanowsky dyes, such as May-Grünwald-Giemsa, Wright-Giemsa, Leishman, and Diff-Quik® stains (see Image. Howell-Jolly Body).[15]
Heinz Bodies
Heinz bodies represent precipitated, denatured hemoglobin deposits within RBCs. These inclusions are seen attached or close to the cellular membrane, may be single or multiple, and are small, round, refractile inclusions measuring 1 to 3µm in size. Heinz bodies may protrude through the cellular membrane; in a wet blood film, they may be seen moving around in the cell. Heinz bodies are typically poorly visualized with Romanowsky dyes; they may appear as pink-brown nodular structures inclusions. Heinz bodies will stain dark purple-blue and are better visualized with supravital stains such as methylene blue, brilliant cresyl blue, and methyl violet.[9][18]
Pappenheimer Bodies
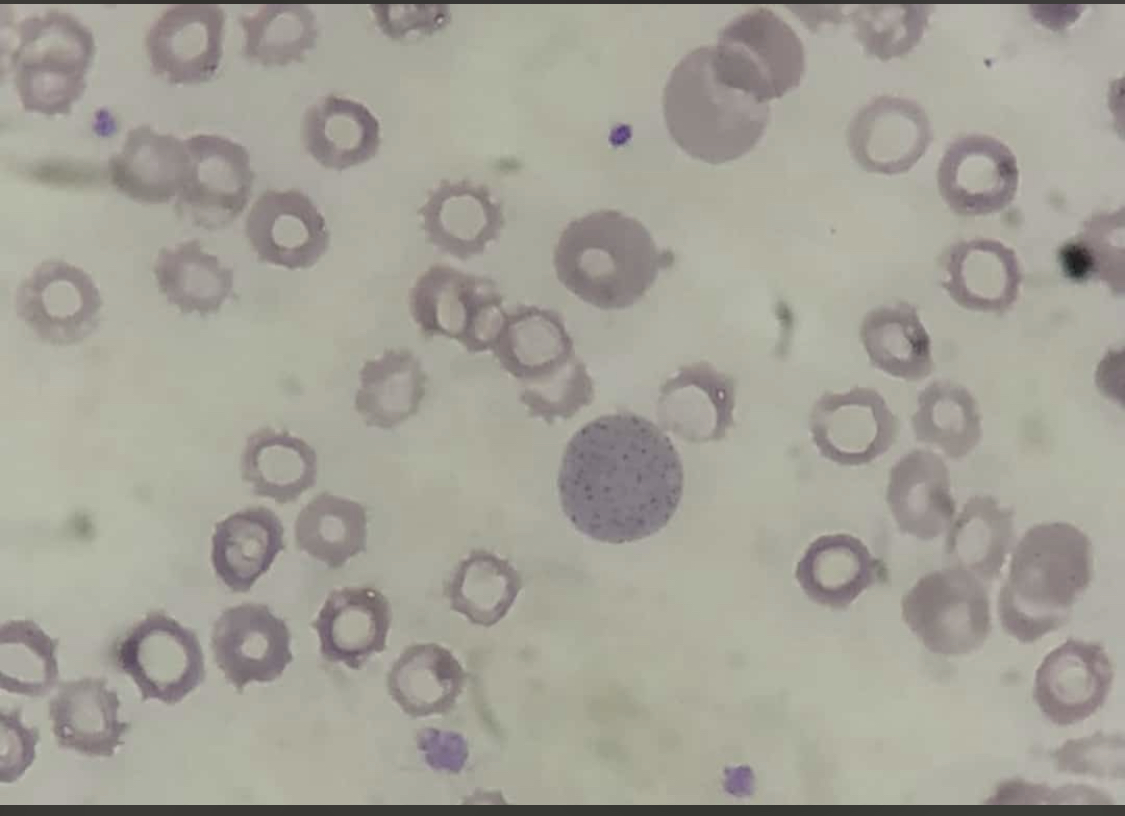
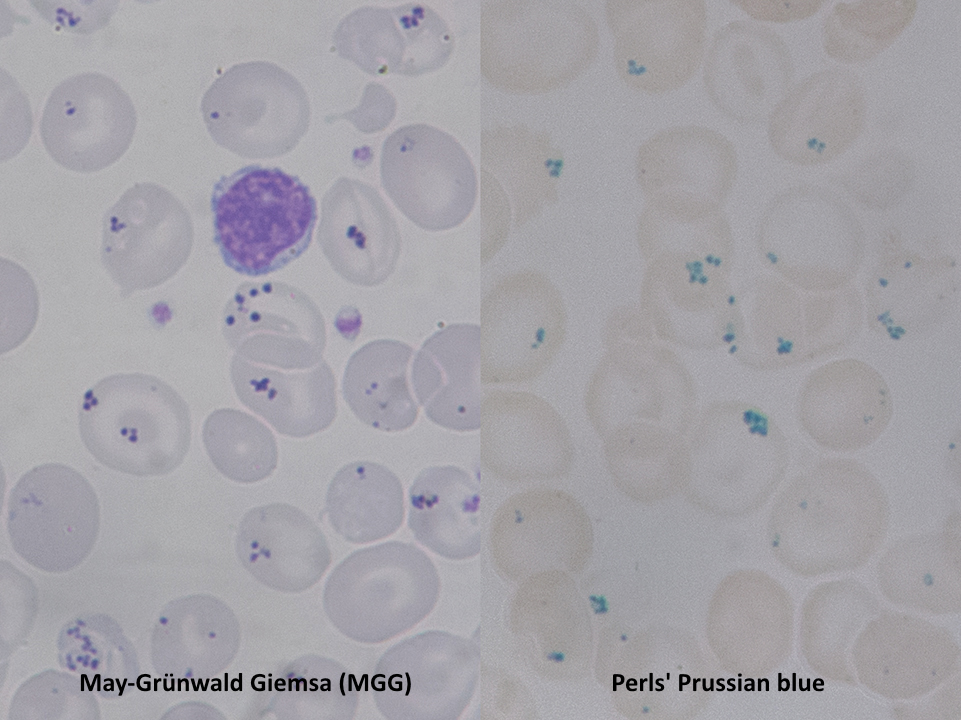
Pappenheimer bodies, or siderotic granules, are hemosiderin-containing, small, round, dark, basophilic or black-colored inclusions situated at the periphery of RBCs. Pappenheimer bodies are not related to iron-overload conditions but the result of sideroblastic erythropoiesis producing siderotic, non-heme iron intracytoplasmic granules. The granules contain ferric iron, lipids, proteins, and carbohydrates. Pappenheimer bodies can be visualized with Romanowsky dyes and are much smaller than Howell-Jolly bodies. While multiple intracellular inclusions may be seen, cells usually contain only a few. If many Pappenheimer bodies are present, this finding can easily be confused with punctate basophilia. However, because Pappenheimer bodies contain hemosiderin, they will stain blue with Perls Prussian blue (see Image. Pappenheimer Bodies). In contrast, punctate basophilia will appear pink.[19]
Basophilic Stippling
Basophilic stippling, or punctate basophilia, indicates disturbed erythropoiesis, such as defective or accelerated heme synthesis. Basophilic stippling is characterized by numerous irregular basophilic granules dispersed throughout the cell. The granules may be fine or coarse. Punctate basophilia is frequently seen in disorders of impaired hemoglobin synthesis and possibly due to the production of an aberrant unstable RNA during maturation. Examples of these disorders include megaloblastic anemia, lead poisoning, and certain hemoglobinopathies. Basophilic stippling is well-visualized on peripheral blood smears using Romanowsky stains (see Image. Basophilic Stippling on Peripheral Blood Smear).[8][16]
Cabot Rings
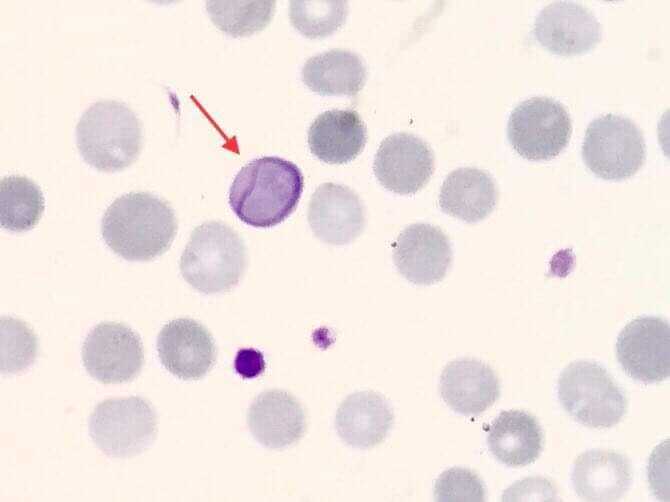
Cabot rings are ring-like or figure-of-eight loop-shaped inclusions composed of microtubule remnants from the mitotic spindle, or possibly nuclear remnants or abnormal histones.[20] Occasionally Cabot rings may appear as concentric lines or inclusions with double or multiple loops. Cabot rings stain red, red-purple, or blue-purple with Romanowsky dyes (see Image. Cabot Ring in the Red Blood Cell). Cabot rings are typically seen in patients with dyserythropoiesis, such as megaloblastic anemia.
Precipitated Hemoglobin Inclusions
Hemoglobin H (HbH) inclusions are precipitated beta-chain tetramers seen in alpha-thalassemia.[7] Alpha-chain inclusions represent alpha-chain precipitation in beta-thalassemia major and appear as irregular nodules close to the nucleus of the marrow erythroblasts when stained with supravital methyl violet or brilliant cresyl blue stains.[21]
Mechanisms
Due to overlapping morphology, RBC inclusions may need to be differentiated from other structures.
Pappenheimer bodies resemble the granules of basophilic stippling. However, in contrast to the dispersed and numerous granules of basophilic stippling, Pappenheimer bodies are single or few and easily distinguished with Prussian blue staining.
Reticulocytes may contain dot-like structures resembling Pappenheimer bodies. Pappenheimer bodies are much darker than the reticulofilamentous dots of reticulocytes; it may be necessary to utilize a Perls stain to differentiate between the two.
HbH may denature and form greenish, round inclusion bodies when staining for reticulocytes with new methylene blue. Heinz bodies may also stain a light blue with new methylene blue used for reticulocyte staining. However, examining the new methylene blue-stained slide with phase contrast microscopy will help differentiate late reticulocytes from erythrocytes with inclusion bodies.
Clinicopathologic Correlations
Howell-Jolly Bodies
Howell-Jolly bodies are seen in significant numbers in patients with splenic atrophy or who have undergone splenectomy. Vitamin B12 deficiency may also result in small numbers of RBCs containing Howell-Jolly bodies, mainly if the deficiency is chronic or severe and megaloblastosis has occurred.[15]
Heinz Bodies
Heinz bodies may be seen in any disorder of unstable hemoglobin, chemical poisoning, drug toxicity, or G6PD deficiency due to the precipitation or denaturation of hemoglobin within the RBC.[9] Heinz bodies may also be detected in severely unstable hemoglobinopathies causing hemolytic anemia; Hemoglobin Köln is the most common example of this disorder.[22] Hemoglobin electrophoresis facilitates the diagnosis of unstable hemoglobinopathies.
Heinz bodies may also be seen after splenectomy and in hemolytic anemias due to acute oxidant-induced hemoglobin denaturation, such as G6PD deficiency.[9] If Heinz bodies are visualized on the peripheral blood smear, appropriate further testing may include hemoglobin electrophoresis, glutathione stability testing, drug-dependent antibody titers, methemoglobin, and sulfhemoglobin.
Basophilic Stippling
Basophilic stippling is seen in various conditions, such as lead and other heavy metal poisonings, infections, liver disease, thalassemia, and some cases of megaloblastic anemia. Basophilic stippling in microcytic, normochromic anemia highly suggests a thalassemia trait.
In patients with lead poisoning, coarse and fine basophilic stippling can be seen. The underlying mechanism of basophilic stippling in lead poisoning appears to be the interaction of lead with thiol, carboxylic, and phosphate groups to form stable complexes with enzymes and block pyrimidine 5′-nucleotidase from removing clumped or denatured intracellular RNA. This clumping of RNA complexes gives rise to the observed basophilic stippling.[23] Additionally, pronounced basophilic stippling affecting more than 5% of erythrocytes may be seen in congenital pyrimidine 5′-nucleotidase deficiency, an autosomal recessive disorder that presents with hereditary non-spherocytic hemolytic anemia.[24]
Pappenheimer Bodies
Pappenheimer bodies in the presence of microcytic anemia suggest sideroblastic erythropoiesis, which may be seen in sideroblastic anemia, lead poisoning, or hemochromatosis. Surgical asplenia may result in the formation of Pappenheimer bodies. The functional asplenia or hyposplenism of sickle cell disease may lead to increased erythrocytes with Pappenheimer bodies.[19] These inclusions may also be seen in myelodysplastic syndrome.
Cabot Rings
These erythrocyte inclusions are commonly seen in megaloblastic anemia, myelodysplastic syndrome, and lead poisoning.[17]
Hemoglobin Inclusions
Inclusions of hemoglobin are abundant in the normoblasts of patients with beta-thalassemia and other thalassemias.[21] However, patients who have undergone splenectomy will also have increased numbers of these inclusions on the peripheral blood smear.[7]
Clinical Significance
Erythrocyte inclusions can be diagnostic for many conditions. After obtaining a comprehensive medical history and performing a thorough physical examination, testing for erythrocyte inclusions can help determine specific hematological diagnoses.
A frequent initial complaint of many hematological pathologies is fatigue, which may be isolated or concurrent with other symptoms. A concern for anemia often prompts laboratory testing. While a complete blood count (CBC) is often helpful, there are times when a peripheral blood smear is needed. The medical history and physical examination may indicate a disease with an associated erythrocyte inclusion.
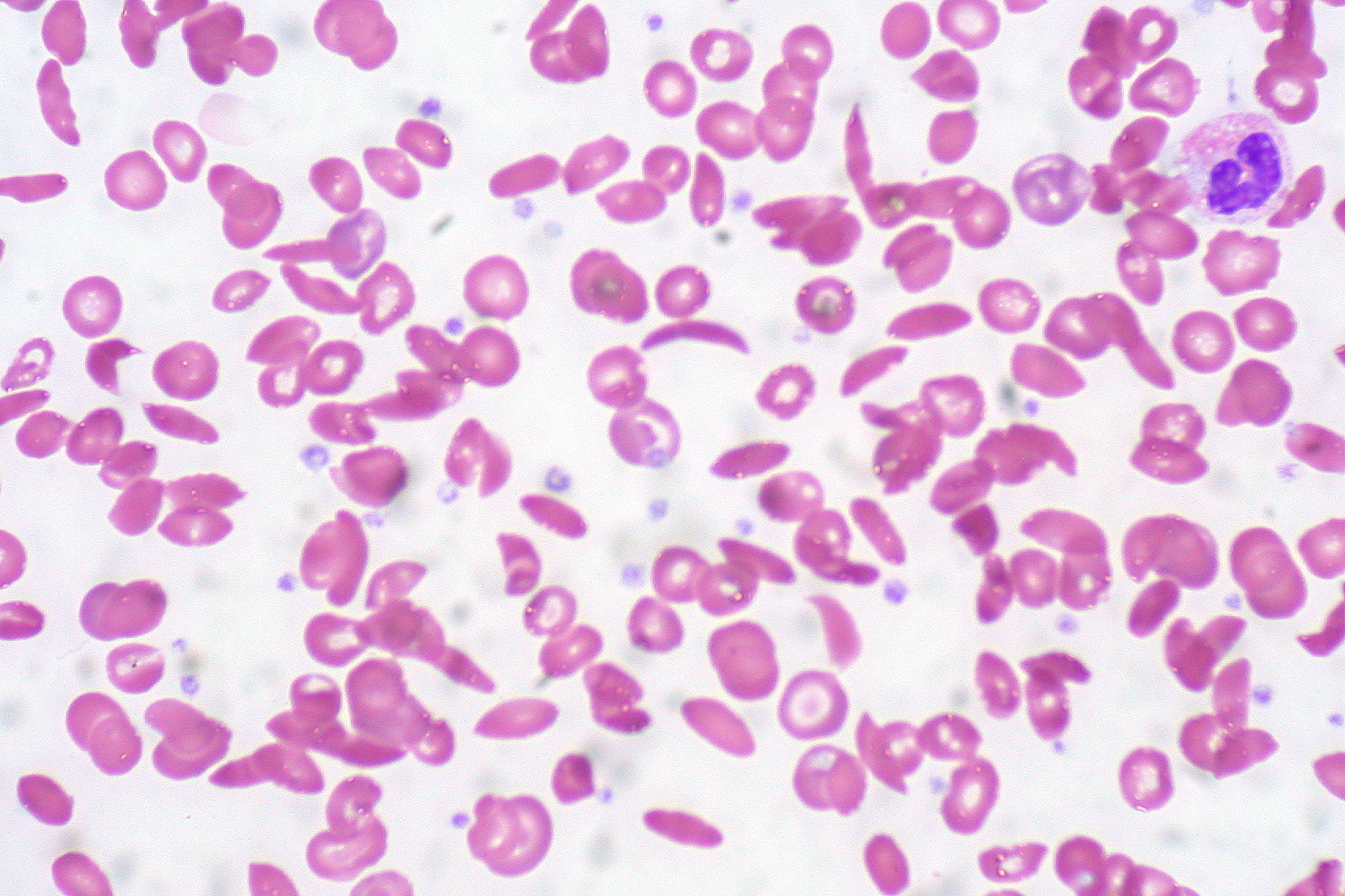
Howell-Jolly bodies on a peripheral blood smear can be observed in sickle cell disease, asplenia, myelodysplastic syndrome, and many other conditions. While most infants born in the United States are screened for sickle cell disease, these screens can be missed or erroneous (see Image. Sickle cells on a peripheral smear. RBC inclusions are not seen). Additionally, screening for sickle cell disease is not performed in all countries. Infants with jaundice or unexplained pain episodes will benefit from a CBC and peripheral blood smear. If the smear reveals Howell-Jolly bodies or sickled erythrocytes, hemoglobin electrophoresis can confirm sickle cell disease. Early diagnosis of sickle cell disease is imperative to avoid the complications of stroke, nephropathy, avascular necrosis of bone, and priapism.[25] Treatments for sickle cell disease include hydroxyurea and prophylactic penicillin. More recently, crizanlizumab, L-glutamine, and voxelotor have become available. Hematopoeitic stem cell transplant can be curative.[25][26]
Heinz bodies are seen in various hemoglobinopathies, the thalassemias, and G6PD deficiency. Although G6PD deficiency is diagnosed in patients worldwide, the deficiency is more common along the malaria belts of Asia, the Mediterranean, sub-Saharan Africa, and the Middle East.[27] The most common form of G6PD deficiency is a genetic disorder, and patients will often report a family history of episodic hemolytic anemia triggered by certain drugs or infections. Qualitative testing for enzymatic activity is diagnostic.[28] Patients with the most common type of G6PD deficiency experience no symptoms unless exposed to fava beans, certain drugs, or infections that precipitate hemolysis of red blood cells.[27] Pharmaceutical triggers of episodic hemolysis include nitrofurantoin, dapsone, primaquine, ribavirin, and rifampin.[28] Hemolysis may also be triggered by infection with the hepatitis A, B, or E viruses, certain streptococcal and staphylococcal infections, or infection with cytomegalovirus.[27] Once diagnosed, prevention of hemolysis includes avoidance of triggers. Treatment of an episode of hemolysis requires supportive care, folic acid, and blood transfusion if the anemia is severe.[28]
Basophilic stippling may be coarse or fine. Coarse basophilic stippling is found in several conditions, including sideroblastic anemia, myelodysplastic syndrome, lead poisoning, and thalassemia. Fine basophilic stippling can be seen in health and disease processes characterized by polychromatophilia, the increased production of red blood cells.[1]
Basophilic stippling on the peripheral blood smear of patients with lead poisoning is very common.[29][30][29] Children are most commonly affected by lead poisoning, although adults may also be affected. Adults with lead poisoning tend to have vague symptoms such as fatigue and chronic abdominal pain. Early childhood screening for lead toxicity is routinely performed; the Centers for Disease Control publish age-based screening guidelines for all children with special guidelines for children at increased risk of lead exposure.. children with lead poisoning may be asymptomatic or complain of constipation, headaches, or abdominal pain. At high levels, lead toxicity may present with appetite loss, drowsiness, difficulty concentrating, anxiety, and seizure.[31] Blood lead levels are confirmatory for the diagnosis.[31] Treatment includes chelation therapy and prevention through reducing exposure to lead by replacing lead-based materials found in paint, building construction materials, foods, water pipes, and more.[31]
Pappenheimer bodies are frequently seen in sideroblastic anemia, lead poisoning, the thalassemias, and hemochromatosis.[32] Primary hereditary hemochromatosis is characterized by disordered iron metabolism. There are four types of hereditary hemochromatosis, the most common of which is an autosomal recessive disorder. Iron deposits in the liver, heart, skin, pancreas, pituitary gland, and joints.[33] Symptoms of hereditary hemochromatosis may include but are not limited to fatigue, joint pain, and erectile dysfunction.[34] Iron overload can result in cardiomyopathy, heart failure, cirrhosis, and hepatocellular carcinoma.[33] Pappenheimer bodies seen on a peripheral blood smear will narrow the diagnostic differential. Further evaluation of suspected hereditary hemochromatosis includes iron studies and liver enzymes; magnetic resonance imaging of the liver or liver elastography can define the degree of liver involvement. Genetic testing is confirmatory.[34] The initial treatment of hereditary hemochromatosis is serial phlebotomy; organ-specific pathology from the iron overload is managed as needed.[33]
Cabot rings are frequently observed in myelodysplastic syndrome, myelofibrosis, chronic myeloid leukemia, and megaloblastic anemia.[17] Megaloblastic anemia is often the result of nutritional deficiencies, including vitamin B12 and folate deficiency. Deficiencies commonly arise from reduced dietary intake, as seen in vegetarian or vegan diets, or impaired absorption.[35] Celiac disease is one of many diseases that can impair the absorption of vitamin B12 and iron. The use of certain medications, inborn errors of metabolism, pernicious anemia, and many other pathologic processes may result in megaloblastic anemia.[35] Early symptoms of vitamin B12 deficiency include fatigue, glossitis, and angular cheilitis, none of which are specific to vitamin B12 deficiency. Later symptoms of vitamin B12 deficiency may include decreased peripheral position and vibratory sensation, and impaired coordination and balance. Neuropsychiatric symptoms are a late finding.[35] Once vitamin B12 deficiency is suspected, a serum vitamin B12 serum level should be assessed. If the level is nondiagnostic, an assessment of methylmalonic acid and homocysteine levels may aid in the diagnosis. Treatment of vitamin B12 deficiency requires vitamin B12 replacement via oral or injectable means and management of any underlying disorder.[36]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Ford J. Red blood cell morphology. International journal of laboratory hematology. 2013 Jun:35(3):351-7. doi: 10.1111/ijlh.12082. Epub 2013 Mar 9 [PubMed PMID: 23480230]
Ford JC, Milner R, Dix DB. Red blood cell morphology reporting: how much is a waste of time? Journal of pediatric hematology/oncology. 2011 Jan:33(1):10-4. doi: 10.1097/MPH.0b013e3181fd6c8b. Epub [PubMed PMID: 21088617]
Lai SK, Yow CM, Benzie IF. Interference of Hb-H disease in automated reticulocyte counting. Clinical and laboratory haematology. 1999 Aug:21(4):261-4 [PubMed PMID: 10583328]
Hinchliffe RF. Errors in automated reticulocyte counts due to Heinz bodies. Journal of clinical pathology. 1993 Sep:46(9):878-9 [PubMed PMID: 8227446]
Level 3 (low-level) evidenceLenti MV, Luu S, Carsetti R, Osier F, Ogwang R, Nnodu OE, Wiedermann U, Spencer J, Locatelli F, Corazza GR, Di Sabatino A. Asplenia and spleen hypofunction. Nature reviews. Disease primers. 2022 Nov 3:8(1):71. doi: 10.1038/s41572-022-00399-x. Epub 2022 Nov 3 [PubMed PMID: 36329079]
Solis M, Perrin J, Guédenet JC, Lesesve JF. [RBCs inclusions after splenectomy: not only Howell-Jolly bodies!]. Annales de biologie clinique. 2013 Mar-Apr:71(2):185-9. doi: 10.1684/abc.2013.0798. Epub [PubMed PMID: 23587584]
Level 3 (low-level) evidenceSpencer-Chapman M, Luqmani A, Layton DM, Bain BJ. Unusual inclusions in hemoglobin H disease post-splenectomy. American journal of hematology. 2018 Jul:93(7):963-964. doi: 10.1002/ajh.25072. Epub 2018 May 6 [PubMed PMID: 29473200]
Sanchez JR, Lynch DT. Histology, Basophilic Stippling. StatPearls. 2023 Jan:(): [PubMed PMID: 31424843]
Herman TF, Killeen RB, Javaid MU. Heinz Body. StatPearls. 2023 Jan:(): [PubMed PMID: 31869086]
Fyfe AJ, Soutar RL. Multiple Pappenheimer bodies. British journal of haematology. 2005 Sep:130(5):651 [PubMed PMID: 16115118]
Level 3 (low-level) evidenceChilds JC, Adelizzi RA, Dabrow MB, Freed N. Splenic hypofunction in systemic lupus erythematosus. The Journal of the American Osteopathic Association. 1994 May:94(5):414-5 [PubMed PMID: 8056631]
Level 3 (low-level) evidenceO'Grady JG, Stevens FM, McCarthy CF. Celiac disease: does hyposplenism predispose to the development of malignant disease? The American journal of gastroenterology. 1985 Jan:80(1):27-9 [PubMed PMID: 3966450]
Tong YT, Nguyen ND, Wahed A. Howell-Jolly Body-Like Inclusions in Neutrophils of Patients With Myelodysplastic Syndrome: A Novel Correlation. Archives of pathology & laboratory medicine. 2019 Jan:143(1):112-114. doi: 10.5858/arpa.2017-0328-OA. Epub 2018 Jul 30 [PubMed PMID: 30059259]
Boyko WJ, Pratt R, Wass H. Functional hyposplenism, a diagnostic clue in amyloidosis. Report of six cases. American journal of clinical pathology. 1982 Jun:77(6):745-8 [PubMed PMID: 7091054]
Level 3 (low-level) evidenceScafidi JM, Amraei R, Gupta V. Histology, Howell Jolly Bodies. StatPearls. 2023 Jan:(): [PubMed PMID: 32491421]
Kano N, Fukui S, Kushiro S, Inui A, Saita M, Kura Y, Sawada U, Naito T. Basophilic stippling in red blood cells in the bone marrow: indication for lead poisoning diagnosis. The Journal of international medical research. 2022 Feb:50(2):3000605221078405. doi: 10.1177/03000605221078405. Epub [PubMed PMID: 35184610]
Erdem N, Berber İ, Aydoğdu İ, Sevinç A. A hundred years after the first article, a recollection: Cabot ring. The Korean journal of internal medicine. 2016 Jan:31(1):199. doi: 10.3904/kjim.2016.31.1.199. Epub 2015 Dec 28 [PubMed PMID: 26767879]
Jacob HS. Mechanisms of Heinz body formation and attachment to red cell membrane. Seminars in hematology. 1970 Jul:7(3):341-54 [PubMed PMID: 5425759]
Dubey A, Dey AK, Nandy K, Garg A, Kadakia M. Congenital Sideroblastic Anaemia- Classic Presentation. Journal of clinical and diagnostic research : JCDR. 2016 Sep:10(9):OJ01-OJ02 [PubMed PMID: 27790505]
Bessis M. [Erythrocyte form and deformability for normal blood and some hereditary hemolytic anemias (author's transl)]. Nouvelle revue francaise d'hematologie; blood cells. 1977:18(1):75-94 [PubMed PMID: 896459]
Bain BJ. Alpha chain inclusions in E/beta thalassemia. American journal of hematology. 2008 Nov:83(11):871. doi: 10.1002/ajh.21223. Epub [PubMed PMID: 18553561]
Level 3 (low-level) evidenceMiller DR, Weed RI, Stamatoyannopoulos G, Yoshida A. Hemoglobin Köln disease occurring as a fresh mutation: erythrocyte metabolism and survival. Blood. 1971 Dec:38(6):715-29 [PubMed PMID: 4942314]
de la Serna FJ, Gilsanz F, Ricard P, Urrutia A. [Hemolytic anemia caused by pyrimidine 5'-nucleotidase (P5N) deficiency 15 years later. Apropos of 2 new cases of hereditary deficit and another one of lead poisoning]. Medicina clinica. 1989 Oct 7:93(10):380-2 [PubMed PMID: 2558262]
Level 3 (low-level) evidenceRees DC, Duley JA, Marinaki AM. Pyrimidine 5' nucleotidase deficiency. British journal of haematology. 2003 Feb:120(3):375-83 [PubMed PMID: 12580951]
Kavanagh PL, Fasipe TA, Wun T. Sickle Cell Disease: A Review. JAMA. 2022 Jul 5:328(1):57-68. doi: 10.1001/jama.2022.10233. Epub [PubMed PMID: 35788790]
Brandow AM, Liem RI. Advances in the diagnosis and treatment of sickle cell disease. Journal of hematology & oncology. 2022 Mar 3:15(1):20. doi: 10.1186/s13045-022-01237-z. Epub 2022 Mar 3 [PubMed PMID: 35241123]
Level 3 (low-level) evidenceLuzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematology/oncology clinics of North America. 2016 Apr:30(2):373-93. doi: 10.1016/j.hoc.2015.11.006. Epub [PubMed PMID: 27040960]
Phillips J, Henderson AC. Hemolytic Anemia: Evaluation and Differential Diagnosis. American family physician. 2018 Sep 15:98(6):354-361 [PubMed PMID: 30215915]
Zhao Y, Lv J. Basophilic Stippling and Chronic Lead Poisoning. Turkish journal of haematology : official journal of Turkish Society of Haematology. 2018 Nov 13:35(4):298-299. doi: 10.4274/tjh.2018.0195. Epub 2018 Sep 5 [PubMed PMID: 30182925]
Zamani A, Kazerooni ES, Kasaee SS, Anbardar MH, Mohammadzadeh S, Shekarkhar G, Soleimani N. And the Oscar Goes to Peripheral Blood Film for the Detection of Lead Poisoning in a Complicated Toxic Patient: A Case Report with a Review of Laboratory Clues. Case reports in medicine. 2022:2022():9238544. doi: 10.1155/2022/9238544. Epub 2022 Feb 23 [PubMed PMID: 35251184]
Level 3 (low-level) evidenceMayans L. Lead Poisoning in Children. American family physician. 2019 Jul 1:100(1):24-30 [PubMed PMID: 31259498]
Tyrrell L, Rose G, Shukri A, Kahwash SB. Morphologic changes in red blood cells: An illustrated review of clinically important light microscopic findings. The Malaysian journal of pathology. 2021 Aug:43(2):219-239 [PubMed PMID: 34448787]
Kane SF, Roberts C, Paulus R. Hereditary Hemochromatosis: Rapid Evidence Review. American family physician. 2021 Sep 1:104(3):263-270 [PubMed PMID: 34523883]
Golfeyz S, Lewis S, Weisberg IS. Hemochromatosis: pathophysiology, evaluation, and management of hepatic iron overload with a focus on MRI. Expert review of gastroenterology & hepatology. 2018 Aug:12(8):767-778. doi: 10.1080/17474124.2018.1496016. Epub 2018 Jul 19 [PubMed PMID: 29966105]
Green R, Datta Mitra A. Megaloblastic Anemias: Nutritional and Other Causes. The Medical clinics of North America. 2017 Mar:101(2):297-317. doi: 10.1016/j.mcna.2016.09.013. Epub 2016 Dec 14 [PubMed PMID: 28189172]
Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. American family physician. 2017 Sep 15:96(6):384-389 [PubMed PMID: 28925645]