Introduction
A thorough bone marrow evaluation is critical for diagnosing neoplastic and certain nonneoplastic hematopoietic conditions and monitoring patients undergoing bone marrow transplantation and chemotherapy. A comprehensive laboratory bone marrow examination includes a complete blood count (CBC) and analysis of the peripheral blood smear (PBS), bone marrow aspirate, core biopsy specimen, clot section, and cytometric flow. Integrating advanced diagnostic techniques, such as cytogenetics, fluorescence in situ hybridization (FISH), and molecular profiling in clinical practice is essential in diagnosing, classifying, and prognosticating bone marrow pathologies.[1] Establishing uniform reporting systems and enhancing laboratory advancement awareness can promote efficient interprofessional collaboration and improve patient care quality.
Etiology and Epidemiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology and Epidemiology
Bone marrow examination is indicated in a range of clinical scenarios, including the following:
- Unexplained cytosis, cytopenias, or atypical cells on PBS
- Diagnosing and staging of hematopoietic neoplasms
- Evaluation of radiologically detected osseous lesions concerning for metastases
- Investigating hepatosplenomegaly of unknown origin
- Pyrexia of unknown origin, especially when infection or occult hematologic disease is suspected
- Assessment of storage disorders, including glycogen storage disorders and iron deposition [2]
Specimen Requirements and Procedure
The iliac crest is the primary site for obtaining bone marrow specimens. The specimen collection should include BMA, bone marrow cores for trephine biopsy, and blood clots following biopsy. Following the guidelines established by the College of American Pathologists (CAP) and the International Society of Laboratory Hematology (ISLH) helps ensure specimen reporting consistency and accuracy.[3][4]
Testing Procedures
Bone marrow specimen collection for evaluation typically involves 2 procedures: bone marrow aspiration (BMA) and bone marrow biopsy (BMB). These procedures are typically performed in an outpatient setting and are generally well-tolerated. BMA involves using a thin needle to withdraw a liquid bone marrow sample from the posterior iliac crest or, less commonly, sternum or other bones. BMB involves using a larger needle to obtain a solid core sample of bone marrow tissue along with a thin core of bone.
A complete bone marrow laboratory examination requires different specimen types to yield complementary results. These specimen types include the following:
Bone Marrow Aspirates
The World Health Organization (WHO) recommends immediate bedside preparation of aspirate smears using the pull technique or crush film smear to reduce drying artifacts from the initial specimen obtained.[5] Aspirates for flow cytometry should be collected separately in sodium heparin tubes. In contrast, aspirates for molecular studies should be collected in ethylenediaminetetraacetic acid (EDTA) or sodium heparin tubes. Romanoswky stains such as Wright or Giemsa must be used on the aspirates before methanol preservation for ancillary studies. Beyond 2 hours, EDTA-fixed aspirates lose their original morphology and become less suitable for evaluating morphologic dysplasia.[6]
Bone Marrow Cores for Trephine Biopsy
Core biopsy specimens are best obtained after BMA to prevent aspirate contamination with peripheral blood. The same skin entry site may be used, though the aspiration area should be avoided. Imprint smears are obtained by touching the core biopsy specimens onto glass slides. These imprints are especially helpful when aspiration yields a dry tap, and quick preliminary staining is needed after the biopsy procedure.
The most common fixative used during core biopsy sampling is neutral buffered formalin. Zinc formaldehyde and Bouin’s fixative are good alternatives. Calcium salts can make tissues hard and brittle, making sectioning and staining difficult. Decalcification is thus necessary. EDTA is the preferred decalcification agent, as it can preserve nuclear acids for immunohistochemical staining. However, urgent cases, ie, needing a 6-hour decalcification time, require the Hammersmith protocol using 10% formic acid and 5% formaldehyde.[7]
Post-Biopsy Blood Clot
Clots obtained after trephine biopsy may provide additional insights. These clots are fixed in formalin and processed similarly to trephine biopsy specimens. Blood clots help assess marrow cellularity, cellular morphology, and atypical infiltrates, enabling immunohistochemical staining. As decalcification is not required, these samples are better suited for cytogenetics, FISH, and molecular testing. However, flow cytometric analysis cannot be performed on clot samples.
Results, Reporting, and Critical Findings
BMA and BMB are often performed together to provide complementary information about the bone marrow's cellular composition and architecture. Below is a systematic approach to examining marrow aspirates and biopsies for a thorough bone marrow evaluation.
Bone Marrow Aspirate Analysis
Bone marrow aspirate is better suited for assessing cytomorphology, differential cell counts, and performing ancillary studies like flow cytometry, cytogenetics, and molecular testing. A stepwise approach to examining bone marrow aspirates is discussed below.
Low-power magnification
Low-power lenses typically provide 10 times object magnification. The adequacy and overall cellularity of the bone marrow can be appreciated in this setting.
- Adequacy: An adequate bone marrow smear is characterized by numerous particles with trails of cytomorphologically well-defined and well-stained blood cells. The presence of megakaryocytes indicates a successful marrow tap. However, the tap is considered dilute if megakaryocytes are present without particles. In contrast, smears with stromal fragments lacking particles, megakaryocytes, and hematopoietic marrow elements are deemed inadequate and noted as “peripheral blood” or “bloody tap” on the report (see Image. Stromal Fragments).
- Cellular clusters: Metastatic carcinomas and benign marrow cells like plasma cells, osteoblasts, and macrophages can cluster in marrow aspirates. Identifying and further examining these clusters under high-power magnification allows for a detailed analysis of their cytologic features and accurate identification of specific cell types.
High-power magnification
High-power lenses have a magnifying ability of at least 40 times. High-power magnification is ideal for assessing finer cellular morphological details, including nuclear-cytoplasmic ratio, chromatin pattern, and the presence of any abnormal inclusions, cytoplasmic granules, or parasites. High-power magnification is essential for identifying specific cell types, assessing cell maturation, and detecting morphologic dysplasia and abnormal cells like blasts.
- Cellularity: "Cellularity" refers to the proportion of different cell types present in a bone marrow aspirate. CAP recommends conducting a differential count of 500 cells. However, research suggests that a count of 300 cells may also suffice.[8] The differential cell count includes myeloid (granulocytic and monocytic cells) and erythroid progenitors, lymphoid elements, and plasma cells. Megakaryocytes, histiocytes, mast cells, and nonhematopoietic cells are excluded from this count. A normal myeloid-erythroid ratio falls between 2:1 and 4:1, but this ratio must be interpreted alongside PBS findings for a comprehensive assessment.
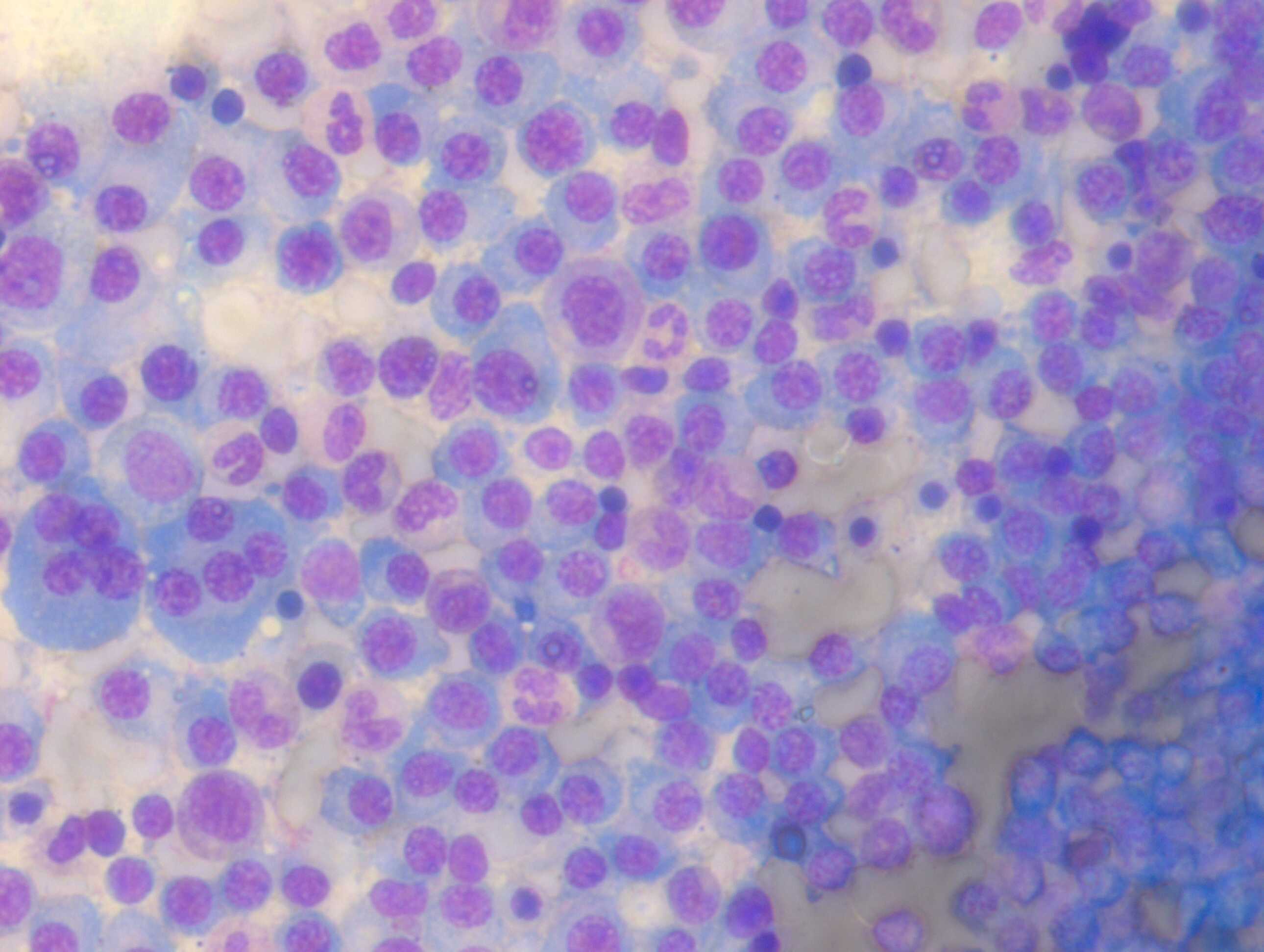
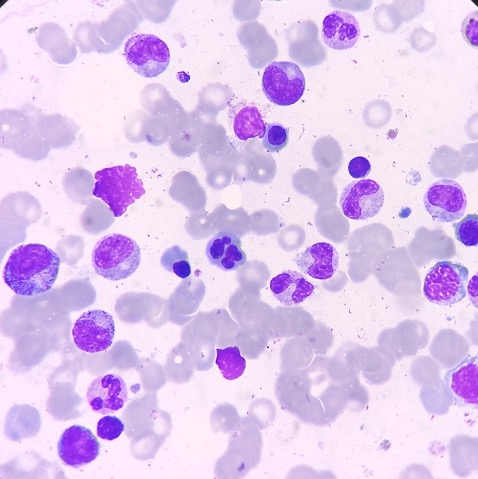
- Maturation: Cell maturation in the bone marrow is a continuous process. Increased immature cells, including blasts and blast equivalents such as promyelocytes and promonocytes, suggest a neoplastic process and warrant further investigation (see Image. Bone Marrow Aspirate with Blasts). Acute leukemias are defined by more than 20% blasts or blast equivalents in the bone marrow in the absence of leukemia-defining genetic abnormalities. If the blast count is less than 20%, WHO 2022 guidelines recommend that acute myeloid leukemia (AML) can be diagnosed if defined molecular aberrations accompany this finding. Different AML subtypes have differing morphologic features, immunophenotypes, and prognoses (see Table. Features of Genetically Defined AML).[9] Despite well-defined molecular characteristics, certain AML subtypes with BCR-ABL1 fusion and CEBPA mutation still require more than 20% blasts for diagnosis. Although the boundaries between myelodysplastic neoplasms (MDS) and AML have softened, retaining a blast cutoff of 20% prevents overtreatment.[10][11]
Table 1. Features of Genetically Defined AML
|
AML-defining genetic abnormality (< 20% blasts required for diagnosis) |
Morphologic features |
Immunophenotype |
Prognosis |
|
AML with RUNX1:: RUNX1T1 fusion |
|
|
Adults: Favorable prognosis Children: Poor prognosis |
|
Acute promyelocytic leukemia (APL) with PML::RARA fusion |
|
|
Excellent prognosis with prompt all-trans retinoic acid (ATRA) treatment |
|
AML with CBFB::MYH11 fusion |
|
|
Favorable |
|
AML with KMT2A rearrangements |
|
|
Poor |
|
AML with DEK::NUP214 fusion |
|
|
Poor |
|
AML with MECOM rearrangements |
|
|
Poor |
|
AML with NPM1 fusion |
|
|
Without FLT3-ITD-Favorable With FLT3-ITD- Intermediate |
|
AML with RBM15::MRTFA fusion |
|
|
Intermediate |
|
AML with NUP98 rearrangement |
|
|
Poor |
Dysplasia
Erythroid dysplasia is characterized by morphological abnormalities, including nuclear budding and lobation, internuclear bridges, karyorrhexis, binuclear and multinuclearity, cytoplasmic vacuolation, and megaloblastic changes, and the presence of ringed sideroblasts (see Image. Nuclear Budding).[12] Nonneoplastic conditions like megaloblastic anemia, congenital dyserythropoietic anemia, copper deficiency, and zinc excess may also exhibit features of erythroid dysplasia. Ringed sideroblasts may be detected by iron-staining aspirate smears with particles. A semiquantitative score of 1 to 4 is used to evaluate the abundance of ringed sideroblasts.
Granulocytic dysplasia’s morphologic features include nuclear hypersegmentation and hyposegmentation (pseudo-Pelger-Huët neutrophils), decreased cytoplasmic granularity, Döhle bodies, and Auer rods.[13] Nonneoplastic conditions, including vitamin B12 and folic acid deficiency, hepatitis C and human immunodeficiency virus (HIV) infection, autoimmune diseases like systemic lupus erythematosus, medications like granulocyte macrophage-colony stimulating factor, and hereditary Pelger-Huët anomaly can show morphologic features of granulocytic dysplasia. Using EDTA as an anticoagulant—which lavender-top tubes contain—can give rise to pseudo-dysplastic granulocytes but erythroid dyspoiesis.[14]
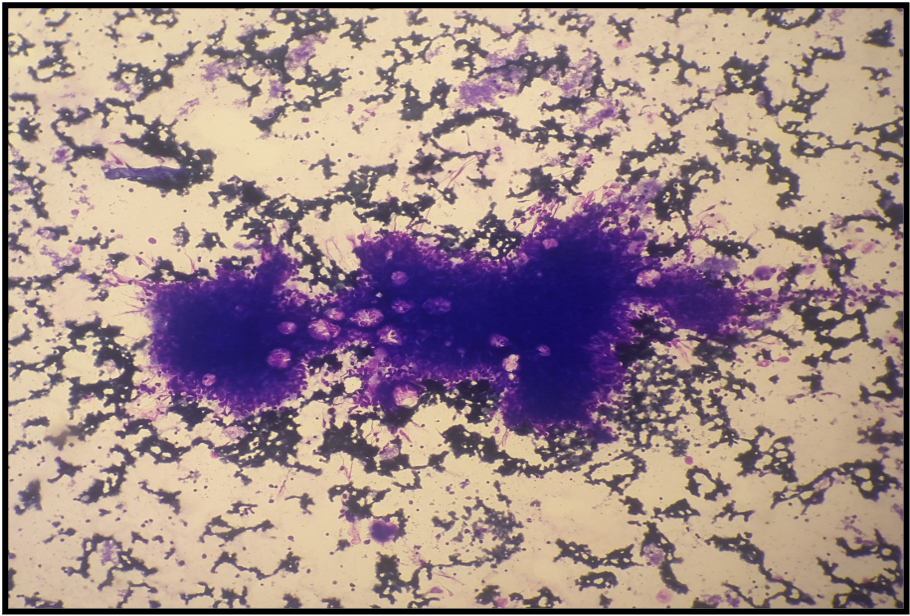
Megakaryocytic dysplasia manifests with micromegakaryocytosis and megakaryocyte hypolobation, multinucleation, and clustering (aggregates with >6 megakaryocytes)(see Images. Dysplastic Megakaryocytes and Dysplastic Megakaryocytes, Hyperlobated). Neoplastic causes of megakaryocytic dysplasia include myeloproliferative neoplasms (MPN), myelodysplastic neoplasms (MDS), and MDS/MPN.
Importantly, nonneoplastic causes should be ruled out in cases presenting with erythroid or granulocytic dysplasia before making a diagnosis of MDS. The dysplasia evaluation includes a comprehensive approach, starting with a detailed clinical history, CBC to assess for cytopenias, PBS examination, and biochemical and serological tests, including vitamin and serologic markers. Further investigations include a bone marrow examination, flow cytometry, cytogenetics, and molecular tests.
In the 5th edition of WHO’s 2022 myelodysplastic neoplasm classification, the term "myelodysplastic neoplasms" has replaced "myelodysplastic syndromes" while retaining the “MDS” abbreviation, aligning the nomenclature with myeloproliferative neoplasms for consistency. Lineage dysplasia can be progressive, indicating disease evolution. Distinguishing between unilineage and multilineage dysplasia is now optional.
Accurate blast percentage determination in bone marrow and peripheral blood helps distinguish between various morphologically defined MDS subgroups. According to the WHO 2022 guidelines, MDS with specific genetic abnormalities are classified as follows:
- MDS with low blasts and isolated del(5q): blasts <5% in the bone marrow and <2% in the peripheral blood
- MDS with low blasts and SF3B1 mutation: blasts <5% in the bone marrow and <2% in the peripheral blood; the presence of >15% ringed sideroblasts obviates the need to find an SF3B1 mutation
- MDS with bi-allelic TP53 inactivation: <20% in bone marrow and peripheral blood
A summary of morphologically defined MDS subgroups is provided below (see Table. Morphologically Defined MDS).[15]
Table 2. Morphologically Defined MDS
|
Morphologically defined MDS, category |
Blast percentage |
|
MDS with low blasts (MDS-LB) |
<5% bone marrow and <2% peripheral blood |
|
MDS, hypoplastic (MDS-h) |
<5% bone marrow and <2% peripheral blood |
|
MDS with increased blasts (MDS-IB) |
|
|
5-9% bone marrow or 2-4% peripheral blood |
|
10-19% bone marrow or 5-19% peripheral blood or Auer rods |
|
5-19% bone marrow or 2-19% peripheral blood |
Lymphoma
Bone marrow examination is routinely used to stage lymphoid neoplasms. The presence of cells with blastoid morphology in the background of marrow lymphoglandular bodies should raise the possibility of lymphoma rather than a myeloid neoplasm.[16]
Plasma cell neoplasms
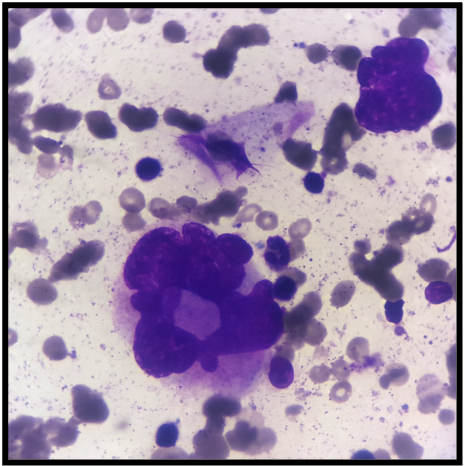
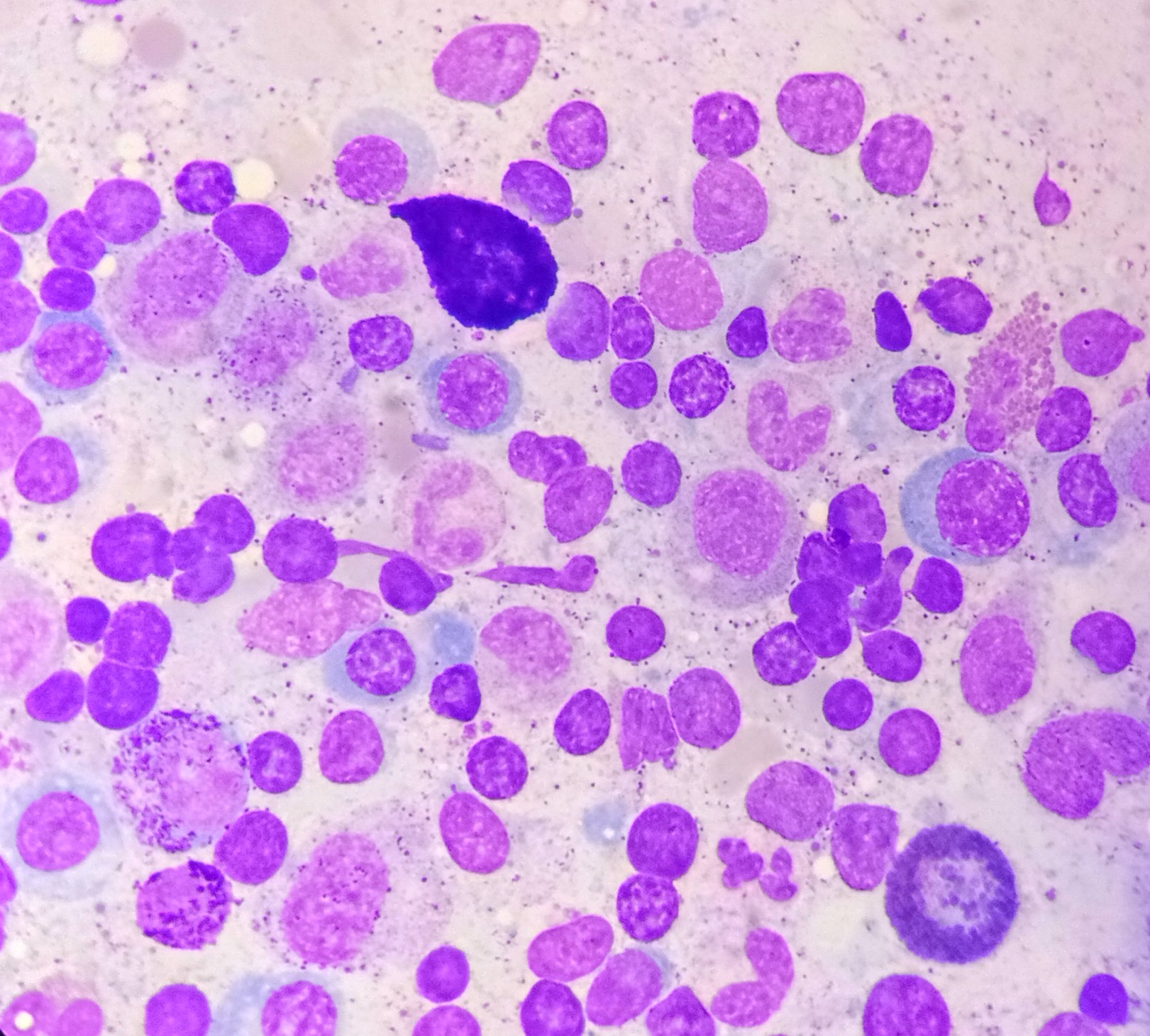
According to the WHO 2022 guidelines, multiple myeloma is diagnosed when at least 10% of clonal plasma cells are detected in the bone marrow, accompanied by one or more myeloma-defining events. These events include clonal bone marrow plasmacytosis exceeding 60%, a serum-free light chain ratio of at least 100, or more than 1 focal lesion on imaging (see Image. Bone Marrow Aspirate with Plasma Cells). Cytogenetic marrow aspirate evaluation is used for myeloma risk stratification. High-risk multiple myelomas are identified by the presence of t(14;16), t(14;20), t(4;14), del(17p), gain(1q), or p53 mutation. Myelomas with 2 or 3 of these high-risk cytogenetics are categorized as double- or triple-hit myelomas, respectively.[17]
Significance of other cell types
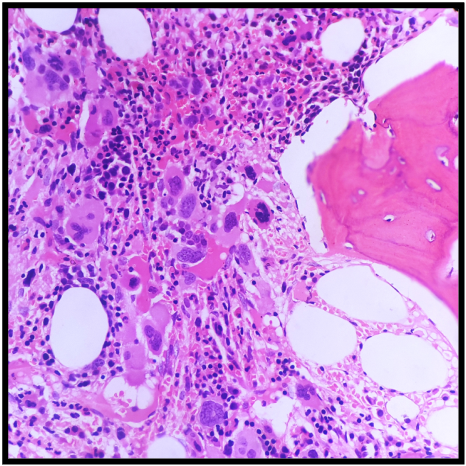
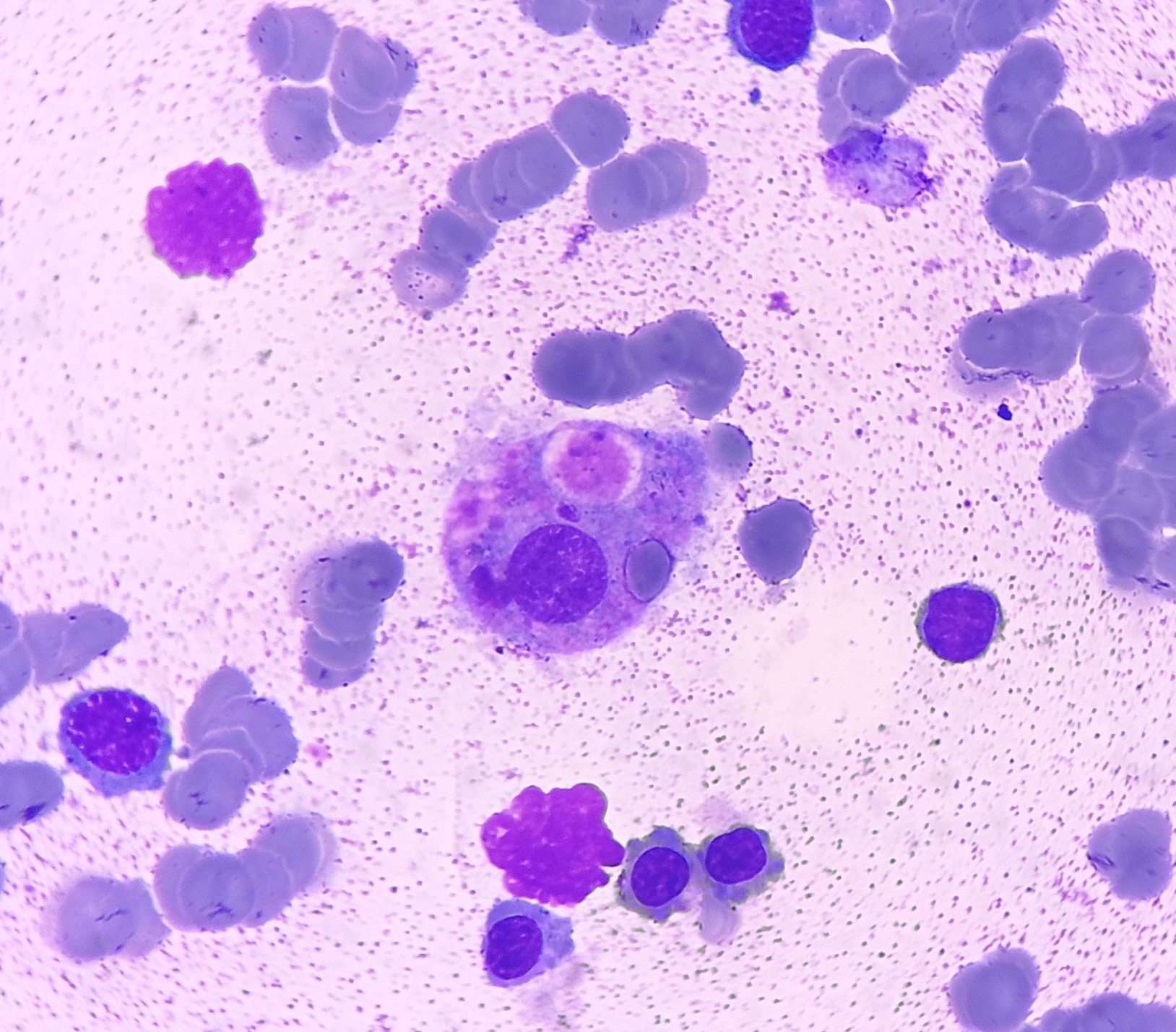
Mast cells may occasionally be observed in bone marrow aspirates and are discussed further below. Macrophages, usually rare, are primarily associated with erythroid islands and may contain hemosiderin.[18] Increasing macrophages might indicate hemolytic anemias, inflammatory conditions, or neoplastic disorders such as myeloproliferative neoplasms.[19] Erythrophagocytosis can be observed in immune hemolysis, hemophagocytic lymphohistiocytosis, and histiocytic neoplasms (see Image. Eyrthrophagocytosis). In lysosomal storage disorders like Gaucher disease, macrophages can accumulate lysosomal products, leading to a distinctive ''wrinkled tissue paper'' appearance.[20]
Bone Marrow Biopsy
Bone marrow trephine biopsy helps assess overall marrow cellularity and architecture, which are especially sensitive in detecting focal lesions. Core biopsy provides better insight into the relationship of bone marrow elements to each other.[21] Below is a systematic approach for evaluating both marrow aspirates and biopsies for a thorough bone marrow evaluation.
Marrow cellularity
Bone marrow cellularity indicates the proportion of hematopoietic elements to both hematopoietic and mature adipose tissue. This value decreases with age. Neonatal marrows are highly cellular (90-100%), declining to 50% by age 30 and further diminishing to 30% by age 70. Subcortical bone marrow is physiologically hypocellular and not optimal for evaluating cellularity.[22]
The correlation between trephine biopsy specimen and bone marrow aspirate cellularity is essential, as a hypercellular aspirate with unremarkable biopsy warrants further investigation. Conversely, a dry tap may indicate marrow fibrosis or infiltration, which is better appreciated on trephine biopsy.
Cellular distribution
The distribution of maturing hematopoietic elements is better discerned in trephine biopsy sections. Erythroid cells typically cluster in the interstitium. Meanwhile, immature myeloid precursors reside in the paratrabecular spaces, progressing toward the interstitium as they mature. Megakaryocytes normally have a perisinusoidal distribution. Paratrabecular megakaryocyte growth signifies abnormal hematopoiesis.[23]
Megakaryocytic clustering is a diagnostic feature indicative of marrow regeneration after bone marrow transplantation or chemotherapy. This feature is also a hallmark of various neoplastic conditions, particularly myeloproliferative neoplasms like primary myelofibrosis (PMF), essential thrombocythemia (ET), and polycythemia vera (PV). Thus, this finding warrants further investigation.
Normal bone marrow contains few interstitial lymphocytes and occasional well-circumscribed reactive lymphoid aggregates in non-paratrabecular locations. However, neoplastic lymphoid aggregates exhibit features such as paratrabecular and perisinusoidal distribution, B-cell predominance and infiltration, and adipose tissue involvement (see Image. Paratrabecular Lymphoid Aggregate). Marrow samples with increased benign lymphoid aggregation have increased plasmacytosis and lipid granuloma formation. These findings may be associated with autoimmune conditions like rheumatoid arthritis.[24]
Diagnosing residual disease post-rituximab therapy becomes challenging due to resulting CD20 downregulation. PAX-5 and CD79a are alternative B-cell markers for evaluating residual disease following therapy.[25][26] Lymphoid aggregates may be present in MDS, myeloproliferative neoplasms, and lymphomatous marrow infiltration.
Lymphomatous bone marrow involvement can exhibit different patterns: focal (paratrabecular) pattern observed in follicular lymphomas and mantle cell lymphomas and non-paratrabecular pattern in chronic lymphocytic leukemia (CLL), small lymphocytic leukemia (SLL), and Hodgkin lymphoma (HL). Diffuse solid and interstitial patterns are seen in aggressive and blastic lymphomas. Intravascular patterns are found in splenic marginal zone lymphoma and intrasinusoidal large cell lymphoma.[27] Studies indicate that 11.4% to 37% of B-cell lymphomas, 16% to 25% of T-cell lymphomas, and up to 11.8% of HL involve the bone marrow.[28][29]
Mast cells are identifiable by their metachromatic granules, best visualized using Giemsa and toluidine-blue stains (see Image. Mast Cells). Atypical mast cells appear as spindle cells with a monocytoid nucleus, hypogranular cytoplasm, and paratrabecular location.
Increased mast cells in the marrow are observed in conditions such as aplastic anemia, CLL, hairy cell leukemia, impending blast crisis in chronic myeloid leukemia, systemic mastocytosis, mast cell sarcoma, and lymphoplasmacytic lymphoma. Neoplastic mast cells show aberrant CD25, CD30, and CD2 expression. Systemic mastocytosis is associated with an activating KITD816V mutation present in more than 90% of cases.[30]
Focal pathology detection
Neoplastic lymphoid aggregates, plasma cells, clusters of mast cells, and metastases can be focal processes. (see Image. Metastatic Carcinoma). Examining multiple tissue levels helps evaluate for focal pathology, especially when clinically indicated.
Nonhematopoietic malignancies that most commonly metastasize to the bone marrow originate from the breast, prostate, kidney, thyroid, and gastrointestinal tract.[31] To identify subtle tumor cell infiltration, a keratin stain is recommended in patients with a known epithelial cancer history and suspected bone metastases.
Dysplasia
Megakaryocytic dysplasia is often characterized by atypical megakaryocyte morphology and clustering and is best evaluated through trephine biopsies. In the normal marrow, megakaryocytes are usually found solitary in non-paratrabecular locations. However, megakaryocytes cluster in paratrabecular and perisinusoidal locations in myeloproliferative neoplasms and PMF but are more scattered in ET (see Image. Dysplastic Megakaryocytes).
A highly specific dysplastic MDS feature is micromegakaryocytosis, considered pathognomonic for this condition. Morphologically, ET megakaryocytes appear large with a staghorn-like nucleus, while those in PMF exhibit cloud-like, hypolobated nuclei with hyperchromasia and a decreased nuclear-cytoplasmic ratio (see Image. Primary Myelofibrosis).[32]
Overlapping clinicopathologic features among Philadelphia-negative myeloproliferative neoplasms (eg, PV, ET, and PMF) suggest a common JAK/STAT genetic pathway, with JAK2, CALR, and MPL being 3 of the most common driver mutations. Distinguishing PMF from PV and ET is crucial, as PMF is associated with a higher fibrosis incidence and acute leukemia progression. Bone marrow biopsy is critical in diagnosing and detecting leukemic transformation and fibrotic progression. Immunohistochemical stains such as CD34 are highly useful for determining blast counts, particularly in cases of marrow fibrosis.[33]
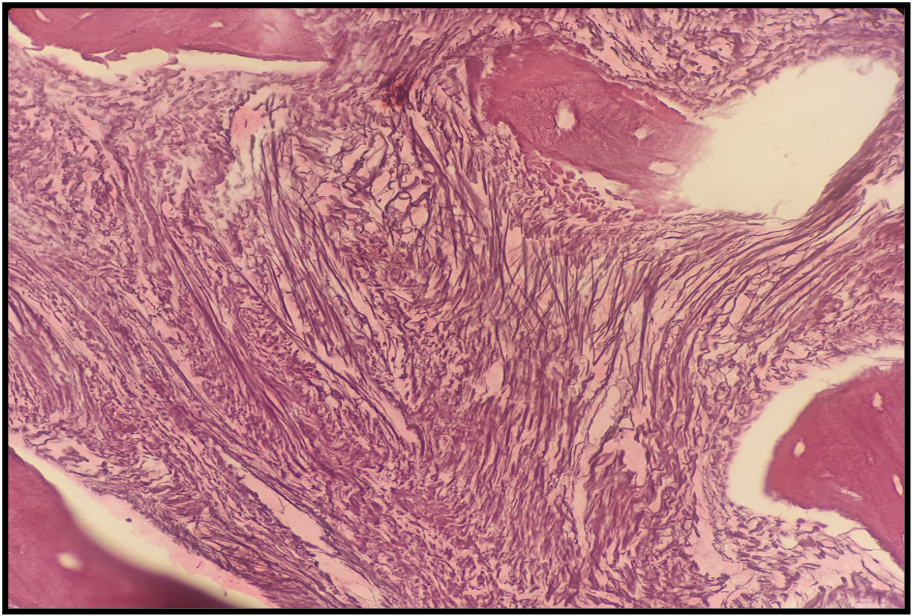
Fibrosis
Bone marrow fibrosis is a common cause of dry tap and contributes to discordances between aspirate and marrow findings. Fibrosis can arise from nonneoplastic conditions like bone marrow necrosis, metastatic nonhematopoietic malignancies, lymphoma infiltration, and myeloproliferative neoplasms, including PMF, post-ET, and PV.
Reticulin staining helps determine the extent of fibrosis in the marrow microenvironment, which is graded as follows:[34]
- MF-0: Normal bone marrow with a scattered linear reticulin pattern but without intersections
- MF-1: Loose reticulin network with numerous intersections, predominantly in perivascular regions
- MF-2: Dense and diffuse increased reticulin with extensive intersections, occasionally interspersed with focal collagen bundles or focal osteosclerosis
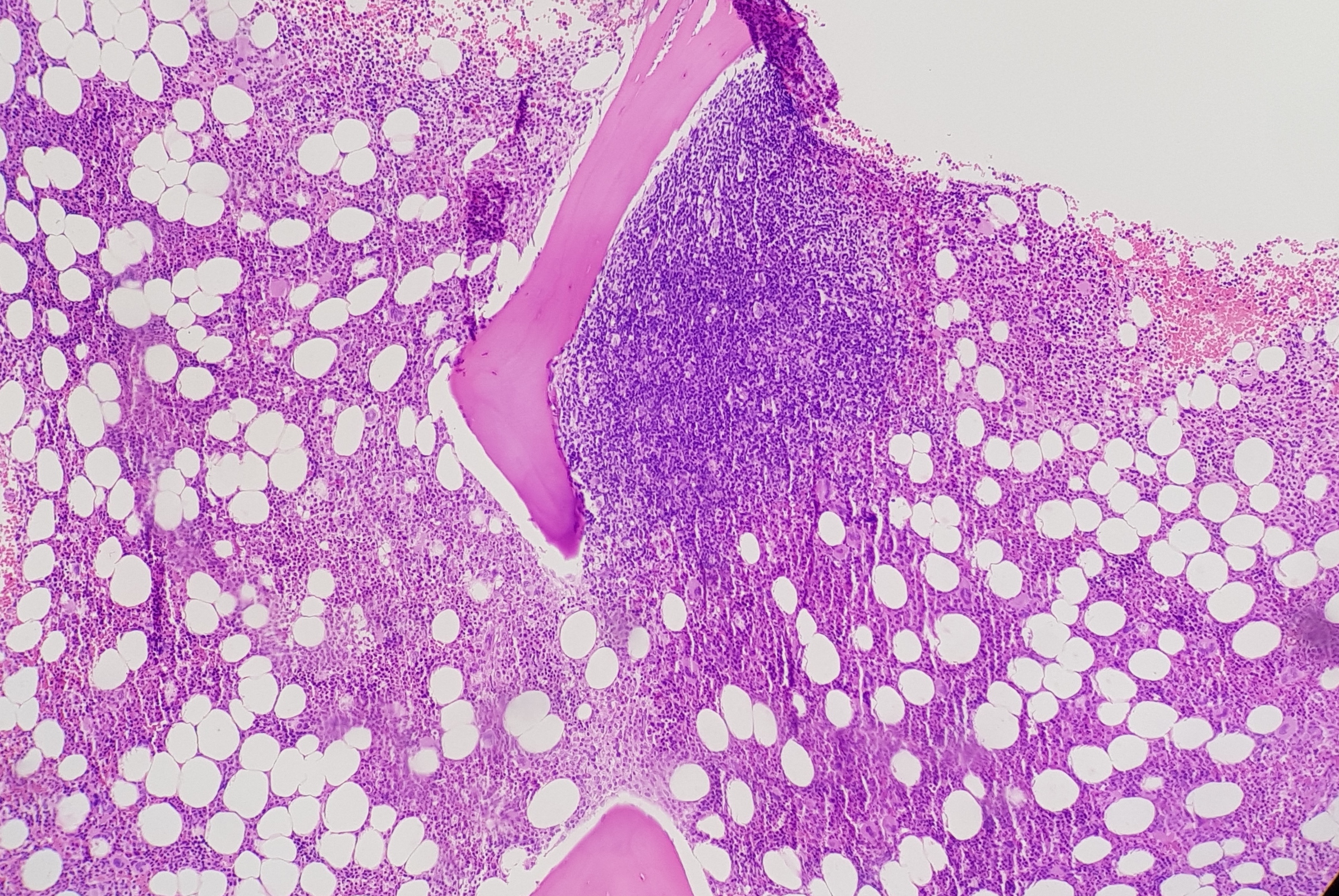
- MF-3: Dense and diffuse increased reticulin with extensive intersections, coarse collagen bundles, and significant osteosclerosis (see Image. Grade 3 Bone Marrow Fibrosis)
Necrosis
Bone marrow necrosis is a rare, potentially fatal complication that may arise from hematopoietic malignancies like myeloproliferative neoplasms, leukemias, lymphomas, and plasma cell myelomas. Chemotherapy and treatment with retinoic acid and granulocyte colony-stimulating factor increase the risk of bone marrow necrosis (see Image. Bone Marrow Necrosis). This condition can also occur after infections like parvovirus (especially in sickle cell patients) and HIV or the use of medications like sulfasalazine, interferon, and imatinib.[35] Distinguishing bone marrow necrosis from conditions like aplastic anemia, extensive amyloid deposition, and gelatinous transformation is vital to patient management.[36]
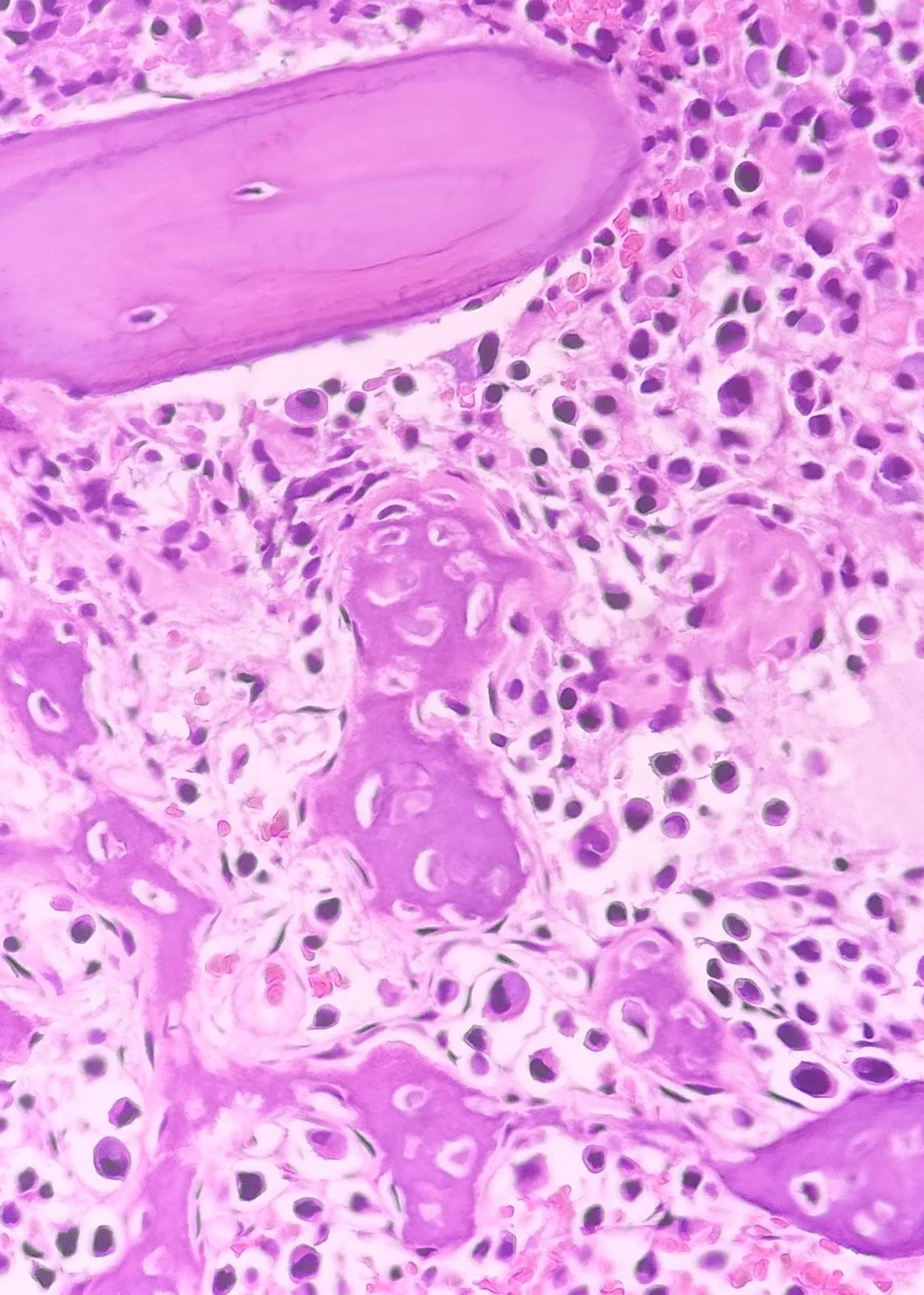
Granulomas
Macrophage infiltration and granulomatous inflammation often pose diagnostic challenges. Exclusion of CD68-expressing mast cells mimicking granulomas is essential. Granulomas may be associated with diseases like HL but do not necessarily indicate direct lymphomatous bone marrow infiltration.[26]
Discordance Between Aspirate and Bone Marrow Trephine Biopsy
Discrepancies between the results of bone marrow aspirate and trephine biopsy samples occur frequently. Bone marrow aspirate criteria for the morphological assessment of blasts and erythroid or granulocytic dysplasia are more clearly defined than biopsy criteria. Focal lesions such as lymphoid and plasma cell aggregation, metastatic carcinomas, and dysplastic megakaryocyte growth can be underrepresented in aspirates and better appreciated on trephine biopsies. Dry marrow taps attributed to pathologies like myelofibrosis, aplastic anemia, and metastatic carcinoma infiltration are also better visualized on trephine biopsy.
Correlating BMA and BMB findings is crucial. The general guideline is that pathologies visible on the aspirate should also be identifiable on biopsies. However, the reverse may not always be accurate, emphasizing the importance of thorough clinicopathologic correlation in diagnosis.[26]
Bone Marrow Evaluation in Clonal Hematopoiesis
Clonal hematopoiesis (CH) denotes the presence of cells arising from a mutated progenitor cell and exhibiting a propensity for proliferation in the absence of unexplained cytopenias, hematologic malignancies, or other clonal disorders.[11] The WHO 2022 has defined 2 categories:
- "Clonal hematopoiesis of indeterminate potential" (CHIP) refers to cases where CH with myeloid-malignancy-associated somatic gene mutations is observed at a variant allele fraction of more than 2%. Individuals with CHIP lack a diagnosed hematologic malignancy or unexplained cytopenia. The significance of a variant allele frequency (VAF) of less than 2% is currently unclear.
- "Clonal cytopenia of undetermined significance" (CCUS) refers to CH with at least 1 persistent cytopenia lasting longer than 4 months. These cytopenias are unexplained by any hematologic or systemic condition and do not meet the diagnostic criteria for defined myeloid malignancies. The peripheral blood criteria for CCUS, MDS, and MDS/MPN include the following:
- Hemoglobin less than 13 g/dL in male individuals and less than 12 g/dL in female individuals
- Absolute neutrophil count less than 1,800/mm³
- Platelet count less than 150,000/mm³
Bone marrow evaluation in CH requires a thorough understanding of the patient's clinical presentation and close collaboration between hematology and hematopathology teams. Excluding an underlying hematologic myeloid neoplasm requires an interprofessional approach.
Ancillary Studies
Ancillary bone marrow studies include immunohistochemistry, flow cytometry, cytogenetics, FISH, polymerase chain reaction (PCR), and next-generation sequencing (NGS). The upcoming sections delve into these studies’ substantial impact on patient management.
Clinical Significance
Molecular genetics have become pivotal for diagnosing, classifying, prognosticating, and treating hematopoietic malignancies. Certain neoplasms are now defined by molecular alterations, making molecular testing indispensable. Cytogenetics and FISH were traditionally used merely for chromosomal studies. Presently, NGS may be employed to detect significant somatic mutations.
Summarized below are the various hematopoietic conditions’ primary defining molecular pathways and mutations and their prognostic and clinical significance (see Table. Myeloproliferative, Eosinophilic, and Mast Cell Neoplasms).[37][38]
Table 3. Myeloproliferative, Eosinophilic, and Mast Cell Neoplasms
|
Disease |
Major genetic pathway/defining mutation |
Mutations with prognostic significance |
Clinical and therapeutic implications |
|
Chronic myeloid leukemia (CML) |
BCR::ABL1 fusion resulting from t(9;22)(q34;q11) |
FISH or RT-PCR in PB samples Myeloid panel testing for non-tyrosine kinase mutations (IKZH1 deletions, RUNX1 and ASXL1 mutations) conferring drug resistance |
BCR::ABL1 transcript levels determine response to treatment and therapeutic decisions |
|
Polycythemia vera (PV) |
JAK2 p.V617F or JAK2 exon 12 mutations |
An increase in VAF from baseline indicates progression to post-PV myelofibrosis. |
Higher JAK2 VAF is associated with older age, high hemoglobin, low platelet count, and leukocytosis. Therapies like interferon need mutation burden serial measurements to predict treatment outcomes, so VAF reporting is recommended. |
|
Essential thrombocythemia (ET) |
JAK2, MPL, CALR |
An increase in VAF from baseline indicates progression to post-ET myelofibrosis. |
NGS is recommended in JAK2-, MPL-, and CALR-negative patients to identify rare somatic variants (eg, noncanonical somatic MPL and JAK2 mutations). |
|
Primary myelofibrosis |
JAK2, MPL, CALR |
EZH2, IDH1, IDH2, CBL, KRAS, NRAS, TP53 associated with poor prognosis |
NGS is recommended in JAK2-, MPL-, and CALR-negative patients to identify rare somatic variants (eg, noncanonical somatic MPL and JAK2 mutations). |
|
Chronic neutrophilic leukemia (CNL) |
CSF3R mutations |
|
Dasatinib is sensitive in CNLs with CSF3R mutations outside the proximal membrane region. |
|
Chronic eosinophilic leukemia, NOS |
Lack of criteria for any other myeloid or lymphoid neoplasm |
|
PDGFRA (4q12), PDGFRB(5q31-33), FLT3 (13q12), FGFR1 (8p11) and JAK2(9p24) rearrangements should be absent |
|
Juvenile myelomonocytic leukemia (now classified as MPN) |
90% involve somatic activation of the RAS signaling pathway, including PTPN11, KRAS, and NRAS. |
Germline NF1 mutation: aggressive behavior Germline CBL mutation: spontaneous regression |
|
|
Systemic mastocytosis |
KITpD816V mutation |
SRSF, ASXL1, or RUNX1 somatic mutations are identified by NGS and are associated with a poor prognosis. Bone marrow samples used for greater sensitivity |
|
|
Neoplasms with eosinophilia |
FIP1L1::PDGFRA (most common) PDGFRA (4q12), PDGFRB (5q31-33), FLT3 (13q12), FGFR1(8p11) and JAK2(9p24) rearrangements |
ETKN1, RUNX1, ASXL1, EZH2 have a poor prognosis FIP1L1::PDGFRA can be detected by FISH, NGS, or RT-PCR |
KITD816V suggestive of systemic mastocytosis as a cause of eosinophilia |
Table 4. Myelodysplastic neoplasms and MDS/MPNs
|
Disease |
Primary genetic pathway/defining mutation |
Mutations with prognostic significance |
Clinical/Therapeutic implications |
|
MDS with low blasts and isolated del 5q |
5q deletion alone or with one other cytogenetic abnormality besides 7q deletion or monosomy 7 |
Biallelic TP53 inactivation places this mutation in the TP53 inactivation subtype |
Lenalidomide licensed for use in MDS with del(5q) |
|
MDS with low blasts and SF3B1 mutation |
Presence of SF3B1 mutation Absence of monosomy 7, 5q deletion, or a complex karyotype |
Biallelic TP53 inactivation places this mutation in the TP53 inactivation subtype >15% sideroblasts negates the need to find SF3B1 mutation |
|
|
MDS with biallelic TP53 inactivation |
Two or more TP53 mutations or the presence of one TP53 copy number loss Usually complex karyotype |
|
This category can be considered as an AML equivalent from a therapeutic standpoint. TP53 mutations are associated with increased therapeutic response to hypomethylating agents and shorter survival following allogenic stem-cell transplantation. |
|
Chronic Myelomonocytic Leukemia |
The presence of a clonal mutation is part of the supporting diagnostic criteria, with TET2 and SRSF2 biallelic variants showing high specificity. |
|
SETBP1, RUNX1, NRAS, and ASXL1 analyses are strongly recommended for transplantation-eligible individuals. |
The prognosis of various genetically defined AMLs has been stated above (see Table 1. Features of Genetically Defined AML). Optimizing treatment strategies requires identifying targetable mutations like FLT3-ITD, IDH-1, and IDH2 by techniques such as PCR or capillary electrophoresis. AML cases with TP53 mutation, specifically with a VAF exceeding 10%, are now denoted as "AML with mutated TP53." The mutation is associated with an unfavorable prognosis despite aggressive therapeutic interventions.[10]
Posttreatment minimal residual disease (MRD) monitoring identifies impending relapse and facilitates early interventions. Multiparameter flow cytometry and molecular techniques like PCR and NGS can now be performed on peripheral blood and bone marrow samples. Notably, bone marrow samples offer higher sensitivity in MRD evaluation.
NGS can provide information about preleukemic clones like DNMT3A, TET2, and ASXL1. These mutations may not be detectable during initial diagnosis but could become apparent through NGS. Importantly, the presence of these mutations does not necessarily predict disease relapse. Meanwhile, mutations like NPM1, RUNX1::RUNX1T1, and CBFB::MYH11 are usually present at diagnosis and persist through relapse. Mutations like FLT3-ITD, NRAS, and KRAS may exhibit outcome variability following therapeutic interventions.[37]
Acute lymphoid leukemia subtypes are also defined based on molecular genetics in the WHO 2022 classification. Cytogenetics and FISH are efficient diagnostic tools for identifying the defining chromosomal alterations (eg, BCR::ABL1, KMT2A::AFF1, ETVG::RUNX1, TCF3::PBX1, and iAMP21), and prognostic factors like aneuploidy.[37]
The ICSH and CAP advocate formulating an “Integrated Diagnosis,” which combines analysis of the peripheral blood, bone marrow aspirate, and trephine biopsy with ancillary and molecular testing. An integrated diagnosis aims to classify hematopoietic neoplasms as currently defined by the WHO and provide hematologists with pertinent information to help determine management. These insights are instrumental in devising tailored and effective therapeutic strategies and optimizing overall treatment outcomes.
The information that must be included in a comprehensive bone marrow examination report are the following:
- Institutional Information
- Institution’s name
- Patient Details
- Patient's name
- Birth date
- Medical record number
- Requesting Physician
- Physician’s name
- Medical license number
- Procedure Details
- Procedure date
- Indication for bone marrow examination
- Procedure type (aspirate, trephine biopsy)
- Procedure site (iliac crest, sternum, etc)
- Peripheral Blood Smear
- CBC parameters
- Peripheral blood smear description
- Bone Marrow Aspirate Examination
- Morphologic findings: cellularity, nucleated differential cell count, myeloid: erythroid ratio, blast percentages, erythropoiesis, myelopoiesis, megakaryocytes, lymphocytes, plasma cells, other hematopoietic cells, abnormal cells
- Special stains: iron stain, cytochemistry
- Other investigations: cytogenetics, FISH, PCR, and molecular methods, including NGS
- Flow cytometry results (if performed)
- Bone Marrow Trephine Biopsy Examination
- Morphologic findings: length of core biopsy, cellularity, bone architecture, location, number, morphology, and maturation of erythroid, myeloid and megakaryocyte lineages, plasma cells, lymphocytes, and macrophages; abnormal cells or infiltrates.
- Special stains: Reticulin stain
- Immunohistochemistry results
- Final Integrated Diagnosis per WHO Criteria
- Summary of findings
- Note
- Signature and date of reporting
Enhancing Healthcare Team Outcomes
Bone marrow examination is an indispensable tool in the diagnosis and posttreatment monitoring of hematopoietic conditions. Developing a systematic bone marrow evaluation approach and establishing a standardized reporting methodology, including molecular studies, is essential. These practices help promote a better understanding of the terminology and behavior of hematopoietic neoplasms, thus fostering efficient collaboration within interprofessional teams caring for patients with hematopoietic disorders.
The interprofessional team members in these cases include pathologists, hematologists, oncologists, primary care physicians, nurses, and pharmacists. Pathologists provide accurate diagnostic assessments based on bone marrow samples. Hematologists oversee patient management and treatment strategies tailored to specific blood disorders. Oncologists administer specialized cancer care, including chemotherapy regimens.
Primary care physicians coordinate overall patient care and ensure continuity beyond specialty settings. Nurses and pharmacists support patient care, administer treatments, and manage side effects. Laboratory analysts process and evaluate bone marrow samples, contributing to accurate diagnostic reports. Together, these healthcare professionals ensure comprehensive care and optimal treatment outcomes for patients undergoing bone marrow examination.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kaur M, Singh Rana AP, Kapoor S, Puri A. Diagnostic value of bone marrow aspiration and biopsy in routine hematology practice. Journal of clinical and diagnostic research : JCDR. 2014 Aug:8(8):FC13-6. doi: 10.7860/JCDR/2014/9823.4760. Epub 2014 Aug 20 [PubMed PMID: 25302200]
Rindy LJ, Chambers AR. Bone Marrow Aspiration and Biopsy. StatPearls. 2024 Jan:(): [PubMed PMID: 32644658]
Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC, International Council for Standardization In Hematology. ICSH guidelines for the standardization of bone marrow specimens and reports. International journal of laboratory hematology. 2008 Oct:30(5):349-64. doi: 10.1111/j.1751-553X.2008.01100.x. Epub [PubMed PMID: 18822060]
Sever C, Abbott CL, de Baca ME, Khoury JD, Perkins SL, Reichard KK, Taylor A, Terebelo HR, Colasacco C, Rumble RB, Thomas NE. Bone Marrow Synoptic Reporting for Hematologic Neoplasms: Guideline From the College of American Pathologists Pathology and Laboratory Quality Center. Archives of pathology & laboratory medicine. 2016 Sep:140(9):932-49. doi: 10.5858/arpa.2015-0450-SA. Epub 2016 Feb 23 [PubMed PMID: 26905483]
Level 2 (mid-level) evidenceSharma P, Sachdeva MU, Varma N. Bone marrow aspirate smear preparation: morphological superiority of the timely wedge smear and the importance of imprints. Annals of hematology. 2014 Jun:93(6):1063-4. doi: 10.1007/s00277-013-1927-6. Epub 2013 Oct 19 [PubMed PMID: 24141334]
Level 3 (low-level) evidenceRiley RS, Gandhi P, Harley SE, Garcia P, Dalton JB, Chesney A. A Synoptic Reporting System to Monitor Bone Marrow Aspirate and Biopsy Quality. Journal of pathology informatics. 2021:12():23. doi: 10.4103/jpi.jpi_53_20. Epub 2021 May 25 [PubMed PMID: 34447603]
Level 2 (mid-level) evidenceTorlakovic EE, Brynes RK, Hyjek E, Lee SH, Kreipe H, Kremer M, McKenna R, Sadahira Y, Tzankov A, Reis M, Porwit A, International Council for Standardization in Haematology. ICSH guidelines for the standardization of bone marrow immunohistochemistry. International journal of laboratory hematology. 2015 Aug:37(4):431-49. doi: 10.1111/ijlh.12365. Epub 2015 May 15 [PubMed PMID: 25977137]
Abdulrahman AA, Patel KH, Yang T, Koch DD, Sivers SM, Smith GH, Jaye DL. Is a 500-Cell Count Necessary for Bone Marrow Differentials?: A Proposed Analytical Method for Validating a Lower Cutoff. American journal of clinical pathology. 2018 May 31:150(1):84-91. doi: 10.1093/ajcp/aqy034. Epub [PubMed PMID: 29757362]
Falini B, Martelli MP. Comparison of the International Consensus and 5th WHO edition classifications of adult myelodysplastic syndromes and acute myeloid leukemia. American journal of hematology. 2023 Mar:98(3):481-492. doi: 10.1002/ajh.26812. Epub 2023 Jan 5 [PubMed PMID: 36606297]
Level 3 (low-level) evidenceBhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. Journal of hematology & oncology. 2023 Mar 25:16(1):29. doi: 10.1186/s13045-023-01424-6. Epub 2023 Mar 25 [PubMed PMID: 36966300]
Level 3 (low-level) evidenceKhoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul:36(7):1703-1719. doi: 10.1038/s41375-022-01613-1. Epub 2022 Jun 22 [PubMed PMID: 35732831]
Goasguen JE, Bennett JM, Bain BJ, Brunning R, Vallespi MT, Tomonaga M, Zini G, Renault A, (The International Working Group on Morphology of MDS). Dyserythropoiesis in the diagnosis of the myelodysplastic syndromes and other myeloid neoplasms: problem areas. British journal of haematology. 2018 Aug:182(4):526-533. doi: 10.1111/bjh.15435. Epub 2018 Jun 19 [PubMed PMID: 29917221]
Shekhar R, Srinivasan VK, Pai S. How I investigate dysgranulopoiesis. International journal of laboratory hematology. 2021 Aug:43(4):538-546. doi: 10.1111/ijlh.13607. Epub 2021 May 25 [PubMed PMID: 34031992]
Lee SH, van der Weyden C, Mayson E, Desai S. Excessive EDTA induces morphologic changes in bone marrow smears that mimic specific features of dysplasia. International journal of laboratory hematology. 2013 Apr:35(2):163-9. doi: 10.1111/ijlh.12015. Epub 2012 Oct 13 [PubMed PMID: 23062172]
Hoff FW, Madanat YF. Molecular Drivers of Myelodysplastic Neoplasms (MDS)-Classification and Prognostic Relevance. Cells. 2023 Feb 15:12(4):. doi: 10.3390/cells12040627. Epub 2023 Feb 15 [PubMed PMID: 36831294]
Jain S, Gajendra S, Sachdev R. Lymphoglandular bodies in bone marrow aspirate smears. Blood research. 2017 Mar:52(1):7. doi: 10.5045/br.2017.52.1.7. Epub 2017 Mar 27 [PubMed PMID: 28401093]
Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. American journal of hematology. 2022 Aug:97(8):1086-1107. doi: 10.1002/ajh.26590. Epub 2022 May 23 [PubMed PMID: 35560063]
Heideveld E, van den Akker E. Digesting the role of bone marrow macrophages on hematopoiesis. Immunobiology. 2017 Jun:222(6):814-822. doi: 10.1016/j.imbio.2016.11.007. Epub 2016 Nov 17 [PubMed PMID: 27890297]
Sadahira Y, Wada H, Manabe T, Yawata Y. Immunohistochemical assessment of human bone marrow macrophages in hematologic disorders. Pathology international. 1999 Jul:49(7):626-32 [PubMed PMID: 10504523]
Ferreira CR, Gahl WA. Lysosomal storage diseases. Translational science of rare diseases. 2017 May 25:2(1-2):1-71. doi: 10.3233/TRD-160005. Epub 2017 May 25 [PubMed PMID: 29152458]
Merzianu M, Groman A, Hutson A, Cotta C, Brynes RK, Orazi A, Reddy V, Teruya-Feldstein J, Amre R, Balasubramanian M, Brandao G, Cherian S, Courville E, Czuchlewski D, Fan G, Grier D, Hoehn D, Inamdar KV, Juskevicius R, Kaur P, Lazarchick J, Lewis MR, Miles RR, Myers JB, Nasr MR, Qureishi HN, Olteanu H, Robu VG, Salaru G, Vajpayee N, Vos J, Zhang L, Zhang S, Aye L, Brega E, Coad JE, Grantham J, Ivelja S, McKenna R, Sultan K, Wilding G, Hutchison R, Peterson L, Cheney RT. Trends in Bone Marrow Sampling and Core Biopsy Specimen Adequacy in the United States and Canada: A Multicenter Study. American journal of clinical pathology. 2018 Oct 1:150(5):393-405. doi: 10.1093/ajcp/aqy066. Epub [PubMed PMID: 30052721]
Level 2 (mid-level) evidence. BONE MARROW, THYMUS AND BLOOD: CHANGES ACROSS THE LIFESPAN. Aging health. 2009 Jun 1:5(3):385-393 [PubMed PMID: 20072723]
Brown DC, Gatter KC. The bone marrow trephine biopsy: a review of normal histology. Histopathology. 1993 May:22(5):411-22 [PubMed PMID: 8344653]
Johnston A,Brynes RK,Naemi K,Reisian N,Bhansali D,Zhao X,Rezk SA, Differentiating benign from malignant bone marrow B-cell lymphoid aggregates: a statistical analysis of distinguishing features. Archives of pathology [PubMed PMID: 25611106]
Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Applied immunohistochemistry & molecular morphology : AIMM. 2013 Mar:21(2):116-31. doi: 10.1097/PAI.0b013e31825d550a. Epub [PubMed PMID: 22820658]
Wilkins BS. Pitfalls in bone marrow pathology: avoiding errors in bone marrow trephine biopsy diagnosis. Journal of clinical pathology. 2011 May:64(5):380-6. doi: 10.1136/jcp.2010.080838. Epub 2011 Feb 15 [PubMed PMID: 21325142]
Zhang QY, Foucar K. Bone marrow involvement by hodgkin and non-hodgkin lymphomas. Hematology/oncology clinics of North America. 2009 Aug:23(4):873-902. doi: 10.1016/j.hoc.2009.04.014. Epub [PubMed PMID: 19577173]
Jeong SY, Chang YH, Lee JK, Hong YJ, Hong SI, Lee SS. [Incidence and histologic patterns of bone marrow involvement of malignant lymphoma based on the World Health Organization classification: a single institution study]. The Korean journal of laboratory medicine. 2007 Dec:27(6):383-7 [PubMed PMID: 18160826]
Level 2 (mid-level) evidenceKalra SK, Sancheti S, Somal PK, Sali AP, Sharma A, Goel A, Jain S, Dora TK, Gulia A, Divetia JV. Challenges Encountered and Pattern-Based Analysis of Bone Marrow Biopsy in Lymphomas: An Institutional Experience. Journal of laboratory physicians. 2023 Mar:15(1):69-77. doi: 10.1055/s-0042-1751318. Epub 2022 Aug 17 [PubMed PMID: 37064982]
Pardanani A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. American journal of hematology. 2021 Apr 1:96(4):508-525. doi: 10.1002/ajh.26118. Epub 2021 Feb 21 [PubMed PMID: 33524167]
Jayarangaiah A, Kemp AK, Theetha Kariyanna P. Bone Metastasis. StatPearls. 2024 Jan:(): [PubMed PMID: 29939688]
Goasguen JE, Bennett JM, Bain BJ, Brunning RD, Vallespí MT, Tomonaga M, Zini G, Renault A, International Working Group on Morphology of MDS IWGM-MDS. Quality control initiative on the evaluation of the dysmegakaryopoiesis in myeloid neoplasms: Difficulties in the assessment of dysplasia. Leukemia research. 2016 Jun:45():75-81. doi: 10.1016/j.leukres.2016.04.009. Epub 2016 Apr 11 [PubMed PMID: 27107657]
Level 2 (mid-level) evidenceLi N, Chen M, Yin CC. Advances in molecular evaluation of myeloproliferative neoplasms. Seminars in diagnostic pathology. 2023 May:40(3):187-194. doi: 10.1053/j.semdp.2023.04.007. Epub 2023 Apr 13 [PubMed PMID: 37087305]
Level 3 (low-level) evidenceThiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005 Aug:90(8):1128-32 [PubMed PMID: 16079113]
Level 3 (low-level) evidenceSharma R, Gajendra S, Jain S, Gupta SK, Sachdev R. Multiple Myeloma: An Unusual Cause of Extensive Bone Marrow Necrosis. Journal of clinical and diagnostic research : JCDR. 2016 Jun:10(6):XL01-XL02. doi: 10.7860/JCDR/2016/18035.7914. Epub 2016 Jun 1 [PubMed PMID: 27504393]
Deucher A, Wool GD. How I investigate bone marrow necrosis. International journal of laboratory hematology. 2019 Oct:41(5):585-592. doi: 10.1111/ijlh.13091. Epub 2019 Aug 19 [PubMed PMID: 31424633]
Duncavage EJ, Bagg A, Hasserjian RP, DiNardo CD, Godley LA, Iacobucci I, Jaiswal S, Malcovati L, Vannucchi AM, Patel KP, Arber DA, Arcila ME, Bejar R, Berliner N, Borowitz MJ, Branford S, Brown AL, Cargo CA, Döhner H, Falini B, Garcia-Manero G, Haferlach T, Hellström-Lindberg E, Kim AS, Klco JM, Komrokji R, Lee-Cheun Loh M, Loghavi S, Mullighan CG, Ogawa S, Orazi A, Papaemmanuil E, Reiter A, Ross DM, Savona M, Shimamura A, Skoda RC, Solé F, Stone RM, Tefferi A, Walter MJ, Wu D, Ebert BL, Cazzola M. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood. 2022 Nov 24:140(21):2228-2247. doi: 10.1182/blood.2022015853. Epub [PubMed PMID: 36130297]
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh ML, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022 Sep 15:140(11):1200-1228. doi: 10.1182/blood.2022015850. Epub [PubMed PMID: 35767897]
Level 3 (low-level) evidence