Managing Fresh-Frozen Plasma Transfusion Adverse Effects: Allergic Reactions, TACO, and TRALI
Managing Fresh-Frozen Plasma Transfusion Adverse Effects: Allergic Reactions, TACO, and TRALI
Introduction
Fresh-frozen plasma transfusion is a vital component of modern medical practice, employed in various clinical scenarios to manage coagulation disorders and support patients in need. While fresh-frozen plasma is undeniably beneficial, it is crucial to recognize that, like any medical intervention, it carries potential risks and adverse effects that healthcare providers must be well-prepared to manage. Among the spectrum of adverse effects, this discussion mainly focuses on 3 significant categories: allergic reactions, transfusion-associated circulatory overload, and transfusion-related acute lung injury. While varying in their presentation and mechanisms, these complications underscore the importance of thorough understanding and vigilant monitoring in the administration of fresh-frozen plasma to ensure both its effectiveness and the safety of transfusion recipients.
Blood is a circulating specialized body fluid containing components such as plasma, red blood cells, platelets, and white blood cells. Whole blood is separated into components by centrifugation due to the components' different densities and sedimentation rates. Centrifugation of whole blood leads to red cells settling at the bottom and white cells settling above the red cells. Platelets form a layer above the white cells. A refrigerated centrifuge is a device used to separate blood and its components. Whole blood is subjected to a heavy spin (5000G), which yields packed red blood cells, platelet-poor plasma, buffy coat, and fresh-frozen plasma. The light spin (1500G) of the centrifuge is utilized to yield platelet-rich plasma.[1] The components are also separated using apheresis. Apheresis is a procedure used to collect blood components from a single donor in one session. Whole blood is collected from a voluntary donor and stored in bags with CPDA (citrate-phosphate-dextrose-adenine) solution.
Fresh-frozen plasma is a part of whole blood that is fractionated and frozen within 8 hours to preserve the coagulation factors. Plasma was one of the first blood components developed during World War II military trauma resuscitation. In 1936, Dr. John Elliott mentioned that plasma could be used as a blood substitute in traumatic shock. Dr. Max Strumia developed freeze-dried plasma for military use.[2] Post World War II, plasma transfusion became more common in medical management. Over time, increased screening of blood-borne diseases and improved safety measures have reduced complications related to plasma transfusion.[3]
Fresh-frozen plasma can be stored at -25 °C or below for up to 36 months. Fresh-frozen plasma comprises labile and stable coagulation factors, plasma proteins, fibrinogen, and factor VIII. The temperature helps in preserving the labile coagulation factors. The factors present are fibrinogen, factor II, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, protein S, protein C, antithrombin III, and Von-Willebrand factor antigen.[4] Fresh-frozen plasma is thawed using a water bath at 37°C. Once thawed, the plasma should be transfused promptly. If it is not possible to administer the transfusion immediately, the plasma should be stored and used within 4 hours maintained at a temperature of 22 ±2°C, or a maximum of 120 hours if stored at a temperature of 4 ±2°C.
Transfusion is indicated in the context of single or multiple coagulation factor deficiencies, acute disseminated intravascular coagulation, immediate reversal of warfarin effect, thrombotic thrombocytopenic purpura, massive transfusion, liver disease, special pediatric considerations, and cardiopulmonary bypass surgeries. Transfusion reactions with fresh-frozen plasma can range from minor to life-threatening. They can be classified as acute or delayed, immunologic and non-immunologic.[5]
Acute transfusion reactions include acute hemolytic, allergic, anaphylactic, febrile nonhemolytic, bacterial contamination, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO). Delayed transfusion reactions include delayed hemolytic transfusion reaction, transfusion-transmitted infection, and post-transfusion purpura. If a reaction is suspected, transfusion must be stopped immediately, and the blood bank and treating physician must be notified.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Acute transfusion reactions may be immune- or nonimmune-mediated reactions that occur immediately or within 24 hours of fresh-frozen plasma transfusion. The different types of reactions can be attributed to various etiologies.[7]
Fresh-frozen plasma transfusion reactions have been attributed to factors such as human leukocyte antigen (anti-HLA) and human neutrophil antigen(anti-HNA) antibodies. Class II HLA antibodies are more commonly associated.
Allergic transfusion reactions are due to the preformed antibodies binding to transfused donor allergens. Identification of specific proteins to which recipients react has been challenging. Commonly implicated are human immunoglobulin (IgA) and haptoglobin. IgA antibodies have been implicated in many anaphylactic transfusion reactions, primarily observed in IgA-deficient individuals. Methylene blue-treated plasma has also been involved in anaphylactic reactions associated with plasma.[7]
Febrile nonhemolytic transfusion reaction (FNHTR) associated with plasma transfusion is low as fresh-frozen plasma is considered acellular. FNHTR is thought to be due to the immune response to leukocytes in the blood. Instances of FNHTR can be attributed to white blood cells contaminating plasma that has survived the freeze-thaw process.[8] Acute hemolytic transfusion reactions (AHTR) with fresh-frozen plasma are commonly seen due to ABO-incompatible plasma transfusion with higher titers of isohemagglutinins. Red cell fragments within plasma can also cause red blood cell allo-immunization.[6]
TRALI is caused due to damage to pulmonary endothelium mediated by activated neutrophils. A "2-hit" model is also considered: The first hit is the priming of neutrophils on the pulmonary endothelium due to the preexisting medical condition of the patient, and the second hit is the transfusion (containing antibodies) activating the primed neutrophils causing endothelial damage. The commonly implicated antibodies in the donor plasma include HLA and HNA. Plasma from a female donor with a history of pregnancy is a risk factor for TRALI.[9]
The etiology of TACO is multifactorial, with several risk factors contributing to its development. These risks include cardiac failure, renal failure, positive fluid balance, advanced age, suboptimal fluid management, and rapid transfusion rates. Elevated levels of cytokines, such as interleukin 8 (IL-8) and interleukin 10 (IL-10), have been observed in patients with TACO. The exact mechanism for fluid accumulation in the lungs in TACO is being investigated.[10]
Nonimmunologic reactions include bacterial contamination, circulatory overload, and chemical reactions like citrate toxicity. Infection risk with plasma transfusion is minimal due to extensive screening and testing. Transmissible infections include HIV, hepatitis B, and C. Bacterial contamination is rare, but contamination by water baths during thawing has been reported. Staphylococcus, Klebsiella, Propionibacterium, and Pseudomonas are some of the implicated organisms.[11]
Delayed transfusion reactions occur between 24 hours and 30 days. These reactions are due to amnestic responses to previously exposed foreign antigens. They include hemolytic reactions and transfusion-associated graft versus host disease (TA-GVHD). Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare condition, primarily because thawed units of fresh-frozen plasma typically do not contain viable lymphocytes, essential for developing the graft-versus-host disease reaction.[7]
Epidemiology
In the United States, 282 transfusion reactions are reported for every 100,000 transfusions administered. The incidence of adverse reactions related to fresh-frozen plasma transfusion shows substantial variation. Average reported rates of transfusion reactions with fresh-frozen plasma are as follows:
- Allergic reactions: 92 for every 100,000 units transfused.
- Anaphylactic reactions: 0.8 for every 100,000 units transfused.
- Febrile nonhemolytic transfusion reactions (FNHTRs): 12 for every 100,000 units transfused.
- Transfusion-associated circulatory overload (TACO): 6 for every 100,000 units transfused.
- Transfusion-related acute lung injury (TRALI): 1.8 for every 100,000 units transfused.[12]
Posttransfusion risk of HBV infection is estimated to be about 1:357,000 to 1:280,000 per transfusion. HIV and HCV are estimated to be 1:1,467,000 and 1:1,149,000, respectively, in the United States.[13]
Pathophysiology
Acute Transfusion Reactions
Allergic transfusion reactions can manifest as urticarial or anaphylactic responses. These reactions occur when the recipient's antibodies react against foreign proteins in the donor plasma. In most cases, the reactions are mild and present with symptoms like urticaria, pruritus, flushing, and isolated fever. Anaphylactic reactions present with symptoms such as rashes, angioedema, upper and lower airway obstruction, and hypotension. Individuals with IgA deficiency are at a higher risk of anaphylactic transfusion reactions due to the presence of alloantibodies against IgA. Deficiencies in other plasma proteins, such as haptoglobin, can also result in anaphylaxis when exposed through fresh-frozen plasma transfusion. An ongoing discussion revolves around the potential involvement of immunoglobulin E (IgE) in urticarial transfusion reactions. Some studies suggest a contribution of IgE in these reactions, while others do not, highlighting the complexity of the mechanisms involved in allergic responses during transfusions.[14][15]
Methylene-blue treated plasma has caused allergic transfusion reactions. In cases of allergen-independent pathways, biological response modifiers (BRMs), including inflammatory cytokines and chemokines, which accumulate during storage in blood components, have been identified as potential triggers for allergic reactions. These components can lead to immune responses in recipients, even in the absence of specific allergens.[16]
An FNHTR is a febrile response caused by cytokines released from blood donor leukocytes. Incompatible donor antibodies recognizing recipient antigens as foreign are also attributed to FNHTR pathology. FNHTR can also be mediated by bioactive mediators released by the remaining leukocytes during the freeze-thaw process. FNHTRs can also be influenced by the number of viable leukocytes present after the freeze-thaw process in blood products. The presence of viable leukocytes can contribute to these febrile reactions during transfusions. Leucoreducation has been shown to decrease the rates of FNHTR.[8]
Hemolytic transfusion reactions (HTR) encompass two primary types: immediate and delayed hemolytic transfusion reactions (DHTR). These reactions involve the destruction of red blood cells and can occur immediately during the transfusion or be delayed, manifesting days to weeks after the transfusion. ABO-incompatible fresh-frozen plasma units have been shown to cause hemolytic reactions based on the titers of isohemagglutinins in the donor plasma. Whenever possible, ABO-compatible plasma is preferable to avoid HTR; if unavailable, it is essential to consider plasma with low titers of anti-A and anti-B.[17]
Viruses and bacteria can cause transfusion-transmitted infections. Notable transfusions-associated pathogens include human immunodeficiency virus (HIV), hepatitis B, and hepatitis C. These infections can be transmitted through contaminated blood products, emphasizing the importance of rigorous screening and safety measures in blood transfusion practices. Cytomegalovirus and human T-cell lymphotropic virus are typically not transmitted through fresh-frozen plasma because fresh-frozen plasma is an acellular blood component. Safety measures include retesting of plasma, known as donor-retested plasma, where units are quarantined until a follow-up negative infectious disease test from the donor, and pathogen-reduced plasma, treated with UV light or chemicals like methylene blue, to curb lipid-enveloped virus transmission, particularly outside the United States. Solvent/detergent (S/D) plasma is a method that eliminates the risk of transmission of enveloped viruses in blood products, further bolstering the safety of plasma transfusions. These techniques are crucial for reducing the potential transmission of infectious agents in blood components.[18]
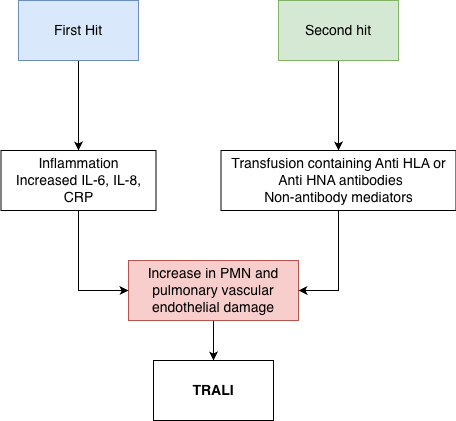
In transfusion-related acute lung injury (TRALI), the primary mechanism involves generating and activating neutrophils within the pulmonary endothelium. Two main pathophysiological mechanisms are implicated in TRALI: antibody-mediated and nonantibody-mediated (see Image. Pathophysiology of TRALI). Both mechanisms can lead to acute lung injury following a blood transfusion. A 2-hit hypothesis often explains TRALI. The first hit is the preexisting clinical condition of the patient, which may prime or sensitize the pulmonary endothelium and neutrophils. The second hit is the transfusion of blood or blood components, which can activate these primed neutrophils, ultimately leading to the development of acute lung injury. This dual mechanism underscores the complexity of TRALI's pathophysiology.
Risk factors for the first hit include shock, liver surgery, current smoking, chronic alcohol abuse, higher peak airway pressure, positive fluid balance, low IL-10 levels, and systemic inflammation. In cases of TRALI, elevated C-reactive protein levels are often observed, reflecting an inflammatory response. Transient leukopenia, a temporary decrease in white blood cell count, may also be part of the immune reaction. Additionally, donor antibodies against human leukocyte antigens (HLA) and human neutrophil antigens (HNA) are commonly implicated in TRALI, contributing to immune-mediated lung injury. Nonantibody-mediated TRALI occurs when no detectable anti-HLA or HNA antibodies are present, but the reaction is still triggered by other unidentified antigens or biological reactive molecules (BRM).[10][19]
Transfusion-Aassociated circulatory overload is characterized by pulmonary hydrostatic edema, which results in the accumulation of protein-poor edema fluid in the lungs. The specific pathways leading to this condition are still a subject of ongoing research. A 2-hit hypothesis is also considered a pathophysiological mechanism for TACO similar to TRALI. TACO is associated with several risk factors, including positive fluid balance, congestive heart failure, and renal failure. When these risk factors are combined with fluid overload or the rapid infusion of blood products, it can lead to TACO. During TACO, an increase in inflammatory cytokines such as IL-6 is often observed, and fever is a common clinical finding. These factors contribute to the pathophysiology and clinical presentation of TACO.[20]
Delayed Transfusion Reactions
Delayed hemolytic transfusion reactions result from an anamnestic response to a foreign protein, which can be non-ABO, such as the Duffy blood group antigen. Sensitization to this foreign protein often occurs due to prior transfusion or pregnancy, leading to an immune response upon reexposure during a subsequent transfusion. In contrast, transfusion-associated graft-versus-host disease (TA-GVHD) is rare in cases of plasma transfusion, as plasma is typically devoid of viable lymphocytes, which are necessary for developing TA-GVHD. Due to the freeze-thaw process, the number of viable lymphocytes capable of causing TA-GVHD is negligible. Posttransfusion purpura is an uncommon condition caused by an immune response to alloantibodies, which leads to severe thrombocytopenia. This condition typically develops within 5 to 10 days following the transfusion of plasma components, and it can result in bleeding and purpura, or small purple or red spots on the skin caused by bleeding into the skin.[21]
History and Physical
Before beginning a transfusion, assessing the patient's medical history and vital signs is crucial. Previous medical history of IgA deficiency, haptoglobin deficiency, congestive heart failure, renal failure, transfusion history, and family and social history must be elicited. Verification of the patient details and details on the blood/blood component bag before initiation of transfusion is essential. Vitals should be recorded every 15 minutes or put on a constant monitor. The patient must be evaluated for signs of fever, chills, urticaria, angioedema, hypotension, and respiratory distress. Slight variation in vitals signs is normal. In trauma and critically ill patients, closely monitoring the transfusion rate is vital. Remaining vigilant for the signs and symptoms of TRALI and TACO, as early detection and prompt intervention are crucial to ensuring patient safety during transfusion therapy.[22]
Allergic transfusion reactions present with urticaria, pruritus, and flushing. Anaphylactic reactions can present with more severe symptoms and signs of dyspnea, wheezing, angioedema, chest tightness, nausea, vomiting, tachycardia, hypotension, and shock. In FNHTR, patients may present with fever and chills. [23]
Hemolytic transfusion reactions can manifest with a range of symptoms, including fever, abdominal pain, and the presence of red or brown-colored urine. Patients experiencing these reactions may also exhibit chills, hypotension, and back pain. In severe cases, patients may exhibit signs of renal failure or disseminated intravascular coagulation, reflecting the systemic consequences of red blood cell destruction and hemoglobin release. Early recognition and immediate intervention are essential to prevent further complications in these cases.[24]
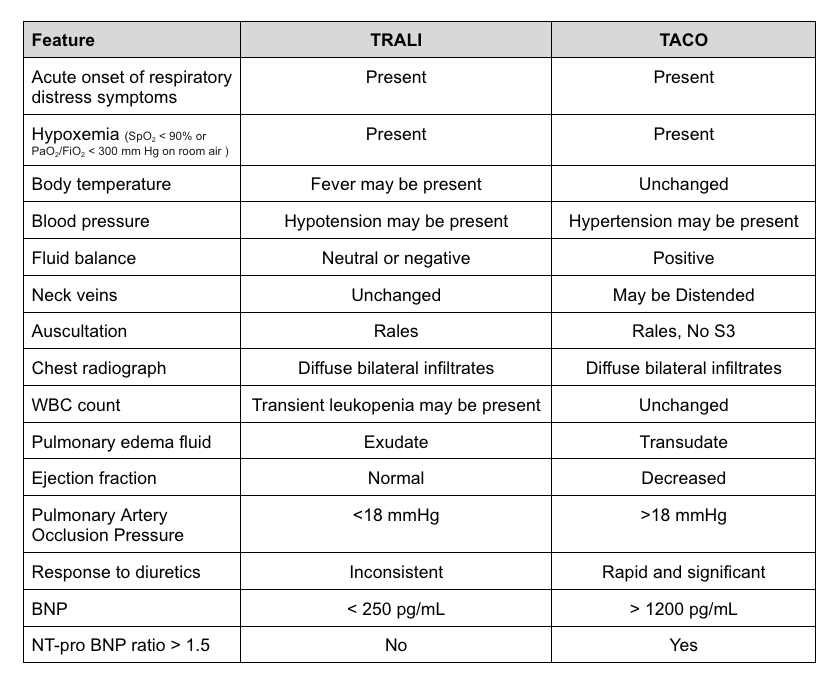
Both TACO and TRALI are characterized by acute onset of respiratory distress within 6 hours of receiving a blood or blood component transfusion. They show features of bilateral infiltrates on a chest x-ray indicative of pulmonary edema and hypoxemia with SpO2 less than 90% on room air and hypotension. See Table. TACO vs TRALI Signs and Symptoms for a comparison of the signs and symptoms of the 2 conditions. The clinical criteria for TRALI as published by an expert panel in 2019:[25]
- TRALI Type I. Patients with no risk factors for ARDS and satisfy the following criteria:
- Acute onset, hypoxemia (P/F ratio ≤ 300 or SpO2 < 90% on room air), evidence of bilateral pulmonary edema on imaging, no evidence of left atrial hypertension or, if present, not the main contributor to the hypoxemia
- Onset during or within 6 hours of transfusion
- No alternative risk factor for acute respiratory distress syndrome (ARDS)
- TRALI Type II. Patients with risk factors for ARDS (but who have not been diagnosed with ARDS) or who have existing mild ARDS but whose respiratory status deteriorates due to transfusion and with both of the following:
- Acute onset, with hypoxemia, bilateral pulmonary edema, no evidence of left atrial hypertension
- Stable respiratory status in the 12 hours before transfusion
In TACO cases, acute respiratory distress, distended neck veins, rales on auscultation, the presence of S3 heart sound on auscultation, hypertension, and fever may be present. The clinical definition of TACO, as outlined by the National Healthcare Safety Network in 2016, is as follows:[10]
- New onset or exacerbation of ≥3 symptoms within 6 hrs of transfusion:
- Acute respiratory distress (dyspnea, orthopnea, and cough)
- Evidence of positive fluid balance
- Elevated brain natriuretic peptide
- Radiographic evidence of pulmonary edema
- Evidence of left heart failure
- Elevated central venous pressure
Delayed transfusion reactions have signs of fever, jaundice, and a decline in hemoglobin concentration 2 to 4 weeks after transfusion. Posttransfusion purpura presents with sudden severe bleeding from mucous membranes, gastrointestinal tract, urinary tract, or intracranial hemorrhage due to severe thrombocytopenia within 2 weeks of transfusion. Other symptoms include fever, chills, and platelet transfusion refractoriness.[21]
Evaluation
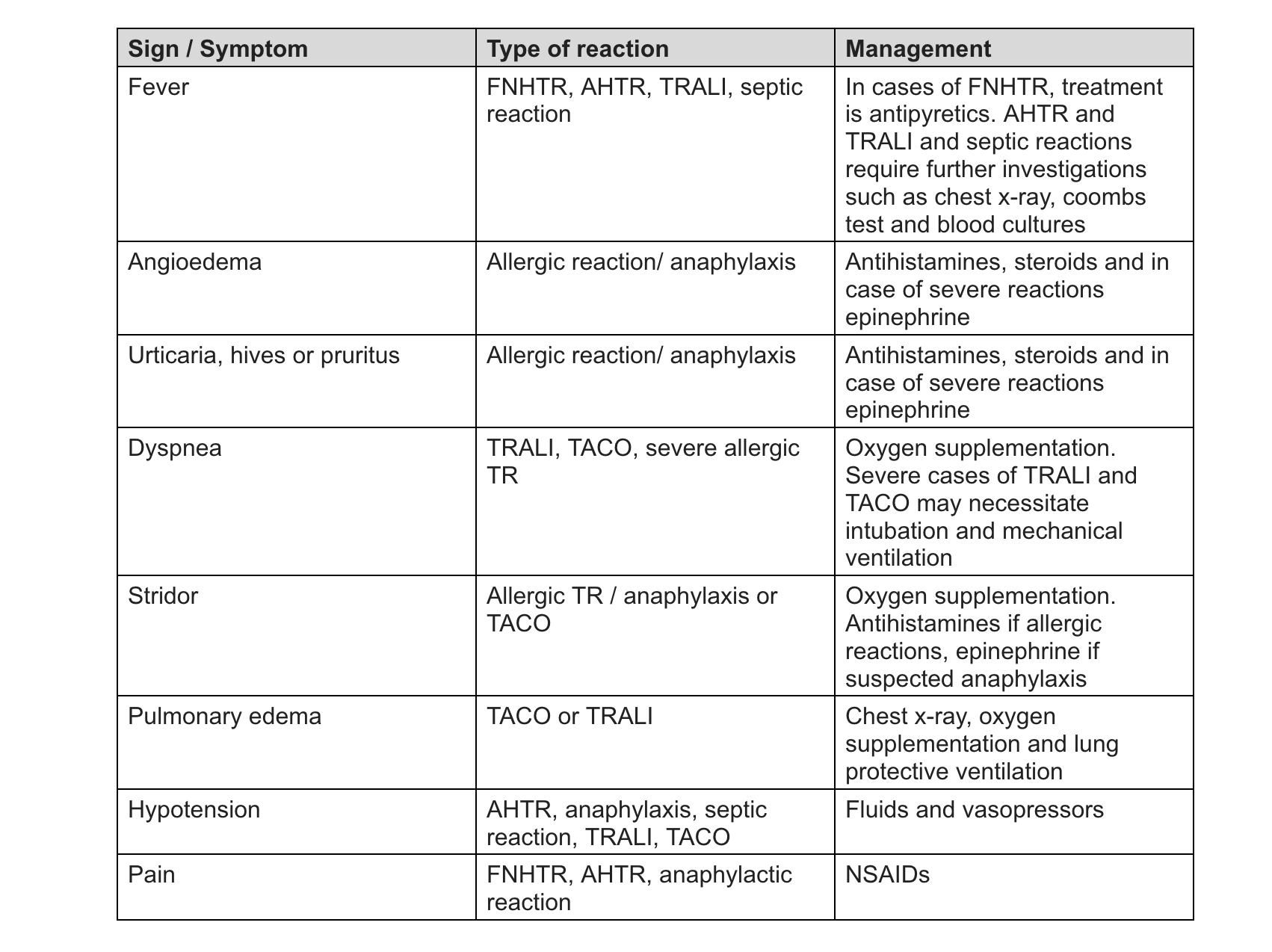
Identification of signs and symptoms plays a crucial role in treating transfusion reactions. An initial assessment must be performed to determine the reaction severity. Symptoms include fever, chills, urticaria, itching, respiratory distress, or hypotension. Patients with mild transfusion reactions generally display minimal alterations in their vital signs and clinical symptoms. These mild reactions can involve a slight increase in temperature, usually within the range of 1 to 2 degrees Celsius from their baseline, and transient urticaria (hives) without any associated systemic symptoms or significant physiological changes.[26]
In case of a reaction, the transfusion must be stopped immediately. Transfusion reaction protocol, if present, must be activated. The blood bank should be notified. The label on the plasma component and all associated records must be checked for possible clerical errors. Examination of the unit to check for discoloration and clumps must be undertaken. Venous access must be maintained. Assess the airway, breathing, and circulation of the patient. The patient must be evaluated for hypotension and shock, severe respiratory distress, sepsis, severe rigors or pain, fever, rash, or urticaria. After initial stabilization of the patient, additional investigations must be undertaken, and a decision to resume or discontinue the transfusion must be made.[26]
Investigations on the pre-reaction and post-reaction plasma transfusions are performed to detect hemolysis or jaundice. Blood cultures are collected if a septic reaction is suspected. In cases of allergic and anaphylactic reactions, basophil activation test (BAT) results and tryptase levels can be evaluated. If IgA or Haptoglobin deficiency is suspected, plasma levels can be investigated. In AHTR investigation, peripheral smear, bilirubin, haptoglobin, lactate dehydrogenase, coombs test, and total counts can be evaluated. In the cases of TRALI and TACO, chest X-ray, B-type natriuretic peptide (BNP), and NT-proBNP can be obtained. It is essential to determine if there is circulatory overload. Total blood counts can be used to evaluate DHTR and posttransfusion purpura.[27]
Treatment / Management
After the clinical assessment of the patient, symptomatic management is undertaken. If hypotension and shock are present, fluids and vasopressors are administered. In cases of severe respiratory distress, oxygen supplementation, epinephrine, antihistamines, and corticosteroids are administered. If signs of volume overload are present, administer IV diuretics and oxygen. In cases of sepsis, obtain blood cultures and administer broad-spectrum antibiotics. Treat fever with antipyretics and rash or urticaria with antihistamines.[26]
Allergic transfusion reactions are managed depending on the reaction severity. For mild acute transfusion reactions, systemic antihistamines are used. If the patient's symptoms improve with the initial management, it may be possible to resume the transfusion under careful monitoring. In case the symptoms persist, transfusion must be stopped. In cases of severe allergic reaction and anaphylaxis, the airway, breathing, and circulation must be assessed. Epinephrine (1:1000) is a first-line drug. Oxygen supplementation, IV fluids, methylprednisolone or prednisolone, and antihistamines are other supportive measures. In cases of bronchospasm, bronchodilators or β2-agonists must be administered. In cases of IgA deficiency, transfusions from IgA-deficient donors must be sought.[23]
FNHTRs are transient. Treatment is by administering paracetamol/acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs). In cases of suspected transfusion-transmitted infections, blood cultures should be drawn from the patient, and broad-spectrum IV antibiotics should be started. The transfusion of ABO-compatible plasma can avoid AHTR.[8](B3)
If TRALI is suspected, immediate cessation of the transfusion is essential. The airway is assessed, and high-flow O2 therapy is initiated. Mechanical ventilation should be considered when improvements do not occur with high-flow oxygen therapy. The patient is evaluated for fluid overload, and a chest x-ray is obtained. Lung protective ventilation strategies are used with low tidal volume and high positive end-expiratory pressure (PEEP) as the pathology is similar to ARDS. The management is mainly supportive. Research in transfusion medicine explores various potential therapies for managing and preventing transfusion-related adverse events. These experimental approaches include IL-10 therapy, blocking IL-8 receptors, and downregulating C-reactive protein (CRP) levels.[10][28]
In the case of TACO, the elevation of the head-end of the bed, oxygen supplementation, and administration of diuretics lead to rapid improvement. Noninvasive positive pressure ventilation can improve respiratory status. Optimal fluid management and treatment of underlying conditions are essential in management. In case of worsening respiratory symptoms, intubation and mechanical ventilation must be initiated. Lung-protective ventilation strategies must be implemented.[10]
See Table. FFP Reactions for the signs, symptoms, and management of fresh-frozen plasma transfusion reactions.
Differential Diagnosis
Depending on the type of transfusion reaction, various differential diagnoses should be considered:
- ARDS
- Anaphylaxis
- Disseminated intravascular coagulation
- Septicemia
- Cardiogenic pulmonary edema
- Infections
TRALI and TACO have similar presentations. Differentiating TRAIL and TACO from ARDS is necessary. All 3 conditions present with respiratory distress and have similar findings on chest x-ray. Echocardiography helps in the identification of volume status and diastolic dysfunction. Pulmonary artery occlusion pressure and protein concentration in pulmonary edema fluid are assessed, but findings are not definitive. B-type natriuretic peptide (BNP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) biomarker levels in conjunction with clinical signs and symptoms are used to establish the diagnosis of TACO.[29]
Toxicity and Adverse Effect Management
Adverse effects range from simple allergic reactions to life-threatening reactions such as TACO and TRALI. Simple reactions can be handled in the inpatient unit, whereas more severe reactions warrant ICU care. TRALI and TACO may require intubation and mechanical ventilation. Multidisciplinary management with the involvement of transfusion specialists, intensivists, nurses, and respiratory therapists is essential for improvement in patient outcomes. Mitigation strategies in blood collection and processing are necessary to prevent reactions like TRALI. These strategies include checking for antibodies (HLA, HNA), excluding female donors with a history of pregnancy, and avoiding multiparous donors.[5]
Preventing adverse effects in blood transfusions begins at the collection stage. Male donors are often preferred, as female donors may possess antibodies due to pregnancy that are capable of causing adverse effects. Other measures include checking the bag to avoid clerical errors, monitoring during transfusion, and early identification of symptoms and treatment.
Prognosis
Allergic transfusion reactions, febrile nonhemolytic transfusion reactions, and hemolytic transfusion reactions associated with plasma transfusions have a good prognosis. Trauma patients who receive fresh-frozen plasma transfusions may be at increased risk of developing additional conditions, such as ARDS and multiorgan failure, following their initial injuries.[30] Critically ill patients are at an increased risk of experiencing complications associated with fresh-frozen plasma transfusion.[31]
Patients with TRALI generally have a favorable prognosis. They often experience clinical improvement within 3 to 4 days following the initial event. Pulmonary infiltrates typically resolve relatively quickly. However, in some cases, these infiltrates might persist for more than 7 days, highlighting the variability in the recovery process among TRALI patients.[32]
Complications
Plasma transfusion can lead to various complications, from mild allergic reactions to severe and life-threatening conditions like TACO and TRALI. Allergic transfusion reactions, while generally not causing significant clinical sequelae, can necessitate additional workup and prolong the hospital stay for the affected patient. On the other hand, FNHTRs typically resolve quickly with the administration of antipyretic therapy. Prompt recognition and appropriate management are essential in addressing these complications and ensuring patient safety.
TRALI often worsens the patient's respiratory status and is primarily managed with supportive measures. TRALI is associated with a mortality rate that typically ranges from 5 to 10%, underscoring the severity of this condition.[33] TACO can have even more significant mortality and morbidity. One study reported mortality rates in the range of 14-29%, signifying the potentially life-threatening nature of this condition.[34]
Deterrence and Patient Education
Engaging in a patient-centered conversation about the need for plasma transfusion is crucial. This discussion should cover the reasons for the transfusion, its potential advantages and drawbacks, and the safety precautions, including crossmatching and vigilant monitoring. Patients should also be educated about common symptoms linked to transfusion reactions and encouraged to report any unusual sensations promptly. Providing patients with the opportunity to ask questions and seek additional information ensures they are well informed and empowered to make informed decisions about their healthcare.[26]
Pearls and Other Issues
Fresh-frozen plasma is collected using a citrate-containing anticoagulation solution, which can lead to citrate toxicity when administered. Citrate acts by chelating calcium, resulting in hypocalcemia, which can produce a range of symptoms. These symptoms may encompass hypotension, arrhythmias, alterations in mental status, increased central venous pressure (CVP), and clinical signs like Chvostek and Trousseau. The treatment for citrate toxicity involves calcium supplementation to correct the hypocalcemia and alleviate these symptoms.[35]
To mitigate the risk of TRALI, several key strategies are employed. These encompass donor screening for antibodies like anti-HLA and anti-neutrophil antibodies and the preference for plasma and platelet donations from only male donors or females who haven't experienced pregnancy. Utilizing pathogen-reduced, washed, or volume-reduced blood components also helps minimize TRALI risk. Alongside these measures, close monitoring during and after transfusions is essential, coupled with comprehensive healthcare provider education, to ensure early recognition and timely treatment of TRALI cases, collectively enhancing the overall safety of blood transfusions.[36]
Mitigation strategies for TACO encompass a multifaceted approach, including pretransfusion risk assessment and administering volume-reduced, leukoreduced, and washed blood products. Maintaining slow transfusion rates, administering diuretics before and after transfusion, and ensuring optimal fluid management are critical components of TACO prevention and management.[20]
Enhancing Healthcare Team Outcomes
Fresh-frozen plasma transfusions have increased steadily over the years. Even with evidence-based guidelines guiding usage, transfusion has increased in patients with abnormal coagulation studies who are not bleeding. All interprofessional healthcare team members, including physicians, advanced practice practitioners, nursing staff, and pharmacists, should know the indications and adverse reactions associated with fresh-frozen plasma administration. During fresh-frozen plasma transfusion patients, the patient's vital signs, including pulse, blood pressure, oxygen saturation, and temperature, must be monitored before, during, and after transfusion. Healthcare workers should familiarize themselves with the symptomatology of adverse events and act immediately if they suspect a transfusion reaction.
The blood and blood products must be checked with the patient's records before transfusion to avoid clerical errors. If a previous history of transfusion reaction is present, precautions must be taken to prevent a repeat reaction. If a transfusion reaction is suspected during transfusion, the transfusion must be stopped immediately, and the blood bank must be notified. Life-threatening reactions such as TRALI and TACO must be identified swiftly to provide supportive management and improve outcomes.
Local and national hematovigilance networks must be utilized as a source of information and contacted to report adverse events. Fresh-frozen plasma transfusion is safer now than in previous years, but clinicians must be prepared for the potential risks of transfusion.
The interprofessional healthcare team enhances patient care and outcomes when addressing adverse reactions and complications from fresh-frozen plasma transfusions. Physicians, advanced practitioners, nurses, pharmacists, and other allied healthcare professionals collaborate seamlessly to ensure a well-coordinated response. Clinicians provide clinical expertise to promptly diagnose and treat adverse events, tailoring interventions based on the patient's needs. Nursing staff closely monitor vital signs and observe for early warning signs of adverse reactions, immediately reporting any concerns. Pharmacists offer valuable insights into medication management and compatibility during the transfusion process. Effective communication and collaboration within the team are fundamental, allowing for a swift and comprehensive response to minimize patient harm and optimize transfusion outcomes. This coordinated effort ensures that patient safety remains at the forefront of fresh-frozen plasma transfusion management.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Basu D, Kulkarni R. Overview of blood components and their preparation. Indian journal of anaesthesia. 2014 Sep:58(5):529-37. doi: 10.4103/0019-5049.144647. Epub [PubMed PMID: 25535413]
Level 3 (low-level) evidenceStrumia MM, Wagner JA, Monaghan JF. THE INTRAVENOUS USE OF SERUM AND PLASMA, FRESH AND PRESERVED. Annals of surgery. 1940 Apr:111(4):623-9 [PubMed PMID: 17857568]
Watson JJ, Pati S, Schreiber MA. Plasma Transfusion: History, Current Realities, and Novel Improvements. Shock (Augusta, Ga.). 2016 Nov:46(5):468-479 [PubMed PMID: 27380536]
von Heymann C, Keller MK, Spies C, Schuster M, Meinck K, Sander M, Wernecke KD, Kiesewetter H, Pruss A. Activity of clotting factors in fresh-frozen plasma during storage at 4 degrees C over 6 days. Transfusion. 2009 May:49(5):913-20. doi: 10.1111/j.1537-2995.2008.02063.x. Epub 2009 Jan 21 [PubMed PMID: 19159416]
Khawar H, Kelley W, Stevens JB, Guzman N. Fresh Frozen Plasma (FFP). StatPearls. 2023 Jan:(): [PubMed PMID: 30020719]
Suddock JT, Crookston KP. Transfusion Reactions. StatPearls. 2023 Jan:(): [PubMed PMID: 29489247]
Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012 May:52 Suppl 1(Suppl 1):65S-79S. doi: 10.1111/j.1537-2995.2012.03663.x. Epub [PubMed PMID: 22578374]
Shmookler AD, Flanagan MB. Educational Case: Febrile Nonhemolytic Transfusion Reaction. Academic pathology. 2020 Jan-Dec:7():2374289520934097. doi: 10.1177/2374289520934097. Epub 2020 Jul 14 [PubMed PMID: 32728618]
Level 3 (low-level) evidenceRoubinian N. TACO and TRALI: biology, risk factors, and prevention strategies. Hematology. American Society of Hematology. Education Program. 2018 Nov 30:2018(1):585-594. doi: 10.1182/asheducation-2018.1.585. Epub 2018 Dec 14 [PubMed PMID: 30570487]
Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019 Apr 25:133(17):1840-1853. doi: 10.1182/blood-2018-10-860809. Epub 2019 Feb 26 [PubMed PMID: 30808638]
Hillyer CD, Josephson CD, Blajchman MA, Vostal JG, Epstein JS, Goodman JL. Bacterial contamination of blood components: risks, strategies, and regulation: joint ASH and AABB educational session in transfusion medicine. Hematology. American Society of Hematology. Education Program. 2003:():575-89 [PubMed PMID: 14633800]
Saadah NH, van Hout FMA, Schipperus MR, le Cessie S, Middelburg RA, Wiersum-Osselton JC, van der Bom JG. Comparing transfusion reaction rates for various plasma types: a systematic review and meta-analysis/regression. Transfusion. 2017 Sep:57(9):2104-2114. doi: 10.1111/trf.14245. Epub 2017 Aug 2 [PubMed PMID: 28766723]
Level 1 (high-level) evidenceEpstein JS, Holmberg JA. Progress in monitoring blood safety. Transfusion. 2010 Jul:50(7):1408-12. doi: 10.1111/j.1537-2995.2010.02728.x. Epub [PubMed PMID: 20636529]
Homburger HA, Smith JR, Jacob GL, Laschinger C, Naylor DH, Pineda AA. Measurement of anti-IgA antibodies by a two-site immunoradiometric assay. Transfusion. 1981 Jan-Feb:21(1):38-44 [PubMed PMID: 7008284]
Level 3 (low-level) evidenceShimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002 Jun:42(6):766-73 [PubMed PMID: 12147031]
Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. British journal of haematology. 2013 Feb:160(4):434-44. doi: 10.1111/bjh.12150. Epub 2012 Dec 6 [PubMed PMID: 23215650]
Level 3 (low-level) evidenceBerséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013 Jan:53 Suppl 1():114S-123S. doi: 10.1111/trf.12045. Epub [PubMed PMID: 23301963]
Marietta M, Franchini M, Bindi ML, Picardi F, Ruggeri M, De Silvestro G. Is solvent/detergent plasma better than standard fresh-frozen plasma? A systematic review and an expert consensus document. Blood transfusion = Trasfusione del sangue. 2016 Jul:14(4):277-286. doi: 10.2450/2016.0168-15. Epub 2016 Feb 17 [PubMed PMID: 27136429]
Level 1 (high-level) evidenceTung JP, Chiaretti S, Dean MM, Sultana AJ, Reade MC, Fung YL. Transfusion-related acute lung injury (TRALI): Potential pathways of development, strategies for prevention and treatment, and future research directions. Blood reviews. 2022 May:53():100926. doi: 10.1016/j.blre.2021.100926. Epub 2022 Jan 5 [PubMed PMID: 35065815]
Bulle EB, Klanderman RB, Pendergrast J, Cserti-Gazdewich C, Callum J, Vlaar APJ. The recipe for TACO: A narrative review on the pathophysiology and potential mitigation strategies of transfusion-associated circulatory overload. Blood reviews. 2022 Mar:52():100891. doi: 10.1016/j.blre.2021.100891. Epub 2021 Oct 2 [PubMed PMID: 34627651]
Level 3 (low-level) evidenceHawkins J, Aster RH, Curtis BR. Post-Transfusion Purpura: Current Perspectives. Journal of blood medicine. 2019:10():405-415. doi: 10.2147/JBM.S189176. Epub 2019 Dec 9 [PubMed PMID: 31849555]
Level 3 (low-level) evidenceLotterman S, Sharma S. Blood Transfusion. StatPearls. 2023 Jan:(): [PubMed PMID: 29762999]
Savage WJ, Tobian AA, Savage JH, Wood RA, Schroeder JT, Ness PM. Scratching the surface of allergic transfusion reactions. Transfusion. 2013 Jun:53(6):1361-71. doi: 10.1111/j.1537-2995.2012.03892.x. Epub 2012 Sep 24 [PubMed PMID: 22998777]
Harewood J, Ramsey A, Master SR. Hemolytic Transfusion Reaction. StatPearls. 2023 Jan:(): [PubMed PMID: 28846280]
Vlaar APJ, Toy P, Fung M, Looney MR, Juffermans NP, Bux J, Bolton-Maggs P, Peters AL, Silliman CC, Kor DJ, Kleinman S. A consensus redefinition of transfusion-related acute lung injury. Transfusion. 2019 Jul:59(7):2465-2476. doi: 10.1111/trf.15311. Epub 2019 Apr 16 [PubMed PMID: 30993745]
Level 3 (low-level) evidenceBakdash S, Yazer MH. What every physician should know about transfusion reactions. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2007 Jul 17:177(2):141-7 [PubMed PMID: 17638948]
Soutar R, McSporran W, Tomlinson T, Booth C, Grey S. Guideline on the investigation and management of acute transfusion reactions. British journal of haematology. 2023 Jun:201(5):832-844. doi: 10.1111/bjh.18789. Epub 2023 Apr 26 [PubMed PMID: 37211954]
Semple JW, McVey MJ, Kim M, Rebetz J, Kuebler WM, Kapur R. Targeting Transfusion-Related Acute Lung Injury: The Journey From Basic Science to Novel Therapies. Critical care medicine. 2018 May:46(5):e452-e458. doi: 10.1097/CCM.0000000000002989. Epub [PubMed PMID: 29384784]
Tobian AA, Sokoll LJ, Tisch DJ, Ness PM, Shan H. N-terminal pro-brain natriuretic peptide is a useful diagnostic marker for transfusion-associated circulatory overload. Transfusion. 2008 Jun:48(6):1143-50. doi: 10.1111/j.1537-2995.2008.01656.x. Epub 2008 Feb 22 [PubMed PMID: 18298592]
Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, Cuschieri J, Maier RV, Billiar TR, Peitzman AB, Inflammation and Host Response to Injury Investigators. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. The Journal of trauma. 2009 Aug:67(2):221-7; discussion 228-30. doi: 10.1097/TA.0b013e3181ad5957. Epub [PubMed PMID: 19667872]
Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007 May:131(5):1308-14 [PubMed PMID: 17400669]
Level 2 (mid-level) evidenceWebert KE, Blajchman MA. Transfusion-related acute lung injury. Transfusion medicine reviews. 2003 Oct:17(4):252-62 [PubMed PMID: 14571393]
Popovsky MA. Transfusion-Related Acute Lung Injury: Incidence, Pathogenesis and the Role of Multicomponent Apheresis in Its Prevention. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2008:35(2):76-79 [PubMed PMID: 21512631]
Roubinian NH, Hendrickson JE, Triulzi DJ, Gottschall JL, Chowdhury D, Kor DJ, Looney MR, Matthay MA, Kleinman SH, Brambilla D, Murphy EL, NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Incidence and clinical characteristics of transfusion-associated circulatory overload using an active surveillance algorithm. Vox sanguinis. 2017 Jan:112(1):56-63. doi: 10.1111/vox.12466. Epub 2016 Dec 21 [PubMed PMID: 28001313]
Li K, Xu Y. Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. International journal of clinical and experimental medicine. 2015:8(4):6578-84 [PubMed PMID: 26131288]
Otrock ZK, Liu C, Grossman BJ. Transfusion-related acute lung injury risk mitigation: an update. Vox sanguinis. 2017 Nov:112(8):694-703. doi: 10.1111/vox.12573. Epub 2017 Sep 25 [PubMed PMID: 28948604]