Nuclear Medicine Applications in Prostate Cancer
Nuclear Medicine Applications in Prostate Cancer
Introduction
In recent years, the incidence of prostate cancer has been the highest ever recorded due to earlier and more accurate detection and an increase in the average lifetime. Prostate cancer is now the second most common malignancy amongst all cancers in men and the fourth most common overall.
The American Cancer Society has identified prostate cancer as the second leading cause of mortality from oncological causes in the United States, accounting for an estimated 268,490 newly diagnosed cases and 34,500 deaths in 2022. The National Cancer Institute predicts that the average American man has an 11% risk of being diagnosed with prostate cancer at some point in life and a 2.5% overall risk of dying from the disease.
Globally, there were more than 1.4 million new cases of prostate cancer diagnosed worldwide in 2020, accounting for 375,304 deaths.[1][2][3] Prostate cancer has been identified as the most commonly diagnosed malignancy in 112 countries worldwide and the leading cause of cancer mortality in 48.[2][4]
Timely diagnosis leading to earlier and more effective treatment is required to reduce the significant morbidity and mortality rates. Nuclear medicine has several new and revolutionary prostate cancer detection, localization, staging, and treatment tools utilizing targeted therapy.[5][6] The expanding role nuclear medicine plays in managing prostate cancer will be discussed in detail in the following review.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The prostate is a glandular and muscular organ located just below the neck of the bladder and at the base of the urethra. The prostate's muscular function helps regulate urinary flow and prevents retrograde ejaculation, but its primary function is to produce a prostatic secretion that is added to the semen. Prostatic secretions include acid phosphatase, a prostatic growth regulator that helps release chemical energy from compounds in the semen; prostate-specific antigen, a protease that acts to liquefy the semen after ejaculation; transglutaminase, an enzyme that promotes liquefaction of semen; citric acid; zinc; and calcitonin, which improves sperm motility.[7]
The prostate gland is situated within the pelvic cavity, immediately anterior to the rectum, below the inferior edge of the symphysis pubis, above the triangular ligament but below the inferior margin of the symphysis pubis. The prostate's peak contacts the triangular ligament and is oriented downwards, while its base is below the bladder's neck.[8] The base of the structure is oriented superiorly. The gland's anterior surface is attached to the pubis by the puboprostatic ligaments, while its posterior surface is in contact with the rectum's second section. The levator ani muscles contact the lateral surfaces of the gland.[8][9][10]
The prostate comprises 5 lobes: 2 equally sized lateral lobes and the anterior, posterior, and median lobes. The prostate is separated from the rectovesical fascia by a plexus of veins and is encased in a thin capsule composed of fibrous tissue. Muscular tissue can be found immediately below the capsule and surrounding the urethra.
The arterial supply to the prostate is principally from the internal iliac circulation, specifically, the internal pudendal, inferior vesical, and hemorrhoidal or middle rectal arteries. The prostatic artery is the terminal branch of the inferior vesical artery. The prostatic artery is typically only 1 to 2 cm long before dividing into capsular and urethral branches. The venous drainage of the prostate is from the prostatic venous plexus and the dorsal vein of the penis into the internal iliac veins. The prostatic nerve supply is via the pelvic plexus and inferior hypogastric plexus.[8][9][10][11]
Indications
After the early discovery of high-risk cancer (>Gleason 7, T3a or higher, PSA=20 or higher, ISUP=4 or higher), the most effective, definitive therapy for localized prostatic malignancies is radical prostatectomy and/or definitive radiation therapy. Approximately 80% of patients are cancer-free for 7 years, but 27% to 53% will eventually develop a recurrence.[12] The 5-year survival rate for patients with locally advanced prostate cancer is close to 100%; this drops to 31% for those with metastatic disease. Unfortunately, 12% of patients already have metastases to lymph nodes or other organs at diagnosis.[1] Those with metastases have a significantly shorter survival time and are not likely to benefit from definitive, curative therapy.[13][14]
CT and MRI are generally poor in detecting small lymph node metastases as they rely primarily on anatomical findings and nodal size characteristics.[15] Nuclear medicine bone scans typically require PSA levels of 20 ng/mL or more before reliably detecting metastatic bone lesions.[16] New nuclear medicine imaging modalities offer much more sensitive and specific detection of metastatic disease, allowing improved prostate cancer staging and restaging accuracy. This facilitates more appropriate and effective therapeutic choices while avoiding unnecessary morbidity from overtreatment, such as eliminating the need for definitive local therapy if there is already evidence of metastatic spread.
Therapeutic radioligands designed for prostate cancer are also part of managing metastatic disease.
Historically, radiotherapeutic isotopes have played a significant role in the palliative treatment of painful bone metastases. However, in recent years, therapeutic radioligands targeting prostate-specific membrane antigen (PSMA), which is overexpressed on the cell surface membrane of most prostate cancers, have also been utilized successfully in several clinical trials.[1][12]
PSMA is a transmembrane cell surface glycoprotein highly expressed in up to 95% of prostate cancer cells, including castrate-resistant, aggressive, metastatic disease.[12] Emerging androgen resistance enhances PSMA overexpression (up to 1,000 times the normal level).[17] These characteristics make PSMA an excellent target for antibody-based tracers and small-molecule radiopharmaceuticals, including hormone-refractory disease progression, recurrence, or suspected metastases.
The first available PSMA-based PET imaging radioligand was capromab pendetide radiolabeled with indium-111, known commercially as Prostascint which was FDA-approved in 1996. Although a breakthrough, it could only bind to intracellular PSMA and had poor tissue penetration. This meant it could only effectively image nonviable cells with membrane damage exposing their intracellular contents. Imaging was also degraded by high background activity. These factors significantly limited its clinical utility and gave PSMA an initial bad reputation for prostate cancer imaging.[18]
This changed radically on December 1, 2020, when the FDA approved the first successful small-molecule PSMA-based radioligand that could highlight surface PSMA targets on living cancer cells. The development of small ligands which could reliably target active cell surface membrane PSMA sites has allowed widespread availability of prostate cancer radioligand-based imaging for staging and restaging, including castration-resistant tumors, even with very small tumor volumes and low PSA levels.
PSMA-binding radionuclides are now widely used and recommended for primary staging, restaging, and early cancer detection. Therapeutic radioligands can be used in selected high-risk and advanced cases.[12] About 5% of prostate cancers are PSMA-negative, so PSMA-binding radioligands will not be helpful or therapeutic in these cases.[19][20][21][22]
Such tumors typically have a poor prognosis. Fibroblast activation protein (FAP) is overexpressed in many solid tumors, including prostate cancer. Preliminary investigational studies suggest it may become a useful alternative PET tracer in PSMA-negative prostate cancer patients for both diagnostic imaging and theranostic treatment.[23]
Technique or Treatment
Commonly used radiopharmaceuticals and the scans used for imaging purposes are summarised below. Current good radiopharmaceutical practices should be considered when making and administering these agents. All pertinent regulations and guidelines should be followed in their creation, preparation, handling, storage, transport, dosing, and administration.[24][25][26]
CT and MRI are essentially equivalent in the evaluation of lymph node metastases. However, MRI is superior to CT imaging with regard to contrast enhancement in the evaluation of prostatic and pelvic anatomy. Both imaging modalities identify bone metastases well.
F-18-FDG is a commonly used radioisotope scan for many malignancies, but it is not optimal or generally recommended for prostate cancer.[27] F-18-FDG is a glucose analog that accumulates in cells and tissues with increased metabolic activity.[28] However, when used in prostate cancer, it demonstrates generally low overall sensitivity.[29] This is caused by the relatively slow rate of glucose metabolism in prostate cancer compared to other malignancies.
Urinary excretion of the radioisotope can interfere with the imaging of prostatic lesions. Also, several benign conditions can cause false positives with increased F18-FDG uptake in the prostate, such as benign prostatic hyperplasia, prostatitis, and various cystic malformations.[27][29] For all of these reasons and a lack of specific data showing a benefit, F-18-FDG scans are not currently recommended by the National Comprehensive Cancer Network (NCCN) for the routine staging of prostate cancer.
Radiopharmaceuticals for Bone Scan Imaging
Bone scans are designed to identify skeletal metastases in prostate cancer. The national comprehensive cancer network (NCCN) recommends an initial bone scan for patients at high risk for skeletal metastases (PSA equal or >20 ng/mL; clinical stage at least T2c; or Gleason 8, 9, or 10).[30]
They are also indicated in evaluating patients at high risk for metastasis after radical prostatectomy surgery if PSA levels do not become undetectable or if subsequent PSA levels become detectable on at least 2 occasions.
Bone scans may be considered in patients who become symptomatic, have an increasing PSA, or have a positive digital rectal examination after definitive radiation therapy. They can be used to monitor the response to androgen deprivation therapy, for which the NCCN recommends repeating the bone scan every 6 to 12 months. The NCCN recommends a bone scan interval of 8 to 12 weeks to monitor castrate-resistant prostate cancer.
Ga-68-PSMA-11, F-18-piflufolastat (DCFPyL), C-11-choline, and F-18-fluciclovine may all be considered to clarify equivocal results on initial bone scans.
A separate bone scan is not required if a Ga-68 PSMA-11 or F-18 piflufolastat (DCFPyL) PSMA PET/CT scan is performed.
Tc-99m Diphosphonates (99mTc-MDP) Bone Scan
Gamma camera-based bone scans are widely available in clinical practice and have a long history of staging bone metastases in prostate cancer. Patients do not require specific preparation for this procedure. The imaging typically takes approximately 45 minutes, but patients require injection approximately 3 hours before scanning.
The introduction of 3D fusion hybrid imaging with single photon emission computed tomography and computerized X-ray tomography (SPECT-CT) has improved the resolution and anatomic localization of the acquired images. To identify bone metastases with the traditional Tc-99m bone scan, PSA levels typically have to be 20 ng/mL or higher, while PET scans typically require only 0.2 ng/mL.
- Indication: Detection and evaluation of bone metastases utilizing increased bone turnover.
- Scanner Used: Gamma camera/SPECT-CT.
- Tracer: [99m Tc] technetium hydroxy diphosphonate is the most commonly used tracer.
- Tracer Pharmacokinetics: Diphosphonates function as phosphate analogs that, through chemisorption, combine with crystalline hydroxyapatite in bone.
- Acquisition protocol
- Injected dose: 730-1110 MBq injected intravenously
- Injection to imaging time: 2 to 4 hours
- Protocol: whole body image acquisition with anterior and posterior projections
- SPECT/CT of regions of interest
- Reconstructions: performed utilizing iterative reconstructive methods [objective substitute expectation maximization (OSEM), maximum likelihood expectation maximization (MLEM)]
- Sensitivity: 79%
- Specificity: 86%
- Positive predictive value: 45%
- Negative predictive value: 96%[31]
- FDA-approved for suspected bone metastases
[F-18] F-18-Sodium Fluoride Bone Scan
F-18-sodium fluoride positron emission tomography (PET) has a high specificity and sensitivity for bone metastases. This scan has become a frequent replacement for conventional diphosphonate bone scans as PET/CT cameras become more common in clinical practice. Medical cyclotrons can produce f-18-sodium fluoride. The scans are more sensitive to bone turnover than diphosphonate scintigraphy, involve less uptake time, and offer 3D whole-body hybrid imaging with CT.
Patients generally fast for 4 hours before intravenous tracer administration and are advised to stay hydrated before and during the uptake time post-injection.
Whole body images with CT fusion help to identify and localize individual bone metastases in 3D volumes.
- Indication: suspected bone metastases due to advanced prostate cancer; a replacement and upgrade for the traditional Tc-99 MDP bone scan.
- Scanner Used: PET-CT.
- Tracer Pharmacokinetics: uptake based on the chemisorption of fluoride ions on the surface of hydroxyapatite, which exchanges hydroxyl ions in the crystal to make fluorapatite.
- Acquisition protocol
- Injected dose: 1.5-3.7 MBq/kg with a max of 370 MBq doses for large patients
- Radiopharmaceuticals: injected intravenously
- Injection to imaging time: 30 to 45 minutes
- Scan acquired: typically a whole-body image
- CT protocol: 35 mA, 120 kVp, with a rotation time of 0.55 seconds, Pitch 0.85 with a FOV of 780 mm
- PET protocol: 256 X 256 matrix, Zoom 1.0, 2 to 5 minutes/bed position scan duration; reconstruction should be performed via iterative-based methods with a setting of 2 iterations and 21 subsets.
- Sensitivity and specificity: sensitivity - 77 to 94%, specificity - 92 to 99%.[32]
- Positive predictive value (PPV) for bone metastasis: 82 to 97%[32][33]
- Equivalent to F-18-PSMA piflufolastat (DCFPyL) for the detection of bony metastases.[34]
- FDA-approved for suspected bone metastases only
- Recommended by the NCCN for bone metastases only
Radiopharmaceuticals for Prostate Cancer Imaging
[C-11] Carbon-11-Choline PET Scan
Choline has numerous nutritional functions. Prostate cancer cells have markedly increased choline uptake, which concentrates in these cells. Carbon-11 (C-11) radioisotope labeled choline was used for early detection of recurrent malignant disease but is not currently recommended for initial staging. Carbon-11 has a short half-life (20 minutes), requiring an on-site cyclotron, limiting its availability. F-18-fluorocholine PET/CT is more widely used in clinical practice due to its longer half-life. Ga-68 radioligands have a longer half-life and do not require a cyclotron on-site.[35]
Choline-based scans are less sensitive than PSMA-binding radioligand imaging, and their indications have now been superseded in most nuclear medicine practices.
- Indications: detecting recurrent disease and metastases for biochemical recurrence or progression only.
- Scanner Used: PET-CT.
- Tracer pharmacokinetics: malignant prostate cells have higher kinase expression, increasing choline uptake and phosphatidylcholine concentrations; high phosphatidylcholine concentrations can be detected using radiotracer-tagged choline.
- Acquisition protocol
- Injected dose: 350 MB
- Injected intravenously
- Injection to imaging time: 10 minutes.
- Position: position patient supine with the arms elevated above the head
- Scan: cover from the head to mid-thigh level
- CT protocol: 35 mA, 120 kVp, with a rotation time of 0.55 seconds; pitch is 0.85 with a FOV of 780 mm
- PET protocol: 256 X 256 matrix, Zoom 1.0, 1.5 to 2.5 minutes/bed position scan duration; reconstruction should be performed via iterative-based methods with a setting of 2 iterations and 21 subsets
- Attenuation correction: performed with the help of CT scan data
- Positive predictive value for biochemical recurrence: 53 to 96%.
- FDA-approved: biochemical recurrence or progression only.
- Recommended by the NCCN: biochemical recurrence or progression only.
[F-18] F-Fluciclovine (FACBC) PET Scan
F-18-fluciclovine is an analog of the amino acid leucine. Prostate cancer has increased amino acid transport and utilization, which can be seen in PET/CT imaging using this agent. F-18-fluciclovine also has increased uptake in osteoblastic and osteolytic lesions, giving it approximate equivalency to bone scintigraphy. This analog has been shown to find more bone metastases than Tc-99m MDP bone scans and offers the advantage of finding tissue lesions as well.[36]
However, it has relatively poor specificity for primary prostate cancer and nodal disease. F-18-fluciclovine has high sensitivity but low specificity and a moderate positive predictive value for recurrent involvement in the prostate bed. F-18-fluciclovine is superior to CT alone, In-111-Prostascint, and C-11-choline, plus its limited urinary excretion improves the visualization of lesions near the bladder. However, it is less sensitive than PSMA-binding tracers, and its indications have also been largely superseded in clinical practice. It is FDA-approved for biochemical recurrence and progression but not for initial staging.
- Indications: Detection of recurrent disease and metastases. Biochemical recurrence.
- Scanner Used: PET-CT.
- Tracer Pharmacokinetics: A leucine analog, F-18-fluciclovine, shows increased uptake in bone and metastases.
- Acquisition Protocol:
- The injected dose is 370 MB.
- Injected intravenously.
- Injection to imaging time is 3 to 5 minutes.
- The patient should be positioned supine with the arms elevated above the head after the injection.
- The injection should be given to the right arm.
- The scan should cover from the head to mid-thigh level.
- Correct Localization Rate for Biochemical Recurrence: 87%–91%.
- FDA-Approved for Biochemical Recurrence or Progression only.
- Recommended by the NCCN for Biochemical Recurrence or Progression only.
PSMA-Binding Radionuclide Imaging
Ga-68 and F-18 labeled PSMA-binding radioligands have the advantage of high sensitivity and specificity for metastatic prostate cancer lesions. With increasing pathological grade and the onset of androgen independence, PSMA expression appears to increase. Therefore, a PSMA-radioligand scan can effectively show bone, lymph node, and visceral metastatic lesions and primary disease at the prostate gland or local recurrence. In addition, the possibility of in-house production of Ga-68 with the help of a Ga-68 generator makes it a practical choice in facilities without a cyclotron.
Cyclotron-produced F-18 PSMA binding radioligands have shown equally high specificity for metastatic prostate lesions compared to the more commonly used Ga-68 agents. Overall, PSMA-based PET-CT imaging now plays an essential role in detecting metastatic prostate cancer. Generally, a PSA level greater than 0.2 ng/mL is needed for good results from a PSMA-based PET scan. PSMA imaging should ideally be performed starting antiandrogen hormonal therapy as imaging sensitivity may be affected.
Ga-68-PSMA PET/CT is currently the gold standard for PSMA imaging in prostate cancer, providing high sensitivity and specificity for detecting and staging the disease. However, Ga-68-PSMA PET/CT may be unavailable, particularly in resource-limited settings. In these situations, Tc-99m-PSMA single-photon emission computed tomography/computed tomography (SPECT/CT) and Tc-99m methyl diphosphonate (Tc-99m MDP) bone scans are commonly used for staging.
[Ga-68] Ga-68-PSMA-11
Ga-68-PSMA-11 radioligands were first described in 2012 and are now the most widely used radioisotope tracer for prostate cancer PET imaging.[37] They can detect very small metastases or recurrences due to high binding affinity, rapid rate of intracellular accumulation, and rapid elimination from non-target tissues. Ga-68 PSMA is highly concentrated in the salivary and lacrimal glands with moderate uptake in the liver, spleen, bowel, and sympathetic ganglia and only minimal concentration in normal prostate cells. It is superior (greater sensitivity) to choline PET imaging for prostate cancer and can be used for both initial staging and biochemical recurrences.[38][39] The kidneys excrete Ga-PSMA-11, which accumulates in the bladder and may obscure adjacent lesions.
Ga-68 has a relatively short half-life (68 minutes). In-house generators for Ga-68 help mitigate the drawbacks of its short half-life.[1] Current commercially available Ga-68 radioligands for PSMA imaging are Ga-68-PSMA-11 and Ga-68-PSMA-I&T. Ga-PSMA-11 is the more commonly used in clinical practice.
- Sensitivity: 40%.
- Specificity: 95% for nodal involvement.
- Positive Predictive Value for Biochemical Recurrence: 92%.
- The half-life is 68 minutes.
- Requires a local generator.
- FDA-Approved for initial staging and biochemical recurrence or progression.
- Scanner Used: PET-CT.
- Patient preparation: patients fast for 3-4 hours before injection and remain hydrated to improve target to background uptake and reduce radiation dose to the urinary bladder.
- Tracer Pharmacokinetics: PSMA is present in almost all prostate adenocarcinomas, including primary tumors and metastatic lesions.
- PSMA is significantly overexpressed in hormone-refractory malignant lesions.
- Radioligands are directed against the antigen's intracellular (necrotic or apoptotic cells) or extracellular (live cell) motifs.
- Acquisition Protocol:
- The injected dose is 1.8 to 2.2 MBq/Kg.
- Injected intravenously.
- Injection to imaging time is 60 minutes.
- The patient should be positioned supine with the arms elevated above the head.
- The scan should generally cover from the head to mid-thigh level.
- CT protocol: 35 mA, 120 kVp, with a rotation time of 0.55 seconds, Pitch 0.85 with a FOV of 780 mm.
- PET protocol: 256 X 256 matrix, zoom 1.0, 3 - 4 minutes/bed position scan duration, reconstruction should be performed via iterative-based methods with a setting of 2 iterations and 21 subsets.
- Attenuation correction must be performed with the help of CT scan data.
- FDA-Approved for Initial Staging and Biochemical Recurrence or Progression.
- Recommended by the NCCN for Initial Staging and Biochemical Recurrence or Progression.
[F-18] F-18-PSMA piflufolastat (DCFPyL)
[F-18] F-PSMA radioligands have similar performance characteristics to Ga-68-PSMA-11 in PET scans for prostate cancer but have a longer half-life at 2 hours. F-18 can be easily produced in a regional cyclotron.[40] Two commercially available F-18 radioligands are available: [F-18] F-PSMA-DCFPyl and [F-18] F-PSMA-1007. [F-18] F-PSMA-1007 has non-urinary (hepatobiliary) excretion, while [F-18] F-PSMA-DCFPyL is rapidly cleared from the urinary tract. Therefore both agents have advantages over current Gallium-based agents, which are excreted in the urine, for detecting lesions adjacent to the bladder that may be obscured in scans using Ga-68.[1][41] Additional tips, suggestions, procedural details, and interpretation assistance can be found in the published F-18-DCFPyL PET/CT guidelines.[42][43]
- Sensitivity: 31 to 42%.
- Specificity: 96 to 99% for nodal metastasis.
- Correct Localization Rate (CLR): 85 to 87% for biochemical recurrence.
- The half-life is 2 hours.
- Produced by a regional cyclotron.
- Equivalent to the F-18-Sodium Fluoride bone scan for the detection of skeletal metastases.[34]
- Overall, roughly equivalent to Ga-68-PSMA-11 PET scans.
- FDA-Approved for Initial Staging and Biochemical Recurrence.
- Recommended by the NCCN for Initial Staging and Biochemical Recurrence or Progression.
Tc-99m-PSMA Radioligands
PSMA-binding radioligands using technetium 99m are in clinical use for the staging of prostate cancer. Because they use the gamma emitter technetium 99m, they allow planar imaging and SPECT/CT in most nuclear medicine departments using a gamma camera.
Ga-68-PSMA PET/CT reveals a considerably higher average number of lesions and is more effective in detecting prostatic bed lesions than Tc-99m-PSMA SPECT/CT. But at higher PSA levels (>4 ng/ml), Tc-99m-PSMA SPECT/CT still provides high overall detection rates for prostate cancer metastases, and there is no significant difference in the detection of lymph nodes or bony metastases.[44]
When compared head-to-head, patients with a PSA >2.1 ng/mL showed consistent results between the two imaging modalities.[44]
Tc-99m-PSMA SPECT/CT does not detect small lesions well, so it is not recommended in patients with small-volume disease.[45] The total cost is substantially less than a Ga-68-PSMA PET/CT scan, making it a cost-effective alternative with no discernible difference in cancer detection at higher PSA levels between the two imaging modalities.[45]
In a prospective, comparative trial, 99m-Tc PSMA was found to be far superior to 99m-Tc-MDP SPECT/CT with regards to sensitivity, specificity, and PSA cutoff (2.6 ng/mL vs. 15 ng/mL) in detecting bone metastases.[46]
Ga-68-PSMA PET/CT provides higher spatial resolution and has a higher sensitivity and specificity for detecting metastases, particularly in the early stages of the disease. Although its sensitivity cannot match the positron-emitting PSMA radioligands in a PET/CT camera, Tc-99m-PSMA SPECT/CT offers an effective, valuable, cost-effective alternative to centers or patients without access to a PET/CT facility.[45]
Indium-111 Capromab Pendetide Imaging
Capromab pendetide radiolabeled with indium-111 (indium-111 capromab pendetide), formerly used for imaging prostate cancer, was approved by the US Food and Drug Administration (FDA) in 1996. Indium-111 capromab pendetide is presented here primarily for historical context as it has been supplanted by newer imaging tracers as outlined above.
Indium-111 capromab pendetide comprises a monoclonal antibody that targets an intracellular prostate-specific membrane antigen (PSMA) glycoprotein and a radioisotope, indium 111, which emits gamma radiation that a gamma camera can detect. Indium-111 capromab pendetide is injected into the patient's bloodstream, and the monoclonal antibody binds to the PSMA. Since only intracellular PSMA is targeted, the radiolabeled antibody can only identify necrotic prostate cancer cells, dramatically limiting its sensitivity and usefulness.[47] The gamma radiation emitted by the indium 111 is detected by a gamma camera, which produces an image of the prostate and any cancerous lesions.[48][49]
The technical protocol for indium-111 capromab pendetide imaging involves several steps. Before the injection, the patient must fast for at least 4 hours. The imaging procedure takes place over 2 days, with the injection of indium-111 capromab pendetide on day 1 and imaging on day 2. On day 1, the patient is injected with the radiolabeled antibody, prepared in a sterile, pyrogen-free environment. The recommended dose is 5 mCi (millicuries) or 185 MBq for patients with a mass under 90 kg and 7 mCi/225 MBq for patients with a mass over 90 kg. After injection, the patient must remain still for at least 30 minutes to allow for the distribution of the radiopharmaceutical throughout the body.
The patient is then monitored for adverse reactions, such as allergies or injection site reactions. The patient returns for imaging on the second day (24-hour delay). The patient is positioned on the gamma camera/SPECT-CT table, and images are acquired, including the whole body (anterior and posterior view) and SPECT-CT from head to mid-thigh.
Indium-111 capromab pendetide imaging has several significant drawbacks. One major limitation is its low sensitivity for detecting small tumors. This radiolabeled antibody can only detect tumors larger than 1 cm in size, which means that smaller tumors may be missed. This limitation is due to the limited spatial resolution of gamma cameras, which cannot detect the tiny amounts of radiation emitted from small tumors.
Another limitation is its low specificity. Furthermore, indium-111 capromab pendetide imaging is not recommended for patients with a rising prostate-specific antigen (PSA) level after radical prostatectomy or radiation therapy. This imaging may detect residual prostate tissue or inflammation rather than cancerous lesions. In addition, the long half-life of the indium-111 radioisotope increases the radiation burden on the patient.
While indium-111 capromab pendetide imaging has been used for over 2 decades to image prostate cancer, it has major and significant limitations. This imaging is best used with other imaging modalities, such as MRI or CT, to improve its sensitivity and specificity. Overall, the performance of the indium-111 capromab pendetide PSMA-based scan has been very disappointing, and the NCCN no longer recommends it.[35][50][51][52] New radiopharmaceutical agents, such as PSMA-targeted positron emission tomography (PET) tracers, are now available. They are far superior and preferred due to their high sensitivity and specificity for imaging prostate cancer.[35]
Tips for PSMA-PET Interpretation
PSMA-PET imaging is a highly sensitive imaging modality used to detect prostate-specific membrane antigen (PSMA) expression in prostate cancer cells. Here are some tips and guidance for radiologists to help them interpret Ga-68-PSMA imaging and identify false positives:
- Understand the limitations of Ga-68 PSMA imaging: Ga-68 PSMA imaging is a highly sensitive modality, but it is not 100% specific. False positives can occur for various reasons, such as inflammation, benign prostatic hyperplasia, prostatitis, new (within 90 days of starting) hormonal therapy, or even normal physiological uptake. Radiologists should be aware of these limitations and interpret the imaging in the context of the patient's clinical history and other findings.
- Be familiar with the normal distribution of Ga-68 PSMA uptake: Ga-68 PSMA uptake is commonly seen in the prostate gland, ureters, bladder, and salivary glands. Radiologists should be familiar with the normal distribution of Ga-68 PSMA uptake and recognize these structures on the imaging.
- Using multimodality imaging to confirm suspicious findings: False positives on Ga-68 PSMA imaging can be confirmed using other imaging modalities, such as CT or MRI. Radiologists should use multimodality imaging to confirm suspicious findings and rule out false positives.
- Look for uptake patterns: False positives on Ga-68 PSMA imaging can be identified by looking for uptake patterns that do not fit the typical distribution of PSMA expression. For example, diffuse or asymmetric prostate uptake in the prostate gland is more likely to indicate inflammation rather than malignancy.
- Consider the clinical history: The patient's clinical history can provide valuable information to help identify false positives on Ga-68 PSMA-PET imaging. For example, a patient with a history of a recent biopsy, radiation therapy, trauma, or surgery may have increased PSMA uptake due to inflammation or healing without any active malignancy.[53][54]
- The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) has published standards on the indications, technical procedures, and acquisition as well as interpretation guidance for multiple PSMA PET/CT scans for prostate cancer imaging which is highly recommended, particularly for facilities new to these modern, state-of-the-art scanning modalities.[55]
- Additional guidance and tips on interpreting PSMA-PET scans have been published and are available.[42][56][57][58][59]
Challenges to PSMA-PET Interpretation
Interpreting radioisotope PSMA-PET scans can be challenging due to false positives, the "flare response," trapping of the tracer in nontargeted tissues, and urinary excretion, which interferes with visualization.[60]
Short-term androgen deprivation therapy may increase PSMA expression leading to increased radioisotope concentrations, while long-term hormonal therapy reduces PSMA exposure and uptake.[60]
An essential aspect of Ga-68-PSMA scans is the phenomenon of "flare," which can occur shortly after the injection of the radioactive tracer. "Flare" is a transient increase in PSMA uptake in the prostate, metastatic lesions, or elsewhere occurring during the first hour after the radioisotope injection. While the exact cause of the flare is not fully understood, it is thought to be related to an immune response triggered by the injection.
There are several possible interpretations of "flare" on a Ga-68-PSMA scan. Fare could indicate active prostate cancer as malignant cells rapidly take up PSMA and its attached radioactive isotope. The transient increase may also be a sign of a positive therapeutic response.
Patients undergoing treatment for prostate cancer may experience an initial, transient increase in PSMA uptake, followed by a subsequent decrease. This indicates that the treatment is working to reduce tumor size and malignant cell activity. This is known as a "PSMA flare response" and is considered a positive indicator of treatment efficacy.
However, flare can occur even in patients who have previously undergone definitive treatment for prostate cancer and have no evidence of active malignant disease. Such false-positive results may occur in benign conditions with inflammation or a localized cellular immune response, such as in prostatitis, untreated urinary tract infections, after starting antiandrogen therapy, and bone healing after radiation therapy or trauma.[61][62] For this reason, PET scans should not be performed any earlier than 3 months after the start of antiandrogen hormonal therapy.[62]
The patient's clinical history and other imaging findings must be considered when interpreting a possible flare. Further evaluation and follow-up may be necessary to confirm the presence of cancer and determine the appropriate course of treatment.[63]
Interpreting radioisotope-PSMA PET scans can be challenging due to false positives, the flare response, trapping of the tracer in nontargeted tissues, and urinary excretion, which interferes with visualization.[60] Short-term androgen deprivation therapy may increase PSMA expression leading to high radioisotope concentrations, while long-term hormonal therapy reduces PSMA exposure and uptake.[60]
Summary: PET-Based Radionuclide Imaging in Prostate Cancer
Only 2 PET tracers are FDA-approved and recommended by the National Comprehensive Cancer Network (NCCN) for use in the initial staging of prostate cancer for patients with suspected metastatic disease AND biochemical recurrence or progression.
- [F-18] F-PSMA piflufolastat (DCFPyL)
- [Ga-68] Ga-PSMA-11
Five PET tracers are FDA-Approved and recommended by the NCCN for use only in prostate cancer patients with biochemical recurrence or progression.
- [C-11] C-Choline
- [F-18] F-Fluciclovine
- [F-18] F-PSMA piflufolastat (DCFPyL)
- [F-18] F-Sodium fluoride (alternative for a bone scan only)
- [Ga-68] Ga-PSMA
Targeted Radiopharmaceutical Therapy for Prostate Cancer (Theranostics)
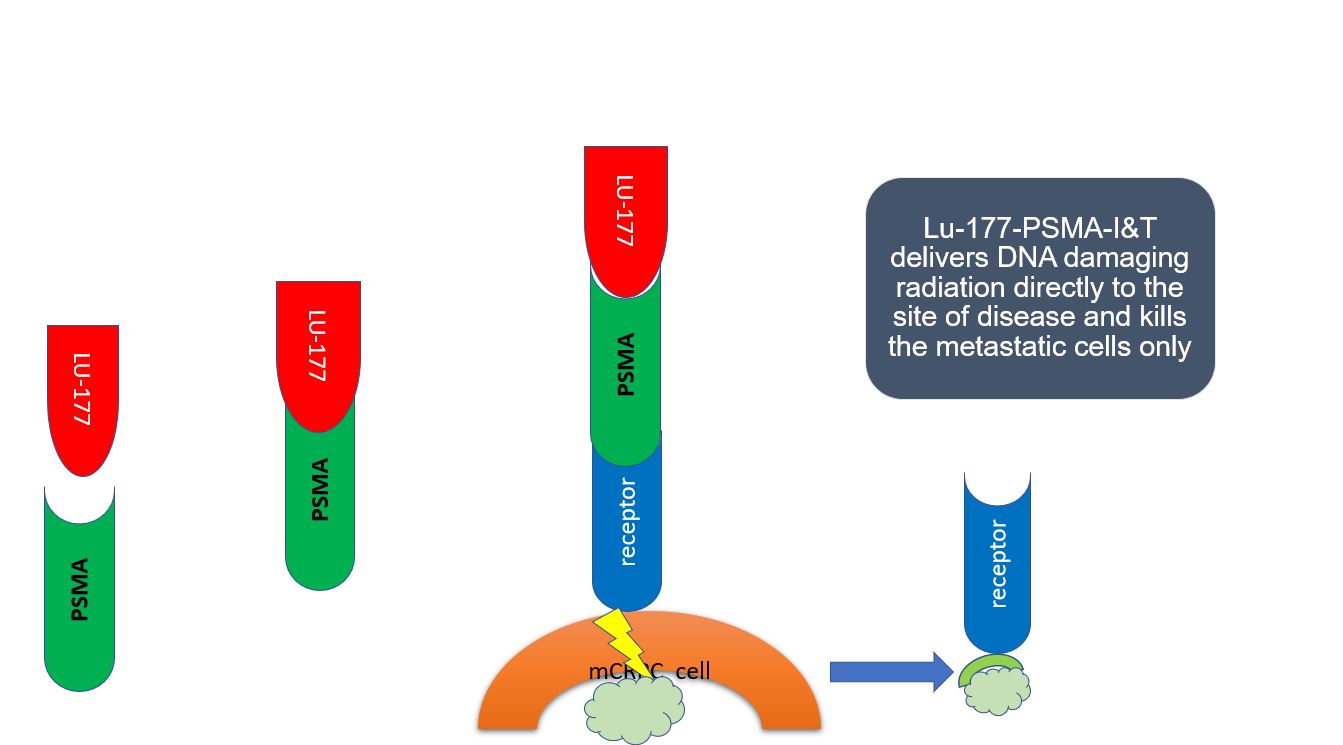
Radiolabeled PSMA-binding agents can also be used for therapy. The branch of nuclear medicine where imaging radioligands demonstrate tissue that can be targeted with similar therapy radioligands is known as theranostics.
The use of radioligands for therapy requires specialist knowledge and training. Such treatment should be performed only by qualified staff in a certified facility.
Theranostics for prostate cancer target PSMA overexpression to concentrate therapeutic radioligands to those specific locations.
Although there has been an explosion of interest in treating prostate cancer with targeted radiopharmaceuticals and multiple trials are in progress, there is only a single proven indication that such therapy has been shown to improve survival.[64] The only currently approved indication for PSMA-binding radioligand therapy is in metastatic castrate-resistant prostate cancer (mCRPC) patients with PSMA-positive disease progressing after first-line taxane treatment or who cannot receive chemotherapy. (See Patient Selection for Lu-177-PSMA Treatment below.)
Current radioligand therapies are typically better tolerated than chemotherapy.[65] Two large, prospective clinical trials (Thera-P and VISION) have shown comparable or improved outcomes with targeted radioligand treatment compared to standard-of-care therapies.[64][66][67]
- The Thera-P trial showed a higher PSA response and fewer grade 3 or 4 adverse events compared with cabazitaxel in men with metastatic castration-resistant prostate cancer.[66]
- The VISION trial demonstrated prolonged imaging-based progression-free survival and overall survival when added to standard care in patients with advanced PSMA-positive metastatic castration-resistant prostate cancer.[64][67]
Lutetium-177 Radioligand Therapy
The following factors and characteristics make Lu-177 the currently preferred radionuclide for PSMA-targeted therapeutic purposes:[65]
- The mean penetration range of β− particles emitted by Lu-177 in soft tissue is 670 μm, and maximal tissue penetration is 2 mm, localizing cytotoxic radiation to target lesions and reducing non-target injury to adjacent, bystander areas.
- Lu-177 also emits low-energy γ-rays during its decay (Gamma radiation), which allows scintigraphy with gamma cameras and subsequent dosimetry.
- Lu-177 has +3 oxidation states simplifying chemical bonds with targeting agents.
- Lu-177 has a long half-life (6.73 days), making it an easily transportable radionuclide and helpful in delivering radiation to the target tissue.[68]
Current commercially available Lu-177 labeled PSMA ligands are [Lu-177] Lu-PSMA-617 and [Lu-177] Lu-PSMA-I&T. Other PSMA binding radioligands, in particular those using alpha emitter Actinium-225 [Ac-225], remain experimental but are seeing increasing interest.[69][70][71]
Patient Selection for Lu-177-PSMA Treatment
Patients are usually referred by their medical oncologist, radiation therapist, or urologist. In this rapidly changing area of oncology, it is suggested that nuclear medicine practitioners and other specialists who regularly take care of prostate cancer patients periodically check the latest NCCN guidelines and recommendations, as the indications for this therapy are changing rapidly.
Patient suitability for treatment may require a multidisciplinary team assessment, but ultimately it is the nuclear medicine specialist who assumes primary responsibility for the final decision regarding patient suitability and education, treatment planning, response assessment, and management of treatment-related complications as well as adverse effects.
Current clinical indications include metastatic, castrate-resistant, progressive prostate cancer following conventional systemic, taxane-based chemotherapy. (Patients who cannot take or tolerate the chemotherapy for any reason are also candidates.) The tumor must show adequate expression of PSMA on a baseline [Ga-68] Ga-PSMA PET/CT scan. In addition, patients should have a life expectancy of at least six months, adequate bone marrow and renal function without renal outflow obstruction, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 2 or less.
Diffuse bone marrow disease with a high risk of marrow failure will exclude patients from therapy.
Most institutions also assess for the presence of active disease that does not express PSMA and is, therefore, unlikely to respond to this treatment. This is done by performing an FDG PET/CT scan before approving the patient for [Lu-177] Lu-PSMA-617 radioligand therapy.
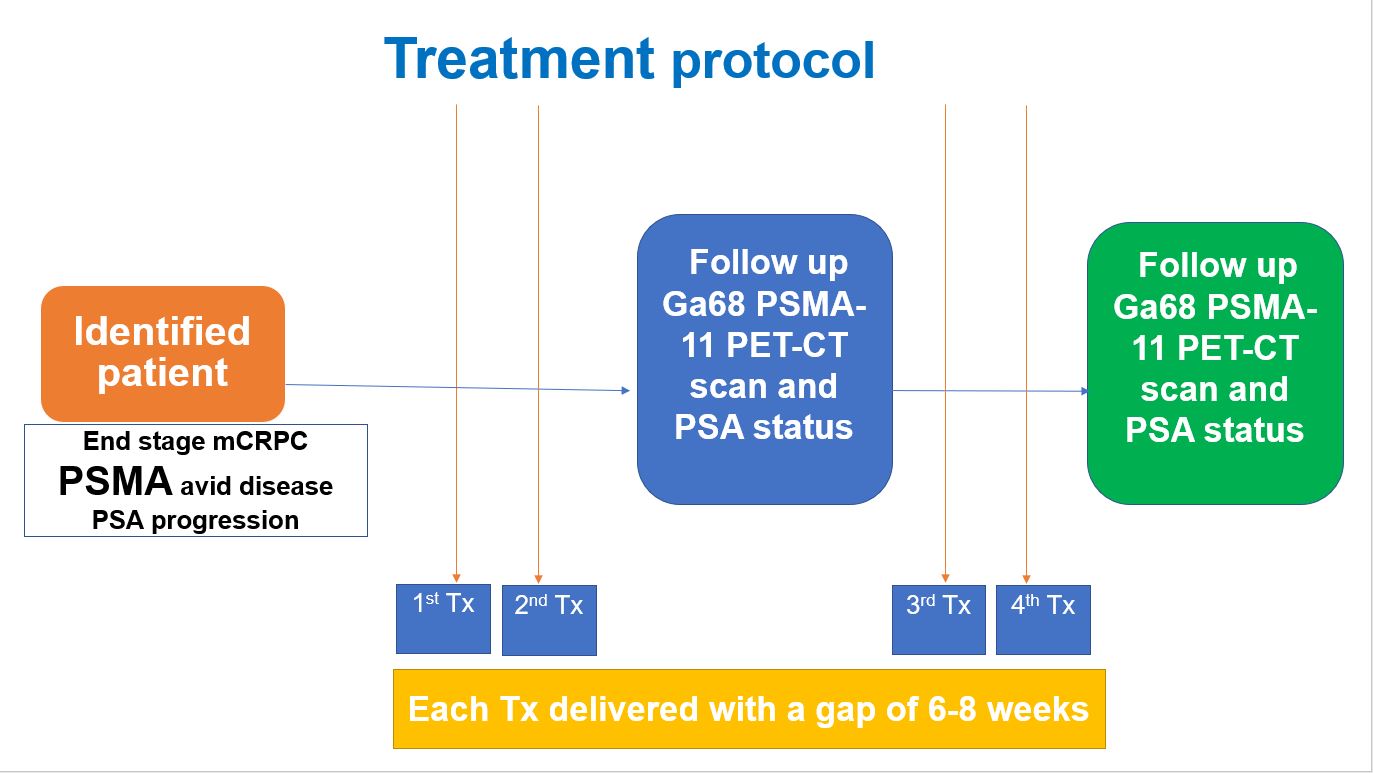
Treatment Protocols for Lu-177-PSMA Therapy
Patients typically receive 4 to 6 treatment cycles at 6- to 8-week intervals. Administered doses may be scaled or adjusted due to disease burden, renal impairment, or after dosimetry testing, but a typical administration is 7.4 GBq (200 mCi).
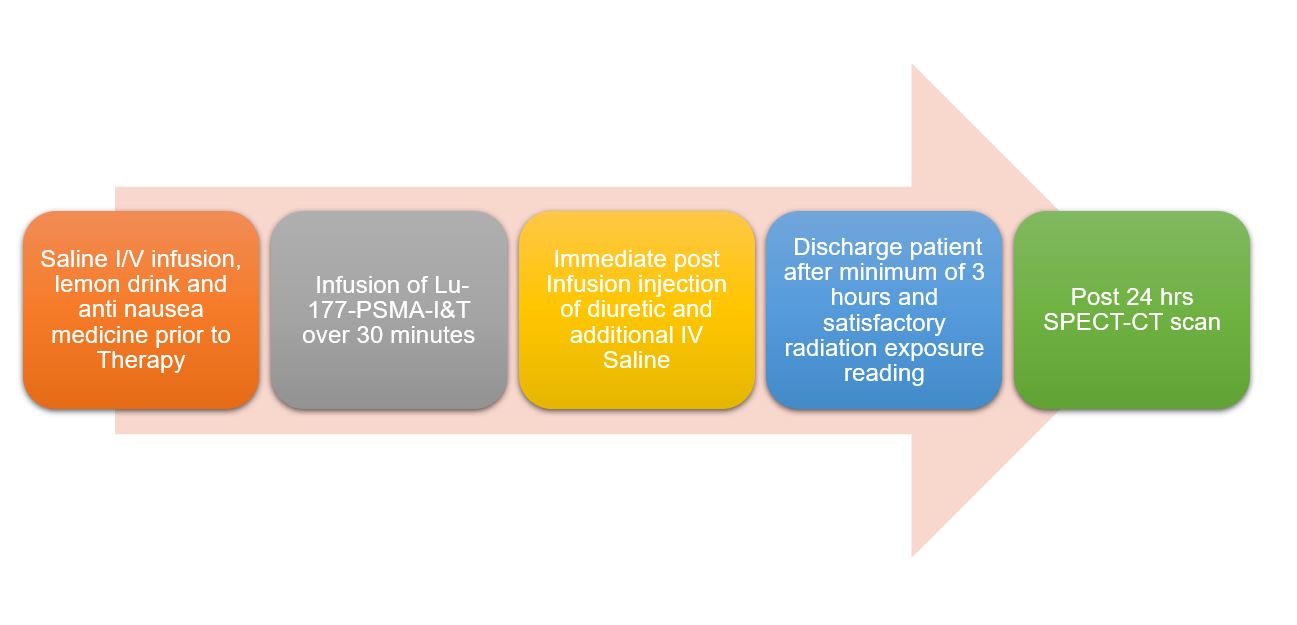
Patients spend up to 4 hours in the nuclear medicine department for therapy. They are discharged when their radiation levels fall to a safe level for other contacts, per local radiation safety regulations, and any adverse effects have been assessed and managed. Treated patients must understand and follow strict radiation protection procedures for up to 7 days due to the half-life of Lu-177 and radiopharmaceutical retention.
Patients usually fast before treatment but with oral hydration. They receive intravenous hydration and antiemetics in the treatment facility before therapy administration. Measures to reduce radiation dose to PSMA expressing normal salivary glands may be applied.
Infusions of Lu-177 radioligands are typically performed over 5 to 30 minutes. Therefore, monitoring vital signs pre- and post-infusion of [Lu-177] Lu-PSMA is required.
Approximately 13% of patients experience serious adverse effects. Patients may suffer nausea both during and after treatment. Additionally, fatigue may develop in the days following therapy. Diarrhea and drowsiness are mentioned relatively commonly. Xerostomia due to physiologic PSMA expression by salivary glands and dry eyes due to PSMA expression by the lacrimal glands may occur and may be irreversible. Hematuria is occasionally reported. The most commonly reported adverse effects are fatigue (43%), dry mouth (39%), and anemia (32%).
Following treatment, marrow suppression may occur, usually in patients with preceding marrow impairment, and should be carefully monitored. Renal function also requires monitoring, but renoprotective amino acid infusions are unnecessary for current PSMA therapies, unlike similar radioligand therapy with [Lu-177] Lu-dotatate, which is used for somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors.
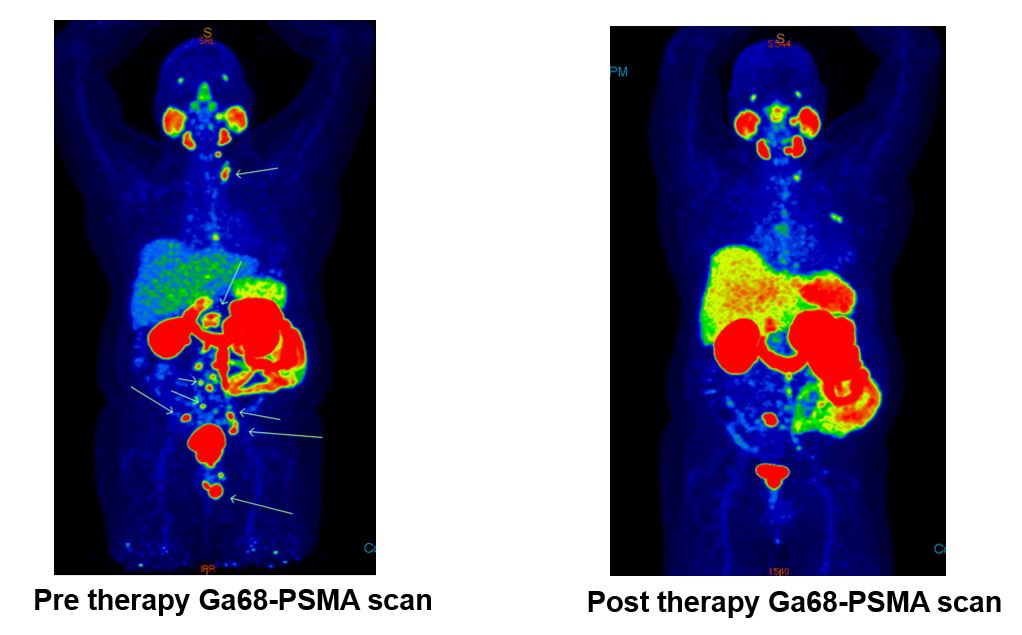
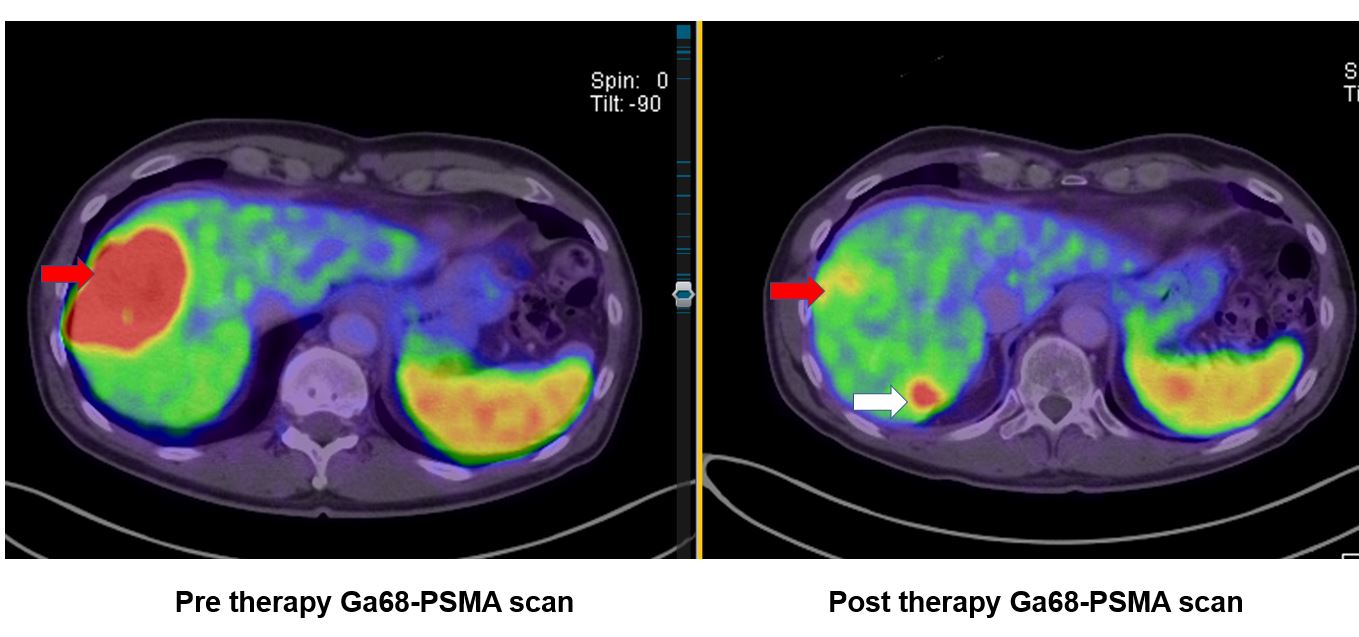
Imaging the gamma emissions from [Lu-177] Lu-PSMA radioligands following therapy can be performed using a gamma camera. Whole body and SPECT images allow subjective uptake assessment to target and non-target tissue. If required, multiple timepoint imaging can be performed for dosimetry.
Treatment response may be assessed using the tenets of the prostate cancer workgroup (PCWG3) but should reasonably include PSA response, follow-up imaging at PSMA PET, and diagnostic CT. In addition, patient symptom response and tolerance of therapy should also be key factors in assessing treatment. Quantitative pain assessments and quality of life should be performed before, during, and after therapy.
Other PSMA Ligand Therapies
Radioisotopes emitting alpha particles are also used in cancer treatment. Alpha particles typically travel about 6 cell diameters, with minimal tissue penetration.[72] However, compared to other radionuclide therapies, they have high linear energy transfer within this range.[73] Alpha particles cause double-strand damage to DNA within the nucleus, and cluster breaks independent of oxygenation and active cellular reproduction processes, in contrast to the single-strand damage of the beta-emitting Lu-177 radioligands, which is less likely to cause cell death but has a greater range.[74]
The alpha emitter Actinium-225 [Ac-225] application is increasing in radionuclide-based therapies. PSMA-binding radioligands of Ac-225 have been assessed in advanced metastatic castrate-resistant prostate cancer, which is Lu-177 resistant, with some early success.[75] Xerostomia is common, and hematologic side effects may limit or stop therapy.[75]
Tandem therapy (using both Lu-177 and Ac-225 radioligands synchronously or serially) and other applications of Ac-225 radioligands in prostate cancer are currently under investigation.[76]
Targeted α-therapy using astatine (211-At)-labeled PSMA appears promising in animal models for treating metastatic castration-resistant prostate cancer. Astatine has a half-life of 7.2 hours and can be produced by 30-MeV cyclotrons.[77]
Multimodal radiobioconjugates of magnetic nanoparticles labeled with 44-Sc and 47-Sc (scandium) for theranostic use in prostate cancer are also being studied experimentally.[78]
Other Nuclear Medicine Therapies in Prostate Cancer
Beta-emitting radioisotopes samarium-153 [Sm-153] and strontium-89 [Sr-89] are used to manage pain from bone metastases in prostate and other cancers. [Sr-89] is administered as chloride salt and [Sm-153] as an EDTMP complex. Both bind to sites of osteogenesis, usually indicated by the uptake of other bone-seeking radiopharmaceuticals in imaging bone scans.
Metastasis irradiation is an indirect effect of osteoblastic bone turnover rather than due to direct binding to tumor cells. Both agents have shown effective pain relief from bone metastases in clinical trials, but unfortunately, they provide no survival advantage. Both agents exhibit myelotoxicity as the dose-limiting factor.
Radium-223 (Ra-223) is an alpha-emitting radioisotope given to people with osteoblastic metastases from prostate cancer. This isotope has the same benefits and limitations similar to other alpha emitters used in nuclear medicine therapy. Ra-223 is not only effective in the relief of pain from bone metastases but also shows a survival benefit compared to beta-emitting agents. Concomitant use with denosumab or zoledronic acid is recommended and will not interfere with the survival benefits of radium-223.
Radium-223 has not been shown to provide a survival benefit in patients with visceral metastases or bulky nodal disease (>3–4 cm). The isotope is also limited by myelotoxicity, despite a theoretical advantage compared to the longer path lengths of the beta-emitting agents. Radium-223 should not be used in combination with myelosuppressive chemotherapy or docetaxel. It can also cause an increased risk of fractures if used with abiraterone.
Before starting treatment with radium-223, the patient must have a neutrophil count of ≥1,500,000/mL, a platelet count of ≥100,000,000/mL, and a hemoglobin level of ≥10 g/dL. The neutrophil count must be ≥1,000,000/mL for subsequent doses with a platelet count of ≥50,000,000/mL. If these hematological minimums cannot be reached after the Radium-223 therapy has been discontinued for 8 weeks, the treatment should be discontinued.
Other bone-seeking agents in clinical and experimental use include radioisotopes of rhenium and [Lu-177] Lu-EDTMP.
Summary of Principal Therapeutic Targeted Radiopharmaceuticals
Lutetium-177 ([Lu-177] Lu-PSMA-617, [Lu-177] Lu-PSMA-I&T)
- Physical characteristics
- Beta and gamma emitters
- 497 keV Beta max
- 113 (6.6%) and 210 (11%) keV of gamma radiation
- Half-life of 6.73 days
- Mean penetration of beta particles - 670 microns
Actinium-225 ([Ac-225] Ac-PSMA-617, [Ac-225] Ac-PSMA-I&T)
- Physical characteristics
- Alpha emitter
- Limited beta and gamma emitter
- 410 keV alpha energy
- Half-life of 9.920 days[79]
- Minimal tissue penetration
Radium-223 ([Ra-223] Ra-Chloride)
- Physical characteristics
- Alpha emitter
- 667 Mev/decay total alpha energy
- Half-life of 11.43 days
- Mean range of penetration in soft tissue - 43 microns[80]
Strontium-89
- Physical characteristics
- Beta emitter
- Average beta energy 1.46 Mev
- Half-life of 50.5 days
- Mean range of penetration in soft tissue - 2.4 mm[81]
Samarium-153
- Physical characteristics
More Radioligand Diagnostic and Therapeutic Options
Experimentally, PSMA PET/CT has improved the accuracy of staging prostate biopsies.[84][85]
- PSMA-based PET studies can be helpful with MRI scans in evaluating questionable PIRADS 3 lesions and selected PIRADS 1 or 2 nodules.[19][86][87]
- Hybrid PET/MRI cameras can improve the detection rate of extracapsular extension, seminal vesicle involvement, infiltration of the neurovascular bundle, and neighboring organ spread when assessing primary disease.[86][88][89]
- PSMA-based immunotherapy, without radiation, is also being investigated.
- These studies include immune checkpoint inhibitors, chimeric antigen receptors, bispecific antibodies, and tri-specific T-cell activating molecules.
Clinical Significance
Modern radionuclide PET scan imaging and targeted radiopharmaceutical therapy can significantly change management decisions in over 50% of prostate cancer patients tested and offer substantially improved survival in those with advanced disease.[90][91]
PSMA PET imaging is superior to CT imaging and standard bone scans for the staging of prostate cancer.[92][93]
PSMA-based imaging should be performed before antiandrogen hormonal therapy is started (NCCN recommendation.)
Ga-68-PSMA-11 and F-18-piflufolastat (DCFPyL) PET imaging have each demonstrated higher sensitivity than C-11 choline or F-18 fluciclovine PET imaging, especially at very low PSA levels.
No separate bone scan is necessary when performing a Ga-68-PSMA-11 or F-18 piflufolastat (DCFPyL) PSMA PET/CT scan.
Tc-99m-PSMA single-photon emission computed tomography/computed tomography (SPECT/CT) can be a cost-effective alternative to more expensive PET scans.
Various new imaging techniques are now available to evaluate better each patient's prostate cancer staging, course, and progress.
Nuclear medicine-based diagnostic imaging and therapeutics can significantly affect treatment planning, therapeutic decisions, quality of life, and overall survival.
Enhancing Healthcare Team Outcomes
Updated standards and guidelines covering the indications, technical procedures, proper handling of radiotracers, and interpretation of PSMA PET/CT scans for prostate cancer are available for multiple PSMA radioligands.[55]
Initiating a new radioligand therapy center requires the interest, diligence, and cooperation of an interprofessional team, including all the involved clinical services and administrative input and support. An organizational plan that clearly delineates the responsibilities of each clinical team member in covering all of the required tasks is essential. Scheduling services, reimbursement, care coordination, patient education, counseling, safety considerations, and financial support are also necessary for establishing a first-class center.[94]
- Every patient diagnosed with prostate cancer faces unique difficulties, concerns, and worries related to understanding the diagnosis, staging, prognosis, and treatment of their disease.
- Patients and their families receive abundant information about prostate cancer from multiple sources, some of which may be conflicting or outdated. They may find much of this information confusing and challenging to understand fully.
- Patients and families who require extra time or help with communication, teaching, or understanding should receive appropriate, comprehensive, and knowledgeable counseling.
- Several imaging techniques can be used to figure out the stage and progression of the disease. To get the best results, each team member must interact with patients effectively and consistently about the available imaging options.
- Because a thorough and consistent approach is necessary, an informed multidisciplinary team is critical when discussing all aspects of disease management, prognosis, and imaging modalities with patients.
- Every staff member plays an essential role in communicating, educating, and caring for these patients, who are often quite physically and emotionally fragile, to achieve optimal outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kaewput C, Vinjamuri S. Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends. Journal of clinical medicine. 2022 May 12:11(10):. doi: 10.3390/jcm11102738. Epub 2022 May 12 [PubMed PMID: 35628867]
Jain MA, Leslie SW, Sapra A. Prostate Cancer Screening. StatPearls. 2024 Jan:(): [PubMed PMID: 32310541]
Rawla P. Epidemiology of Prostate Cancer. World journal of oncology. 2019 Apr:10(2):63-89. doi: 10.14740/wjon1191. Epub 2019 Apr 20 [PubMed PMID: 31068988]
US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M, Epling JW Jr, Kemper AR, Krist AH, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Siu AL, Tseng CW. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 May 8:319(18):1901-1913. doi: 10.1001/jama.2018.3710. Epub [PubMed PMID: 29801017]
Farolfi A, Mei R, Ali S, Castellucci P. Theragnostics in prostate cancer. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of.... 2021 Dec:65(4):333-341. doi: 10.23736/S1824-4785.21.03419-1. Epub [PubMed PMID: 35133097]
Brunello S, Salvarese N, Carpanese D, Gobbi C, Melendez-Alafort L, Bolzati C. A Review on the Current State and Future Perspectives of [(99m)Tc]Tc-Housed PSMA-i in Prostate Cancer. Molecules (Basel, Switzerland). 2022 Apr 19:27(9):. doi: 10.3390/molecules27092617. Epub 2022 Apr 19 [PubMed PMID: 35565970]
Level 3 (low-level) evidenceNg M, Leslie SW, Baradhi KM. Benign Prostatic Hyperplasia. StatPearls. 2025 Jan:(): [PubMed PMID: 32644346]
Singh O, Bolla SR. Anatomy, Abdomen and Pelvis, Prostate. StatPearls. 2024 Jan:(): [PubMed PMID: 31082031]
De Silva F, Alcorn J. A Tale of Two Cancers: A Current Concise Overview of Breast and Prostate Cancer. Cancers. 2022 Jun 15:14(12):. doi: 10.3390/cancers14122954. Epub 2022 Jun 15 [PubMed PMID: 35740617]
Level 3 (low-level) evidenceWu EH, De Cicco FL. Anatomy, Abdomen and Pelvis, Male Genitourinary Tract. StatPearls. 2023 Jan:(): [PubMed PMID: 32965962]
Pletcher A, Shibata M. Prostate organogenesis. Development (Cambridge, England). 2022 Jun 15:149(12):. doi: 10.1242/dev.200394. Epub 2022 Jun 21 [PubMed PMID: 35726824]
Rasul S, Haug AR. Clinical Applications of PSMA PET Examination in Patients with Prostate Cancer. Cancers. 2022 Aug 2:14(15):. doi: 10.3390/cancers14153768. Epub 2022 Aug 2 [PubMed PMID: 35954432]
Duan H, Iagaru A, Aparici CM. Radiotheranostics - Precision Medicine in Nuclear Medicine and Molecular Imaging. Nanotheranostics. 2022:6(1):103-117. doi: 10.7150/ntno.64141. Epub 2022 Jan 1 [PubMed PMID: 34976584]
Koziorowski J, Ballinger J. Theragnostic radionuclides: a clinical perspective. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of.... 2021 Dec:65(4):306-314. doi: 10.23736/S1824-4785.21.03424-5. Epub 2021 Dec 9 [PubMed PMID: 34881851]
Level 3 (low-level) evidenceHövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clinical radiology. 2008 Apr:63(4):387-95. doi: 10.1016/j.crad.2007.05.022. Epub 2008 Feb 4 [PubMed PMID: 18325358]
Level 1 (high-level) evidenceSuh CH, Shinagare AB, Westenfield AM, Ramaiya NH, Van den Abbeele AD, Kim KW. Yield of bone scintigraphy for the detection of metastatic disease in treatment-naive prostate cancer: a systematic review and meta-analysis. Clinical radiology. 2018 Feb:73(2):158-167. doi: 10.1016/j.crad.2017.08.004. Epub 2017 Sep 25 [PubMed PMID: 28958581]
Level 1 (high-level) evidencePerner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, Hautmann RE, Gschwend JE, Kuefer R, Rubin MA. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Human pathology. 2007 May:38(5):696-701 [PubMed PMID: 17320151]
Level 2 (mid-level) evidenceWilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. The Journal of urology. 2004 Jul:172(1):133-6 [PubMed PMID: 15201753]
Level 2 (mid-level) evidenceEiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, Beer AJ, Wester HJ, Gschwend J, Schwaiger M, Maurer T. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. European urology. 2016 Nov:70(5):829-836. doi: 10.1016/j.eururo.2015.12.053. Epub 2016 Jan 18 [PubMed PMID: 26795686]
Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathology oncology research : POR. 2009 Jun:15(2):167-72. doi: 10.1007/s12253-008-9104-2. Epub 2008 Sep 18 [PubMed PMID: 18802790]
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kübler H, Beer AJ, Schwaiger M, Eiber M. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. The Journal of urology. 2016 May:195(5):1436-1443. doi: 10.1016/j.juro.2015.12.025. Epub 2015 Dec 9 [PubMed PMID: 26682756]
Fendler WP, Schmidt DF, Wenter V, Thierfelder KM, Zach C, Stief C, Bartenstein P, Kirchner T, Gildehaus FJ, Gratzke C, Faber C. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016 Nov:57(11):1720-1725 [PubMed PMID: 27261520]
Laudicella R, Spataro A, Crocè L, Giacoppo G, Romano D, Davì V, Lopes M, Librando M, Nicocia A, Rappazzo A, Celesti G, Torre F, Pagano B, Garraffa G, Bauckneht M, Burger IA, Minutoli F, Baldari S. Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics. Diagnostics (Basel, Switzerland). 2023 Mar 19:13(6):. doi: 10.3390/diagnostics13061175. Epub 2023 Mar 19 [PubMed PMID: 36980482]
Ma L, Zhang WC, Hao YX. Current state of prostate-specific membrane antigen PET/CT imaging-targeted biopsy techniques for detection of clinically significant prostate cancer. Journal of medical imaging and radiation oncology. 2022 Sep:66(6):776-780. doi: 10.1111/1754-9485.13369. Epub 2021 Dec 16 [PubMed PMID: 34914195]
Schöder H, Hope TA, Knopp M, Kelly WK, Michalski JM, Lerner SP, Tawab-Amiri A, Mann BS, Lin DW, Yu EY, Chen RC, Beach GC, Reeves SA, Members of the Working Group, Shankar LK. Considerations on Integrating Prostate-Specific Membrane Antigen Positron Emission Tomography Imaging Into Clinical Prostate Cancer Trials by National Clinical Trials Network Cooperative Groups. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022 May 1:40(13):1500-1505. doi: 10.1200/JCO.21.02440. Epub 2022 Jan 11 [PubMed PMID: 35015566]
Li Y, Zheng R, Zhang Y, Huang C, Tian L, Liu R, Liu Y, Zhang Z, Han H, Zhou F, He L, Dong P. Special issue "The advance of solid tumor research in China": 68Ga-PSMA-11 PET/CT for evaluating primary and metastatic lesions in different histological subtypes of renal cell carcinoma. International journal of cancer. 2023 Jan 1:152(1):42-50. doi: 10.1002/ijc.34189. Epub 2022 Jul 16 [PubMed PMID: 35751420]
O'Connor E, Teh J, Bolton D. Pitfalls of FDG-PET in the prostate for the surgical oncologist. Urology case reports. 2020 Nov:33():101262. doi: 10.1016/j.eucr.2020.101262. Epub 2020 May 22 [PubMed PMID: 32489895]
Level 3 (low-level) evidenceHess S, Blomberg BA, Zhu HJ, Høilund-Carlsen PF, Alavi A. The pivotal role of FDG-PET/CT in modern medicine. Academic radiology. 2014 Feb:21(2):232-49. doi: 10.1016/j.acra.2013.11.002. Epub [PubMed PMID: 24439337]
Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Positron emission tomography and molecular imaging of the prostate: an update. BJU international. 2006 May:97(5):923-31 [PubMed PMID: 16643472]
D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16:280(11):969-74 [PubMed PMID: 9749478]
Level 2 (mid-level) evidenceChang CY, Gill CM, Joseph Simeone F, Taneja AK, Huang AJ, Torriani M, Bredella MA. Comparison of the diagnostic accuracy of 99 m-Tc-MDP bone scintigraphy and 18 F-FDG PET/CT for the detection of skeletal metastases. Acta radiologica (Stockholm, Sweden : 1987). 2016 Jan:57(1):58-65. doi: 10.1177/0284185114564438. Epub 2014 Dec 22 [PubMed PMID: 25533313]
Broos WAM, van der Zant FM, Wondergem M, Knol RJJ. Accuracy of 18F-NaF PET/CT in bone metastasis detection and its effect on patient management in patients with breast carcinoma. Nuclear medicine communications. 2018 Apr:39(4):325-333. doi: 10.1097/MNM.0000000000000807. Epub [PubMed PMID: 29351123]
Bénard F, Harsini S, Wilson D, Zukotynski K, Abikhzer G, Turcotte E, Cossette M, Metser U, Romsa J, Martin M, Mar C, Saad F, Soucy JP, Eigl BJ, Black P, Krauze A, Burrell S, Nichol A, Tardif JC. Intra-individual comparison of (18)F-sodium fluoride PET-CT and (99m)Tc bone scintigraphy with SPECT in patients with prostate cancer or breast cancer at high risk for skeletal metastases (MITNEC-A1): a multicentre, phase 3 trial. The Lancet. Oncology. 2022 Dec:23(12):1499-1507. doi: 10.1016/S1470-2045(22)00642-8. Epub 2022 Nov 4 [PubMed PMID: 36343655]
Rowe SP, Li X, Trock BJ, Werner RA, Frey S, DiGianvittorio M, Bleiler JK, Reyes DK, Abdallah R, Pienta KJ, Gorin MA, Pomper MG. Prospective Comparison of PET Imaging with PSMA-Targeted (18)F-DCFPyL Versus Na(18)F for Bone Lesion Detection in Patients with Metastatic Prostate Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2020 Feb:61(2):183-188. doi: 10.2967/jnumed.119.227793. Epub 2019 Aug 26 [PubMed PMID: 31451492]
Leslie SW, Soon-Sutton TL, Skelton WP. Prostate Cancer. StatPearls. 2024 Jan:(): [PubMed PMID: 29261872]
Chen B, Wei P, Macapinlac HA, Lu Y. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nuclear medicine communications. 2019 Sep:40(9):940-946. doi: 10.1097/MNM.0000000000001051. Epub [PubMed PMID: 31343613]
Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W, Haberkorn U, Eisenhut M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate chemistry. 2012 Apr 18:23(4):688-97. doi: 10.1021/bc200279b. Epub 2012 Mar 13 [PubMed PMID: 22369515]
Level 3 (low-level) evidenceAfshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, Holland-Letz T, Hadaschik BA, Giesel FL, Debus J, Haberkorn U. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. European journal of nuclear medicine and molecular imaging. 2014 Jan:41(1):11-20. doi: 10.1007/s00259-013-2525-5. Epub 2013 Sep 27 [PubMed PMID: 24072344]
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, Hruby G, Fogarty G, Jagavkar R, Kneebone A, Hickey A, Fanti S, Tarlinton L, Emmett L. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015 Aug:56(8):1185-90. doi: 10.2967/jnumed.115.160382. Epub 2015 Jun 25 [PubMed PMID: 26112024]
Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, Schmidt M, Dietlein F, Zlatopolskiy BD, Krapf P, Richarz R, Neubauer S, Drzezga A, Neumaier B. Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Molecular imaging and biology. 2015 Aug:17(4):575-84. doi: 10.1007/s11307-015-0866-0. Epub [PubMed PMID: 26013479]
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, Kesch C, Tolstov Y, Singer S, Grabe N, Duensing S, Schäfer M, Neels OC, Mier W, Haberkorn U, Kopka K, Kratochwil C. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. European journal of nuclear medicine and molecular imaging. 2017 Apr:44(4):678-688. doi: 10.1007/s00259-016-3573-4. Epub 2016 Nov 26 [PubMed PMID: 27889802]
Level 1 (high-level) evidenceGutiérrez Cardo AL, Vallejo Casas JA, García Garzón JR, Tirado Hospital JL, Medina López R, Freire Macías JM, Rodríguez Fernández A. (18)F-DCFPyL PET/CT guidelines. Revista espanola de medicina nuclear e imagen molecular. 2023 May-Jun:42(3):203-208. doi: 10.1016/j.remnie.2023.02.009. Epub 2023 Mar 5 [PubMed PMID: 36878314]
Rowe SP, Voter AF, Werner RA, Zukotynski KA, Pomper MG, Gorin MA, Solnes LB. Image acquisition and interpretation of 18F-DCFPyL (piflufolastat F 18) PET/CT: How we do it. The Canadian journal of urology. 2023 Feb:30(1):11432-11437 [PubMed PMID: 36779950]
Albalooshi B, Al Sharhan M, Bagheri F, Miyanath S, Ray B, Muhasin M, Zakavi SR. Direct comparison of (99m)Tc-PSMA SPECT/CT and (68)Ga-PSMA PET/CT in patients with prostate cancer. Asia Oceania journal of nuclear medicine & biology. 2020 Winter:8(1):1-7. doi: 10.22038/aojnmb.2019.43943.1293. Epub [PubMed PMID: 32064277]
Lawal IO, Ankrah AO, Mokgoro NP, Vorster M, Maes A, Sathekge MM. Diagnostic sensitivity of Tc-99m HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with Ga-68 PSMA PET/CT. The Prostate. 2017 Aug:77(11):1205-1212. doi: 10.1002/pros.23379. Epub 2017 Jun 26 [PubMed PMID: 28649735]
Level 2 (mid-level) evidenceZhang Y, Lin Z, Li T, Wei Y, Yu M, Ye L, Cai Y, Yang S, Zhang Y, Shi Y, Chen W. Head-to-head comparison of (99m)Tc-PSMA and (99m)Tc-MDP SPECT/CT in diagnosing prostate cancer bone metastasis: a prospective, comparative imaging trial. Scientific reports. 2022 Sep 26:12(1):15993. doi: 10.1038/s41598-022-20280-x. Epub 2022 Sep 26 [PubMed PMID: 36163353]
Level 2 (mid-level) evidenceGusman M, Aminsharifi JA, Peacock JG, Anderson SB, Clemenshaw MN, Banks KP. Review of (18)F-Fluciclovine PET for Detection of Recurrent Prostate Cancer. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 May-Jun:39(3):822-841. doi: 10.1148/rg.2019180139. Epub [PubMed PMID: 31059396]
Sengupta S, Asha Krishnan M, Chattopadhyay S, Chelvam V. Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer reports (Hoboken, N.J.). 2019 Aug:2(4):e1169. doi: 10.1002/cnr2.1169. Epub 2019 Apr 2 [PubMed PMID: 32721116]
Wong WW, Schild SE, Vora SA, Ezzell GA, Nguyen BD, Ram PC, Roarke MC. Image-guided radiotherapy for prostate cancer: a prospective trial of concomitant boost using indium-111-capromab pendetide (ProstaScint) imaging. International journal of radiation oncology, biology, physics. 2011 Nov 15:81(4):e423-9. doi: 10.1016/j.ijrobp.2011.01.048. Epub 2011 Apr 7 [PubMed PMID: 21477947]
Morigi JJ, Anderson J, DE Nunzio C, Fanti S. Prostate specific membrane antigen positron emission tomography/computed tomography and staging high risk prostate cancer: a non-systematic review of high clinical impact literature. Minerva urology and nephrology. 2021 Feb:73(1):32-41. doi: 10.23736/S2724-6051.20.03739-X. Epub 2020 Jun 16 [PubMed PMID: 32550630]
Level 1 (high-level) evidenceKoschel S, Murphy DG, Hofman MS, Wong LM. The role of prostate-specific membrane antigen PET/computed tomography in primary staging of prostate cancer. Current opinion in urology. 2019 Nov:29(6):569-577. doi: 10.1097/MOU.0000000000000677. Epub [PubMed PMID: 31567440]
Level 3 (low-level) evidenceHerlemann A,Wenter V,Kretschmer A,Thierfelder KM,Bartenstein P,Faber C,Gildehaus FJ,Stief CG,Gratzke C,Fendler WP, {sup}68{/sup}Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. European urology. 2016 Oct; [PubMed PMID: 26810345]
Qin ZQ, Pan GJ, Xu Z, Wang H, Xu LW, Jia RP. The performance of (18)F-PSMA PET/CT in the detection of prostate cancer: a systematic review and meta-analysis. Asian journal of andrology. 2022 Jul-Aug:24(4):373-379. doi: 10.4103/aja202162. Epub [PubMed PMID: 34747721]
Level 2 (mid-level) evidenceZinzani PL, Gherlinzoni F, Lauria F, Mazza P, Barbieri E, Emiliani E, Tabanelli M, Tura S. Intensive chemotherapy regimen in high-grade non Hodgkin's lymphomas. Tumori. 1987 Apr 30:73(2):121-6 [PubMed PMID: 3576707]
Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, Cho SY, Fanti S, Giesel FL, Goffin K, Haberkorn U, Jacene H, Koo PJ, Kopka K, Krause BJ, Lindenberg L, Marcus C, Mottaghy FM, Oprea-Lager DE, Osborne JR, Piert M, Rowe SP, Schöder H, Wan S, Wester HJ, Hope TA, Herrmann K. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. European journal of nuclear medicine and molecular imaging. 2023 Apr:50(5):1466-1486. doi: 10.1007/s00259-022-06089-w. Epub 2023 Jan 5 [PubMed PMID: 36604326]
Pomykala KL, Herrmann K, Lalumera E, Fanti S. Positive Prostate-specific Membrane Antigen Findings: How To Interpret Them. European urology oncology. 2023 Apr:6(2):113-115. doi: 10.1016/j.euo.2022.10.007. Epub 2022 Nov 22 [PubMed PMID: 36428201]
Murad V, Kulanthaivelu R, Ortega C, Veit-Haibach P, Metser U. Standardized classification schemes in reporting oncologic PET/CT. Frontiers in medicine. 2022:9():1051309. doi: 10.3389/fmed.2022.1051309. Epub 2023 Jan 26 [PubMed PMID: 36777163]
Phelps TE, Harmon SA, Mena E, Lindenberg L, Shih JH, Citrin DE, Pinto PA, Wood BJ, Dahut WL, Gulley JL, Madan RA, Choyke PL, Turkbey B. Predicting Outcomes of Indeterminate Bone Lesions on (18)F-DCFPyL PSMA PET/CT Scans in the Setting of High-Risk Primary or Recurrent Prostate Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2023 Mar:64(3):395-401. doi: 10.2967/jnumed.122.264334. Epub 2022 Oct 20 [PubMed PMID: 36265908]
Kase AM, Tan W, Copland JA 3rd, Cai H, Parent EE, Madan RA. The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer. Cancers. 2022 Mar 8:14(6):. doi: 10.3390/cancers14061361. Epub 2022 Mar 8 [PubMed PMID: 35326513]
Wang Y, Galante JR, Haroon A, Wan S, Afaq A, Payne H, Bomanji J, Adeleke S, Kasivisvanathan V. The future of PSMA PET and WB MRI as next-generation imaging tools in prostate cancer. Nature reviews. Urology. 2022 Aug:19(8):475-493. doi: 10.1038/s41585-022-00618-w. Epub 2022 Jul 4 [PubMed PMID: 35789204]
Messiou C, Cook G, deSouza NM. Imaging metastatic bone disease from carcinoma of the prostate. British journal of cancer. 2009 Oct 20:101(8):1225-32. doi: 10.1038/sj.bjc.6605334. Epub 2009 Sep 29 [PubMed PMID: 19789531]
Esen B, Herrmann K, Bavbek S, Kordan Y, Tilki D, Esen T. Prostate-specific Membrane Antigen Positron Emission Tomography as a Biomarker to Assess Treatment Response in Patients with Advanced Prostate Cancer. European urology focus. 2023 Jul:9(4):596-605. doi: 10.1016/j.euf.2023.02.001. Epub 2023 Feb 25 [PubMed PMID: 36842919]
Malaspina S, Ettala O, Tolvanen T, Rajander J, Eskola O, Boström PJ, Kemppainen J. Flare on [(18)F]PSMA-1007 PET/CT after short-term androgen deprivation therapy and its correlation to FDG uptake: possible marker of tumor aggressiveness in treatment-naïve metastatic prostate cancer patients. European journal of nuclear medicine and molecular imaging. 2023 Jan:50(2):613-621. doi: 10.1007/s00259-022-05970-y. Epub 2022 Sep 26 [PubMed PMID: 36161511]
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ, VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. The New England journal of medicine. 2021 Sep 16:385(12):1091-1103. doi: 10.1056/NEJMoa2107322. Epub 2021 Jun 23 [PubMed PMID: 34161051]
Shah H, Ravi P, Sonpavde G, Jacene H. Lutetium Lu 177 vipivotide tetraxetan for metastatic castration-resistant prostate cancer. Expert review of anticancer therapy. 2022 Nov:22(11):1163-1175. doi: 10.1080/14737140.2022.2139679. Epub 2022 Nov 3 [PubMed PMID: 36305305]
Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, Francis RJ, Zhang AY, Scott AM, Lee ST, Azad AA, McJannett MM, Stockler MR, Williams SG, Davis ID, Hofman MS, TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. The Lancet. Oncology. 2022 Nov:23(11):1389-1397. doi: 10.1016/S1470-2045(22)00605-2. Epub 2022 Oct 16 [PubMed PMID: 36261050]
Level 1 (high-level) evidenceRohith G. VISION trial: (177)Lu-PSMA-617 for progressive metastatic castration-resistant prostate cancer. Indian journal of urology : IJU : journal of the Urological Society of India. 2021 Oct-Dec:37(4):372-373. doi: 10.4103/iju.iju_292_21. Epub 2021 Oct 1 [PubMed PMID: 34759536]
Filippi L, Chiaravalloti A, Schillaci O, Cianni R, Bagni O. Theranostic approaches in nuclear medicine: current status and future prospects. Expert review of medical devices. 2020 Apr:17(4):331-343. doi: 10.1080/17434440.2020.1741348. Epub 2020 Mar 19 [PubMed PMID: 32157920]
Miederer M. Alpha emitting nuclides in nuclear medicine theranostics. Nuklearmedizin. Nuclear medicine. 2022 Jun:61(3):273-279. doi: 10.1055/a-1650-9995. Epub 2022 Oct 8 [PubMed PMID: 34624903]
Ferdinandus J, Fendler WP, Morigi JJ, Fanti S. Theranostics in oncology: What radiologists want to know. European journal of radiology. 2021 Sep:142():109875. doi: 10.1016/j.ejrad.2021.109875. Epub 2021 Jul 26 [PubMed PMID: 34391057]
Ahmadzadehfar H, Matern R, Baum RP, Seifert R, Kessel K, Bögemann M, Kratochwil C, Rathke H, Ilhan H, Svirydenka H, Sathekge M, Kabasakal L, Yordanova A, Garcia-Perez FO, Kairemo K, Maharaj M, Paez D, Virgolini I, Rahbar K. The impact of the extent of the bone involvement on overall survival and toxicity in mCRPC patients receiving [(177)Lu]Lu-PSMA-617: a WARMTH multicentre study. European journal of nuclear medicine and molecular imaging. 2021 Nov:48(12):4067-4076. doi: 10.1007/s00259-021-05383-3. Epub 2021 May 25 [PubMed PMID: 34031719]
de Kruijff RM, Wolterbeek HT, Denkova AG. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals (Basel, Switzerland). 2015 Jun 10:8(2):321-36. doi: 10.3390/ph8020321. Epub 2015 Jun 10 [PubMed PMID: 26066613]
Lassmann M, Nosske D, Reiners C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: dosimetry and risk considerations. Radiation and environmental biophysics. 2002 Sep:41(3):173-8 [PubMed PMID: 12373325]
Morgenstern A, Bruchertseifer F, Apostolidis C. Bismuth-213 and actinium-225 -- generator performance and evolving therapeutic applications of two generator-derived alpha-emitting radioisotopes. Current radiopharmaceuticals. 2012 Jul:5(3):221-7 [PubMed PMID: 22642390]
Level 3 (low-level) evidenceFeuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, Bruchertseifer F, Pickhard A, Gafita A, Kratochwil C, Retz M, Gschwend JE, Weber WA, D'Alessandria C, Morgenstern A, Eiber M. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. European urology. 2021 Mar:79(3):343-350. doi: 10.1016/j.eururo.2020.11.013. Epub 2020 Dec 5 [PubMed PMID: 33293081]
Birindelli G, Drobnjakovic M, Morath V, Steiger K, D'Alessandria C, Gourni E, Afshar-Oromieh A, Weber W, Rominger A, Eiber M, Shi K. In silico study on radiobiological efficacy of Ac-225 and Lu-177 for PSMA-guided radiotherapy. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2021 Nov:2021():4497-4500. doi: 10.1109/EMBC46164.2021.9630297. Epub [PubMed PMID: 34892217]
Watabe T, Kaneda-Nakashima K, Shirakami Y, Kadonaga Y, Ooe K, Wang Y, Haba H, Toyoshima A, Cardinale J, Giesel FL, Tomiyama N, Fukase K. Targeted α-therapy using astatine ((211)At)-labeled PSMA1, 5, and 6: a preclinical evaluation as a novel compound. European journal of nuclear medicine and molecular imaging. 2023 Feb:50(3):849-858. doi: 10.1007/s00259-022-06016-z. Epub 2022 Nov 8 [PubMed PMID: 36344651]
Tan Y, Fang Z, Tang Y, Liu K, Zhao H. Clinical advancement of precision theranostics in prostate cancer. Frontiers in oncology. 2023:13():1072510. doi: 10.3389/fonc.2023.1072510. Epub 2023 Feb 2 [PubMed PMID: 36816956]
Sanli Y, Kuyumcu S, Simsek DH, Büyükkaya F, Civan C, Isik EG, Ozkan ZG, Basaran M, Sanli O. 225Ac-Prostate-Specific Membrane Antigen Therapy for Castration-Resistant Prostate Cancer: A Single-Center Experience. Clinical nuclear medicine. 2021 Dec 1:46(12):943-951. doi: 10.1097/RLU.0000000000003925. Epub [PubMed PMID: 34593693]
Sindhu KK, Nehlsen AD, Stock RG. Radium-223 for Metastatic Castrate-Resistant Prostate Cancer. Practical radiation oncology. 2022 Jul-Aug:12(4):312-316. doi: 10.1016/j.prro.2022.03.004. Epub [PubMed PMID: 35717046]
Yamada K, Kaise H, Taguchi T, Horiguchi J, Takao S, Suzuki M, Kubota T, Miura D, Narui K, Tawaraya K, Machida Y, Akazawa K, Kohno N, Ishikawa T, JONIE Study Group. Strontium-89 plus zoledronic acid versus zoledronic acid for patients with painful bone metastatic breast cancer. Journal of bone and mineral metabolism. 2022 Nov:40(6):998-1006. doi: 10.1007/s00774-022-01366-y. Epub 2022 Aug 30 [PubMed PMID: 36042056]
Correa-González L, Arteaga de Murphy C, Pichardo-Romero P, Pedraza-López M, Moreno-García C, Correa-Hernández L. (153)Sm-EDTMP for pain relief of bone metastases from prostate and breast cancer and other malignancies. Archives of medical research. 2014 May:45(4):301-8. doi: 10.1016/j.arcmed.2014.03.006. Epub 2014 Mar 28 [PubMed PMID: 24681187]
Van de Voorde M, Duchemin C, Heinke R, Lambert L, Chevallay E, Schneider T, Van Stenis M, Cocolios TE, Cardinaels T, Ponsard B, Ooms M, Stora T, Burgoyne AR. Production of Sm-153 With Very High Specific Activity for Targeted Radionuclide Therapy. Frontiers in medicine. 2021:8():675221. doi: 10.3389/fmed.2021.675221. Epub 2021 Jul 19 [PubMed PMID: 34350194]
Caracciolo M, Castello A, Urso L, Borgia F, Ortolan N, Uccelli L, Cittanti C, Castellani M, Bartolomei M, Lazzeri M, Lopci E. The Role of [(68)Ga]PSMA PET/CT for Clinical Suspicion of Prostate Cancer in Patients with or without Previous Negative Biopsy: A Systematic Review. Cancers. 2022 Oct 14:14(20):. doi: 10.3390/cancers14205036. Epub 2022 Oct 14 [PubMed PMID: 36291820]
Level 1 (high-level) evidencePepe P, Pepe L, Tamburo M, Marletta G, Pennisi M, Fraggetta F. Targeted prostate biopsy: 68Ga-PSMA PET/CT vs. mpMRI in the diagnosis of prostate cancer. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica. 2022 Sep 26:94(3):274-277. doi: 10.4081/aiua.2022.3.274. Epub 2022 Sep 26 [PubMed PMID: 36165469]
Kawada T, Yanagisawa T, Rajwa P, Sari Motlagh R, Mostafaei H, Quhal F, Laukhtina E, Aydh A, König F, Pallauf M, Pradere B, Ceci F, Baltzer PAT, Hacker M, Rasul S, Karakiewicz PI, Araki M, Nasu Y, Shariat SF. Diagnostic Performance of Prostate-specific Membrane Antigen Positron Emission Tomography-targeted biopsy for Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. European urology oncology. 2022 Aug:5(4):390-400. doi: 10.1016/j.euo.2022.04.006. Epub 2022 Jun 15 [PubMed PMID: 35715320]
Level 1 (high-level) evidenceAl-Bayati M, Grueneisen J, Lütje S, Sawicki LM, Suntharalingam S, Tschirdewahn S, Forsting M, Rübben H, Herrmann K, Umutlu L, Wetter A. Integrated 68Gallium Labelled Prostate-Specific Membrane Antigen-11 Positron Emission Tomography/Magnetic Resonance Imaging Enhances Discriminatory Power of Multi-Parametric Prostate Magnetic Resonance Imaging. Urologia internationalis. 2018:100(2):164-171. doi: 10.1159/000484695. Epub 2018 Jan 25 [PubMed PMID: 29393268]
Grubmüller B, Baltzer P, Hartenbach S, D'Andrea D, Helbich TH, Haug AR, Goldner GM, Wadsak W, Pfaff S, Mitterhauser M, Balber T, Berroteran-Infante N, Grahovac M, Babich J, Seitz C, Kramer G, Susani M, Mazal P, Kenner L, Shariat SF, Hacker M, Hartenbach M. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018 Dec 15:24(24):6300-6307. doi: 10.1158/1078-0432.CCR-18-0768. Epub 2018 Aug 23 [PubMed PMID: 30139879]
Muehlematter UJ, Burger IA, Becker AS, Schawkat K, Hötker AM, Reiner CS, Müller J, Rupp NJ, Rüschoff JH, Eberli D, Donati OF. Diagnostic Accuracy of Multiparametric MRI versus (68)Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients with Prostate Cancer. Radiology. 2019 Nov:293(2):350-358. doi: 10.1148/radiol.2019190687. Epub 2019 Sep 10 [PubMed PMID: 31502937]
Abiodun-Ojo OA, Jani AB, Akintayo AA, Akin-Akintayo OO, Odewole OA, Tade FI, Joshi SS, Master VA, Fielder B, Halkar RK, Zhang C, Goyal S, Goodman MM, Schuster DM. Salvage Radiotherapy Management Decisions in Postprostatectomy Patients with Recurrent Prostate Cancer Based on (18)F-Fluciclovine PET/CT Guidance. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Aug 1:62(8):1089-1096. doi: 10.2967/jnumed.120.256784. Epub 2021 Jan 30 [PubMed PMID: 33517323]
Dewes S, Schiller K, Sauter K, Eiber M, Maurer T, Schwaiger M, Gschwend JE, Combs SE, Habl G. Integration of (68)Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiation oncology (London, England). 2016 May 26:11():73. doi: 10.1186/s13014-016-0646-2. Epub 2016 May 26 [PubMed PMID: 27229485]
Level 2 (mid-level) evidenceJani AB, Schreibmann E, Goyal S, Halkar R, Hershatter B, Rossi PJ, Shelton JW, Patel PR, Xu KM, Goodman M, Master VA, Joshi SS, Kucuk O, Carthon BC, Bilen MA, Abiodun-Ojo OA, Akintayo AA, Dhere VR, Schuster DM. (18)F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet (London, England). 2021 May 22:397(10288):1895-1904. doi: 10.1016/S0140-6736(21)00581-X. Epub 2021 May 7 [PubMed PMID: 33971152]
Level 1 (high-level) evidenceHofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, Rutherford N, Martin JM, Frydenberg M, Shakher R, Wong LM, Taubman K, Ting Lee S, Hsiao E, Roach P, Nottage M, Kirkwood I, Hayne D, Link E, Marusic P, Matera A, Herschtal A, Iravani A, Hicks RJ, Williams S, Murphy DG, proPSMA Study Group Collaborators. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet (London, England). 2020 Apr 11:395(10231):1208-1216. doi: 10.1016/S0140-6736(20)30314-7. Epub 2020 Mar 22 [PubMed PMID: 32209449]
Level 1 (high-level) evidenceCalais J, Eulau SM, Gardner L, Hauke RJ, Kendi AT, Shore ND, Zhao S. Incorporating radioligand therapy in clinical practice in the United States for patients with prostate cancer. Cancer treatment reviews. 2023 Apr:115():102524. doi: 10.1016/j.ctrv.2023.102524. Epub 2023 Feb 11 [PubMed PMID: 36933329]