Indications
Osteoporosis affects more than 10 million individuals in the United States (US) and causes more than 2 million fractures every year.[1] Romosozumab is a humanized monoclonal antibody against sclerostin that is US Food and Drug Administration-approved to treat osteoporosis in postmenopausal women. These patients are at an increased risk of fracture and often have a history of osteoporotic fractures, multiple risk factors, or cannot be prescribed other medications to treat osteoporosis (see Image. Romosozumab Mechanism of Action). Romosozumab's safety and effectiveness in treating osteoporosis and increasing bone mass density have been evaluated in numerous studies. One phase 2 study found the recommended 210 mg monthly dose of romosozumab suppressed bone resorption while increasing bone mineral density in the lumbar spine by 11.3%.[2]
The Fracture Study in Postmenopausal Women with Osteoporosis trial confirmed the ability of romosozumab to prevent vertebral fractures in postmenopausal women. At 12 months, 1.8% of patients in the placebo group had sustained a new vertebral fracture versus 0.5% in the treatment group.[3] The trial also revealed a progressive reduction in new vertebral fractures at 24 months for patients who transitioned to denosumab after completing the 12-month regimen of romosozumab.[3] The Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at a High-Risk trial demonstrated the effectiveness of 12 months of romosozumab followed by alendronate versus alendronate alone. At 24 months, patients treated with romosozumab and alendronate had a 48% lower risk of a new vertebral fracture, 19% lower risk of nonvertebral fractures, and 38% lower risk of hip fracture compared to patients treated with alendronate alone.[4]
The STRUCTURE trial compared the effectiveness of romosozumab versus teriparatide in patients transitioning from ineffective bisphosphonate therapy. This trial revealed greater increases in bone mass density in the romosozumab treatment group than in the teriparatide group.[5] One study showed that procollagen type 1 N-telopeptide (P1NP) (a bone formation marker) increased 66% to 147% from baseline compared to placebo 2 weeks after initiating romosozumab therapy. Romosozumab also reduced type 1 collagen C-telopeptide (CTX) (a bone resorption marker) by 15% to 50% from baseline compared to placebo two weeks after initiation. After 12 months of treatment, romosozumab reduced the concentrations of P1NP and CTX below the levels achieved with a placebo. Both markers returned to baseline levels within 12 months after discontinuing romosozumab.[4][6][7] Micro-computed tomography and histological analyses revealed that romosozumab contributed to increased bone mass, bone volume, cortical thickness, and trabecular thickness. Additionally, computed tomography analysis showed improvements in trabecular connectivity, significantly reduced levels of trabecular bone pattern factor, and increased trabecular tissue bone mineral density.[8]
FDA-Approved Indications
The FDA has approved romosozumab to treat osteoporosis in postmenopausal women at high risk for fracture, including those with multiple risk factors for fracture, a history of osteoporotic fractures, or who have failed or are intolerant to other osteoporosis treatments.[1] The American College of Physicians recommends romosozumab (moderate-certainty evidence) or teriparatide (low-certainty evidence), followed by a bisphosphonate, to reduce the fracture risk in women with primary osteoporosis and a very high risk of fractures.[9]
Off-Label Uses
Several studies have demonstrated romosozumab's potential for men with osteoporosis; as of 2024, the FDA has not approved the use of romosozumab for this patient population.[10][11][12] The BRIDGE study (phase 3 trial) revealed that romosozumab significantly increased bone mineral density (BMD) in the lumbar spine and hip in men with osteoporosis between 55 to 90 compared to the placebo. The safety profiles were comparable between groups, with romosozumab demonstrating a slight increase in the risk of serious cardiovascular adverse events.[13][8]
A recent meta-analysis of placebo-controlled trials involving 7990 patients with osteoporosis, including postmenopausal women and men, found that administration of romosozumab (210 mg subcutaneous monthly) over 6 to 12 months increased BMD in the lumbar spine, hip, and femoral neck. Additionally, romosozumab was associated with a reduced incidence of falls and major osteoporotic fractures. In another study, romosozumab demonstrated promising efficacy in increasing bone mineral density in men with osteoporosis compared to standard treatment (eg, bisphosphonates, denosumab).
Further research is needed to validate its effectiveness and safety profile in the broader male population.[14][15] Another study involved women who could not ambulate due to chronic spinal cord injuries. This study found a 12-month romosozumab regimen significantly improved bone mineral density in the lumbar spine and hip, potentially reducing fracture risk.[16] Similarly, the use of romosozumab to treat osteoporosis in premenopausal women is currently under investigation but is not FDA-approved. One meta-analysis demonstrated romosozumab's superior efficacy to denosumab (particularly within the first 12 months of treatment) using bone mineral density and bone turnover markers.[8]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Romosozumab is the first anabolic medication to increase the formation and reduce the resorption of bone by inhibiting sclerostin.[1][2][10] Sclerostin was first recognized as a possible therapeutic target for the treatment of osteoporosis when its role was established in sclerostin deficiency. This genetic condition is associated with increased bone mass and fracture resistance.[3] Further research revealed sclerostin to be a negative regulator of bone formation.[17]
Sclerostin is a secreted, osteocyte-derived glycoprotein that binds to low-density lipoprotein receptor proteins 5 and 6 (LRP5 and LRP6), preventing the activation of the Wnt/β-catenin pathway that normally stimulates osteoblasts.[17] Preventing this activation promotes the ubiquitination and degradation of β-catenin, which blocks nuclear import and inhibits the osteogenic function of osteoblasts, resulting in decreased bone formation. Additionally, sclerostin stimulates the production of RANKL (receptor activator of nuclear factor-κB ligand) and suppresses the production of OPG (osteoprotegerin) in osteoblasts and pre-osteoblasts. Increased RANKL and reduced OPG activates and induces differentiation of osteoclasts.
The study of sclerostin's role led to the development of romosozumab, a humanized monoclonal sclerostin-neutralizing antibody (Scl-Ab) that binds to and inhibits sclerostin, preventing it from binding to the LRP5 and LRP6 receptor proteins. Romosozumab allows the Wnt/β-catenin pathway to proceed, promoting osteogenesis and inhibiting bone resorption to a lesser extent. This increases cortical and trabecular bone mass, which is beneficial in treating osteoporosis. Blocking sclerostin results in dual effects: 1) increased osteoblastic activity, thereby increasing bone formation, and 2) reduced osteoclastic activity, thereby decreasing bone resorption.
Pharmacokinetics
Absorption: Romosozumab reaches its maximum concentration (Tmax) in 5 days (± 3 days). A single dose of romosozumab (210 mg) achieves a maximum average serum concentration of 22.2 (5.8) μg/mL and a steady concentration state 3 months after monthly administration in postmenopausal women.[1] The pharmacokinetics of romosozumab are nonlinear as dose-increased clearance decreases.
Metabolism: The metabolism of romosozumab is not fully understood. As a monoclonal antibody, it is thought to be broken down into proteins and amino acids, similar to catabolic pathways for immunoglobulin G.[18]
Distribution: Romosozumab has an estimated volume distribution of 3.92 L at a steady state.[1]
Elimination: Romosozumab's systemic clearance occurs at 0.38 mL/kg/h. Romosozumab has a half-life of 12.8 days after 3 consecutive monthly doses.[1] No significant pharmacokinetic differences have been observed; however, increased body weight decreases romosozumab exposure.[7] One study found that 17.6% of patients in the romosozumab group developed binding antibodies, with 2.9% developing neutralizing antibodies by month 9. These findings corroborate a previous study involving postmenopausal women with osteoporosis. Neither binding nor neutralizing antibodies significantly affected the efficacy, safety, or drug exposure of romosozumab.[19][3]
Administration
Available Dosage Forms and Strengths
Romosozumab is available as a prefilled syringe containing a preservative-free solution for subcutaneous administration. Romosozumab should be stored at 36 to 46 °F (2.2 to 7.7 °C).[10] A romosozumab syringe should be examined for damage and discarded if it has cracks, breaks, a missing or loose needle cap, or an expiration date that has passed. An undamaged syringe should be discarded if dropped on a hard surface. The romosozumab solution should be clear to opalescent and colorless to light yellow. Do not use a syringe containing a solution that looks cloudy, discolored, or contains particles. A romosozumab syringe should be given 30 minutes to equilibrate to room temperature before administration and should not be warmed using any other method.
Adult Dosage
Romosozumab is administered as a monthly subcutaneous injection for 12 months. The recommended monthly dose is 210 mg, regardless of body weight. Each prefilled syringe contains 105 mg romosozumab in 1.17 mL solution; 2 prefilled syringes provide the recommended dose of 210 mg. Potential injection sites include the patient’s thigh, abdomen, or upper arm and should be altered each month. Patients receiving romosozumab should also receive calcium and vitamin D supplementation.[1] Romosozumab is less effective after 12 monthly injections. Studies have shown that bone mineral density levels return to baseline 12 months after discontinuing romosozumab; it is recommended that after discontinuing romosozumab, the patient continues osteoporosis treatment with antiresorptive therapy to maintain and continue increased bone mass density.[10][20] If a dose is missed or delayed, romosozumab should be administered immediately, with subsequent monthly injections from the new date.
Specific Patient Populations
Hepatic impairment: The product label does not specify adjustments in romosozumab dosage for patients with liver conditions.
Renal impairment: Romosozumab dosage remains unchanged for individuals with renal impairment. However, individuals with severe renal impairment (estimated glom filtration rate of 15 to 29 mL/min/1.73 m² or receiving dialysis) are at increased risk of hypocalcemia.[1][10] Providers must monitor calcium levels and administer calcium and vitamin D supplementation when appropriate. A recent meta-analysis evaluated the efficacy of romosozumab, teriparatide, denosumab, and raloxifene for reducing vertebral fractures in patients with kidney disease. Romosozumab demonstrated efficacy in improving bone mineral density across all stages of chronic kidney disease stages.[21]
Pregnancy considerations: Romosozumab is not indicated for women of reproductive potential. The administration of romosozumab to pregnant rats during organogenesis at exposures exceeding 31 times the recommended clinical dose resulted in skeletal abnormalities in offspring. Romosozumab doses 1.4 to 54 times the standard human dose in rats before mating and lactation resulted in minor reductions in offspring femoral bone mineral density and cortical circumferences. In rat reproductive toxicity studies, skeletal malformations such as syndactyly and polydactyly were observed in 1 out of 75 litters. These deformities occurred at doses much higher than the maximum recommended human dose.
Breastfeeding considerations: Romosozumab is not approved for women of reproductive potential. In animal studies with pregnant rats, romosozumab administration beginning 6 weeks before mating and lactation resulted in detectable serum levels in offspring, confirming dose-dependent exposure during gestation and lactation.
Pediatric patients: The safety and efficacy of romosozumab have not been established in pediatric patients.
Older patients: In clinical studies of romosozumab involving postmenopausal women with osteoporosis, most patients were 65 or older, with a significant subset at 75 or older. No variances in safety and efficacy were observed between the older and younger patients. In one study, older age and higher baseline calcium concentrations correlated with a greater reduction in calcium levels following the administration of romosozumab.[22]
Adverse Effects
A literature review performed by Miller et al determined that the most commonly reported adverse events occurring in less than 5% of subjects during clinical trials with romosozumab were arthralgia and headache.[1] This review also noted injection site reactions in approximately 5% of subjects but found that these reactions did not seem to recur with subsequent injections.[1] Other reported adverse events include nasopharyngitis, back pain, erythema multiforme, rash, dermatitis, and angioedema.[1][5][10][3][23]
Several trials found cardiovascular and cerebrovascular events increased with romosozumab use.[4][11][23] During the ARCH trial, cardiovascular adverse events occurred in 2.5% of patients in the romosozumab group versus 1.9% in the alendronate group.[4] During the BRIDGE trial, cardiovascular adverse effects occurred at a rate of 4.9% in the romosozumab group versus 2.5% in the placebo group.[11]
While these trials indicated differences in cardiovascular events, the FRAME and STRUCTURE trials found no difference in the rate of cardiovascular events between the romosozumab and placebo groups. However, due to the increase in reported cardiovascular and cerebrovascular events in the ARCH and BRIDGE trials, it is recommended not to use romosozumab in patients who have had a myocardial infarction or stroke in the past year. Evaluating the benefits versus risks in patients with cardiovascular risk factors is recommended on an individual basis.[1]
Drug-Drug Interactions
Concurrent administration of romosozumab with medications known to be associated with osteonecrosis of the jaw, such as bisphosphonates, chemotherapy, angiogenesis inhibitors, denosumab, and corticosteroids, may elevate the risk of developing osteonecrosis of the jaw. Providers should avoid concurrent administration of these medications and romosozumab.[24]
Contraindications
Romosozumab is contraindicated for patients with pre-existing hypocalcemia and hypersensitivity. Patients with documented serum calcium imbalances require correction before initiating treatment. Additionally, a history of systemic hypersensitivity to romosozumab or its excipients precludes its use. This risk is due to the potential for angioedema and erythema multiforme.[25]
Box Warnings
Romosozumab has been associated with an increased risk of myocardial infarction, stroke, and cardiovascular mortality. Romosozumab should not be initiated in patients who have had a myocardial infarction or stroke within the past year. Physicians should carefully evaluate the risk-benefit profile in patients with other cardiovascular risk factors. If the patient has a myocardial infarction or stroke during treatment, romosozumab must be immediately discontinued.
Warning and precautions
- Romosozumab is not recommended for patients who have completed romosozumab therapy for 12 months. Despite ongoing studies, romosozumab is not currently FDA-approved for use in premenopausal patients.[10][23] As per the American Association of Clinical Endocrinology (AACE), treatment with an anabolic agent (eg, romosozumab, teriparatide, abaloparatide) should be followed with bisphosphonates or denosumab to prevent bone density decline and maintain efficacy.[26]
- Serious adverse events associated with romosozumab administration include osteonecrosis of the jaw and atypical femur fractures. However, it should be noted that osteonecrosis of the jaw was seen in patients who had possible inciting events such as poor-fitting dentures and recent tooth extraction. Due to this, clinicians should perform precautionary routine oral examinations before initiating romosozumab. Furthermore, there were 2 reported cases of atypical fracture of the femur in the ARCH trial and 1 in the FRAME trial in the romosozumab treatment groups.[1][3][4] The risk of osteonecrosis of the jaw and atypical femur fractures appears to be low for patients on romosozumab.[23]
Monitoring
The development of hypocalcemia is a significant concern during romosozumab therapy. Hypocalcemia should be corrected before initiating romosozumab treatment.[10] Combining this medication with other drugs may increase the risk of hypocalcemia. Serum calcium levels should be monitored if a patient takes other medications that could cause hypocalcemia with concomitant romosozumab use.[23] Patients with severe renal impairment or who are on dialysis are at increased risk for hypocalcemia and should be monitored closely. Patients should be counseled about supplementing calcium and vitamin D to mitigate the potential risk of hypocalcemia.[1][10]
According to AACE, clinicians should assess bone mineral density at the lumbar spine, total hip, or femoral neck with dual-energy x-ray absorptiometry scans. Providers may consider using bone turnover markers (BTMs) to assess patient compliance and treatment response. Antiresorptive therapies typically reduce BTMs significantly and are associated with reduced fracture risk, while anabolic therapies generally lead to notable BTM increases, indicating a favorable treatment response.[26] BTMs such as procollagen type 1 N-telopeptide (P1NP) for bone formation and type 1 collagen C-telopeptide for bone resorption can be used to evaluate response to treatment.
Toxicity
Signs and Symptoms of Overdose
A study assessing the carcinogenicity risk of romosozumab concluded that romosozumab administration would not pose a carcinogenic risk to humans based on the weight of evidence factors and results of a rat lifetime study on tumor incidence.[27] Furthermore, additional animal studies have demonstrated no effects on mortality.[28] In an accidental overdose of romosozumab, serum calcium levels should be monitored.
Management of Overdose
There is no antidote for romosozumab. The poison control center or a medical toxicologist should be contacted for the latest information regarding an overdose.
Enhancing Healthcare Team Outcomes
Romosozumab represents a novel pharmacologic intervention intended for the management of osteoporosis in women with a high risk of bone fractures. Comprehensive education of healthcare professionals regarding the utilization of this medication is imperative, ensuring a detailed understanding of its therapeutic indications, potential adverse effects, and requisite clinical surveillance protocols. To optimize healthcare outcomes, comprehensive awareness and recognition of romosozumab's established adverse effects are essential for all members of the interprofessional healthcare team and patients. Healthcare professionals should educate patients on potential medication adverse events, including cardiovascular events, hypocalcemia, hypersensitivity reactions, osteonecrosis of the jaw, and atypical femoral fractures, ensuring proactive management and patient safety. Clinicians and the team should educate the patient on possible medication adverse events, such as the signs and symptoms of a cardiovascular event, hypocalcemia, hypersensitivity reactions, osteonecrosis of the jaw, and atypical femoral fracture. Due to romosozumab's association with hypocalcemia, healthcare team members should perform a medication review to identify any medications that could exacerbate hypocalcemia before giving the injections.[22] Healthcare professionals also need to obtain serum calcium levels should they be indicated. Patients should be advised to take calcium and vitamin D supplementation as well.
Continuous patient monitoring and ongoing medical education for clinicians are essential to integrate evolving clinical trial data on romosozumab, ensuring informed decisions and optimizing long-term treatment outcomes and safety management. Pharmacists should be consulted to ensure proper dosing and to check for possible drug interactions, particularly drugs that affect calcium levels. Endocrinologists and orthopedic surgeons should be consulted for patients with a high fracture risk for expert management and treatment guidance. Nurses are vital in monitoring treatment adherence to ensure patients follow prescribed therapies consistently and effectively. Dentists and oral surgeons should be consulted for patients with osteonecrosis of the jaw. Emergency medicine plays a crucial role in promptly managing and addressing acute adverse events or emergencies associated with romosozumab therapy, including hypersensitivity reactions and cardiovascular events.
An interprofessional team approach is crucial for addressing barriers to using romosozumab, such as its high cost, the requirement for monthly injections administered by healthcare providers, and managing adverse effects. This collaboration ensures that healthcare team members, including clinicians, endocrinologists, pharmacists, and nurses, work together to identify these potential obstacles and develop strategies to optimize individualized treatment. This approach aims to decrease potential adverse effects, improve disease management and quality of life, and ultimately enhance patient outcomes related to romosozumab therapy.
Media
(Click Image to Enlarge)
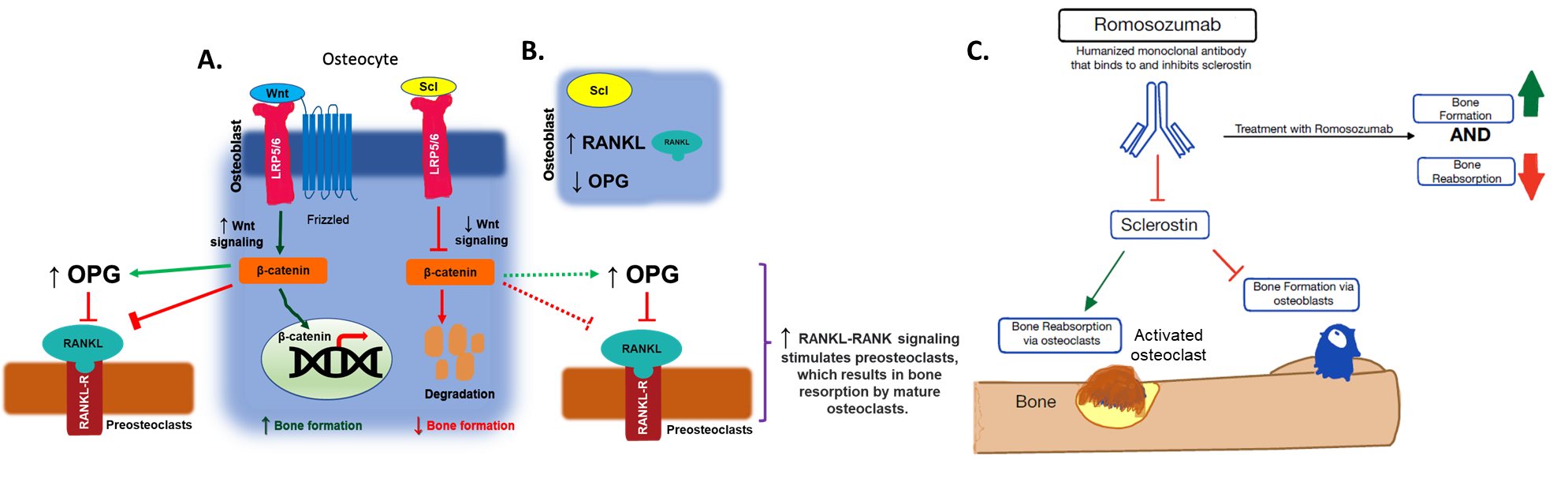
Romosozumab Mechanism of Action. (A) Sclerostin inhibits the Wnt/β-catenin pathway by competitively binding the LRP5 and 6 receptors on osteoblasts. This promotes the ubiquitinated degradation of β-catenin, which blocks the nuclear import to inhibit the osteogenic function of the osteoblasts. (B) Sclerostin increases the RANKL production and decreases the OPG production in osteoblasts and pre-osteoblasts to activate the osteoclasts. (C) Romosozumab binds to sclerostin and inhibits sclerostin's ability to bind to the LRP5 and 6 receptor proteins and allows the Wnt/β -catenin pathway to occur, promoting osteogenesis and inhibiting bone resorption (to a lesser extent).
Contributed by M Parmar, PhD
References
Miller SA, St Onge EL, Whalen KL. Romosozumab: A Novel Agent in the Treatment for Postmenopausal Osteoporosis. The Journal of pharmacy technology : jPT : official publication of the Association of Pharmacy Technicians. 2021 Feb:37(1):45-52. doi: 10.1177/8755122520967632. Epub 2020 Oct 23 [PubMed PMID: 34752536]
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. The New England journal of medicine. 2014 Jan 30:370(5):412-20. doi: 10.1056/NEJMoa1305224. Epub 2014 Jan 1 [PubMed PMID: 24382002]
Level 1 (high-level) evidenceCosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. The New England journal of medicine. 2016 Oct 20:375(16):1532-1543 [PubMed PMID: 27641143]
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. The New England journal of medicine. 2017 Oct 12:377(15):1417-1427. doi: 10.1056/NEJMoa1708322. Epub 2017 Sep 11 [PubMed PMID: 28892457]
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K, Finkelstein JS, Genant HK, Goemaere S, Hyldstrup L, Jodar-Gimeno E, Keaveny TM, Kendler D, Lakatos P, Maddox J, Malouf J, Massari FE, Molina JF, Ulla MR, Grauer A. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet (London, England). 2017 Sep 30:390(10102):1585-1594. doi: 10.1016/S0140-6736(17)31613-6. Epub 2017 Jul 26 [PubMed PMID: 28755782]
Level 1 (high-level) evidenceKerschan-Schindl K. Romosozumab: a novel bone anabolic treatment option for osteoporosis? Wiener medizinische Wochenschrift (1946). 2020 Apr:170(5-6):124-131. doi: 10.1007/s10354-019-00721-5. Epub 2019 Dec 19 [PubMed PMID: 31858345]
Hsu CP, Maddox J, Block G, Bartley Y, Yu Z. Influence of Renal Function on Pharmacokinetics, Pharmacodynamics, and Safety of a Single Dose of Romosozumab. Journal of clinical pharmacology. 2022 Sep:62(9):1132-1141. doi: 10.1002/jcph.2050. Epub 2022 Apr 2 [PubMed PMID: 35304747]
Hu M, Zhang Y, Guo J, Guo C, Yang X, Ma X, Xu H, Xiang S. Meta-analysis of the effects of denosumab and romosozumab on bone mineral density and turnover markers in patients with osteoporosis. Frontiers in endocrinology. 2023:14():1188969. doi: 10.3389/fendo.2023.1188969. Epub 2023 Jul 12 [PubMed PMID: 37529613]
Level 1 (high-level) evidenceQaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, Shamliyan T, Cooney TG, Clinical Guidelines Committee of the American College of Physicians, Cross JT Jr, Fitterman N, Lin JS, Maroto M, Obley AJ, Tice JA, Tufte JE. Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline From the American College of Physicians. Annals of internal medicine. 2023 Feb:176(2):224-238. doi: 10.7326/M22-1034. Epub 2023 Jan 3 [PubMed PMID: 36592456]
Prather C, Adams E, Zentgraf W. Romosozumab: A first-in-class sclerostin inhibitor for osteoporosis. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2020 Nov 16:77(23):1949-1956. doi: 10.1093/ajhp/zxaa285. Epub [PubMed PMID: 32880646]
Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, Miyauchi A, Maddox J, Chen L, Horlait S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. The Journal of clinical endocrinology and metabolism. 2018 Sep 1:103(9):3183-3193. doi: 10.1210/jc.2017-02163. Epub [PubMed PMID: 29931216]
Level 1 (high-level) evidencePadhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. Journal of clinical pharmacology. 2014 Feb:54(2):168-78. doi: 10.1002/jcph.239. Epub 2013 Dec 11 [PubMed PMID: 24272917]
Level 1 (high-level) evidenceWu D, Li L, Wen Z, Wang G. Romosozumab in osteoporosis: yesterday, today and tomorrow. Journal of translational medicine. 2023 Sep 27:21(1):668. doi: 10.1186/s12967-023-04563-z. Epub 2023 Sep 27 [PubMed PMID: 37759285]
Beaudart C, Demonceau C, Sabico S, Veronese N, Cooper C, Harvey N, Fuggle N, Bruyère O, Rizzoli R, Reginster JY. Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis. Aging clinical and experimental research. 2023 Sep:35(9):1789-1806. doi: 10.1007/s40520-023-02478-9. Epub 2023 Jul 3 [PubMed PMID: 37400668]
Level 1 (high-level) evidenceKobayashi T, Hara M, Shimanoe C, Morimoto T, Masaaki M, Ito K, Shimazaki T. Efficacy and safety of romosozumab: a meta-analysis of placebo-controlled trials. Journal of bone and mineral metabolism. 2024 Jul 8:():. doi: 10.1007/s00774-024-01531-5. Epub 2024 Jul 8 [PubMed PMID: 38977437]
Level 1 (high-level) evidenceCrack LE, Simonian N, Schnitzer TJ, Edwards WB. Monthly treatment with romosozumab for 1 year increases bone mineral at the hip, but not the knee, in women with chronic spinal cord injury. JBMR plus. 2024 Jul:8(7):ziae077. doi: 10.1093/jbmrpl/ziae077. Epub 2024 Jun 7 [PubMed PMID: 38911320]
McClung MR. Romosozumab for the treatment of osteoporosis. Osteoporosis and sarcopenia. 2018 Mar:4(1):11-15. doi: 10.1016/j.afos.2018.03.002. Epub 2018 Mar 27 [PubMed PMID: 30775535]
. Romosozumab. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012:(): [PubMed PMID: 34251779]
Baek KH, Chung YS, Koh JM, Kim IJ, Kim KM, Min YK, Park KD, Dinavahi R, Maddox J, Yang W, Kim S, Lee SJ, Cho H, Lim SK. Romosozumab in Postmenopausal Korean Women with Osteoporosis: A Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study. Endocrinology and metabolism (Seoul, Korea). 2021 Feb:36(1):60-69. doi: 10.3803/EnM.2020.848. Epub 2021 Feb 24 [PubMed PMID: 33677928]
Level 1 (high-level) evidence. Romosozumab for osteoporosis. Australian prescriber. 2021 Jun:44(3):109-110. doi: 10.18773/austprescr.2021.021. Epub 2021 Apr 20 [PubMed PMID: 34211250]
Sabaghian T, Delkash P, Rahmannia M, Shahidi Bonjar AH, Centis R, D'Ambrosio L, Sotgiu G, Nasiri MJ, Migliori GB. Efficacy and Safety of Anti-Osteoporotic Agents across CKD Stages: A Meta-Analysis of Randomized Clinical Trials. Kidney & blood pressure research. 2024:49(1):581-587. doi: 10.1159/000540235. Epub 2024 Jul 22 [PubMed PMID: 38972312]
Level 1 (high-level) evidenceInose H, Kato T, Tomizawa S, Ariga A, Motoyoshi T, Fukushima K, Takahashi K, Yoshii T, Okawa A. Impact of romosozumab on serum calcium concentration and factors predicting the fluctuations in calcium concentration upon romosozumab administration: A multicenter retrospective study. Bone reports. 2022 Dec:17():101635. doi: 10.1016/j.bonr.2022.101635. Epub 2022 Nov 7 [PubMed PMID: 36389625]
Level 2 (mid-level) evidenceNealy KL, Harris KB. Romosozumab: A Novel Injectable Sclerostin Inhibitor With Anabolic and Antiresorptive Effects for Osteoporosis. The Annals of pharmacotherapy. 2021 May:55(5):677-686. doi: 10.1177/1060028020952764. Epub 2020 Aug 29 [PubMed PMID: 32862655]
Ahdi HS, Wichelmann TA, Pandravada S, Ehrenpreis ED. Medication-induced osteonecrosis of the jaw: a review of cases from the Food and Drug Administration Adverse Event Reporting System (FAERS). BMC pharmacology & toxicology. 2023 Mar 6:24(1):15. doi: 10.1186/s40360-023-00657-y. Epub 2023 Mar 6 [PubMed PMID: 36879299]
Level 3 (low-level) evidenceTrepanowski N, Yim RM, Wetstone R, MacDonald E, Servattalab S, Jacob-George S, Harris JE. Vitiligo progression in a patient undergoing romosozumab treatment for osteoporosis. JAAD case reports. 2023 Dec:42():26-30. doi: 10.1016/j.jdcr.2023.09.033. Epub 2023 Oct 11 [PubMed PMID: 37965188]
Level 3 (low-level) evidenceCamacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS- 2020 UPDATE EXECUTIVE SUMMARY. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2020 May:26(5):564-570. doi: 10.4158/GL-2020-0524. Epub [PubMed PMID: 32427525]
Level 1 (high-level) evidenceChouinard L, Felx M, Mellal N, Varela A, Mann P, Jolette J, Samadfam R, Smith SY, Locher K, Buntich S, Ominsky MS, Pyrah I, Boyce RW. Carcinogenicity risk assessment of romosozumab: A review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regulatory toxicology and pharmacology : RTP. 2016 Nov:81():212-222. doi: 10.1016/j.yrtph.2016.08.010. Epub 2016 Aug 26 [PubMed PMID: 27569204]
Miller PD, Adachi JD, Albergaria BH, Cheung AM, Chines AA, Gielen E, Langdahl BL, Miyauchi A, Oates M, Reid IR, Santiago NR, Vanderkelen M, Wang Z, Yu Z. Efficacy and Safety of Romosozumab Among Postmenopausal Women With Osteoporosis and Mild-to-Moderate Chronic Kidney Disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2022 Aug:37(8):1437-1445. doi: 10.1002/jbmr.4563. Epub 2022 May 20 [PubMed PMID: 35466448]