Introduction
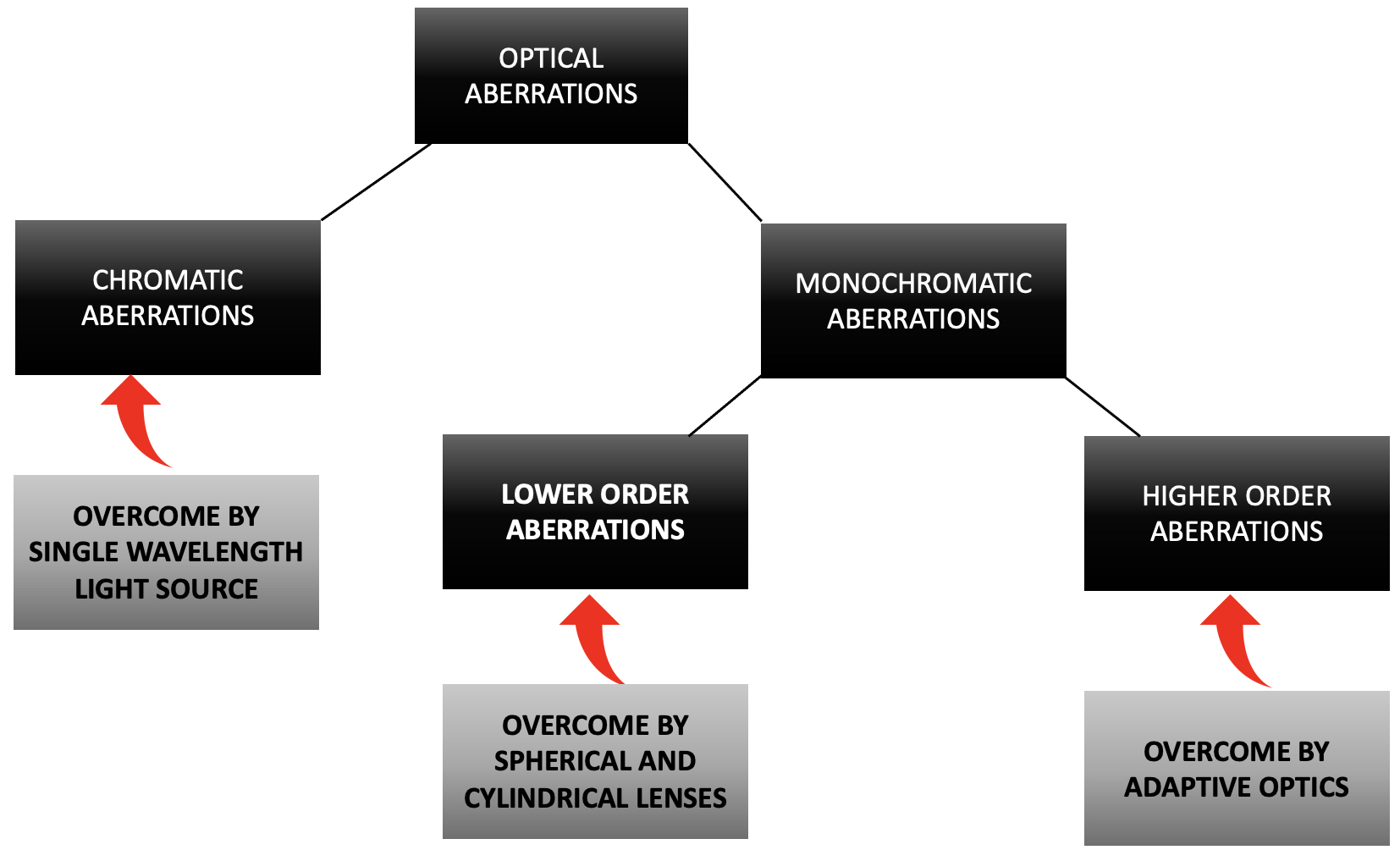
Light traveling through any media other than vacuum undergoes aberrations due to changes in the refractive index and interface of the medium.[1] Similarly, rays exiting the eye also have wavefront aberrations contributed by the differences in the refractive index of ocular tissues. The wavefront aberrations are classified as chromatic and monochromatic aberrations.[2]
The monochromatic aberrations are further classified as lower order (contribute to about 90% of total aberrations) and higher order aberrations (10% of total aberrations). Spherical and cylindrical lenses correct the lower-order aberrations. The higher-order aberrations responsible for limiting the lateral resolution of devices are unique to each patient's eye and were deemed un-rectifiable until recently.[3] In 1971, the Shack-Hartmann wavefront sensor (SHWS) measured the eye's possible optical aberrations.[4]
In 1953, Horace Babcock from the Carnegie laboratories proposed Adaptive optics (AO) for the clear visualization of astronomical bodies, the images distorted by the aberrations in the incident rays due to the turbulence in the earth's atmosphere.[5] In 1997 Liang et al. employed this same principle in retinal imaging to overcome higher-order aberrations.[6] They used the SHWS and deformable mirrors to demonstrate cone photoreceptors in vivo. They concluded AO might help image the live retina at the microscopic level by improving the lateral resolution immensely. Roorda was the first to use AO clinically in a patient with inherited rod-cone dystrophy in 2000.[7]
Though AO technology has evolved in the last two decades, no Food and Drug Administration, USA (FDA) approved devices for clinical use exist. Recently, an AO flood illumination device has been approved for marketing in the European Union, China, and Japan.[8]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The retina is the innermost coat of the eyeball, responsible for processing light energy and generating visual information. The three major types of cells in the retina are the photoreceptors, glial cells, and neuronal cells.[9] The rods are the predominant photoreceptors in the retina and are concentrated more in the retinal peripheries. They are sensitive to photons of light and help in scotopic vision. The cones comprise only 5 to 6 % of the photoreceptors and are concentrated primarily in the center of the macula, the fovea. They are sensitive to a specific wavelength of light and help in photopic and color vision.[10]
The retinal ganglion cells are second-order neurons that relay image-forming and non-image-forming information from the bipolar cells to the lateral geniculate body. Multiple rods converge onto a single ganglion cell, whereas a single cone is connected to a single ganglion cell. At least twenty different types of retinal ganglion cells receive excitatory and inhibitive impulses from the amacrine and bipolar cells.[11]
The retinal pigment epithelium is the outermost layer of the retina, which is made up of a single layer of regularly arranged cells with a polygonal shape. It has many roles, like phagocytosis of photoreceptor outer segments, barrier function, transport of substrates, and protection against phototoxicity, as it contains pigments like melanin and lipofuscin.[12]
Indications
Adaptive optics helps visualize individual cellular components in vivo and demonstrates individual photoreceptors, retinal pigment epithelial cells, ganglion cells, and retinal microvasculature in various acquired and inherited retinochoroidal pathologies. This helps in understanding the basic pathophysiology of these disorders, screening and diagnosing them at the subclinical stage, formulating newer therapeutic interventions, and monitoring cellular-level responses to therapeutic interventions.[13]
The various indications include retinal vascular disorders like hypertensive retinopathy, retinal vasculitis, intraocular inflammation, diabetic retinopathy, macular pathologies like central serous chorioretinopathy, and age-related macular degeneration, inherited retinal dystrophies, drug-induced maculopathies, choroidal pathologies, and glaucoma.[14][15][16]
Contraindications
There are no contraindications to this procedure. Various factors limit the usage of adaptive optics in routine practice.[8] Firstly, studies employing adaptive optics for various retinal pathologies have a limited sample size. Large patient cohorts are essential to generate a normative database comparable to gender, ethnicity, and age. Good-quality image acquisition in patients with nystagmus, corneal opacities, and cataracts is challenging. Poor fixation poses a significant challenge in capturing a good-quality image.
A small area of the retina is imaged, and the time required for imaging is significantly more. It may be difficult for uncooperative and anxious patients. Incorporating eye-tracking software may help acquire better quality information in these patients. Analysis of acquired images is manual and may be laborious and time-consuming. Automated interpretation or development of artificial intelligence software may simplify grading. The enormous cost of the equipment and size has limited its location in research labs. More compact hardware will make its installation and operation practical.
Equipment
Components of the Machine
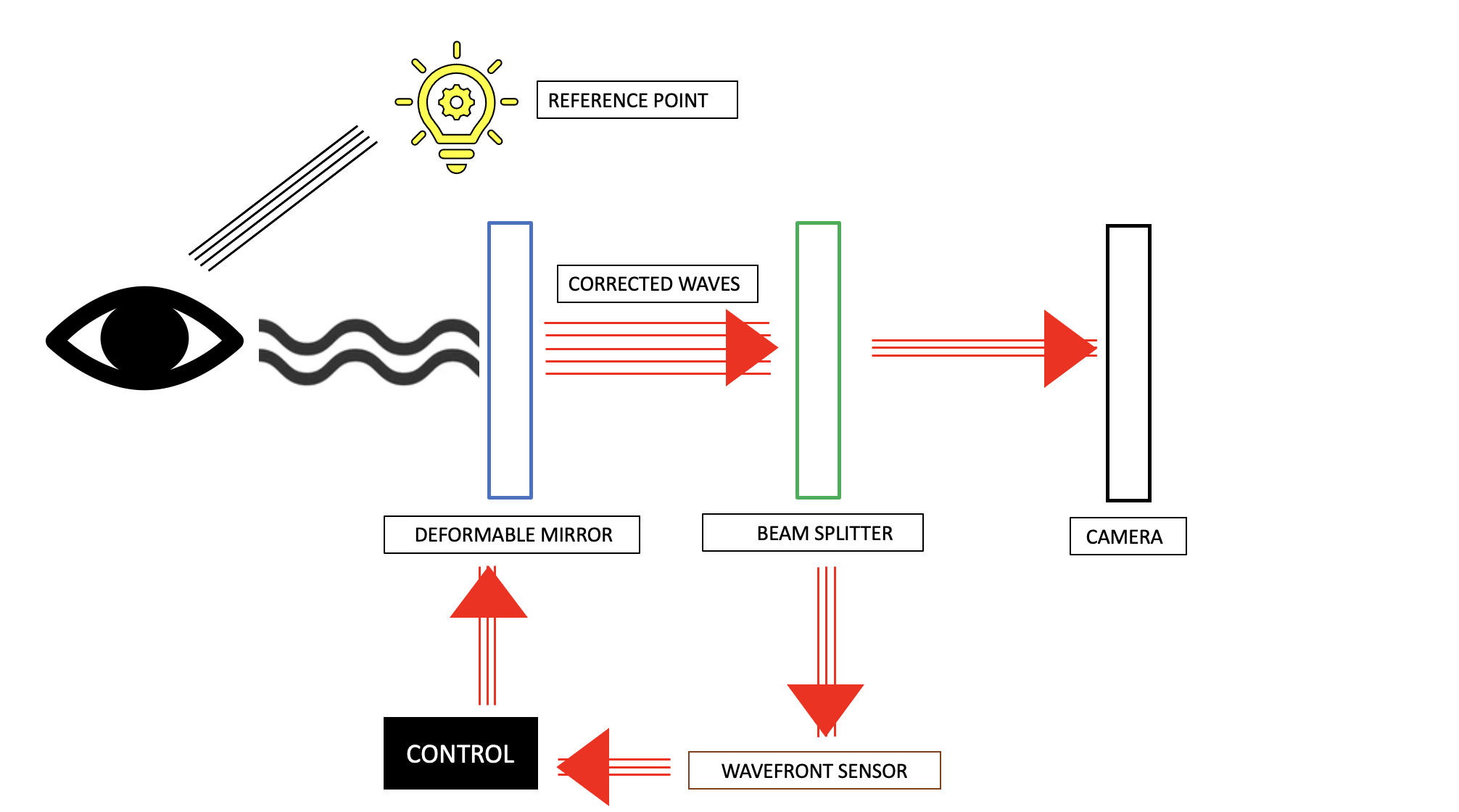
Adaptive optics equipment has four main components.[17] These are the following:
- A wavefront sensor to detect and measure the optical aberrations in the light rays reflected from the eye. It consists of a lenslet array, each of which samples a part of the light wave. Shack- Hartmann wavefront sensor (SHWS) is commonly used.[18]
- A deformable mirror to correct the detected aberrations. The actuator receives inputs from the wavefront sensor and modifies the surface of these mirrors. This helps correct the higher-order and any significant lower-order aberrations in the light beam.[19]
- A control system to calculate the required correction level and receive feedback, and
- Image acquisition and processing device to capture the corrected waveform and generate an image.
Adaptive optics is not a stand-alone technology and has been combined with pre-existing imaging equipment, like the fundus camera, optical coherence tomography, and scanning laser ophthalmoscope.[20]
Adaptive Optics flood Illumination Technology (AO FI)
AO components like the SHWS and deformable mirrors are combined with a high-resolution fundus camera. This setup helps image the spacing and directionality of the cones and pick up any fluctuations in cone reflectance. Since it uses an incoherent light source, the exposure time is brief, reducing the time required for image capture. However, this system is limited by its reduced axial resolution.[21]
Adaptive Optics Scanning Laser Ophthalmoscope (AO SLO)
Dreher et al. were the first to combine AO with SLO devices in 1989.[22] The system offers a retinal image and video with unparalleled lateral and axial resolution. The high-resolution video imaging helps demonstrate the movement of blood columns in retinal capillaries. By using a confocal pinhole, the different retinal layers can be visualized. It is equipped with eye-tracking software and laser-assisted stimulus delivery to the retina.[23]
Adaptive Optics Optical Coherence Tomography (AO-OCT)
This system overcomes monochromatic aberrations and helps in the reduction of speckle size. This enables three-dimensional imaging of individual photoreceptors, ganglion cells, retinal pigment epithelium, lamina cribrosa, and nerve fiber layer.[24]
Personnel
Any trained ophthalmologist, vitreoretinal surgeon, optometrist, or ophthalmic imaging technician can perform this procedure. In addition, technicians and ophthalmologists need to be trained in grading and interpreting images.
Preparation
Adaptive optics is a non-invasive retinal imaging procedure. The procedure of image acquisition is clearly explained to the patient. A pupillary diameter of at least 4 mm is recommended.
Technique or Treatment
The patient is positioned comfortably in a traditional chin rest. They are asked to focus on an internal fixation target, which is mobilized depending on the area of interest. The type of imaging is enface reflectance imaging. Once the site of interest appears on the graphic display in the user interface, the cones are brought into focus. Images are acquired over a 4-degree * 4-degree area at 9.5 frames/ second using a low noise-charged coupled device (CCD) camera. The illumination source is a near-infrared (850 nm) light-emitting diode (LED).
The software acquires 40 images, of which 20 with the best Sobel contrast are chosen. They are summed together using an auto-correlation algorithm to generate a single image with a good signal-to-noise ratio. The final image is obtained by subtracting the computed image from the background image. The brightness and contrast of the resultant image are adjusted while saturating the bright pixels. Every averaged image finally undergoes visual checks to rule out apparent artifacts. Automatic image alignment may be used to track the same retinal area with accuracy in microns for the progression of the disease, the natural history of the disease, or the response to therapy.
Complications
There are no complications associated with Adaptive optics imaging as it is a non-invasive procedure. However, patients with cervical spine pathologies, anxious patients, debilitated individuals, and patients with low vision and ocular motility disorders may find it challenging to maintain fixation for longer.
Clinical Significance
Normal Eyes
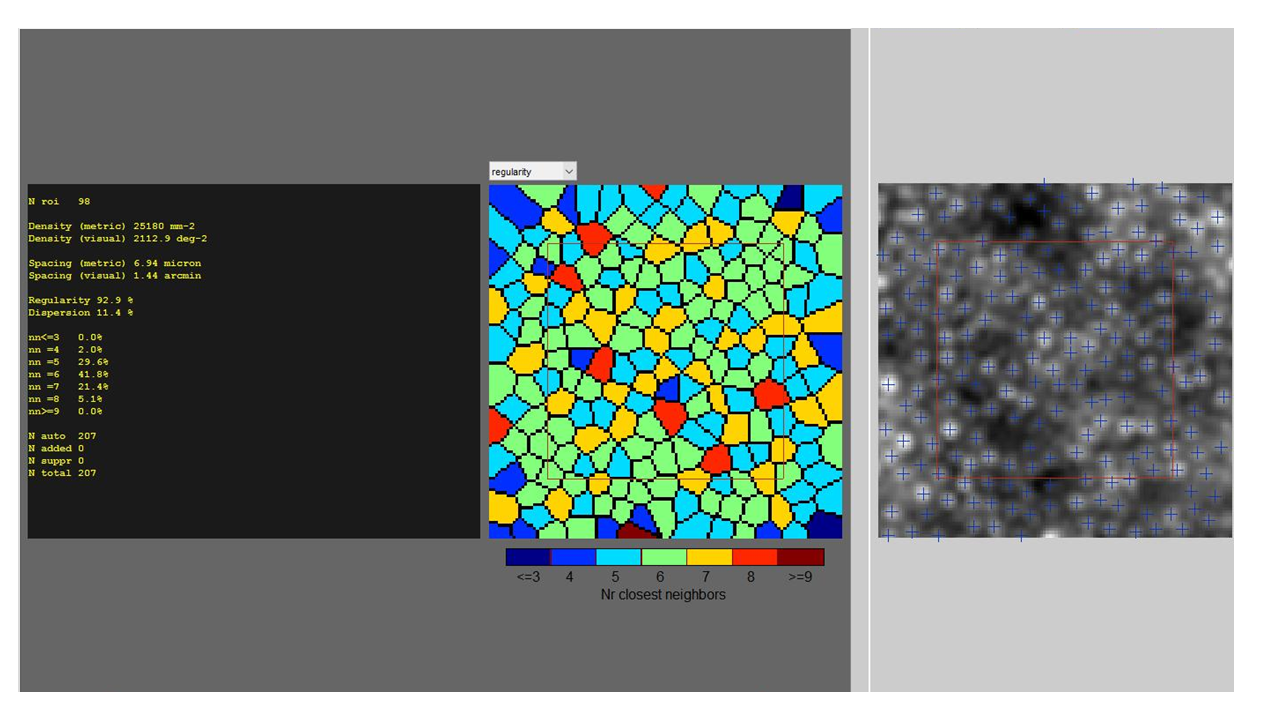
Adaptive optics is still confined to research. Examination of large cohorts of healthy eyes is essential to establish normative databases. This will help spot any deviation from normal and clinch diseases at early stages. The outer photoreceptor segment is visualized as the cone mosaic. Few studies have looked at the cone packing density and the spacing of cones in the foveal cone mosaic. Standardizing this normative data is essential before it's implemented for clinical use.[25][26]
Cone and Rod Photoreceptors
Imaging cones by adaptive optics was easier due to their wave-guiding properties. They are visible as an imperfect hexagonal mosaic. The most significant biomarkers employed in various studies include cone density and spacing.[27] The presence of different functional subtypes of cones - short, medium, and long wavelength was also detected using adaptive optics.[28]
Changes in the reflectance of cone outer segment tips (COST), termed dysflective cones indicate altered function when detected by adaptive optics.[29] Due to their smaller diameter and lesser wave-guiding properties, rods have been imaged sparingly.[30] The visualization of rods may guide therapeutic regimens in many inherited retinal dystrophies.
Retinal Pigment Epithelium (RPE)
The challenge in imaging RPE by adaptive optics was due to the embedding of the photoreceptor outer segment into RPE. This was overcome by imaging RPE at areas of photoreceptor loss and areas of subretinal fluid.[31] Morgan et al. first imaged an RPE mosaic in the healthy retina using double channel fluorescence AO SLO.[32] RPE visualization and computing the RPE - photoreceptor ratio may help follow up on various retinochoroidal pathologies and also help assess treatment outcomes.[32]
Ganglion Cells
Imaging ganglion cells is difficult due to their transparent nature and dense three-dimensional arrangements. Rossi et al. generated a two-dimensional image of the retinal ganglion cells using multi-offset AO-SLO. Adaptive optics could also distinguish between the subtypes of ganglion cells, parasol, and midget types, based on their imaging characteristics.[33][34]
Lamina cribrosa
The visualization of lamina cribrosa, along with quantifying parameters like pore size, shape, and density, has been made possible by adaptive optics with good repeatability. Quantification and standardization of these parameters and correlation with predisposing genes may help screen and detect susceptible individuals at a very early stage.[35]
Retinal Vasculature
AO enables precise visualization and quantification of the retinal microvasculature by various parameters like wall thickness (WT), lumen diameter (LD), wall-to-lumen ratio, and cross-sectional area of the vessel wall.[36] The movement of leucocytes and their velocity in the perifoveal capillaries can also be visualized. These parameters may be significant in patients with retinal vascular diseases like diabetic retinopathy and hypertensive retinopathy.[37] The vessels may be followed up in various vascular disorders, including hypertensive retinopathy, diabetic retinopathy, and retinal vasculitis.
| Disease | Significance and Imaging Features in Adaptive optics |
| Diabetic retinopathy |
AO enables high-resolution imaging of microaneurysms, retinal microvasculature, and cone photoreceptors which are the primary areas of interest in diabetic retinopathy. Experimental studies have shown variable alterations in cone density, especially in patients with type 1 diabetes mellitus.[38] An increase in wall thickness, wall-to-lumen ratio, the cross-sectional area of the vascular wall, tortuosity of the parafoveal arteriovenous plexus, and a decrease in luminal diameter, have been documented in patients with type 2 diabetes mellitus without retinopathy.[39] Hence AO may serve as a screening tool to detect pre-clinical lesions in patients with diabetes mellitus. The AO images of a specific retinal area in follow-up may detect the progression of diabetic retinopathy (increase in the number of microaneurysms). |
| Genetic Retinal diseases |
Retinitis Pigmentosa AO enabled in vivo visualization of cones and showed that cone photoreceptor density was reduced even in the early stages of the disease with the intact ellipsoid zone in optical coherence tomography. It also helps monitor patients receiving experimental therapeutic interventions like ciliary neurotrophic factor (CNTF), where a decelerated photoreceptor loss was documented.[40][41] Stargardt's Disease A study by Song et al. demonstrated that cone photoreceptor loss precedes lipofuscin deposition in patients with macular atrophy phenotype in patients with ABCA4 gene mutation. An increase in photoreceptor spacing was documented in patients with advanced forms of the disease.[42] Other Dystrophies A significant reduction in cone photoreceptors has also been documented in other retinal dystrophies like best macular dystrophy,[43] Bietti crystalline dystrophy, and choroidal dystrophy. The patterns of photoreceptor loss have been demonstrated in animal models.[44][45] |
| Age-related macular degeneration (AMD) | AO demonstrates the presence of hyporeflective clumps (HRCs) in the RPE monolayer in patients with advanced dry AMD. This appearance is due to detached retinal pigment epithelial (RPE) cells, macrophages phagocytosing (RPE) cells or microglia. They are distributed throughout the posterior pole and correlate histologically with melanosomes. The pattern of distribution of these HRCs varies as the disease progresses. AO has also demonstrated the presence of outer retinal tubulations caused by the radial rearrangement of cone photoreceptors and mullers cells.[46] AO imaging may be used to document the progression of geographic atrophy in dry AMD. |
| Central serous chorioretinopathy (CSCR) | Various studies have demonstrated a reduced foveal cone density in patients with CSCR, and the loss of photoreceptors correlated positively with the duration of subretinal fluid. It also demonstrated the loss of foveal cones in the uninvolved eye in patients with unilateral CSCR.[47][48] |
| Hydroxychloroquine (HCQ) toxicity | AO studies have revealed that cone photoreceptors are unaffected in the first two years of HCQ toxicity. Also, AO detected loss of photoreceptors even in patients with no obvious field defects overcoming the shortcoming of current screening technologies, which may detect damage only after irreversible damage has occurred.[49][50] |
| Retinal detachment (RD) | Potic et al. demonstrated an increased cone spacing and a decrease in cone density in patients after the repair of RD, irrespective of macular involvement.[52] Reumueller et al. showed that though cone density was regained within 56 weeks after surgery, microperimetry demonstrated functional impairment.[51] |
| Vogt Koyanagi Harada Syndrome (VKH) | Adaptive optics imaging demonstrated a decrease in cone density in patients with active inflammation. The cone density recovers once inflammation is under control but is still significantly lower compared to healthy eyes. The decreased cone density was significant in eyes with cystoid spaces. Further studies are required to validate these findings in patients with VKH syndrome.[52] |
| White Dot syndromes |
The study conducted by Agarwal et al. showed that photoreceptor density might serve as an early structural biomarker of inflammation. They also showed that cone photoreceptor loss was more in areas of inactive disease than in overactive lesions and areas of ellipsoid zone disruption. AO may have the potential to assess disease activities in these patients.[53] |
| Glaucoma | In the study conducted by Zwillinger et al., AO demonstrated an increase in the size of the pores in the lamina cribrosa in patients with primary open-angle glaucoma (POAG) and normal eyes of their first-degree relatives. This may indicate the individual susceptibility to POAG requiring close intraocular pressure and visual field monitoring. The cone densities were unaffected even in patients with significant field defects.[54][55] |
Enhancing Healthcare Team Outcomes
Adaptive optics helps in non-invasive cellular imaging of the retina. Image acquisition and interpretation require trained personnel to capture good-quality images and interpret them. Image acquisition is time-consuming but has been reduced to 2 seconds in newer machines.
Nurses and ophthalmic photographers play a pivotal role in helping the patient maintain fixation and remain comfortable throughout the procedure. Training of allied ophthalmic personnel may also reduce the burden on treating physicians. It is necessary that all professionals involved in the process of image acquisition are educated and aware of the clinical significance of adaptive optics.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Del Águila-Carrasco AJ,Kruger PB,Lara F,López-Gil N, Aberrations and accommodation. Clinical & experimental optometry. 2020 Jan [PubMed PMID: 31284325]
Hughes RP,Vincent SJ,Read SA,Collins MJ, Higher order aberrations, refractive error development and myopia control: a review. Clinical & experimental optometry. 2020 Jan [PubMed PMID: 31489693]
Bille JF,Jayabalan GS,Bille JF, The Development of Adaptive Optics and Its Application in Ophthalmology. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. 2019; [PubMed PMID: 32091847]
Akondi V,Dubra A, Shack-Hartmann wavefront sensor optical dynamic range. Optics express. 2021 Mar 15; [PubMed PMID: 33820289]
Katz MS,Jonna G, Adaptive optics: astronomy, military might, and retinal imaging. Journal of pediatric ophthalmology and strabismus. 2015 Jan-Feb; [PubMed PMID: 25643366]
Liang J,Williams DR,Miller DT, Supernormal vision and high-resolution retinal imaging through adaptive optics. Journal of the Optical Society of America. A, Optics, image science, and vision. 1997 Nov; [PubMed PMID: 9379246]
Roorda A, Adaptive optics ophthalmoscopy. Journal of refractive surgery (Thorofare, N.J. : 1995). 2000 Sep-Oct; [PubMed PMID: 11019882]
Wynne N,Carroll J,Duncan JL, Promises and pitfalls of evaluating photoreceptor-based retinal disease with adaptive optics scanning light ophthalmoscopy (AOSLO). Progress in retinal and eye research. 2021 Jul; [PubMed PMID: 33161127]
Mahabadi N, Al Khalili Y. Neuroanatomy, Retina. StatPearls. 2024 Jan:(): [PubMed PMID: 31424894]
Lamb TD. Why rods and cones? Eye (London, England). 2016 Feb:30(2):179-85. doi: 10.1038/eye.2015.236. Epub 2015 Nov 13 [PubMed PMID: 26563661]
Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clinical ophthalmology (Auckland, N.Z.). 2008 Dec:2(4):879-89 [PubMed PMID: 19668442]
Yang S, Zhou J, Li D. Functions and Diseases of the Retinal Pigment Epithelium. Frontiers in pharmacology. 2021:12():727870. doi: 10.3389/fphar.2021.727870. Epub 2021 Jul 28 [PubMed PMID: 34393803]
Akyol E,Hagag AM,Sivaprasad S,Lotery AJ, Adaptive optics: principles and applications in ophthalmology. Eye (London, England). 2021 Jan; [PubMed PMID: 33257798]
Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809640]
Battu R,Dabir S,Khanna A,Kumar AK,Sinha Roy A, Adaptive optics imaging of the retina. Indian journal of ophthalmology. 2014 Jan; [PubMed PMID: 24492503]
Mahabadi N, Foris LA, Tripathy K. Open Angle Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 28722917]
Lombardo M,Serrao S,Devaney N,Parravano M,Lombardo G, Adaptive optics technology for high-resolution retinal imaging. Sensors (Basel, Switzerland). 2012 Dec 27; [PubMed PMID: 23271600]
Xu H,Wu J, Extended aperture line-scanning Hartmann wavefront sensor. Applied optics. 2021 Apr 20; [PubMed PMID: 33983245]
Alcock SG,Nistea IT,Badami VG,Signorato R,Sawhney K, High-speed adaptive optics using bimorph deformable x-ray mirrors. The Review of scientific instruments. 2019 Feb; [PubMed PMID: 30831713]
Godara P,Dubis AM,Roorda A,Duncan JL,Carroll J, Adaptive optics retinal imaging: emerging clinical applications. Optometry and vision science : official publication of the American Academy of Optometry. 2010 Dec; [PubMed PMID: 21057346]
Rha J,Jonnal RS,Thorn KE,Qu J,Zhang Y,Miller DT, Adaptive optics flood-illumination camera for high speed retinal imaging. Optics express. 2006 May 15; [PubMed PMID: 19516608]
Dreher AW,Bille JF,Weinreb RN, Active optical depth resolution improvement of the laser tomographic scanner. Applied optics. 1989 Feb 15; [PubMed PMID: 20548563]
Roorda A, Applications of adaptive optics scanning laser ophthalmoscopy. Optometry and vision science : official publication of the American Academy of Optometry. 2010 Apr; [PubMed PMID: 20160657]
Torti C,Povazay B,Hofer B,Unterhuber A,Carroll J,Ahnelt PK,Drexler W, Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Optics express. 2009 Oct 26; [PubMed PMID: 19997159]
Park SP,Chung JK,Greenstein V,Tsang SH,Chang S, A study of factors affecting the human cone photoreceptor density measured by adaptive optics scanning laser ophthalmoscope. Experimental eye research. 2013 Mar; [PubMed PMID: 23276813]
Tumahai P,Moureaux C,Meillat M,Debellemanière G,Flores M,Delbosc B,Saleh M, High-resolution imaging of photoreceptors in healthy human eyes using an adaptive optics retinal camera. Eye (London, England). 2018 Nov; [PubMed PMID: 29993035]
Bergeles C,Dubis AM,Davidson B,Kasilian M,Kalitzeos A,Carroll J,Dubra A,Michaelides M,Ourselin S, Unsupervised identification of cone photoreceptors in non-confocal adaptive optics scanning light ophthalmoscope images. Biomedical optics express. 2017 Jun 1; [PubMed PMID: 28663928]
Roorda A,Williams DR, The arrangement of the three cone classes in the living human eye. Nature. 1999 Feb 11; [PubMed PMID: 10028967]
Duncan JL,Roorda A, Dysflective Cones. Advances in experimental medicine and biology. 2019 [PubMed PMID: 31884601]
Level 3 (low-level) evidenceAlpern M,Ching CC,Kitahara K, The directional sensitivity of retinal rods. The Journal of physiology. 1983 Oct; [PubMed PMID: 6644624]
Roorda A,Zhang Y,Duncan JL, High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Investigative ophthalmology [PubMed PMID: 17460294]
Level 3 (low-level) evidenceMorgan JI,Dubra A,Wolfe R,Merigan WH,Williams DR, In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Investigative ophthalmology [PubMed PMID: 18952914]
Level 3 (low-level) evidenceRossi EA,Granger CE,Sharma R,Yang Q,Saito K,Schwarz C,Walters S,Nozato K,Zhang J,Kawakami T,Fischer W,Latchney LR,Hunter JJ,Chung MM,Williams DR, Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proceedings of the National Academy of Sciences of the United States of America. 2017 Jan 17; [PubMed PMID: 28049835]
Liu Z,Kurokawa K,Zhang F,Lee JJ,Miller DT, Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proceedings of the National Academy of Sciences of the United States of America. 2017 Nov 28; [PubMed PMID: 29138314]
Ivers KM,Li C,Patel N,Sredar N,Luo X,Queener H,Harwerth RS,Porter J, Reproducibility of measuring lamina cribrosa pore geometry in human and nonhuman primates with in vivo adaptive optics imaging. Investigative ophthalmology [PubMed PMID: 21546533]
Level 3 (low-level) evidenceBurns SA,Elsner AE,Chui TY,Vannasdale DA Jr,Clark CA,Gast TJ,Malinovsky VE,Phan AD, In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomedical optics express. 2014 Mar 1; [PubMed PMID: 24688827]
Roorda A,Romero-Borja F,Donnelly Iii W,Queener H,Hebert T,Campbell M, Adaptive optics scanning laser ophthalmoscopy. Optics express. 2002 May 6; [PubMed PMID: 19436374]
Lombardo M,Parravano M,Lombardo G,Varano M,Boccassini B,Stirpe M,Serrao S, Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina (Philadelphia, Pa.). 2014 Mar; [PubMed PMID: 23928676]
Level 2 (mid-level) evidenceLombardo M,Parravano M,Serrao S,Ducoli P,Stirpe M,Lombardo G, Analysis of retinal capillaries in patients with type 1 diabetes and nonproliferative diabetic retinopathy using adaptive optics imaging. Retina (Philadelphia, Pa.). 2013 Sep; [PubMed PMID: 23492950]
Nakatake S,Murakami Y,Funatsu J,Koyanagi Y,Akiyama M,Momozawa Y,Ishibashi T,Sonoda KH,Ikeda Y, Early detection of cone photoreceptor cell loss in retinitis pigmentosa using adaptive optics scanning laser ophthalmoscopy. Graefe [PubMed PMID: 30937533]
Talcott KE,Ratnam K,Sundquist SM,Lucero AS,Lujan BJ,Tao W,Porco TC,Roorda A,Duncan JL, Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Investigative ophthalmology [PubMed PMID: 21087953]
Song H,Rossi EA,Latchney L,Bessette A,Stone E,Hunter JJ,Williams DR,Chung M, Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA ophthalmology. 2015 Oct; [PubMed PMID: 26247787]
Nakanishi A,Ueno S,Hayashi T,Katagiri S,Ito Y,Kominami T,Fujinami K,Tsunoda K,Iwata T,Terasaki H, CHANGES OF CONE PHOTORECEPTOR MOSAIC IN AUTOSOMAL RECESSIVE BESTROPHINOPATHY. Retina (Philadelphia, Pa.). 2020 Jan; [PubMed PMID: 30308565]
Battu R,Akkali MC,Bhanushali D,Srinivasan P,Shetty R,Berendschot TT,Schouten JS,Webers CA, Adaptive optics imaging of the outer retinal tubules in Bietti [PubMed PMID: 26915747]
Gocho K,Akeo K,Itoh N,Kameya S,Hayashi T,Katagiri S,Gekka T,Ohkuma Y,Tsuneoka H,Takahashi H, High-Resolution Adaptive Optics Retinal Image Analysis at Early Stage Central Areolar Choroidal Dystrophy With PRPH2 Mutation. Ophthalmic surgery, lasers [PubMed PMID: 27977834]
Paques M,Meimon S,Rossant F,Rosenbaum D,Mrejen S,Sennlaub F,Grieve K, Adaptive optics ophthalmoscopy: Application to age-related macular degeneration and vascular diseases. Progress in retinal and eye research. 2018 Sep; [PubMed PMID: 30010022]
Wang MS,Sander B,Larsen M, Retinal atrophy in idiopathic central serous chorioretinopathy. American journal of ophthalmology. 2002 Jun; [PubMed PMID: 12036670]
Gerardy M,Yesilirmak N,Legras R,Behar-Cohen F,Bousquet E, CENTRAL SEROUS CHORIORETINOPATHY: High-Resolution Imaging of Asymptomatic Fellow Eyes Using Adaptive Optics Scanning Laser Ophthalmoscopy. Retina (Philadelphia, Pa.). 2022 Feb 1; [PubMed PMID: 34620798]
Ueda-Consolvo T,Oiwake T,Abe S,Nakamura T,Numata A,Hayashi A, Hydroxychloroquine [PubMed PMID: 34527374]
Stepien KE,Han DP,Schell J,Godara P,Rha J,Carroll J, Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic vision loss. Transactions of the American Ophthalmological Society. 2009 Dec; [PubMed PMID: 20126479]
Level 3 (low-level) evidenceReumueller A,Wassermann L,Salas M,Karantonis MG,Sacu S,Georgopoulos M,Drexler W,Pircher M,Pollreisz A,Schmidt-Erfurth U, Morphologic and Functional Assessment of Photoreceptors After Macula-Off Retinal Detachment With Adaptive-Optics OCT and Microperimetry. American journal of ophthalmology. 2020 Jun; [PubMed PMID: 31883465]
Nakamura T,Hayashi A,Oiwake T, Recovery of macular cone photoreceptors in Vogt-Koyanagi-Harada disease. Graefe [PubMed PMID: 29264653]
Agarwal A,Soliman MK,Hanout M,Sadiq MA,Sarwar S,Jack LS,Do DV,Nguyen QD,Sepah YJ, Adaptive Optics Imaging of Retinal Photoreceptors Overlying Lesions in White Dot Syndrome and its Functional Correlation. American journal of ophthalmology. 2015 Oct; [PubMed PMID: 26189087]
Zwillinger S,Paques M,Safran B,Baudouin C, In vivo characterization of lamina cribrosa pore morphology in primary open-angle glaucoma. Journal francais d [PubMed PMID: 26987895]
Hasegawa T,Ooto S,Takayama K,Makiyama Y,Akagi T,Ikeda HO,Nakanishi H,Suda K,Yamada H,Uji A,Yoshimura N, Cone Integrity in Glaucoma: An Adaptive-Optics Scanning Laser Ophthalmoscopy Study. American journal of ophthalmology. 2016 Nov; [PubMed PMID: 27565227]