Introduction
Tonometry involves diagnostic testing to measure the pressure inside the eye or intraocular pressure (IOP). Glaucoma is a silent disease that causes irreversible functional peripheral visual field loss that can ultimately lead to blindness in the very late stages of the disease if not treated. Tonometry should be performed during routine ophthalmic examinations to screen for glaucoma and other ocular diseases. IOP must be monitored periodically during the management of patients with glaucoma, ocular hypertension (OHT), and subjects at risk of developing glaucoma.
Normal IOP measures in the range of 10 to 21 (mmHg), which is based on average IOP levels in populations surveys in normal subjects, and less than 2 % of normal subjects show IOP greater than 21 mmHg.[1]
Possible causes for an IOP under normal rages (hypotonus) include uveitis, ocular traumas, retinal detachment, and post-surgery complications, especially after filtering surgery. Elevated IOP is normally caused by glaucoma. The definition of glaucoma in the past years has evolved from a disease solely defined by IOP>21 mmHg to include the assessments of functional and morphological defects.
The concept of determining and re-evaluating a personalized target IOP is currently an important issue in managing patients. These ranges in mmHg are associated with levels of IOP that are thought to cause a minimal likelihood of optic nerve damage or visual field loss, or progression of an existing lesion due to OHT.[2]
Treatment of OHT and glaucoma involves lowering IOP using local drop therapy, laser, and/or surgery. It is thus of utmost importance that instruments used to measure IOP are properly calibrated, accurate and precise, considering that the treatment options are based on IOP levels, together with visual field results, clinical evaluations, and morphological assessments of the optic nerve and retinal nerve fiber layer.
The true IOP inside the eyeball can be measured by inserting a probe in the anterior chamber to measure the manometric pressure. However, this invasive technique tends to be strictly used in animal models and can surely not be considered in a routine clinical setting.[3]
Numerous instruments and tonometers have been created since the 1800s to measure IOP, which have been designed to provide accurate, reliable, precise, and reproducible measurements of IOP. Each method has advantages, disadvantages, and limits and is more or less influenced by ocular factors, rendering some methods clinically acceptable and practical while others are obsolete.
Tonometers are based on different concepts and principles of physics that define how IOP levels are measured and what factors can theoretically influence these readings. The force needed to applanate, indent, and/or rebound the surface of the eye is used to estimate and calculate the IOP provided by the numerous tonometers used to date. It is important to note that IOP readings can be influenced by numerous factors based on each tonometer used.[4]
These factors can influence accuracy, precision, repeatability, measurement variability, and specificity. The factors that need to be considered include the amount of fluorescein, excessive tear production, corneal astigmatism, scarring, scleral rigidity, corneal edema, central corneal thickness, and arterial perfusion, central venous pressures, eye position, etc.[4]
Goldmann applanation tonometry (GAT) is currently the most widely accepted method used to measure IOP and is considered the gold standard tonometer in clinics.[5] GAT indirectly measures the IOP by assessing the force needed to flatten a predetermined surface area of the cornea. Taken simplistically, if the eyeball is hard, it takes more force to flatten the surface of the cornea, which is directly influenced by the IOP. GAT is based on the principles of applanation tonometry. Other instruments that have been built using the principles of applanation include the Perkins applanation tonometer, non-contact tonometers, and the Ocular Response tonometer (ORA).[6]
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Tonometry is routinely used in a clinical setting to measure IOP. Indications for applanation tonometry include:
- Periodic screening for glaucoma.[7]
- Diagnosis of OHT and all forms of glaucoma.[8]
- Management and follow-up assessments of IOP in patients with OHT and glaucoma.[9]
- Diagnosis, management, and differential diagnosis of numerous ophthalmologic conditions that cause elevated IOP (i.e., Posner-Schlossman syndrome, uveitis, a long-term steroid medication, pigment dispersion syndrome, etc.).[10][11]
- IOP assessment before and after surgery.[12]
- Diagnosis, management, and follow-up of patients with infection, trauma without globe rupture, inflammation, etc.[13]
Contraindications
There are several cases in which applanation should be avoided or limited due to the nature of the testing method, which includes:
- The various methods used in applanation tonometry involve contact of the cornea with the instrument tip or with a column of air. Thus open globe wounds and traumas are contraindications.
- Goldmann and Perkins's applanation tonometry involves administering fluorescein and local topical anesthesia. Patients who do not tolerate these substances should preferably be assessed for IOP with alternative tonometers that are either non-touch or do not require drops or fluorescein.[14][15]
- When performing tonometry, keratoconjunctivitis can be transmitted to the contralateral eye or other patients; thus, contact and air-puff tonometry need to be thoroughly disinfected or preferably avoided.[16]
- GAT and Perkins require assessing the rings formed from the prism in the tip after direct contact with the cornea; thus, patients with central corneal scarring, elevated irregular astigmatism, unhealed corneal abrasions, and/or corneal ulcers should avoid IOP measurements with these tonometers.[17]
- Most applanation tonometers (except for Perkins, which can be used in a supine position and is portable) require cooperative patients in an upright position. Children tend to have limited collaboration.[18] Bedridden and/or non-collaborative patients cannot undergo these types of tonometry.
Equipment
Applanation tonometry is based on the applanation principle described by Imbert and Fick in the late 1800s.[19][20] With regards to applanation tonometry, the law states that pressure (P) within a closed sphere, expressed in mmHg, can be approximated by the force (F) necessary to flatten the cornea in a fixed area, measured in grams, divided by the surface (S) of the flattened area, given in square millimeters. The Imbert-Fick law is defined as P=F/S.[21]
The first applanation tonometer was built by Adolph Weber in 1867, which was later improved and used in clinics in 1885 by Alexei Maklaloff.[22] A more modern version of this applanation tonometer was then later proposed by Posner-Inglima in 1967.[23]
Most of these early instruments did not gain widespread use because of several limitations, which included measurements subject to various errors, difficulties in using the instrument, non-practicability in a clinical setting, etc.
Hans Goldmann invented GAT in 1948, and to this date, it remains the gold standard technique for measuring IOP. Although the Imbert-Fick principle assumes that the sphere is thin-walled without rigidity and elasticity, Goldmann was convinced that corneal elastic properties and thickness were not significantly variable amongst individuals.[20]
Much later, numerous clinical studies showed that corneal properties, including thickness, elasticity, rigidity, and hysteresis, can influence IOP measurements with GAT.[24][25][26] A more complete version of the Imbert-Fick law includes the effect of the surface tension from the tear film (s) and the corneal resistance (b), giving rise to the formula P=F+s-b/S.[27][28]
Eyes with thick corneas tend to show overestimated GAT readings, while those with thin corneas give rise to underestimated IOP readings with GAT, in addition to being a risk for developing glaucoma.[29][30][31]
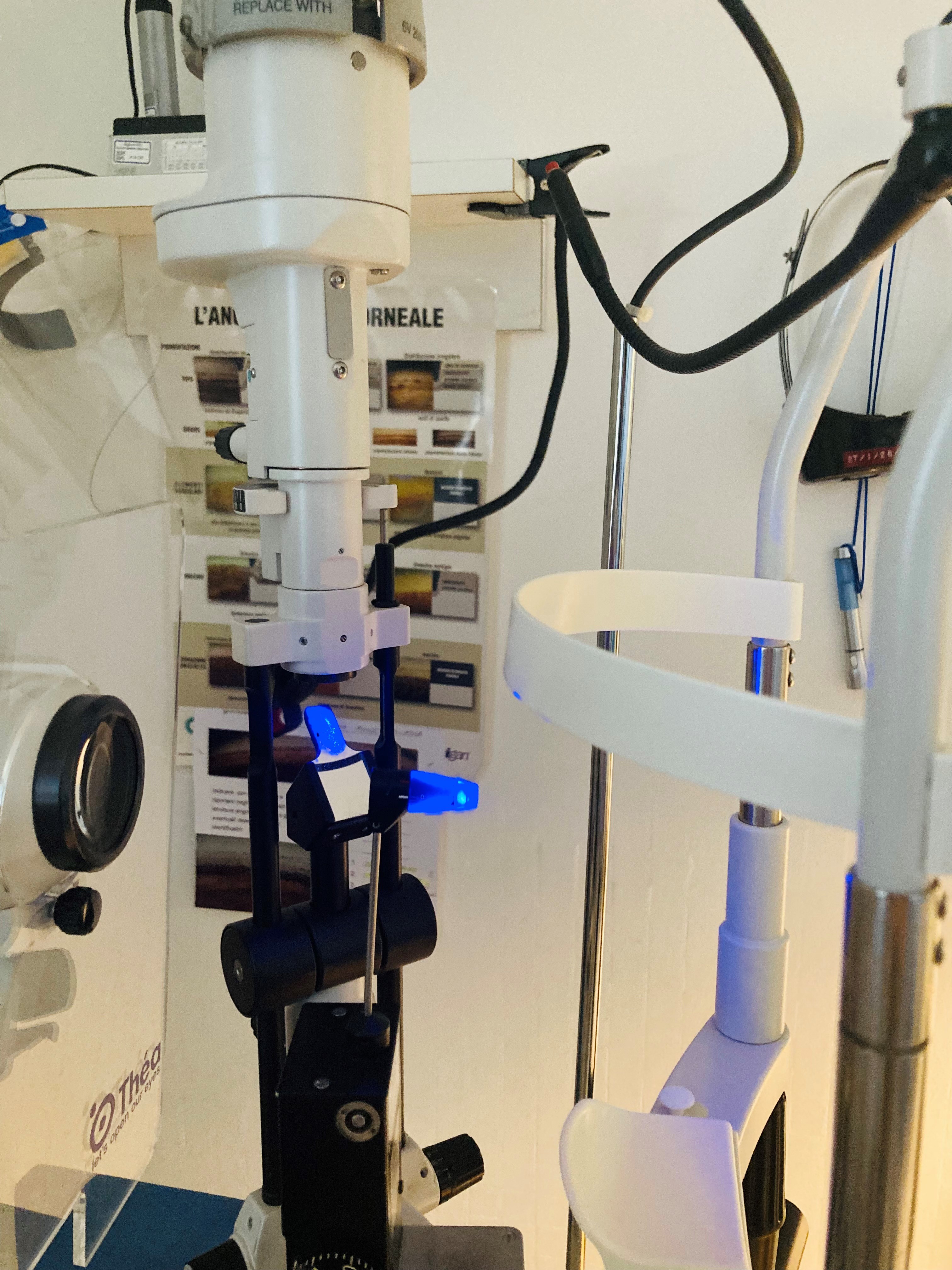
The Goldmann tonometer (See tonometer image) needs to be mounted on a slit lamp, and measurements are taken in an upright position (See slit-lamp image).
Applanation tonometry can be classified as either contact or non-contact. GAT and Perkins are considered as contact applanation tonometers. Air-puff tonometry and ocular response analyzer are defined as non-contact tonometry (NCT). These NCT instruments generate force by air as opposed to direct contact with the cornea and do not require fluorescein and local topical anesthesia.
Goldmann Applanation Tonometer (GAT)
The GAT instrument is composed of a small metal device weighing less than a kilogram, with a height of about 18 cm, a width of 10 cm, and a depth of 4 cm (See tonometer image). The tonometer is made up of a prism holder, a thin metal sensor arm, and a metal weight housing that has a measuring drum equipped with a rotating dial with a range of measurements from 0 to 80 mmHg (See tonometer with dial image).
The tonometer needs to be positioned on a slit-lamp (See slit-lamp image), which can either be permanently fastened and pivoted in front of the eye with a rotating swiveling arm or placed on a metal guided base-plate and used in the retractable version. The tonometer should be calibrated periodically with a control weight bar to ensure accurate measurements.
A truncated plastic cone with an embedded doubling measuring prism (See tip images) having a diameter of 7 mm is mounted on the tip of the rod and used to take the IOP measurements; thus, proper cleaning and disinfection are essential when using GAT. Tonosafe disposable prisms are currently available to limit cross-infection risks in patients.
Perkins Applanation Tonometer
The Perkins tonometer, first described by ES Perkins in 1965, uses the same principles as GAT with the advantage of being portable, thus can be used in patients that cannot be positioned in front of a slit-lamp or in a supine potion (i.e., bedridden subjects or patients under general anesthesia).[32]
The device is equipped with an adjustable forehead rest, prism holder for the same type of cone used in GAT, illumination source, viewing lens, milled thumb-wheel with a scale for IOP measurement, and a battery charging port for wireless use. The instrument, however, is rather difficult to use, has limited positioning stability during use, and has a high learning curve to obtain accurate and precise IOP measurements.
Air-Puff Tonometer (non-contact)
NCT was first proposed in the 1970s.[33] Non-contact air-puff tonometry uses a column of air emitted with increasing intensity to applanate the cornea to measure IOP. Sensors and light beams in the instrument are used to regulate the production of air, which is then halted when the cornea is flattened. The IOP measurements, which are based on the force needed to applanate the cornea, are taken by the waveforms of the reflected lights that are analyzed by the sensor detectors and converted by the internal algorithm of the instrument.[34]
There is no direct contact with the eye, and measuring prisms are not used; thus, anesthesia drops and fluorescein are not required. Several desktop tonometers exist, like the Pulsair model, while others are built-in together with refractometers and pachymeters. The Pulsair and Keeler Air-Puff instruments are also available in portable handheld models. The advantages of NCT include the ease of use and the fact that anesthesia, fluorescein, and slit-lamp are not required, making this type of instrument potentially useful in screening settings, in children and patients with limited collaboration, and administered by paramedical staff.[35][36][37]
The disadvantages, however, are that IOP measurements tend to be less accurate than those taken with GAT, giving rise to underestimated and overestimated IOP measurements in higher and lower levels of IOP, respectively.[38] Moreover, studies have shown that NCT measurements are instrument-dependent, greatly influenced by CCT, and offer limited sensitivity and accuracy compared to GAT.[39][40]
Ocular Response Analyzer (ORA, non-contact)
The ORA is a newer version of NCT proposed by Luce in 2005.[41][42] The dimensions of this countertop instrument are approximately 50 cm x 27 cm x 36 cm, and the weight is about 10 kilograms. The instrument is equipped with an interactive operating display on one side and a testing patient mode on the other side with a forehead rest, illuminating nosepiece, an objective for patient fixation and measurement, and an air tube that generates the air impulses.
The instrument uses air impulses to assess IOP and corneal biomechanics based on corneal deformation. The force of the column of air used in this NCT keeps increasing until the cornea is indented in an inward motion, then decreases slowly until the cornea is again flattened as it moves outwardly.[43]
Corneal elasticity or hysteresis is assessed based on the differences between the forces at the initial and rebound applanation points using internal mathematical calculations. The ORA provides two corneal parameters known as corneal hysteresis and corneal resistance factor, which theoretically provide information regarding the viscoelastic mechanical properties of the cornea.[41]
The ORA provides corneal compensated IOP measurements based on individual biomechanics results that have been reported to be less influenced by corneal properties and thickness measurements when compared to GAT; however, several studies have shown that ORA tends to overestimate IOP at elevated levels, and measurements are affected by corneal thickness and other properties.[44]
Future studies are needed to determine whether or not these new parameters provided by ORA will prove to be of clinical interest in the management of glaucoma, which remains debatable and of little use in current routine practice.
Technique or Treatment
Contact tonometry with GAT and Perkins tonometers involves direct contact with the cornea (Figure 4). The process of measuring IOP according to the Imbert-Fick principle involves the applanation of the anterior central portion of the cornea. GAT and Perkins utilize the same system to measure IOP, differing only in that GAT requires a slit-lamp (See slit-lamp image), while Perkins is portable and can also be taken in a supine position.
In performing contact applanation tonometry, local anesthesia drops and fluorescein dye must be applied to the eyes. The tonometer tip with the split-image prism (See tip images) is gently applied to the surface of the central portion of the cornea. The tonometer measures the force needed the flatten the cornea in a central diameter of 3.06 mm.[20]
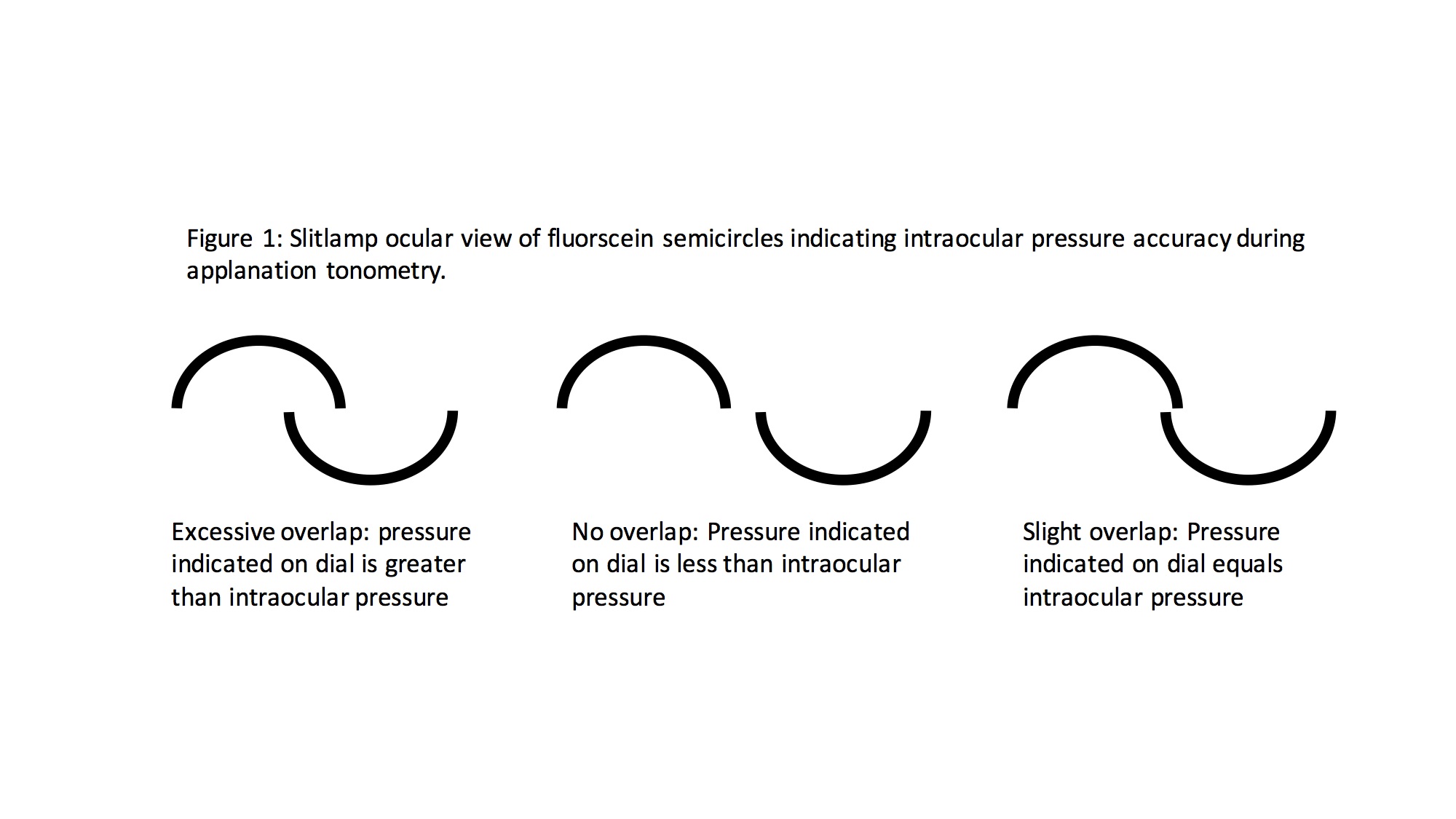
Measurements are taken using a filtered cobalt blue light, which highlights the borders of the semicircles formed by the tip prism (See tip images). The clinician calibrates the force needed on the eye using the rotating dial of the tonometer (See tonometer with dial image) until the superior and inferior arcs align so that the inner margins just touch (See images with arcs). The IOP is measured in mmHg.
Although contact applanation tonometry is considered the gold standard, the disadvantages of GAT include the need for a slit-lamp, upright position of the patient, installation of anesthesia and fluorescein, skilled ability of the clinician, and decreased accuracy in scarred or irregular corneas. Perkins tonometry shows the same disadvantages as GAT, except that it is portable and can be used in a supine position.
Various factors can affect the precision and accuracy of applanation tonometers, which include central corneal thickness, corneal hysteresis, choroidal rigidity, tear film thickness, amount of fluorescein used, high-grade astigmatism, previous refractive surgery, corneal edema, etc.
With regards to NCT, the technique for measuring IOP is rather simple. It involves correctly positioning the patient in front of the instrument and activating the air puff to measure IOP. The force needed to flatten the cornea by the impulse of air is then converted into IOP measurements by the internal software of the tonometers.
The advantages of NCT, when compared to GAT and Perkins, include the fact that it is rapid, simple to perform, and does not require the use of local anesthesia drops and/or fluorescein. NCT can be an interesting tool in select patients and screening; however, it is not recommended in the routine clinical setting, especially in managing patients with elevated IOP and glaucoma.
Complications
Applanation tonometry, especially contact tonometers like GAT and Perkins that involve direct applanation with the cornea, should be avoided or limited to select cases in the presence of eyes with abrasion, severe trauma and/or globe rupture, signs of infection, and intolerance to fluorescein and/or local anesthesia drops.
Clinical Significance
IOP measurements are of utmost importance in diagnosing, screening, and managing patients with glaucoma. Treatment with local drops, laser, and/or surgery to avoid the onset and worsening of glaucomatous optic neuropathy and visual field defect progression involves lowering IOP, which is the only risk factor that can be modified in glaucoma cases.
GAT is considered the gold standard in the routine clinical management of patients. Alternative methods of tonometry can be considered in select cases; however, routine clinical management to date remains IOP with GAT.
Enhancing Healthcare Team Outcomes
Goldmann applanation tonometry (GAT), which is the gold standard method in measuring IOP, is usually performed by the ophthalmologist and optometrist. Other applanation methods that do not require local anesthesia and fluorescein can also be carried out by nurses, ophthalmic technicians, or emergency medicine.
Proper training and communication are needed in tonometry when managing patients, especially considering that accurate IOP measurements at diagnosis and follow-up examinations are of utmost importance in determining therapeutic strategies.
GAT needs to be mounted on a slit lamp and tonometry performed on patients in an upright position. Topical anesthesia and fluorescein are used to obtain accurate measurements. Alternative methods can be considered when GAT may prove to be less suitable, which include: open globe wounds; patients that do not tolerate anesthesia drops or fluorescein; eyes with keratoconjunctivitis or central corneal scarring, elevated irregular astigmatism, unhealed corneal abrasions, and/or corneal ulcers. Alternative tonometers may be used in bedridden and non-collaborative.
Tonometry readings should be accurate and precise, so choosing the correct instrument to measure IOP when managing patients is crucial. Glaucoma and other ophthalmic conditions can give rise to irreversible vision loss and reduced peripheral vision, which is why IOP needs to be properly assessed at periodic examinations to provide thorough management of patients.[45] [Level 1]
Nursing, Allied Health, and Interprofessional Team Interventions
The nursing, allied health, and interprofessional staff help maintain tonometers. The nursing staff is also aware of the calibration and correct method of storage of the tonometers. They also help in the disposal and replacement of the prism of the tonometer.
Nursing, Allied Health, and Interprofessional Team Monitoring
The nursing, allied health, and interprofessional staff assist in monitoring the proper functioning of the tonometers. In case of any error, the maintenance department helps rectify the fault.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
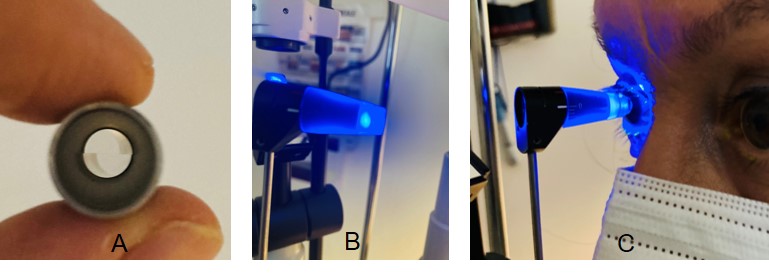
A tip with a split-image prism (A) is used with the Goldmann Applanation Tonometer (GAT), which is mounted on a slit-lamp (B). Filtered cobalt blue light is used during the measurement. The tip is positioned on the center of the eye to gently flatten the cornea (C). Contributed by Marco Zeppieri, MD, PhD.
(Click Image to Enlarge)
References
ALIMUDDIN M. Normal intra-ocular pressure. The British journal of ophthalmology. 1956 Jun:40(6):366-72 [PubMed PMID: 13355942]
Popović-Suić S, Sikić J, Vukojević N, Cerovski B, Nasić M, Pokupec R. Target intraocular pressure in the management of glaucoma. Collegium antropologicum. 2005:29 Suppl 1():149-51 [PubMed PMID: 16193700]
McAllister F, Harwerth R, Patel N. Assessing the True Intraocular Pressure in the Non-human Primate. Optometry and vision science : official publication of the American Academy of Optometry. 2018 Feb:95(2):113-119. doi: 10.1097/OPX.0000000000001171. Epub [PubMed PMID: 29370024]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27 [PubMed PMID: 34501306]
Okafor KC, Brandt JD. Measuring intraocular pressure. Current opinion in ophthalmology. 2015 Mar:26(2):103-9. doi: 10.1097/ICU.0000000000000129. Epub [PubMed PMID: 25594767]
Level 3 (low-level) evidenceKrieglstein GK, Waller WK. Goldmann applanation versus hand-applanation and .schiötz indentation tonometry. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1975:194(1):11-6 [PubMed PMID: 1079117]
Fan T, Do T. Screening for glaucoma. American family physician. 2014 Oct 15:90(8):569-70 [PubMed PMID: 25369645]
Level 3 (low-level) evidenceBlumberg MJ, Varikuti VNV, Weiner A. Real-world comparison between the Tonopen and Goldmann applanation tonometry in a university glaucoma clinic. International ophthalmology. 2021 May:41(5):1815-1825. doi: 10.1007/s10792-021-01742-z. Epub 2021 Mar 2 [PubMed PMID: 33651312]
Wachtl J, Töteberg-Harms M, Frimmel S, Roos M, Kniestedt C. Correlation Between Dynamic Contour Tonometry, Uncorrected and Corrected Goldmann Applanation Tonometry, and Stage of Glaucoma. JAMA ophthalmology. 2017 Jun 1:135(6):601-608. doi: 10.1001/jamaophthalmol.2017.1012. Epub [PubMed PMID: 28494071]
Kamel K, Dervan E, Falzon K, O'Brien C. Difference in intraocular pressure measurements between non-contact tonometry and Goldmann applanation tonometry and the role of central corneal thickness in affecting glaucoma referrals. Irish journal of medical science. 2019 Feb:188(1):321-325. doi: 10.1007/s11845-018-1795-0. Epub 2018 Apr 4 [PubMed PMID: 29616408]
Salvetat ML, Zeppieri M, Tosoni C, Brusini P. Repeatability and accuracy of applanation resonance tonometry in healthy subjects and patients with glaucoma. Acta ophthalmologica. 2014 Feb:92(1):e66-73. doi: 10.1111/aos.12209. Epub 2013 Jul 10 [PubMed PMID: 23837834]
Level 2 (mid-level) evidenceFuest M, Mamas N, Walter P, Plange N. Tonometry in corneal edema after cataract surgery: rebound versus goldmann applanation tonometry. Current eye research. 2014 Sep:39(9):902-7. doi: 10.3109/02713683.2014.888451. Epub 2014 Mar 3 [PubMed PMID: 24588266]
Level 3 (low-level) evidenceGoswami M, Bhattacharya S, Bandyopadhyay M. Ocular manifestation and visual outcomes in herpes zoster ophthalmicus: a prospective study from a tertiary hospital of Eastern India. International journal of ophthalmology. 2021:14(12):1950-1956. doi: 10.18240/ijo.2021.12.21. Epub 2021 Dec 18 [PubMed PMID: 34926213]
Demirci G,Erdur SK,Tanriverdi C,Gulkilik G,Ozsutçu M, Comparison of rebound tonometry and non-contact airpuff tonometry to Goldmann applanation tonometry. Therapeutic advances in ophthalmology. 2019 Jan-Dec [PubMed PMID: 30899901]
Level 3 (low-level) evidenceArend N, Hirneiss C, Kernt M. [Differences in the measurement results of Goldmann applanation tonometry with and without fluorescein]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2014 Mar:111(3):241-6. doi: 10.1007/s00347-013-2843-9. Epub [PubMed PMID: 23604252]
Level 1 (high-level) evidenceYousefi A, Ma Y, Roberts CJ, Moroi SE, Reilly MA. Hydrodynamic Interaction Between Tear Film and Air Puff From Noncontact Tonometry. Translational vision science & technology. 2022 Feb 1:11(2):2. doi: 10.1167/tvst.11.2.2. Epub [PubMed PMID: 35103798]
Stewart RM, Wishart PK, Kaye SB. Overestimation of Intraocular Pressure by Goldmann Applanation Tonometry Without Astigmatic Correction. JAMA ophthalmology. 2016 Mar:134(3):e153691. doi: 10.1001/jamaophthalmol.2015.3691. Epub 2016 Mar 10 [PubMed PMID: 26967752]
Esporcatte BL, Lopes FS, Fonseca Netto C, Rebouças-Santos V, Dias DT, Marujo FI, Rolim-de-Moura C. Rebound tonometry versus Goldmann tonometry in school children: feasibility and agreement of intraocular pressure measurements. Arquivos brasileiros de oftalmologia. 2015 Nov-Dec:78(6):359-62. doi: 10.5935/0004-2749.20150095. Epub [PubMed PMID: 26677038]
Level 2 (mid-level) evidenceMARKIEWITZ HH. The so-called Imbert-Fick law. Archives of ophthalmology (Chicago, Ill. : 1960). 1960 Jul:64():159 [PubMed PMID: 14421255]
Stamper RL. A history of intraocular pressure and its measurement. Optometry and vision science : official publication of the American Academy of Optometry. 2011 Jan:88(1):E16-28. doi: 10.1097/OPX.0b013e318205a4e7. Epub [PubMed PMID: 21150677]
Mark HH. Armand Imbert, Adolf Fick, and their tonometry law. Eye (London, England). 2012 Jan:26(1):13-6. doi: 10.1038/eye.2011.248. Epub 2011 Oct 21 [PubMed PMID: 22020170]
el-Golli M, Parizot H. [Maklakoff's tonometer]. Revue internationale du trachome. International review of trachoma. 1972:49(3):41-9 [PubMed PMID: 4660885]
Gloster J, Martin B. Evaluation of the Posner-Inglima applanometer. The British journal of ophthalmology. 1965 Dec:49(12):617-25 [PubMed PMID: 5853505]
Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. American journal of ophthalmology. 1993 May 15:115(5):592-6 [PubMed PMID: 8488910]
Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta ophthalmologica. 1975 Mar:53(1):34-43 [PubMed PMID: 1172910]
Level 3 (low-level) evidenceGunvant P, O'Leary DJ, Baskaran M, Broadway DC, Watkins RJ, Vijaya L. Evaluation of tonometric correction factors. Journal of glaucoma. 2005 Oct:14(5):337-43 [PubMed PMID: 16148580]
Lu SH, Chong IT, Leung SYY, Lam DCC. Characterization of Corneal Biomechanical Properties and Determination of Natural Intraocular Pressure Using CID-GAT. Translational vision science & technology. 2019 Sep:8(5):10. doi: 10.1167/tvst.8.5.10. Epub 2019 Sep 11 [PubMed PMID: 31579556]
Leung LK, Ko MW, Lam DC. Individual-specific tonometry on porcine eyes. Medical engineering & physics. 2014 Jan:36(1):96-101. doi: 10.1016/j.medengphy.2013.10.001. Epub 2013 Nov 5 [PubMed PMID: 24200347]
Level 3 (low-level) evidenceDoughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Survey of ophthalmology. 2000 Mar-Apr:44(5):367-408 [PubMed PMID: 10734239]
Level 3 (low-level) evidencePark SJ, Ang GS, Nicholas S, Wells AP. The effect of thin, thick, and normal corneas on Goldmann intraocular pressure measurements and correction formulae in individual eyes. Ophthalmology. 2012 Mar:119(3):443-9. doi: 10.1016/j.ophtha.2011.07.058. Epub 2011 Oct 27 [PubMed PMID: 22035576]
Level 2 (mid-level) evidenceBrandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology. 2001 Oct:108(10):1779-88 [PubMed PMID: 11581049]
Level 2 (mid-level) evidenceArora R, Bellamy H, Austin M. Applanation tonometry: a comparison of the Perkins handheld and Goldmann slit lamp-mounted methods. Clinical ophthalmology (Auckland, N.Z.). 2014:8():605-10. doi: 10.2147/OPTH.S53544. Epub 2014 Mar 26 [PubMed PMID: 24707165]
Grolman B. A new tonometer system. American journal of optometry and archives of American Academy of Optometry. 1972 Aug:49(8):646-60 [PubMed PMID: 4506671]
Level 3 (low-level) evidenceAziz K, Friedman DS. Tonometers-which one should I use? Eye (London, England). 2018 May:32(5):931-937. doi: 10.1038/s41433-018-0040-4. Epub 2018 Feb 19 [PubMed PMID: 29456251]
Parker VA, Herrtage J, Sarkies NJ. Clinical comparison of the Keeler Pulsair 3000 with Goldmann applanation tonometry. The British journal of ophthalmology. 2001 Nov:85(11):1303-4 [PubMed PMID: 11673293]
Level 1 (high-level) evidenceJorge J, Díaz-Rey JA, González-Méijome JM, Almeida JB, Parafita MA. Clinical performance of the Reichert AT550: a new non-contact tonometer. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2002 Nov:22(6):560-4 [PubMed PMID: 12477021]
Level 1 (high-level) evidenceJorge J, González-Méijome JM, Díaz-Rey JA, Almeida JB, Ribeiro P, Parafita MA. Clinical performance of non-contact tonometry by Reichert AT550 in glaucomatous patients. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2003 Nov:23(6):503-6 [PubMed PMID: 14622352]
Tonnu PA, Ho T, Sharma K, White E, Bunce C, Garway-Heath D. A comparison of four methods of tonometry: method agreement and interobserver variability. The British journal of ophthalmology. 2005 Jul:89(7):847-50 [PubMed PMID: 15965164]
Level 1 (high-level) evidenceMoseley MJ, Evans NM, Fielder AR. Comparison of a new non-contact tonometer with Goldmann applanation. Eye (London, England). 1989:3 ( Pt 3)():332-7 [PubMed PMID: 2612679]
Farhood QK. Comparative evaluation of intraocular pressure with an air-puff tonometer versus a Goldmann applanation tonometer. Clinical ophthalmology (Auckland, N.Z.). 2013:7():23-7. doi: 10.2147/OPTH.S38418. Epub 2012 Dec 27 [PubMed PMID: 23293511]
Level 2 (mid-level) evidenceLuce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. Journal of cataract and refractive surgery. 2005 Jan:31(1):156-62 [PubMed PMID: 15721708]
Lau W, Pye D. A clinical description of Ocular Response Analyzer measurements. Investigative ophthalmology & visual science. 2011 May 2:52(6):2911-6. doi: 10.1167/iovs.10-6763. Epub 2011 May 2 [PubMed PMID: 21273535]
Qin X, Tian L, Zhang H, Zhang D, Jie Y, Zhang HX, Li L. Determine Corneal Biomechanical Parameters by Finite Element Simulation and Parametric Analysis Based on ORA Measurements. Frontiers in bioengineering and biotechnology. 2022:10():862947. doi: 10.3389/fbioe.2022.862947. Epub 2022 Apr 13 [PubMed PMID: 35497338]
Level 2 (mid-level) evidenceMartinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, Mendez-Hernandez C, Garcia-Sanchez J. Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Investigative ophthalmology & visual science. 2006 Oct:47(10):4410-4 [PubMed PMID: 17003433]
Level 2 (mid-level) evidenceWormald R. Treatment of raised intraocular pressure and prevention of glaucoma. BMJ (Clinical research ed.). 2003 Apr 5:326(7392):723-4 [PubMed PMID: 12676823]