Introduction
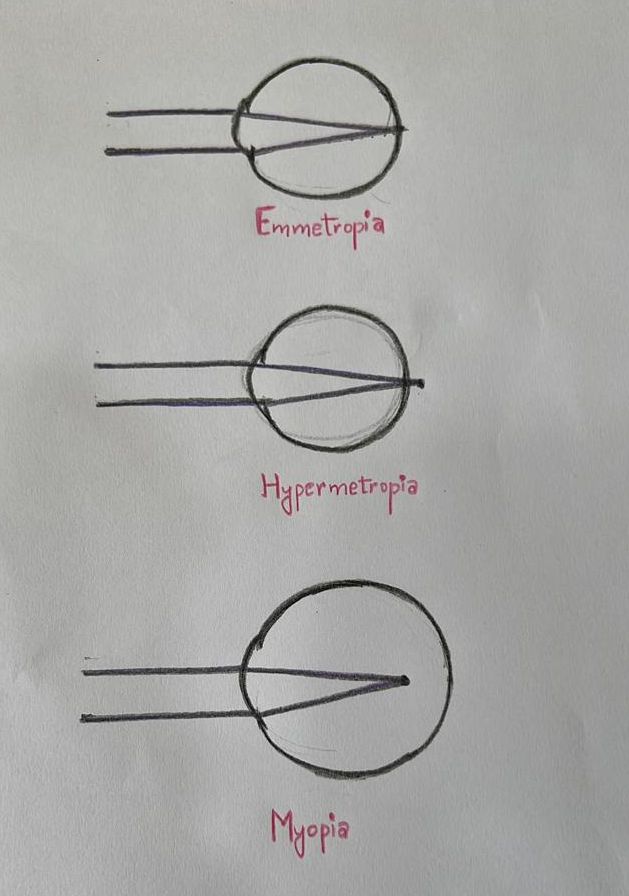
Biometry is the process of measuring the power of the cornea (keratometry or Km), the axial length (AL) of the eye, and other parameters to calculate the ideal intraocular lens (IOL) power.[1] The average normal axial length is 23.5 (22 to 24.5) mm.[2] The axial length is shorter than average in hyperopic eyes and longer than average in myopic eyes (see Image. Refractive Conditions Ray Diagram).[3] An axial length measurement error of 1 mm leads to a 2.5 (2-3) Diopter (D) change in IOL power, though the shift in IOL power is around 3.75 D in the shorter eyes.[4] The postoperative refractive error changes around 2.88 D per 1 mm change in AL.[5] Given the correction to corneal power, this procedure is highly relevant following cataract surgery, which is typically performed by ophthalmologists.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Principle of Ultrasound Biometry
Optical biometry has gained popularity, but ultrasound remained the gold standard for years.[6][7] Ultrasonography is based on the concept of acoustic waves. A piezoelectric crystal embedded in the probe oscillates to generate a high-frequency sound wave penetrating the eye. The probe captures the sound waves reflected by the ocular tissues to calculate the eye's axial length from the sound velocity and time needed to capture the returned sound waves.[8] This gives a one-dimensional amplitude representation of echoes along the path. Most ophthalmic ultrasonic A-scan machines have a frequency of 10 MHz (megahertz).
Methods of Ultrasound Biometry
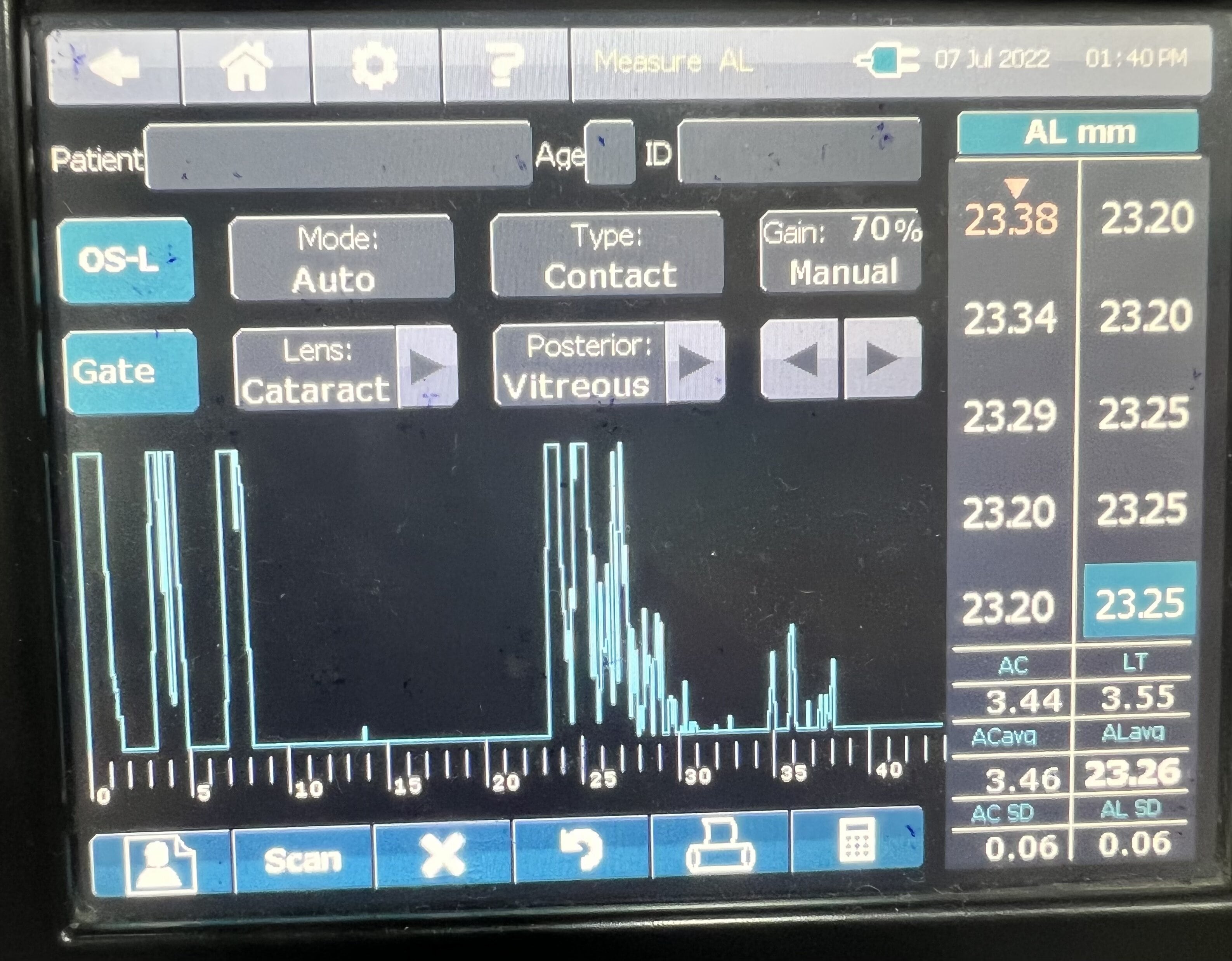
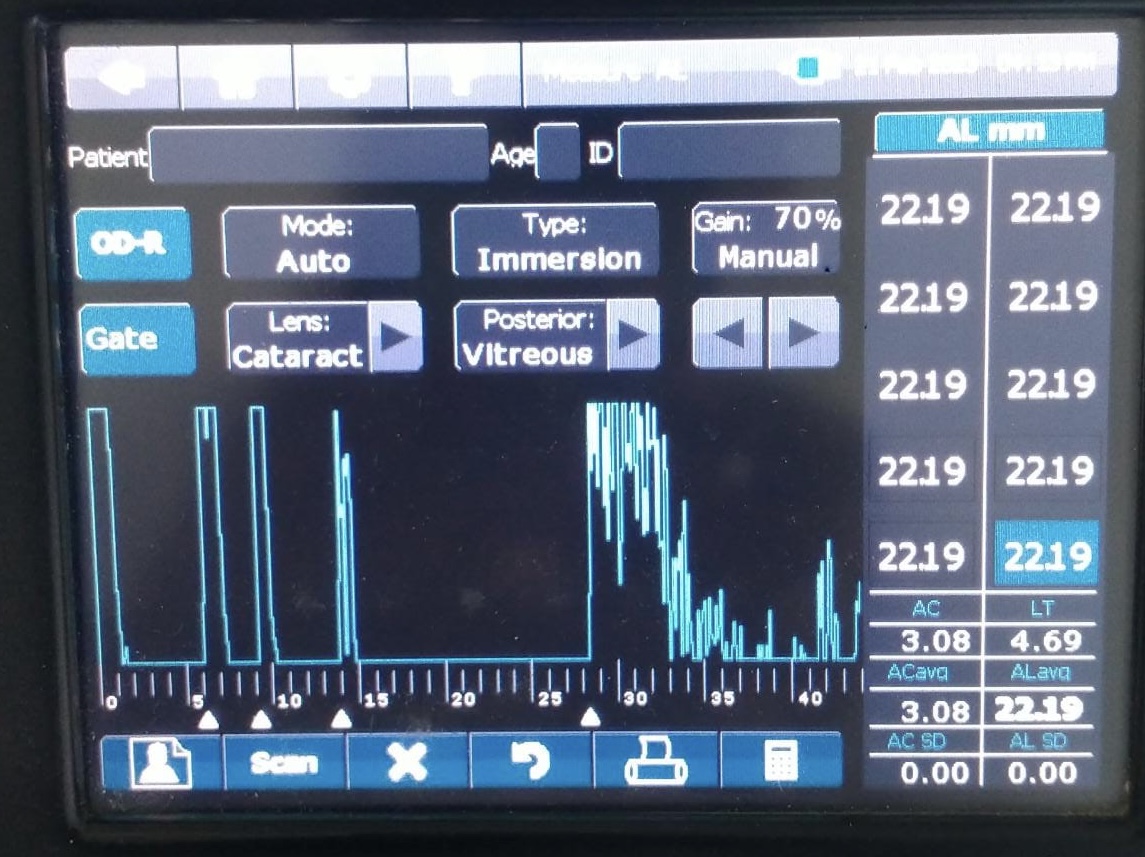
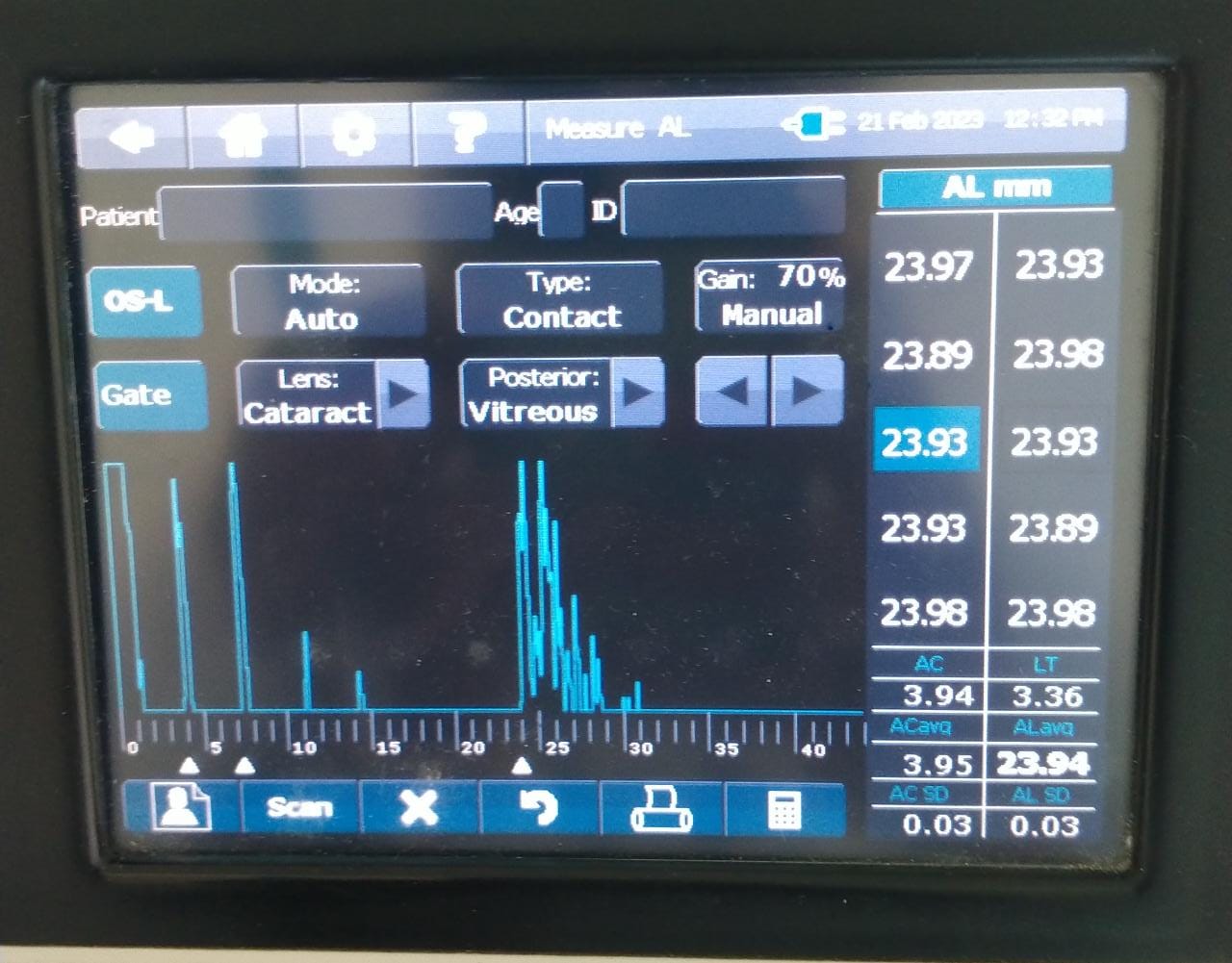
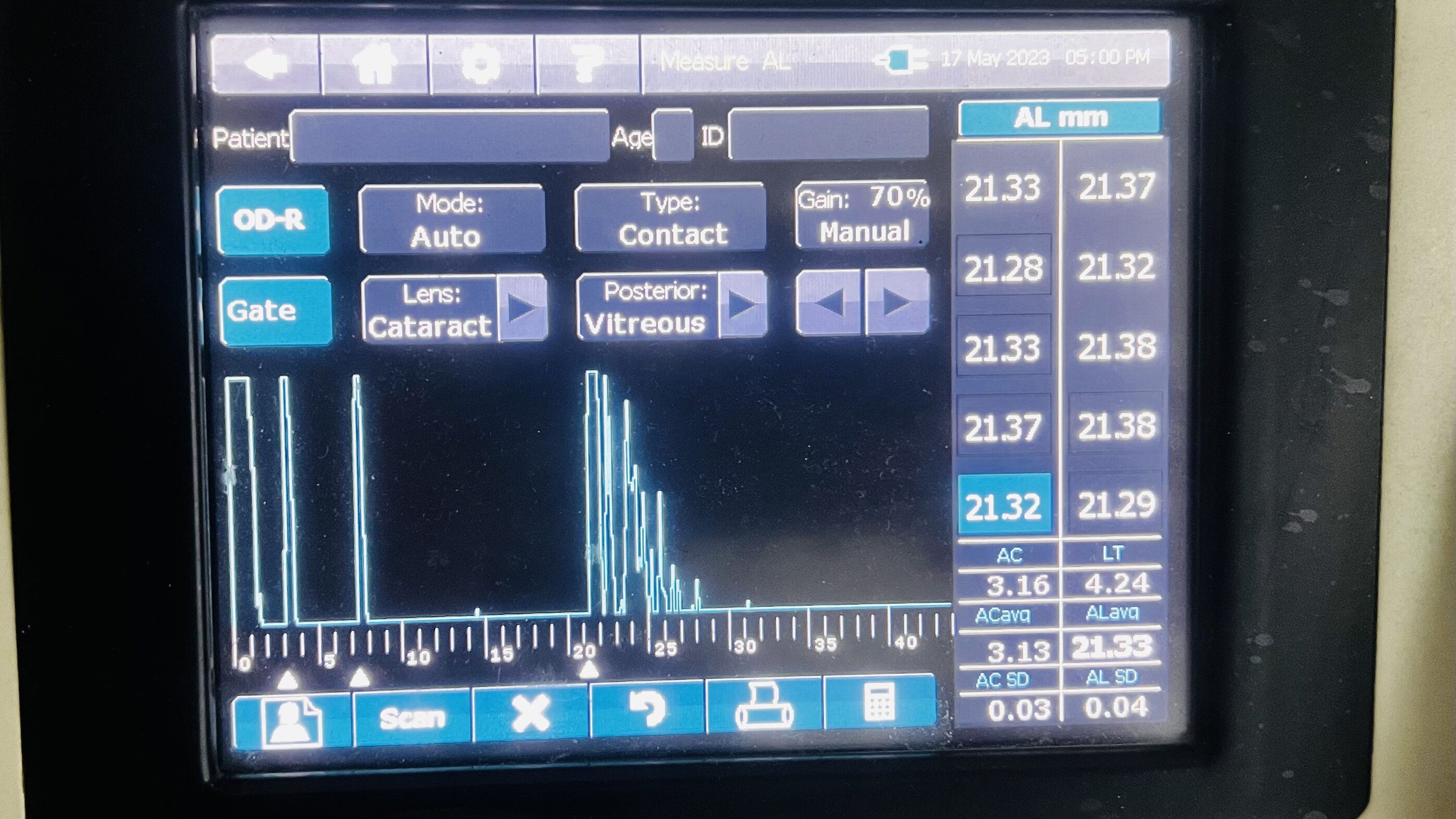
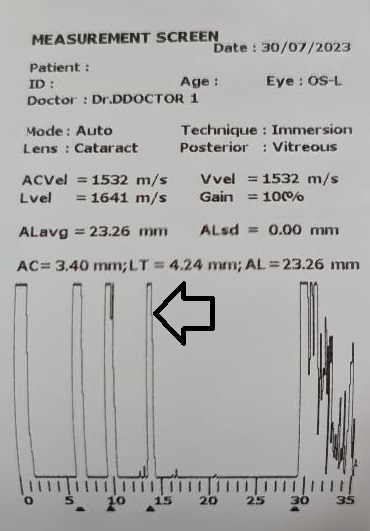
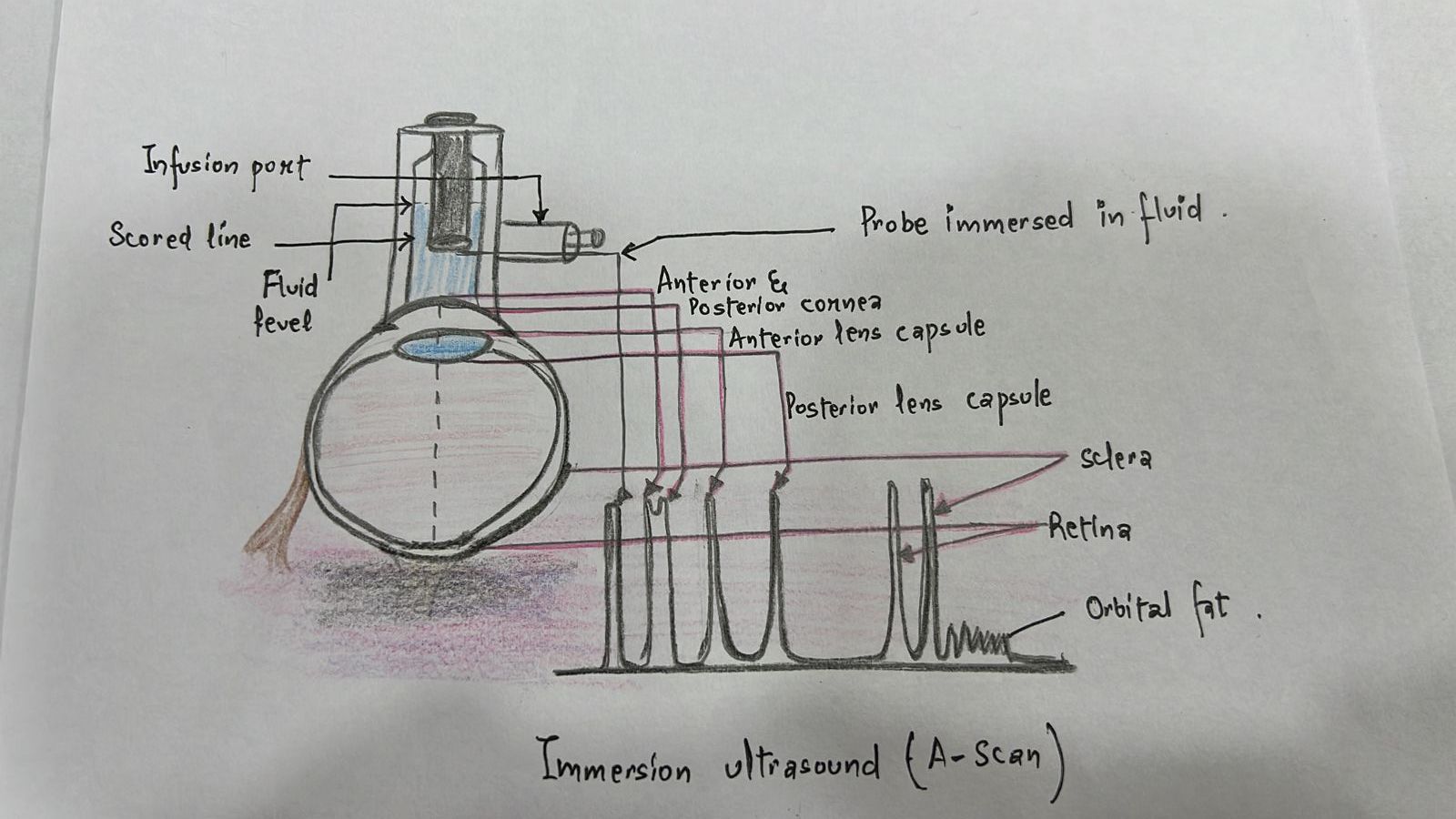
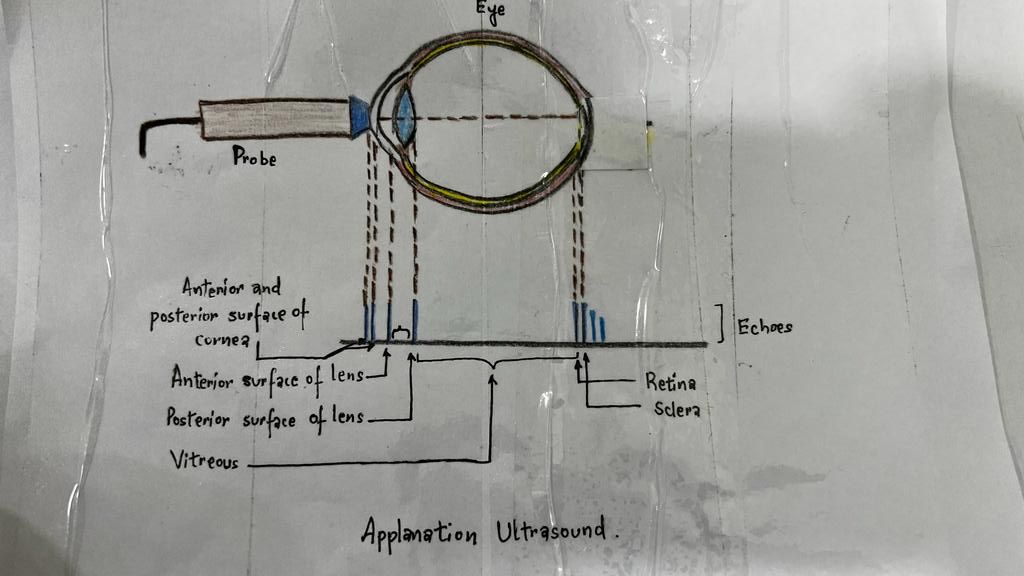
Ultrasound biometry is performed by applanation or contact (with probe touching the cornea) or by immersion technique (using saline-filled shells so that the A-scan probe does not directly touch the cornea)(See Images. Contact Ultrasound Biometry and Ultrasound Biometry Schematic Diagram).[7] The applanation amplitude-scan or A-scan doesn't require a coupling agent as the tear film is the coupling agent between the probe and the cornea. A-scan utilizes the single sound beam sent from a transducer to the eye while the patient fixates with the eye in primary gaze. The probe is placed directly over the cornea in applanation A-scan (see Images. Axial Scan and Contact Ultrasound Biometry).[9] In immersion A-scan, a scleral shell filled with saline is used. Thus, saline is between the probe and the cornea (see Images. Immersion A-scan, Ultrasound Biometry Printout, and Ultrasound Biometry Schematic Diagram). Pediatric cataracts can be measured using contact-type ultrasound biometry or immersion ultrasound biometry. Results from immersion ultrasound biometry are more predictable than contact or applanation ultrasound biometry.[10]
Ideal A-Scan
Ideal axial length measurements or A-scans comprise 3 high-quality readings with no more than 0.1 mm of variation between each other.[9] The A-scan should have steep rising spikes (with no breaks) of the lens and the retina.[8] An ideal A-scan in a phakic eye has spikes for the anterior and posterior surface of the cornea, anterior lens capsule, posterior lens capsule, and retina (see Image. Hyperopic Eye). There should be a scleral echo around 1.5 to 2 mm behind the retinal spike, suggesting that the ultrasound beam was directed toward the macula (see Image. Hyperopic Eye). When the ultrasound beam hits the optic nerve, no scleral spike is seen behind the presumed retinal spike (actually optic nerve spike), and this A-scan is ineffective [11] (see Image. Poor quality A-scan). The vitreous cavity may have echoes due to vitreous opacities or vitreous hemorrhage, which may not necessarily reduce the reliability of the A-scan.[12] The horizontal distance between the registered echoes is equivalent to the depth of the tissue from where the sound wave is reflected.[8]
The Velocity of Sound in Various Ocular Media
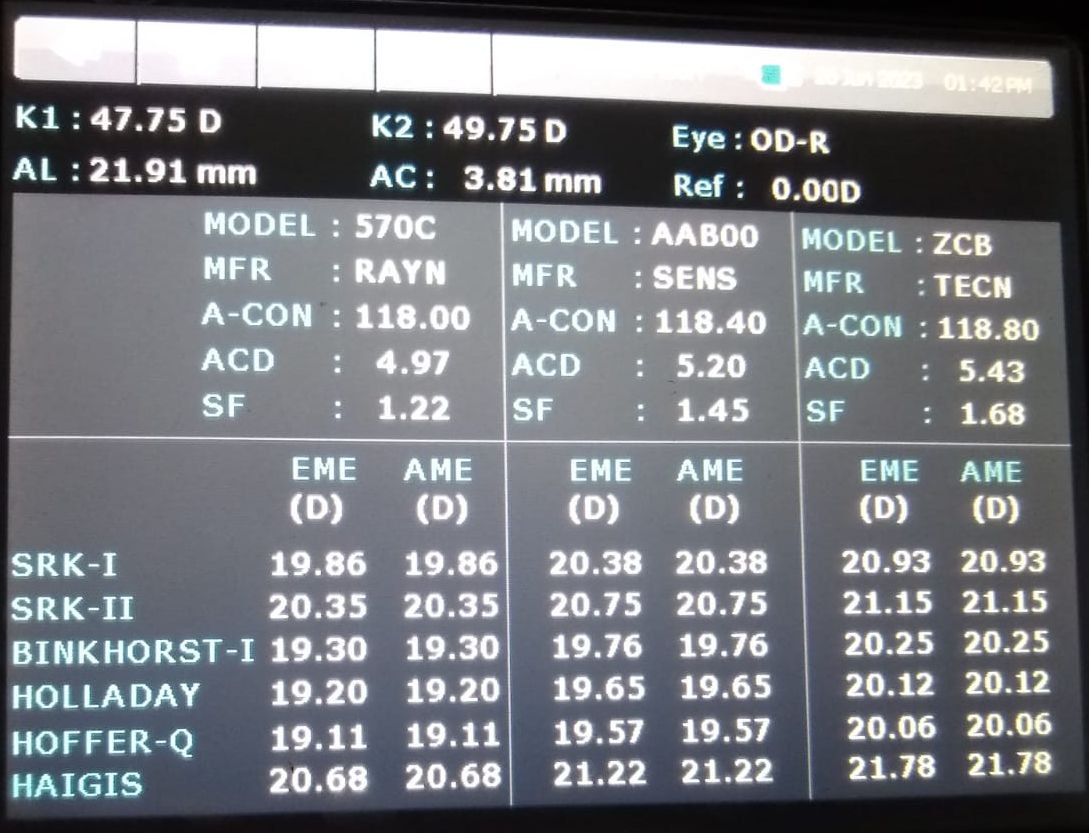
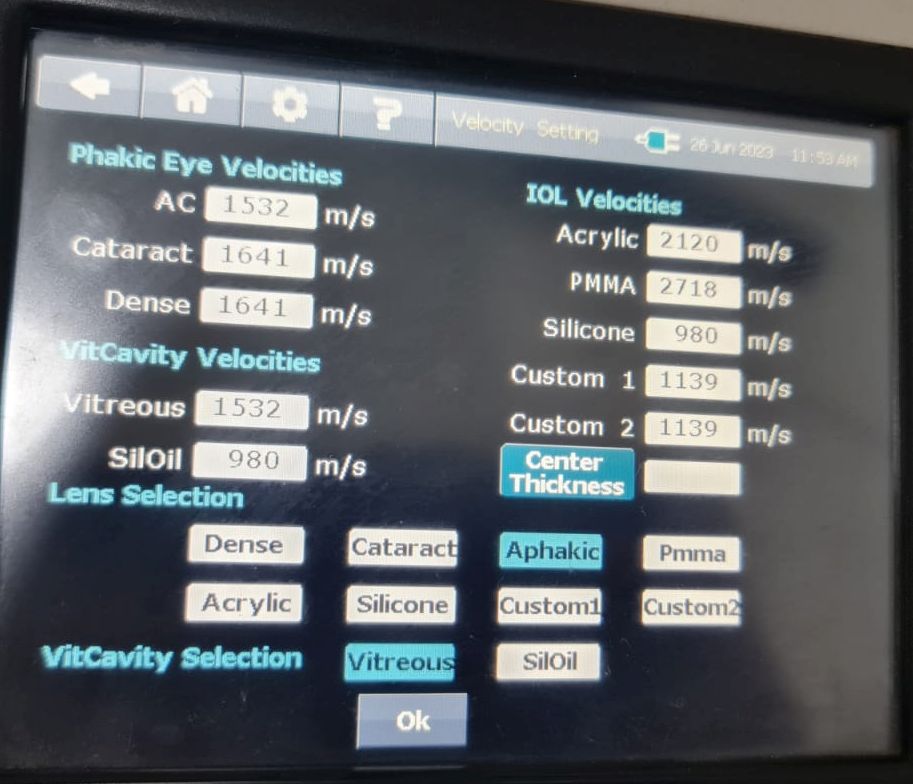
Ultrasonographically, the eye is divided into 4 components: cornea, anterior chamber, lens thickness, and vitreous cavity, and the velocity of sound waves is 1641,1532,1641,1532 m/s respectively (see Images. Ultrasonic Biometry Settings).[13] The average velocity in normal phakic eyes is 1555 m/s. The velocity of sound is 980 m/s in silicone oil for 1000 centistokes and 1040 m/s for 5000 centistokes,[14] 2718 m/s for polymethylmethacrylate,[15] and 2120 m/s for acrylic.[16] In eyes with silicone oil as a tamponade, there is an apparent increase in the axial length because the sound velocity is slower in silicone oil than in vitreous humor. The setting of the A-scan can be changed to select the vitreous cavity as silicone oil in such cases (see Image. Ultrasonic Biometry Settings).
Comparison of Ultrasonic Biometry and Optical Biometry
Comparisons of ultrasound and optical biometry have reported equal or better results with optical biometry, especially in eyes with silicone oil or posterior staphyloma.[17][7][18][19] Principles of measurement are different for ultrasound biometry, where the length of the eye depends upon the signal reflected from the apex of the cornea to the internal limiting membrane.[20] Optical biometry is one of the mainstay elements in biometry, especially supplanting ultrasound biometry in cataract assessment over the past 20 years.[18][21]
Two techniques are widely utilized in optical biometry:
- Laser partial coherence interferometry (PCI) and
- Optical low-coherence reflectometry (OLCR).[22][23]
PCI utilizes the 780 nm (IOL Master 500, Zeiss) infrared diode laser.[24] Interference will occur if the path difference between the beams is smaller than the coherence length, and the photodetector will detect this interference signal. Because the position of the interferometer mirror is known, the machine can measure the optical length between the corneal surface and the retina. It can then derive the geometric intraocular distance based on the refractive indices of the ocular media (cornea, aqueous, lens, vitreous humor), with a resulting resolution of 12 µ.[25][21] Optical biometry using IOL Master 500 measures the axial length, anterior chamber depth (from corneal epithelium), white-to-white diameter, and keratometry. Axial length is measured using the principle of dual-based partial coherence interferometry. The optical path length from the anterior cornea to the retinal pigment epithelium (RPE) is measured by reflecting infrared laser light from tissue interfaces.[26] This technique has inherent limitations based on using wavelengths in the electromagnetic spectrum. Data quality is diminished in cases with dense or posterior subcapsular cataracts; this can be quantified as signal-to-noise (SNR).[18] If the SNR value is >2, there is a minimum difference between optical and ultrasound biometry.[27] The IOL master 500 printout gives the patient's details, identification number, date of examination, date of birth, surgeon name, IOL power calculation formulas, axial length, keratometry, anterior chamber depth, refraction, and lens status. Other instruments utilizing PCI include Galilei G6 and Nidek AL Scan.
OLCR utilizes the 820 nm superluminescent diode in a standard Michelson interferometer setup (Lenstar, Haag Streit). In this biometer, the optical path-length measurements are aligned on the visual axis of the patient’s eye, and a specialized rotating glass system embedded in the machine changes the optical path length. Thus, it measures the axial length, corneal curvature, central corneal thickness, lens thickness, retinal thickness, anterior chamber depth, white-to-white diameter, the center of the visual axis to the center of the cornea, and pupil diameter.[28] Furthermore, the OLCR, since it measures the anatomic length of the eye, makes it a lengthy procedure compared to PCI-based devices. Anterior chamber depth measurement also varies as it measures from the corneal endothelium to lens epithelium compared to PCI, which measures from the corneal epithelium.[29] The Lenstar LS900 uses optical low-coherence reflectometry with an 820-nm superluminescent diode to measure optical path length.[30] The optical path length is converted to geometric path length using a single refractive index for the whole eye.[31] A single refractive index may be suboptimal for the whole eye as different eye segments have variable refractive indices. Instruments utilizing the OLCR include Haag Streit LenStar LS900 and Topcon Aladdin.
The Argos uses swept-source optical coherence tomography using 1060-nm wavelength and 20-nm bandwidth swept-source technology to collect two-dimensional eye data.[32] Instruments utilizing swept-source OCT are Zeiss IOL Master 700, Argos-Movu, OA-2000 by Tomey, and Anterion by Heidelberg Engineering.
Differences Between Ultrasound Biometry and Optical Biometry
| Ultrasound Biometry | Optical Biometry | |
| Principle |
Time sound waves across the eye are measured using an ultrasound transducer. The measured velocity is used to calculate the distance value. |
It utilizes one of the 3 principles: 1. Partial coherence interferometry 2. Optical low coherence reflectometry 3. Swept source optical coherence tomography |
| Signal transmission | Ultrasound waves | Laser |
| Measurement | From corneal apex to internal limiting membrane | From corneal apex to the retinal pigment epithelium |
| Contact procedure | Contact [applanation or immersion] | Non-contact |
| Accuracy |
Less resolution Approximately 0.10-0.12 mm |
More accurate and better resolution Approximately 0.012 mm |
| Dense PSC (posterior subcapsular cataract) or white cataract | Accurate | Accurate in swept-source optical coherence tomographyIn white cataracts, optical biometry cannot be used. |
| Laser | None |
Infrared diode (780 nm) -IOL master 500 830 nm- AL Scan 880 nm- Galilei G6
Superluminescent diode (820 nm)-LenStar, Aladdin 1055 nm- IOL Master 700 1300 nm- Anterion |
| Pediatric cataract | Used | Depends upon the age of the child. |
| Poor fixation | Possible |
Not possible. Possible with swept-source OCT. |
Indications
Precise biometry is essential for accurate outcomes in cataract and refractive surgeries, including phakic intraocular lenses.[33] Biometry helps determine the correct IOL power in cataract surgery. The refractive outcomes of cataract surgery using partial coherence interferometry and ultrasound immersion biometry are comparable.[34] Biometry helps determine IOL power by measuring the axial length, anterior chamber depth, and keratometry values. It helps determine IOL power after post-refractive surgery.[35][21]
Ultrasonic biometry has an additional role in dense cataracts (white cataracts, dense posterior subcapsular cataracts)[36], where the axial length cannot be measured due to the electromagnetic spectrum used in partial coherence interferometry.[37] In corneal edema, scarring, and dense cataracts, ultrasound biometry can measure the axial length and calculate the IOL power using higher gain settings.[1] Axial length measurement is important in determining the IOL power in pediatric populations. An error of 1 mm will lead to a 2.5 D change in IOL power in normal eyes, while it jumps to 3.75 D in small eyes with axial length <20 mm.[4] Axial length measurement using immersion-type ultrasound is most commonly accepted in the pediatric population.[10]
Studies based on preoperative and post-operative ultrasound biometry predicted that 54% of errors in predicted refraction are due to inaccurate axial length measurement, 8% to inaccurate keratometry, and 38% to inaccurate anterior chamber depth measurement.[38] The gating function of the ultrasound biometry machine may be used when the automatic detection of the spikes is faulty. Although the aim of cataract surgery is emmetropia, the standard for refractive outcome after cataract surgery of less or equal to 1 D is accepted by the Royal College of Ophthalmologists.[39] In ocular oncology, A-scan is used as an additional diagnostic tool.[40]
Contraindications
The contraindications of ultrasound biometry include active ocular infection (including but not limited to corneal ulcer and conjunctivitis).
Equipment
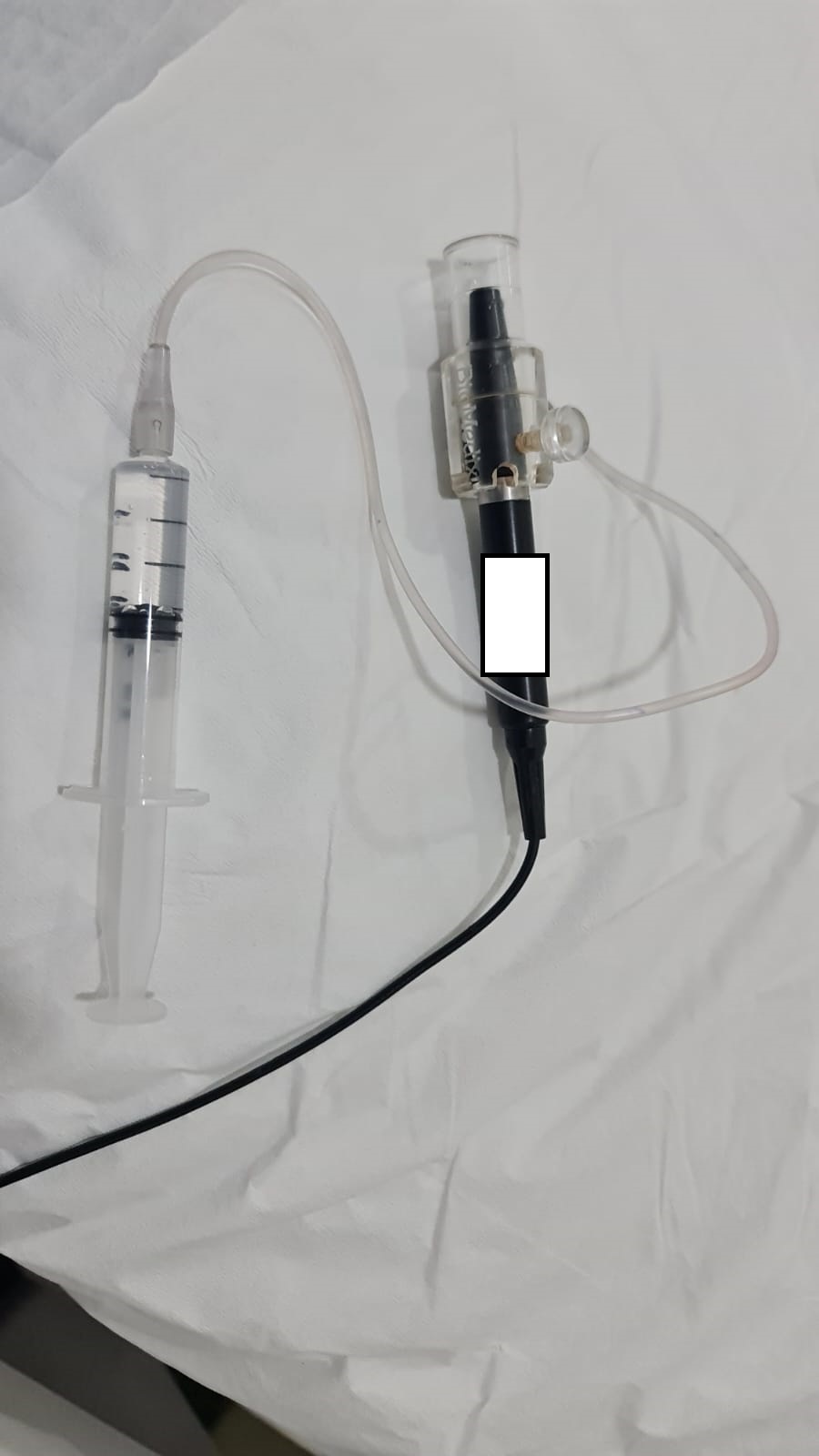
The ultrasound biometry uses an A-scan probe (see Image. Probe of A-scan). In contact ultrasonic biometry, the probe tip touches the central cornea. In immersion ultrasonic biometry, a scleral shell is placed over the eye with the patient in the supine position. Once the scleral shell is filled with saline, the A-scan machine automatically registers the A-scans. An amplitude scan (A-scan) is a one-dimensional acoustic section of the eye. Each peak represents an interface between media with 2 different sound velocities. Depending upon the specific technique used, the first peak represents the probe and the cornea, and the subsequent 2 spikes represent the anterior and posterior surface of the lens, followed by the retinal spike and the scleral spike. The cavities are anechoic, while the retina and sclera show a 100% rise in spikes. The distance between the anterior corneal and retinal spikes gives the eye's axial length.[1]
- An ideal applanation scan should ensure the machine is calibrated and set for the correct velocity settings (eg, cataract, aphakia, pseudophakia) (see Image. Hyperopic Eye).
- The echoes from the cornea, anterior lens surface, and retina should be tall and peaked. The retina spike should be steeply rising without any breaks.
- If the probe is misaligned, there will be an absent scleral spike (when the scan goes through the optic nerve). A strong scleral spike should be around 1.5 to 2 mm behind the retinal spike. The orbital spike creates a series of spikes in descending amplitude.
- Gain should be set at the lowest level at which a good reading is obtained.
- An average of 5 to 10 is the most consistent reading, giving the lowest standard deviation(<0.06 mm).
- If the eyeball is probed with too much force, corneal compression may result, which is contraindicated.
- The scan should be repeated if the difference in axial length between the 2 eyes is more than 0.30 mm or consecutive measurements differ by 0.2 mm.[1]
- The reproducible A-scan measurements with the maximum anterior chamber depth and axial length should be captured. This avoids the compression induced by applanation biometry.
Personnel
A cataract causes visual impairment, affecting the patient physically and mentally. Hence, a patient needs to be well educated and motivated about the nature of the disease, complications associated with the condition, available treatment options, benefits of timely intervention, and the need for long-term follow-up. Patient counseling is crucial due to the chronic nature of the disease and the need for long-term follow-up.
Ultrasound biometry is an approach that requires specific knowledge and skills, as it heavily relies on the operator's expertise. The probe should lie in the center of the cornea in contact ultrasound biometry, and the patient should look at the red light at the tip of the A-scan probe in immersion biometry.[41] Ultrasound biometry (contact or applanation) also requires contact with the cornea, increasing the risk of infections and abrasions. In addition, corneal applanation has a disadvantage to corneal indentation during measurement.[42] After the scan, it has to be visualized for the peaks amounting to the cornea, lens, and retina. Ideal axial length measurements comprise 3 high-quality readings with no more than 0.1 mm of variation between each other[9] and steep, high-rising spikes of the lens and the retina with anechoic cavities.[8]
Any indentation error is compensated using best-quality scans and personalized A constant or fudge factor in calculating IOL power.[8] There is variability between the measurements using applanation and indentation ultrasound biometry.[42] This results in errors in the effective lens position after IOL implantation, which accounts for up to 40% of residual refractive errors.[43] Immersion scans are considered more reliable than applanation ultrasonic biometry as these avoid inadvertent ocular compression and abnormally low value of the axial length. In contact ultrasound biometry, the scan with the highest anterior chamber depth is clinically utilized if other features of ideal scans are present, as it ensures the least eye compression.
Preparation
Before the procedure, the patient must be informed and given topical anesthesia. Both the operator's and patient's cooperation is required for ideal measurements.
Technique or Treatment
A cataract patient presents with complaints of painless, progressive diminution of vision. When patients visit an ophthalmologist, they may be advised to undergo surgery based on the severity of their vision impairment and how it affects their daily lives. The optometrist evaluates the visual impairment by assessing uncorrected and best-corrected visual acuity. Before undergoing the surgical procedure, specific prerequisites must be fulfilled. The technician or nursing staff obtains blood tests and biometry to calculate IOL power. Optical and ultrasound biometry are used for cataract density measurement.
For contact (applanation) ultrasound biometry, the patient sits and looks at a target. A topical anesthetic is applied with the ultrasound A-scan probe before touching the eye. The contact mode of the A-scan is selected in the biometry machine. Care is taken to avoid undue compression on the eye, which causes unintentional lowering of the measured axial length. The patient is asked to close the other eye for immersion ultrasound biometry and lie supine. Then, a scleral shell is applied over the eye, and saline is filled. The immersion mode of the A-scan mode is selected in the machine. The patient is asked to look at the light at the tip of the A-scan probe to ensure fixation. The machine automatically records the scans with a sound. Ideal A-scans are selected, and the non-ideal scans are discarded.
Preoperative optical coherence tomography of the macula is increasingly used as a routine investigation before cataract surgery.[44] On the day of surgery, the ophthalmologist examines the patient before surgery. When the surgery is completed, post-operative medications are prescribed, nursing staff takes care of instillation of topical drops, and patient education is provided. It is a combined effort to help the patient regain vision.
Complications
The complications of contact (applanation) ultrasonic biometry include corneal epithelial defect, discomfort, and transmission of infection. Immersion biometry may be associated with subconjunctival hemorrhage, infection, and discomfort.
Clinical Significance
This procedure helps determine IOL power by measuring the axial length and keratometry values. It helps in the decision of IOL power after post-refractive surgery.[35] It has an additional role in dense cataracts, pediatric cataracts, eyes filled with silicone oil, and uncooperative patients. It is interchangeable with optical biometry. A correction factor of -0.117 should be added to the applanation ultrasound for values clinically similar to axial length with optical biometry.[7] In posterior subcapsular cataracts, ultrasound biometry is superior to optical biometry. Ultrasound biometry is used for dense posterior subcapsular cataracts as electromagnetic waves of partial coherence interferometry cannot pass through.[18] Ultrasound biometry with contact or immersion method is chosen for the pediatric population undergoing cataract surgery.[4] In cases of corneal edema, scarring, and dense cataracts, ultrasound biometry can measure the axial length and calculate the IOL power using higher gain settings.[1][18]
Biometry using ultrasound has been the gold standard in cataract surgery for years. The main problem with A-scan (applanation scan) is poor image resolution because of a relatively long, low-resolution wavelength (frequency: 10 MHz) to measure a relatively short distance. Its major limitation is that it measures the anatomic axial length of the eye, from the anterior pole to the posterior pole, and not the optical axial length along the visual axis.[42] However, during immersion A-scan, the patient is asked to look at the red light at the tip of the A-scan probe, increasing the chances of capturing the visual axis. The optical axis in the typical human eye is tilted approximately 5° horizontally and 1° vertically relative to the anatomic axis; for IOL power calculation, instead of the distance between the vertex and fovea, the anatomic length is calculated, which is longer.[45]
This is of concern in cases of eyes with an axial length greater than 26 mm, where the anatomic axial length is 0.8 mm longer than the optical axial length.[45] In the case of posterior staphyloma, the difference between the measured axial and optical lengths is up to 3 mm, yielding a refractive surprise of approximately 9 D.[46]
Another problem with ultrasound biometry is that it measures the front of the retina, the internal limiting membrane, but the photoreceptors are close to the Bruch membrane. IOL power calculation formulas dating back to the 1970s have added 200 µ to the measured axial length to make up the difference in IOL power calculations.[7] However, 200 µ is average, while retinal thickness varies from 160 to 400 µ. Therefore, addition is not accurate for all patients.[38] Optical biometry is more accurate in eyes without any pathologies than acoustic biometry.[47] In the eyes with retinal detachment, the axial length should be smaller; therefore, the waveform of the detached retina causes an underestimation of axial length.[48]
In eyes that have silicone oil as a tamponade, there is an apparent increase in the axial length because the velocity of sound is slower in silicone oil than in vitreous humor, accounting for 987 m/s in 1000 centistokes, 970 ± 10 m/s in 5000 centistokes.[16] Also, after vitreoretinal surgery (VRSx) with silicone oil injection (SOI), most phakic eyes develop cataracts and need cataract surgery. Many ophthalmic practices prefer biometry before VRSx and SOI to prepare for the possibility of cataract surgery in the future (see Image. Ultrasonic Biometry Settings).
Sometimes, the fovea may be on the slope of the posterior staphyloma, which does not yield a sharp rising spike for the retina, and the measured axial length may be incorrect. Optical biometry is better when the patient looks at the target, ensuring that distance to the fovea is captured. Clinical examination and ultrasound brightness (B) scan will help localize the macula in addition to long and variable successive axial lengths.[49] Highly myopic eyes usually tolerate a bit of residual post-cataract surgery myopia rather than hyperopia, so most cataract surgeons keep the target postoperative refraction to -0.5 to -1 D or -1.5 D. Getting the exact IOL is difficult as such powers may not be available on all IOL designs.
Positioning the probe at the center of the cornea is necessary, which requires operator skill.[41] It also requires contact with the cornea, increasing the risk of infections and abrasions. In addition, corneal application has a disadvantage to corneal indentation during measurement.[42] After the scan, peaks amounting to the cornea, lens, and retina are visualized. Ideal axial length measurements comprise 3 high-quality readings with no more than 0.1 mm of variation between each other[9] and steep rising spikes of the lens and the retina with anechoic cavities.[8]
Conventional ultrasound with a longitudinal resolution of 200 µ gives an accuracy of 100-120 µ, while partial coherence interferometry has a resolution of 12 µ with an unprecedented precision of 10 µ.[47] The optical biometers based either on partial coherence interferometry or swept-source optical coherence tomography give a better resolution. The IOL Master 700 is based on the swept-source optical coherence tomography principle with a resolution of 22 µ and calculates 2000 A-scans per second.[50] On the other hand, ultrasonic A-scan measures the distance between the corneal vertex and the internal limiting membrane, which should theoretically measure a shorter axial length than optical by around 130-200 µ.[51] Power calculations may lead to overestimating IOL power, further amplified in shorter eyes.[52] This problem is overcome to a certain extent by ultrasound immersion biometry.[53] A mean shortening of 0.25 to 0.33 mm with both techniques accounts for around 1 D error in IOL power.[54]
Optical biometry utilizes a laser for signal transmission. The interference phenomenon between the reflected and reference signals is used to determine distances between interfaces. Ideal measurements (for the IOL Master) require a signal-to-noise ratio of at least 2.0, a tall, narrow primary maximum with a thin, well-centered termination, a set of secondary maxima, and a minimum of 4 measurements within 0.02 mm.[7] Optical biometry offers many disadvantages compared to ultrasound-guided biometry, especially in the case of dense media opacities, high axial length, and poor fixation.[18] Optical biometry exhibits systematic errors in axial length measurement that increase linearly. This is because optical biometry assigns a single, global index of refraction to all eyes irrespective of the axial length. Thus, in the case of high myopia, an increased length of the vitreous cavity may change the index of refraction, thereby altering the axial length measurement (see Image. Ultrasound Biometry Schematic Diagram).[55]
Optical biometry is a non-contact approach with accuracy in mild pathology.[51] There was a considerable variation between optical and ultrasound biometry for the cases with a low signal-to-noise ratio SNR ≤1.3.[18] In contrast, the >2.0 SNR optical biometry results were more reliable.[27] In media opacities like corneal scarring or corneal edema, optical biometry based on partial coherence interferometry cannot be used due to the electromagnetic nature of the machine; in these conditions, ultrasound biometry is preferred.
In posterior subcapsular cataracts, ultrasound biometry is superior to optical biometry.[18] The failure rate to accurately measure the axial length using partial coherence interferometry is from 8% to 37.84% due to poor fixation.[56] Ultrasound biometry with contact or immersion method is chosen for the pediatric population undergoing cataract surgery.[4]
In the case of rhegmatogenous retinal detachment patients undergoing cataract surgery, axial length measurement using immersion-type ultrasound biometry is superior to optical biometry.[57]
In cases of individuals undergoing cataract surgery or post-refractive surgeries, measurement with intraoperative biometry using an Optiwave Refractive Analyzer System (ORA) wavefront aberrometer may be superior to ultrasound biometry.[58] After myopic ablation, a false anterior lens position may occur concurrently with a false deep lens position.[35]
Enhancing Healthcare Team Outcomes
An interprofessional team comprising primary clinicians should collaborate to identify any comorbidities associated with cataracts. They need to monitor systemic diseases and provide appropriate specialist referrals for whether surgery is safe. The nursing staff and allied clinicians are responsible for educating the patient about the duration of their hospital stay and any potential complications that may arise. They should also motivate the patient to remain compliant with medical treatment. If surgery is indicated, the ophthalmologist and their team should provide comprehensive care to the patient. When the cataract has progressed, optical biometry is not typically recommended, and the patient should be advised to undergo ultrasound biometry. Accurate biometry plays a vital role in achieving optimal postoperative refractive errors after cataract surgery, and interprofessional collaboration between optometrists, ophthalmic technicians, nurses, and ophthalmologists is ideal.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Astbury N, Ramamurthy B. How to avoid mistakes in biometry. Community eye health. 2006 Dec:19(60):70-1 [PubMed PMID: 17515971]
Oliveira C, Harizman N, Girkin CA, Xie A, Tello C, Liebmann JM, Ritch R. Axial length and optic disc size in normal eyes. The British journal of ophthalmology. 2007 Jan:91(1):37-9 [PubMed PMID: 16987902]
Bhardwaj V, Rajeshbhai GP. Axial length, anterior chamber depth-a study in different age groups and refractive errors. Journal of clinical and diagnostic research : JCDR. 2013 Oct:7(10):2211-2. doi: 10.7860/JCDR/2013/7015.3473. Epub 2013 Oct 5 [PubMed PMID: 24298478]
Wilson ME, Trivedi RH. Axial length measurement techniques in pediatric eyes with cataract. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2012 Jan:26(1):13-7. doi: 10.1016/j.sjopt.2011.11.002. Epub [PubMed PMID: 23960963]
Karabela Y, Eliacik M, Kocabora MS, Erdur SK, Baybora H. Predicting the refractive outcome and accuracy of IOL power calculation after phacoemulsification using the SRK/T formula with ultrasound biometry in medium axial lengths. Clinical ophthalmology (Auckland, N.Z.). 2017:11():1143-1149. doi: 10.2147/OPTH.S136882. Epub 2017 Jun 15 [PubMed PMID: 28670106]
Holzer MP, Mamusa M, Auffarth GU. Accuracy of a new partial coherence interferometry analyser for biometric measurements. The British journal of ophthalmology. 2009 Jun:93(6):807-10. doi: 10.1136/bjo.2008.152736. Epub 2009 Mar 15 [PubMed PMID: 19289385]
Nakhli FR. Comparison of optical biometry and applanation ultrasound measurements of the axial length of the eye. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2014 Oct:28(4):287-91. doi: 10.1016/j.sjopt.2014.04.003. Epub 2014 May 6 [PubMed PMID: 25473345]
Hassani S. Principles of ultrasonography. Journal of the National Medical Association. 1974 May:66(3):205-7, 231 [PubMed PMID: 4827923]
Krimmer JE. A-scan (amplitude ultrasonography) measurement. Insight (American Society of Ophthalmic Registered Nurses). 2001 Apr:26(2):56 [PubMed PMID: 11426209]
Trivedi RH, Wilson ME. Prediction error after pediatric cataract surgery with intraocular lens implantation: Contact versus immersion A-scan biometry. Journal of cataract and refractive surgery. 2011 Mar:37(3):501-5. doi: 10.1016/j.jcrs.2010.09.023. Epub [PubMed PMID: 21333874]
Level 2 (mid-level) evidencePorwal S, Nithyanandam S, Joseph M, Vasnaik AK. Correlation of axial length and peripapillary retinal nerve fiber layer thickness measured by Cirrus HD optical coherence tomography in myopes. Indian journal of ophthalmology. 2020 Aug:68(8):1584-1586. doi: 10.4103/ijo.IJO_1778_19. Epub [PubMed PMID: 32709782]
Sandinha MT,Kotagiri AK,Owen RI,Geenen C,Steel DH, Accuracy of B-scan ultrasonography in acute fundus obscuring vitreous hemorrhage using a standardized scanning protocol and a dedicated ophthalmic ultrasonographer. Clinical ophthalmology (Auckland, N.Z.). 2017; [PubMed PMID: 28794614]
Beers AP, Van der Heijde GL. Presbyopia and velocity of sound in the lens. Optometry and vision science : official publication of the American Academy of Optometry. 1994 Apr:71(4):250-3 [PubMed PMID: 8047337]
Tosi GM, Marigliani D, Bacci T, Romeo N, Balestrazzi A, Martone G, Caporossi T. F6H8 as an Intraoperative Tool and F6H8/Silicone Oil as a Postoperative Tamponade in Inferior Retinal Detachment with Inferior PVR. Journal of ophthalmology. 2014:2014():956831. doi: 10.1155/2014/956831. Epub 2014 Jan 2 [PubMed PMID: 24672710]
Lowery MD, Makker H, Lang A. Effect of the speed of sound in Sensar acrylic lenses on pseudophakic axial length measurements. Journal of cataract and refractive surgery. 2002 Jul:28(7):1269-70 [PubMed PMID: 12106739]
Hoffer KJ. Ultrasound velocities for axial eye length measurement. Journal of cataract and refractive surgery. 1994 Sep:20(5):554-62 [PubMed PMID: 7996413]
Bjeloš Rončević M, Bušić M, Cima I, Kuzmanović Elabjer B, Bosnar D, Miletić D. Comparison of optical low-coherence reflectometry and applanation ultrasound biometry on intraocular lens power calculation. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Jan:249(1):69-75. doi: 10.1007/s00417-010-1509-4. Epub 2010 Sep 18 [PubMed PMID: 20853004]
Chia TMT, Nguyen MT, Jung HC. Comparison of optical biometry versus ultrasound biometry in cases with borderline signal-to-noise ratio. Clinical ophthalmology (Auckland, N.Z.). 2018:12():1757-1762. doi: 10.2147/OPTH.S170301. Epub 2018 Sep 10 [PubMed PMID: 30237695]
Level 3 (low-level) evidenceKhorrami-Nejad M, Khodair AM, Khodaparast M, Babapour Mofrad F, Dehghanian Nasrabadi F. Comparison of the ocular ultrasonic and optical biometry devices in the different quality measurements. Journal of optometry. 2023 Oct-Dec:16(4):284-295. doi: 10.1016/j.optom.2023.05.001. Epub 2023 Aug 9 [PubMed PMID: 37567838]
Level 2 (mid-level) evidenceShen P, Zheng Y, Ding X, Liu B, Congdon N, Morgan I, He M. Biometric measurements in highly myopic eyes. Journal of cataract and refractive surgery. 2013 Feb:39(2):180-7. doi: 10.1016/j.jcrs.2012.08.064. Epub 2012 Dec 7 [PubMed PMID: 23228592]
Rajan MS, Keilhorn I, Bell JA. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye (London, England). 2002 Sep:16(5):552-6 [PubMed PMID: 12194067]
Level 1 (high-level) evidenceHuang J, McAlinden C, Huang Y, Wen D, Savini G, Tu R, Wang Q. Meta-analysis of optical low-coherence reflectometry versus partial coherence interferometry biometry. Scientific reports. 2017 Feb 24:7():43414. doi: 10.1038/srep43414. Epub 2017 Feb 24 [PubMed PMID: 28233846]
Level 1 (high-level) evidenceLee HK, Kim MK. Comparison of a new swept-source optical biometer with a partial coherence interferometry. BMC ophthalmology. 2018 Oct 19:18(1):269. doi: 10.1186/s12886-018-0936-6. Epub 2018 Oct 19 [PubMed PMID: 30340561]
Shammas HJ, Chan S. Precision of biometry, keratometry, and refractive measurements with a partial coherence interferometry-keratometry device. Journal of cataract and refractive surgery. 2010 Sep:36(9):1474-8. doi: 10.1016/j.jcrs.2010.02.027. Epub [PubMed PMID: 20692557]
Santodomingo-Rubido J, Mallen EA, Gilmartin B, Wolffsohn JS. A new non-contact optical device for ocular biometry. The British journal of ophthalmology. 2002 Apr:86(4):458-62 [PubMed PMID: 11914218]
Findl O, Drexler W, Menapace R, Heinzl H, Hitzenberger CK, Fercher AF. Improved prediction of intraocular lens power using partial coherence interferometry. Journal of cataract and refractive surgery. 2001 Jun:27(6):861-7 [PubMed PMID: 11408132]
Olsen T, Thorwest M. Calibration of axial length measurements with the Zeiss IOLMaster. Journal of cataract and refractive surgery. 2005 Jul:31(7):1345-50 [PubMed PMID: 16105605]
Schmid GF. Axial and peripheral eye length measured with optical low coherence reflectometry. Journal of biomedical optics. 2003 Oct:8(4):655-62 [PubMed PMID: 14563204]
Chen YA, Hirnschall N, Findl O. Evaluation of 2 new optical biometry devices and comparison with the current gold standard biometer. Journal of cataract and refractive surgery. 2011 Mar:37(3):513-7. doi: 10.1016/j.jcrs.2010.10.041. Epub 2011 Jan 17 [PubMed PMID: 21244866]
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2000 Sep:238(9):765-73 [PubMed PMID: 11045345]
Level 2 (mid-level) evidenceWang L, Cao D, Weikert MP, Koch DD. Calculation of Axial Length Using a Single Group Refractive Index versus Using Different Refractive Indices for Each Ocular Segment: Theoretical Study and Refractive Outcomes. Ophthalmology. 2019 May:126(5):663-670. doi: 10.1016/j.ophtha.2018.12.046. Epub 2018 Dec 31 [PubMed PMID: 30605743]
Savini G, Hoffer KJ, Shammas HJ, Aramberri J, Huang J, Barboni P. Accuracy of a New Swept-Source Optical Coherence Tomography Biometer for IOL Power Calculation and Comparison to IOLMaster. Journal of refractive surgery (Thorofare, N.J. : 1995). 2017 Oct 1:33(10):690-695. doi: 10.3928/1081597X-20170721-05. Epub [PubMed PMID: 28991337]
Norrby S. Sources of error in intraocular lens power calculation. Journal of cataract and refractive surgery. 2008 Mar:34(3):368-76. doi: 10.1016/j.jcrs.2007.10.031. Epub [PubMed PMID: 18299059]
Kiss B, Findl O, Menapace R, Wirtitsch M, Petternel V, Drexler W, Rainer G, Georgopoulos M, Hitzenberger CK, Fercher AF. Refractive outcome of cataract surgery using partial coherence interferometry and ultrasound biometry: clinical feasibility study of a commercial prototype II. Journal of cataract and refractive surgery. 2002 Feb:28(2):230-4 [PubMed PMID: 11821201]
Level 1 (high-level) evidencePatel RH, Karp CL, Yoo SH, Amescua G, Galor A. Cataract Surgery After Refractive Surgery. International ophthalmology clinics. 2016 Spring:56(2):169-80. doi: 10.1097/IIO.0000000000000106. Epub [PubMed PMID: 26938347]
Neuhann I, Neuhann L, Neuhann T. [Age-related Cataract]. Klinische Monatsblatter fur Augenheilkunde. 2022 Apr:239(4):615-633. doi: 10.1055/a-1758-3451. Epub 2022 Mar 4 [PubMed PMID: 35253130]
González-Godínez S, Saucedo-Urdapilleta R, Mayorquín-Ruiz M, Velasco-Barona C, Moragrega-Adame E, Domínguez-Varela IA, Gonzalez-Salinas R. Ocular biometry in dense cataracts: Comparison of partial-coherence interferometry, swept-source optical coherence tomography and immersion ultrasound. Indian journal of ophthalmology. 2022 Jan:70(1):107-111. doi: 10.4103/ijo.IJO_854_21. Epub [PubMed PMID: 34937218]
Findl O. Biometry and intraocular lens power calculation. Current opinion in ophthalmology. 2005 Feb:16(1):61-4 [PubMed PMID: 15650582]
Level 3 (low-level) evidenceGale RP, Saldana M, Johnston RL, Zuberbuhler B, McKibbin M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (London, England). 2009 Jan:23(1):149-52 [PubMed PMID: 17721503]
Kadakia A, Zhang J, Yao X, Zhou Q, Heiferman MJ. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Experimental biology and medicine (Maywood, N.J.). 2023 May:248(5):371-379. doi: 10.1177/15353702231169539. Epub 2023 May 22 [PubMed PMID: 37212384]
Level 3 (low-level) evidenceBell NP, Feldman RM, Zou Y, Prager TC. New technology for examining the anterior segment by ultrasonic biomicroscopy. Journal of cataract and refractive surgery. 2008 Jan:34(1):121-5. doi: 10.1016/j.jcrs.2007.09.016. Epub [PubMed PMID: 18165091]
Level 1 (high-level) evidenceGiers U, Epple C. Comparison of A-scan device accuracy. Journal of cataract and refractive surgery. 1990 Mar:16(2):235-42 [PubMed PMID: 2329484]
Level 1 (high-level) evidenceHolladay JT. Standardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculations. Journal of cataract and refractive surgery. 1997 Nov:23(9):1356-70 [PubMed PMID: 9423908]
Alizadeh Y, Akbari M, Moghadam RS, Medghalchi A, Dourandeesh M, Bromandpoor F. Macular Optical Coherence Tomography before Cataract Surgery. Journal of current ophthalmology. 2021 Jul-Sep:33(3):317-322. doi: 10.4103/joco.joco_240_20. Epub 2021 Oct 22 [PubMed PMID: 34765821]
Zaldivar R, Shultz MC, Davidorf JM, Holladay JT. Intraocular lens power calculations in patients with extreme myopia. Journal of cataract and refractive surgery. 2000 May:26(5):668-74 [PubMed PMID: 10831895]
Level 2 (mid-level) evidenceZhu XJ, He WW, Du Y, Qian DJ, Dai JH, Lu Y. [Intraocular lens power calculation for high myopic eyes with cataract: comparison of three formulas]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2017 Apr 11:53(4):260-265. doi: 10.3760/cma.j.issn.0412-4081.2017.04.007. Epub [PubMed PMID: 28412798]
Level 2 (mid-level) evidenceDrexler W, Findl O, Menapace R, Rainer G, Vass C, Hitzenberger CK, Fercher AF. Partial coherence interferometry: a novel approach to biometry in cataract surgery. American journal of ophthalmology. 1998 Oct:126(4):524-34 [PubMed PMID: 9780097]
Level 2 (mid-level) evidenceOlsen T. Calculation of intraocular lens power: a review. Acta ophthalmologica Scandinavica. 2007 Aug:85(5):472-85 [PubMed PMID: 17403024]
Montes de Oca I, Gökce SE, Hallahan K, Wang L, Koch DD. IOL Calculations in Short, Long, and Postrefractive Eyes. International ophthalmology clinics. 2016 Summer:56(3):49-70. doi: 10.1097/IIO.0000000000000119. Epub [PubMed PMID: 27257722]
Olsen T. The accuracy of ultrasonic determination of axial length in pseudophakic eyes. Acta ophthalmologica. 1989 Apr:67(2):141-4 [PubMed PMID: 2658459]
Rose LT, Moshegov CN. Comparison of the Zeiss IOLMaster and applanation A-scan ultrasound: biometry for intraocular lens calculation. Clinical & experimental ophthalmology. 2003 Apr:31(2):121-4 [PubMed PMID: 12648044]
Level 2 (mid-level) evidenceLee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation. Current opinion in ophthalmology. 2008 Jan:19(1):13-7 [PubMed PMID: 18090891]
Level 3 (low-level) evidenceAdemola-Popoola DS, Nzeh DA, Saka SE, Olokoba LB, Obajolowo TS. Comparison of ocular biometry measurements by applanation and immersion A-scan techniques. Journal of current ophthalmology. 2015 Sep-Dec:27(3-4):110-4. doi: 10.1016/j.joco.2015.12.002. Epub 2016 Feb 9 [PubMed PMID: 27239588]
Ossoinig KC. Standardized echography: basic principles, clinical applications, and results. International ophthalmology clinics. 1979 Winter:19(4):127-210 [PubMed PMID: 395120]
Wang XG, Dong J, Pu YL, Liu HJ, Wu Q. Comparison axial length measurements from three biometric instruments in high myopia. International journal of ophthalmology. 2016:9(6):876-80. doi: 10.18240/ijo.2016.06.15. Epub 2016 Jun 18 [PubMed PMID: 27366691]
McAlinden C, Wang Q, Pesudovs K, Yang X, Bao F, Yu A, Lin S, Feng Y, Huang J. Axial Length Measurement Failure Rates with the IOLMaster and Lenstar LS 900 in Eyes with Cataract. PloS one. 2015:10(6):e0128929. doi: 10.1371/journal.pone.0128929. Epub 2015 Jun 10 [PubMed PMID: 26061554]
Pongsachareonnont P, Tangjanyatam S. Accuracy of axial length measurements obtained by optical biometry and acoustic biometry in rhegmatogenous retinal detachment: a prospective study. Clinical ophthalmology (Auckland, N.Z.). 2018:12():973-980. doi: 10.2147/OPTH.S165875. Epub 2018 May 23 [PubMed PMID: 29872256]
Ianchulev T, Hoffer KJ, Yoo SH, Chang DF, Breen M, Padrick T, Tran DB. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014 Jan:121(1):56-60. doi: 10.1016/j.ophtha.2013.08.041. Epub 2013 Oct 30 [PubMed PMID: 24183339]