Introduction
Renal infarction is a rare ischemic event caused by the complete or partial occlusion of the main renal artery or its segmental branches, which may ultimately lead to renal ischemia. Renal infarction most commonly occurs due to an embolus originating from the heart or an in situ thrombosis.[1] Other etiological causes of renal infarction are aortic thromboembolism, trauma, renal artery dissection, coagulation disorders, or other atheroembolic disease.
The diagnosis of renal infarction should be considered in patients who develop sudden abdominal or flank pain with reduced renal function, elevated LDH, hematuria, or proteinuria and who do not have urolithiasis or any other diagnosable explanation for their symptoms. The chances increase if the patient has cardiac disease, especially atrial fibrillation, or is aged older than 60 years. Evaluation often starts with a noncontrast abdominal computed tomography (CT) scan, progressing to a contrast-enhanced study if the original CT scan is negative.[2][3] Early diagnosis is essential for initiating revascularization therapy promptly, which optimizes renal function recovery. Treatment options include intravascular thrombolysis, systemic thrombolysis, anticoagulation, and antiplatelet therapy. Clinical presentation, underlying risk factors, length of time between symptom onset and treatment, and partial or total artery occlusion all impact the likelihood of renal recovery.
Given the overlap of clinical symptoms with more common presentations (eg, renal calculi and pyelonephritis), the diagnosis may be missed entirely; this possibility is supported by a higher incidence of renal infarction in autopsy studies compared to retrospective case reviews. An overlooked diagnosis can result in irreversible loss of renal function, as renal reperfusion therapy is ineffective in delayed presentation.[4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The causes of renal infarction can be grouped into the following categories:
- Cardioembolic sources
- Renal artery thrombosis
- Trauma or mechanical injury
- Aortic or renal artery dissection
- Hypercoagulability
- Atheroembolic disease [6][7][8][9][10]
Cardioembolic Disorders
Cardioembolic disorders are the most common etiology of renal infarction, accounting for up to 55% of cases. Within this group, between 64% to 75% are noted to have atrial fibrillation.[7] Renal infarction may be the first sign of atrial fibrillation. Also, many patients with atrial fibrillation who develop renal infarction are found to be subtherapeutic with anticoagulation. Renal infarction due to cardiac emboli tends to occur in the older age group, often in the sixth to eighth decade of life. Other cardiac causes include cardiac emboli due to rheumatic mitral stenosis, myocardial infarction, and prosthetic or native valve endocarditis. Renal infarction can also occur from an embolus originating from an atrial myxoma or thrombus in the left ventricle apex.[11]
Renal Artery Thrombosis
Arterial causes of renal infarction include atherosclerotic thrombosis originating from the aorta or renal artery. Aneurysms of the thoracic or suprarenal abdominal aorta can also increase the risk of renal infarctions by favoring thrombi development. Treatment is aimed at treating risk factors such as hyperlipidemia and hypertension.[12]
Trauma or Mechanical Injury
Renal infarction can occur from a blunt abdominal injury leading to dissection or occlusion of the renal artery, which may result from direct trauma or retroperitoneal bleeding compressing renal vasculature. Surgery or endovascular repair may be indicated depending on the nature of the injury.
Aortic or Renal Artery Dissection
Mechanical instrumentation is the most common cause of renal artery dissection, most often due to endovascular or surgical procedures involving the aorta or its branches. Aortic dissection can also involve 1 or both renal arteries, and endovascular stent placement during aortic repair can also affect the renal artery supply. Spontaneous renal artery dissection is very rare but can have significant sequelae in otherwise healthy patients. Clinicians should assess each patient for risk and individualize surgical or nonsurgical management determinations.[13][14]
Fibromuscular dysplasia occurs primarily in women younger than 50 and usually involves the renal arteries, about half the time, bilaterally.[15] Fibromuscular dysplasia affects small and medium-sized arteries, causing stenosis, aneurysm, or dissection. Renal infarctions can occur due to thrombus formation in the poststenotic, dilated portion of the artery, which usually manifests initially as hypertension.[16][17] See StatPearls' companion reference, "Fibromuscular Dysplasia," for further information.[18] Renal artery dissection can occur spontaneously in fibromuscular dysplasia, Marfan syndrome, and Ehler-Danlos syndrome.[19][20]
Hypercoagulable State
Heritable coagulation disorders like protein C and protein S deficiency, hyperhomocysteinemia, systemic lupus erythematous, polycythemia vera, Factor V Leiden mutation, prothrombin gene mutation, and antithrombin III deficiency can also lead to renal artery blockage. Renal infarction may also be seen with oral contraceptive use, COVID-19 infection, malignancy, sickle cell disease, anabolic steroid abuse, and cocaine use.[21][22]
Atheroembolic Disease
The majority of patients who receive angiography have significant atherosclerotic disease, which puts them at higher risk for atherosclerotic plaque emboli, resulting in renal artery occlusion and infarction. The incidence of acute kidney injury (AKI) in patients older than 60 years due to biopsy-confirmed renal atheroemboli has been reported at 7%, which can be reduced using a brachial rather than an iliofemoral approach for arterial access.[23][24]
In contrast to other causes of AKI, atheroembolic disease can present with renal injury gradually and weeks after the initial insult.[25] Symptoms are generally less severe because atheroemboli often have irregular shapes and do not typically cause complete occlusion, as do thromboemboli.[26] Over time, this results in a narrowed arterial lumen and some degree of renal injury over several weeks.[27][28]
Idiopathic
Despite extensive evaluation, many patients do not have an identifiable cause of renal infarction. About 20% to 30% of cases remain idiopathic, with a median age of approximately 50 years in these patients.[6][7][10] The infarction may represent poor vascular health in general, and all secondary risk factors, including hypertension, hyperlipidemia, and diabetic control, should be aggressively treated.[29] A study found that patients with idiopathic causes were more likely to be younger and smokers, suggesting targets for secondary prevention.[30]
Epidemiology
Autopsy studies suggest the incidence of renal infarction is 14 per 1000, or 1.4%.[6] Other retrospective studies of ER admissions found an incidence of 0.004% to 0.007%.[30][31] Many renal infarctions are diagnosed late or misdiagnosed; therefore, autopsy studies may be more accurate.[30][32] A study found unilateral renal involvement in 81% of cases and bilateral involvement in 19%.[9] Patients with hypercoagulability may be more likely to have multiple and bilateral infarcts.[6] The mean age of patients who present with renal infarction is thought to be approximately 63 years, with no significant difference in sex distribution.[9][19] Risk factors for renal infarction include atrial fibrillation, hypertension, diabetes, and prior embolic infarction.[9][30]
Pathophysiology
Thrombotic mechanisms involve endothelial injury, which activates in-situ thrombus formation. Another ischemic mechanism occurs when emboli from the heart in atrial fibrillation become dislodged and eventually occlude the renal artery. All renal segmental arteries are end-arteries, so a complete or partial reduction in vascular flow due to embolic or thrombotic occlusion leads to renal ischemia and infarction with focal tissue necrosis of the kidney (see Image. Renal Tissue Necrosis). The consequent impairment in renal function can manifest as elevated creatinine levels and decreased glomerular filtration rates (GFR), leading to AKI, chronic kidney disease (CKD), and sometimes end-stage renal failure.
Atheroemboli is more irregular than thrombus-based emboli, but the crystals are smaller. They are, therefore, more likely to produce an incomplete renal arterial blockage than thromboemboli and tend to affect smaller branches.[26] The atheroemboli eventually cause a foreign body reaction with giant cell formation, narrowing the arterial lumen and reducing renal blood flow, resulting in renovascular hypertension and reduced GFR.[27][28]
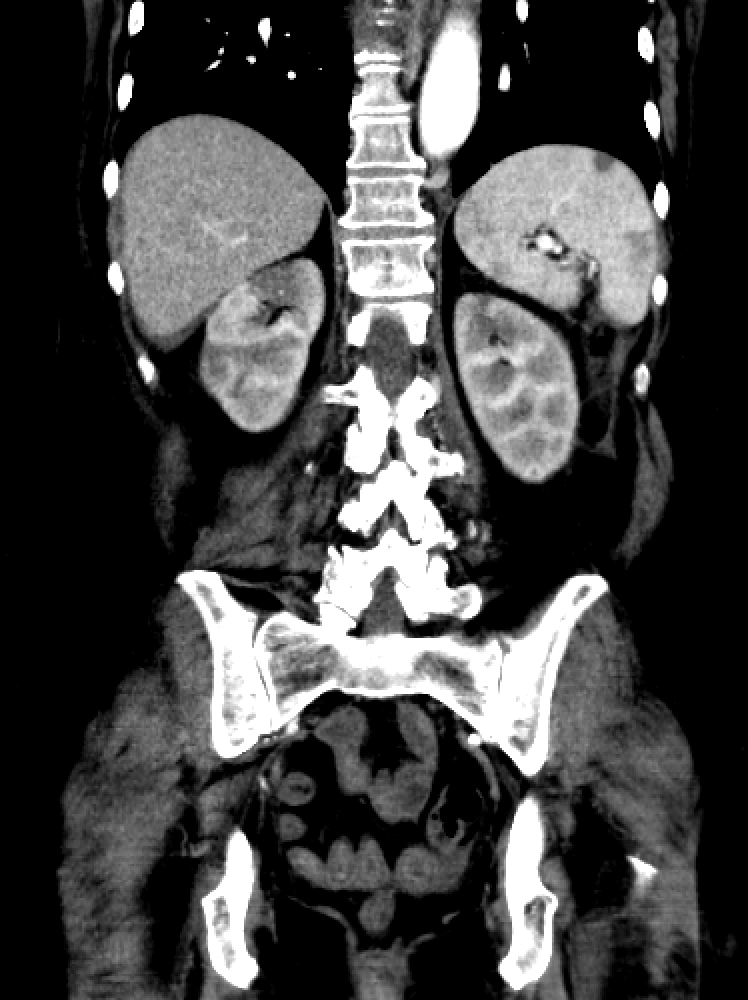
An infarction of the main renal artery jeopardizes the function of the entire organ. Segmental arterial occlusion can lead to reduced function of a large portion of the kidney, which is especially significant when associated with preexisting renal failure or solitary kidneys. Blockage of a subsegmental artery leads to the classic "wedge-shaped" diffusion defect of the renal parenchyma seen on contrast-enhanced CT scans of the abdomen.
History and Physical
Clinical Features
Patients with acute renal infarctions typically present with a sudden onset of abdominal or flank pain along with associated symptoms, including nausea, vomiting, hematuria, and occasionally fever. On physical examination, abdominal or loin tenderness findings may be present.[5][6] Skin color changes are noted in approximately one-third of cases (eg, "blue toe" syndrome or livedo reticularis).[33][34]
Unfortunately, the clinical presentation is often nonspecific or suggestive of alternative, more common disorders, including ureteral colic, a "passed" kidney stone, pyelonephritis, or gastroenteritis. This confusion often leads to a delay, with fewer than 50% of patients receiving the correct diagnosis within 2 days of their initial presentation.[35] Signs of acute kidney injury may only appear several weeks after the actual injurious event. The triad of sudden abdominal or flank pain, elevated lactate dehydrogenase (LDH), and hematuria and proteinuria suggest renal infarction.[31]
Since these cases often present with vague symptoms, a high index of suspicion is necessary for accurate diagnosis. Risk factors for general atherosclerotic disease include male gender, significant smoking history, hypertension, hypercholesterolemia, diabetes, and older age. Atrial fibrillation is a pervasive source of emboli causing renal infarction.[36][37] Fundoscopic examination is suggested in suspected cases of atherosclerotic emboli to identify retinal abnormalities.[38]
Acute loss of renal arterial supply can lead to new-onset, renin-mediated hypertension noted at the time of initial presentation, which usually improves with treatment.[4] Unfortunately, this hypertension is often attributed to a pain response and frequently goes unrecognized.[8] Some patients may be asymptomatic, further contributing to the underdiagnosis of renal infarction. Asymptomatic renal infarction can also be detected incidentally in patients undergoing imaging for unrelated reasons.[39] Atherosclerotic renal emboli as a cause of renal infarction is suggested by the following clinical features:
Evaluation
Laboratory Studies
Laboratory findings in renal infarction include leukocytosis, elevated C-reactive protein (CRP), very high LDH, macroscopic or microscopic hematuria, and proteinuria.[35][40] Other possible lab findings are elevated creatinine and creatine kinase values. The rise in creatinine is more evident in patients with a particularly large renal infarct or bilateral involvement of both kidneys.[6] A careful assessment of coagulation disorders should also be performed if cardiac and renal arterial pathology have been eliminated as causative factors of renal infarction or if surgical revascularization is being considered. LDH is a marker of cell necrosis and can rise to 4 or 5 times the normal value with no similar rise in serum aspartate aminotransferase (AST) and alanine transaminase (ALT) in renal infarction; this does not occur with other etiologies (eg, urolithiasis, renal colic, or pyelonephritis).[4][41][42] Elevated LDH also occurs with myocardial infarction, hemolysis, and renal transplant rejection, which are usually clinically distinguishable from renal infarctions.[42] LDH can remain elevated up to 15 days after the first clinical symptoms.[6]
Cholesterol emboli causing renal infarction are often associated with eosinophilia, eosinophiluria, and low serum complement in the acute phase. However, these findings dissipate after about 1 week from the time of the injury.[43][44][45] Focal neurological defects, mental confusion, localized cyanosis, and retinal Hollenhorst plaques suggest atherosclerotic emboli.[1] Atheroemboli also tend to produce a slow and often subacute decrease in renal function, which typically plateaus at 3 to 8 weeks.[27] Clinical diagnosis of atheroembolic disease is often missed; renal biopsy can be necessary for a definitive diagnosis. Atheroemboli is detected in over 75% of cases. Intraluminal cholesterol crystals appear as biconvex, needle-shaped defects, or "ghosts" as the cholesterol dissolves during the fixation process.[46][47] Significant vascular intimal inflammation and possibly eosinophilic infiltration.[44] Focal glomerulosclerosis may also be seen.[47][48]
Imaging Studies
Noninvasive imaging studies in patients presenting with acute flank pain, vomiting, and hematuria usually start with a noncontrast CT scan of the abdomen and pelvis to rule out urolithiasis and pyelonephritis, which are the most common reasons for such a clinical presentation.[49] However, with renal infarction, noncontrast CT may show only perinephric stranding, mild renal swelling, or no significant abnormality (see Image. Renal Infarction). If a noncontrast CT is not diagnostic, a contrast-enhanced abdominal CT should be performed to look for renal infarction.[19][20] Clinicians should consider renal infarction in patients with a high risk of thromboembolic events, high LDH levels, or hematuria.[30]
CT with contrast often shows a clear hypodensity or wedge-shaped perfusion defect in the kidney, best appreciated in the arterial phase. With a segmental infarct, there may be a thin rim of the renal cortex paralleling the wedge-shaped area of the infarct, presenting with normal enhancement. This finding is called the cortical rim sign, which is seen in about half of the cases of renal infarction.[50][51] The cortical rim sign may become evident several days after a renal infarction due to preserved perfusion of the capsule by perforating branches of the renal capsular artery, receiving collateral circulation from branches of the suprarenal, ureteral, and lumbar vessels.[52] If there is total occlusion of the main renal artery, there can be nonenhancement of the entire kidney. In a traumatic transection of the renal artery, CT of the abdomen may reveal a hematoma starting at the hilum of the renal artery. Intervention is likely ineffective if the kidney is atrophic or scarred. Renal CT arteriography is the gold standard investigation for renal infarction and makes the definitive diagnosis, allowing for proper treatment decision-making.[19] Renal magnetic resonance imaging (MRI) arteriography is equally helpful, but the examination takes much longer, so CT imaging is usually preferred. Other investigations used to confirm the diagnosis of renal infarction are dimercaptosuccinic acid (DMSA) radioisotope scans.
Renal artery dopplers are often used to evaluate for renal artery stenosis but cannot assess nonstenotic lesions and determine etiology.[29] Image quality can also be significantly affected by body habitus. A color Doppler scan may also be beneficial in demonstrating decreased or absent blood flow to the infarcted area. An intravenous pyelogram (IVP) in a renal infarction demonstrates nonvisualization of the infarcted renal parenchyma. A DMSA radioisotope scan will reveal decreased or no tracer uptake in the affected area.[4]
Invasive vascular ultrasound (IVUS) is a newer modality to add diagnostic accuracy for renal infarctions. This method adds information regarding the arterial walls that are not always visualized by arteriography, which primarily images the arterial lumens. IVUS can also help differentiate thrombosis, dissection, and fibromuscular dysplasia. This modality has been used primarily in cardiovascular evaluations, and further study is needed for renal artery imaging.[29][53]
Additional Cardiac Studies
Cardiac studies, including an ECG or Holter monitoring, should evaluate for atrial fibrillation in all patients diagnosed with renal infarction without an apparent cause. A transthoracic or transesophageal echocardiogram can determine the possibility of cardiac thromboembolic disease and thrombi.[52]
Treatment / Management
CT renal angiography is performed to better evaluate the vasculature, identify the specific vessels involved, and determine the degree of occlusion. Revascularization is not useful if imaging demonstrates a shrunken, atrophic kidney or a clearly demarcated, dense wedge-shaped scar suggesting an old infarction. Treating renal infarction can be broadly categorized into catheter-directed thrombolysis, systemic thrombolysis, anticoagulation, surgery, and experimental treatment.
The factors used to determine if a patient would benefit from revascularization procedures include the time since ischemia onset, current kidney function, renal infarct size and extent, the presence of arterial dissection, contralateral kidney function, the precise vessels involved, and whether renal artery occlusion is partial or complete. Determining the time of the infarction onset may not always be straightforward. Acute symptoms (eg, flank pain, nausea, vomiting, and hypertension) suggest a more recent onset of 1 week or less. Typically, even complete occlusions of ≤6 hours are considered potentially reversible. Patients with normal kidney function may not initially show an elevated BUN and creatinine even with complete, acute, unilateral renal arterial occlusion. The degree of renal arterial blockage can be determined by CT renal angiography. Partial occlusion with some degree of visualized parenchymal perfusion suggests potential recovery.
Catheter-Directed Thrombolysis
Catheter-directed thrombolysis, or percutaneous endovascular therapy, is used to revascularize proximal or bilateral renal artery occlusions and restore perfusion in a solitary kidney. Catheter-directed thrombolysis may also be considered for selected patients with significant segmental artery occlusions. Modalities, including local intraarterial thrombolysis, thrombectomy, angioplasty, or stenting, are more effective when performed as close to the onset of clinical symptoms.[54] Most patients show complete or at least partial vascular reperfusion if diagnosed and treated promptly, and case reports have shown successful revascularization for up to 96 hours after presentation.[55][56][57][58] Following catheter-directed thrombolysis, laboratory markers of renal recovery may lag behind functional kidney improvement. One study demonstrated long-term recovery of renal function despite no significant improvement in the BUN and creatinine at the time of hospital discharge. Likely, some renal tissue was reperfused and recovered over time, similar to "myocardial stunning."[37](B3)
One published protocol attempts thrombectomy first; if unsuccessful, a bolus injection of 250,000 IU of urokinase is given directly into the affected artery. If results are not deemed adequate, the catheter is left in place, and a continuous infusion of urokinase at a rate of 50,000 IU per hour is utilized for up to 72 hours. Daily renal angiograms are done while the urokinase infusion is administered, and the patients are carefully monitored for possible complications. If the infarction remains unchanged after 72 hours, the treatment is ineffective and discontinued.[37] Other thrombolytic agents like urokinase, streptokinase, or tissue plasminogen activator have also been used. If thrombolysis is ineffective, angioplasty with or without stenting can be considered, but no definitive guidelines exist. The complications of catheter-directed thrombolysis include bleeding, pseudoaneurysm formation, and acute mesenteric embolism with ischemia.[59] Typically, patients who undergo angioplasty or stenting receive 3 to 6 months of aspirin and clopidogrel. Ultrasound-enhanced catheter-directed thrombolysis is a novel treatment option for renal artery thrombosis, wherein the ultrasound waves allow better penetration of thrombolytic agents by acoustic streaming.[60] Following endovascular therapy, patients are maintained on aspirin and clopidogrel; the clopidogrel can be discontinued after 3 months.[60](B3)
Systemic Thrombolysis
Systemic thrombolysis can be attempted if catheter-directed thrombolysis is not available.[61][62] However, the risk of systemic bleeding is higher in systemic fibrinolytic therapy than in catheter-directed thrombolysis.[4] Compared to systemic fibrinolysis, catheter-directed thrombolysis has the advantage of a lower dosage of thrombolytic agents and easier deliverability to the target blood vessels.[60] Reperfusion injury is a potential complication of thrombolysis. (B3)
Anticoagulation or antiplatelet agents are considered the treatment of choice for patients who present several days or longer after a renal infarction, as revascularization therapy is not likely to be beneficial. Long-term anticoagulation is initiated for those with underlying hypercoagulation disorders or cardioembolic disease. The duration of anticoagulation is typically at least 6 months if the patient does not have risk factors for repeat embolization. Intravenous heparin, followed by warfarin, with a target INR of 2 to 3, is commonly used. In patients already on warfarin with a therapeutic INR before their renal infarct, an increased INR target of 2.5 to 3.5 is suggested. Direct oral anticoagulants (eg, Factor Xa inhibitors) are frequently used for anticoagulation indications and do not require the frequent monitoring associated with long-term warfarin therapy. If anticoagulants are stopped after 6 months, life-long aspirin has been recommended for patients with renal infarction, even if no specific hypercoagulable state has been identified. Aspirin may also benefit those with asymptomatic remote renal infarctions who present with an atrophic kidney.
Surgical Therapy
Open surgery is mostly attempted in the event of trauma-related renal artery injury and aortic dissection extending into the renal artery. Various surgical options include arteriotomy with embolectomy, aorto-renal, ileo-renal, or splenorenal bypass procedures.[40] Clinicians should refer patients with underlying fibromuscular dysplasia to specialists for possible percutaneous transluminal angioplasty or vascular reconstructive surgery. Surgical intervention has not proven effective in thrombotic and embolic etiologies and is associated with significant risks.[37](B3)
Antihypertensive Medications
Antihypertensive medications inhibiting the renin-angiotensin axis are preferred for renovascular hypertension, which often develops in the first week after a renal infarction due to increased renin levels. The associated hypertension frequently subsides in the following weeks unless underlying essential hypertension exists.[37][39] Long-term management of high blood pressure should be the same as for any hypertensive patient.[37]
Atherosclerotic Emboli Therapy
Atherosclerotic emboli treatment in the renal arteries is primarily supportive.[63][64] Scheduled angiographic and vascular procedures should be canceled, postponed, or delayed. Patients with renal infarcts due to atheroemboli have a relatively poor overall prognosis due to the severity of their underlying atherosclerotic disease. However, patients without significant comorbidities or complications can see their renal function improve over time, as shown in a study where 24% fully recovered.[27] Patients should follow general measures, including aspirin use, smoking cessation, hypertension control, weight management, and glycemic control.[1][65] (B2)
Surveillance Recommendations
Long-term follow-up for patients with thromboembolic renal infarctions has not been standardized. Monitoring patients for complications related to ongoing therapy (eg, bleeding episodes) and regularly checking renal function is reasonable. Lifelong aspirin therapy is typically recommended as well.[10][66]
Follow-up imaging is recommended, and some experts have suggested serial abdominal imaging, preferably with MRI, at 6 and 12 months. If no new lesions or concerns are found, further surveillance imaging may not be necessary. Patients with underlying renal or cardiac issues will require closer and longer-term monitoring.
Differential Diagnosis
Diagnoses in the differential for renal infarction include the following: aortic aneurysm, aortic dissection, appendicitis, diverticulitis, gastroenteritis, mesenteric ischemia, nephrolithiasis, pyelonephritis, renal cell carcinoma, and gynecologic disorders. Nephrolithiasis and pyelonephritis are commonly confused for renal infarction. Failure to consider renal infarction as a differential in a case of flank pain initially suspected as renal colic could delay diagnosis and make renal function unsalvageable with reperfusion therapy. Significant LDH elevation is not seen in pyelonephritis or nephrolithiasis and can be used to help distinguish these disorders from renal infarction, especially if serum transaminases are low. A contrast-enhanced CT scan of the abdomen is crucial to help differentiate renal infarction from extrarenal causes of abdominal pain. Clinicians should exclude mesenteric ischemia because systemic embolization in a patient with atrial fibrillation or hypercoagulable disorder could potentially occlude the blood vessels supplying the intestine, leading to acute bowel infarction; angiography may be required for evaluation.
Prognosis
One large retrospective review found approximately 20% of patients diagnosed with renal infarction had died at 40 months; however, only 2% of these patients progressed to ESRD. The poor prognosis suggests that renal infarction is indicative of cardiac and other mortality. [29] Prognosis is improved with early diagnosis. Warm ischemia time, collateral flow, and preexisting kidney disease tend to influence prognosis following treatment with catheter-directed thrombolysis.[55] Prognosis can also be influenced by embolic infarction in other organs like the spleen, intestine, liver, and lungs.[19] The presence of extrarenal embolisms increases the duration of hospital stay as well as the overall morbidity and mortality.[11]
Complications
Common complications include acute kidney injury, chronic kidney disease, and hypertension. The progression to CKD from AKI correlates with the magnitude of the infarction.[67] The main risk factors for CKD are advanced age and a higher degree of AKI. Up to 58% of renal infarction patients developed CKD, as detected in follow-up renal scintigraphy.[67][68][69]
Consultations
Nephrologists, hematologists, and cardiologists may require consultation.[5] Patients also need follow-up with vascular surgery or interventional radiology if recurrent vascular lesions like fibromuscular dysplasia are discovered. Follow-up is necessary because inflammation may prevent the accurate detection of fibromuscular dysplasia in the acute phase of renal infarction. Clinicians must also assess the carotid and other extra-renal arteries if suspected of fibromuscular dysplasia or other vasculopathies like Ehler-Danlos syndrome.[52]
Deterrence and Patient Education
Patients should be educated regarding anticoagulation and INR range if warfarin is used. Those developing repeated infarcts, even with anticoagulant therapy, must be educated regarding medication compliance and reactivity with certain food items. Clinicians should also counsel patients to address other risk factors (eg, smoking cessation) with lifestyle modifications.
Pearls and Other Issues
Key factors to bear in mind when managing renal infarction include:
- Given the rarity of this condition, clinicians must have a high suspicion for clinical diagnosis, especially in patients with significant risk factors.
- Patients with a history of atrial fibrillation, older age, and recent vascular instrumentation are at exceptionally high risk for renal infarction.
- A renal infarction should be suspected whenever a patient presents with acute flank pain and hematuria without evidence of a UTI, hydronephrosis, or urinary calculi.
- If the initial noncontrast CT is negative, immediate contrast-enhanced CT of the abdomen should be performed to allow the opportunity for effective revascularization.
- Elevated LDH with a normal AST/ALT is suggestive of renal infarction.
- A CT angiogram is the definitive test to confirm the diagnosis.
- Laboratory work for patients diagnosed with a renal infarction should include a urinalysis, serum aminotransferases, creatinine, LDH levels, and an EKG and coagulation studies.
- Rapid diagnosis allows for possible immediate catheter-directed thrombolysis therapy, the optimal recommended treatment, ideally administered within 6 hours of the infarction.
- There is controversy regarding when catheter-directed thrombolysis can be administered, but there are anecdotal reports of a benefit even after the usually recommended time limits.
- If catheter-directed thrombolysis therapy is unavailable, systemic thrombolytic therapy should be considered.
- Patients may also require secondary prevention in the form of antiplatelet therapy, and most, if not all, should be on some permanent anticoagulation or antiplatelet therapy.
Enhancing Healthcare Team Outcomes
The involvement of an interprofessional team composed of primary clinicians (eg, physicians, nurse practitioners, and physician assistants), interventional radiologists, nephrologists, cardiologists, hematologists, and vascular surgeons are required to manage renal infarction. Nursing staff and pharmacists round out the interprofessional team. While a clinician diagnoses the case of renal infarction, endovascular procedures are referred to an interventional radiologist or vascular surgeon. Complications such as acute kidney injury may need evaluation from a nephrologist. The etiology of renal infarction may require consultation with a cardiologist and hematologist. A combined approach by all these clinicians helps identify and tackle the rare and challenging diagnosis of renal infarction.
Nursing supports patient examination and counseling, surgical preparation and assistance during surgery, and postoperative care. Pharmacists will be critical in all these cases, particularly with anticoagulation regimens, medication reconciliation, and patient counseling. Any interprofessional healthcare team member who notes a change in patient status, performs an intervention, or notes an adverse event should immediately record their findings in the patient's chart and notify other team members to implement corrective action. This interprofessional information sharing and collaborative action will optimize patient outcomes in renal infarction cases and help prevent adverse results.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
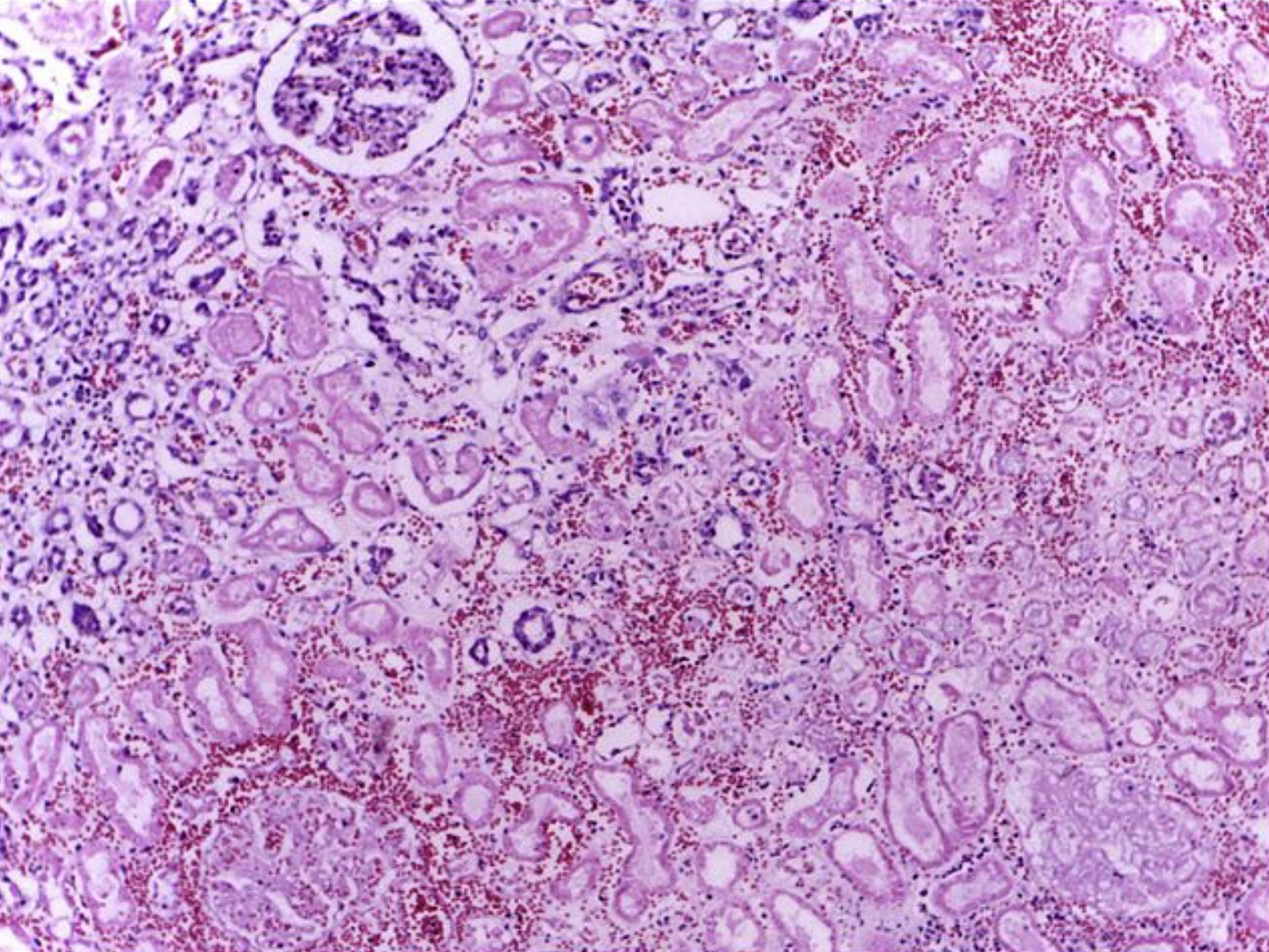
Renal Tissue Necrosis. The histologic image shows the edge of a renal infarct of an otherwise normal kidney (upper left) with necrotic tubules and glomeruli in the infarct area having preserved cellular outlines with loss of nuclei. Interstitial hemorrhage and mild inflammatory infiltrates can be seen at the border of the infarct. (H&E stain 4X)
Department of Pathology, Calicut Medical College, (CC By-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0)
References
Scolari F, Ravani P, Gaggi R, Santostefano M, Rollino C, Stabellini N, Colla L, Viola BF, Maiorca P, Venturelli C, Bonardelli S, Faggiano P, Barrett BJ. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007 Jul 17:116(3):298-304 [PubMed PMID: 17606842]
Level 2 (mid-level) evidenceMarkabawi D, Singh-Gambhir H. Acute renal infarction: A diagnostic challenge. The American journal of emergency medicine. 2018 Jul:36(7):1325.e1-1325.e2. doi: 10.1016/j.ajem.2018.04.018. Epub 2018 Apr 19 [PubMed PMID: 29699899]
Khokhar S, Garcia D, Thirumaran R. A rare case of renal infarction due to heroin and amphetamine abuse: case report. BMC nephrology. 2022 Jan 12:23(1):28. doi: 10.1186/s12882-021-02642-1. Epub 2022 Jan 12 [PubMed PMID: 35021999]
Level 3 (low-level) evidenceSaeed K. Renal infarction. International journal of nephrology and renovascular disease. 2012:5():119-23. doi: 10.2147/IJNRD.S33768. Epub 2012 Sep 3 [PubMed PMID: 22969301]
Level 3 (low-level) evidenceSeetho IW, Bungay PM, Taal MW, Fluck RJ, Leung JC. Renal infarction in patients presenting with suspected renal colic. NDT plus. 2009 Oct:2(5):362-4. doi: 10.1093/ndtplus/sfp074. Epub 2009 Jun 26 [PubMed PMID: 25949343]
Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, Karras A, Amoura Z, Jourde-Chiche N, Izzedine H, François H, Boffa JJ, Hummel A, Bernadet-Monrozies P, Fouque D, Canouï-Poitrine F, Lang P, Daugas E, Audard V. Acute renal infarction: a case series. Clinical journal of the American Society of Nephrology : CJASN. 2013 Mar:8(3):392-8. doi: 10.2215/CJN.05570612. Epub 2012 Nov 30 [PubMed PMID: 23204242]
Level 2 (mid-level) evidencePizzarossa AC, Mérola V. [Etiology of renal infarction. A systematic review]. Revista medica de Chile. 2019 Jul:147(7):891-900. doi: 10.4067/S0034-98872019000700891. Epub [PubMed PMID: 31859988]
Level 1 (high-level) evidenceMesiano P, Rollino C, Beltrame G, Ferro M, Quattrocchio G, Fenoglio R, Pozzato M, Cecere P, Forneris G, Bazzan M, Macchia G, Roccatello D. Acute renal infarction: a single center experience. Journal of nephrology. 2017 Feb:30(1):103-107. doi: 10.1007/s40620-015-0259-0. Epub 2016 Jan 7 [PubMed PMID: 26743079]
Yang J, Lee JY, Na YJ, Lim SY, Kim MG, Jo SK, Cho W. Risk factors and outcomes of acute renal infarction. Kidney research and clinical practice. 2016 Jun:35(2):90-5. doi: 10.1016/j.krcp.2016.04.001. Epub 2016 May 11 [PubMed PMID: 27366663]
Oh YK, Yang CW, Kim YL, Kang SW, Park CW, Kim YS, Lee EY, Han BG, Lee SH, Kim SH, Lee H, Lim CS. Clinical Characteristics and Outcomes of Renal Infarction. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016 Feb:67(2):243-50. doi: 10.1053/j.ajkd.2015.09.019. Epub 2015 Nov 4 [PubMed PMID: 26545635]
Caravaca-Fontán F, Pampa Saico S, Elías Triviño S, Galeano Álvarez C, Gomis Couto A, Pecharromán de las Heras I, Liaño F. Acute renal infarction: Clinical characteristics and prognostic factors. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2016:36(2):141-8. doi: 10.1016/j.nefro.2015.09.015. Epub 2015 Dec 15 [PubMed PMID: 26698927]
Hall SK. Acute renal vascular occlusion: an uncommon mimic. The Journal of emergency medicine. 1993 Nov-Dec:11(6):691-700 [PubMed PMID: 8157906]
Level 3 (low-level) evidenceHenry CM, MacEneaney P, Browne G. Spontaneous renal artery dissection: an elusive diagnosis. BMJ case reports. 2021 Sep 27:14(9):. doi: 10.1136/bcr-2021-245949. Epub 2021 Sep 27 [PubMed PMID: 34580135]
Level 3 (low-level) evidenceKuo TT, Huang CY, Chen PL, Chen IM, Shih CC. Impact of Renal Artery Stent-Graft Placement on Renal Function in Chronic Aortic Dissection. Journal of vascular and interventional radiology : JVIR. 2019 Jul:30(7):979-986. doi: 10.1016/j.jvir.2018.12.015. Epub 2019 Apr 11 [PubMed PMID: 30982639]
Varennes L, Tahon F, Kastler A, Grand S, Thony F, Baguet JP, Detante O, Touzé E, Krainik A. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights into imaging. 2015 Jun:6(3):295-307. doi: 10.1007/s13244-015-0382-4. Epub 2015 Apr 30 [PubMed PMID: 25926266]
Van den Driessche A, Van Hul E, Ichiche M, Verpooten GA, Bosmans JL. Fibromuscular dysplasia presenting as a renal infarction: a case report. Journal of medical case reports. 2010 Jun 30:4():199. doi: 10.1186/1752-1947-4-199. Epub 2010 Jun 30 [PubMed PMID: 20591148]
Level 3 (low-level) evidenceAbi Doumet A, Bustos B, Garrell J, Salman M, Haider L. Fibromuscular Dysplasia Presenting as Acute Unilateral Renal Infarction: A Case Report and Review of Two Diseases. Cureus. 2023 Mar:15(3):e35933. doi: 10.7759/cureus.35933. Epub 2023 Mar 9 [PubMed PMID: 37038580]
Level 3 (low-level) evidenceBaradhi KM, Bream P. Fibromuscular Dysplasia. StatPearls. 2024 Jan:(): [PubMed PMID: 29630256]
Antopolsky M, Simanovsky N, Stalnikowicz R, Salameh S, Hiller N. Renal infarction in the ED: 10-year experience and review of the literature. The American journal of emergency medicine. 2012 Sep:30(7):1055-60. doi: 10.1016/j.ajem.2011.06.041. Epub 2011 Aug 25 [PubMed PMID: 21871764]
Level 2 (mid-level) evidenceSaarinen HJ, Palomäki A. Acute renal infarction resulting from fibromuscular dysplasia: a case report. Journal of medical case reports. 2016 May 10:10(1):118. doi: 10.1186/s13256-016-0895-6. Epub 2016 May 10 [PubMed PMID: 27159998]
Level 3 (low-level) evidenceBemanian S, Motallebi M, Nosrati SM. Cocaine-induced renal infarction: report of a case and review of the literature. BMC nephrology. 2005 Sep 22:6():10 [PubMed PMID: 16176587]
Level 3 (low-level) evidenceAmmous A, Ghaffar MA, El-Charabaty E, El-Sayegh S. Renal infarction in COVID-19 patient. Journal of nephrology. 2021 Feb:34(1):267-268. doi: 10.1007/s40620-020-00866-2. Epub 2020 Oct 29 [PubMed PMID: 33119839]
Haas M, Spargo BH, Wit EJ, Meehan SM. Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000 Mar:35(3):433-47 [PubMed PMID: 10692269]
Level 2 (mid-level) evidenceRudnick MR, Berns JS, Cohen RM, Goldfarb S. Nephrotoxic risks of renal angiography: contrast media-associated nephrotoxicity and atheroembolism--a critical review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1994 Oct:24(4):713-27 [PubMed PMID: 7942832]
Scolari F, Ravani P. Atheroembolic renal disease. Lancet (London, England). 2010 May 8:375(9726):1650-60. doi: 10.1016/S0140-6736(09)62073-0. Epub 2010 Apr 8 [PubMed PMID: 20381857]
Mannesse CK, Blankestijn PJ, Man in 't Veld AJ, Schalekamp MA. Renal failure and cholesterol crystal embolization: a report of 4 surviving cases and a review of the literature. Clinical nephrology. 1991 Nov:36(5):240-5 [PubMed PMID: 1752074]
Level 3 (low-level) evidenceThadhani RI, Camargo CA Jr, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine. 1995 Nov:74(6):350-8 [PubMed PMID: 7500898]
Level 2 (mid-level) evidenceLi X, Bayliss G, Zhuang S. Cholesterol Crystal Embolism and Chronic Kidney Disease. International journal of molecular sciences. 2017 May 24:18(6):. doi: 10.3390/ijms18061120. Epub 2017 May 24 [PubMed PMID: 28538699]
Ivanes F, Dewaele J, Touboul C, Gatault P, Sautenet B, Barbet C, Büchler M, Quilliet L, Angoulvant D, Halimi JM. Renal arteriography with endovascular ultrasound for the management of renal infarction patients. BMC nephrology. 2020 Jul 14:21(1):273. doi: 10.1186/s12882-020-01929-z. Epub 2020 Jul 14 [PubMed PMID: 32664890]
Domanovits H, Paulis M, Nikfardjam M, Meron G, Kürkciyan I, Bankier AA, Laggner AN. Acute renal infarction. Clinical characteristics of 17 patients. Medicine. 1999 Nov:78(6):386-94 [PubMed PMID: 10575421]
Level 3 (low-level) evidenceHuang CC, Lo HC, Huang HH, Kao WF, Yen DH, Wang LM, Huang CI, Lee CH. ED presentations of acute renal infarction. The American journal of emergency medicine. 2007 Feb:25(2):164-9 [PubMed PMID: 17276805]
Level 2 (mid-level) evidenceKomolafe B, Dishmon D, Sultan W, Khouzam RN. Successful aspiration and rheolytic thrombectomy of a renal artery infarct and review of the current literature. The Canadian journal of cardiology. 2012 Nov-Dec:28(6):760.e1-3. doi: 10.1016/j.cjca.2012.06.020. Epub 2012 Aug 18 [PubMed PMID: 22906801]
Level 3 (low-level) evidenceRaja A, Karch J, Shih AF, De La Garza H, De Zepeda Diaz AJ, Maymone MBC, Phillips TJ, Secemsky E, Vashi N. Part II: Cutaneous manifestations of peripheral vascular disease. Journal of the American Academy of Dermatology. 2023 Aug:89(2):211-226. doi: 10.1016/j.jaad.2021.05.077. Epub 2022 Apr 30 [PubMed PMID: 35504485]
Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology. 1987 Oct:38(10):769-84 [PubMed PMID: 3310742]
Level 3 (low-level) evidenceKorzets Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. The Israel Medical Association journal : IMAJ. 2002 Oct:4(10):781-4 [PubMed PMID: 12389340]
Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A, Cholesterol Embolism Study(CHEST) Investigators. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. Journal of the American College of Cardiology. 2003 Jul 16:42(2):211-6 [PubMed PMID: 12875753]
Lin JW, Tsai JH, Huang CH, Lin TK. Early Diagnosis and Intervention of Acute Renal Infarction with Catheter-Directed Thrombolytic Therapy. Acta Cardiologica Sinica. 2022 Mar:38(2):134-140. doi: 10.6515/ACS.202203_38(2).20210925A. Epub [PubMed PMID: 35273434]
Kaufman EJ, Mahabadi N, Munakomi S, Patel BC. Hollenhorst Plaque. StatPearls. 2024 Jan:(): [PubMed PMID: 29261979]
Paris B, Bobrie G, Rossignol P, Le Coz S, Chedid A, Plouin PF. Blood pressure and renal outcomes in patients with kidney infarction and hypertension. Journal of hypertension. 2006 Aug:24(8):1649-54 [PubMed PMID: 16877969]
Gasparini M, Hofmann R, Stoller M. Renal artery embolism: clinical features and therapeutic options. The Journal of urology. 1992 Mar:147(3):567-72 [PubMed PMID: 1538430]
Level 3 (low-level) evidenceBolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S. Idiopathic renal infarction. The American journal of medicine. 2006 Apr:119(4):356.e9-12 [PubMed PMID: 16564787]
Level 2 (mid-level) evidenceWinzelberg GG, Hull JD, Agar JW, Rose BD, Pletka PG. Elevation of serum lactate dehydrogenase levels in renal infarction. JAMA. 1979 Jul 20:242(3):268-9 [PubMed PMID: 448917]
Wilson DM, Salazer TL, Farkouh ME. Eosinophiluria in atheroembolic renal disease. The American journal of medicine. 1991 Aug:91(2):186-9 [PubMed PMID: 1867244]
Cosio FG, Zager RA, Sharma HM. Atheroembolic renal disease causes hypocomplementaemia. Lancet (London, England). 1985 Jul 20:2(8447):118-21 [PubMed PMID: 2862317]
Level 3 (low-level) evidenceKasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Archives of internal medicine. 1987 Aug:147(8):1384-5 [PubMed PMID: 3632147]
Haqqie SS, Urizar RE, Singh J. Nephrotic-range proteinuria in renal atheroembolic disease: report of four cases. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1996 Oct:28(4):493-501 [PubMed PMID: 8840937]
Level 3 (low-level) evidenceFries C, Roos M, Gaspert A, Vogt P, Salomon F, Wüthrich RP, Vavricka SR, Fehr T. Atheroembolic disease--a frequently missed diagnosis: results of a 12-year matched-pair autopsy study. Medicine. 2010 Mar:89(2):126-132. doi: 10.1097/MD.0b013e3181d5eb39. Epub [PubMed PMID: 20517183]
Level 2 (mid-level) evidenceGreenberg A, Bastacky SI, Iqbal A, Borochovitz D, Johnson JP. Focal segmental glomerulosclerosis associated with nephrotic syndrome in cholesterol atheroembolism: clinicopathological correlations. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997 Mar:29(3):334-44 [PubMed PMID: 9041208]
Level 2 (mid-level) evidenceHazanov N, Somin M, Attali M, Beilinson N, Thaler M, Mouallem M, Maor Y, Zaks N, Malnick S. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine. 2004 Sep:83(5):292-299. doi: 10.1097/01.md.0000141097.08000.99. Epub [PubMed PMID: 15342973]
Level 2 (mid-level) evidenceMuro K, Kobayashi T, Yanagita M. The cortical rim sign in graft renal infarction. Clinical and experimental nephrology. 2019 Sep:23(9):1169-1170. doi: 10.1007/s10157-019-01757-y. Epub 2019 Jun 18 [PubMed PMID: 31214873]
Piccoli GB, Priola AM, Vigotti FN, Guzzo G, Veltri A. Renal infarction versus pyelonephritis in a woman presenting with fever and flank pain. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014 Aug:64(2):311-4. doi: 10.1053/j.ajkd.2014.02.027. Epub 2014 Apr 22 [PubMed PMID: 24767880]
Level 3 (low-level) evidenceLengelé JP, Christophe JL, Persu A. Renal infarction management: towards an etiological approach? Journal of hypertension. 2018 Mar:36(3):490-492. doi: 10.1097/HJH.0000000000001629. Epub [PubMed PMID: 29384986]
Kang Y, Wu Q, Xu J, Hong M, Ma Y, Tang X, Zhu L, Gao P, Wang J. Intravascular ultrasound provides additional insights in the hypertensive patients with focal renal artery fibromuscular dysplasia. Hypertension research : official journal of the Japanese Society of Hypertension. 2023 Jun:46(6):1407-1416. doi: 10.1038/s41440-023-01216-y. Epub 2023 Feb 10 [PubMed PMID: 36765108]
Greenberg JM, Steiner MA, Marshall JJ. Acute renal artery thrombosis treated by percutaneous rheolytic thrombectomy. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2002 May:56(1):66-8 [PubMed PMID: 11979537]
Level 3 (low-level) evidenceSilverberg D, Menes T, Rimon U, Salomon O, Halak M. Acute renal artery occlusion: Presentation, treatment, and outcome. Journal of vascular surgery. 2016 Oct:64(4):1026-32. doi: 10.1016/j.jvs.2016.04.043. Epub 2016 Jun 23 [PubMed PMID: 27345378]
Lim JL, Lau KK, Lim AKH. Successful rescue from kidney failure with delayed catheter-directed intervention after catastrophic bilateral kidney paradoxical thromboembolism. BMJ case reports. 2022 Feb 10:15(2):. doi: 10.1136/bcr-2021-246885. Epub 2022 Feb 10 [PubMed PMID: 35144961]
Level 3 (low-level) evidenceKoivuviita N, Tertti R, Heiro M, Manner I, Metsärinne K. Thromboembolism as a cause of renal artery occlusion and acute kidney injury: the recovery of kidney function after two weeks. Case reports in nephrology and urology. 2014 Jan:4(1):82-7. doi: 10.1159/000362538. Epub 2014 Apr 17 [PubMed PMID: 24847350]
Level 3 (low-level) evidenceHeidemann F, Kölbel T, Debus ES, Diener H, Carpenter SW, Rohlffs F, Tsilimparis N. Renal Function Salvage After Delayed Endovascular Revascularization of Acute Renal Artery Occlusion in Patients With Fenestrated-Branched Endovascular Aneurysm Repair or Visceral Debranching. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2018 Aug:25(4):466-473. doi: 10.1177/1526602818783506. Epub 2018 Jun 29 [PubMed PMID: 29956578]
Robinson S, Nichols D, Macleod A, Duncan J. Acute renal artery embolism: a case report and brief literature review. Annals of vascular surgery. 2008 Jan:22(1):145-7 [PubMed PMID: 18083341]
Level 3 (low-level) evidenceHassanein M, Saleh Y, Randhawa M, Karve M. Renal artery embolism successfully managed by ultrasound enhanced catheter directed thrombolysis. The Egyptian heart journal : (EHJ) : official bulletin of the Egyptian Society of Cardiology. 2018 Dec:70(4):447-450. doi: 10.1016/j.ehj.2018.10.002. Epub 2018 Oct 29 [PubMed PMID: 30591770]
Karakurt A. New Thrombolytic Infusion Application of Dissolving Renal Artery Embolic Thrombosis: Low-Dose Slow-Infusion Thrombolytic Therapy. Case reports in nephrology. 2018:2018():1609025. doi: 10.1155/2018/1609025. Epub 2018 May 2 [PubMed PMID: 29854504]
Level 3 (low-level) evidenceChondros K, Karpathakis N, Tsetis D, Sofras F, Mamoulakis C. Systemic thrombolysis with the use of tenecteplase for segmental acute renal in-farction potentially associated with multiple thrombophilic gene polymorphisms. Hippokratia. 2014 Jan:18(1):67-70 [PubMed PMID: 25125956]
Kim H, Zhen DB, Lieske JC, McBane RD, Grande JP, Sandhu GS, Melduni RM. Treatment of Cholesterol Embolization Syndrome in the Setting of an Acute Indication for Anticoagulation Therapy. Journal of medical cases. 2014 Jun 1:5(6):376-379 [PubMed PMID: 25197328]
Level 3 (low-level) evidenceBelenfant X, Meyrier A, Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999 May:33(5):840-50 [PubMed PMID: 10213638]
Scolari F, Ravani P, Pola A, Guerini S, Zubani R, Movilli E, Savoldi S, Malberti F, Maiorca R. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. Journal of the American Society of Nephrology : JASN. 2003 Jun:14(6):1584-90 [PubMed PMID: 12761259]
Level 2 (mid-level) evidenceColburn S, Childers WK, Chacon A, Swailes A, Ahmed FM, Sahi R. The cost of seeking an edge: Recurrent renal infarction in setting of recreational use of anabolic steroids. Annals of medicine and surgery (2012). 2017 Feb:14():25-28. doi: 10.1016/j.amsu.2017.01.015. Epub 2017 Jan 12 [PubMed PMID: 28127424]
Bae EJ, Hwang K, Jang HN, Kim MJ, Jeon DH, Kim HJ, Cho HS, Chang SH, Park DJ. A retrospective study of short- and long-term effects on renal function after acute renal infarction. Renal failure. 2014 Oct:36(9):1385-9. doi: 10.3109/0886022X.2014.947514. Epub 2014 Aug 12 [PubMed PMID: 25112371]
Level 2 (mid-level) evidenceKwon JH, Oh BJ, Ha SO, Kim DY, Do HH. Renal Complications in Patients with Renal Infarction: Prevalence and Risk Factors. Kidney & blood pressure research. 2016:41(6):865-872. doi: 10.1159/000452589. Epub 2016 Nov 21 [PubMed PMID: 27871081]
Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Current opinion in nephrology and hypertension. 2012 Nov:21(6):628-34. doi: 10.1097/MNH.0b013e3283588f24. Epub [PubMed PMID: 23010757]
Level 3 (low-level) evidence