Introduction
In exudative retinal detachment, subretinal fluid accumulates without retinal breaks or tractional forces.[1][2] The underlying cause is a blood-retinal barrier disruption.[1] Processes that either actively or passively allow for excessive fluid accumulation in the subretinal space between the retinal pigment epithelium and neurosensory retina lead to retinal detachment.[3] During a retinal detachment, the retina separates from the underlying retinal pigment epithelium and choroid, resulting in retinal ischemia and photoreceptor degeneration.
The subretinal space originates from the remnant of the embryonic optic vesicle.[3] Retinal detachment is classified into three groups based on the etiology of subretinal fluid accumulation: rhegmatogenous, tractional, or exudative.[1][4] Some cases may involve a combination of these categories. Rhegmatogenous retinal detachment occurs when retinal tears or holes allow fluid to seep into the subretinal space.[2] Tractional retinal detachment happens when traction on the retina occurs due to fibrovascular proliferation over the retina, often resulting from ischemic or hypoxic stimuli or other factors.[2]
No anatomical adhesion exists between the retinal pigment epithelium and the neurosensory retina.[3] Neural cell adhesion molecules expressed on the apical surface of retinal pigment epithelial cells facilitate adhesion between the retinal pigment epithelium and photoreceptor cells.[3][5] Various etiologic factors, including inflammation, idiopathy, infection, surgery, neoplasia, vascular issues, or drug effects, can trigger ischemic-hypoxic stimuli, compromising the integrity of the blood-retinal barrier.[6][1]
Timely diagnosis and treatment offer the possibility of reasonably good vision in patients with exudative retinal detachment.[1] Exudative retinal detachments are primarily managed medically. Surgical intervention with scleral buckling or vitrectomy is rarely considered, typically only if all medical interventions prove ineffective.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Exudative retinal detachment occurs either due to hypersecretion of fluid into the subretinal space or defective fluid transport from the subretinal space. Various factors, such as inflammation, idiopathy, infection, surgery, neoplasia, vascular issues, or drug effects, can compromise the integrity of the blood-retinal barrier, ultimately resulting in exudative retinal detachment.[1][6]
Inflammatory Causes
- Vogt-Koyanagi-Harada syndrome (VKH) [7]
- Sympathetic ophthalmia [8][9][10]
- Orbital inflammation
- Orbital pseudotumor
- Idiopathic orbital inflammatory disease [11]
- Posterior scleritis [12]
- Benign reactive lymphoid hyperplasia [13]
- Acute posterior multifocal placoid pigment epitheliopathy (APMPPE) [14]
- Serpiginous choroiditis [15]
- Behçet disease [16]
- Intermediate uveitis [17]
- Relapsing polychondritis [18]
- Inflammatory bowel disease [19]
- Sarcoidosis [20]
- Unilateral acute idiopathic maculopathy [21]
HLA-DR53 is associated with VKH syndrome in individuals of Chinese and Japanese descent. VKH syndrome in those of Chinese, Japanese, Native American, Hispanic, and European descent is associated with HLA-DR4.[22] HLA-DR1 is associated with VKH syndrome in individuals of Hispanic descent, while HLA-DQ4 is associated with VKH syndrome in the Japanese population.[23] Mutations in KIF11, observed in 5% to 8% of familial exudative vitreoretinopathy cases, are connected with microcephaly with or without chorioretinopathy, lymphedema, or intellectual disability (MCLID). MCLID is an autosomal dominant condition.[24]
Idiopathic Causes
Infectious Causes
- Tuberculosis [27]
- Dengue [28]
- Syphilis [29]
- Cat-scratch disease [30]
- Cytomegalovirus (CMV) retinitis [31]
- Lyme disease [32]
- Fungal infection [33]
- Nematode infection
- Diffuse unilateral subacute neuroretinitis [34][35]
- Toxoplasmosis [36]
- Herpes zoster [37]
Surgical or Postsurgical Causes
Neoplastic or Paraneoplastic Causes
- Metastatic disease [41]
- Choroidal melanoma and nevus [42]
- Retinoblastoma [43]
- Choroidal hemangioma [44]
- Retinal or optic disc capillary hemangioblastoma or Von Hippel-Lindau syndrome [45]
- Choroidal osteoma [46]
- Lymphoma [47]
- Bilateral diffuse uveal melanocytic proliferation [48][49]
- Multiple myeloma [50]
- Leukemia [51]
- Lymphomatoid granulomatosis [52]
- Cancer-associated retinopathy [53]
Patients suspected of having bilateral diffuse uveal melanocytic proliferation should undergo evaluation for ovarian, lung carcinoma, and urogenital cancer.[54][55][56] These patients should also have regular ophthalmologic monitoring for early detection and management of ocular complications.
Vascular or Hematologic Causes
- Preeclampsia [57][58]
- Eclampsia
- Malignant hypertension [59][60][61]
- Disseminated intravascular coagulation [62]
- Thrombotic thrombocytopenic purpura [63]
- Granulomatosis with polyangiitis [64]
- HELLP syndrome (hemolysis, elevated liver enzymes, low platelets) [65]
- Systemic lupus erythematosus [66]
- Antiglomerular basement membrane disease [67]
- Organ transplantation and hemodialysis [68]
- Severe diabetic retinopathy
- Retinal vein occlusion in the systemically unstable patient [69][70]
- Renal diseases like IGA nephropathy, chronic renal failure, type II membranoproliferative glomerulonephritis, and crescentic membranous nephropathy [71]
- Choroidal neovascularization due to various causes, including age-related macular degeneration [72]
- Idiopathic polypoidal choroidal vasculopathy [73]
- Coats disease [74]
- Retinal vein occlusion [70]
- Venous overload choroidopathy due to carotid obstruction [75]
- Familial exudative vitreoretinopathy [76]
- Retinopathy of prematurity [77]
- Norrie disease
- Retinal artery macroaneurysm [78][79]
Norrie disease is a rare X-linked recessive disorder typically manifesting as bilateral leukocoria in children. Ocular findings in Norrie disease include microphthalmos, cataracts, leukokoria, and retinal detachment.[80] Systemic findings encompass intellectual disabilities, behavioral difficulties, growth and developmental delays, hearing loss, peripheral vascular disease, and disruptions in sleep-wake cycles.
Miscellaneous Causes
- Acute exudative polymorphous vitelliform maculopathy [81]
- Medications like interferon-α, ribavirin, checkpoint inhibitors like atezolizumab and ipilimumab, mitogen-activated protein kinase kinase (MEK) inhibitors, and topiramate [82][83][84][85]
- Systemic steroids
- Due to the worsening of central serous chorioretinopathy[86]
Epidemiology
Age predilection in exudative retinal detachment varies across different diseases. Retinal detachment due to Coats disease is evident in younger patients, while exudative retinal detachment secondary to malignancy and age-related macular degeneration is more prevalent among older patients. Exudative retinal detachment secondary to preeclampsia, eclampsia, HELLP syndrome, VKH syndrome, and central serous chorioretinopathy tends to occur more frequently in patients who are middle-aged.[87]
Choroidal malignancies and exudative age-related macular degeneration are more common in White patients.[88][89] Black patients have a higher incidence of inflammatory ocular diseases.[90] VKH syndrome (see Image. Vogt-Koyanagi-Harada Syndrome) is more common in patients of Asian descent.[6] Due to the multifactorial origin of exudative retinal detachment, previous data lacks data on the frequency. Additionally, sex predilection in exudative retinal detachment varies across different diseases. Central serous chorioretinopathy, Coats disease, and uveal effusion syndrome are more commonly diagnosed in males.[26][91][92] Individuals with a stressful lifestyle, type A personality, and pregnancy have an elevated risk of central serous chorioretinopathy.[86]
Pathophysiology
Typically, water flows from the vitreous cavity to the choroid. Any excess inflow or insufficient outflow from the vitreous cavity beyond what standard compensatory mechanisms can handle results in fluid accumulation in the subretinal space, culminating in an exudative retinal detachment.
The amount of fluid in the subretinal space is influenced by various factors. These include the relative hyperosmolarity of the choroid compared to the vitreous, as well as the active pumping of ions and water from the vitreous into the choroid by the retinal pigment epithelium. Choroidal vascular permeability directly affects the choroidal interstitial fluid content, and damage to the retinal pigment epithelium can disrupt its pumping function, resulting in fluid accumulation in the subretinal space.
The retinal pigment epithelium serves as the primary component of the blood-retinal barrier.[93] Comprising a single polarized monolayer of cells, the retinal pigment epithelium forms the outer blood-retinal barrier, crucial for regulating fluid and molecular transport between retinal vasculature and retinal layers.[94][95] The blood-retinal barrier consists of 2 segments: the inner and outer blood-retinal barrier. Tight junctions between the retinal pigment epithelial cells form the outer blood-retinal barrier.[96] No anatomical attachment is present between the retinal pigment epithelium and the neurosensory retina. However, neural cell adhesion molecules expressed on the apical surface of the retinal pigment epithelial cells facilitate attachment between the two structures.[96] Tight junctions between endothelial cells of the capillaries form the inner blood-retinal barrier.[96]
The tight junctions formed by cells comprising the blood-retinal barrier play a crucial role in its integrity and function.[95] Disruption of these junctions, whether due to infectious, inflammatory, vascular, infiltrative, neoplastic, or degenerative factors, leads to blood-retinal barrier failure, causing fluid accumulation in the subretinal space.[93][95] Furthermore, the loss of polarity in the retinal pigment epithelium can cause the reversal of pumping mechanisms, contributing to fluid accumulation in the subretinal space.[86]
Histopathology
During the acute phases of exudative retinal detachment, patients experience a loss of photoreceptor outer segments. In chronic cases, notable findings include proliferation of retinal pigment epithelium, formation of cysts, increased leakage into the subretinal space, and retinoschisis.[1]
History and Physical
A retinal detachment patient may present with various symptoms and a pertinent medical history. They may report decreased visual acuity, floaters, eye pain, visual field defects, redness, leukocoria, and metamorphopsia. It is essential for the clinician to note the duration of symptoms, inquire about any prior intraocular surgeries or systemic illnesses, and review the patient's use of systemic or topical medications. Additionally, excessive weight loss or loss of appetite may raise concerns about malignancy, warranting a thorough evaluation. A history of a flu-like illness during the prodromal phase may indicate the acute uveitis phase of VKH syndrome.[97]
A comprehensive physical examination is crucial for assessing potential systemic causes of exudative retinal detachment.[1] Measuring the patient's blood pressure is essential. The ophthalmic examination should include the best-corrected visual acuity, intraocular pressure, and evaluation for longstanding amblyopia. Ptosis, proptosis, and pain with ocular movements may indicate posterior scleritis and orbital inflammation.[98][99]
Anterior Segment Examination
Anterior segment examination may be unremarkable, but in cases of inflammatory etiologies, specific manifestations may include circumcorneal congestion, aqueous cells, anterior chamber flare, posterior synechiae, complicated cataracts, keratic precipitates, hypopyon, and retrolental or anterior vitreous cells on slit-lamp examination.[100] Raised intraocular pressure may be observed due to secondary angle closure associated with ciliary congestion and anterior rotation of the ciliary body, as seen in conditions such as scleritis, panretinal photocoagulation, and VKH syndrome.[14]
Neovascularization of the iris and angle may occur in patients with VKH syndrome.[101] Evaluating the pupil for a relative afferent pupillary defect is important, as it can provide insight into the severity of posterior segment involvement.[102] Additionally, features indicative of rhegmatogenous retinal detachment, such as retrolental pigments or Shafer sign, as well as hypotony, should also be noted during the examination.[103]
Posterior Segment Examination
Shifting fluid across the retina is a characteristic feature of exudative retinal detachment.[104] As the patient changes position, the subretinal fluid accumulates in the most dependent area of the retina. Previously, shifting fluid was the hallmark finding associated with exudative retinal detachment.[105] However, shifting fluid is also present with aphakic and longstanding rhegmatogenous retinal detachment in patients with small retinal holes.[106]
Other characteristic features of exudative retinal detachment include a smooth retinal surface without retinal folds or corrugations, and the presence of vitreous cells. In cases with inflammatory etiologies, optic disc hyperemia may be observed.[105] Additionally, choroidal neovascularization can develop in chronic nonresolving cases of exudative retinal detachment.[107]
Choroidal metastases can present as an exudative retinal detachment, with the metastases typically being small while the detachment appears relatively larger.[41][108] Unlike rhegmatogenous retinal detachment, exudative retinal detachment lacks associated Shaffer sign, posterior vitreous detachment, and vitreous hemorrhage.[1] Detailed discussion on the distinctions between exudative and rhegmatogenous retinal detachments is provided in the "Differential Diagnosis" section.
Assessing for Retinal Break or Tractional Bands
Exudative retinal detachment is a diagnosis of exclusion. A thorough central and peripheral fundal examination is necessary to exclude the presence of any fibrovascular proliferation causing traction over the retina, resulting in a tractional retinal detachment, or the presence of any retinal tear, hole, or dialysis that may lead to a rhegmatogenous retinal detachment.[109][110] In cases of inferior bullous retinal detachment with shifting fluid, evaluating for superior small breaks is crucial.[109] The presence of proliferative vitreoretinopathy suggests a likely rhegmatogenous retinal detachment. This differentiation is essential as both rhegmatogenous and tractional retinal detachments require surgical management.[111]
Evaluation
Diagnosis of exudative retinal detachment is primarily clinical, relying on a thorough ophthalmic examination. However, additional testing may be necessary to determine the underlying etiology.
Laboratory and General Imaging
The evaluation is tailored to each patient based on medical history, associated symptoms, and examination findings. Depending on clinical presentation, tests may include a combination of the following:
- Complete blood cell count with a differential
- Erythrocyte sedimentation rate
- Mantoux test
- Chest radiography
- Rheumatoid factor
- Antinuclear antibody
- Interferon-gamma release assay
- Venereal disease research laboratory (VDRL) test and fluorescent treponemal antibody absorption test (FTA-ABS) test
- Serum homocysteine levels
- Serum cortisol levels
- Renal function tests
- Liver function test
- Toxoplasma, rubella, cytomegalovirus, herpes (TORCH) markers
- Prothrombin time, bleeding time, and clotting time
- Fasting blood sugar, postprandial blood sugar, and glycosylated hemoglobin [112]
The Amsler grid provides a simple method for detecting metamorphopsia and scotoma.[113] It is a valuable tool for monitoring changes in central vision over time and can aid in assessing retinal function.
Ocular Imaging
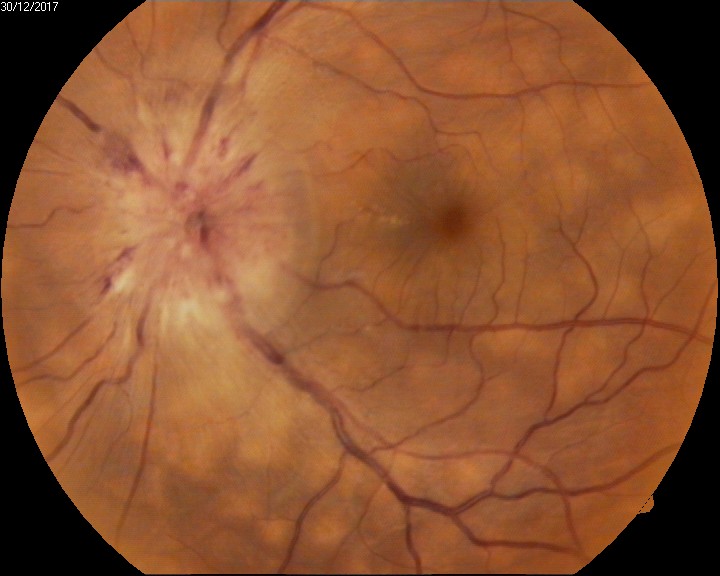
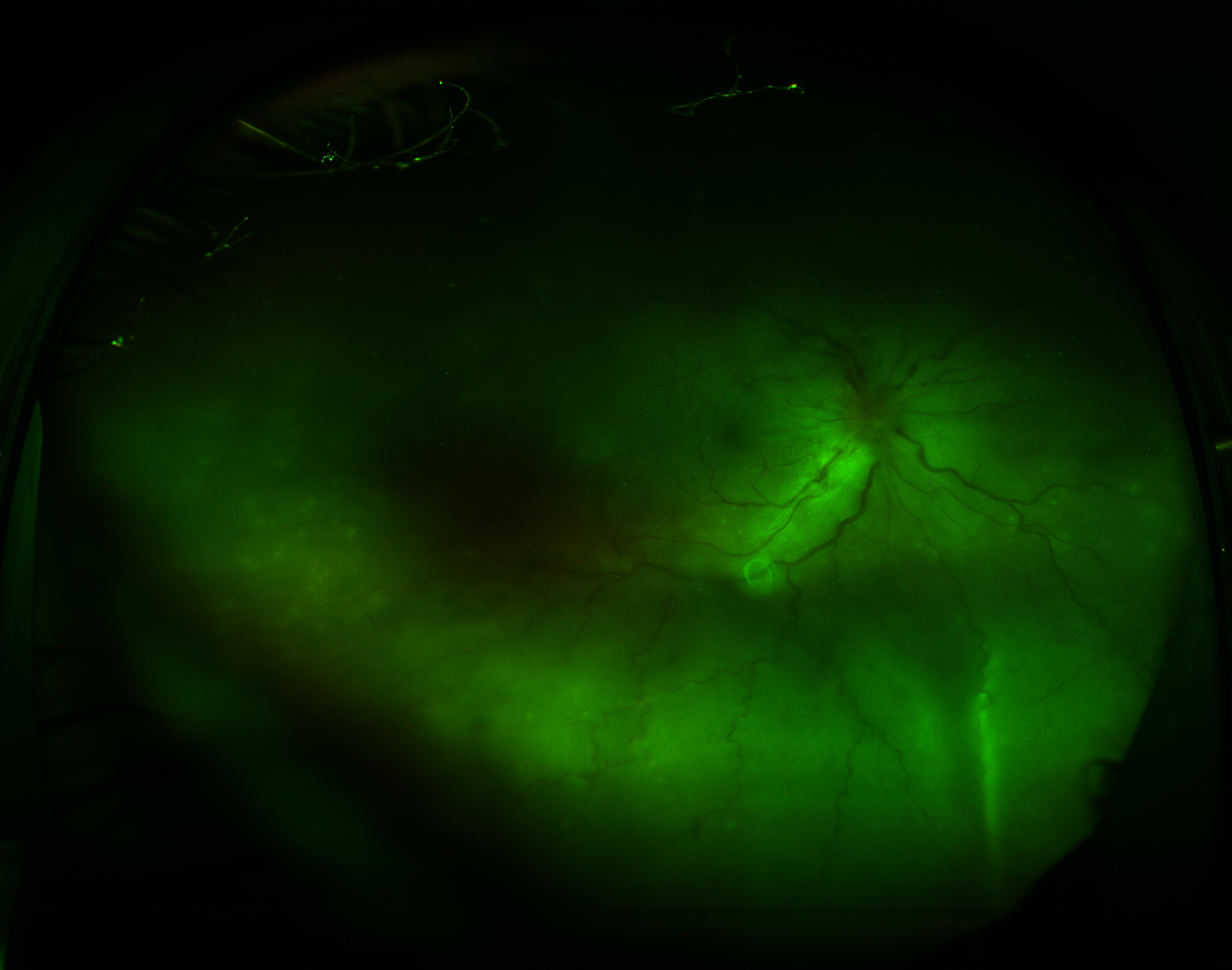
Imaging studies, such as optical coherence tomography (OCT), fundus fluorescein angiography (FFA), and ultrasonography, may be necessary to investigate the etiology and guide the management of an exudative retinal detachment. These imaging modalities provide detailed information about the retinal anatomy, vascular perfusion, and underlying pathology, aiding in accurate diagnosis and treatment planning (see Image. Exudative Retinal Detachment).
Optical coherence tomography of the macula
OCT scanning is invaluable for delineating the macula's serous detachment, assessing the septae's presence, and measuring choroidal thickness. Additionally, it allows for the precise visualization of any associated structural changes in the retina and choroid.
Choroidal thickness decreases as the primary inflammatory insults subside with or without treatment.[114][115] Hence, choroidal thickness is a useful OCT biomarker for disease prognostication.[116] Increased choroidal thickness is noted on enhanced depth imaging OCT in patients affected with various conditions, such as the following:
- VKH syndrome
- Posterior scleritis
- Uveal effusion syndrome
- Central serous chorioretinopathy [117]
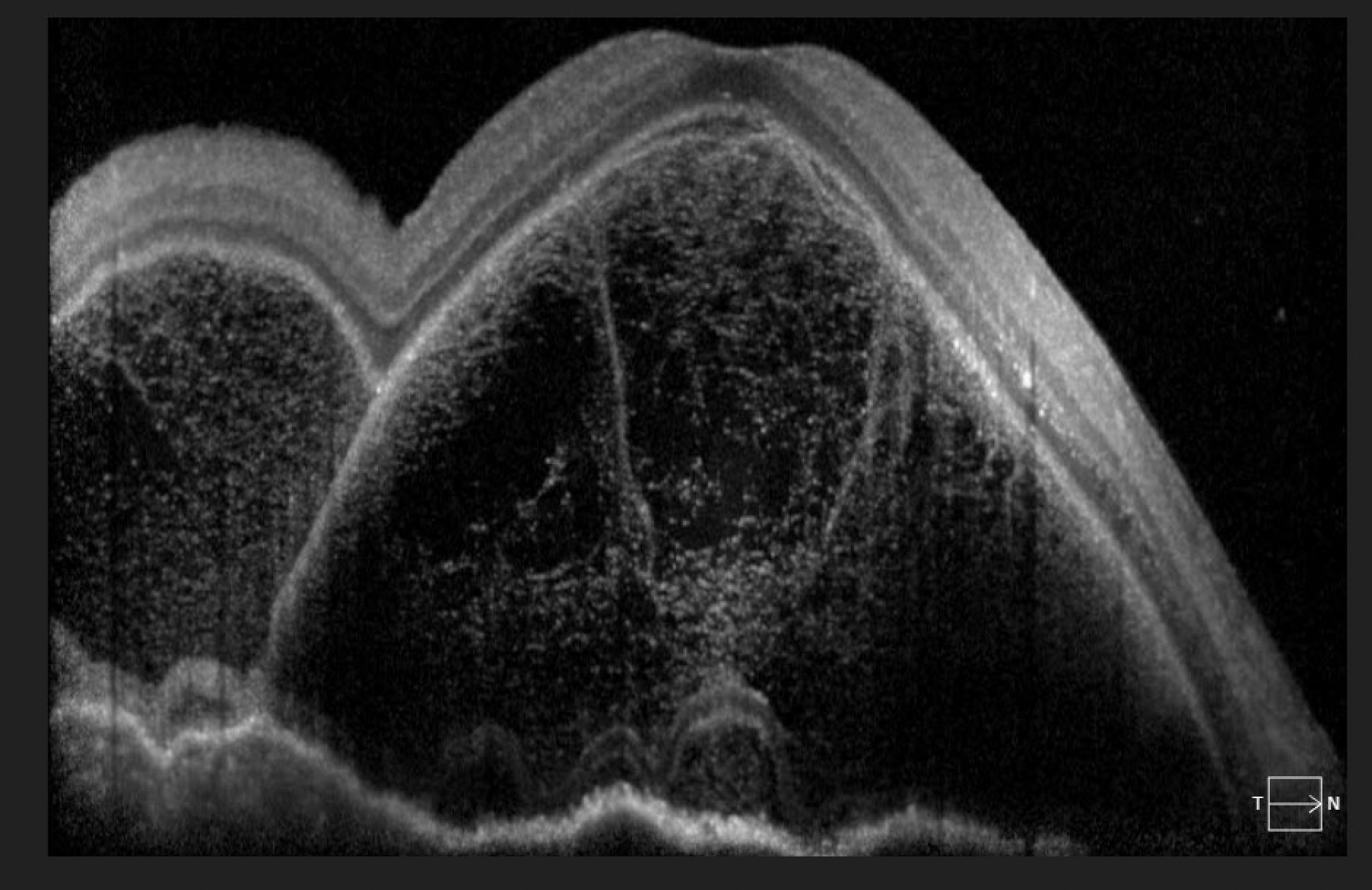
Patients with bacillary layer detachment may present symptoms such as metamorphopsia, scotoma, and decreased visual acuity. Additionally, they may exhibit characteristic findings on OCT, including disruption or elevation of the outer retinal layers and detachment of the photoreceptor outer segments from the retinal pigment epithelium (see Image. Bacillary Layer Detachment). This can be seen in conditions such as:
- VKH syndrome
- Sympathetic ophthalmia
- Acute posterior multifocal placoid pigment epitheliopathy
- Hematological disorders
- Posterior scleritis
- Choroidal metastases
- Central serous chorioretinopathy [118]
Fundus fluorescein angiography
FFA utilizes intravenous fluorescein to examine the choroid and retinal vascular supply. Patients with an exudative retinal detachment may have areas of hyper- and hypofluorescence, multiple window defects, dye pooling in serous retinal detachment areas, and disc staining.[119] Angiography is also beneficial for detecting choroidal and disc neovascularization, neovascularization elsewhere, retinochoroidal and arteriovenous anastomoses, and identifying the peripheral ischemic zone, aiding in further management planning.[120]
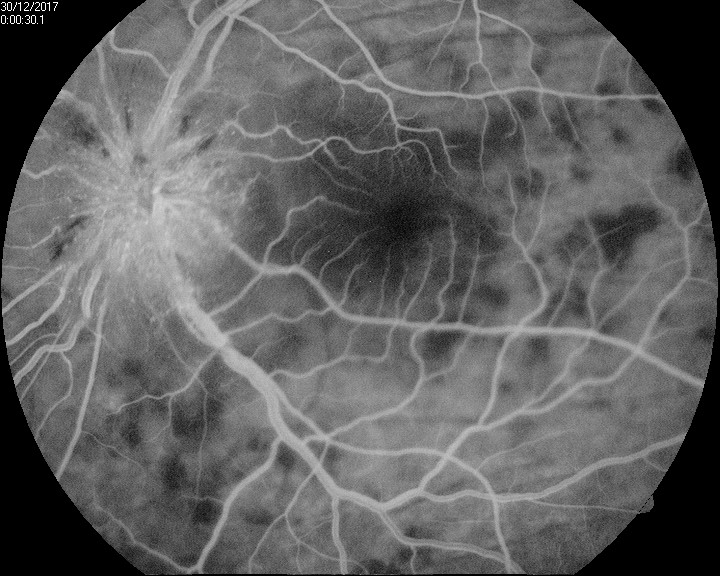
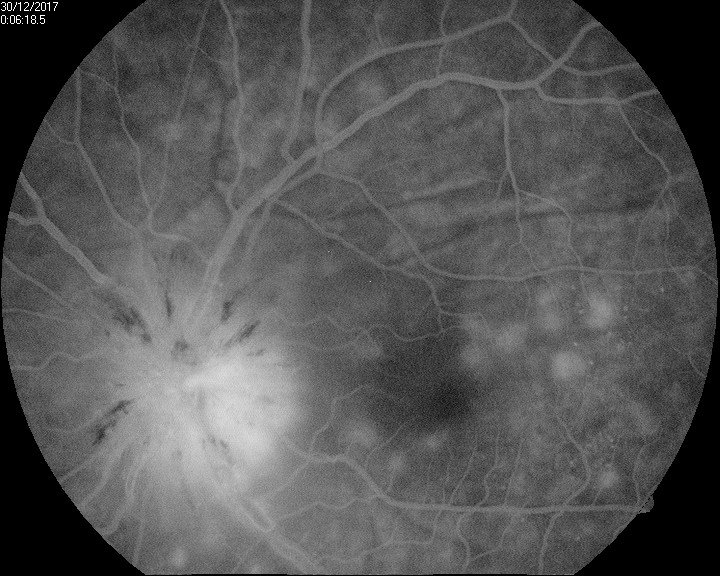
In patients with VKH syndrome, FFA imaging reveals hypofluorescent dots in the early phase (see Image. Early Phase Vogt-Koyanagi Harada Syndrome), followed by multiple focal areas of leakage and subretinal fluid accumulation in the late phase (see Image. Late Phase Vogt-Koyanagi Harada Syndrome).[115] These findings indicate the choroidal inflammatory process and associated disruption of the blood-retinal barrier seen in VKH syndrome.
In patients with central serous chorioretinopathy, characteristic patterns such as the inkblot or, less commonly, the smokestack pattern may be observed. The inkblot pattern begins as a pinpoint leakage in the early phase, which concentrically enlarges in the late phase. Conversely, with the smokestack pattern, leakage from the choroid begins as a pinpoint and gradually expands to form an umbrella- or tree-like appearance.[86]
In patients with posterior scleritis, FFA typically reveals blocked fluorescence during the initial arteriovenous phase and diffuse hyperfluorescence in the late phase without any evident leak. Choroidal folds appear as alternating hyperfluorescent and hypofluorescent bands.[121][122] Uveal effusion syndrome appears as hyperfluorescence in a leopard-spot pattern on FFA.[123]
In patients with acute posterior multifocal placoid pigment epitheliopathy, early-phase FFA typically demonstrates hypofluorescence due to choroidal fluorescence blockage. Subsequently, staining of the lesions becomes evident later in the angiogram. As the disease becomes inactive, hyperfluorescence corresponding to window defects in the mottled retinal pigment epithelium develops.[124] The early phase of FFA early phase reveals a reticular pattern of hypofluorescence surrounded by a background of choroidal hyperfluorescence in patients with bilateral diffuse uveal melanocytic proliferation.[125]
Indocyanine green angiography
Indocyanine green (ICG) angiography uses indocyanine green dye instead of fluorescein. Unlike fluorescein, indocyanine green fluoresces in the infrared or nonvisible light spectrum. The infrared wavelengths can penetrate the retinal layers, rendering the circulation in deeper layers visible when photographed with an infrared-sensitive camera.
In patients with central serous chorioretinopathy, early-phase indocyanine green angiography (ICG) typically reveals hypocyanescence, suggesting nonperfusion or delayed choriocapillaris filling. Subsequently, mid-phase hypercyanescence is observed, indicating choroidal vessel hyperpermeability. The hypercyanescence gradually diminishes in the late phase.[126][127][128]
In VKH syndrome, ICG typically demonstrates early hyperfluorescence accompanied by leakage and hypofluorescent dark dots at the choroid level.[129][130] These findings indicate choroidal inflammation and disruption of the blood-retinal barrier seen in VKH syndrome.
In idiopathic polypoidal choroidal vasculopathy, ICG typically reveals interconnecting channels with an abnormal vascular network that becomes visible within 1 minute of dye injection. Additionally, the polyp and feeder vessels associated with the lesion are often identified on ICG imaging.[131][132]
In acute posterior multifocal placoid pigment epitheliopathy, the early lesions typically exhibit hypofluorescence on fluorescein angiography. As the angiogram progresses into the late phases, these lesions become more defined in shape and size, often appearing larger than the placoid lesions seen clinically. As the lesions heal, the hypofluorescence in the late phases tends to become smaller and less distinct.[133][134][135][136]
Ultrasonography
Ultrasound is invaluable for detecting choroidal thickness, assessing the presence or absence of choroidal masses, determining the size and location of choroidal masses, and evaluating scleral thickness.[98] It mainly benefits patients with hazy media that preclude a precise posterior segment examination.
Autofluorescence
Fundus autofluorescence maps naturally and pathologically occurring fluorophores in the posterior pole. In patients with cancer-associated retinopathy, a parafoveal ring of hyper-autofluorescence is typically observed, with normal autofluorescence within the ring and hypo-autofluorescence outside the ring.[137] In bilateral diffuse uveal melanocytic proliferation, ultra-widefield color fundus photography often reveals greater pigmentary changes and lesions in the periphery. Ultra-widefield autofluorescence imaging demonstrates the characteristic giraffe-pattern fundal changes associated with bilateral diffuse uveal melanocytic proliferation.[138]
In acute central serous chorioretinopathy, observers often detect focal areas of hypoautofluorescence, which may correspond to the leakage observed on FFA. In chronic central serous chorioretinopathy, hyperautofluorescent tracks are present due to the accumulation of photoreceptor pigments.[86] In Best disease, autofluorescence imaging shows uniform hyperfluorescence of vitelliform lesions, while sub-retinal pigment epithelium fibrosis or atrophy shows hypofluorescence.[139][140] The increased autofluorescence is attributed to the lipofuscin content.[141][142][143]
Electrophysiological tests
Electroretinography (ERG) measures the retina's electrical response to light stimuli. In patients with cancer-associated retinopathy, full-field ERG may reveal attenuated or absent photopic and scotopic responses, indicating dysfunction of both cones and rods. However, in some cases of cancer-associated retinopathy, only the cones are affected, and full-field ERG may appear normal. In contrast, multifocal ERG is typically abnormal in cancer-associated retinopathy, reflecting localized retinal dysfunction.[144][145]
Treatment / Management
Exudative retinal detachment has a multifactorial origin, and the management approach is individualized depending on the type, severity, and presentation stage. Medical management is the primary approach for treating exudative retinal detachment. Surgical intervention is rarely necessary unless all other measures fail.[76]
Inflammatory diseases like VKH syndrome and posterior scleritis should be treated with intravenous methylprednisolone pulse therapy at 1 g/d for 3 to 5 days, followed by a shift to oral corticosteroids according to weight.[97] The involvement of inflammation and the use of steroids in the management of uveal effusion may be subject to debate.[26][146](B3)
In successfully treating VKH syndrome, clinicians have utilized immunosuppressive agents such as methotrexate, azathioprine, cyclosporine A, mycophenolate mofetil, and alkylating agents, alongside corticosteroids.[147][148][149][150] These immunosuppressive therapies aim to suppress the autoimmune response and reduce inflammation associated with VKH syndrome.(B2)
The American Uveitis Society and the International Uveitis Study Group recommend immunosuppressive agents as mandatory in treating VKH syndrome to prevent recurrences. While high-dose corticosteroids also affect recurrences, as outlined above, careful consideration must be given to balance the initial treatment with the subsequent need for immunosuppressive agents.[97]
Oral nonsteroidal anti-inflammatory medications can be effective with various appropriate regimens, such as ibuprofen 600 to 800 mg 4 times daily, piroxicam 20 mg daily, and naproxen 375 mg twice daily. In cases unresponsive to oral nonsteroidal anti-inflammatory medications, high-dose systemic corticosteroids are often used, with typical doses of 1 mg/kg/d.[151][152][153] (B3)
These steroids are tapered slowly over several weeks, carefully considering potential side effects such as weight gain, mood instability, blood-sugar abnormalities, and adrenal insufficiency. If the patient continues to demonstrate active disease or cannot tolerate corticosteroids, prompt corticosteroid-sparing immunosuppression is warranted.
Antimetabolites such as methotrexate and mycophenolate mofetil are employed, often to their maximal doses of 25 mg/week of methotrexate or 1,500 mg twice daily of mycophenolate.[154][152] These medications may take up to 6 months for full effect, and some patients may require a faster-acting solution. Biologics such as tumor necrosis factor inhibitors (TNF-inhibitors) like adalimumab and infliximab may be necessary in these cases.[155][156](B3)
The management of malignant hypertensive retinopathy involves controlling blood pressure and addressing any secondary causes of hypertension. Timely intervention and close monitoring of blood pressure levels are crucial to prevent further retinal damage and preserve visual function in patients with malignant hypertensive retinopathy.
Management Options of Central Serous Chorioretinopathy
Management of central serous chorioretinopathy includes observation, laser, photodynamic therapy, and anti-vascular endothelial growth factor (anti-VGEF) agents. Laser photocoagulation can treat the retinal pigment epithelium leakage sites identified on angiography, effectively sealing the leakage point and promoting the resolution of subretinal fluid.[157] (B2)
Central serous chorioretinopathy with a subfoveal leak, juxtafoveal leak, multiple leaks, and chronic cases with diffuse decompensation of the retinal pigment epithelium often benefit from laser photodynamic therapy as a management strategy.[158][159] Anti-VEGF therapy has been proposed to reduce choroidal hyperpermeability by upregulating the tight junctions between endothelial cells and reducing vascular fenestrations.[160][161][162] (A1)
Surgical intervention is considered the last resort for managing exudative retinal detachment after exhausting all medical treatment options.[4] Surgical management options include:[163]
- Scleral buckling with external drainage of the subretinal fluid with laser of the nonperfused retina
- Pars plana vitrectomy with internal drainage of the subretinal fluid with laser delimitation of the nonperfused retina with or without endotamponade with expansile gases or silicone oil [164][165] (B3)
Management of Familial Exudative Vitreoretinopathy
The management of familial exudative vitreoretinopathy depends on the stage at the time of presentation:
- Stage 1: Observation
- Stage 2: Laser photocoagulation of the areas with abnormal vessels and ischemia or the avascular zone [166]
- Advanced stage: In cases of retinal detachment, a surgical approach is necessary. Scleral buckling, vitrectomy, and lasering of the avascular zone are recommended.[167]
Screening family members for genetic predisposition to certain retinal conditions is advisable. The use of anti-VEGF therapy remains a topic of debate in the management of retinal neovascularization. According to a study by Tagami M et al, regression of neovascularization occurs after a single intravitreal injection of bevacizumab.[168] A study by Henry CR et al reveals favorable results with intravitreal bevacizumab use along with laser or surgical treatment.[169](B3)
There is a risk of worsening tractional forces, potentially causing tractional retinal detachment with the administration of anti-VEGF injections. Therefore, clear guidelines are essential for appropriately using anti-VEGF as an adjunct to laser or surgical therapy.[167][169]
Management of Coats Disease
The management of Coats disease varies depending on the stage of the condition. Care for these patients is outlined as follows:
- Direct laser photocoagulation of the telangiectatic vessels is a beneficial treatment option in mild to moderate cases of exudation.[92] (B2)
- Laser therapy will not be effective in cases of massive subretinal exudation and exudative retinal detachment. In such cases, practitioners perform cryotherapy over the diseased retina using a double freeze-thaw technique.[170] (B2)
- Anti-VEGF medications and intravitreal corticosteroids can be used as adjunctive therapy before laser or surgical management, as they also help to reduce macular edema associated with exudation.[171][172] (B3)
- Advanced stages of Coats disease are associated with inferior visual prognosis. Treatment in advanced stages focuses on avoiding a painful blind eye and enucleation. Scleral buckling with external drainage or pars plana vitrectomy with or without endotamponade may save the eye.[173][163]
- Transscleral diode laser photocoagulation is a treatment option for neovascular glaucoma. If all measures to save the eye fail, practitioners should enucleate an endstage painful blind eye.[92][170] (B2)
Exudative retinal detachment due to wound leak or hypotony after ocular surgery responds satisfactorily with timely wound suturing and anti-inflammatory agents.[1] Similarly, exudative detachments secondary to panretinal photocoagulation or scleral buckling may benefit from topical and systemic anti-inflammatory or steroid therapy.[38] In cases where the scleral buckle exerts excessive pressure, readjustment is required to alleviate stress on the vascular system.[39](B3)
Radiation therapy, particularly proton beam radiation, is frequently employed in the treatment of malignant uveal melanoma.[42][174] However, post-radiation therapy, there is an elevated risk of exacerbating retinal detachment, particularly with proton beam radiation.[174] Treatment of choroidal tumors with exudative retinal detachment includes proton beam radiation or brachytherapy using a plaque, transretinal tumor biopsy for prognostication of the disease, and surgical intervention as the last measure with may entail pars plana vitrectomy and drainage of subretinal fluid with endotamponade.[42]
A variety of treatment options exist for choroidal hemangioma, including laser photocoagulation, low-dose external beam radiation, proton beam irradiation, gamma knife radiosurgery, radiotherapy, cryotherapy, photodynamic therapy with verteporfin, transpupillary thermotherapy, oral β-blockers, or intravitreal anti–VEGF.[175] Patients with metastatic tumors should receive chemotherapy or focal radiation therapy.[176]
Patients with infectious causes leading to exudative retinal detachment should be promptly treated with appropriate antibiotics.[177] Tuberculous exudative detachment requires a comprehensive approach involving antitubercular drugs in conjunction with glucocorticoids to manage the infection effectively.[178] Syphilitic exudative detachment necessitates treatment with the regimen typically used for neurosyphilis, while viral etiologies warrant urgent initiation of antiviral drugs alongside corticosteroids to mitigate inflammation and viral replication.[179][180][181] (B3)
Pregnancy-induced hypertension can lead to marked vision loss, but these changes are typically reversible in the postpartum period.[59] Adequate blood pressure control is the most critical intervention. Compared to malignant hypertension, the visual prognosis is generally more favorable in cases of pregnancy-induced hypertension.[59]
Secondary exudative retinal detachment in patients with systemic hematologic and vascular diseases like disseminated intravascular coagulation, malignant hypertension, anti-glomerular basement membrane disease, thrombotic thrombocytopenic purpura, organ transplant recipients, renal failure, systemic lupus erythematosus, and granulomatosis with polyangiitis can resolve once the underlying systemic condition is controlled and treated.[182][183][184] Timely and comprehensive management of the primary systemic disease is crucial in achieving resolution of secondary exudative retinal detachment in these patients.(B3)
Exudative retinal detachment in hepatitis C patients treated with interferon-α and ribavirin improves upon discontinuation of the offending medications and initiation of corticosteroid treatment.[85] Close monitoring of visual symptoms and prompt intervention are essential to managing exudative retinal detachment in patients undergoing these treatments.(B3)
Differential Diagnosis
Exudative retinal detachments can arise from various causes, including inflammatory, infectious, neoplastic, and other conditions. Clinicians must rule out rhegmatogenous and tractional retinal detachments during the diagnostic process.
Exudative retinal detachment, rhegmatogenous retinal detachment, and tractional retinal detachment have distinct underlying causes. Therefore, it is essential to differentiate exudative retinal detachment from rhegmatogenous retinal detachment and tractional retinal detachment (see Table. Comparison of Different Forms of Retinal Detachment).
Table. Comparison of Different Forms of Retinal Detachment
| Rhegmatogenous Retinal Detachment | Tractional Retinal Detachment | Exudative Retinal Detachment | |
| History |
Aphakia, myopia, blunt trauma,photopsia, floaters, and visual field defect Symptoms are progressive, generally healthy individuals |
Diabetes mellitus, prematurity,penetrating trauma, sickle celldisease, venous occlusions | Systemic factors such as malignanthypertension, eclampsia, and renal failure |
| Retinal Break | Identified in 90% to 95% of cases |
No primary break May develop asecondary break |
No break or coincidental |
| Extent of Detachment |
Extends ora serrata to the optic nerve head early Has convex borders and surfaces Gravity-dependent |
Frequently does not extend to ora May be central or peripheral |
Volume and gravity-dependent Extension toora is variable May be central or peripheral |
| Retinal Mobility | Undulating bullae or folds |
Taut retina Concave borders andsurfaces Peaks to traction points |
Smoothly elevated bullae, usually withoutfolds |
| Evidence ofChronicity |
Demarcation lines, Intraretinal macrocysts Atrophic retina |
Demarcation lines | Usually none |
| Pigment in Vitreous | Present in 70% of cases | Present in trauma cases | Not present |
| Vitreous Changes |
Frequently syncretic Posteriorvitreous detachment Traction onflap of tear |
Vitreoretinal traction | Usually clear, except in uveitis |
| Subretinal Fluid | Clear | Clear, no shift | Maybe turbid and shift rapidly to dependentlocation with changes in head position |
| Choroidal Mass | None | None | May be present |
| Intraocular Pressure | Frequently low | Usually normal | Varies |
| Transillumination | Normal | Normal |
Normal Blocked transillumination ifpigmented choroidal lesion is present |
| Examples ofConditions CausingDetachment | Retinal break |
Proliferative diabetic retinopathy Retinopathy of prematurity Toxocariasis Sickle cell retinopathy Posttraumatic vitreous traction |
Uveitis Metastatic tumor Malignant melanoma Coats disease Vogt-Koyanagi-Haradasyndrome Retinoblastoma Choroidal hemangioma Senile exudative maculopathy Exudative detachment after cryotherapy or diathermy |
Patients with a hemorrhagic retinal detachment are distinguished from those with an exudative retinal detachment by subretinal blood instead of serous fluid. Causes of hemorrhagic retinal detachment include conditions such as peripheral exudative hemorrhagic chorioretinopathy, often considered a variant of idiopathic polypoidal choroidal vasculopathy, trauma, leukemia, and age-related macular degeneration, particularly in individuals taking blood thinners.
Prognosis
The prognosis of exudative retinal detachment depends on the underlying condition. Exudative retinal detachment secondary to uveal effusion and VKH syndrome may improve with high-dose systemic steroids. However, the recurrence risk is high.[97][185] Recurrence risk is also high in patients with acute central serous chorioretinopathy. Once central serous chorioretinopathy becomes chronic, the permanent loss of cones leads to a less promising visual recovery.[186] Exudative retinal detachment in patients with preeclampsia and eclampsia is usually self-limiting and resolves entirely with time.[187]
The prognosis for other underlying conditions like Coats disease, choroidal tumors, infection, uveitis, retinal vein occlusion, and retinopathy of prematurity depends on the severity of the underlying condition, the response to treatment, the amount of macular involvement, and complications secondary to treatment.[42][86][166][185][188][189] Long-standing pathology causes permanent damage to photoreceptors and the retinal pigment epithelium, often leading to a poor prognosis despite comprehensive treatment efforts.[86][185][188][185][86][190]
Complications
If not treated promptly, secondary complications may develop in patients with an exudative retinal detachment. The following is a list of potential complications:
Deterrence and Patient Education
A retinal detachment is the separation of the retina from the underlying retinal pigment epithelium and choroid, resulting in retinal ischemia and photoreceptor degeneration. Without treatment, a retinal detachment can lead to blindness. Retinal detachments occur when a retinal hole or tear allows fluid accumulation between the layers, with vitreous traction or an exudative process. Patients with an exudative retinal detachment have fluid that enters the subretinal space from retinal or choroidal blood vessels. The adhesive forces between the neurosensory retina and retinal pigment epithelium become overwhelmed. Potential causes are choroidal tumors, uveitis, hypertension, ocular vascular disorders, medications, other systemic inflammatory conditions, collagen vascular diseases, or infectious diseases.
The differential diagnosis for exudative retinal detachment is extensive. Differentiating exudative, tractional, and rhegmatogenous retinal detachment is paramount and can be a clinical diagnosis. Patients with exudative retinal detachment should undergo a thorough history and physical examination to determine the underlying etiology of their retinal detachment. Diagnostic laboratory studies and imaging are individualized based on the patient's history and examination findings. Appropriate and timely intervention by the treating clinician may improve the visual and systemic status of the patient.
Pearls and Other Issues
Key points to keep in mind about exudative retinal detachments include the following:
- Exudative retinal detachment refers to the accumulation of fluid in the subretinal space without the presence of retinal breaks or tractional forces.
- It can be caused by various factors, including inflammatory conditions, infectious diseases, systemic diseases (such as hypertension), ocular vascular disorders, medications, and ocular tumors.
- Patients may present with decreased visual acuity, floaters, visual field defects, and metamorphopsia.
- They may have signs of retinal detachment on fundoscopic examination.
- Diagnosis is primarily clinical, but additional testing, such as OCT, FFA, and ICG, may be necessary to determine the underlying etiology.
- Treatment depends on the underlying cause and may include medical management with corticosteroids or immunosuppressive agents, laser photocoagulation, photodynamic therapy, anti-VEGF therapy, or surgical intervention in refractory cases.
- Prognosis varies depending on the underlying condition, treatment response, and macular involvement extent. Prompt diagnosis and treatment are crucial to prevent permanent vision loss.
Enhancing Healthcare Team Outcomes
Caring for patients with an exudative retinal detachment necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes. Ophthalmology, primary care, emergency medicine, oncology, rheumatology, hematology, dermatology, genetics, and infectious disease clinicians, along with any other healthcare professionals involved in the care of these patients, must possess the essential clinical skills and knowledge to diagnose and manage exudative retinal detachments. This knowledge includes the ability to differentiate exudative retinal detachments from other retinal detachment forms and recognize the multitude of potential underlying etiologies.
A strategic, evidence-based evaluation is necessary to promptly diagnose the retinal detachment and any underlying condition to prevent morbidity, vision loss, and recurrence. Each healthcare professional should contribute their expertise and participate in seamless interprofessional communication to enable collaborative decision-making among the team members. By embracing the principles of skill, strategy, responsibilities, and interprofessional communication, healthcare professionals can deliver patient-centered care, enhancing overall quality of life and decreasing morbidity, ultimately improving patient outcomes and team performance in managing exudative retinal detachment.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Amer R, Nalcı H, Yalçındağ N. Exudative retinal detachment. Survey of ophthalmology. 2017 Nov-Dec:62(6):723-769. doi: 10.1016/j.survophthal.2017.05.001. Epub 2017 May 13 [PubMed PMID: 28506603]
Level 3 (low-level) evidenceBlair K, Czyz CN. Retinal Detachment. StatPearls. 2024 Jan:(): [PubMed PMID: 31855346]
Kolb H, Fernandez E, Nelson R, Kolb H. Facts and Figures Concerning the Human Retina. Webvision: The Organization of the Retina and Visual System. 1995:(): [PubMed PMID: 21413409]
Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. American family physician. 2004 Apr 1:69(7):1691-8 [PubMed PMID: 15086041]
Gundersen D, Powell SK, Rodriguez-Boulan E. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. The Journal of cell biology. 1993 Apr:121(2):335-43 [PubMed PMID: 8468350]
Level 3 (low-level) evidenceShah DN, Al-Moujahed A, Newcomb CW, Kaçmaz RO, Daniel E, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Sen HN, Suhler EB, Bhatt NP, Kempen JH, Systemic Immunosuppressive Therapy for Eye Diseases Research Group. Exudative Retinal Detachment in Ocular Inflammatory Diseases: Risk and Predictive Factors. American journal of ophthalmology. 2020 Oct:218():279-287. doi: 10.1016/j.ajo.2020.06.019. Epub 2020 Jul 2 [PubMed PMID: 32621891]
Tripathy K, Sengupta T. Is there a link between hyperhomocysteinemia and Vogt-Koyanagi-Harada syndrome? Medical hypotheses. 2017 Jul:104():116. doi: 10.1016/j.mehy.2017.06.003. Epub 2017 Jun 2 [PubMed PMID: 28673567]
Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clinical ophthalmology (Auckland, N.Z.). 2021:15():4201-4218. doi: 10.2147/OPTH.S289688. Epub 2021 Oct 20 [PubMed PMID: 34707340]
Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic Ophthalmia: Experience from a Tertiary Care Center in Northern India. Journal of ophthalmic & vision research. 2018 Oct-Dec:13(4):439-446. doi: 10.4103/jovr.jovr_86_17. Epub [PubMed PMID: 30479714]
Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ case reports. 2016 Mar 14:2016():. doi: 10.1136/bcr-2016-214344. Epub 2016 Mar 14 [PubMed PMID: 26976837]
Level 3 (low-level) evidenceYuen KS, Lai CH, Chan WM, Lam DS. Bilateral exudative retinal detachments as the presenting features of idiopathic orbital inflammation. Clinical & experimental ophthalmology. 2005 Dec:33(6):671-4 [PubMed PMID: 16402970]
Level 3 (low-level) evidenceMazumdar S, Tripathy K. The Clinical Entity Called Panscleritis. The Journal of emergency medicine. 2020 Jul:59(1):e37. doi: 10.1016/j.jemermed.2019.12.035. Epub [PubMed PMID: 32900464]
Chang TS, Byrne SF, Gass JD, Hughes JR, Johnson RN, Murray TG. Echographic findings in benign reactive lymphoid hyperplasia of the choroid. Archives of ophthalmology (Chicago, Ill. : 1960). 1996 Jun:114(6):669-75 [PubMed PMID: 8639077]
Level 3 (low-level) evidenceLee GE, Lee BW, Rao NA, Fawzi AA. Spectral domain optical coherence tomography and autofluorescence in a case of acute posterior multifocal placoid pigment epitheliopathy mimicking Vogt-Koyanagi-Harada disease: case report and review of literature. Ocular immunology and inflammation. 2011 Feb:19(1):42-7. doi: 10.3109/09273948.2010.521610. Epub 2010 Oct 31 [PubMed PMID: 21034311]
Level 3 (low-level) evidenceBalakrishnan D, Mathai A, Gogte P, Tibra N, Chhablani J. Serpiginous choroiditis with atypical presentation treated with intravenous methyl prednisolone. Seminars in ophthalmology. 2015 Mar:30(2):157-9. doi: 10.3109/08820538.2013.835836. Epub 2013 Oct 11 [PubMed PMID: 24117451]
Level 3 (low-level) evidenceVrabec TR. Exudative retinal detachment in Behçet disease. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Sep:119(9):1383-6 [PubMed PMID: 11545652]
Level 3 (low-level) evidenceChauhan K, Tripathy K. Pars Planitis. StatPearls. 2024 Jan:(): [PubMed PMID: 28613790]
Bhagat N, Green RL, Feldon SE, Lim JI. Exudative retinal detachment in relapsing polychondritis : case report and literature review. Ophthalmology. 2001 Jun:108(6):1156-9 [PubMed PMID: 11382646]
Level 3 (low-level) evidenceLee HJ, Song HJ, Jeong JH, Kim HU, Boo SJ, Na SY. Ophthalmologic manifestations in patients with inflammatory bowel disease. Intestinal research. 2017 Jul:15(3):380-387. doi: 10.5217/ir.2017.15.3.380. Epub 2017 Jun 12 [PubMed PMID: 28670235]
Watts PO, Mantry S, Austin M. Serous retinal detachment at the macula in sarcoidosis. American journal of ophthalmology. 2000 Feb:129(2):262-4 [PubMed PMID: 10682988]
Level 3 (low-level) evidenceYannuzzi LA, Jampol LM, Rabb MF, Sorenson JA, Beyrer C, Wilcox LM Jr. Unilateral acute idiopathic maculopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1991 Oct:109(10):1411-6 [PubMed PMID: 1929931]
Shi T, Lv W, Zhang L, Chen J, Chen H. Association of HLA-DR4/HLA-DRB1*04 with Vogt-Koyanagi-Harada disease: a systematic review and meta-analysis. Scientific reports. 2014 Nov 10:4():6887. doi: 10.1038/srep06887. Epub 2014 Nov 10 [PubMed PMID: 25382027]
Level 2 (mid-level) evidenceNg JY, Luk FO, Lai TY, Pang CP. Influence of molecular genetics in Vogt-Koyanagi-Harada disease. Journal of ophthalmic inflammation and infection. 2014:4():20. doi: 10.1186/s12348-014-0020-1. Epub 2014 Jul 22 [PubMed PMID: 25097674]
Balikova I, Robson AG, Holder GE, Ostergaard P, Mansour S, Moore AT. Ocular manifestations of microcephaly with or without chorioretinopathy, lymphedema or intellectual disability (MCLID) syndrome associated with mutations in KIF11. Acta ophthalmologica. 2016 Feb:94(1):92-8. doi: 10.1111/aos.12759. Epub 2015 May 21 [PubMed PMID: 25996076]
Venkatesh P, Chawla R, Tripathy K, Singh HI, Bypareddy R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye and vision (London, England). 2016:3(1):23. doi: 10.1186/s40662-016-0055-5. Epub 2016 Sep 9 [PubMed PMID: 27617266]
Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Survey of ophthalmology. 2010 Mar-Apr:55(2):134-45. doi: 10.1016/j.survophthal.2009.05.003. Epub [PubMed PMID: 20159229]
Level 3 (low-level) evidenceSong JH, Koreishi AF, Goldstein DA. Tuberculous Uveitis Presenting with a Bullous Exudative Retinal Detachment: A Case Report and Systematic Literature Review. Ocular immunology and inflammation. 2019:27(6):998-1009. doi: 10.1080/09273948.2018.1485958. Epub 2018 Jul 3 [PubMed PMID: 29969330]
Level 3 (low-level) evidenceChan DP, Teoh SC, Tan CS, Nah GK, Rajagopalan R, Prabhakaragupta MK, Chee CK, Lim TH, Goh KY, Eye Institute Dengue-Related Ophthalmic Complications Workgroup. Ophthalmic complications of dengue. Emerging infectious diseases. 2006 Feb:12(2):285-9 [PubMed PMID: 16494756]
Level 2 (mid-level) evidenceAguilar-González M, Fernández-Santodomingo AS, Marín-Payá E, Rahhal-Ortuño M, Udaondo P. Bilateral exudative retinal detachment in undiagnosed ocular syphilis after treatment with corticosteroids. European journal of ophthalmology. 2021 Mar:31(2):NP86-NP90. doi: 10.1177/1120672119889007. Epub 2019 Nov 20 [PubMed PMID: 31746221]
Matsuo T, Kato M. Submacular exudates with serous retinal detachment caused by cat scratch disease. Ocular immunology and inflammation. 2002 Jun:10(2):147-50 [PubMed PMID: 12778351]
Level 3 (low-level) evidenceYen M, Chen J, Ausayakhun S, Kunavisarut P, Vichitvejpaisal P, Ausayakhun S, Jirawison C, Shantha J, Holland GN, Heiden D, Margolis TP, Keenan JD. Retinal detachment associated with AIDS-related cytomegalovirus retinitis: risk factors in a resource-limited setting. American journal of ophthalmology. 2015 Jan:159(1):185-92. doi: 10.1016/j.ajo.2014.10.014. Epub 2014 Oct 22 [PubMed PMID: 25448999]
Level 2 (mid-level) evidenceGrinager HS, Krason DA, Olsen TW. Lyme disease: resolution of a serous retinal detachment and chorioretinal folds after antibiotic therapy. Retinal cases & brief reports. 2012 Summer:6(3):232-4. doi: 10.1097/ICB.0b013e3182247783. Epub [PubMed PMID: 25389719]
Level 3 (low-level) evidenceSawant SD, Biswas J. Fungal scleritis with exudative retinal detachment. Ocular immunology and inflammation. 2010 Dec:18(6):457-8. doi: 10.3109/09273948.2010.507129. Epub 2010 Sep 16 [PubMed PMID: 20846054]
Level 3 (low-level) evidencePoppert S, Heideking M, Agostini H, Fritzenwanker M, Wüppenhorst N, Muntau B, Henneke P, Kern W, Krücken J, Junker B, Hufnagel M. Diffuse Unilateral Subacute Neuroretinitis Caused by Ancylostoma Hookworm. Emerging infectious diseases. 2017 Feb:23(2):343-344. doi: 10.3201/eid2302.142064. Epub [PubMed PMID: 28098549]
Goodart RA, Riekhof FT, Beaver PC. Subretinal nematode. An unusual etiology for uveitis and retinal detachment. Retina (Philadelphia, Pa.). 1985 Spring-Summer:5(2):87-90 [PubMed PMID: 4048662]
Level 3 (low-level) evidenceAl-Zahrani YA, Al-Dhibi HA, Al-Abdullah AA. Atypical Presentation of Ocular Toxoplasmosis: A Case Report of Exudative Retinal Detachment and Choroidal Ischemia. Middle East African journal of ophthalmology. 2016 Jan-Mar:23(1):150-2. doi: 10.4103/0974-9233.164624. Epub [PubMed PMID: 26957857]
Level 3 (low-level) evidenceLe TD, Weisbrod D, Mandelcorn ED. Chorioretinitis with exudative retinal detachment secondary to varicella zoster virus. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2015 Oct:50(5):e91-3. doi: 10.1016/j.jcjo.2015.06.006. Epub [PubMed PMID: 26455991]
Reddy SV, Husain D. Panretinal Photocoagulation: A Review of Complications. Seminars in ophthalmology. 2018:33(1):83-88. doi: 10.1080/08820538.2017.1353820. Epub 2017 Nov 27 [PubMed PMID: 29172937]
Matsuo T, Eguchi K, Matsuo N. Unusual exudative retinal detachment 9 months after scleral buckling surgery. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1990:201(2):79-82 [PubMed PMID: 2234819]
Level 3 (low-level) evidenceSolberg T, Ytrehus T, Ringvold A. Hypotony and retinal detachment. Acta ophthalmologica. 1986 Feb:64(1):26-32 [PubMed PMID: 3962615]
Vicini G, Nicolosi C, Pieretti G, Mazzini C. Large choroidal metastasis with exudative retinal detachment as presenting manifestation of small cell lung cancer: A case report. Respiratory medicine case reports. 2020:30():101074. doi: 10.1016/j.rmcr.2020.101074. Epub 2020 May 4 [PubMed PMID: 32420018]
Level 3 (low-level) evidenceGibran SK, Kapoor KG. Management of exudative retinal detachment in choroidal melanoma. Clinical & experimental ophthalmology. 2009 Sep:37(7):654-9. doi: 10.1111/j.1442-9071.2009.02127.x. Epub [PubMed PMID: 19788660]
Rowlands MA, Mondesire-Crump I, Levin A, Mauguen A, Francis JH, Dunkel IJ, Brodie SE, Gobin YP, Abramson DH. Total retinal detachments due to retinoblastoma: Outcomes following intra-arterial chemotherapy/ophthalmic artery chemosurgery. PloS one. 2018:13(4):e0195395. doi: 10.1371/journal.pone.0195395. Epub 2018 Apr 26 [PubMed PMID: 29698399]
Anaya-Pava EJ, Saenz-Bocanegra CH, Flores-Trejo A, Castro-Santana NA. Diffuse choroidal hemangioma associated with exudative retinal detachment in a Sturge-Weber syndrome case: photodynamic therapy and intravitreous bevacizumab. Photodiagnosis and photodynamic therapy. 2015 Mar:12(1):136-9. doi: 10.1016/j.pdpdt.2014.12.002. Epub 2015 Jan 3 [PubMed PMID: 25560419]
Level 3 (low-level) evidenceRuppert MD, Gavin M, Mitchell KT, Peiris AN. Ocular Manifestations of von Hippel-Lindau Disease. Cureus. 2019 Aug 4:11(8):e5319. doi: 10.7759/cureus.5319. Epub 2019 Aug 4 [PubMed PMID: 31588386]
Padhy SK, Mandal S, Gagrani M. Bone inside eye: choroidal osteoma presenting as exudative retinal detachment: a challenge to diagnosis. BMJ case reports. 2018 Jun 17:2018():. pii: bcr-2018-225393. doi: 10.1136/bcr-2018-225393. Epub 2018 Jun 17 [PubMed PMID: 29914906]
Level 3 (low-level) evidenceKormann BA, Holzgreve H, Wolff-Kormann PG, Riedel KG. Systemic malignant lymphoma presenting as bilateral exudative retinal detachment. Klinische Wochenschrift. 1990 Oct 17:68(20):1027-31 [PubMed PMID: 2283792]
Level 3 (low-level) evidenceKlemp K, Kiilgaard JF, Heegaard S, Nørgaard T, Andersen MK, Prause JU. Bilateral diffuse uveal melanocytic proliferation: Case report and literature review. Acta ophthalmologica. 2017 Aug:95(5):439-445. doi: 10.1111/aos.13481. Epub 2017 Jun 21 [PubMed PMID: 28636126]
Level 3 (low-level) evidenceBreazzano MP, Bacci T, Wang H, Francis JH, Yannuzzi LA. Bacillary Layer Detachment in Bilateral Diffuse Uveal Melanocytic Proliferation Masquerading as Neovascular AMD. Ophthalmic surgery, lasers & imaging retina. 2020 Jul 1:51(7):413-417. doi: 10.3928/23258160-20200702-07. Epub [PubMed PMID: 32706900]
Brody JM, Butrus SI, Ashraf MF, Rabinowitz AI, Whitmore PV. Multiple myeloma presenting with bilateral exudative macular detachments. Acta ophthalmologica Scandinavica. 1995 Feb:73(1):81-2 [PubMed PMID: 7627765]
Level 3 (low-level) evidenceYoshida K, Hasegawa D, Takusagawa A, Kato I, Ogawa C, Echizen N, Ohkoshi K, Yamaguchi T, Hosoya R, Manabe A. Bullous exudative retinal detachment due to infiltration of leukemic cells in a child with acute lymphoblastic leukemia. International journal of hematology. 2010 Oct:92(3):535-7. doi: 10.1007/s12185-010-0683-9. Epub 2010 Sep 14 [PubMed PMID: 20838956]
Level 3 (low-level) evidenceCameron JR, Cackett P. Lymphomatoid granulomatosis associated with bilateral exudative retinal detachments. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 May:125(5):712-3 [PubMed PMID: 17502519]
Level 3 (low-level) evidenceZarzecki M, Saeed E, Mariak Z, Konopińska J. Recurrent monocular exudative retinal detachment as the first manifestation of squamous cell lung cancer: A case report. Medicine. 2021 Mar 19:100(11):e25189. doi: 10.1097/MD.0000000000025189. Epub [PubMed PMID: 33726010]
Level 3 (low-level) evidenceMiles SL, Niles RM, Pittock S, Vile R, Davies J, Winters JL, Abu-Yaghi NE, Grothey A, Siddiqui M, Kaur J, Hartmann L, Kalli KR, Pease L, Kravitz D, Markovic S, Pulido JS. A factor found in the IgG fraction of serum of patients with paraneoplastic bilateral diffuse uveal melanocytic proliferation causes proliferation of cultured human melanocytes. Retina (Philadelphia, Pa.). 2012 Oct:32(9):1959-66 [PubMed PMID: 22791177]
Jampol LM, Leskov I, Lyon AT. Diffuse Uveal Melanocytic Proliferation With Primary Vitreoretinal Lymphoma-Reply. JAMA ophthalmology. 2019 Dec 1:137(12):1466-1467. doi: 10.1001/jamaophthalmol.2019.4263. Epub [PubMed PMID: 31647494]
Pefkianaki M, Agrawal R, Desai P, Pavesio C, Sagoo MS. Bilateral Diffuse Uveal Melanocytic Proliferation (BDUMP) associated with B-cell lymphoma: report of a rare case. BMC cancer. 2015 Jan 30:15():23. doi: 10.1186/s12885-015-1020-8. Epub 2015 Jan 30 [PubMed PMID: 25633015]
Level 3 (low-level) evidenceSingalavanija A, Dangosintr N, Namatra C. Retinal detachment in toxemia of pregnancy. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 1989 Oct:72(10):597-600 [PubMed PMID: 2584907]
Level 3 (low-level) evidenceTripathy K, Chawla R, Mutha V, Selvan H. Spontaneous suprachoroidal haemorrhage with exudative retinal detachment in pregnancy-induced hypertension. BMJ case reports. 2018 Mar 9:2018():. pii: bcr-2017-223907. doi: 10.1136/bcr-2017-223907. Epub 2018 Mar 9 [PubMed PMID: 29523618]
Level 3 (low-level) evidenceLee CS, Choi EY, Lee M, Kim H, Chung H. Serous retinal detachment in preeclampsia and malignant hypertension. Eye (London, England). 2019 Nov:33(11):1707-1714. doi: 10.1038/s41433-019-0461-8. Epub 2019 May 14 [PubMed PMID: 31089238]
Tripathy K, Chawla R. Bilateral exudative retinal detachment with choroidopathy in malignant hypertension. The National medical journal of India. 2015 Sep-Oct:28(5):261 [PubMed PMID: 27132968]
Tripathy K, Chaudhuri A. Comment on: Dramatic response to intravitreal bevacizumab in hypertensive retinopathy. Indian journal of ophthalmology. 2019 Jan:67(1):180. doi: 10.4103/ijo.IJO_1654_18. Epub [PubMed PMID: 30574946]
Level 3 (low-level) evidenceHoines J, Buettner H. Ocular complications of disseminated intravascular coagulation (DIC) in abruptio placentae. Retina (Philadelphia, Pa.). 1989:9(2):105-9 [PubMed PMID: 2672208]
Level 3 (low-level) evidenceSampo M, Yin GH, Hoffart L, Denis D, Soler V, Matonti F. Exudative Retinal Detachment Treatment in a Patient with Thrombotic Thrombocytopenic Purpura. Case reports in ophthalmology. 2016 Jan-Apr:7(1):90-5. doi: 10.1159/000444291. Epub 2016 Feb 20 [PubMed PMID: 27293407]
Level 3 (low-level) evidenceJaben SL, Norton EW. Exudative retinal detachment in Wegener's granulomatosis: case report. Annals of ophthalmology. 1982 Aug:14(8):717-20 [PubMed PMID: 7125466]
Level 3 (low-level) evidenceSchönfeld CL. Bilateral Exudative Retinal Detachment in HELLP Syndrome. Case reports in ophthalmology. 2012 Jan:3(1):35-7. doi: 10.1159/000336151. Epub 2012 Jan 31 [PubMed PMID: 22615699]
Level 3 (low-level) evidenceHannouche D, Korobelnik JF, Cochereau I, Hayem G, Beaudreuil J, Meyer O, Hoang-Xuan T. Systemic lupus erythematosus with choroidopathy and serous retinal detachment. International ophthalmology. 1995:19(2):125-7 [PubMed PMID: 8586496]
Level 3 (low-level) evidenceHoscheit AM, Austin JK, Jones WL. Nonrhegmatogenous retinal detachment in Goodpasture's syndrome: a case report and discussion of the clinicopathologic entity. Journal of the American Optometric Association. 1993 Aug:64(8):563-7 [PubMed PMID: 8409194]
Level 3 (low-level) evidenceOtuka OAI, Eweputanna LI, Okoronkwo NC, Kalu A. Bilateral Exudative Retinal Detachment in a Young Patient with Chronic Renal Failure. International medical case reports journal. 2021:14():139-144. doi: 10.2147/IMCRJ.S283565. Epub 2021 Mar 3 [PubMed PMID: 33716512]
Level 3 (low-level) evidenceNentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World journal of diabetes. 2015 Apr 15:6(3):489-99. doi: 10.4239/wjd.v6.i3.489. Epub [PubMed PMID: 25897358]
Ferencz JR, Rosen E, Tam G, Gilady G, Rubowich A, Assia EI, Korzets Z. Treatment of total exudative retinal detachment due to central retinal vein occlusion by intravitreal bevacizumab in a patient with p-ANCA vasculitis. Clinical ophthalmology (Auckland, N.Z.). 2007 Sep:1(3):347-51 [PubMed PMID: 19668494]
Level 3 (low-level) evidenceChang YS, Weng SF, Chang C, Wang JJ, Chen HI, Ko SY, Tu IT, Chien CC, Wang JJ, Wang CM, Jan RL. Risk of serous retinal detachment in patients with end-stage renal disease on dialysis. PloS one. 2017:12(6):e0180133. doi: 10.1371/journal.pone.0180133. Epub 2017 Jun 28 [PubMed PMID: 28658289]
AlAli A, Bourgault S, Clark I, Lam WC. EXUDATIVE RETINAL DETACHMENT CAUSED BY A CHOROIDAL NEOVASCULAR MEMBRANE IN HALLERMANN-STREIFF SYNDROME. Retinal cases & brief reports. 2018 Winter:12(1):45-47. doi: 10.1097/ICB.0000000000000411. Epub [PubMed PMID: 27648586]
Level 3 (low-level) evidenceYannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina (Philadelphia, Pa.). 1990:10(1):1-8 [PubMed PMID: 1693009]
Khairil-Ridzwan KK, Azian A, Hanizasurana H, Shatriah I. Exudative Retinal Detachment due to Coats Disease in a Teenager with Senior-Loken Syndrome: Case Report and Review of Literature. Cureus. 2019 Apr 15:11(4):e4460. doi: 10.7759/cureus.4460. Epub 2019 Apr 15 [PubMed PMID: 31205846]
Level 3 (low-level) evidenceSpaide RF, Gemmy Cheung CM, Matsumoto H, Kishi S, Boon CJF, van Dijk EHC, Mauget-Faysse M, Behar-Cohen F, Hartnett ME, Sivaprasad S, Iida T, Brown DM, Chhablani J, Maloca PM. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Progress in retinal and eye research. 2022 Jan:86():100973. doi: 10.1016/j.preteyeres.2021.100973. Epub 2021 May 21 [PubMed PMID: 34029721]
Tauqeer Z, Yonekawa Y. Familial Exudative Vitreoretinopathy: Pathophysiology, Diagnosis, and Management. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 May-Jun:7(3):176-182. doi: 10.22608/APO.201855. Epub 2018 Apr 9 [PubMed PMID: 29633588]
Moshfeghi DM, Silva RA, Berrocal AM. Exudative retinal detachment following photocoagulation in older premature infants for retinopathy of prematurity: description and management. Retina (Philadelphia, Pa.). 2014 Jan:34(1):83-6. doi: 10.1097/IAE.0b013e3182993d5f. Epub [PubMed PMID: 23881225]
Level 3 (low-level) evidenceTakahashi K, Kishi S. Serous macular detachment associated with retinal arterial macroaneurysm. Japanese journal of ophthalmology. 2006 Sep-Oct:50(5):460-464. doi: 10.1007/s10384-006-0347-0. Epub [PubMed PMID: 17013700]
Level 2 (mid-level) evidenceSingh D, Tripathy K. Retinal Macroaneurysm. StatPearls. 2024 Jan:(): [PubMed PMID: 35015432]
Mozo Cuadrado M, Tabuenca Del Barrio L, Zubicoa Enériz A, Antonia Ardanaz Aldave M. Ocular manifestations of Norrie disease. Journal francais d'ophtalmologie. 2020 May:43(5):439-441. doi: 10.1016/j.jfo.2019.09.023. Epub 2020 May 4 [PubMed PMID: 32381368]
Chan CK, Gass JD, Lin SG. Acute exudative polymorphous vitelliform maculopathy syndrome. Retina (Philadelphia, Pa.). 2003 Aug:23(4):453-62 [PubMed PMID: 12972754]
Level 3 (low-level) evidenceFeroze KB, Tripathy K, Wang J. Interferon-Induced Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 28722892]
Mahendradas P, Parab S, Sasikumar R, Kawali A, Shetty BK. Topiramate-induced acute angle closure with severe panuveitis: A challenging case report. Indian journal of ophthalmology. 2018 Sep:66(9):1342-1344. doi: 10.4103/ijo.IJO_1192_17. Epub [PubMed PMID: 30127167]
Level 3 (low-level) evidenceMazumdar S, Tripathy K, Sarma B, Agarwal N. Acquired myopia followed by acquired hyperopia due to serous neurosensory retinal detachment following topiramate intake. European journal of ophthalmology. 2019 Jan:29(1):NP21-NP24. doi: 10.1177/1120672118797286. Epub 2018 Sep 3 [PubMed PMID: 30175623]
Modorati G, Matteo DF, Miserocchi E, Colucci A, Bandello F. Serous Retinal Detachments Complicating Interferon-α and Ribavirin Treatment in Patients with Hepatitis C. Case reports in ophthalmology. 2011 Jan:2(1):105-10. doi: 10.1159/000326747. Epub 2011 Mar 12 [PubMed PMID: 22110438]
Level 3 (low-level) evidenceGupta A, Tripathy K. Central Serous Chorioretinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32644399]
Chee SP, Jap A, Bacsal K. Prognostic factors of Vogt-Koyanagi-Harada disease in Singapore. American journal of ophthalmology. 2009 Jan:147(1):154-161.e1. doi: 10.1016/j.ajo.2008.07.044. Epub 2008 Oct 2 [PubMed PMID: 18834575]
Level 2 (mid-level) evidenceShields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Current opinion in ophthalmology. 2014 May:25(3):177-85. doi: 10.1097/ICU.0000000000000041. Epub [PubMed PMID: 24614143]
Level 3 (low-level) evidenceStahl A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Deutsches Arzteblatt international. 2020 Jul 20:117(29-30):513-520. doi: 10.3238/arztebl.2020.0513. Epub [PubMed PMID: 33087239]
Egwuagu CE, Sun L, Kim SH, Dambuza IM. Ocular Inflammatory Diseases: Molecular Pathogenesis and Immunotherapy. Current molecular medicine. 2015:15(6):517-28 [PubMed PMID: 26238372]
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta ophthalmologica. 2008 Mar:86(2):126-45 [PubMed PMID: 17662099]
Shapiro MJ, Chow CC, Karth PA, Kiernan DF, Blair MP. Effects of green diode laser in the treatment of pediatric Coats disease. American journal of ophthalmology. 2011 Apr:151(4):725-731.e2. doi: 10.1016/j.ajo.2010.10.024. Epub 2011 Jan 22 [PubMed PMID: 21257148]
Level 2 (mid-level) evidenceAnderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. The American journal of physiology. 1995 Oct:269(4 Pt 1):G467-75 [PubMed PMID: 7485497]
Level 3 (low-level) evidenceMarmor MF. Control of subretinal fluid: experimental and clinical studies. Eye (London, England). 1990:4 ( Pt 2)():340-4 [PubMed PMID: 2199242]
Level 3 (low-level) evidenceAijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. International review of cytology. 2006:248():261-98 [PubMed PMID: 16487793]
Level 3 (low-level) evidenceCunha-Vaz JG. The blood-retinal barriers. Documenta ophthalmologica. Advances in ophthalmology. 1976 Oct 15:41(2):287-327 [PubMed PMID: 1009819]
Level 3 (low-level) evidenceAndreoli CM, Foster CS. Vogt-Koyanagi-Harada disease. International ophthalmology clinics. 2006 Spring:46(2):111-22 [PubMed PMID: 16770158]
Vermeirsch S, Testi I, Pavesio C. Choroidal involvement in non-infectious posterior scleritis. Journal of ophthalmic inflammation and infection. 2021 Oct 27:11(1):41. doi: 10.1186/s12348-021-00269-9. Epub 2021 Oct 27 [PubMed PMID: 34705127]
Yeşiltaş YS, Gündüz AK. Idiopathic Orbital Inflammation: Review of Literature and New Advances. Middle East African journal of ophthalmology. 2018 Apr-Jun:25(2):71-80. doi: 10.4103/meajo.MEAJO_44_18. Epub [PubMed PMID: 30122852]
Level 3 (low-level) evidenceZhu M, Tang A, Amatya N, Qiu L. Exudative retinal detachment. The Netherlands journal of medicine. 2011 Nov-Dec:69(11):527, 530 [PubMed PMID: 22173366]
Level 3 (low-level) evidenceMagliyah MS, Al-Fakhri AS, Al-Dhibi HA. Proliferative retinopathy as a feature of Vogt Koyanagi Harada Disease: a report of two cases. BMC ophthalmology. 2020 Dec 1:20(1):470. doi: 10.1186/s12886-020-01736-y. Epub 2020 Dec 1 [PubMed PMID: 33261580]
Level 3 (low-level) evidenceSimakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2024 Jan:(): [PubMed PMID: 32491607]
García-Arumí J, Martínez-Castillo V, Boixadera A, Blasco H, Marticorena J, Zapata MÁ, Macià C, Badal J, Distéfano L, Rafart JM, Berrocal M, Zambrano A, Ruíz-Moreno JM, Figueroa MS. Rhegmatogenous retinal detachment treatment guidelines. Archivos de la Sociedad Espanola de Oftalmologia. 2013 Jan:88(1):11-35. doi: 10.1016/j.oftal.2011.10.013. Epub 2012 Sep 20 [PubMed PMID: 23414946]
Obuchowska I, Mariak Z. [Choroidal detachment--pathogenesis, etiology and clinical features]. Klinika oczna. 2005:107(7-9):529-32 [PubMed PMID: 16417015]
Spaide RF, Goldbaum M, Wong DW, Tang KC, Iida T. Serous detachment of the retina. Retina (Philadelphia, Pa.). 2003 Dec:23(6):820-46; quiz 895-6 [PubMed PMID: 14707834]
Kirkby GR, Chignell AH. Shifting subretinal fluid in rhegmatogenous retinal detachment. The British journal of ophthalmology. 1985 Sep:69(9):654-5 [PubMed PMID: 4041411]
Sartini F, Menchini M, Posarelli C, Casini G, Figus M. Bullous Central Serous Chorioretinopathy: A Rare and Atypical Form of Central Serous Chorioretinopathy. A Systematic Review. Pharmaceuticals (Basel, Switzerland). 2020 Aug 28:13(9):. doi: 10.3390/ph13090221. Epub 2020 Aug 28 [PubMed PMID: 32872388]
Level 1 (high-level) evidenceLin SH, Xu YG, Zhao JH, Cui H, Jin H, Jia YJ, Zhao J, Li YJ. Choroidal metastasis with retinal detachment: A case report. Medicine. 2021 Dec 23:100(51):e28009. doi: 10.1097/MD.0000000000028009. Epub [PubMed PMID: 34941041]
Level 3 (low-level) evidenceFeltgen N, Walter P. Rhegmatogenous retinal detachment--an ophthalmologic emergency. Deutsches Arzteblatt international. 2014 Jan 6:111(1-2):12-21; quiz 22. doi: 10.3238/arztebl.2014.0012. Epub [PubMed PMID: 24565273]
Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian journal of ophthalmology. 2018 Dec:66(12):1751-1762. doi: 10.4103/ijo.IJO_1217_18. Epub [PubMed PMID: 30451175]
Liao L, Zhu XH. Advances in the treatment of rhegmatogenous retinal detachment. International journal of ophthalmology. 2019:12(4):660-667. doi: 10.18240/ijo.2019.04.22. Epub 2019 Apr 18 [PubMed PMID: 31024823]
Level 3 (low-level) evidenceKirchhof B. [The diseased vitreous body: Malformations, developmental disorders and opacities]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2015 Jul:112(7):559-63. doi: 10.1007/s00347-015-0059-x. Epub [PubMed PMID: 26149492]
Tripathy K, Salini B. Amsler Grid. StatPearls. 2024 Jan:(): [PubMed PMID: 30844168]
Benson WE. Posterior scleritis. Survey of ophthalmology. 1988 Mar-Apr:32(5):297-316 [PubMed PMID: 3043740]
Level 3 (low-level) evidenceJoye A, Suhler E. Vogt-Koyanagi-Harada disease. Current opinion in ophthalmology. 2021 Nov 1:32(6):574-582. doi: 10.1097/ICU.0000000000000809. Epub [PubMed PMID: 34545845]
Level 3 (low-level) evidenceWong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2011 Jul:42 Suppl():S75-84. doi: 10.3928/15428877-20110627-07. Epub [PubMed PMID: 21790115]
Laviers H, Zambarakji H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Dec:252(12):1871-83. doi: 10.1007/s00417-014-2840-y. Epub 2014 Nov 4 [PubMed PMID: 25363655]
Ramtohul P, Engelbert M, Malclès A, Gigon E, Miserocchi E, Modorati G, Cunha de Souza E, Besirli CG, Curcio CA, Freund KB. BACILLARY LAYER DETACHMENT: MULTIMODAL IMAGING AND HISTOLOGIC EVIDENCE OF A NOVEL OPTICAL COHERENCE TOMOGRAPHY TERMINOLOGY: Literature Review and Proposed Theory. Retina (Philadelphia, Pa.). 2021 Nov 1:41(11):2193-2207. doi: 10.1097/IAE.0000000000003217. Epub [PubMed PMID: 34029276]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Cavallerano AA. Ophthalmic fluorescein angiography. Optometry clinics : the official publication of the Prentice Society. 1996:5(1):1-23 [PubMed PMID: 8963072]
Norton EW. A characteristic fluorescein angiographic pattern in choriodal folds. Proceedings of the Royal Society of Medicine. 1969 Feb:62(2):119-28 [PubMed PMID: 5775225]
Biswas J, Mittal S, Ganesh SK, Shetty NS, Gopal L. Posterior scleritis: clinical profile and imaging characteristics. Indian journal of ophthalmology. 1998 Dec:46(4):195-202 [PubMed PMID: 10218301]
Level 2 (mid-level) evidenceFriedel S, Polak A. [Leopard-spot pattern in fluorescein angiography]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2013 Apr:110(4):360-4. doi: 10.1007/s00347-012-2771-0. Epub [PubMed PMID: 23338531]
Level 3 (low-level) evidenceGass JD. Acute posterior multifocal placoid pigment epitheliopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1968 Aug:80(2):177-85 [PubMed PMID: 5661882]
Chen TY, Bhagat S, Bhagat N. Melanocytic Lesions in Buccal Mucosa in BDUMP. Ophthalmology. 2020 Aug:127(8):1063. doi: 10.1016/j.ophtha.2020.04.002. Epub [PubMed PMID: 32703386]
Pierce KK, Lane RG. Central serous chorioretinopathy associated with the use of ephedra. Retinal cases & brief reports. 2009 Fall:3(4):376-8. doi: 10.1097/ICB.0b013e31818ad3ce. Epub [PubMed PMID: 25389852]
Level 3 (low-level) evidencePark DW, Schatz H, Gaffney MM, McDonald HR, Johnson RN, Schaeffer D. Central serous chorioretinopathy in two families. European journal of ophthalmology. 1998 Jan-Mar:8(1):42-7 [PubMed PMID: 9590595]
Level 3 (low-level) evidenceMuraleedharan S, Tripathy K. Indocyanine Green (ICG) Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35593804]
Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. International ophthalmology. 2007 Apr-Jun:27(2-3):173-82 [PubMed PMID: 17457515]
Miyanaga M, Kawaguchi T, Miyata K, Horie S, Mochizuki M, Herbort CP. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Japanese journal of ophthalmology. 2010 Sep:54(5):377-82. doi: 10.1007/s10384-010-0853-6. Epub 2010 Nov 5 [PubMed PMID: 21052896]
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina (Philadelphia, Pa.). 2012 Sep:32(8):1453-64 [PubMed PMID: 22426346]
Level 1 (high-level) evidenceJapanese Study Group of Polypoidal Choroidal Vasculopathy. [Criteria for diagnosis of polypoidal choroidal vasculopathy]. Nippon Ganka Gakkai zasshi. 2005 Jul:109(7):417-27 [PubMed PMID: 16050460]
Schneider U, Inhoffen W, Gelisken F. Indocyanine green angiography in a case of unilateral recurrent posterior acute multifocal placoid pigment epitheliopathy. Acta ophthalmologica Scandinavica. 2003 Feb:81(1):72-5 [PubMed PMID: 12631024]
Level 3 (low-level) evidenceYuzawa M, Kawamura A, Matsui M. Indocyanine green video angiographic findings in acute posterior multifocal placoid pigment epitheliopathy. Acta ophthalmologica. 1994 Feb:72(1):128-33 [PubMed PMID: 8017187]
Level 3 (low-level) evidenceMontero JA, Ruiz-Moreno JM, Fernandez-Munoz M. Spectral domain optical coherence tomography findings in acute posterior multifocal placoid pigment epitheliopathy. Ocular immunology and inflammation. 2011 Feb:19(1):48-50. doi: 10.3109/09273948.2010.530733. Epub [PubMed PMID: 21250924]
Level 3 (low-level) evidenceCheung CM, Yeo IY, Koh A. Photoreceptor changes in acute and resolved acute posterior multifocal placoid pigment epitheliopathy documented by spectral-domain optical coherence tomography. Archives of ophthalmology (Chicago, Ill. : 1960). 2010 May:128(5):644-6. doi: 10.1001/archophthalmol.2010.48. Epub [PubMed PMID: 20457992]
Level 3 (low-level) evidenceLima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, Yannuzzi LA, Tsang SH. Hyperautofluorescent ring in autoimmune retinopathy. Retina (Philadelphia, Pa.). 2012 Jul:32(7):1385-94. doi: 10.1097/IAE.0b013e3182398107. Epub [PubMed PMID: 22218149]
Level 3 (low-level) evidenceNaysan J, Pang CE, Klein RW, Freund KB. Multimodal imaging of bilateral diffuse uveal melanocytic proliferation associated with an iris mass lesion. International journal of retina and vitreous. 2016:2():13 [PubMed PMID: 27847631]
von Rückmann A, Fitzke FW, Bird AC. In vivo fundus autofluorescence in macular dystrophies. Archives of ophthalmology (Chicago, Ill. : 1960). 1997 May:115(5):609-15 [PubMed PMID: 9152128]
Boon CJ, Theelen T, Hoefsloot EH, van Schooneveld MJ, Keunen JE, Cremers FP, Klevering BJ, Hoyng CB. Clinical and molecular genetic analysis of best vitelliform macular dystrophy. Retina (Philadelphia, Pa.). 2009 Jun:29(6):835-47. doi: 10.1097/IAE.0b013e31819d4fda. Epub [PubMed PMID: 19357557]
Level 2 (mid-level) evidenceO'Gorman S, Flaherty WA, Fishman GA, Berson EL. Histopathologic findings in Best's vitelliform macular dystrophy. Archives of ophthalmology (Chicago, Ill. : 1960). 1988 Sep:106(9):1261-8 [PubMed PMID: 3415551]
Level 3 (low-level) evidenceWeingeist TA, Kobrin JL, Watzke RC. Histopathology of Best's macular dystrophy. Archives of ophthalmology (Chicago, Ill. : 1960). 1982 Jul:100(7):1108-14 [PubMed PMID: 7092654]
Level 3 (low-level) evidenceBakall B, Radu RA, Stanton JB, Burke JM, McKay BS, Wadelius C, Mullins RF, Stone EM, Travis GH, Marmorstein AD. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Experimental eye research. 2007 Jul:85(1):34-43 [PubMed PMID: 17477921]
Dutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian journal of ophthalmology. 2020 Sep:68(9):1829-1837. doi: 10.4103/ijo.IJO_786_20. Epub [PubMed PMID: 32823399]
Mohamed Q, Harper CA. Acute optical coherence tomographic findings in cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Aug:125(8):1132-3 [PubMed PMID: 17698766]
Level 3 (low-level) evidencePatil YB, Garg R, Rajguru JP, Sirsalmath M, Bevinakatti VA, Kumar M, Sharma S. Vogt-Koyanagi-Harada (VKH) syndrome: A new perspective for healthcare professionals. Journal of family medicine and primary care. 2020 Jan:9(1):31-35. doi: 10.4103/jfmpc.jfmpc_787_19. Epub 2020 Jan 28 [PubMed PMID: 32110561]
Level 3 (low-level) evidenceBaltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003 May:110(5):1061-5 [PubMed PMID: 12750115]
Level 2 (mid-level) evidenceAgarwal M, Ganesh SK, Biswas J. Triple agent immunosuppressive therapy in Vogt-Koyanagi-Harada syndrome. Ocular immunology and inflammation. 2006 Dec:14(6):333-9 [PubMed PMID: 17162603]
Level 3 (low-level) evidenceBykhovskaya I, Thorne JE, Kempen JH, Dunn JP, Jabs DA. Vogt-Koyanagi-Harada disease: clinical outcomes. American journal of ophthalmology. 2005 Oct:140(4):674-8 [PubMed PMID: 16226518]
Level 2 (mid-level) evidenceJap A, Luu CD, Yeo I, Chee SP. Correlation between peripapillary atrophy and corticosteroid therapy in patients with Vogt-Koyanagi-Harada disease. Eye (London, England). 2008 Feb:22(2):240-5 [PubMed PMID: 16980924]
Level 2 (mid-level) evidenceAbdel-Aty A, Gupta A, Del Priore L, Kombo N. Management of noninfectious scleritis. Therapeutic advances in ophthalmology. 2022 Jan-Dec:14():25158414211070879. doi: 10.1177/25158414211070879. Epub 2022 Jan 21 [PubMed PMID: 35083421]
Level 3 (low-level) evidenceStem MS, Todorich B, Faia LJ. Ocular Pharmacology for Scleritis: Review of Treatment and a Practical Perspective. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2017 May:33(4):240-246. doi: 10.1089/jop.2016.0127. Epub 2017 Mar 29 [PubMed PMID: 28355124]
Level 3 (low-level) evidenceLavric A, Gonzalez-Lopez JJ, Majumder PD, Bansal N, Biswas J, Pavesio C, Agrawal R. Posterior Scleritis: Analysis of Epidemiology, Clinical Factors, and Risk of Recurrence in a Cohort of 114 Patients. Ocular immunology and inflammation. 2016:24(1):6-15. doi: 10.3109/09273948.2015.1005240. Epub 2015 Jul 2 [PubMed PMID: 26134101]
Dutta Majumder P, Agrawal R, McCluskey P, Biswas J. Current Approach for the Diagnosis and Management of Noninfective Scleritis. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2020 Dec 7:10(2):212-223. doi: 10.1097/APO.0000000000000341. Epub 2020 Dec 7 [PubMed PMID: 33290287]
Sota J, Girolamo MM, Frediani B, Tosi GM, Cantarini L, Fabiani C. Biologic Therapies and Small Molecules for the Management of Non-Infectious Scleritis: A Narrative Review. Ophthalmology and therapy. 2021 Dec:10(4):777-813. doi: 10.1007/s40123-021-00393-8. Epub 2021 Sep 2 [PubMed PMID: 34476773]
Level 3 (low-level) evidenceWakefield D, Di Girolamo N, Thurau S, Wildner G, McCluskey P. Scleritis: Immunopathogenesis and molecular basis for therapy. Progress in retinal and eye research. 2013 Jul:35():44-62. doi: 10.1016/j.preteyeres.2013.02.004. Epub 2013 Feb 26 [PubMed PMID: 23454614]
Level 3 (low-level) evidenceLim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. The British journal of ophthalmology. 2011 Apr:95(4):514-7. doi: 10.1136/bjo.2010.182121. Epub 2010 Jul 19 [PubMed PMID: 20644214]
Level 2 (mid-level) evidenceChan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008 Oct:115(10):1756-65. doi: 10.1016/j.ophtha.2008.04.014. Epub 2008 Jun 5 [PubMed PMID: 18538401]
Level 1 (high-level) evidenceSchmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Archives of ophthalmology (Chicago, Ill. : 1960). 2002 Jun:120(6):835-44 [PubMed PMID: 12049594]
Witkin AJ, Brown GC. Update on nonsurgical therapy for diabetic macular edema. Current opinion in ophthalmology. 2011 May:22(3):185-9. doi: 10.1097/ICU.0b013e3283459724. Epub [PubMed PMID: 21427573]
Level 3 (low-level) evidenceChiang A, Regillo CD. Preferred therapies for neovascular age-related macular degeneration. Current opinion in ophthalmology. 2011 May:22(3):199-204. doi: 10.1097/ICU.0b013e32834597d9. Epub [PubMed PMID: 21427571]
Level 3 (low-level) evidenceLondon NJ, Brown G. Update and review of central retinal vein occlusion. Current opinion in ophthalmology. 2011 May:22(3):159-65. doi: 10.1097/ICU.0b013e3283459737. Epub [PubMed PMID: 21460724]
Level 3 (low-level) evidenceKusaka S. Surgical Management of Coats Disease. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 May-Jun:7(3):156-159. doi: 10.22608/APO.201867. Epub 2018 Mar 27 [PubMed PMID: 29664241]
Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: An overview of classification, management and outcomes. Indian journal of ophthalmology. 2019 Jun:67(6):763-771. doi: 10.4103/ijo.IJO_841_19. Epub [PubMed PMID: 31124484]
Level 3 (low-level) evidenceRugwizangoga B, Mwabili T, Scanlan T, Meyer P, Kitinya J. Coats' disease in Tanzania: first case report and literature review. African health sciences. 2014 Sep:14(3):763-8. doi: 10.4314/ahs.v14i3.37. Epub [PubMed PMID: 25352900]
Level 3 (low-level) evidenceGilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye (London, England). 2015 Jan:29(1):1-14. doi: 10.1038/eye.2014.70. Epub 2014 Oct 17 [PubMed PMID: 25323851]
Sızmaz S, Yonekawa Y, T Trese M. Familial Exudative Vitreoretinopathy. Turkish journal of ophthalmology. 2015 Aug:45(4):164-168 [PubMed PMID: 27800225]
Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008 Dec:246(12):1787-9. doi: 10.1007/s00417-008-0949-6. Epub 2008 Sep 16 [PubMed PMID: 18795314]
Level 3 (low-level) evidenceHenry CR, Sisk RA, Tzu JH, Albini TA, Davis JL, Murray TG, Berrocal AM. Long-term follow-up of intravitreal bevacizumab for the treatment of pediatric retinal and choroidal diseases. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2015 Dec:19(6):541-8. doi: 10.1016/j.jaapos.2015.09.006. Epub [PubMed PMID: 26691034]
Schefler AC, Berrocal AM, Murray TG. Advanced Coats' disease. Management with repetitive aggressive laser ablation therapy. Retina (Philadelphia, Pa.). 2008 Mar:28(3 Suppl):S38-41. doi: 10.1097/IAE.0b013e318163cd7c. Epub [PubMed PMID: 18317343]
Level 2 (mid-level) evidenceEntezari M, Ramezani A, Safavizadeh L, Bassirnia N. Resolution of macular edema in Coats' disease with intravitreal bevacizumab. Indian journal of ophthalmology. 2010 Jan-Feb:58(1):80-2. doi: 10.4103/0301-4738.58482. Epub [PubMed PMID: 20029156]
Level 3 (low-level) evidenceLei S, Lam WC. Efficacy and safety of dexamethasone intravitreal implant for refractory macular edema in children. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2015 Jun:50(3):236-41. doi: 10.1016/j.jcjo.2015.01.007. Epub [PubMed PMID: 26040225]
Spitznas M, Joussen F, Wessing A. Treatment of Coats' disease with photocoagulation. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1976 Apr 1:199(1):31-7 [PubMed PMID: 1083682]
Finger PT. Radiation therapy for exudative choroidal hemangioma. Indian journal of ophthalmology. 2019 May:67(5):579-581. doi: 10.4103/ijo.IJO_707_19. Epub [PubMed PMID: 31007211]
Singh AD, Kaiser PK, Sears JE. Choroidal hemangioma. Ophthalmology clinics of North America. 2005 Mar:18(1):151-61, ix [PubMed PMID: 15763200]
Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian journal of ophthalmology. 2015 Feb:63(2):122-7. doi: 10.4103/0301-4738.154380. Epub [PubMed PMID: 25827542]
Song W, Du C, Zhang Y. A case report of exudative retinal detachment derived from orbital cellulitis in mainland China. BMC ophthalmology. 2020 Aug 17:20(1):332. doi: 10.1186/s12886-020-01596-6. Epub 2020 Aug 17 [PubMed PMID: 32807110]
Level 3 (low-level) evidenceTesti I, Agrawal R, Mehta S, Basu S, Nguyen Q, Pavesio C, Gupta V. Ocular tuberculosis: Where are we today? Indian journal of ophthalmology. 2020 Sep:68(9):1808-1817. doi: 10.4103/ijo.IJO_1451_20. Epub [PubMed PMID: 32823397]
Schulz DC, Orr SMA, Johnstone R, Devlin MK, Sheidow TG, Bursztyn LLCD. The many faces of ocular syphilis: case-based update on recognition, diagnosis, and treatment. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2021 Oct:56(5):283-293. doi: 10.1016/j.jcjo.2021.01.006. Epub 2021 Feb 4 [PubMed PMID: 33549544]
Level 3 (low-level) evidenceKoundanya VV, Tripathy K. Syphilis Ocular Manifestations. StatPearls. 2024 Jan:(): [PubMed PMID: 32644383]
Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, Ehlers JP. Diagnosis and Treatment of Acute Retinal Necrosis: A Report by the American Academy of Ophthalmology. Ophthalmology. 2017 Mar:124(3):382-392. doi: 10.1016/j.ophtha.2016.11.007. Epub 2017 Jan 13 [PubMed PMID: 28094044]
Jampol LM, Lahov M, Albert DM, Craft J. Ocular clinical findings and basement membrane changes in Goodpasture's syndrome. American journal of ophthalmology. 1975 Mar:79(3):452-63 [PubMed PMID: 1092172]
Diddie KR, Aronson AJ, Ernest JT. Chorioretinopathy in a case of systemic lupus erythematosus. Transactions of the American Ophthalmological Society. 1977:75():122-31 [PubMed PMID: 349823]
Level 3 (low-level) evidenceParis GL, Macoul KL. Reversible bullous retinal detachment in chronic renal disease. American journal of ophthalmology. 1969 Feb:67(2):249-51 [PubMed PMID: 4885012]
Suzani M, Moore AT. Intraoperative fluorescein angiography-guided treatment in children with early Coats' disease. Ophthalmology. 2015 Jun:122(6):1195-202. doi: 10.1016/j.ophtha.2015.02.002. Epub 2015 Mar 29 [PubMed PMID: 25824326]
Gass JD, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995 May:102(5):737-47 [PubMed PMID: 7777273]
Level 3 (low-level) evidenceVigil-De Gracia P, Ortega-Paz L. Retinal detachment in association with pre-eclampsia, eclampsia, and HELLP syndrome. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011 Sep:114(3):223-5. doi: 10.1016/j.ijgo.2011.04.003. Epub 2011 Jun 29 [PubMed PMID: 21719013]
Level 3 (low-level) evidenceShakarchi FI. Ocular tuberculosis: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2015:9():2223-7. doi: 10.2147/OPTH.S65254. Epub 2015 Nov 26 [PubMed PMID: 26648690]
Level 3 (low-level) evidenceDaruich A, Bremond-Gignac D, Behar-Cohen F, Kermorvant E. [Retinopathy of prematurity: from prevention to treatment]. Medecine sciences : M/S. 2020 Oct:36(10):900-907. doi: 10.1051/medsci/2020163. Epub 2020 Oct 7 [PubMed PMID: 33026333]
Dutta Majumder P, Chen EJ, Shah J, Ching Wen Ho D, Biswas J, See Yin L, Gupta V, Pavesio C, Agrawal R. Ocular Syphilis: An Update. Ocular immunology and inflammation. 2019:27(1):117-125. doi: 10.1080/09273948.2017.1371765. Epub 2017 Oct 11 [PubMed PMID: 29020491]
Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Seminars in ophthalmology. 2018:33(6):788-803. doi: 10.1080/08820538.2018.1477966. Epub 2018 Jun 14 [PubMed PMID: 29902388]
Level 3 (low-level) evidenceShields JA, Shields CL. Review: coats disease: the 2001 LuEsther T. Mertz lecture. Retina (Philadelphia, Pa.). 2002 Feb:22(1):80-91 [PubMed PMID: 11884883]