Introduction
Acute or subacute loss of visual acuity caused by circulating antibodies formed against the retinal proteins in the presence of systemic cancer is called cancer-associated retinopathy or carcinoma-associated retinopathy (CAR).[1][2][3] It is described under the broad spectrum of autoimmune retinopathy (AIR) diseases.[4] Autoimmune retinopathy can be paraneoplastic (pAIR) and nonparaneoplastic (npAIR).[5]
Sawyer et al first described visual loss along with retinal degeneration in patients suffering from lung carcinoma in 1976.[6] The term paraneoplastic retinopathy (PR) was coined by Klingele et al to describe autoimmune retinopathy associated with a distant neoplasm.[7] They described PR as "a nonmetastatic remote effect of carcinoma and is characterized by rapid visual deterioration accompanied by narrow arterioles seen on ophthalmoscopic examination and an extinguished electroretinogram." In AIR, autoantibodies against retinal proteins are found in the serum of patients without a known malignancy, whereas, in PR, retinal antibodies are seen in the presence of an underlying malignancy.[8]
CAR is a rare type of retinal paraneoplastic retinopathy. Paraneoplastic syndrome is defined as "rare clinical syndromes due to the systemic effects of tumors; they are unrelated to tumor size, invasiveness or metastases."[9] CAR is characterized by sudden and progressive vision loss.[10] Other entities included in the pAIR visual syndromes are melanoma-associated retinopathy (MAR), paraneoplastic optic neuropathy (PON), and bilateral diffuse uveal melanocytic proliferation (BDUMP).
Often, the loss of visual acuity from CAR can occur even before diagnosing cancer.[11] It has been reported that the diagnosis of CAR (confirmed by the presence of antiretinal antibodies in the sera) precedes the diagnosis of cancer in up to 50% of patients.[12] Management options for CAR include systemic steroids, intravenous immunoglobulin, and various monoclonal antibodies.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Small-cell lung carcinoma (SCLC) is the most important condition associated with npAIR syndrome.[13] Associations of CAR include:
- Non-SCLC [1]
- Breast cancer
- Endometrial cancer [14]
- Invasive thymoma [12]
- Lymphoma [15]
- Uterine cervical cancer [16]
- Endometroid sarcoma [6]
- Myeloma
- Basal cell carcinoma [17]
- Colon cancer
- Kidney cancer [18]
- Leukemia
- Mixed Müllerian tumor
- Prostate cancer
- Melanoma
- Squamous cell carcinoma
- Pancreatic cancer
- Laryngeal carcinoma [19]
- Urinary bladder carcinoma [19]
Epidemiology
AIR accounted for less than 1% of all the cases seen at a tertiary eye center.[20] The pAIR syndrome can be seen in 1 out of 10,000 cancer patients.[21] Braithwaite et al showed that pAIR syndrome eventually develops in approximately 10% to 15% of all cancer patients.[22]
CAR is more common in females compared with males. Misiuk-Hojlo et al noted that the sera of 6 out of 295 breast cancer patients had immunoreactivity to retinal antigens, although only 2 cases showed ocular features of CAR. The mean age of onset of CAR ranges from 55 to 65 years.[23][24]
In a case series of 209 patients, Adamus showed that the mean age of CAR was between 40 and 85 years. Adamus also reported that CAR was mainly associated with malignancy of the breast (31%), followed by lung (16%) and hematological malignancies (15%). SCLC accounts for approximately 29,000 diagnosed cases in the United States annually.[25]
The time interval between the onset of retinopathy and the cancer diagnosis can vary from weeks to months (lymphoma and lung cancer) to even years (breast and prostate cancer).
Pathophysiology
The autoimmune theory was postulated by Keltner et al in 1983.[11] According to the theory, antibodies against retinal photoreceptors were present in the serum of lymphoma patients who developed acute loss of vision and retinal degeneration. The pathological changes seen in CAR result from an interaction between the retinal antigen expression in the cancerous tissues and their systemic immune response. These antigens trigger an autoimmune response within the host to form antibodies that cross-react with the retinal antigen, ultimately causing cell death/apoptosis with retinal degeneration.
Thirkill and colleagues, in 1987, first observed the appearance of autoantibodies against the 23 kDa retinal protein in patients with SCLC and CAR.[1] The 23 kDa protein implicated as the CAR-antigen was noted to be a "photoreceptor cell-specific protein" or "recoverin-like protein."[26]
Recoverin is regarded as the most commonly involved antigen seen in CAR patients.[27] It is a calcium-dependent activator of guanylate cyclase[28] responsible for light and dark adaptation of the photoreceptors.[29] The conformational changes induced by activation of the calcium-binding domains present in recoverin protein play a crucial role in the binding of the antirecoverin antibodies.[30][31][32]
In cancer, the level of vascular endothelial growth factor (VEGF) increases, which causes retinal pericyte loss.[33] This leads to increased permeability and vascular attenuation with subsequent blood–retina barrier breakdown.[34][35] Antibodies then penetrate the membranes of the living photoreceptors by the process of endocytosis and react with recoverin.[35] Injection of recoverin in Lewis rats resulted in high antibody titer and cell-mediated immunity against recoverin, ultimately leading to features of uveoretinitis, including perivasculitis, vitreous cells, retinal lesions, and loss of retinal photoreceptors.[36]
Ohguro et al demonstrated that recoverin dysfunction leads to increased phosphorylation of rhodopsin and opening of cyclic guanosine monophosphate (cGMP)-gated channels, causing a rise in the intracellular calcium.[37] This further leads to the activation of Bcl-2 proteins and caspases 3 and 9, initiating the apoptosis cascade with the breakdown of DNA and cell degeneration.
The gene for recoverin is present on chromosome 17p13.1.[38] In patients with lung cancer, increased recoverin protein expression caused by hypomethylation of DNA within the promoter region is seen.[39] Recoverin protein within the cancer tissues with antirecoverin antibodies can be demonstrated in cancer patients with no evidence of CAR.[40] Recoverin antibodies may have an antitumor effect and may denote a longer survival rate with lesser recurrence in patients with SCLC.[41]
Recoverin may aid in cancer treatment by causing cancer cells to become more sensitive to the chemotherapy or by becoming the target for cancer treatment. Bazhin AV et al analyzed serial samples of tumor tissues and sera from 143 patients with lung cancer and showed that on account of a high occurrence of aberrant expression of recoverin in lung tumors, targeting recoverin as a paraneoplastic antigen may play a role in immunotherapy of lung cancer.[42] The autoimmune reactions that occur against the cancerous cells expressing recoverin can also contribute to the systemic control of malignancy.[43]
Adamus et al showed that antirecoverin antibodies can be detected in only 10% of patients with visual symptoms and identified the presence of autoantibodies against α-enolase (46 kDa) and transducin-α (40 kDa) in the serum of patients with CAR and nonneoplastic retinopathy.[44][45] Enolase is a glycolytic enzyme found in rods, cones, ganglion cells, and Müller cells. Three different isoforms of enolase have been described, but the anti–α-enolase antibodies have mostly been reported in CAR.[46]
Vision loss in enolase-associated retinopathy is less severe as compared to recoverin-associated retinopathy. Recoverin-associated retinopathy has an acute onset with faster progression. Anti-enolase antibodies have been associated with breast, prostate, and hematologic cancers.[47] Antibodies against α-enolase can also be found in about 11% of healthy individuals.[48] Other conditions showing autoantibodies against α-enolase include Behçet disease, mixed cryoglobulinemia, inflammatory bowel disease, primary sclerosing cholangitis, systemic lupus erythematosus (SLE), multiple sclerosis, and systemic sclerosis.[49][50]
Ohguro et al suggested that humoral autoimmunity against recoverin and heat shock cognate protein 70 (65 kDa) may play a role in the pathogenesis of CAR.[51] Other antigens associated with CAR include:
- Anticarbonic anhydrase II (30 kDa) [52]
- Anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
- Interphotoreceptor retinoid-binding protein (145 kDa) [53]
- Neurofilament proteins [35]
- Tubby-like protein 1 [TULP1] [54]
- Arrestin
- Heat shock cognate (HSC) protein 70
- Guanylyl cyclase–activating proteins (GCAP) 1 and 2
- Photoreceptor-specific nuclear receptor 9PNR)
- Rab6A GTPase (Rab6A) and other unidentified antibodies formed against the retinal proteins
T-cells are also involved in the pathogenesis of CAR. CAR enhancement occurs by blockade of the negative T-cell signaling via the cytotoxic T-lymphocyte antigen-4 (CTLA-4).[55] The CTLA-4 receptor is expressed by the regulatory T cells, which block the interleukin-2 (IL-2) expression and inhibit T-cell activation. This leads to a downregulation of the immune response. T-cell activation caused by the antagonism of the CTLA-4 is instrumental in various autoimmune diseases and in causing immune-related cancer regression.[56][57] Therefore, for CAR to develop, exposure to recoverin or other retinal antigens, along with immune modulation in the form of blockade of CTLA-4 signaling, is required.
Histopathology
Histopathological examination reveals patchy photoreceptor loss.[35] Immunohistochemical analysis shows that serum from patients with CAR selectively stains the outer retina (inner and outer segment of most photoreceptors, outer nuclear layer, and outer plexiform layer). Though both the inner and outer segments of rods appeared to stain, many of the cone inner segments did not stain in a study.[58] The occurrence of cross-reactivity with the optic nerve tissue has also been noted.[59]
The retinal pigment epithelium and choroid are usually not involved in CAR, contrary to retinitis pigmentosa. Immunohistochemical staining of the retina varies depending upon the specific type of antibody involved in the pathogenesis. Recoverin-mediated CAR shows predominant photoreceptor staining. Sawyer et al showed photoreceptor degeneration of rods and cones and scattered melanophages in the outer retina and sparing of the ganglion cells in the inner retinal layer.[6]
History and Physical
CAR leads to progressive and rapid visual loss caused by rod and cone dysfunction with retinal degeneration. It is characterized by acquired, acute, or subacute sudden, bilateral, progressive, painless visual deterioration, which can sometimes have a chronic presentation. Even though CAR is usually bilaterally symmetrical, it can be asymmetric and sequential or rarely even have a unilateral presentation.[60]
Early cases of CAR may mimic various other conditions and may be difficult to diagnose. Nonspecific complaints are not uncommon and include glare, photosensitivity, reduced vision, especially in scotopic conditions, floaters, photopsia, apparent graying or darkening of the surrounding environment, transient visual obscurations, and bizarre visual sensations. Clinical features of cone dysfunction are decreased visual acuity, abnormal color vision, metamorphopsia, central scotoma, and abnormal cone-mediated electroretinogram (ERG).[61][62]
Rod dysfunction leads to prolonged dark adaptation, nyctalopia, constricted visual fields, mid-peripheral or ring scotomas, and abnormal rod-mediated ERG. Loss of vision in patients with CAR can occur over weeks to months. Patients with recoverin antibodies in their serum have a diffuse involvement of both rods and cones and suffer from profound visual impairment compared to patients with anti–α-enolase antibodies. Patients with anti–α-enolase antibodies are also less likely to develop nyctalopia due to more limited central cone involvement.[63]
Visual symptoms in CAR may manifest even months or years before the diagnosis of the neoplasm and occur due to the remote effect of the neoplasm. The interval may be as long as 11 years.[64] Jacobson and colleagues suggested that pAIR should be suspected in patients with the triad of "photosensitivity, ring scotomatous visual field loss, and attenuated retinal arteriole caliber."[65]
Recent-onset acquired nyctalopia in an elderly patient with no family history of retinitis pigmentosa or no evidence of vitamin A deficiency are other clinical clues for diagnosing CAR. A slit-lamp examination may reveal the presence of low-grade inflammation in the anterior chamber or cellular debris in the anterior vitreous. The fundus may appear normal, but some patients may have retinal pigment abnormalities in the form of bony spicules or atrophy of the retinal pigment epithelium, vascular attenuation, and optic disc pallor. Other fundus findings include vascular sheathing, macular edema, periphlebitis, chorioretinal atrophy, and vitritis. Vitritis may be seen in up to 20% of patients. Cancer patients also develop cataracts at an early age.[66]
Malignancy is associated with the overproduction of reactive oxygen species (ROS).[67] This oxidative stress may damage the lens proteins, resulting in lens opacification and early-onset cataracts.[68]
Evaluation
Various modalities are used to confirm the diagnosis of CAR.
Visual Fields
Visual field testing usually shows constriction of fields. Other visual field defects include central, cecocentral, or equatorial scotomas and enlargement of the blind spot.[22][69]
Fundus Autofluorescence
Fundus autofluorescence (FAF) is an important noninvasive tool used to analyze patients with CAR; it uses a scanning laser ophthalmoscope and is based on the evaluation of lipofuscin.[70] Lipofuscin is a fluorophore derived from photoreceptor outer segments.[71] Photoreceptor death and retinal pigment epithelium (RPE) atrophy cause hypoautofluorescence (decreased autofluorescence), whereas increased RPE function causes hyperautofluorescence (increased autofluorescence).[72][73]
CAR is characterized by a hyperautofluorescent parafoveal ring with normal autofluorescence within the ring and hypoautofluorescence outside the ring, primarily centered in the macular and peripapillary region. Hyperautofluorescent rings can also be seen in multiple disorders, including retinitis pigmentosa, X-linked retinoschisis, Leber congenital amaurosis, and cone dystrophy.[74][75][76] In Retinitis Pigmentosa, a parafoveal ring of increased autofluorescence (Robson-Holder ring) can be seen in up to 59% of patients.[77]
The Robson-Holder ring represents an area of lipofuscin accumulation around a preserved subfoveal RPE.[78] FAF can detect even minute structural changes in patients with CAR, which are difficult to identify on an ophthalmoscope. Hence, it should be incorporated as a part of the standard imaging protocol for such patients.
Fundus Fluorescein Angiography
Fundus Fluorescein Angiography (FFA) is usually normal. Infrequent findings include optic nerve head staining, perivascular leakage in vasculitis, and petaloid leak at the macula in cystoid macular edema. Peripheral subtle window defects corresponding to retinal degeneration may also be seen.[64][79]
Spectral-Domain Optical Coherence Tomography
Spectral-Domain Optical Coherence Tomography (SD-OCT) provides an objective measure of the amount of retinal damage that has occurred and is also helpful in diagnosing CAR. It may detect the presence of CAR in the early stages.[80][81] See Image. Cancer-Associated Retinopathy.
SD-OCT shows loss of the outer retinal complex, including disruption of the ellipsoid zone (EZ) and thinning of the photoreceptor layer. Abazari et al demonstrated the presence of outer retinal abnormalities and decreased central macular thickness on SD-OCT in CAR patients.[81] Similarly, Sepah et al showed a statistically significant loss of the photoreceptor layer in CAR patients.[82]
OCT findings depend upon both the severity and the duration of the disease. Diagnostic delay accounts for deterioration in the visual acuity and imminent reduction in the subfoveal EZ. Cystic spaces or the presence of cystoid macular edema (CME) is also a frequent finding. Larson et al, in a study among 17 patients with autoimmune retinopathy, showed CME to be the most prevalent finding seen in 24% of the patients. Patients with CME at initial presentation have a greater rate of EZ loss than those without CME.[83]
The condition is also more aggressive in patients with CME, which manifests in reduced a and b waves in the electroretinogram.[84] Vitreomacular traction induced by an epiretinal membrane has also been reported in patients with CAR.[85] Occasionally, mild schisis-like changes can also be seen. Intact EZ and external limiting membrane on OCT in patients before treatment may denote good visual recovery.[86]
Electrophysiological Tests
These measure the electrical activity generated by the eye, the optic pathways, and the visual cortex. The full-field electroretinogram (ffERG) provides information about activity in the rod and cone systems and other neural elements.[87] ERG may be a more sensitive tool than OCT, and ffERG is abnormal in the early stage of the disease. However, some cases may have involvement of the central cones, which is only evident in multifocal ERG (mfERG).
The mfERG indicates the topographical location of the response within the retina. Measurement of the integrity of the retinal pigment epithelium is given by the electrooculogram (EOG). The a-wave of ffERG indicates the response of the photoreceptors. The b-wave can be photopic and scotopic. The photopic, cone-driven b-wave reflects the activity of the on and off bipolar cells, whereas the scotopic, rod-driven b-wave reflects the activity of the on-type bipolar cells.
Photoreceptor damage affects both the a and b-waves, whereas conditions affecting the bipolar cells result in an absent b-wave with a wave showing negative deflection. Oscillatory potentials (OPs) represent the activity of a complex feedback circuit that includes amacrine cells, bipolar cells, and interplexiform cells.[88] Responses such as delayed b-waves, the extinguishing of a- and b-waves involving the rods and cones, reduced b-waves, and an electronegative ERG may be seen in patients with CAR.[89][90]
Electronegative ERG is more typical of melanoma-associated retinopathy. ffERG is almost always abnormal, showing absent or attenuation flash responses in photopic and scotopic conditions. When cones are mainly affected, ffERG might be normal, but the multifocal ERG is abnormal.[91]
In a case series of 10 patients by Thirkill et al, loss of rod and cone amplitudes with normal or prolonged implicit times were seen in patients with antirecoverin antibody–associated CAR.[1] Weleber et al, in a case series of 12 patients, showed that anti–enolase-associated retinopathy is associated with more central or global cone dysfunction than rod dysfunction.[63] In a case series of 39 patients, Adamus et al showed abnormal electroretinogram findings in patients with anti–rod transducin-α associated with npAIR and CAR.[44] The patients associated with anti–rod transducin-α were usually females (female-to-male ratio, 2:1), had abnormal rod function, and typically, there was no evidence of cancer.[44]
Antibody Testing
The presence of antiretinal antibodies in cancer patients was first shown by Kornguth et al[92]. Testing can be done using immunohistochemistry, western blotting, enzyme-linked immunosorbent assay (ELISA, or multiplex assay systems. Adamus et al first showed that clinical manifestations in CAR vary based on the triggering antigens and the circulating antibodies. Diffuse rod and cone involvement is seen in CAR patients with antirecoverin antibodies.[63]
Patients with anti–α-enolase antibodies have limited involvement of the central cones. CAR associated with transducin-α antibodies has more rod degeneration. Serum antibody levels may be found to be elevated even after completion of treatment of the primary neoplasm, and it may also be associated with the progression of CAR. However, fluctuating autoantibody levels in the serum do not indicate cancer recurrence or progression.
The role of serial serum antiretinal antibody levels for longitudinal monitoring requires further research, and technical difficulty and cost limit its regular use. It is important to note that antiretinal antibodies can be found even in the normal population and patients with nonparaneoplastic autoimmune retinopathy. Ko et al showed the presence of antiretinal antibodies in 42% of normal individuals.[93] Hence, positive antiretinal antibodies alone are not diagnostic of CAR. Antiretinal antibodies can also be found in the sera of patients with systemic autoimmune diseases like Behçet disease, SLE, and age-related macular degeneration.[94] Khanna et al, in a review of 20 eyes of 15 patients with autoimmune retinopathy, showed a higher percentage of anti-enolase antibodies in the serum and staining of the photoreceptor layer in patients that developed CME.[83]
As CAR can precede the underlying cancer diagnosis by several months, running a systemic survey facilitated by the primary care physician is essential. This includes a thorough medical history with physical examination, complete blood investigations, MRI of the brain, CT of the chest, abdomen, and pelvis, whole-body PET, and other appropriate tests such as mammogram, colonoscopy, prostate and genitourinary system evaluation, and others. This extensive methodology is pivotal in determining the treatment protocol and prognosis of the patient.[35]
There are no set diagnostic criteria for CAR. The diagnosis is made by combining the patient's clinical symptoms, exam findings, diagnosis of systematic cancer, and positive antibodies against retinal proteins. A patient's visual symptoms may precede cancer diagnosis, making the diagnosis of CAR difficult initially.
Treatment / Management
Early initiation of treatment for CAR is critical for the preservation of vision. CAR associated with untreated antirecoverin antibodies may lead to severe vision loss and even no perception of light.[63] The treatment must be targeted toward the ocular condition because the removal of underlying cancer does not affect the course of CAR. However, regression of the primary neoplasm brings about a significant decline in the circulating autoantibodies.[95] No standardized approach or protocol has been laid for treating paraneoplastic retinopathy. The management of CAR is often difficult, and visual loss may progress despite therapy.(B3)
Systemic steroids can be used for treatment, but they have variable outcomes. The use of corticosteroids has been seen to be more efficient in the short-term management of CAR associated with recoverin-mediated auto-antibodies. Keltner et al first used serum auto-antibody–guided prednisone to control intraocular disease in a cancer patient.[2] The dose of initial oral prednisone may be as high as 1 to 2 mg/kg/day. Other treatment options include intravitreal triamcinolone and subtenon triamcinolone (40-60 mg).[96][97] However, periocular or intraocular steroids can cause a rise in intraocular pressure, which has to be controlled with topical medications.[98] (B2)
Intravenous methylprednisolone (500 mg/day for 3 days), when integrated with resection of the primary neoplasm, can lay down notable short-term improvement, as reported by Dot et al.[14] The use of intravenous methylprednisolone is associated with more favorable outcomes as compared to oral prednisone and hence can be used to initiate the treatment.[99] Plasmapheresis coupled with prednisone may also show beneficial effects in patients with CAR.[95](B3)
Multiple steroid-sparing immunomodulatory agents have been used to treat CAR, but no single therapy has shown consistency or long-term efficacy. Systemic azathioprine (100 mg/day), cyclosporine (100 mg/day), and mycophenolate mofetil (2 g/day) have been used in the management of CAR. Parallel and synchronous use of multiple agents can be done to hold up the visual decline. Ferreyra et al showed that supplementation of immunosuppressive agents (eg, azathioprine [100 mg/day] and cyclosporine [100 mg/day]) causes improvement in visual acuity and visual fields with stabilization in ERG in patients with CAR associated with recoverin autoantibodies.[98] (B2)
Guy et al showed stabilization in patients with CAR using intravenous immunoglobulins (IVIG). IVIG (400 mg/kg/d for 5 days) can improve visual acuity and field.[100] However, CAR associated with anti–α-enolase antibodies is challenging to manage even with IVIG. The use of IVIG and plasmapheresis in the CAR treatment is appropriate if given before the onset of irreversible neuronal degeneration.(B3)
Newer treatment modalities include the use of newer monoclonal antibodies such as alemtuzumab (anti-CD52, pan-lymphocyte) dosed at 30 mg intravenously 3 times a week for 4 months) and rituximab (anti-CD20, B-cell) in patients who have failed prednisone and IVIG.[35][101](B3)
Rituximab dosed at 1000 mg twice a week of 2 doses or 375 mg once a week for 4 weeks, when combined with immunosuppressive agents (eg, prednisone, azathioprine, infliximab, cyclosporine, and mycophenolate), gave beneficial effects in the form of stable or improved visual outcomes in 77% of eyes in a retrospective case series of 16 patients (30 eyes) with autoimmune retinopathy (including 1 melanoma-associated retinopathy, 6 CAR, and 9 npAIR patients).[102]
Ohguro et al showed improvement in ERG and a delay in the progression of the disease by pharmacological reduction of intracellular calcium (nilvadipine) in experimental animals. The antirecoverin antibodies get localized in the photoreceptor cells to block the recoverin function and regulate rhodopsin, resulting in the enhancement of phosphorylation of rhodopsin and induction of apoptotic cell death.[103] (B3)
Retinal dysfunction may be prevented by decreasing the phosphorylation of light-dependent rhodopsin or by antagonism of the intracellular calcium levels. AdamIn an experimental CAR model, a study demonstrated that nifedipine might protect from apoptosis.[104] However, there might be progressive loss in visual acuity despite calcium channel blocker monotherapy in humans. Hence, the effectiveness of using calcium channel blockers or tinted glasses to reduce the ambient light in curbing the pathogenesis of CAR in human beings needs further exploration.(B3)
Antioxidants and vitamins like lutein, vitamin C, vitamin E, and beta carotene (in nonsmokers) may help stabilize retinal degeneration and the disease course. However, their exact role may need further evaluation. Future treatment for CAR is directed towards inhibiting vascular endothelial growth factor (VEGF) receptor 1 to prevent the penetration of antibodies. Calcium modulation and ciliary neurotrophic factor (CNTF) gene transfer may cause a delay in apoptosis.[105] Monoclonal antibodies targeted towards B-cell inhibition can also be used as a safer mode of treatment for patients with CAR.(B3)
Differential Diagnosis
It is important to differentiate CAR from a number of other conditions.
Retinitis Pigmentosa
Retinitis pigmentosa is associated with family history and is inherited by various modes (autosomal dominant, X-linked, autosomal recessive, or sporadic).[106][107] Antiretinal antibodies can be found circulating in around 10% to 37% of patients with retinitis pigmentosa.[108] Features like retinal degeneration may also be seen secondary to retinitis pigmentosa; however, in CAR, minimal inflammation occurs, and usually, there is sparing of the retinal pigment epithelium and choroid. The reason for the similarity is sharing a common target in the form of the S-antigen and interphotoreceptor retinoid-binding protein.[109]
In retinitis pigmentosa, there is a predominant rod photoreceptor dysfunction with a later cone photoreceptor and retinal pigment epithelium dysfunction. The disease manifests during adolescence with the chief complaint of night blindness and mid-peripheral visual loss (due to rod cell damage) and the development of tunnel vision at later stages.[110] Typical features of retinitis pigmentosa include arteriolar attenuation, changes in the retinal pigment epithelium (RPE), and a waxy disc pallor.[111] Other features such as RPE depigmentation and atrophy, cystic macular lesions, posterior subcapsular cataracts, vitreous cells, and refractive errors (myopia and astigmatism) may also be seen.[112] CAR is differentiated by acquired nyctalopia, which usually starts at or after the 6th decade; usually, there is no family history.
npAIR
npAIR was first described in 1997. The phenotype and electrophysiological tests seen in npAIR are similar to those in CAR. It is usually associated with an autoimmune family history, and hence, it needs more intense immunosuppressive therapy. npAIR has a slower progression rate than CAR, the visual changes are more subtle, and the age of presentation is usually younger. npAIR can be associated with photopsia, dyschromatopsia, and nyctalopia. The presence of macular edema and chorioretinal atrophy also points towards npAIR.[113]
The antirecoverin antibodies are usually found in the sera of patients with suspected npAIR. Other antiretinal antibodies associated with npAIR include anticarbonic anhydrase II, anti–α-enolase, antirecoverin, and anti–rod transducin-α antibodies. Adamus et al, after analyzing 193 patients with retinopathy with or without neoplasia, reported that antibodies against recoverin are found in the serum of patients with cancer, whereas α-enolase antibodies were found in all the other patients.[23] Systemic conditions associated with npAIR include hypothyroidism, rheumatoid arthritis, multiple sclerosis, autoimmune hepatitis, Hashimoto thyroiditis, Myasthenia gravis, Graves disease, and bullous pemphigoid.
Melanoma-associated Retinopathy
CAR is most commonly associated with SCLC, whereas MAR is commonly associated with cutaneous malignant melanoma. CAR usually precedes the diagnosis of neoplasm, whereas MAR usually presents after the diagnosis of melanoma. MAR typically has a normal fundus but can also present with diffuse RPE changes, white spots, granular macular appearance, optic disc pallor, retinal vascular attenuation, vitelliform material, and multiple serous retinal detachments.[114] Vitreous cells may be present and may denote the severity of the autoimmune response.[115]
MAR stems from a similar origin as the neuroectoderm of the melanocytes and the retinal cells.[115] Age at presentation varies from 30 to 78 years, and males are predominantly affected.[115] Additionally, a family history of autoimmune diseases may be present.
The typical triad of MAR consists of visual symptoms including night blindness, positive visual phenomena (flickering, pulsating, or shimmering lights that may get worse on exposure to bright light), visual field defects, reduction in b-wave amplitude in ERG, and presence of serum antibody reacting to retinal bipolar cells. The diagnosis of melanoma may precede MAR by 2 months to 19 years, though the interval is usually shorter in metastatic melanoma.
Usually, both eyes are affected within 2 months, though unilateral cases have also been reported.[116] A characteristic feature is the preservation of central visual acuity, with most cases having at least 20/60 vision.[115] Color vision is usually preserved, though subtle discrimination of color may be affected. Reported visual field defects include generalized depression, central or paracentral defects, arcuate scotoma, or constriction of peripheral visual fields.
Electroretinogram in MAR reveals reduced b-wave and preserved dark-adapted a-wave (electronegative ERG) showing bipolar cell dysfunction. Circulating autoantibodies against transient receptor potential cation channel, subfamily M, member 1 (TRPM1), and bestrophin-1 can be found in patients with MAR. Other antibodies associated with MAR include anti-aldolase A and C, anti–α-enolase, anti-interphotoreceptor retinoid-binding protein, antirecoverin, antirhodopsin, antimitofilin, antititin, and antitransducin antibodies.
Therapy for MAR includes cytoreduction and immunotherapy. Cytoreduction aims to reduce tumor burden and stimulus for antibody production. Methods of cytoreduction include surgery, chemotherapy, and radiation. Immunotherapy aims to reduce circulating autoantibody levels. Options for immunotherapy include steroids, intravenous immunoglobulin, plasmapheresis, and various immunosuppressive therapies.[115]
Acute Zonal Outer Occult Retinopathy and Other White Dot Syndromes
Although acute zonal outer occult retinopathy (AZOOR) can present with symptoms, electroretinogram findings, and visual fields showing an enlarged blind spot similar to CAR, AZOOR is typically a bilateral but asymmetric entity. Hypoautofluorescence is observed in the majority of eyes with AZOOR. Patients with AZOOR show partial recovery or stabilization even without treatment. Multiple evanescent white dot syndrome (MEWDS) shows unilateral retinopathy associated with optic nerve edema, afferent pupillary defect, and spontaneous recovery.[117][118]
Cone Dystrophy
In cone dystrophy, the rods usually remain normal or are at least partly spared, even during the late stages.[119] Features include dyschromatopsia, photophobia, loss of visual acuity, and exclusive cone involvement in the ERG characterized by diminished or nonrecordable photopic ERGs and normal or reduced amplitudes in the scotopic ERGs.[120]
Toxic Retinopathy or Toxic Optic Neuropathy
In toxic retinopathy or toxic optic neuropathy, visual impairment occurs due to optic nerve damage caused by a toxin.[121] This entity can occur at any age, with both genders and all races being affected equally. It mainly affects the ganglion cell axons, particularly the papillomacular nerve fiber bundle. Clinically, toxic retinopathy or toxic optic neuropathy presents as bilaterally symmetrical and progressive loss of vision with dyschromatopsia and the presence of central or cecocentral scotoma on visual fields. Toxins causing retinopathy can be found in the blood, urine, and even hair. The most common causes of toxic optic neuropathy include ethambutol, isoniazid, methanol, amiodarone, tobacco, and alcohol.[122]
Leber Hereditary Optic Neuropathy
Leber hereditary optic neuropathy (LHON) is a rare mitochondrial disorder affecting young males between 15 and 35 years of age. Sequential vision loss is seen in both eyes by optic neuropathy.[123][124] It has a predominant maternal inheritance pattern.[125] Degeneration of the retinal ganglionic cells and their axons results in acute or subacute central vision loss. Presymptomatic features include telangiectatic vessels around the optic discs and variable degrees of retinal nerve fiber layer edema.[126][127]
Red–green color vision abnormalities suggesting optic nerve dysfunction reduced contrast sensitivity, and subnormal visual electrophysiological parameters can also be seen.[128] In acute phases, central retinal vessel tortuosity, retinal nerve fiber layer edema, and circumpapillary telangiectatic microangiopathy are seen.[129] The optic disc can appear normal in the acute phase in approximately 20% of cases. The chronic phase is characterized by retinal nerve fiber layer degeneration and optic atrophy seen after 6 months after the onset of the condition.[130]
Nutritional Optic Neuropathy
In nutritional optic neuropathy, a visual impairment results from optic nerve damage occurring secondary to nutritional deficiency (primarily a vitamin deficiency). Deficiency of vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin), and folic acid is associated with nutritional optic neuropathy. The clinical features and pathophysiology involved are similar to toxic retinopathy. Nutritional optic neuropathy can present as a nonspecific retrobulbar optic neuropathy.[121]
BDUMP
BDUMP is a rare paraneoplastic disorder first described by Machemer et al in 1966.[131] The term BDUMP was coined by Barr et al in 1982.[132] The usual age of presentation ranges between 34 to 89 years, with the median age being 64 years.[133] It presents with acute onset, rapid, bilateral (rarely unilateral), and painless loss of visual acuity with a female predilection. The primary malignancy usually associated with BDUMP in females is urogenital cancer (most commonly ovarian cancer), and in males is lung cancer.[134]
The cardinal signs of BDUMP, as described by Gass et al include multiple, subtle, round, or oval, orange–red patches at the level of RPE; multifocal early hyperfluorescence; multiple, focally elevated, pigmented, and nonpigmented uveal melanocytic tumors with diffuse uveal tract thickening; the presence of an exudative type of retinal detachment; and rapid cataract progression.[135] Fundus autofluorescence usually shows nummular changes, giving a giraffe pattern with inverse changes compared to fluorescein angiogram.[136]
Treatment options available for BDUMP include radiation therapy, the use of local and systemic corticosteroids, plasmapheresis, drainage of the subretinal fluid, and vitrectomy. However, BDUMP has a very poor visual prognosis and survival rate. Most of the patients become legally blind within 1 year or succumb to death within 3 years of initial presentation secondary to the systemic malignancy.[137]
Chronic Central Serous Chorioretinopathy
Chronic central serous chorioretinopathy (CSCR) is a retinal disorder seen in young males between the ages of 20 and 45 years.[138] It is usually unilateral; however, a bilateral presentation has been noted in 40% of cases.[139] Chronic CSCR (or diffuse retinal epitheliopathy) is characterized by the persistence of a well-defined, circular, or oval area of localized serous retinal detachment over the posterior pole beyond 3 to 6 months of initial presentation. It can be seen in older patients or those on long-term steroid therapy.[140]
Presenting complaints in acute CSCR include the presence of micropsia, blurring of vision, metamorphopsia, disturbances in color vision, relative scotoma, and temporary hyperopia. whereas chronic form is associated with a more severe or even permanent loss of visual acuity. The extent of visual damage depends on the damage to the photoreceptor-outer segments and the external limiting membrane.[141] FAF in chronic CSCR shows vertical tracks in the inferior retina.[142]
Patchy areas of hyperfluorescence (areas of RPE atrophy) can be seen on FFA. Optical coherence tomography (OCT) findings in chronic CSCR comprise elongation of photoreceptor outer segments, the presence of intraretinal cysts, and hyperreflective dots.[142] Chronic CSCR with retinal pigment epithelial detachments can develop an RPE rip with secondary exudative retinal detachment.[142]
PON
PON is a rare paraneoplastic entity affecting middle-aged patients with malignancies, including thyroid cancer, thymoma, cervical cancer, testicular seminoma, and lung cancer.[143] It is characterized by bilateral, subacute, progressive, painless, and profound loss of visual acuity.[143] It is caused by the cross-reactivity of antibodies generated by the tumor-associated antigens with the neuronal and glial proteins.[144] The fundus examination reveals optic disc edema.[143]
Other findings include an abnormal ERG, visual field defects (tubular vision, enlargement of blind spot, and an inferior scotoma), and visual evoked potential (VEP) changes (decreased amplitude and a delayed implicit period).[145] Important serological tests include:
- Anti-Hu or antineuronal nuclear antibody type-1 (AANA-1)
- anti-Tr (anti-Purkinje cell antibody)
- anti-Yo (anti-Purkinje cell cytoplasmic antibody type 1)
- anti-CV2/CRMP5 (anti-collapsin response mediator protein-5)
- anti-Ri (antineuronal nuclear antibody type-2)
- anti-Ma2/TA (anti-PNMA2 or anti-paraneoplastic antigen Ma2), and
- anti-amphiphysin antibody testing.
Of these, anti-Hu and anti-CV2/CRMP5, antibodies are more consistently associated with paraneoplastic neuropathy.[146][147] Antibodies to aquaporin-4 antibody may be present.[143]
PON leads to irreversible optic nerve changes, and hence, the visual prognosis of PON remains poor as even immunosuppressive therapy and steroids have not been very effective in management. Prompt treatment of the underlying malignancy is the only way to halt the progression of the disease.[148]
Vitamin A Deficiency
Vitamin A is a fat-soluble vitamin required for vision, overall cellular development, immunity, metabolism, and reproductive functions.[149][150] The recommended dietary allowance (RDA) of vitamin A for an adult male is 900 mcg/day, and for an adult female is 700 mcg/day.[151] The RDA of vitamin A for children is 300 to 900 mcg/day; for pregnant and lactating women, it is 770 mcg/day and 1300 mcg/day, respectively.[152]
Serum retinol concentrations of less than 20 mcg/dL are regarded as vitamin A deficiency. Ocular symptoms of vitamin A deficiency develop when the serum retinol concentration drops below 10 mcg/dL.[153] It usually occurs following malabsorption, infections, or poor nutrition. Night blindness or nyctalopia is the first presenting feature of vitamin A deficiency caused by profound rod dysfunction. Fundus examination may reveal multiple white or grey-white spots or dots in the peripheral retina (Uyemura fundus).[154]
ERG shows undetectable or reduced rod response with reduced amplitudes and increased latency of cone response. OCT may show hyperreflective elevated lesions just below the ellipsoid zone corresponding to the white dots.[155] Other early external ocular findings include Bitot spots (oval or triangular, foamy lesions of the conjunctiva) and conjunctival xerosis (wrinkling of the conjunctiva). Late signs of vitamin A deficiency, such as corneal xerosis, corneal ulceration, and, eventually, keratomalacia, may lead to permanent visual damage. Prompt management of vitamin A deficiency at a subclinical stage has a very good prognosis.[152] Permanent vision loss or blindness may be seen in the case of severe vitamin A deficiency.[156]
Hence, to diagnose CAR, it is important to correlate the findings of the sera with the electroretinogram and clinical features with due help from other investigative modalities.
Prognosis
Visual decline and even loss of visual acuity due to CAR may occur even before the diagnosis of cancer. When CAR is associated with recoverin antibodies, the condition can progress to profound vision loss to even no perception of light.[63] Early diagnosis and initiation of treatment are prerequisites for the preservation of vision. However, a targeted decimation of the photoreceptors and the support cells occurs; thus, despite even immunomodulatory therapy, the visual prognosis remains poor. Hence, in any patient, if there is an acute or subacute loss of visual acuity, visual field alterations, and vascular attenuation in the fundus without any other etiology, a suspicion for CAR should be made. Visual prognosis is not affected by the treatment of underlying cancer. Overall survival of the patient depends upon the underlying tumor and stage at which it was diagnosed and the available treatment options.[157]
Adamus et al, after examining 209 cancer patients, concluded that patients who are at risk for developing CAR could be identified early using the antiretinal autoantibodies.[158] This early detection may prevent vision loss in such vulnerable patients.
Complications
Choroidal neovascularization can occur secondary to CAR.[53] Other causes of vision loss in patients with CAR include cataracts, macular edema, foveal thinning, and secondary optic atrophy.[65] The mortality depends on the cause of CAR, and the primary malignancy should be managed optimally.
Deterrence and Patient Education
Patients with CAR need proper guidance, in-depth evaluation, and education about this condition and the various treatment options available. Patients need to understand that CAR is a potentially blinding and devastating condition, and the features of the same may develop even before the neoplasm is clinically evident. Further, patients must understand that although there is no standard treatment protocol for this entity, proper compliance with the treatment is needed to stall or rarely reverse this condition.[35]
Enhancing Healthcare Team Outcomes
A comprehensive approach is crucial for managing patients with CAR. An appropriately high index of suspicion and early diagnosis and treatment play an imperative role in curtailing the risk of irreversible immunological damage to the retinal cells in these patients. Hence, a multidisciplinary approach for complete evaluation and treatment is vital for properly managing this condition, even though there is no consensus on the standard treatment protocol. For instance, when a patient is suspected of having CAR, a prompt referral by the treating physician or oncologist to the ophthalmologist is recommended. This is to be followed by collaborative planning between the oncologist, ophthalmologist, rheumatologist, dermatologist, radiologist, or even a uveitis specialist accustomed to administering the immunomodulatory agents.
The interprofessional teamwork required to treat this condition includes evaluation by the physician/clinician or oncologist, the ophthalmologist, dispensing of medicines by the pharmacist, aiding in patient education for treatment compliance as well as monitoring the vitals by the nurses, and thorough evaluation of the visual acuity by the optometrist. This united interprofessional approach is decisive in managing the patients and acquiring favorable outcomes.
Media
(Click Image to Enlarge)
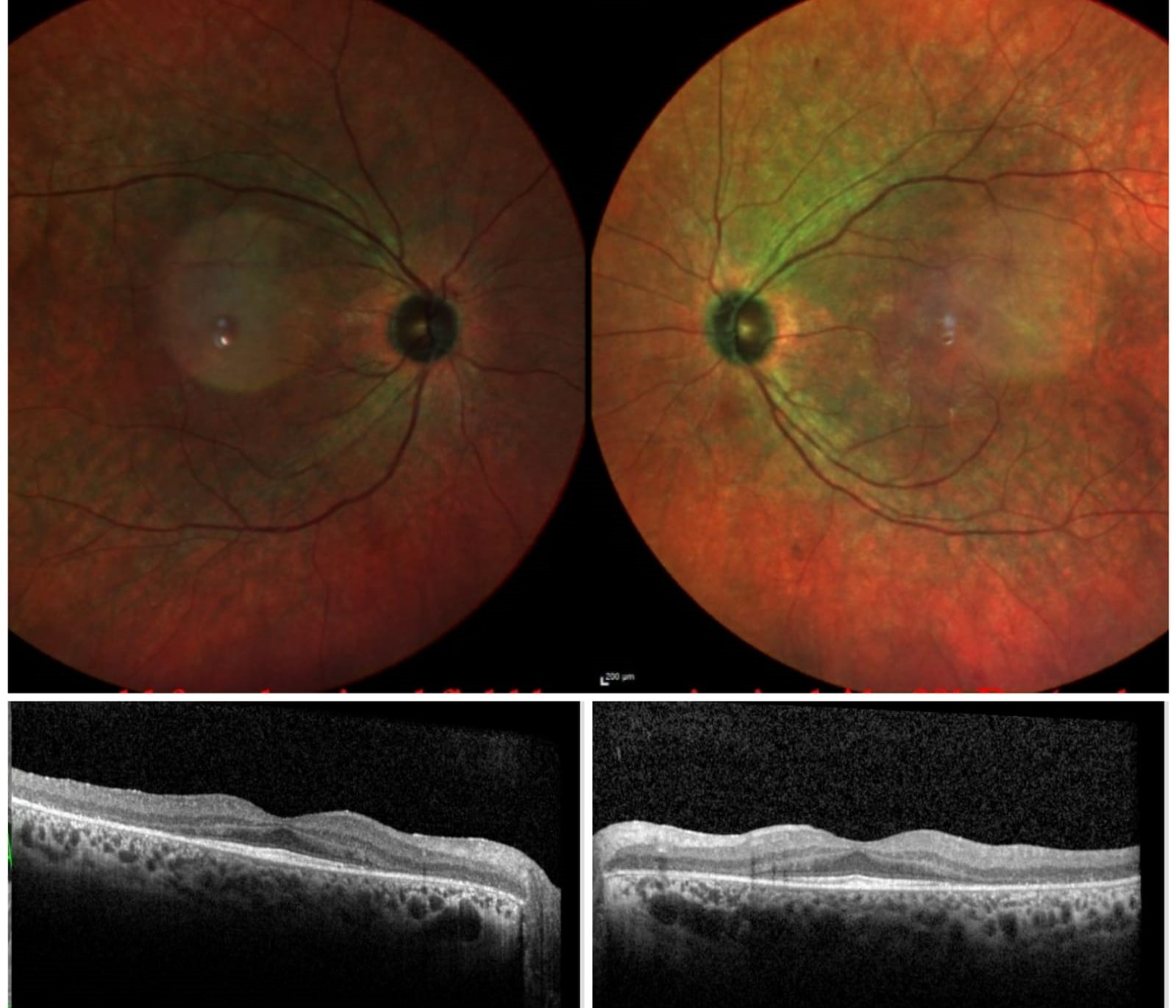
Cancer-Associated Retinopathy. Multicolor fundus images of the right and the left eye show retinal pigmentary changes seen during the early phase of cancer-associated retinopathy. Optical coherence tomography (OCT) of both eyes shows the absence of the ellipsoid zone (EZ) outside the fovea with thinning of the photoreceptor layer.
Contributed by Koushik Tripathy, MD
References
Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1987 Mar:105(3):372-5 [PubMed PMID: 2950846]
Level 3 (low-level) evidenceKeltner JL, Thirkill CE, Tyler NK, Roth AM. Management and monitoring of cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1992 Jan:110(1):48-53 [PubMed PMID: 1310001]
Level 3 (low-level) evidenceThirkill CE, FitzGerald P, Sergott RC, Roth AM, Tyler NK, Keltner JL. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. The New England journal of medicine. 1989 Dec 7:321(23):1589-94 [PubMed PMID: 2555714]
Level 3 (low-level) evidenceHeckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Seminars in immunopathology. 2008 Apr:30(2):127-34. doi: 10.1007/s00281-008-0114-7. Epub 2008 Apr 12 [PubMed PMID: 18408929]
Level 3 (low-level) evidenceDutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian journal of ophthalmology. 2020 Sep:68(9):1829-1837. doi: 10.4103/ijo.IJO_786_20. Epub [PubMed PMID: 32823399]
Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. American journal of ophthalmology. 1976 May:81(5):606-13 [PubMed PMID: 179323]
Level 3 (low-level) evidenceKlingele TG, Burde RM, Rappazzo JA, Isserman MJ, Burgess D, Kantor O. Paraneoplastic retinopathy. Journal of clinical neuro-ophthalmology. 1984 Dec:4(4):239-45 [PubMed PMID: 6240497]
Level 3 (low-level) evidenceMizener JB, Kimura AE, Adamus G, Thirkill CE, Goeken JA, Kardon RH. Autoimmune retinopathy in the absence of cancer. American journal of ophthalmology. 1997 May:123(5):607-18 [PubMed PMID: 9152066]
Level 3 (low-level) evidenceHenry K. Paraneoplastic syndromes: Definitions, classification, pathophysiology and principles of treatment. Seminars in diagnostic pathology. 2019 Jul:36(4):204-210. doi: 10.1053/j.semdp.2019.01.002. Epub 2019 Feb 1 [PubMed PMID: 30876820]
Naramala S, Ahmad J, Adapa S, Gavini F, Konala VM. Case Series of Cancer-associated Retinopathy (CAR). Cureus. 2019 Jun 10:11(6):e4872. doi: 10.7759/cureus.4872. Epub 2019 Jun 10 [PubMed PMID: 31417817]
Level 2 (mid-level) evidenceKeltner JL, Roth AM, Chang RS. Photoreceptor degeneration. Possible autoimmune disorder. Archives of ophthalmology (Chicago, Ill. : 1960). 1983 Apr:101(4):564-9 [PubMed PMID: 6838414]
Level 3 (low-level) evidenceKatsuta H, Okada M, Nakauchi T, Takahashi Y, Yamao S, Uchida S. Cancer-associated retinopathy associated with invasive thymoma. American journal of ophthalmology. 2002 Sep:134(3):383-9 [PubMed PMID: 12208250]
Level 3 (low-level) evidenceChan JW. Paraneoplastic retinopathies and optic neuropathies. Survey of ophthalmology. 2003 Jan-Feb:48(1):12-38 [PubMed PMID: 12559325]
Level 3 (low-level) evidenceDot C, Guigay J, Adamus G. Anti-alpha-enolase antibodies in cancer-associated retinopathy with small cell carcinoma of the lung. American journal of ophthalmology. 2005 Apr:139(4):746-7 [PubMed PMID: 15808190]
Level 3 (low-level) evidenceAdamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmunity reviews. 2009 Mar:8(5):410-4. doi: 10.1016/j.autrev.2009.01.002. Epub 2009 Jan 23 [PubMed PMID: 19168157]
Level 3 (low-level) evidenceMisiuk-Hojło M, Ejma M, Gorczyca WA, Szymaniec S, Witkowska D, Fortuna W, Miedzybrodzki R, Rogozińska-Szczepka J, Bartnik W. Cancer-associated retinopathy in patients with breast carcinoma. Archivum immunologiae et therapiae experimentalis. 2007 Jul-Aug:55(4):261-5 [PubMed PMID: 17659379]
Raghunath A, Adamus G, Bodurka DC, Liu J, Schiffman JS. Cancer-associated retinopathy in neuroendocrine carcinoma of the fallopian tube. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2010 Sep:30(3):252-4. doi: 10.1097/WNO.0b013e3181e22ef0. Epub [PubMed PMID: 20724944]
Level 3 (low-level) evidenceWagley S, Tran TM, Mallory PW, Lee MS, Armbrust KR, Trautman B, Montezuma SR. Cancer-Associated Retinopathy due to Clear Cell Renal Carcinoma. Ocular oncology and pathology. 2021 Mar:7(1):31-35. doi: 10.1159/000511189. Epub 2020 Dec 18 [PubMed PMID: 33796514]
Matsui Y, Mehta MC, Katsumi O, Brodie SE, Hirose T. Electrophysiological findings in paraneoplastic retinopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1992:230(4):324-8 [PubMed PMID: 1505762]
Level 3 (low-level) evidenceGrange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. American journal of ophthalmology. 2014 Feb:157(2):266-272.e1. doi: 10.1016/j.ajo.2013.09.019. Epub 2013 Sep 29 [PubMed PMID: 24315290]
Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet journal of rare diseases. 2007 May 4:2():22 [PubMed PMID: 17480225]
Braithwaite T, Vugler A, Tufail A. Autoimmune retinopathy. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2012:228(3):131-42 [PubMed PMID: 22846442]
Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC ophthalmology. 2004 Jun 4:4():5 [PubMed PMID: 15180904]
Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2001 Sep:21(3):173-87 [PubMed PMID: 11725182]
Level 3 (low-level) evidenceCarrera W, Tsamis KA, Shah R. A case of cancer-associated retinopathy with chorioretinitis and optic neuritis associated with occult small cell lung cancer. BMC ophthalmology. 2019 May 2:19(1):101. doi: 10.1186/s12886-019-1103-4. Epub 2019 May 2 [PubMed PMID: 31046716]
Level 3 (low-level) evidenceThirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JL. The cancer-associated retinopathy antigen is a recoverin-like protein. Investigative ophthalmology & visual science. 1992 Sep:33(10):2768-72 [PubMed PMID: 1388144]
Level 3 (low-level) evidenceOhguro H, Yokoi Y, Ohguro I, Mamiya K, Ishikawa F, Yamazaki H, Metoki T, Takano Y, Ito T, Nakazawa M. Clinical and immunologic aspects of cancer-associated retinopathy. American journal of ophthalmology. 2004 Jun:137(6):1117-9 [PubMed PMID: 15183799]
Level 2 (mid-level) evidenceMcGinnis JF, Stepanik PL, Baehr W, Subbaraya I, Lerious V. Cloning and sequencing of the 23 kDa mouse photoreceptor cell-specific protein. FEBS letters. 1992 May 11:302(2):172-6 [PubMed PMID: 1386025]
Level 3 (low-level) evidencePolans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proceedings of the National Academy of Sciences of the United States of America. 1995 Sep 26:92(20):9176-80 [PubMed PMID: 7568096]
Level 3 (low-level) evidenceAdamus G, Amundson D. Epitope recognition of recoverin in cancer associated retinopathy: evidence for calcium-dependent conformational epitopes. Journal of neuroscience research. 1996 Sep 15:45(6):863-72 [PubMed PMID: 8892098]
Level 3 (low-level) evidenceAdamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Investigative ophthalmology & visual science. 1997 Feb:38(2):283-91 [PubMed PMID: 9040460]
Level 3 (low-level) evidenceMaeda T, Maeda A, Maruyama I, Ogawa KI, Kuroki Y, Sahara H, Sato N, Ohguro H. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Investigative ophthalmology & visual science. 2001 Mar:42(3):705-12 [PubMed PMID: 11222531]
Level 3 (low-level) evidenceCao R, Xue Y, Hedlund EM, Zhong Z, Tritsaris K, Tondelli B, Lucchini F, Zhu Z, Dissing S, Cao Y. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jan 12:107(2):856-61. doi: 10.1073/pnas.0911661107. Epub 2009 Dec 22 [PubMed PMID: 20080765]
Level 3 (low-level) evidenceSuzuki T, Obara Y, Sato Y, Saito G, Ichiwata T, Uchiyama T. Cancer-associated retinopathy with presumed vasculitis. American journal of ophthalmology. 1996 Jul:122(1):125-7 [PubMed PMID: 8659589]
Level 3 (low-level) evidenceShildkrot Y, Sobrin L, Gragoudas ES. Cancer-associated retinopathy: update on pathogenesis and therapy. Seminars in ophthalmology. 2011 Jul-Sep:26(4-5):321-8. doi: 10.3109/08820538.2011.588657. Epub [PubMed PMID: 21958182]
Adamus G, Ortega H, Witkowska D, Polans A. Recoverin: a potent uveitogen for the induction of photoreceptor degeneration in Lewis rats. Experimental eye research. 1994 Oct:59(4):447-55 [PubMed PMID: 7859820]
Level 3 (low-level) evidenceOhguro H, Ogawa K, Maeda T, Maruyama I, Maeda A, Takano Y, Nakazawa M. Retinal dysfunction in cancer-associated retinopathy is improved by Ca(2+) antagonist administration and dark adaptation. Investigative ophthalmology & visual science. 2001 Oct:42(11):2589-95 [PubMed PMID: 11581204]
Level 3 (low-level) evidenceMcGinnis JF, Austin B, Klisak I, Heinzmann C, Kojis T, Sparkes RS, Bateman JB, Lerious V. Chromosomal assignment of the human gene for the cancer-associated retinopathy protein (recoverin) to chromosome 17p13.1. Journal of neuroscience research. 1995 Feb 1:40(2):165-8 [PubMed PMID: 7745609]
Level 3 (low-level) evidenceBazhin AV, De Smet C, Golovastova MO, Schmidt J, Philippov PP. Aberrant demethylation of the recoverin gene is involved in the aberrant expression of recoverin in cancer cells. Experimental dermatology. 2010 Nov:19(11):1023-5. doi: 10.1111/j.1600-0625.2010.01126.x. Epub 2010 Aug 31 [PubMed PMID: 20812967]
Level 3 (low-level) evidenceBazhin AV, Shifrina ON, Savchenko MS, Tikhomirova NK, Goncharskaia MA, Gorbunova VA, Senin II, Chuchalin AG, Philippov PP. Low titre autoantibodies against recoverin in sera of patients with small cell lung cancer but without a loss of vision. Lung cancer (Amsterdam, Netherlands). 2001 Oct:34(1):99-104 [PubMed PMID: 11557119]
Level 3 (low-level) evidenceKobayashi M, Ikezoe T, Uemura Y, Ueno H, Taguchi H. Long-term survival of a patient with small cell lung cancer associated with cancer-associated retinopathy. Lung cancer (Amsterdam, Netherlands). 2007 Sep:57(3):399-403 [PubMed PMID: 17397962]
Level 3 (low-level) evidenceBazhin AV, Savchenko MS, Shifrina ON, Demoura SA, Chikina SY, Jaques G, Kogan EA, Chuchalin AG, Philippov PP. Recoverin as a paraneoplastic antigen in lung cancer: the occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung cancer (Amsterdam, Netherlands). 2004 May:44(2):193-8 [PubMed PMID: 15084384]
Maeda A, Maeda T, Ohguro H, Palczewski K, Sato N. Vaccination with recoverin, a cancer-associated retinopathy antigen, induces autoimmune retinal dysfunction and tumor cell regression in mice. European journal of immunology. 2002 Aug:32(8):2300-7 [PubMed PMID: 12209643]
Level 3 (low-level) evidenceAdamus G, Brown L, Weleber RG. Molecular biomarkers for autoimmune retinopathies: significance of anti-transducin-alpha autoantibodies. Experimental and molecular pathology. 2009 Dec:87(3):195-203. doi: 10.1016/j.yexmp.2009.08.003. Epub 2009 Sep 8 [PubMed PMID: 19744478]
Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clinical immunology and immunopathology. 1996 Feb:78(2):120-9 [PubMed PMID: 8625554]
Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. Journal of clinical immunology. 2007 Mar:27(2):181-92 [PubMed PMID: 17235687]
Level 3 (low-level) evidenceGrewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina (Philadelphia, Pa.). 2014 May:34(5):827-45. doi: 10.1097/IAE.0000000000000119. Epub [PubMed PMID: 24646664]
Forooghian F, Adamus G, Sproule M, Westall C, O'Connor P. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2007 Aug:245(8):1077-84 [PubMed PMID: 17219105]
Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmunity reviews. 2007 Jan:6(3):176-82 [PubMed PMID: 17289554]
Level 3 (low-level) evidencePratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. The Journal of rheumatology. 2000 Jan:27(1):109-15 [PubMed PMID: 10648026]
Ohguro H, Ogawa K, Nakagawa T. Recoverin and Hsc 70 are found as autoantigens in patients with cancer-associated retinopathy. Investigative ophthalmology & visual science. 1999 Jan:40(1):82-9 [PubMed PMID: 9888430]
Level 3 (low-level) evidenceAdamus G, Karren L. Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. Journal of autoimmunity. 2009 Mar:32(2):133-9. doi: 10.1016/j.jaut.2009.02.001. Epub 2009 Mar 6 [PubMed PMID: 19269136]
Querques G, Thirkill CE, Hagege H, Soubrane G, Souied EH. Choroidal neovascularization associated with cancer-associated retinopathy. Acta ophthalmologica. 2010 Aug:88(5):571-5. doi: 10.1111/j.1755-3768.2008.01456.x. Epub 2008 Dec 24 [PubMed PMID: 19141145]
Level 3 (low-level) evidenceKikuchi T, Arai J, Shibuki H, Kawashima H, Yoshimura N. Tubby-like protein 1 as an autoantigen in cancer-associated retinopathy. Journal of neuroimmunology. 2000 Feb 1:103(1):26-33 [PubMed PMID: 10674986]
Maeda A, Maeda T, Liang Y, Yenerel M, Saperstein DA. Effects of cytotoxic T lymphocyte antigen 4 (CTLA4) signaling and locally applied steroid on retinal dysfunction by recoverin, cancer-associated retinopathy antigen. Molecular vision. 2006 Aug 10:12():885-91 [PubMed PMID: 16917481]
Level 3 (low-level) evidenceGough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunological reviews. 2005 Apr:204():102-15 [PubMed PMID: 15790353]
Level 3 (low-level) evidenceSerrano NC, Millan P, Páez MC. Non-HLA associations with autoimmune diseases. Autoimmunity reviews. 2006 Mar:5(3):209-14 [PubMed PMID: 16483921]
Rizzo JF 3rd, Gittinger JW Jr. Selective immunohistochemical staining in the paraneoplastic retinopathy syndrome. Ophthalmology. 1992 Aug:99(8):1286-95 [PubMed PMID: 1325045]
Level 3 (low-level) evidenceAdamus G, Guy J, Schmied JL, Arendt A, Hargrave PA. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Investigative ophthalmology & visual science. 1993 Aug:34(9):2626-33 [PubMed PMID: 7688357]
Level 3 (low-level) evidenceAlmeida DR, Chin EK, Niles P, Kardon R, Sohn EH. Unilateral manifestation of autoimmune retinopathy. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2014 Aug:49(4):e85-7. doi: 10.1016/j.jcjo.2014.04.007. Epub 2014 Jul 17 [PubMed PMID: 25103665]
Level 3 (low-level) evidenceOhguro H, Maruyama I, Nakazawa M, Oohira A. Antirecoverin antibody in the aqueous humor of a patient with cancer-associated retinopathy. American journal of ophthalmology. 2002 Oct:134(4):605-7 [PubMed PMID: 12383822]
Level 3 (low-level) evidenceWhitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. American journal of ophthalmology. 1998 Aug:126(2):230-7 [PubMed PMID: 9727517]
Level 3 (low-level) evidenceWeleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, Adamus G. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. American journal of ophthalmology. 2005 May:139(5):780-94 [PubMed PMID: 15860281]
Level 3 (low-level) evidenceSaito W, Kase S, Ohguro H, Furudate N, Ohno S. Slowly progressive cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Oct:125(10):1431-3 [PubMed PMID: 17923561]
Level 3 (low-level) evidenceJacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Annals of neurology. 1990 Aug:28(2):162-7 [PubMed PMID: 2171418]
Level 3 (low-level) evidenceChiang CC, Lin CL, Peng CL, Sung FC, Tsai YY. Increased risk of cancer in patients with early-onset cataracts: a nationwide population-based study. Cancer science. 2014 Apr:105(4):431-6. doi: 10.1111/cas.12360. Epub 2014 Mar 11 [PubMed PMID: 24450445]
Level 2 (mid-level) evidenceKlaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicologic pathology. 2010 Jan:38(1):96-109. doi: 10.1177/0192623309356453. Epub 2009 Dec 17 [PubMed PMID: 20019356]
Level 3 (low-level) evidenceMichael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011 Apr 27:366(1568):1278-92. doi: 10.1098/rstb.2010.0300. Epub [PubMed PMID: 21402586]
Ruia S, Tripathy K. Humphrey Visual Field. StatPearls. 2025 Jan:(): [PubMed PMID: 36256759]
Lima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, Yannuzzi LA, Tsang SH. Hyperautofluorescent ring in autoimmune retinopathy. Retina (Philadelphia, Pa.). 2012 Jul:32(7):1385-94. doi: 10.1097/IAE.0b013e3182398107. Epub [PubMed PMID: 22218149]
Level 3 (low-level) evidenceEldred GE, Katz ML. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Experimental eye research. 1988 Jul:47(1):71-86 [PubMed PMID: 3409988]
von Rückmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. The British journal of ophthalmology. 1995 May:79(5):407-12 [PubMed PMID: 7612549]
Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye (London, England). 1995:9 ( Pt 6)():763-71 [PubMed PMID: 8849547]
Aguirre-Lamban J, Riveiro-Alvarez R, Maia-Lopes S, Cantalapiedra D, Vallespin E, Avila-Fernandez A, Villaverde-Montero C, Trujillo-Tiebas MJ, Ramos C, Ayuso C. Molecular analysis of the ABCA4 gene for reliable detection of allelic variations in Spanish patients: identification of 21 novel variants. The British journal of ophthalmology. 2009 May:93(5):614-21. doi: 10.1136/bjo.2008.145193. Epub 2008 Nov 21 [PubMed PMID: 19028736]
Tsang SH, Vaclavik V, Bird AC, Robson AG, Holder GE. Novel phenotypic and genotypic findings in X-linked retinoschisis. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Feb:125(2):259-67 [PubMed PMID: 17296904]
Level 3 (low-level) evidenceScholl HP, Chong NH, Robson AG, Holder GE, Moore AT, Bird AC. Fundus autofluorescence in patients with leber congenital amaurosis. Investigative ophthalmology & visual science. 2004 Aug:45(8):2747-52 [PubMed PMID: 15277500]
Pichi F, Abboud EB, Ghazi NG, Khan AO. Fundus autofluorescence imaging in hereditary retinal diseases. Acta ophthalmologica. 2018 Aug:96(5):e549-e561. doi: 10.1111/aos.13602. Epub 2017 Nov 2 [PubMed PMID: 29098804]
Murakami T, Akimoto M, Ooto S, Suzuki T, Ikeda H, Kawagoe N, Takahashi M, Yoshimura N. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. American journal of ophthalmology. 2008 Apr:145(4):687-94. doi: 10.1016/j.ajo.2007.11.018. Epub 2008 Feb 1 [PubMed PMID: 18242574]
Level 2 (mid-level) evidenceRuia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Pepple KL, Cusick M, Jaffe GJ, Mruthyunjaya P. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. The British journal of ophthalmology. 2013 Feb:97(2):139-44. doi: 10.1136/bjophthalmol-2012-302524. Epub 2012 Dec 5 [PubMed PMID: 23221966]
Level 2 (mid-level) evidenceAbazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. American journal of ophthalmology. 2012 Apr:153(4):750-6, 756.e1. doi: 10.1016/j.ajo.2011.09.012. Epub 2012 Jan 14 [PubMed PMID: 22245461]
Sepah YJ, Sadiq MA, Hassan M, Hanout M, Soliman M, Agarwal A, Afridi R, Coupland SG, Nguyen QD. Assessment of Retinal Structural and Functional Characteristics in Eyes with Autoimmune Retinopathy. Current molecular medicine. 2015:15(6):578-86 [PubMed PMID: 26238366]
Khanna S, Martins A, Oakey Z, Mititelu M. Non-paraneoplastic autoimmune retinopathy: multimodal testing characteristics of 13 cases. Journal of ophthalmic inflammation and infection. 2019 Feb 26:9(1):6. doi: 10.1186/s12348-019-0171-1. Epub 2019 Feb 26 [PubMed PMID: 30806850]
Level 3 (low-level) evidenceFinn AP, Thomas AS, Stinnett SS, Keenan RT, Grewal DS, Jaffe GJ. The role of cystoid macular edema as a marker in the progression of non-paraneoplastic autoimmune retinopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018 Oct:256(10):1867-1873. doi: 10.1007/s00417-018-4084-8. Epub 2018 Aug 20 [PubMed PMID: 30128606]
Igarashi N, Sawamura H, Kaburaki T, Aihara M. Cancer-associated Retinopathy Developing After 10 Years of Complete Breast Cancer Remission. Neuro-ophthalmology (Aeolus Press). 2019 Feb:43(1):36-42. doi: 10.1080/01658107.2018.1460761. Epub 2018 May 23 [PubMed PMID: 30723523]
Neena R, Jain A, Anantharaman G, Antony MA. Carcinoma -associated Retinopathy(CAR): Role of Electroretinography(ERG) and Optical coherence Tomography(OCT) in diagnosis and predicting treatment outcome. American journal of ophthalmology case reports. 2021 Mar:21():101008. doi: 10.1016/j.ajoc.2020.101008. Epub 2021 Jan 13 [PubMed PMID: 33511304]
Level 3 (low-level) evidenceHolopigian K, Hood DC. Electrophysiology. Ophthalmology clinics of North America. 2003 Jun:16(2):237-51 [PubMed PMID: 12809161]
Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Progress in retinal and eye research. 1998 Oct:17(4):485-521 [PubMed PMID: 9777648]
Level 3 (low-level) evidenceLink B, Schlötzer-Schrehardt U, Jünemann A. Carcinoma-associated retinopathy-an electrophysiological and immunohistochemical correlation. Retina (Philadelphia, Pa.). 2009 Jan:29(1):69-72. doi: 10.1097/IAE.0b013e3181853d06. Epub [PubMed PMID: 18728619]
Level 3 (low-level) evidenceGoetgebuer G, Kestelyn-Stevens AM, De Laey JJ, Kestelyn P, Leroy BP. Cancer-associated retinopathy (CAR) with electronegative ERG: a case report. Documenta ophthalmologica. Advances in ophthalmology. 2008 Jan:116(1):49-55 [PubMed PMID: 17721792]
Level 3 (low-level) evidenceMohamed Q, Harper CA. Acute optical coherence tomographic findings in cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 2007 Aug:125(8):1132-3 [PubMed PMID: 17698766]
Level 3 (low-level) evidenceKornguth SE, Klein R, Appen R, Choate J. Occurrence of anti-retinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer. 1982 Oct 1:50(7):1289-93 [PubMed PMID: 6286090]
Level 3 (low-level) evidenceKo AC, Brinton JP, Mahajan VB, Zimmerman B, Brinton GS, Stone EM, Folk JC, Mullins RF. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Archives of ophthalmology (Chicago, Ill. : 1960). 2011 Apr:129(4):415-20. doi: 10.1001/archophthalmol.2011.65. Epub [PubMed PMID: 21482867]
Forooghian F, Cao S, Cui J, Matsubara JA. The enigma of autoimmune retinopathy. International ophthalmology clinics. 2015 Spring:55(2):81-91. doi: 10.1097/IIO.0000000000000063. Epub [PubMed PMID: 25730621]
Murphy MA, Thirkill CE, Hart WM Jr. Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell lung carcinoma. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 1997 Jun:17(2):77-83 [PubMed PMID: 9176775]
Level 3 (low-level) evidenceHuynh N, Shildkrot Y, Lobo AM, Sobrin L. Intravitreal triamcinolone for cancer-associated retinopathy refractory to systemic therapy. Journal of ophthalmic inflammation and infection. 2012 Sep:2(3):169-71. doi: 10.1007/s12348-012-0067-9. Epub 2012 Mar 14 [PubMed PMID: 22415770]
Javaid Z, Rehan SM, Al-Bermani A, Payne G. Unilateral cancer-associated retinopathy: a case report. Scottish medical journal. 2016 Aug:61(3):155-159 [PubMed PMID: 26246524]
Level 3 (low-level) evidenceFerreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Archives of ophthalmology (Chicago, Ill. : 1960). 2009 Apr:127(4):390-7. doi: 10.1001/archophthalmol.2009.24. Epub [PubMed PMID: 19365013]
Level 2 (mid-level) evidenceJacobzone C, Cochard-Marianowski C, Kupfer I, Bettembourg S, Dordain Y, Misery L, Cochener B, Sassolas B. Corticosteroid treatment for melanoma-associated retinopathy: effect on visual acuity and electrophysiologic findings. Archives of dermatology. 2004 Oct:140(10):1258-61 [PubMed PMID: 15492190]
Level 3 (low-level) evidenceGuy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Apr:117(4):471-7 [PubMed PMID: 10206574]
Level 3 (low-level) evidenceMahdi N, Faia LJ, Goodwin J, Nussenblatt RB, Sen HN. A case of autoimmune retinopathy associated with thyroid carcinoma. Ocular immunology and inflammation. 2010 Aug:18(4):322-3. doi: 10.3109/09273941003802379. Epub [PubMed PMID: 20662664]
Level 3 (low-level) evidenceDavoudi S, Ebrahimiadib N, Yasa C, Sevgi DD, Roohipoor R, Papavasilieou E, Comander J, Sobrin L. Outcomes in Autoimmune Retinopathy Patients Treated With Rituximab. American journal of ophthalmology. 2017 Aug:180():124-132. doi: 10.1016/j.ajo.2017.04.019. Epub 2017 May 5 [PubMed PMID: 28483493]
Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. Journal of autoimmunity. 1998 Oct:11(5):523-33 [PubMed PMID: 9802939]
Level 3 (low-level) evidenceAdamus G, Webb S, Shiraga S, Duvoisin RM. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. Journal of autoimmunity. 2006 Mar:26(2):146-53 [PubMed PMID: 16426815]
Level 3 (low-level) evidenceAdamus G, Sugden B, Shiraga S, Timmers AM, Hauswirth WW. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. Journal of autoimmunity. 2003 Sep:21(2):121-9 [PubMed PMID: 12935781]
Level 3 (low-level) evidenceFishman GA. Retinitis pigmentosa. Visual loss. Archives of ophthalmology (Chicago, Ill. : 1960). 1978 Jul:96(7):1185-8 [PubMed PMID: 307377]
Fishman GA, Farber MD, Derlacki DJ. X-linked retinitis pigmentosa. Profile of clinical findings. Archives of ophthalmology (Chicago, Ill. : 1960). 1988 Mar:106(3):369-75 [PubMed PMID: 3257866]
Heckenlively JR, Aptsiauri N, Nusinowitz S, Peng C, Hargrave PA. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Transactions of the American Ophthalmological Society. 1996:94():179-200; discussion 200-6 [PubMed PMID: 8981696]
Level 3 (low-level) evidenceTakeuchi M, Usui Y, Okunuki Y, Zhang L, Ma J, Yamakawa N, Hattori T, Kezuka T, Sakai J, Goto H. Immune responses to interphotoreceptor retinoid-binding protein and S-antigen in Behcet's patients with uveitis. Investigative ophthalmology & visual science. 2010 Jun:51(6):3067-75. doi: 10.1167/iovs.09-4313. Epub 2010 Jan 20 [PubMed PMID: 20089879]
Naik A, Ratra D, Banerjee A, Dalan D, Jandyal S, Rao G, Sen P, Bhende M, Jayaprakash V, Susvar P, Walinjkar J, Rao C. Enhanced S-cone syndrome: Clinical spectrum in Indian population. Indian journal of ophthalmology. 2019 Apr:67(4):523-529. doi: 10.4103/ijo.IJO_1480_18. Epub [PubMed PMID: 30900587]
Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Seminars in ophthalmology. 2017:32(2):237-250. doi: 10.3109/08820538.2015.1045150. Epub 2015 Jul 6 [PubMed PMID: 26148300]
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet (London, England). 2006 Nov 18:368(9549):1795-809 [PubMed PMID: 17113430]
Level 3 (low-level) evidenceHeckenlively JR, Fawzi AA, Oversier J, Jordan BL, Aptsiauri N. Autoimmune retinopathy: patients with antirecoverin immunoreactivity and panretinal degeneration. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Nov:118(11):1525-33 [PubMed PMID: 11074809]
Nieuwendijk TJ, Hooymans JM. Paraneoplastic vitelliform retinopathy associated with metastatic choroidal melanoma. Eye (London, England). 2007 Nov:21(11):1436-7 [PubMed PMID: 17693995]
Level 3 (low-level) evidenceElsheikh S, Gurney SP, Burdon MA. Melanoma-associated retinopathy. Clinical and experimental dermatology. 2020 Mar:45(2):147-152. doi: 10.1111/ced.14095. Epub 2019 Nov 19 [PubMed PMID: 31742740]
Janáky M, Pálffy A, Kolozsvári L, Benedek G. Unilateral manifestation of melanoma-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 2002 Jun:120(6):866-7 [PubMed PMID: 12049605]
Level 3 (low-level) evidenceFujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina (Philadelphia, Pa.). 2010 Sep:30(8):1206-16. doi: 10.1097/IAE.0b013e3181e097f0. Epub [PubMed PMID: 20661173]
Level 2 (mid-level) evidenceYeh S, Forooghian F, Wong WT, Faia LJ, Cukras C, Lew JC, Wroblewski K, Weichel ED, Meyerle CB, Sen HN, Chew EY, Nussenblatt RB. Fundus autofluorescence imaging of the white dot syndromes. Archives of ophthalmology (Chicago, Ill. : 1960). 2010 Jan:128(1):46-56. doi: 10.1001/archophthalmol.2009.368. Epub [PubMed PMID: 20065216]
Hamel CP. Cone rod dystrophies. Orphanet journal of rare diseases. 2007 Feb 1:2():7 [PubMed PMID: 17270046]
Iijima H, Yamaguchi S, Kogure S, Hosaka O, Shibutani T. Electroretinogram in cone dystrophy. Japanese journal of ophthalmology. 1991:35(4):453-66 [PubMed PMID: 1821435]
Sharma P, Sharma R. Toxic optic neuropathy. Indian journal of ophthalmology. 2011 Mar-Apr:59(2):137-41. doi: 10.4103/0301-4738.77035. Epub [PubMed PMID: 21350283]
Margolin E, Blair K, Shemesh A. Toxic and Nutritional Optic Neuropathy. StatPearls. 2025 Jan:(): [PubMed PMID: 29763154]
Sajjadi H, Poorsalman H. Previously Diagnosed Leber's Hereditary Optic Neuropathy with Clinical Signs of Idiopathic Intracranial Hypertension Responsive to Acetazolamide Therapy. Journal of ophthalmic & vision research. 2019 Jan-Mar:14(1):109-113. doi: 10.4103/jovr.jovr_85_18. Epub [PubMed PMID: 30820297]
Mauri E, Dilena R, Boccazzi A, Ronchi D, Piga D, Triulzi F, Gagliardi D, Brusa R, Faravelli I, Bresolin N, Magri F, Corti S, Comi GP. Subclinical Leber's hereditary optic neuropathy with pediatric acute spinal cord onset: more than meets the eye. BMC neurology. 2018 Dec 27:18(1):220. doi: 10.1186/s12883-018-1227-9. Epub 2018 Dec 27 [PubMed PMID: 30591017]
Glover JM, Casmaer ML, April MD. An uncommon cause of vision loss: Leber hereditary optic neuropathy. JAAPA : official journal of the American Academy of Physician Assistants. 2018 Nov:31(11):32-34. doi: 10.1097/01.JAA.0000546478.56818.50. Epub [PubMed PMID: 30358677]
Savini G, Barboni P, Valentino ML, Montagna P, Cortelli P, De Negri AM, Sadun F, Bianchi S, Longanesi L, Zanini M, Carelli V. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber's hereditary optic neuropathy mutations. Ophthalmology. 2005 Jan:112(1):127-31 [PubMed PMID: 15629832]
Level 2 (mid-level) evidenceQuiros PA, Torres RJ, Salomao S, Berezovsky A, Carelli V, Sherman J, Sadun F, De Negri A, Belfort R, Sadun AA. Colour vision defects in asymptomatic carriers of the Leber's hereditary optic neuropathy (LHON) mtDNA 11778 mutation from a large Brazilian LHON pedigree: a case-control study. The British journal of ophthalmology. 2006 Feb:90(2):150-3 [PubMed PMID: 16424523]
Level 2 (mid-level) evidenceSadun AA, Salomao SR, Berezovsky A, Sadun F, Denegri AM, Quiros PA, Chicani F, Ventura D, Barboni P, Sherman J, Sutter E, Belfort R Jr, Carelli V. Subclinical carriers and conversions in Leber hereditary optic neuropathy: a prospective psychophysical study. Transactions of the American Ophthalmological Society. 2006:104():51-61 [PubMed PMID: 17471325]
Level 3 (low-level) evidenceYu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF. Inherited mitochondrial optic neuropathies. Journal of medical genetics. 2009 Mar:46(3):145-58. doi: 10.1136/jmg.2007.054270. Epub 2008 Nov 10 [PubMed PMID: 19001017]
Riordan-Eva P, Harding AE. Leber's hereditary optic neuropathy: the clinical relevance of different mitochondrial DNA mutations. Journal of medical genetics. 1995 Feb:32(2):81-7 [PubMed PMID: 7760326]
Machemer R. [On the pathogenesis of the flat malignant melanoma]. Klinische Monatsblatter fur Augenheilkunde. 1966:148(5):641-52 [PubMed PMID: 5996537]
Barr CC, Zimmerman LE, Curtin VT, Font RL. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms. A recently recognized syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1982 Feb:100(2):249-55 [PubMed PMID: 7065942]
Level 3 (low-level) evidencevan Noort BC, Keunen JEE, Schlingemann RO, Marinkovic M. Long Survival and Preservation of Good Visual Acuity in a Patient with Bilateral Diffuse Uveal Melanocytic Proliferation. Ocular oncology and pathology. 2019 Jan:5(1):75-78. doi: 10.1159/000488454. Epub 2018 Jun 19 [PubMed PMID: 30675481]
Klemp K, Kiilgaard JF, Heegaard S, Nørgaard T, Andersen MK, Prause JU. Bilateral diffuse uveal melanocytic proliferation: Case report and literature review. Acta ophthalmologica. 2017 Aug:95(5):439-445. doi: 10.1111/aos.13481. Epub 2017 Jun 21 [PubMed PMID: 28636126]
Level 3 (low-level) evidenceGass JD, Gieser RG, Wilkinson CP, Beahm DE, Pautler SE. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Archives of ophthalmology (Chicago, Ill. : 1960). 1990 Apr:108(4):527-33 [PubMed PMID: 2322154]
Level 3 (low-level) evidenceRahimy E, Soheilian M. Giraffe Pattern of Bilateral Diffuse Uveal Melanocytic Proliferation. Ophthalmology. 2016 Mar:123(3):483. doi: 10.1016/j.ophtha.2015.12.015. Epub [PubMed PMID: 26902560]
Chahud F, Young RH, Remulla JF, Khadem JJ, Dryja TP. Bilateral diffuse uveal melanocytic proliferation associated with extraocular cancers: review of a process particularly associated with gynecologic cancers. The American journal of surgical pathology. 2001 Feb:25(2):212-8 [PubMed PMID: 11176070]
Level 3 (low-level) evidenceLiu B, Deng T, Zhang J. RISK FACTORS FOR CENTRAL SEROUS CHORIORETINOPATHY: A Systematic Review and Meta-Analysis. Retina (Philadelphia, Pa.). 2016 Jan:36(1):9-19. doi: 10.1097/IAE.0000000000000837. Epub [PubMed PMID: 26710181]
Level 1 (high-level) evidenceGäckle HC, Lang GE, Freissler KA, Lang GK. [Central serous chorioretinopathy. Clinical, fluorescein angiography and demographic aspects]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1998 Aug:95(8):529-33 [PubMed PMID: 9782727]
Level 2 (mid-level) evidenceTittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, Freund B, Guyer DR, Slakter JS, Sorenson JA. Systemic findings associated with central serous chorioretinopathy. American journal of ophthalmology. 1999 Jul:128(1):63-8 [PubMed PMID: 10482095]
Level 2 (mid-level) evidenceYalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina (Philadelphia, Pa.). 2014 Apr:34(4):705-12. doi: 10.1097/IAE.0000000000000001. Epub [PubMed PMID: 24100708]
Level 2 (mid-level) evidenceGupta A, Tripathy K. Central Serous Chorioretinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32644399]
Xu Q, Du W, Zhou H, Zhang X, Liu H, Song H, Wang X, Wei S. Distinct clinical characteristics of paraneoplastic optic neuropathy. The British journal of ophthalmology. 2019 Jun:103(6):797-801. doi: 10.1136/bjophthalmol-2018-312046. Epub 2018 Jul 18 [PubMed PMID: 30021813]
Malik S, Furlan AJ, Sweeney PJ, Kosmorsky GS, Wong M. Optic neuropathy: a rare paraneoplastic syndrome. Journal of clinical neuro-ophthalmology. 1992 Sep:12(3):137-41 [PubMed PMID: 1328306]
Level 3 (low-level) evidencePaul R, Ghosh AK, Sinha A, Bhattacharya R. Paraneoplastic optic neuritis as the first manifestation of periampullary carcinoma. International journal of applied & basic medical research. 2015 Jan-Apr:5(1):73-5. doi: 10.4103/2229-516X.149255. Epub [PubMed PMID: 25664276]
Level 3 (low-level) evidenceRosencher L, Maisonobe T, Lavole A, Milleron B, Ferroir JP. [Neurologic paraneoplastic syndrome with anti-CV2/CRMP5 antibodies revealing a small cell lung cancer. Effectiveness of the lung cancer treatment]. Revue neurologique. 2012 Apr:168(4):371-4. doi: 10.1016/j.neurol.2011.07.016. Epub 2012 Mar 3 [PubMed PMID: 22387203]
Level 3 (low-level) evidenceLanger JE, Lopes MB, Fountain NB, Pranzatelli MR, Thiele EA, Rust RS, Goodkin HP. An unusual presentation of anti-Hu-associated paraneoplastic limbic encephalitis. Developmental medicine and child neurology. 2012 Sep:54(9):863-6. doi: 10.1111/j.1469-8749.2012.04221.x. Epub 2012 Feb 9 [PubMed PMID: 22320677]
Level 3 (low-level) evidenceDamek DM. Paraneoplastic Retinopathy/Optic Neuropathy. Current treatment options in neurology. 2005 Jan:7(1):57-67 [PubMed PMID: 15610708]
Wiseman EM, Bar-El Dadon S, Reifen R. The vicious cycle of vitamin a deficiency: A review. Critical reviews in food science and nutrition. 2017 Nov 22:57(17):3703-3714. doi: 10.1080/10408398.2016.1160362. Epub [PubMed PMID: 27128154]
D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011 Jan:3(1):63-103. doi: 10.3390/nu3010063. Epub [PubMed PMID: 21350678]
Level 3 (low-level) evidencePfeiffer CM, Sternberg MR, Schleicher RL, Haynes BM, Rybak ME, Pirkle JL. The CDC's Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. The Journal of nutrition. 2013 Jun:143(6):938S-47S. doi: 10.3945/jn.112.172858. Epub 2013 Apr 17 [PubMed PMID: 23596164]
Hodge C, Taylor C. Vitamin A Deficiency. StatPearls. 2024 Jan:(): [PubMed PMID: 33620821]
Miller M, Humphrey J, Johnson E, Marinda E, Brookmeyer R, Katz J. Why do children become vitamin A deficient? The Journal of nutrition. 2002 Sep:132(9 Suppl):2867S-2880S. doi: 10.1093/jn/132.9.2867S. Epub [PubMed PMID: 12221263]
McBain VA, Egan CA, Pieris SJ, Supramaniam G, Webster AR, Bird AC, Holder GE. Functional observations in vitamin A deficiency: diagnosis and time course of recovery. Eye (London, England). 2007 Mar:21(3):367-76 [PubMed PMID: 16341129]
Level 3 (low-level) evidenceAleman TS, Garrity ST, Brucker AJ. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Documenta ophthalmologica. Advances in ophthalmology. 2013 Dec:127(3):239-43. doi: 10.1007/s10633-013-9403-0. Epub 2013 Jul 31 [PubMed PMID: 23900584]
Level 3 (low-level) evidenceGilbert C. The eye signs of vitamin A deficiency. Community eye health. 2013:26(84):66-7 [PubMed PMID: 24782581]
Goldstein SM, Syed NA, Milam AH, Maguire AM, Lawton TJ, Nichols CW. Cancer-associated retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Dec:117(12):1641-5 [PubMed PMID: 10604671]
Level 3 (low-level) evidenceAdamus G, Brown L, Schiffman J, Iannaccone A. Diversity in autoimmunity against retinal, neuronal, and axonal antigens in acquired neuro-retinopathy. Journal of ophthalmic inflammation and infection. 2011 Sep:1(3):111-21. doi: 10.1007/s12348-011-0028-8. Epub 2011 Jul 10 [PubMed PMID: 21744285]