Introduction
Morning glory syndrome (MGS) is a congenital optic disc pathology.[1] It was first described in 1970 by Kindler.[2] He reported ten cases of congenital optic disc anomaly. The optic nerve head was funnel-shaped. It had a central whitish fibrous tissue and was surrounded by a ring-shaped area of chorioretinal pigmentary disturbance. The retinal vessels originated as multiple straight narrow branches at the edge of the optic disc. He called it the morning glory disc because of its resemblance to a morning glory flower.[2] It is a rare sporadic disorder.[3]
This review summarizes the etiology, epidemiology, histopathology, clinical features, differential diagnoses, associations, complications, evaluation, and the management of MGS.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The pathogenesis of the MGS is not fully understood.[4] It might be due to a primary mesenchymal abnormality, partial development of the lamina cribrosa, and incomplete closure of the posterior scleral wall.[5] The central gliosis and the abnormal vascular pattern may point toward primary neuroectodermal dysgenesis.[6] Azuma and associates reported eight patients with optic nerve anomalies and PAX6 gene mutations. This gene is involved in ocular development and is expressed in multiple ocular tissues and the central nervous system.[7] However, Nallathambi et al. failed to find PAX6 mutations in their patients.[8]
Nagy et al. suggested hereditary association and reported a mother and daughter with MGS.[9] Traboulsi et al. also suggested a hereditary association as they described a case with aniridia, morning glory disc in one eye, and optic disc pit the fellow eye.[10] The patient’s mother also had aniridia but no other ocular abnormalities. The MGS can be a variation of a spectrum that includes optic disc pit and optic disc coloboma.[11]
Pedler suggested that there can be an improper fusion of the posterior sclera, which can cause herniation of the disc and the adjacent retina.[12] Dempster suggested that anomalous mesodermal differentiation can result in abnormal closure of the posterior sclera and lamina cribrosa.[13] This would result in herniation of the retina and optic nerve head. Cavitary optic disc anomalies (CODA) have been reported to be autosomal dominantly inherited diseases caused by the mutation in the MMP19 gene located at chromosome 12q13.2. CODA is characterized by a spectrum of diseases, including optic disc coloboma, optic disc pit, megalopapilla, and MGS.[14][15]
Epidemiology
The prevalence of MGS has been reported to be 2.6 per 100,000.[16] Both sexes are equally affected. In the USA, it occurs less commonly in black people. It is mostly unilateral.[17] Bilaterality is seen in approximately 16% of cases.[18] It is usually diagnosed by two years of age. Sometimes late presentations can be seen around the age of 5 years.[19] This is due to a lack of awareness as patients are from rural areas and of lower socioeconomic status.[20] The familial CODA is usually bilateral (around 65%).
Histopathology
Dempster suggested that abnormal mesodermal differentiation can result in abnormal closure of the posterior sclera and herniation of the retina and optic nerve head.[13] One histopathological study shows an uninterrupted monolayer retinal pigment epithelium lining of the inner wall of the staphyloma. If there was a defect in the embryonic fissure, this continuous layer would not be present. Other histological studies have found abnormal blood vessels in the optic nerve head, abnormal fibrous adipose tissue in the optic nerve meninges, and persistent hyaloid remnants.[21]
These all are mesodermal derivatives. In another case, the histopathology demonstrated smooth-muscle cells and fat cells in the peripapillary staphylomatous scleral tissue.[22] These smooth muscle cells can cause contractile movement of the optic disc.[23]
History and Physical
The mean age at presentation was 8.8 years (range 0.25 to 46 years) in a large study of 51 eyes of 44 patients—most patients present with either vision loss, strabismus, or leukocoria. Unilaterality is seen in around 85% of cases, while bilateral findings are seen in 15%. Gender predisposition is not seen. Family history is usually not present, though familial cases of CODA have been reported.[15][14] Visual acuity usually ranges from 20/200 to counting fingers.[16][18] The patient is usually myopic and astigmatic.[24] Strabismus may be present in 80% of cases.[25][26] The affected eye may have esotropia or exotropia.[27][28]
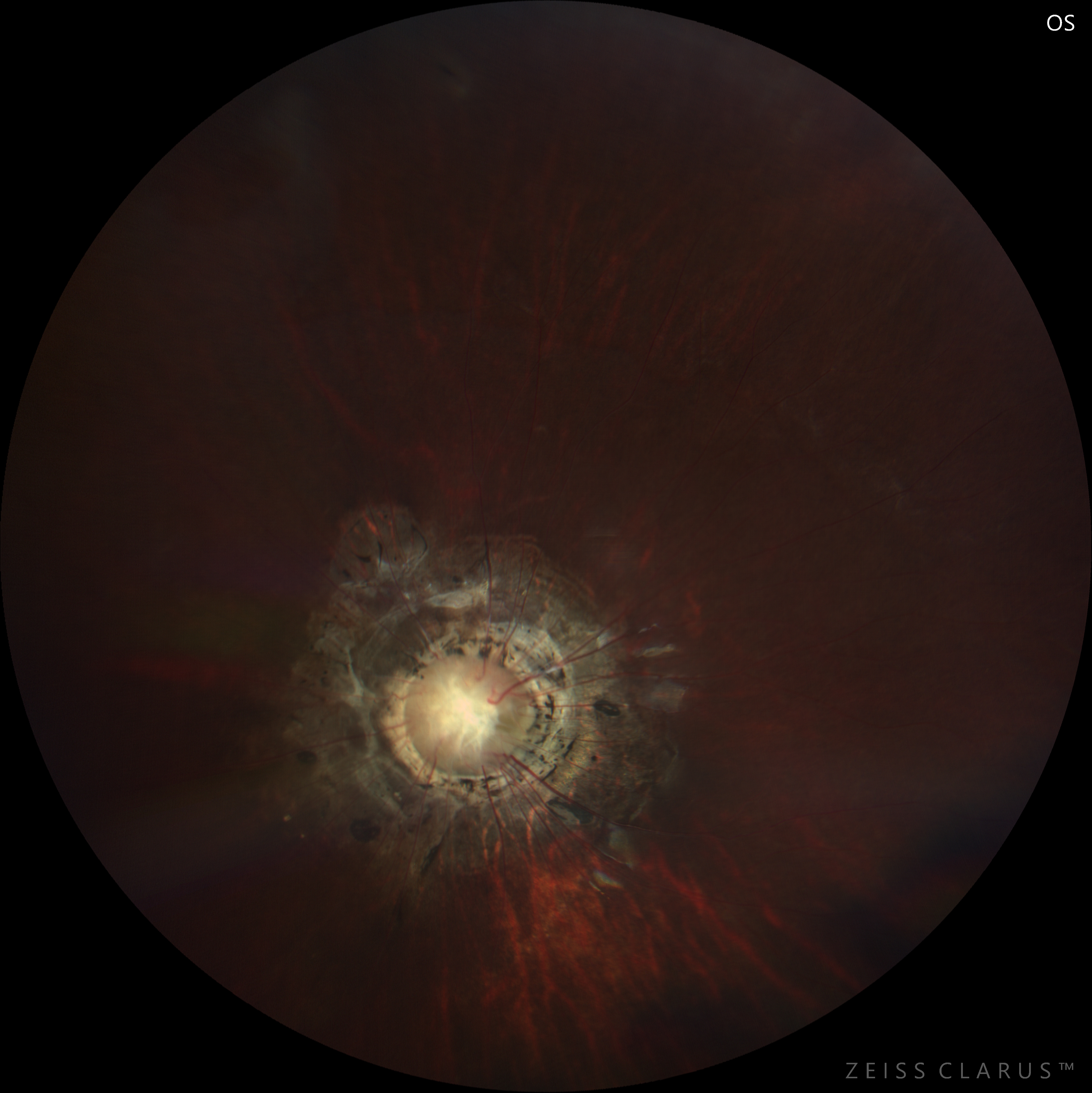
Fundus evaluation reveals an enlarged disc situated centrally within an excavated area.[29] The peripapillary area is elevated with an annular zone of pigmentary changes. The junction between the disc and the peripapillary area is often obscured. A whitish glial tissue is present in the center of the optic disc.[30]
The retinal blood vessels arise from the peripheral disc and course radially towards the retinal periphery. They are abnormally straight and branch at acute angles. It is often difficult to differentiate an artery from a vein. Vascular sheathing may be present. The macula may be incorporated in the funnel-shaped excavated area, a finding known as "macular capture." Persistent hyaloid remnants may be found in the fellow or the affected eye.[31] It can be present in 26% of cases, as reported by Fei et al. Persistent fetal vasculature may be a primary condition and might also have a genetic basis.[32] It can be present in various forms like anterior, posterior, and combined persistent fetal vasculature.[33]
Retinal detachment may be present. It may be found in 38% of cases.[34] It generally involves the posterior pole, but some cases may progress to total bullous retinal detachment.[30][35] It is hypothesized that small breaks can form due to the contractile nature of the glial tissue, allowing the accumulation of subretinal fluid.[36] The retinal break may be present in the peripapillary retina or at the junction of the disc and retina.[37] In some cases, a retinal hole was hypothesized to be present in the tissue overlying the optic disc.[38][36]
In other cases, there may be a communication between the subretinal space and the subarachnoid space allowing the cerebrospinal fluid in the optic nerve to reach the subretinal space and cause a retinal detachment. In one such case of MGS with retinal detachment, metrizamide was injected intrathecally. It was radiographically found to move into the subretinal space. Reattachment happened after optic nerve sheath fenestration, thus confirming the existence of this connection.[39]
Irvine and associates were able to reattach the retina through optic nerve sheath fenestration. They also observed the gas bubbling out of the optic nerve sheath defect when injected into the vitreous. They suggested the possibility of continuity between the subretinal space and perineural space.[40]
In another histopathological study, Manschot described clear fluid leaking from a hole in the optic nerve during sectioning.[22] The posterior pole retinal detachment could also be secondary to subretinal exudation, though exudative detachments are rare. Akamine et al. reported a case of a 5-month-old girl with MGS and retinal detachment. There was neovascularization and retinal exudation. Exudation was confirmed on fluorescein angiography. No tears or vitreoretinal traction was noted.[41] In another case, neovascularization was present in juxtafoveal and subfoveal areas.[42] Fellow eye anomalies can be present in 16 to 25% of patients.[19][16]
The fellow eye can have Mittendorf's dot, cataract, microphthalmos, and retinal detachment. Close monitoring of the fellow eye is important as retinal complications may occur when a disc or retinochoroidal coloboma is present.
Evaluation
Optical coherence tomography (OCT) helps in evaluating the optic disc anomaly. Studies have shown a well-defined epiretinal membrane pulling the retina centripetally.[43] Few cases have reported optic disc contraction in cases of MGS. OCT helps in studying these movements. Yoshida et al. described the swept-source OCT features.[44] They reported a decrease in the size of the area at the bottom of the peripapillary excavation during contraction.[44]
Rajendran and Kumar described the spectral domain OCT features and observed that the dominant movement during contraction was the anterior movement of the walls of the peripapillary excavation.[45] The optic disc contraction lasted for 4 to 5 seconds and occurred 2 or 3 times in a minute. The margins of the optic disc remained stationary. Similar findings were reported by Sawada et al.[46]
Cennamo et al. have described the OCT angiography features in patients with contractile optic discs in MGS. They found a dense microvascular network in the peripapillary capillary layer with no difference between the superficial vascular plexus and the deep vascular plexus around the optic nerve.[47] In cases with retinal detachment, OCT can detect subtle slit-like breaks at the margin of the excavation.[48] It can detect shallow retinal detachments and may show direct communication between the vitreous and the subarachnoid space. It can evaluate peripapillary vitreous traction.
Lytvynchuk LM et al. reported a case of intraoperative OCT-assisted pars plana vitrectomy for retinal detachment in MGS. There was no break. They found strong vitreous traction and adhesion above the optic disc and macula, which was the cause of the retinal detachment. They induced a vitreous detachment, peeled the epiretinal and internal limiting membrane, and gave an air tamponade, leading to retinal reattachment.[49]
Peripheral capillary nonperfusion has been reported in MGS.[50] Fundus fluorescein angiography helps in detecting those areas.[51] She et al. have classified the severity of nonperfusion into four categories: mild, moderate, severe, and extreme.[52] They noted leakage at the junction of perfused and non perfused retina. They also noted fibrovascular proliferation and tractional retinal detachment in a few cases. Thus peripheral retinal nonperfusion should be evaluated in all cases of MGS, as it can lead to tractional retinal detachment. Fluorescein angiography has also revealed arteriovenous communications near the optic disc.[53]
The diagnosis of MGS is mainly clinical, but sometimes the diagnosis can be confusing. MGS should be distinguished from optic disc coloboma. Optic disc coloboma may be associated with multisystemic congenital syndromes.[54][55][56] Ellika et al. demonstrated three findings in MGS: 1) funnel-shaped posterior optic disc and elevation of the adjacent retina; 2) abnormal tissue in the distal intraorbital segment of the affected optic nerve, with effacement of the subarachnoid space; and 3) discontinuity of the uveoscleral coat. These findings were not observed in normal eyes.[57]
MGS can be associated with systemic anomalies. The abnormalities of the face and central nervous system should be recognized. Facial abnormalities could be capillary hemangiomas, hypertelorism, cleft lip, and cleft palate.[58][59][60][61] Another association is basal encephalocele. It is the herniation of neural tissue through a defect in the basal skull. MGS is usually associated with the trans-sphenoidal type. Other reported basal encephaloceles include sphenoethmoidal, sphenopharyngeal, and trans-ethmoidal.[62][63][64]
These defects can be misdiagnosed as nasal polyps; it is thus important not to biopsy such lesions. These encephaloceles can be treated surgically. Another associated central nervous system abnormality is agenesis of the corpus callosum.[65] Razeghinejad et al and reported a case of Chiari type 1 malformation.[66] Endocrine abnormality of the pituitary gland has been reported.[67] Diabetes insipidus and abnormal levels of prolactin, thyroid-stimulating hormone, gonadotropins, and growth hormone are most common.[68]
Moyamoya disease may be associated with MGS. It is characterized by abnormal narrowing of the cerebral arteries, which can result in cerebral ischemia or hemorrhage. This association was first described by Hanson et al. in 1985. Since then, many cases have been reported.[69][70][71] Magnetic resonance angiography or computed tomography angiography can diagnose these vascular anomalies. They are usually found in the anterior cerebral circulation and can range from mild narrowing to bilateral narrowing of the supraclinoid internal carotid artery.[72] The complete absence of the internal carotid arteries has also been described.[71]
Moyamoya disease can cause stroke, seizures, intellectual impairment, and transient ischemic attacks. Patients can present with decreased vision before the onset of cerebral dysfunction. Thus all patients with MGS should be evaluated for Moyamoya disease. Also, in patients with Moyamoya disease, a retinal examination should be done. It is essential for both neurologists and ophthalmologists to be aware of the association between cerebrovascular disease and ocular manifestations to achieve a diagnosis.
Renal coloboma syndrome (RCS) or papillorenal syndrome is an autosomal dominant condition characterized by optic nerve dysplasia and renal hypodysplasia.[73] The eye anomalies consist of a dysplastic disc, microcornea, retinal coloboma, posterior staphyloma, optic nerve cyst, and pigmentary maculopathy. The kidney is hypodysplastic and has fewer glomeruli. As a result, the patient develops hypertension, proteinuria, and end-stage kidney disease.[74] Early recognition of this rare condition leads to improved management. Thus patients with MGS should be evaluated for kidney disease and hypertension.
Treatment / Management
Early diagnosis and treatment in MGS are essential. The strabismus and anisometropia should be corrected to prevent amblyopia. Full cycloplegic refraction is done, and spectacles are given. Squint may be managed surgically. Retinal detachment is an associated condition that requires management surgically. Pars plans vitrectomy with or without encircling band is employed. First, a core vitrectomy is done. Then triamcinolone-assisted posterior vitreous detachment is induced. Meticulous base shaving is done. All breaks are identified and diathermized. If an epiretinal membrane is present, membrane peeling has to be done. Fluid air exchange is done, and subretinal fluid is drained. All breaks are lasered, and a peripheral barrage laser is done. Silicone oil or gas tamponade is given. Chang et al. and Zhang et al. reported retinal reattachment in all cases with pars plana vitrectomy.[75][76] (B3)
In some cases of retinal detachment, spontaneous attachment has been noticed. Haik et al. reported spontaneous attachment in four patients over seven and half years. Thus it is important to consider this factor prior to any surgical treatment.[77](B3)
Close monitoring of the fellow eye in unilateral cases of MGS is essential. The fellow eye may develop a cataract or retinal detachment. Accordingly, phacoemulsification and lens implantation is done, or vitreoretinal surgery is undertaken.
Differential Diagnosis
The MGS closely resembles an optic nerve head coloboma. Optic nerve coloboma is characterized by a large excavation which is usually decentred inferiorly, corresponding to the position of the embryonic fissure.[78] The excavation can involve the contiguous retina and choroid. The superior neuroretinal rim is normal, but the inferior neuroretinal rim is thinned out. It is frequently associated with microcornea, microphthalmia, iris, or fundus coloboma.[79] It can be unilateral or bilateral.[80] Some patients can have optic nerve cysts.[81]
Coloboma may occur with systemic conditions such as CHARGE syndrome (ocular Coloboma, Heart defects, choanal Atresia, growth Retardation, Genital abnormalities, and Ear abnormalities), Walker-Warburg syndrome, Aicardi syndrome, Goldenhar syndrome, and Goltz dermal hypoplasia.[82][80]
MGS is characterized by a central excavated or conical optic disc. It is not decentred inferiorly. It has a central glial tissue and peripapillary pigmentary disturbance, not present in optic coloboma. The retinal vessels exit radially from the morning glory disc, while in optic coloboma, they exit in a branching fashion. Microcornea and iris coloboma are not seen in MGS. It is associated with persistent hyaloid tissue, lid hemangiomas, retinal gliosis, peripapillary contractile staphyloma, optic disc pit, lens coloboma, glaucoma, and Duane’s retraction.[76]
Optic nerve head avulsion (ONHA) can mimic MGS. ONHA is seen after severe blunt trauma.[83] Fundus examination will reveal an area of the optic nerve head excavation filled with blood with or without vitreous hemorrhage.[84] Other signs of injury like lid laceration, ecchymoses, or subconjunctival hemorrhage may be present. OCT will show avulsion at the optic nerve head section. Ultrasonography (USG) B scan will show an area of hypoechogenicity at the optic nerve head section.[85]
Optic nerve aplasia (ONA) is another differential to consider. ONA is a congenital anomaly characterized by the absence of the optic nerve head, optic nerve fibers, retinal vessels, and retinal ganglion cells. The patient presents with nil perception of light and afferent pupillary defect. Fundus evaluation reveals an absent optic nerve head and retinal blood vessels. USG B-scan shows an absent optic nerve shadow.[86]
MGS can be confused with optic disc pit (ODP). ODP is a unilateral, single, oval, grey-white excavation in the inferotemporal quadrant of the optic disc. The size of the pit is 1/8 -1/4 of the optic disc size.[87] Vision is generally unaffected unless complicated by a serous retinal detachment, macular schisis, or macular edema.[88][89]
Advanced glaucomatous optic neuropathy (AGON) can mimic MGS. AGON is characterized by a generalized or focal enlargement of the cup with a vertical cup disc ratio of 0.9. There is thinning of the neuroretinal rim generally at superior and inferior poles. There is nasalization of vessels and baring of circumlinear vessels. Parapapillary atrophy and laminar dots are present.[90]
Peripapillary staphyloma is another differential that needs to be considered. It is a congenital anomaly in which a deep fundus excavation is present around a normal optic nerve head.[91] Unlike MGS, it is not associated with other congenital defects or systemic diseases.[92]
Prognosis
The visual prognosis in individuals with MGS is usually poor. These patients have high refractive errors and strabismus that cause amblyopia. The chances of retinal detachment are high as the optic disc is abnormal.[19] Children may not complain of decreased vision, and a significant number are discovered incidentally due to chronic retinal detachment.[93]
After a successful retinal surgery, final vision depends upon the duration of retinal detachment and preexisting amblyopia. Longstanding retinal schisis leads to atrophic changes, which further limit visual recovery. Visual acuity generally ranges from 20/200 to no perception of light. Even in patients without retinal detachments, vision is poor, usually 20/200 or worse.[35]
Complications
Longstanding strabismus and the uncorrected refractive error may cause amblyopia which may be resistant to treatment.[19] Traction from the peripapillary tissue over the retina can cause retinal breaks. Retinal breaks may occur in tissues within the optic disc or at the margin of the excavation.[36]
Low contrast between the white scleral tissue in the optic nerve and the thin retina makes it challenging to visualize the break clinically or intraoperatively.[76] Thus the rate of retinal detachment and retinal redetachment is higher in MGS. Longstanding retinal schisis can lead to atrophic retinal changes due to chronic intraretinal and subretinal fluid.[49] Retinal detachment along with retinoschisis are the complications that impair vision.[38]
Subretinal neovascularization in the peripapillary retina and macula is a known complication.[42] Peripheral retinal non-perfusion can lead to fibrovascular proliferation and tractional retinal detachment.[45]
Deterrence and Patient Education
The patient should be counseled properly regarding the prognosis and complications of MGS. These cases require a thorough ocular and systemic evaluation. Family members should also be evaluated by an ophthalmologist.
Enhancing Healthcare Team Outcomes
Any patient presenting with MGS should receive an interprofessional team evaluation by an ophthalmologist, a neurologist, and a physician. A review by a vitreoretina specialist and neuro-ophthalmologist may be necessary. The patient most often presents to the primary clinician with ocular problems, and these professionals should be aware of the condition. Systemic anomalies like moyamoya disease or encephalocele can be associated with MGS; therefore, prompt referral to a neurologist is necessary. These patients can then be followed by their primary clinicians.
Compliance with treatment should be ensured. The nursing staff is the first in the department to come in contact with patients on follow-up. They can evaluate compliance with medication and lifestyle measures and report any issues to the primary care clinician. This collaborative and interprofessional approach to care can ensure optimal patient outcomes.
Media
References
Chan RT, Chan HH, Collin HB. Morning glory syndrome. Clinical & experimental optometry. 2002 Nov:85(6):383-8 [PubMed PMID: 12452790]
Level 3 (low-level) evidenceKindler P. Morning glory syndrome: unusual congenital optic disk anomaly. American journal of ophthalmology. 1970 Mar:69(3):376-84 [PubMed PMID: 5418855]
Harasymowycz P, Chevrette L, Décarie JC, Hanna N, Aroichane M, Jacob JL, Milot J, Homsy M. Morning glory syndrome: clinical, computerized tomographic, and ultrasonographic findings. Journal of pediatric ophthalmology and strabismus. 2005 Sep-Oct:42(5):290-5 [PubMed PMID: 16250218]
Level 2 (mid-level) evidenceGolnik KC. Cavitary anomalies of the optic disc: neurologic significance. Current neurology and neuroscience reports. 2008 Sep:8(5):409-13 [PubMed PMID: 18713577]
Fei P, Zhang Q, Li J, Zhao P. Clinical characteristics and treatment of 22 eyes of morning glory syndrome associated with persistent hyperplastic primary vitreous. The British journal of ophthalmology. 2013 Oct:97(10):1262-7. doi: 10.1136/bjophthalmol-2013-303565. Epub 2013 Jul 22 [PubMed PMID: 23878133]
Level 2 (mid-level) evidencePollock S. The morning glory disc anomaly: contractile movement, classification, and embryogenesis. Documenta ophthalmologica. Advances in ophthalmology. 1987 Apr:65(4):439-60 [PubMed PMID: 3319466]
Level 3 (low-level) evidenceAzuma N, Yamaguchi Y, Handa H, Tadokoro K, Asaka A, Kawase E, Yamada M. Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. American journal of human genetics. 2003 Jun:72(6):1565-70 [PubMed PMID: 12721955]
Level 3 (low-level) evidenceDubey SK, Mahalaxmi N, Vijayalakshmi P, Sundaresan P. Mutational analysis and genotype-phenotype correlations in southern Indian patients with sporadic and familial aniridia. Molecular vision. 2015:21():88-97 [PubMed PMID: 25678763]
Level 2 (mid-level) evidenceNagy V, Kettesy B, Toth K, Vamosi P, Damjanovich J, Berta A. [Morning glory syndrome -- a clinical study of two cases]. Klinische Monatsblatter fur Augenheilkunde. 2002 Nov:219(11):801-5 [PubMed PMID: 12494371]
Level 3 (low-level) evidenceTraboulsi EI, Jurdi-Nuwayhid F, Torbey NS, Frangieh GT. Aniridia, atypical iris defects, optic pit and the morning glory disc anomaly in a family. Ophthalmic paediatrics and genetics. 1986 Aug:7(2):131-5 [PubMed PMID: 3097598]
Level 3 (low-level) evidenceCennamo G, Liguori G, Pezone A, Iaccarino G. Morning glory syndrome associated with marked persistent hyperplastic primary vitreous and lens colobomas. The British journal of ophthalmology. 1989 Aug:73(8):684-6 [PubMed PMID: 2765452]
Level 3 (low-level) evidencePedler C. UNUSUAL COLOBOMA OF THE OPTIC NERVE ENTRANCE. The British journal of ophthalmology. 1961 Dec:45(12):803-7 [PubMed PMID: 18170740]
Level 3 (low-level) evidenceDempster AG, Lee WR, Forrester JV, McCreath GT. The 'morning glory syndrome' - a mesodermal defect? Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1983:187(4):222-30 [PubMed PMID: 6657186]
Level 3 (low-level) evidenceHonkanen RA, Jampol LM, Fingert JH, Moore MD, Taylor CM, Stone EM, Alward WL. Familial cavitary optic disk anomalies: clinical features of a large family with examples of progressive optic nerve head cupping. American journal of ophthalmology. 2007 May:143(5):788-794 [PubMed PMID: 17362864]
Slusher MM, Weaver RG Jr, Greven CM, Mundorf TK, Cashwell LF. The spectrum of cavitary optic disc anomalies in a family. Ophthalmology. 1989 Mar:96(3):342-7 [PubMed PMID: 2710526]
Ceynowa DJ, Wickström R, Olsson M, Ek U, Eriksson U, Wiberg MK, Fahnehjelm KT. Morning glory disc anomaly in childhood - a population-based study. Acta ophthalmologica. 2015 Nov:93(7):626-34. doi: 10.1111/aos.12778. Epub 2015 Jul 14 [PubMed PMID: 26173377]
Altun A, Altun G, Kurna SA, Olcaysu OO, Aki SF. Unilateral morning glory optic disc anomaly in a case with Down syndrome. BMC ophthalmology. 2014 Apr 13:14():48. doi: 10.1186/1471-2415-14-48. Epub 2014 Apr 13 [PubMed PMID: 24725623]
Level 3 (low-level) evidenceCennamo G, de Crecchio G, Iaccarino G, Forte R, Cennamo G. Evaluation of morning glory syndrome with spectral optical coherence tomography and echography. Ophthalmology. 2010 Jun:117(6):1269-73. doi: 10.1016/j.ophtha.2009.10.045. Epub 2010 Feb 16 [PubMed PMID: 20163868]
Kumar J, Adenuga OO, Singh K, Ahuja AA, Kannan NB, Ramasamy K. Clinical characteristics of morning glory disc anomaly in South India. Taiwan journal of ophthalmology. 2021 Jan-Mar:11(1):57-63. doi: 10.4103/tjo.tjo_52_20. Epub 2020 Oct 19 [PubMed PMID: 33767956]
Singh U, Katoch D, Kaur S, Dogra MR, Bansal D, Kapoor R. Retinoblastoma: A Sixteen-Year Review of the Presentation, Treatment, and Outcome from a Tertiary Care Institute in Northern India. Ocular oncology and pathology. 2017 Dec:4(1):23-32. doi: 10.1159/000477408. Epub 2017 Jul 5 [PubMed PMID: 29344495]
Hope-Ross M, Johnston SS. The Morning Glory syndrome associated with sphenoethmoidal encephalocele. Ophthalmic paediatrics and genetics. 1990 Jun:11(2):147-53 [PubMed PMID: 2198507]
Level 3 (low-level) evidenceManschot WA. Morning glory syndrome: a histopathological study. The British journal of ophthalmology. 1990 Jan:74(1):56-8 [PubMed PMID: 2306447]
Level 3 (low-level) evidenceChuman H, Nao-i N, Sawada A. [A case of morning glory syndrome associated with contractile movement of the optic disc and subretinal neovascularization]. Nippon Ganka Gakkai zasshi. 1996 Sep:100(9):705-9 [PubMed PMID: 8905968]
Level 3 (low-level) evidenceAl-Tamimi ER, Shakeel A, Yassin SA, Ali SI, Khan UA. A clinic-based study of refractive errors, strabismus, and amblyopia in pediatric age-group. Journal of family & community medicine. 2015 Sep-Dec:22(3):158-62. doi: 10.4103/2230-8229.163031. Epub [PubMed PMID: 26392796]
Quah BL, Hamilton J, Blaser S, Héon E, Tehrani NN. Morning glory disc anomaly, midline cranial defects and abnormal carotid circulation: an association worth looking for. Pediatric radiology. 2005 May:35(5):525-8 [PubMed PMID: 15480611]
Level 3 (low-level) evidenceMelbourne-Chambers R, Singh Minott I, Mowatt L, Johnson P, Thame M. Aicardi syndrome associated with anterior cephalocele in a female infant. Developmental medicine and child neurology. 2007 Jun:49(6):464-6 [PubMed PMID: 17518934]
Level 3 (low-level) evidenceKim MR, Park SE, Oh SY. Clinical feature analysis of congenital optic nerve abnormalities. Japanese journal of ophthalmology. 2006 May-Jun:50(3):250-5 [PubMed PMID: 16767381]
Level 2 (mid-level) evidenceDe Laey JJ, Ryckaert S, Leys A. The 'morning glory' syndrome. Ophthalmic paediatrics and genetics. 1985 Feb:5(1-2):117-24 [PubMed PMID: 3932911]
Level 3 (low-level) evidenceBrodsky MC. Congenital optic disk anomalies. Survey of ophthalmology. 1994 Sep-Oct:39(2):89-112 [PubMed PMID: 7801227]
Level 3 (low-level) evidenceLee BJ, Traboulsi EI. Update on the morning glory disc anomaly. Ophthalmic genetics. 2008 Jun:29(2):47-52. doi: 10.1080/13816810801901876. Epub [PubMed PMID: 18484308]
Beyer WB, Quencer RM, Osher RH. Morning glory syndrome. A functional analysis including fluorescein angiography, ultrasonography, and computerized tomography. Ophthalmology. 1982 Dec:89(12):1362-7 [PubMed PMID: 7162781]
Tripathy K, Sharma V. Thin posterior capsule in persistent fetal vasculature causing an appearance of spontaneous posterior capsular rupture. Indian journal of ophthalmology. 2018 Nov:66(11):1616-1617. doi: 10.4103/ijo.IJO_590_18. Epub [PubMed PMID: 30355876]
Tartarella MB, Takahagi RU, Braga AP, Fortes Filho JB. Persistent fetal vasculature: ocular features, management of cataract and outcomes. Arquivos brasileiros de oftalmologia. 2013 May-Jun:76(3):185-8 [PubMed PMID: 23929081]
Level 2 (mid-level) evidenceInoue M. Retinal complications associated with congenital optic disc anomalies determined by swept source optical coherence tomography. Taiwan journal of ophthalmology. 2016 Jan-Mar:6(1):8-14. doi: 10.1016/j.tjo.2015.05.003. Epub 2015 Jul 15 [PubMed PMID: 29018703]
Lit ES, D'Amico DJ. Retinal manifestations of morning glory disc syndrome. International ophthalmology clinics. 2001 Winter:41(1):131-8 [PubMed PMID: 11198140]
Bartz-Schmidt KU, Heimann K. Pathogenesis of retinal detachment associated with morning glory disc. International ophthalmology. 1995:19(1):35-8 [PubMed PMID: 8537194]
Level 3 (low-level) evidencevon Fricken MA, Dhungel R. Retinal detachment in the Morning Glory syndrome. Pathogenesis and management. Retina (Philadelphia, Pa.). 1984 Spring-Summer:4(2):97-9 [PubMed PMID: 6463401]
Level 3 (low-level) evidenceColl GE, Chang S, Flynn TE, Brown GC. Communication between the subretinal space and the vitreous cavity in the morning glory syndrome. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1995 Jul:233(7):441-3 [PubMed PMID: 7557510]
Level 3 (low-level) evidenceChang S, Haik BG, Ellsworth RM, St Louis L, Berrocal JA. Treatment of total retinal detachment in morning glory syndrome. American journal of ophthalmology. 1984 May:97(5):596-600 [PubMed PMID: 6720839]
Level 3 (low-level) evidenceIrvine AR, Crawford JB, Sullivan JH. The pathogenesis of retinal detachment with morning glory disc and optic pit. Retina (Philadelphia, Pa.). 1986 Summer-Fall:6(3):146-50 [PubMed PMID: 3797832]
Level 3 (low-level) evidenceAkamine T, Doi M, Takahashi H, Mori K, Uji Y. Morning glory syndrome with peripheral exudative retinal detachment. Retina (Philadelphia, Pa.). 1997:17(1):73-4 [PubMed PMID: 9051849]
Level 3 (low-level) evidenceSobol WM, Bratton AR, Rivers MB, Weingeist TA. Morning glory disk syndrome associated with subretinal neovascular membrane formation. American journal of ophthalmology. 1990 Jul 15:110(1):93-4 [PubMed PMID: 1695063]
Level 3 (low-level) evidenceLee KM, Woo SJ, Hwang JM. Evaluation of congenital excavated optic disc anomalies with spectral-domain and swept-source optical coherence tomography. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Nov:252(11):1853-60. doi: 10.1007/s00417-014-2680-9. Epub 2014 Jun 7 [PubMed PMID: 24906342]
Yoshida T, Katagiri S, Yokoi T, Nishina S, Azuma N. Optical coherence tomography and video recording of a case of bilateral contractile peripapillary staphyloma. American journal of ophthalmology case reports. 2019 Mar:13():66-69. doi: 10.1016/j.ajoc.2018.12.002. Epub 2018 Dec 5 [PubMed PMID: 30582075]
Level 3 (low-level) evidenceRajendran A, Kumar J. CONTRACTILE OPTIC DISK AND PERIPHERAL AVASCULAR RETINA IN A CASE OF MORNING GLORY DISK ANOMALY. Retinal cases & brief reports. 2022 Jul 1:16(4):426-429. doi: 10.1097/ICB.0000000000000998. Epub 2020 Apr 1 [PubMed PMID: 32243283]
Level 3 (low-level) evidenceSawada Y, Fujiwara T, Yoshitomi T. Morning glory disc anomaly with contractile movements. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2012 Nov:250(11):1693-5. doi: 10.1007/s00417-012-2115-4. Epub 2012 Aug 4 [PubMed PMID: 22865260]
Level 3 (low-level) evidenceCennamo G, Montorio D, Breve MA, Morra VB, Cennamo G. Optical coherence tomography angiography in contractile morning glory syndrome. European journal of ophthalmology. 2021 Jan:31(1):NP13-NP16. doi: 10.1177/1120672119864010. Epub 2019 Jul 17 [PubMed PMID: 31313592]
Ho TC, Tsai PC, Chen MS, Lin LL. Optical coherence tomography in the detection of retinal break and management of retinal detachment in morning glory syndrome. Acta ophthalmologica Scandinavica. 2006 Apr:84(2):225-7 [PubMed PMID: 16637841]
Level 3 (low-level) evidenceLytvynchuk LM, Glittenberg CG, Ansari-Shahrezaei S, Binder S. Intraoperative optical coherence tomography assisted analysis of pars Plana vitrectomy for retinal detachment in morning glory syndrome: a case report. BMC ophthalmology. 2017 Aug 1:17(1):134. doi: 10.1186/s12886-017-0533-0. Epub 2017 Aug 1 [PubMed PMID: 28764684]
Level 3 (low-level) evidenceRojanaporn D, Kaliki S, Shields CL, Shields JA. Morning glory disc anomaly with peripheral retinal nonperfusion in 4 consecutive cases. Archives of ophthalmology (Chicago, Ill. : 1960). 2012 Oct:130(10):1327-30. doi: 10.1001/archophthalmol.2012.505. Epub [PubMed PMID: 23044951]
Level 3 (low-level) evidenceRuia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
She K, Zhang Q, Fei P, Peng J, Lyu J, Li Y, Huang Q, Zhao P. Peripheral Retinal Nonperfusion in Pediatric Patients With Morning Glory Syndrome. Ophthalmic surgery, lasers & imaging retina. 2018 Sep 1:49(9):674-679. doi: 10.3928/23258160-20180831-04. Epub [PubMed PMID: 30222801]
Brodsky MC, Wilson RS. Retinal arteriovenous communications in the morning glory disc anomaly. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Apr:113(4):410-1 [PubMed PMID: 7654274]
Level 3 (low-level) evidenceMafee MF, Jampol LM, Langer BG, Tso M. Computed tomography of optic nerve colobomas, morning glory anomaly, and colobomatous cyst. Radiologic clinics of North America. 1987 Jul:25(4):693-9 [PubMed PMID: 3602361]
Level 3 (low-level) evidenceTraboulsi EI. Morning glory disc anomaly or optic disc coloboma? Archives of ophthalmology (Chicago, Ill. : 1960). 1994 Feb:112(2):153 [PubMed PMID: 8311757]
Level 3 (low-level) evidenceEustis HS, Sanders MR, Zimmerman T. Morning glory syndrome in children. Association with endocrine and central nervous system anomalies. Archives of ophthalmology (Chicago, Ill. : 1960). 1994 Feb:112(2):204-7 [PubMed PMID: 8311773]
Level 3 (low-level) evidenceEllika S, Robson CD, Heidary G, Paldino MJ. Morning glory disc anomaly: characteristic MR imaging findings. AJNR. American journal of neuroradiology. 2013 Oct:34(10):2010-4. doi: 10.3174/ajnr.A3542. Epub 2013 May 9 [PubMed PMID: 23660287]
Level 3 (low-level) evidenceHodgkins P, Lees M, Lawson J, Reardon W, Leitch J, Thorogood P, Winter RM, Taylor DS. Optic disc anomalies and frontonasal dysplasia. The British journal of ophthalmology. 1998 Mar:82(3):290-3 [PubMed PMID: 9602627]
Level 3 (low-level) evidenceLeitch RJ, Winter RM. Midline craniofacial defects and morning glory disc anomaly. A distinct clinical entity. Acta ophthalmologica Scandinavica. Supplement. 1996:(219):16-9 [PubMed PMID: 8741108]
Level 3 (low-level) evidenceWilson DC, Cunningham MJ, Reid MM, Johnston SS, Fannin TF. Meningitis and midline facial deformity. Acta paediatrica (Oslo, Norway : 1992). 1992 Jan:81(1):84-5 [PubMed PMID: 1600312]
Level 3 (low-level) evidenceHolmström G, Taylor D. Capillary haemangiomas in association with morning glory disc anomaly. Acta ophthalmologica Scandinavica. 1998 Oct:76(5):613-6 [PubMed PMID: 9826051]
Level 3 (low-level) evidenceChen CS, David D, Hanieh A. Morning glory syndrome and basal encephalocele. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2004 Feb:20(2):87-90 [PubMed PMID: 14691642]
Level 3 (low-level) evidenceCaprioli J, Lesser RL. Basal encephalocele and morning glory syndrome. The British journal of ophthalmology. 1983 Jun:67(6):349-51 [PubMed PMID: 6849854]
Level 3 (low-level) evidenceBakri SJ, Siker D, Masaryk T, Luciano MG, Traboulsi EI. Ocular malformations, moyamoya disease, and midline cranial defects: a distinct syndrome. American journal of ophthalmology. 1999 Mar:127(3):356-7 [PubMed PMID: 10088755]
Level 3 (low-level) evidenceKoenig SB, Naidich TP, Lissner G. The morning glory syndrome associated with sphenoidal encephalocele. Ophthalmology. 1982 Dec:89(12):1368-73 [PubMed PMID: 7162782]
Level 3 (low-level) evidenceRazeghinejad MR, Masoumpour M. Chiari type capital I, Ukrainian malformation associated with morning glory disc anomaly. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2006 Dec:26(4):279-81 [PubMed PMID: 17204923]
Level 3 (low-level) evidenceKomiyama M, Yasui T, Sakamoto H, Fujita K, Sato T, Ota M, Sugita M. Basal meningoencephalocele, anomaly of optic disc and panhypopituitarism in association with moyamoya disease. Pediatric neurosurgery. 2000 Aug:33(2):100-4 [PubMed PMID: 11070437]
Level 3 (low-level) evidencePierre-Filho Pde T, Limeira-Soares PH, Marcondes AM. Morning glory syndrome associated with posterior pituitary ectopia and hypopituitarism. Acta ophthalmologica Scandinavica. 2004 Feb:82(1):89-92 [PubMed PMID: 14738491]
Level 3 (low-level) evidenceMassaro M, Thorarensen O, Liu GT, Maguire AM, Zimmerman RA, Brodsky MC. Morning glory disc anomaly and moyamoya vessels. Archives of ophthalmology (Chicago, Ill. : 1960). 1998 Feb:116(2):253-4 [PubMed PMID: 9488287]
Level 3 (low-level) evidenceKrishnan C, Roy A, Traboulsi E. Morning glory disk anomaly, choroidal coloboma, and congenital constrictive malformations of the internal carotid arteries (moyamoya disease). Ophthalmic genetics. 2000 Mar:21(1):21-4 [PubMed PMID: 10779846]
Level 3 (low-level) evidenceSabti K, Hajj BA, Hwang JM, Traboulsi EI, Reid J. Congenital third nerve palsy, moyamoya disease and optic nerve head staphyloma. The British journal of ophthalmology. 2005 Jun:89(6):778-9 [PubMed PMID: 15923525]
Level 3 (low-level) evidenceLenhart PD, Lambert SR, Newman NJ, Biousse V, Atkinson DS Jr, Traboulsi EI, Hutchinson AK. Intracranial vascular anomalies in patients with morning glory disk anomaly. American journal of ophthalmology. 2006 Oct:142(4):644-50 [PubMed PMID: 17011858]
Level 2 (mid-level) evidenceSchimmenti LA. Renal coloboma syndrome. European journal of human genetics : EJHG. 2011 Dec:19(12):1207-12. doi: 10.1038/ejhg.2011.102. Epub 2011 Jun 8 [PubMed PMID: 21654726]
Level 3 (low-level) evidenceDureau P, Attie-Bitach T, Salomon R, Bettembourg O, Amiel J, Uteza Y, Dufier JL. Renal coloboma syndrome. Ophthalmology. 2001 Oct:108(10):1912-6 [PubMed PMID: 11581073]
Zhang Y, Ou H, Zhu T. Surgical treatment for the proliferative retinal detachment associated with macular hole in the morning glory syndrome. Eye science. 2013 Mar:28(1):7-10 [PubMed PMID: 24404661]
Chang S, Gregory-Roberts E, Chen R. Retinal detachment associated with optic disc colobomas and morning glory syndrome. Eye (London, England). 2012 Apr:26(4):494-500. doi: 10.1038/eye.2011.354. Epub 2012 Jan 13 [PubMed PMID: 22241012]
Level 3 (low-level) evidenceHaik BG, Greenstein SH, Smith ME, Abramson DH, Ellsworth RM. Retinal detachment in the morning glory anomaly. Ophthalmology. 1984 Dec:91(12):1638-47 [PubMed PMID: 6441134]
Level 3 (low-level) evidenceZinn KM. Bilateral complete colobomas with a unilateral optic pit and recurrent retinal detachment: a case report. The Mount Sinai journal of medicine, New York. 1979 Jul-Aug:46(4):419-23 [PubMed PMID: 314583]
Level 3 (low-level) evidenceVedantham V. Double optic discs, optic disc coloboma, and pit: spectrum of hybrid disc anomalies in a single eye. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Oct:123(10):1450-2 [PubMed PMID: 16219745]
Level 3 (low-level) evidenceLingam G, Sen AC, Lingam V, Bhende M, Padhi TR, Xinyi S. Ocular coloboma-a comprehensive review for the clinician. Eye (London, England). 2021 Aug:35(8):2086-2109. doi: 10.1038/s41433-021-01501-5. Epub 2021 Mar 21 [PubMed PMID: 33746210]
Vegunta S, Patel BC. Optic Nerve Coloboma. StatPearls. 2023 Jan:(): [PubMed PMID: 30422472]
Jeng-Miller KW, Cestari DM, Gaier ED. Congenital anomalies of the optic disc: insights from optical coherence tomography imaging. Current opinion in ophthalmology. 2017 Nov:28(6):579-586. doi: 10.1097/ICU.0000000000000425. Epub [PubMed PMID: 28817389]
Level 3 (low-level) evidenceHillman JS, Myska V, Nissim S. Complete avulsion of the optic nerve. A clinical, angiographic, and electrodiagnostic study. The British journal of ophthalmology. 1975 Sep:59(9):503-9 [PubMed PMID: 1203238]
Level 3 (low-level) evidenceTripathy K, Kumawat B, Chawla R, Sharma YR, Bypareddy R. Acute Vision Loss Due to Central Retinal Arterial Occlusion, Partial Optic Nerve Avulsion, and Hemorrhage "Spurting Out" from Optic Disc after Blunt Trauma. Journal of ophthalmic & vision research. 2017 Jul-Sep:12(3):351-352. doi: 10.4103/jovr.jovr_4_15. Epub [PubMed PMID: 28791073]
Borel A, Bonnin N, Porte C, Chiambaretta F, Bacin F. [Optic nerve trauma: case report of partial optic nerve avulsion]. Journal francais d'ophtalmologie. 2013 Apr:36(4):372-7. doi: 10.1016/j.jfo.2012.08.003. Epub 2013 Jan 29 [PubMed PMID: 23375246]
Level 3 (low-level) evidenceSadasivan KS, Pawar N, Ravindran M, Rengappa R. Optic nerve aplasia: A case series. Indian journal of ophthalmology. 2018 May:66(5):717-719. doi: 10.4103/ijo.IJO_1108_17. Epub [PubMed PMID: 29676329]
Level 2 (mid-level) evidenceGordon R, Chatfield RK. Pits in the optic disc associated with macular degeneration. The British journal of ophthalmology. 1969 Jul:53(7):481-9 [PubMed PMID: 5804034]
FERRY AP. MACULAR DETACHMENT ASSOCIATED WITH CONGENITAL PIT OF THE OPTIC NERVE HEAD. PATHOLOGIC FINDINGS IN TWO CASES SIMULATING MALIGNANT MELANOMA OF THE CHOROID. Archives of ophthalmology (Chicago, Ill. : 1960). 1963 Sep:70():346-57 [PubMed PMID: 14048795]
Level 3 (low-level) evidenceTripathy K. Spontaneous Resolution of Optic Disc Pit Maculopathy. Turkish journal of ophthalmology. 2017 Jun:47(3):184-185. doi: 10.4274/tjo.01047. Epub 2017 Jun 1 [PubMed PMID: 28630797]
Bourne RR. The optic nerve head in glaucoma. Community eye health. 2006 Sep:19(59):44-5 [PubMed PMID: 17491717]
Gottlieb JL, Prieto DM, Vander JF, Brown GC, Tasman WS. Peripapillary staphyloma. American journal of ophthalmology. 1997 Aug:124(2):249-51 [PubMed PMID: 9262555]
Level 3 (low-level) evidenceBlair MP, Blair NP, Rheinstrom SD, Jednock NJ. A case of peripapillary staphyloma. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Aug:118(8):1138-9 [PubMed PMID: 10922217]
Level 3 (low-level) evidenceAl-Zaaidi S, Al-Rashaed S, Al-Harthi E, Al-Kahtani E, Abu El-Asrar AM. Rhegmatogenous retinal detachment in children 16 years of age or younger. Clinical ophthalmology (Auckland, N.Z.). 2013:7():1001-14. doi: 10.2147/OPTH.S40056. Epub 2013 Jun 17 [PubMed PMID: 23818752]