Introduction
The auditory steady-state response (ASSR) is an objective electrophysiological test to estimate hearing thresholds. ASSR is a newer procedure than other auditory evoked potential (AEP) tests, such as auditory brainstem response (ABR); both ASSR and ABR provide similar hearing threshold results, but ASSR provides more consistent, statistically valid results in less time.[1][2]
ASSR was first described by Galambos et al in 1981, highlighting a prominent neural response to 40-Hz tonal stimuli in adults with normal hearing. Initially termed the 40-Hz event-related potential, or steady-state evoked potential, Galambos et al observed its distinctive and consistent presence near the behavioral hearing threshold, suggesting its potential as a predictor of objective hearing threshold.[3]
Subsequent studies revealed that the reliable recording of the 40-Hz response was achievable mainly in awake adults. This posed a serious limitation, as this type of objective testing is mostly needed in the pediatric population, often in a sleeping or sedated state. Further work demonstrated that this limitation could be overcome by altering the stimulus parameters.[2][4]
Currently, ASSR represents a vital addition to the audiologist's electrophysiological test battery, along with otoacoustic emissions (OAE) and ABR. The ability of the ASSR to test multiple frequencies simultaneously makes it more efficient to perform when infants are asleep. However, it is an emerging technology without widely accepted standardization of testing protocols and equipment, and its results must be interpreted with circumspection.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Detectable ASSR waveforms are attributed to the activation of various anatomic neural signal generators, each responding to distinct frequency stimuli.[2]
- Stimulation below 20 Hz elicits responses originating from the primary auditory cortex.
- Stimuli ranging from 20 to 60 Hz evoke neural responses across the primary auditory cortex, auditory midbrain, and thalamic regions.
- Stimulation exceeding 60 Hz predominantly incites responses from the superior olivary complex, inferior colliculus, and cochlear nucleus.
These diverse neural generators are responsible for the challenges Galambos et al encountered in the initial stages of ASSR research. The prominent response at 40 Hz emerged reliably in awake adults with developed neural systems; in contrast, stimulation at 70 Hz evoked brainstem responses independent of subject condition and age, comparable to the ABR.[2]
Indications
ASSR is most commonly used to measure hearing thresholds for individuals who cannot or will not cooperate with conventional behavioral or voluntary response audiometry. Thus, ASSR is especially important for measuring objective hearing thresholds in children. The ASSR can also be performed in individuals with inconsistent cooperation, such as those with cognitive or physical disabilities or inorganic hearing loss.[5][6] ASSRs can provide objective hearing thresholds in patients liable to demonstrate worse hearing thresholds with conventional audiometry testing.[7]
ASSR facilitates hearing aid fittings, especially in very young children whose hearing rehabilitation should be initiated as early as possible for reasons of speech development. In the past, the fitting of amplification in young children was often delayed. Free-field behavioral audiometry is unreliable in hearing aid fitting because it does not provide independent hearing thresholds for each ear.[8][9] The use of ABR for hearing assessment in young children can also pose challenges due to the need for multiple appointments. ABR examination often lasts upwards of 60 minutes, longer than many children's natural sleep duration; patients often wake up before the test is finished.[10]
In addition to hearing loss, recent studies have investigated the use of ASSR with 40-Hz stimuli to evaluate neural response and synchrony in fetuses and conditions such as bipolar disorder, schizophrenia, autism spectrum disorder, and Alzheimer disease.[11][12][13][14][15][16][17] Using 40-Hz stimuli evaluates activity and provides the most prominent response within the primary auditory and prefrontal cortices in response to sounds. The primary auditory and prefrontal cortices contribute to various cognitive functions impaired in neuropsychiatric and developmental disorders.[4][18]
Contraindications
ASSR is not usually performed for patients who are able and willing to participate in conventional behavioral audiometry. While there are no absolute contraindications for ASSR, children with potential hearing loss may also have comorbidities that render sedation more dangerous. For example, a 2022 study by Urfali et al revealed that patients with mucopolysaccharidosis have a higher risk of respiratory distress during sedation than healthy controls.[19]
Equipment
Interpreting the results of ASSR testing requires knowledge and understanding of specific terminology.[2] Some basic terminology includes:
- Carrier frequency (CF): The specific frequency of a tonal stimulus that generates a response in the cochlea. CF is analogous to the frequency used in pure-tone audiometry. Common CF values include 0.5, 1, 2, and 4 kHz.
- Modulation frequency (MF): The frequency at which the auditory stimulus is altered or modulated. MF is similar to the stimulus rate in other AEP tests. For instance, an MF of 100 Hz indicates that the response peaks occur every 10 msec.
- Amplitude modulation (AM): Pure tone stimuli whose amplitudes modulate over time.
- Frequency modulation (FM): Pure tone stimuli whose frequencies modulate over time.
- Mixed modulation (MM): Pure tone stimuli that change or modulate in amplitude and frequency over time.
- Fast Fourier transform (FFT): FFT is an analytical method to convert the ASSR waveform recorded, which usually consists of multiple frequencies, into individual frequency components to produce an estimated pure-tone audiogram.
- F-test: The F-test is a statistical analysis used to determine the presence of a response to auditory stimuli with a certain degree of accuracy, with the often-used p-value cut-off of <0.05.
ASSR equipment and testing methodologies continue to evolve. Newer algorithms employ a specialized "q-sample" statistical assessment, which evaluates the phase and amplitude of responses across several harmonics or multiples of the MF.[20] This methodology departs from the past practice of focusing solely on the first harmonic when neural responses may be seen at several provided stimuli, with the response amplitude diminishing as the harmonic order increases. Using this multi-harmonic technique has improved the detection rates and shortened the identification times of responses during ASSR assessments. Additionally, next-generation systems offer the advantage of testing multiple frequencies simultaneously, thereby enhancing test efficiency.[10][20]
Stimuli
ASSRs utilize single continuous or steady-state tones instead of the transient tones utilized in other AEP tests. This arrangement allows testing higher-intensity sounds up to 120 dB, thereby permitting testing of residual hearing even in patients with profound hearing loss.[1][5][8][21][22] The most commonly utilized stimuli are in the 75- to 110-Hz range; these frequencies are not affected by age and sleep state.[1][5][8] ASSR can diagnose conductive or sensorineural hearing loss by presenting stimuli delivered through air conduction or bone conduction, similar to the capabilities of pure-tone audiometry.[2]
ASSR testing employs varied stimuli, mainly AM, FM, and MM tones. AM tones are characterized by changes in their amplitude over a defined period, and these tones are the most commonly employed stimuli in auditory testing because of their frequency specificity. The extent of AM is quantified as a percentage, where a larger value, such as 90% to 100%, signifies a more substantial amplitude alteration than smaller values, such as 30% to 40%.[2]
FM tones exhibit changes only in the frequency of the stimulus during stimulation. These tones are generated by simultaneously modulating both the frequency and phase of the CF tone. For instance, with a CF of 4000 Hz and frequency modulation of 20%, the maximum and minimum frequency values deviate by ±20% from the CF, resulting in a range spanning from 3200 to 4800 Hz.[2]
MM stimuli combine aspects of both AM and FM. MM stimuli demonstrate less frequency specificity than AM stimuli but exhibit a larger response amplitude. Notably, when AM and FM are out of phase by 180 degrees, the peak of the response waves shifts toward lower frequencies, causing a reduction in response amplitude. Conversely, when AM and FM are in phase, with their peaks aligning temporally, the peak of the MM response waves shifts toward higher frequencies, resulting in an increased response amplitude. Importantly, in-phase AM and FM generate 20% higher amplitude than each modulation alone while remaining reasonably frequency-specific, making MM the preferred method of delivering stimuli for ASSR.[2]
Personnel
The interprofessional team for performing ASSR testing typically consists of the following personnel:
- Audiologist
- Otorhinolaryngologist
- Anesthesia provider
- Nurse.
Preparation
Young children typically struggle to remain still during audiology testing. Although conducting testing during natural sleep is feasible, interruptions are common. ASSR during natural sleep is most effective for children younger than 6 months of age.[10] Safe sedation options like phenobarbital-alimemazine or chloral hydrate have facilitated testing in older children.[23][24] ASSR was found reliable under total intravenous anesthesia and useful for cases requiring deeper sedation.[10] To increase the chance of successful natural sleep or sedation, parents may be advised to wake their children early on the morning of the procedure and keep them awake until the examination to deprive them of sleep.[25]
Technique or Treatment
ASSR testing procedures involve the presentation of auditory stimuli, typically in the form of tones or modulated sounds. The stimuli are relayed using either supra-aural headphones or insert headphones. Electrodes are placed on the scalp to record the electrical responses generated by the brain in response to these stimuli. Analysis of brain electrical activity permits audiologists and researchers to gather information about auditory thresholds, frequency-specific hearing abilities, and potential auditory processing disorders.[2]
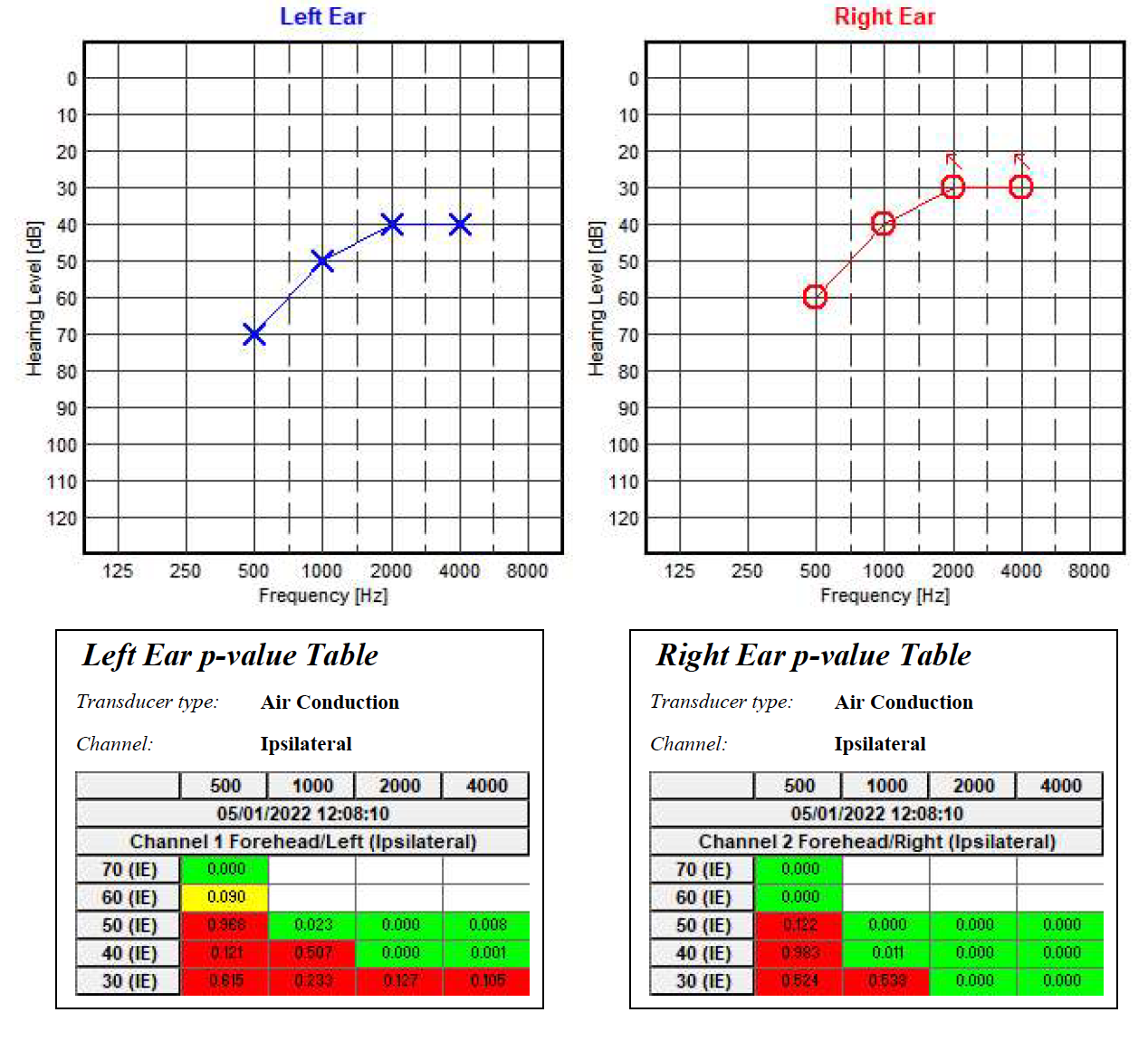
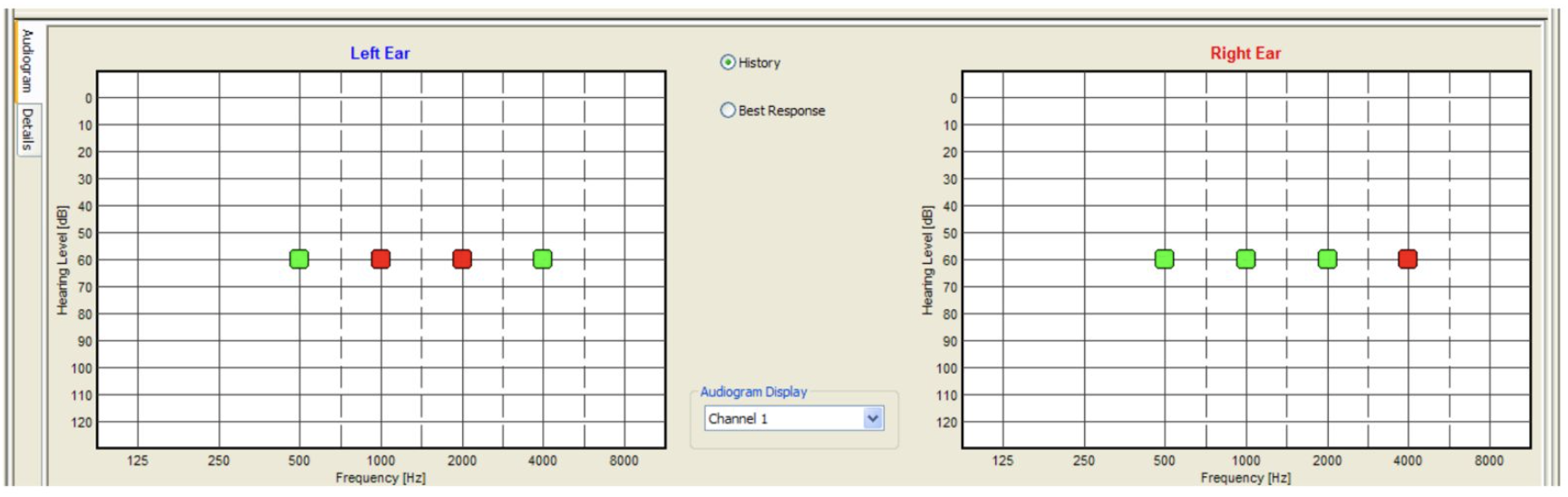
The electrode placement for ASSR mimics that of 2-channel ABR: ground on the nasion at Fpz, noninverting on the vertex at Cz, and inverting on the earlobe or second mastoid. Interelectrode impedances should be checked before beginning the test and maintained below 3 kΩ.[26] ASSR enables frequency-specific response evaluation based on the CF. The most commonly evaluated CFs are 0.5 kHz, 1 kHz, 2 kHz, and 4 kHz.[2] (see Image. Audiogram. Auditory steady-state response ongoing examination using MASTER application.)
Hearing thresholds provided from prior tests, such as non–frequency-specific ABR, can provide the starting intensity or loudness points for the ASSR test. The remainder of the testing procedure is similar to conventional audiometry, focused on finding the hearing threshold at each tested frequency.[2][26] For patients undergoing testing during natural sleep, larger steps of 20 dB up and down in intensity may be necessary to expedite the examination and prevent patients from awakening before the test is completed.
ASSR testing offers the advantage of simultaneous multifrequency binaural testing, reducing testing time compared to that required for ABR.[2] This advantage is particularly valuable for assessing children who may prematurely wake from sleep or sedation. Advanced, next-generation ASSR equipment enables individual frequency stimulus intensity adjustments while other frequencies are still being tested.[10] In traditional first-generation ASSR, stimuli at the same intensity are given for multiple frequencies to both ears, and intensity changes are made only after completing tests for all frequencies. With newer systems, individual intensity adjustments are possible before completing testing for all frequencies, resulting in a shorter overall testing duration. Multiple studies have indicated no significant differences in hearing thresholds between single-frequency and multifrequency or monaural and binaural testing.[2][10][26] The average testing time for ASSR is 20 to 25 min to obtain 8 thresholds, significantly shorter than the ABR test duration of 32 to 60 min.[10]
ASSR testing also differs from ABR when evaluating the presence of response. While ABR requires the examiners to inspect the presence of wave V visually with each stimulus, ASSR minimizes this subjectivity by using the F-test after the wave undergoes FFT analysis. This analysis is performed with each sweep, ensuring objective and unbiased data. The number of sweeps required is generally greater as the sound intensities decrease.[2]
The outcomes of ASSR testing in children are influenced by various factors, including the ambient noise levels, with higher noise associated with smaller amplitudes, recording time, with longer durations reducing residual noise in the electroencephalogram and aiding the recognition of near-threshold responses, and the signal-to-noise ratio, which compares response amplitude in ASSR to background EEG amplitude. Infants typically exhibit smaller ASSR response amplitudes than adults, necessitating noise reduction and stringent criteria for detection. The age of the infant also plays a role, as ASSR results are less reliable in younger infants and exhibit greater variability; ASSR is more effective when conducted on healthy, full-term infants older than 2 weeks of age.[2]
Complications
The complications of ASSR testing are directly due to sedation or general anesthesia and include vomiting, agitation, prolonged sedation, hypoxia, respiratory distress, respiratory obstruction, bradycardia, neurological problems, and apnea. Patient vital signs must be monitored throughout the examination if conducted under anesthesia.[25][19]
Clinical Significance
The results of ASSR testing are formatted into a graph resembling the widely recognizable and easy-to-read conventional behavioral audiogram.[8] (see Image. Audiogram. Auditory steady-state response examination using MASTER application.) However, ASSR thresholds do not always correspond exactly to those obtained via pure-tone audiometry. A 1999 study by Rance et al found differences of 0 to 20 dB in patients with normal hearing and mild hearing loss and a difference of up to 10 dB in patients with moderate or severe hearing loss.[27] Similarly, in 2016, François et al found ASSR thresholds to be typically lower than conventional behavioral thresholds, with a difference of 8 to 15 dB.[28] Compared to conventional audiometry, ASSR threshold estimates tend to be higher for individuals with little-to-mild hearing impairment than those with more severe losses. This is due to the recruitment phenomenon, reflecting abnormal increases in response amplitude with stimuli above threshold intensities, a common characteristic of AEP testing.[29][30][31][8]
Van Maanen and Stapells proposed normal ASSR thresholds for different frequencies: 50 dBHL at 500 Hz, 45 dBHL at 1000 Hz, 40 dBHL at 2000 Hz, and 40 dBHL at 4000 Hz. These thresholds provide insight into the expected hearing sensitivity of individuals when using ASSR testing. However, further research is needed to refine and expand these findings, ensuring a comprehensive understanding of ASSR thresholds across a broader range of frequencies and population groups.[32]
Various studies in auditory electrophysiology have utilized 30 dB as the threshold for normal hearing, with the upper limit of mild-to-moderate hearing loss ranging from 60 to 75 dB.[29][33][30][34] There is a 16- to 25-dB difference between bone conduction and behavioral thresholds at frequencies ranging from 500 to 4000 Hz.[2] Due to variations in hearing threshold differences between ASSR and behavioral audiometry with different equipment, it is important to use the correction factors provided by each manufacturer when estimating hearing thresholds based on ASSR.
ASSR results in higher hearing thresholds at low frequencies ≤0.5 kHz compared to high frequencies; this is attributed to various factors, including higher response amplitudes at higher frequencies, partially due to a lower EEG noise floor at these frequencies.[33][35][10] Misplaced earphones can also attenuate lower-frequency stimuli, and maturational changes can affect the transmission of low-frequency energy in infants' middle ears. Stimulation at lower frequencies affects a broader portion of the basilar membrane due to longer travel time for waves to reach the apical region of the cochlea, potentially leading to delayed jitter between receptors and stimulations. Additionally, there may be immature neural synchronization in low-frequency responses.[35][33]
The primary limitation of ASSR is its inability to diagnose auditory neuropathy spectrum disorder (ANSD), a disorder at the auditory nerve. ANSD patients demonstrate abnormal or absent ABR in the presence of intact OAE or cochlear microphonics. A 2010 study by Emara and Gabr showed that ASSR generates hearing thresholds that correlate poorly at any frequency with pure tone audiometry in ANSD patients.[36] Moreover, ASSR can detect non-neural responses, stimulus artifacts, and short-latency vestibular responses, leading to apparent near-normal thresholds in ANSD patients even when ABR recordings are abnormal.[36][37]
ASSR responses are smaller in children younger than 1 year of age than in adults, suggesting that ASSR hearing thresholds are 10 to 15 dB higher in infants. Various studies indicate that ASSR should only be used in outpatient clinics for infants older than 2 weeks of age due to ongoing maturation processes in the human auditory system during early life.[2] For neonatal assessments, the initial electrophysiological testing should include OAE and ABR.[9] Once ANSD is ruled out, subsequent diagnosis and management can be guided by combining information from both ABR and ASSR results.[37]
ASSR holds promise as an objective electrophysiological measure of hearing thresholds in infants, but because of its novelty, caution is warranted when performing the test and interpreting results. Recommendations regarding ASSR use vary. Francois et al advised performing ASSR before ABR when the goal is to determine hearing threshold rather than latency.[28] Farinetti et al reported that ASSR has not been adopted for routine practice partially due to the high cost. They also advised against using ASSR as the primary testing method in infants with an increased risk of ANSD.[38] Meanwhile, the French Society of Otorhinolaryngology advocates using ASSR in routine hearing diagnostic evaluation.[22]
Van Maanen and Stapells suggested that ASSR should not be relied on as the sole measure for assessing hearing thresholds in infants.[32] This suggestion also aligns with the cross-check principle advised for the pediatric population, wherein a battery of hearing tests is preferred to relying on a single test.[39] Thus, ASSR should be employed as one of the test battery components, accompanying other modalities, such as ABR, in evaluating hearing thresholds in children.[22] As technology advances and more comprehensive data are gathered, the role of ASSR in infant hearing assessment may become clearer, further elucidating auditory development and hearing disorders during early life.[32][40]
Enhancing Healthcare Team Outcomes
The 1-3-6 plan for pediatric hearing loss suggests that hearing loss, if present, should be identified via screening by 1 month of age, a diagnostic evaluation should be completed by 3 months of age, and intervention should be initiated by 6 months of age.[41] This is best achieved by coordination between specialists, particularly pediatricians and otorhinolaryngologists. Children diagnosed with hearing loss should be fitted for hearing amplification immediately, ideally within 1 month after diagnosis. Speech-language pathologists also play a role in rehabilitating patients with hearing-related speech development delays. Further routine follow-up for developmental milestones with pediatricians and audiological evaluation with otorhinolaryngologists are also recommended.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rabelo CM, Schochat E. Sensitivity and specificity of auditory steady-state response testing. Clinics (Sao Paulo, Brazil). 2011:66(1):87-93 [PubMed PMID: 21437442]
Korczak P, Smart J, Delgado R, Strobel TM, Bradford C. Auditory steady-state responses. Journal of the American Academy of Audiology. 2012 Mar:23(3):146-70. doi: 10.3766/jaaa.23.3.3. Epub [PubMed PMID: 22436114]
Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proceedings of the National Academy of Sciences of the United States of America. 1981 Apr:78(4):2643-7 [PubMed PMID: 6941317]
Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. International journal of audiology. 2003 Jun:42(4):177-219 [PubMed PMID: 12790346]
Szymańska A, Gryczyński M, Pajor A. [Auditory steady-state responses--the state of art]. Otolaryngologia polska = The Polish otolaryngology. 2010 Sep-Oct:64(5):274-80. doi: 10.1016/S0030-6657(10)70606-5. Epub [PubMed PMID: 21166136]
Resende LM, Carvalho SA, Dos Santos TS, Abdo FI, Romão M, Ferreira MC, Tierra-Criollo CJ. Auditory steady-state responses in school-aged children: a pilot study. Journal of neuroengineering and rehabilitation. 2015 Feb 10:12(1):13. doi: 10.1186/s12984-015-0003-y. Epub 2015 Feb 10 [PubMed PMID: 25884712]
Level 3 (low-level) evidenceLin YH, Chen PR, Hsu CJ, Wu HP. Validation of multi-channel auditory steady-state response in adults with sensorineural hearing loss. The Journal of laryngology and otology. 2009 Jan:123(1):38-44. doi: 10.1017/S0022215108002351. Epub 2008 May 1 [PubMed PMID: 18452631]
Level 1 (high-level) evidenceCanale A, Lacilla M, Cavalot AL, Albera R. Auditory steady-state responses and clinical applications. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2006 Jun:263(6):499-503 [PubMed PMID: 16557415]
Aimoni C, Crema L, Savini S, Negossi L, Rosignoli M, Sacchetto L, Bianchini C, Ciorba A. Hearing threshold estimation by auditory steady state responses (ASSR) in children. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2018 Aug:38(4):361-368. doi: 10.14639/0392-100X-1463. Epub [PubMed PMID: 30197427]
Sininger YS, Hunter LL, Hayes D, Roush PA, Uhler KM. Evaluation of Speed and Accuracy of Next-Generation Auditory Steady State Response and Auditory Brainstem Response Audiometry in Children With Normal Hearing and Hearing Loss. Ear and hearing. 2018 Nov/Dec:39(6):1207-1223. doi: 10.1097/AUD.0000000000000580. Epub [PubMed PMID: 29624540]
Xiao W, Manyi G, Khaleghi A. Deficits in auditory and visual steady-state responses in adolescents with bipolar disorder. Journal of psychiatric research. 2022 Jul:151():368-376. doi: 10.1016/j.jpsychires.2022.04.041. Epub 2022 May 2 [PubMed PMID: 35551068]
Niepel D, Krishna B, Siegel ER, Draganova R, Preissl H, Govindan RB, Eswaran H. A pilot study: Auditory steady-state responses (ASSR) can be measured in human fetuses using fetal magnetoencephalography (fMEG). PloS one. 2020:15(7):e0235310. doi: 10.1371/journal.pone.0235310. Epub 2020 Jul 22 [PubMed PMID: 32697776]
Level 3 (low-level) evidenceThuné H, Recasens M, Uhlhaas PJ. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA psychiatry. 2016 Nov 1:73(11):1145-1153. doi: 10.1001/jamapsychiatry.2016.2619. Epub [PubMed PMID: 27732692]
Level 1 (high-level) evidenceRoach BJ, Hirano Y, Ford JM, Spencer KM, Mathalon DH. Phase Delay of the 40 Hz Auditory Steady-State Response Localizes to Left Auditory Cortex in Schizophrenia. Clinical EEG and neuroscience. 2023 Jul:54(4):370-378. doi: 10.1177/15500594221130896. Epub 2022 Oct 10 [PubMed PMID: 36213937]
O'Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. Supplements to Clinical neurophysiology. 2013:62():101-12 [PubMed PMID: 24053034]
Level 3 (low-level) evidenceArutiunian V, Arcara G, Buyanova I, Davydova E, Pereverzeva D, Sorokin A, Tyushkevich S, Mamokhina U, Danilina K, Dragoy O. Neuromagnetic 40 Hz Auditory Steady-State Response in the left auditory cortex is related to language comprehension in children with Autism Spectrum Disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2023 Mar 2:122():110690. doi: 10.1016/j.pnpbp.2022.110690. Epub 2022 Dec 5 [PubMed PMID: 36470421]
Osipova D, Pekkonen E, Ahveninen J. Enhanced magnetic auditory steady-state response in early Alzheimer's disease. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006 Sep:117(9):1990-5 [PubMed PMID: 16887381]
Parciauskaite V, Bjekic J, Griskova-Bulanova I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain sciences. 2021 Feb 10:11(2):. doi: 10.3390/brainsci11020217. Epub 2021 Feb 10 [PubMed PMID: 33579014]
Level 1 (high-level) evidenceUrfali S, Urfali B, Sarac ET, Koyuncu O. Safety and Complications of Sedation Anesthesia during Pediatric Auditory Brainstem Response Testing. ORL; journal for oto-rhino-laryngology and its related specialties. 2022:84(3):188-192. doi: 10.1159/000517156. Epub 2021 Jul 12 [PubMed PMID: 34252904]
Cebulla M, Stürzebecher E, Elberling C. Objective detection of auditory steady-state responses: comparison of one-sample and q-sample tests. Journal of the American Academy of Audiology. 2006 Feb:17(2):93-103 [PubMed PMID: 16640063]
Level 2 (mid-level) evidenceKandogan T, Dalgic A. Reliability of Auditory Steady-State Response (ASSR): Comparing Thresholds of Auditory Steady-State Response (ASSR) with Auditory Brainstem Response (ABR) in Children with Severe Hearing Loss. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2013 Dec:65(Suppl 3):604-7. doi: 10.1007/s12070-012-0581-y. Epub 2012 Oct 25 [PubMed PMID: 24427722]
Favier V, Vincent C, Bizaguet É, Bouccara D, Dauman R, Frachet B, Le Her F, Meyer-Bisch C, Tronche S, Sterkers-Artières F, Venail F. French Society of ENT (SFORL) guidelines (short version): Audiometry in adults and children. European annals of otorhinolaryngology, head and neck diseases. 2018 Oct:135(5):341-347. doi: 10.1016/j.anorl.2018.05.009. Epub 2018 Jun 19 [PubMed PMID: 29929777]
François M, Teissier N, Barthod G, Nasra Y. Sedation for children 2 to 5 years of age undergoing auditory brainstem response and auditory steady state responses recordings. International journal of audiology. 2012 Apr:51(4):282-6. doi: 10.3109/14992027.2011.601469. Epub 2011 Sep 22 [PubMed PMID: 21936745]
Avlonitou E, Balatsouras DG, Margaritis E, Giannakopoulos P, Douniadakis D, Tsakanikos M. Use of chloral hydrate as a sedative for auditory brainstem response testing in a pediatric population. International journal of pediatric otorhinolaryngology. 2011 Jun:75(6):760-3. doi: 10.1016/j.ijporl.2011.02.010. Epub 2011 Apr 29 [PubMed PMID: 21531030]
Level 2 (mid-level) evidenceNecula V, Stamate MC, Blebea C, Cozma S. Safety and effectiveness of chloral hydrate in outpatient paediatric sedation for objective hearing tests. International journal of pediatric otorhinolaryngology. 2019 Nov:126():109605. doi: 10.1016/j.ijporl.2019.109605. Epub 2019 Jul 26 [PubMed PMID: 31369972]
Sininger YS, Hunter LL, Roush PA, Windmill S, Hayes D, Uhler KM. Protocol for Rapid, Accurate, Electrophysiologic, Auditory Assessment of Infants and Toddlers. Journal of the American Academy of Audiology. 2020 Jun:31(6):455-468. doi: 10.3766/jaaa.19046. Epub 2020 Aug 3 [PubMed PMID: 31870467]
Rance G, Beer DE, Cone-Wesson B, Shepherd RK, Dowell RC, King AM, Rickards FW, Clark GM. Clinical findings for a group of infants and young children with auditory neuropathy. Ear and hearing. 1999 Jun:20(3):238-52 [PubMed PMID: 10386850]
Level 3 (low-level) evidenceFrançois M, Dehan E, Carlevan M, Dumont H. Use of auditory steady-state responses in children and comparison with other electrophysiological and behavioral tests. European annals of otorhinolaryngology, head and neck diseases. 2016 Nov:133(5):331-335. doi: 10.1016/j.anorl.2016.07.008. Epub 2016 Aug 5 [PubMed PMID: 27502823]
Wang X, Cheng Y, Shi J, Sheng X, Wu D, Zhao Y, Li D, He D, Wang H. Comparison of auditory steady-state response and click-evoked auditory brain response in infants with different types and degrees of hearing loss. Acta oto-laryngologica. 2020 Feb:140(2):116-121. doi: 10.1080/00016489.2019.1697463. Epub 2019 Dec 11 [PubMed PMID: 31825723]
Swanepoel D, Ebrahim S. Auditory steady-state response and auditory brainstem response thresholds in children. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009 Feb:266(2):213-9. doi: 10.1007/s00405-008-0738-1. Epub 2008 Jun 17 [PubMed PMID: 18560866]
Level 2 (mid-level) evidenceChen M, Wei Y, Wang X, Liu L, Liu M, Jiang G, Wei F. Assessing Agreement between Frequency-Specific Chirp Auditory Steady-State Response and Pure Tone Audiometry in Adults by Intraclass Correlation Coefficient. ORL; journal for oto-rhino-laryngology and its related specialties. 2022:84(1):30-38. doi: 10.1159/000515237. Epub 2021 May 12 [PubMed PMID: 33979798]
Van Maanen A, Stapells DR. Normal multiple auditory steady-state response thresholds to air-conducted stimuli in infants. Journal of the American Academy of Audiology. 2009 Mar:20(3):196-207 [PubMed PMID: 19927690]
Çelik O, Eskiizmir G, Uz U. A Comparison of Thresholds of Auditory Steady-State Response and Auditory Brainstem Response in Healthy Term Babies. The journal of international advanced otology. 2016 Dec:12(3):277-281. doi: 10.5152/iao.2016.2397. Epub 2016 Nov 28 [PubMed PMID: 27897125]
McCreery RW, Kaminski J, Beauchaine K, Lenzen N, Simms K, Gorga MP. The impact of degree of hearing loss on auditory brainstem response predictions of behavioral thresholds. Ear and hearing. 2015 May-Jun:36(3):309-19. doi: 10.1097/AUD.0000000000000120. Epub [PubMed PMID: 25470369]
Level 2 (mid-level) evidenceLee HS, Ahn JH, Chung JW, Yoon TH, Lee KS. Clinical comparison of the auditory steady-state response with the click auditory brainstem response in infants. Clinical and experimental otorhinolaryngology. 2008 Dec:1(4):184-8. doi: 10.3342/ceo.2008.1.4.184. Epub 2008 Dec 26 [PubMed PMID: 19434265]
Emara AA, Gabr TA. Auditory steady state response in auditory neuropathy. The Journal of laryngology and otology. 2010 Sep:124(9):950-6. doi: 10.1017/S0022215110000630. Epub 2010 Apr 14 [PubMed PMID: 20388244]
Level 2 (mid-level) evidenceJafari Z, Malayeri S, Ashayeri H, Farahani MA. Adults with auditory neuropathy: comparison of auditory steady-state response and pure-tone audiometry. Journal of the American Academy of Audiology. 2009 Nov-Dec:20(10):621-8 [PubMed PMID: 20503800]
Level 1 (high-level) evidenceFarinetti A, Raji A, Wu H, Wanna B, Vincent C. International consensus (ICON) on audiological assessment of hearing loss in children. European annals of otorhinolaryngology, head and neck diseases. 2018 Feb:135(1S):S41-S48. doi: 10.1016/j.anorl.2017.12.008. Epub 2018 Feb 1 [PubMed PMID: 29366866]
Level 3 (low-level) evidenceJerger JF, Hayes D. The cross-check principle in pediatric audiometry. Archives of otolaryngology (Chicago, Ill. : 1960). 1976 Oct:102(10):614-20 [PubMed PMID: 971134]
Level 3 (low-level) evidenceRodrigues GR, Lewis DR, Fichino SN. Steady-state auditory evoked responses in audiological diagnosis in children: a comparison with brainstem evoked auditory responses. Brazilian journal of otorhinolaryngology. 2010 Jan-Feb:76(1):96-101 [PubMed PMID: 20339696]
Level 2 (mid-level) evidenceAwad R, Oropeza J, Uhler KM. Meeting the Joint Committee on Infant Hearing Standards in a Large Metropolitan Children's Hospital: Barriers and Next Steps. American journal of audiology. 2019 Jun 10:28(2):251-259. doi: 10.1044/2019_AJA-18-0001. Epub 2019 May 14 [PubMed PMID: 31084570]